Abstract
Institutional delivery has been proposed as a method for reducing maternal morbidity and mortality, but little is known about how referral hospitals in low-resource settings can best manage the expected influx of patients. In this study, we assess the impact of an obstetric triage improvement programme on reducing hospital-based delay in a referral hospital in Accra, Ghana. An Active Implementation Framework is used to describe a 5-year intervention to introduce and monitor obstetric triage capabilities. Baseline data, collected from September to November 2012, revealed significant delays in patient assessment on arrival. A triage training course and monitoring of quality improvement tools occurred in 2013 and 2014. Implementation barriers led to the construction of a free-standing obstetric triage pavilion, opened January 2015, with dedicated midwives. Data were collected at three time intervals following the triage pavilion opening and compared with baseline including: referral indications, patient and labour characteristics, waiting time from arrival to assessment and the documentation of a care plan. An obstetric triage improvement programme reduced the median (IQR) patient waiting time from facility arrival to first assessment by a midwife from 40 min (15–100) to 5 min (2–6) (p<0.001) over the 5-year intervention. The triage pavilion enhanced performance resulting in the elimination of previous delays associated with the time of admission and disease acuity. Care plan documentation increased from 51% to 96%. Obstetric triage, when properly implemented, reduced delay in a busy, low-resource hospital. The implementation process was sustained under local leadership during transition to a new hospital.
Keywords: maternal health, obstetrics, cohort study
Summary box.
What is already known about the topic?
There has been a shift towards facility-based obstetric care in low-resource countries, yet many hospitals are unprepared to receive additional patients.
Delay of receiving care within hospitals, also known as ‘the third delay’, results in preventable maternal and newborn mortality worldwide.
There are no descriptions of obstetric triage systems in low-resource countries and little is known about how long patients wait for care.
What are the new findings?
An implementation science approach can be used to turn innovative ideas into sustained, practical improvements in healthcare.
An obstetric triage improvement programme was successfully established at a large, referral hospital in Accra, Ghana, and the results have been sustained.
Risk acuity systems can be applied and patient waiting times reduced in low-resource referral hospitals when triage principles are correctly employed.
Other interventions aiming to minimise the third delay should be evaluated and scaled up if found to be effective.
How might this influence practice?
Other low-resource hospitals can identify patients at risk for complications and reduce delay when obstetric triage principles are applied.
Partnerships in the global health community should be encouraged to achieve novel and sustained improvements in healthcare.
Introduction
In 2010, the United Nations stated that ‘we know what works’ to provide adequate healthcare to prevent maternal mortality.1 However, approximately 300 000 women, predominantly in low-income and middle-income countries (LMIC), died last year due to pregnancy-related complications.2 The lifetime risk of maternal death has fallen from 1 in 16 to 1 in 36 pregnancies in sub-Saharan Africa (SSA), but these women still face risks of death 500–1000 times higher than women in high-income countries.2 This disparity continues due to failure to implement what we know works into clinical practice.
During the Millennium Development Goal era, access to skilled birth attendance improved. Greater recognition of complicated pregnancies, however, shifts the burden of care to facilities that may be unprepared to provide the signal functions of comprehensive emergency obstetric care (CEmOC). It is estimated that at least 15% of births will require transfer of care to CEmOC facilities.3 4 The actions taken by these facilities to quickly assess and treat already compromised women are critical to improving survival. Existing reports on delay within facilities, termed ‘the third delay’ suggest that women who need CEmOC often experience long treatment delays after reaching LMIC health facilities.5–9 Yet, there are inconsistent definitions of what constitutes delay, differing study methodologies and little evidence on the effect of delay on birth outcomes.9 One review states, ‘the third delay is likely to be a source of considerable inequity in access to emergency obstetric care in developing countries’.5
Ghana has experienced significant increases in institutional deliveries over the past decade without sufficiently addressing the quality of care.10 Consequently, mortality rates in regional hospitals are significantly higher than the national average.7 11–15 Resources required to treat high-risk patients are often lacking.9 16 Regional hospitals often have lower staff-to-patient ratios and higher acuity patients.17 An increasing work load has been found to be detrimental to morale, attentiveness and outcomes.18 Obstetric triage practices are unknown and many hospitals appear to operate on a first-come, first-served basis irrespective of patient risk. International guidelines recommend that assessment begin within 10 min of patient arrival to the hospital, to stratify care based on risk and imminence of delivery.19 Developing strategies to strengthen the initial assessment process is one step towards improving healthcare quality in high-volume obstetric facilities in low-resource countries, yet there are little published data in this context. In this report, we describe the impact of an obstetric triage improvement programme (TIP) implemented at The Greater Accra Regional Hospital (GARH), a major referral hospital in Accra, Ghana.
Setting
A 5-year quality improvement (QI) intervention (2007–2011) at GARH was conducted between Kybele, a non-governmental organisation (NGO) (www.kybeleworldwide.org) and the Ghana Health Service (GHS). The project focused on an integrated systems strengthening approach that improved leadership, work processes and clinical protocols leading to significant, cost-effective reductions in maternal mortality.16 17 20 An emerging priority was to address delay occurring due to the growing number of obstetric transfers from other facilities accounting for approximately 70% of deliveries at GARH.17
Annual deliveries had rapidly increased from 4000 in 2006 to over 11 000 in 2012, and the process of receiving, assessing and treating high-risk women became overwhelming for the labour ward staff, typically consisting of 3–4 midwives caring for 10 delivery beds. As women arrived, they waited in queue on a bench along a corridor. When a midwife became available, women were evaluated in a small adjacent ‘triage’ room which was poorly equipped and only accommodated one patient. Patients frequently waited long periods of time, often without prioritisation based on acuity. Conditions such as eclamptic seizures, antepartum haemorrhage or imminent delivery would advance a woman through the queue, but these events could not be anticipated or prevented.
Implementation
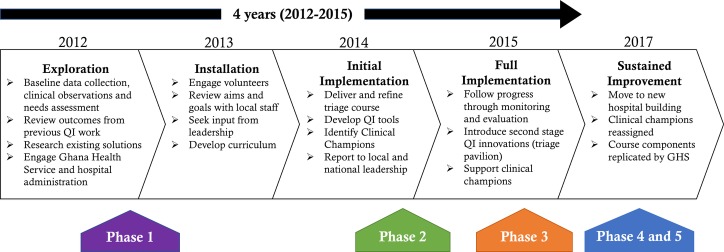
Systematic implementation approaches help to close gaps between innovations and their sustainable adaptation into standard practice.21 Researchers in implementation science, which is defined as the study of methods to promote the integration of research findings and evidence into healthcare policy and practice, have developed a number of frameworks to inform the implementation process.22 Active Implementation Frameworks, developed by the National Implementation Research Network, recommend that implementation takes place in four overlapping stages: exploration, installation, initial implementation and full implementation.23 24 We use this framework to describe the successful implementation of an obstetric TIP conducted at GARH from 2012 to 2015 (figure 1).24
Figure 1.
Active Implementation Framework for an obstetric triage improvement programme, adapted with permission from Metz.20
Exploration
The exploration stage involves preparatory activities for implementation, such as understanding the local context and creating or adapting an innovation for local fit. Stakeholder involvement is critical at this stage. From January to June 2012, the Kybele team consisting of experienced clinicians (obstetricians, midwives, obstetric anaesthesiologists and nurses from the USA and UK) made observations of clinical practice and referral patterns at GARH during three 10–14 day visits. A needs assessment was conducted with the midwifery staff and senior doctors to identify deficiencies in skills that they felt needed to be addressed.25 Clinical observations noted poor compliance to the hospitals original protocols, no consideration of patient waiting time and time consuming, narrative charting. This evaluation has been described and serves as the baseline for this study.8
Installation
This stage involves the acquisition of resources to support the implementation of the innovation. The GARH senior leadership ensured that training facilities and staff would be available. The training team reviewed several well-established short courses for CEmOC that have been adapted for LMICs, but instruction about triage was not found. The triage training course was developed by the Kybele trainers who were clinical content experts, entrenched in the local environment along with significant local engagement.25 The course uniquely focused on the obstetric triage process including the principles of triage, the role of the midwife, professional accountability, local and international statistics, clinical management of high-risk conditions, hands-on exercises and QI principles.25
Initial implementation
Once an innovation is implemented, process data are used to monitor implementation barriers and evaluate adaptation for local fit. The didactic portion of the obstetric triage course was taught at GARH over eight sessions with approximately eight participants each between January 2013 and September 2014. Two clinical champions were selected to be the facilitators of triage implementation. The clinical champions were frontline midwives chosen during the initial training due to their enthusiasm and quick grasp of the triage concepts. They were provided additional training in leadership and QI, after which they led the monitoring and evaluation activities. The last course was delivered by the GARH triage clinical champions, with support from the Kybele trainers.
The triage course resulted in three jointly developed QI interventions. First, the ‘triage’ room at the labour ward entrance was outfitted with monitors, supplies for primary assessment and initial treatment. Second, red, yellow and green patient wristbands were introduced to identify women as high, intermediate or low risk based on the triage assessment.25 The bands were worn for the duration of the hospital stay and alerted staff of a woman’s acuity. Third, a triage assessment form was created as a time-saving tool that guided midwives towards making a diagnosis and treatment plan.25 Although just a simple form, the process of identifying risk and creating a plan, empowered midwives to take responsibility for making an initial diagnosis and management plan since doctors were often unavailable. Actions such as writing the assessor’s name on the form, increased accountability in accordance with the course content. It was hypothesised that having a better equipped room to assess patients, a way of quickly distinguishing high-risk patients and a rubric for developing an initial treatment plan would optimise the triage process and reduce the wait time for evaluation and treatment.
Using data to assess implementation and drive decision-making is a hallmark of initial implementation. This involves the use of Plan-Do-Study-Act cycles to iteratively improve implementation. Clinical champions were trained in a QI approach based on the Institute for Healthcare Improvement’s ‘Model for Improvement’.26 Ward managers tracked wristband usage at the beginning of each shift and the clinical champions conducted weekly random chart reviews to determine whether the bands were correctly applied.25 Additionally, they were sponsored on a 2-week visit to England to observe obstetric triage practices in action.
Full implementation
Full implementation involves integrating the innovation into practice and creating the conditions for sustainability. During the initial implementation, process monitoring identified that the proximity of the triage room to the labour ward made it too easy for the midwife to be pulled away from triage duties to attend to problems on the labour ward. In addition, the hallway and single, small examination room were inadequate for managing the large number of incoming patients. The GARH administration and the Ghanaian Ministry of Health decided to fund the construction of a ‘triage pavilion’, which was a locally led QI innovation. A simple, free-standing metal building was built behind the hospital just outside the labour ward. It provided an additional four beds for dedicated midwives to assess and stabilise women on arrival and provided a place for first-line treatment including oxygen tanks, magnesium sulfate and antihypertensive medications. A triage protocol booklet and wall posters were created based on common conditions encountered and treatments.
Sustained implementation
Significant changes occurred for the hospital in May 2017. Staff moved into a new hospital building with a different physical layout and the triage pavilion was dismantled. This occurred without the assistance of or reinforcement from Kybele. In addition, the original clinical champions were relocated and no longer provide patient care at GARH. The triage concepts became embedded in the system and were not dependent on physical space, external or local trainers.
The triage training course has been adapted for smaller hospitals and training is being conducted across Ghana. The GHS and the United States Agency for International Development Systems for Health have trained over 4000 providers in 50 hospitals since inception. The clinical champions served as technical advisors and Kybele was uninvolved.
Outcomes
Data were collected on obstetric patient admissions in five phases over 5 years: Phase 1 (9 September–11 November 2012) represented the baseline period prior to triage training.8 Phase 2 (15–31 December 2014) followed training but was prior to moving to the triage pavilion. Phase 3 (15 September–19 November 2015) and Phase 4 (1 December 2016–28 February 2017) represented time frames during the pavilion utilisation and Phase 5 (1 September–31 October 2017) followed moving to the new hospital. Data included patient and pregnancy characteristics, referral information and the waiting time, in minutes, from arrival to first assessment by a midwife. Sample sizes for time interval data for each phase were 926, 162, 770, 869 and 542, respectively, which represented 69%, 40%, 53%, 55% and 54% of patients admitted. For Phases 2–5, patient arrival times were recorded by an attendant at the admission desk and assessment times were recorded by the examining midwife on the triage assessment form. For Phases 1, 4 and 5, paid data collectors assisted in additional data acquisition from patient chart review. Data were collected during similar months representing an intermediate patient volume to reduce the potential influence of seasonal variation during low and peak periods. Waiting times were non-parametrically distributed and, therefore, represented as median (IQR). Differences across phases were compared with the Kruskal-Wallis test and median regression was used to assess differences for each phase. Data were analysed using Stata V.14.1.
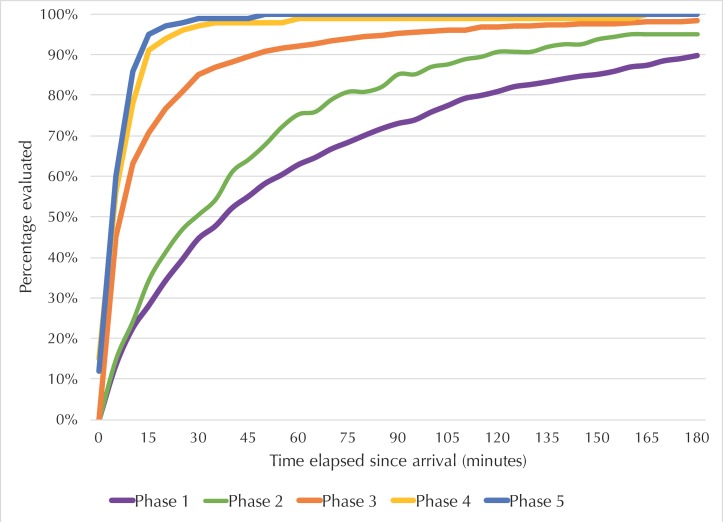
Patient demographic data (table 1) and reasons for referral (table 2) are similar across phases. The median (IQR) waiting time from hospital arrival to first evaluation by a midwife decreased from 40 min (15–100)8 to 5 min (2–6) (p<0.001) over the course of the analysis. With Phase 1 as the reference,8 Phase 2 achieved an 11 min reduction (p<0.001) and Phase 3, a 33 min reduction (p=0.002) in waiting time (table 1). Improvements were sustained during Phase 4 and Phase 5 with 35 min reductions (p<0.001) in waiting time compared with baseline (table 1). In Phase 1, 45% (414/926) of women were evaluated in less than 30 min; the hospital’s target time for patient assessment. This increased to 51% (82/162) in Phase 2, 85% (656/770) in Phase 3, 97% (843/869) in Phase 4 and 99% (535/542) in Phase 5 (p<0.001) (figure 2). In addition, the documentation of a treatment plan by the midwives as part of their triage assessment increased from 51% (552/1082) in Phase 1 to 96% (751/784) in Phase 3 (p<0.001).
Table 1.
Patient demographics and waiting time from arrival to assessment by phase
| Variable | Phase 1 | Phase 2 | Phase 3 | Phase 4 | Phase 5 |
| Dates of collection | 9 September– 11 November 2012 |
15 December– 31 December 2014 |
15 September– 19 November 2015 |
1 December 2016– 28 February 2017 |
1 September– 31 October 2017 |
| Number of complete time interval observations | 926 | 162 | 770 | 869 | 542 |
| Number of hospital deliveries | 1351 | 405 | 1465 | 1589 | 1008 |
| Age (years) | 28.1 (5.7) | 30.0 (5.8) | 28.2 (5.8) | 28.8 (7.4) | 30.0 (5.4) |
| Estimated GA (weeks) | 39+1 (3.5) | 39+5 (2.4) | 39+2 (2.4) | 37+5 (4.3) | 37.2 (4.9) |
| Gravida | 2.6 (1.6) | 3.1 (1.7) | 2.9 (1.8) | 2.9 (1.9) | 3.0 (1.9) |
| Para | 1.4 (1.5) | 1.2 (1.6) | 1.3 (1.4) | 1.3 (1.5) | 1.6 (2.3) |
| Median (IQR) waiting time (min)* | 40 (15,100) | 29 (11,60) | 7 (2,19) | 5 (2,8) | 5 (2,6) |
| Minimum (min) | 0 | 0 | 0 | 0 | 0 |
| Maximum (min) | 1545 | 530 | 790 | 1107 | 236 |
Data for age, gestational age, gravida and parity are presented as mean (SD).
Phase 1 included 1082 patients evaluated from 9 September to 11 November 2012.8
Phase 2 included 162 patients evaluated from 15 December to 31 December 2014.
Phase 3 included 784 patients evaluated from 15 September to 19 November 2015.
Phase 4 included 901 patients evaluated from 1 December 2016 to 28 February 2017.
Phase 5 included 552 patients evaluated from 1 September to 31 October 2017.
GA, gestational age.
Table 2.
Reason for referral by phase
| Reason | Phase 1 | Phase 3 | Phase 4 | Phase 5 |
| Dates of collection | 9 September 2012–11 November 2012 | 15 September 2015–19 November 2015 | 1 December 2016–28 February 2017 | 1 September– 31 October 2017 |
| Number of hospital deliveries | 1351 | 1465 | 1589 | 1008 |
| Fetal pelvic disproportion* | 346 (24.3) | 193 (21.0) | 276 (24.3) | 181 (26.4) |
| Hypertensive disorder† | 139 (9.8) | 77 (8.4) | 91 (8.0) | 86 (12.6) |
| Prior uterine scar‡ | 129 (9.1) | 54 (5.9) | 114 (10.0) | 80 (11.7) |
| Maternal miscellaneous§ | 115 (8.1) | 60 (6.5) | 100 (8.9) | 53 (7.7) |
| Anemia¶ | 103 (7.2) | 40 (4.4) | 64 (5.7) | 38 (5.5) |
| Self-referral/no indication | 92 (6.5) | 205 (22.3) | 29 (2.6) | 42 (6.1) |
| Fetal distress** | 69 (4.8) | 47 (5.1) | 49 (4.3) | 37 (5.4) |
| Fetal malpresentation†† | 62 (4.4) | 44 (4.8) | 65 (5.7) | 24 (3.5) |
| Rupture of membranes‡‡ | 54 (3.8) | 42 (4.6) | 44 (3.9) | 21 (3.1) |
| Labour | 45 (3.2) | 30 (3.3) | 71 (6.2) | 36 (5.3) |
| Lack of resources§§ | 43 (3.0) | 14 (1.5) | 17 (1.5) | 7 (1.0) |
| Infectious causes¶¶ | 39 (2.7) | 2 (0.2) | 12 (1.1) | 3 (0.4) |
| Obstetric hemorrhage*** | 39 (2.7) | 9 (1.0) | 33 (3.9) | 12 (1.8) |
| Prematurity††† | 29 (2.0) | 29 (3.2) | 67 (5.9) | 25 (3.6) |
| Poor obstetric history‡‡‡ | 27 (1.9) | 20 (2.2) | 11 (1.0) | 4 (0.6) |
| Multiple gestation§§§ | 26 (1.8) | 14 (1.5) | 28 (2.5) | 11 (1.6) |
| Record illegible | 22 (1.5) | 4 (0.4) | 6 (0.5) | 0 (0) |
| Age <16 or >35 | 18 (1.3) | 8 (1.0) | 21 (1.8) | 5 (0.7) |
| Fetal demise | 14 (1.0) | 7 (1.0) | 16 (1.4) | 3 (0.4) |
| Poor/non-attendant | 12 (0.8) | 5 (0.5) | 3 (0.3) | 0 (0) |
| Fetal miscellaneous¶¶¶ | 2 (0.1) | 0 (0) | 19 (1.7) | 4 (0.6) |
| Uterine rupture | 0 (0) | 1 (0.1) | 0 (0) | 2 (0.3) |
| Total | 1425(100) | 946(100) | 1136(100) | 685(100) |
| One referral indication | 739 (68.3) | 782 (82.7) | 897 (80.0) | 551 (80.3) |
| Two referral indications | 315 (29.1) | 152 (16.0) | 207 (18.2) | 134 (19.6) |
| Three referral indications | 28 (2.6) | 12 (1.3) | 32 (2.8) | 3 (0.4) |
Data are shown as number (%) of responses for each reason referred.
Phase 1 included 1082 patients evaluated from 9 September to 11 November 2012.8
Phase 3 included 784 patients evaluated from 15 September to 19 November 2015.
Phase 4 included 901 patients evaluated from 1 December 2016 to 28 February 2017.
Phase 5 included 552 patients evaluated from 1 September to 31 October 2017.
*Cephalopelvic disproportion, fetal macrosomia, large maternal abdomen, post-term pregnancy, over 40 weeks estimated gestational age, borderline pelvis, contracted pelvis, failure to progress (delayed or prolonged labour, arrest of labour, slow progress, failed induction, unfavourable cervix, high head in labour, obstructed labour).
†Chronic hypertension, pregnancy-induced hypertension, pre-eclampsia, severe pre-eclampsia or eclampsia.
‡Previous caesarean delivery, prior myomectomy or previous uterine rupture.
§Maternal asthma, diabetes, gestational diabetes, prior abdominal surgery, uterine fibroids, vaginal/vulvar growth or discharge, proteinuria, urinary tract infection, fever, generalised oedema, short/long pregnancy interval, short maternal stature, maternal distress, sterilisation request, grand multiparty, seizure disorder, mental illness, obesity, patient refusal for care, patient lacks laboratory or scan information, crippled, rhesus negative.
¶Maternal anaemia or sickle cell disease.
**Abnormal cardiotocography, fetal tachycardia, fetal distress, oligohydramnios, meconium stained amniotic fluid, decreased fetal movement, intrauterine growth restricition, umbilical cord prolapse.
††Face/mentum posterior, brow, breech/footling breech, oblique, transverse, unstable lie, arm prolapse, leading twin breech, compound presentation.
‡‡Rupture of membranes, prolonged rupture of membranes, losing liquor, gestations >37 weeks.
§§No electricity, no bed, no gloves, no water, no doctor, no anaesthetist.
¶¶Hepatitis B, malaria, syphilis, HIV.
***Placenta previa, placental abruption, placenta accreta, antepartum, intrapartum and postpartum bleeding, unclassified haemorrhage.
†††Gestation <37 weeks, prematurity, preterm labour or preterm premature rupture of membranes.
‡‡‡Bad obstetric history, prior stillbirth, prior ectopic pregnancy, unexplained history of intrauterine fetal death, previous failure to progress, prior cervical cerclage, previous peripartum haemorrhage.
§§§Twin pregnancy, triplet pregnancy.
¶¶¶Anencephaly, severe hydrocephalus, polyhydramnios, fetal deformity.
Figure 2.
Obstetric patient waiting time from arrival to assessment at the Greater Accra Regional Hospital between 2012 and 2017. Phase 1 included 1082 patients evaluated from 9 September to 11 November 2012.8 Phase 2 included 162 patients evaluated from 15 December to 31 December 2014. Phase 3 included 784 patients evaluated from 15 September to 19 November 2015. Phase 4 included 901 patients evaluated from 1 December 2016 to 28 February 2017. Phase 5 included 552 patients evaluated from 1 September to 31 October 2017. *Complete time interval data (arrival and assessment times) were available for 926, 162, 770, 869 and 542 patients in Phases 1–5, respectively.
Performance was analysed based on work shift, day of the week, daily patient volume and the presence of a time-sensitive condition as a reason for referral. Time-sensitive conditions were defined as signs of sepsis, obstetric haemorrhage, hypertensive disorders of pregnancy or fetal distress. In Phase 1, the median wait time during the night shift was 55 min (15–120) compared with 35 min (10–83) in the morning shift and 28 min (12–51) in the evening shift (p<0.01).8 In Phase 3 onwards, this difference was eliminated with wait times of 8 (3–20), 6 (2–16) and 8 (3–21) min during the morning, evening and night shifts, respectively (p=0.104) in Phase 3 and 5 (2–7), 5 (3–5), 4 (1–7.5) min in Phase 5 (p=0.54). There was no difference based on day of the week or weekday versus weekend shifts. The daily volume of patients in Phases 3, 4 and 5 ranged from 1 to 38, similar to Phase 1, and did not impact triage performance. In all phases, most days involved managing 10–19 transferred women. In Phase 1, having a time-sensitive condition (haemorrhage, hypertension or sepsis) led to a 10 min (1–19) shorter wait time (p=0.034), but by Phase 3 onwards, this difference was eliminated.
Reflection
By analysing this intervention, we found that a systematically implemented programme to introduce obstetric triage to a high-volume referral hospital in a low-income country can achieve international standards for performance and thereby reduce the third delay. International guidelines recommend that women and fetuses be evaluated within 10 min of hospital arrival.19 Initially, this standard seemed unachievable, so the hospital set a modified goal of 30 min, however, a higher standard was achieved. By the end of the project 99% of women were evaluated within 30 min compared with only 45% initially, and the median wait time from arrival to evaluation and initial treatment was reduced from 40 to 5 min.
Training is commonly offered by NGOs and multinational organisations, but it represents only a small part of implementation. Research by Wandersman and others has shown that in addition to training, an implementation support system that consists of tools, ongoing coaching and QI is needed.27 This programme utilised a systematic methodology which included mentoring, monitoring and evaluation conducted through a data-driven continuous QI approach. Data were collected prospectively and change monitored throughout the process. Implementation included jointly developed tools such as the triage assessment form, colour-coded wristbands and triage guidelines. The hospital leadership was committed to process improvement over a multiyear programme. Local leadership buy-in is critical to the success of implementation. Furthermore, the entire programme was conducted at GARH, without per diem expenditures, which maximised staff involvement and minimised cost.
Our data capture rates for time intervals was limited by the difficulties associated with providing care in a low-resource, high-volume environment. In Phases 1, 4 and 5, local data collectors were hired for data capture; however, in Phases 2 and 3, midwives on duty collected the triage assessment forms. Missing time data occurred in 1%–15% of records. In addition, data may not have been captured on patients due to the following reasons. Some women were admitted directly from the emergency room or the antenatal clinic, thus, bypassing labour triage. Second, deliveries occurred on patients admitted prior to the data collection periods. Third, there were occasional gaps in research assistant coverage and in one instance a set of forms was misplaced. Finally, in Phases 4 and 5, data concentrated more on referred patients; thus, GARH originating patients may have been excluded. We were, therefore, unable to capture more than 53% (770/1465) of the time interval data for patients admitted during Phase 3, 55% (869/1589) for Phase 4 and 54% (542/1008) for Phase 5, compared with 69% (926/1351) for the baseline interval. Therefore, we cannot exclude the possibility of selection bias in either direction. The triage system and data collection were monitored by a local consultant obstetrician to ensure uniformity to the extent possible. In addition, the sample sizes were still sufficiently large with patient arrival distributed similarly across days and work shifts, compared with baseline.8
Only one other paper specifically attempts to improve obstetric triage in SSA. In 2016, Forshaw and coauthors analysed obstetric triage processes at a large, urban hospital in Uganda that had similarly employed a red, yellow, and green ‘Traffic Light System’ for identifying high-risk women.7 Observations by external researchers captured only 98 (14%) of the approximately 700 patients admitted over 10 days that included 12-hour day shifts and one night shift. They reported that triage was conducted ‘informally’ in 46% of observations and that no patients were allocated by the traffic light system. Furthermore, the average wait time from arrival to assessment was 194 min and significantly longer at night.7 The authors speculated that the lack of dedicated triage personnel, equipment, and a suitable examination area likely impeded the triage process.7 We had similar limitations during our Phase 2 implementation, but overcame these in Phase 3. Their method of identifying high-risk women was similar to the wrist banding system used in this obstetric TIP, but the educational component was not supported by continuous QI, infrastructural improvements, and leadership engagement. A 2014 review of obstetric triage practices searched English language articles from 1998 to 2013 and of 33 relevant articles, none were from LMICs.28
Two systematic reviews, published in 2013, assessed health facility responsiveness in providing emergency obstetric care.5 9 Cavallaro and Marchant found 26 studies that addressed delay in receiving care within LMIC health facilities but defining and measuring delay were inconsistent among reports.9 The most commonly cited barriers to providing timely care were unavailability of treatment supplies, surgical capability and qualified staff.9 Similarly, Knight published a systematic review, analysing contributors to delay once women reached CEmOCs.5 They identified six themes including: drugs and equipment, policy and guidelines, human resources, facility infrastructure, patient related and referral related. There remains insufficient knowledge regarding the impact of the third delay on birth outcomes.9 Although the current study has shown improvements in obstetric triage times, it is not possible to make a causal link to mortality improvement. Further research into this topic is needed.
Conclusion
The implementation of an obstetric triage system designed for a high-volume, high-risk obstetric referral hospital in Ghana resulted in a significant and locally sustained reduction in patient waiting time. This programme was based on QI methodology, clinical skills building and leadership and uniquely focused on the triage process. The median waiting time from hospital arrival to evaluation and initial treatment decreased from 40 min to 5 min. The lack of triage processes in many CEmOC centres should be recognised as a factor that contributes to ‘the third delay’ potentially increasing untimely maternal and neonatal deaths. Reducing the third delay begins at the hospital door but it does not stop there. Process improvement and timeliness of care must be orchestrated throughout a woman’s hospitalisation to ensure quality of the services rendered and better outcomes.
Acknowledgments
The authors would like to acknowledge the contributions of the Ministry of Health in Ghana for providing funds to construct and equip the triage pavilion at Greater Accra Regional Hospital. We wish to thank Dr Mariam Batakji, Nana Kwame Ampofo, Michael Asare, Sung Min Kim, Lynn Harris, Sebnem Ucer, Erin Pfeiffer for data entry, cleaning and analysis.
Footnotes
Handling editor: Seye Abimbola
Contributors: DMG made significant contribution to data analysis and interpretation and wrote the initial manuscript draft. EKS made significant contribution to study conception and design and acquisition of data, envisioned and sourced the triage pavilion and led the triage implementation. RR oversaw the quality improvement aspects of the project and contributed to manuscript writing. FB conducted observations of clinical care, developed and taught the triage training course and provided clinical coaching. LF conducted observations of clinical care, developed and taught the triage training course and provided clinical coaching. AO maintained an electronic patient database, facilitated the training and provided edits to the manuscript draft. CT was a clinical champion leading the triage implementation and conducted data collection for Phase 3. MDO made significant contributions to study conception, design, acquisition and interpretation of data and was heavily involved in manuscript writing and revision. All authors agreed to be accountable for the accuracy and integrity of the work. All authors have read and approved the final version of this manuscript.
Funding: This work was part of the ‘Making Every Baby Count Initiative’ awarded to PATH by the Children’s Investment Fund Foundation. Kybele received a subaward (CIF.1838-01-705622-SUB) to improve maternal and newborn care capacity in regional hospitals in Ghana. Funding for Phase 4 and Phase 5 data collection was provided by a USAID Systems for Health Innovation Grant (Subagreement No. FY16-CR05-6017). Activities within the awards underwent review; however, the funders played no role in conducting the research, analysing the results or writing the manuscript.
Competing interests: All authors report grants from PATH, during the conduct of the study and non-financial support from Kybele, outside the submitted work. MDO is the founder and president of Kybele.
Patient consent: Not required.
Ethics approval: The study was granted Institutional Review Board’s approval by Wake Forest University Health Sciences, Winston-Salem, North Carolina, USA, on 24 September 2012 (IRB#00021947) and the Ghana Health Service, Accra, Ghana, on 30 November 2012 (IRB#GHS/DGS/G-27). The study qualified for expedited review and met the criteria to waive informed consent.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Original data are available upon request to the corresponding author.
References
- 1. The Partnership for Maternal, Newborn, and Child Health. Global strategy for women’s and children’s health 2010. http://www.who.int/pmnch/knowledge/publications/fulldocument_globalstrategy/en/ (accesssed 16 Aug 2017).
- 2. Alkema L, Chou D, Hogan D, et al. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Maternal Mortality Estimation Inter-Agency Group. Lancet 2016;387:462–74. 10.1016/S0140-6736(15)00838-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Otolorin E, Gomez P, Currie S, et al. Essential basic and emergency obstetric and newborn care: from education and training to service delivery and quality of care. Int J Gynaecol Obstet 2015;130(Suppl 2):S46–53. 10.1016/j.ijgo.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization. Geneva, Switzerland. Trends in maternal mortality: 1990-2010. 2012. http://whqlibdoc.who.int/publications/2012/9789241503631_eng.pdf?ua=1 (accessed 16 Aug 2017).
- 5. Knight HE, Self A, Kennedy SH. Why are women dying when they reach hospital on time? A systematic review of the ’third delay'. PLoS One 2013;8:e63846 10.1371/journal.pone.0063846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gabrysch S, Campbell OM. Still too far to walk: literature review of the determinants of delivery service use. BMC Pregnancy Childbirth 2009;9:34 10.1186/1471-2393-9-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Forshaw J, Raybould S, Lewis E, et al. Exploring the third delay: an audit evaluating obstetric triage at Mulago National Referral Hospital. BMC Pregnancy Childbirth 2016;16:300 10.1186/s12884-016-1098-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goodman DM, Srofenyoh EK, Olufolabi AJ, et al. The third delay: understanding waiting time for obstetric referrals at a large regional hospital in Ghana. BMC Pregnancy Childbirth 2017;17:216 10.1186/s12884-017-1407-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cavallaro FL, Marchant TJ. Responsiveness of emergency obstetric care systems in low- and middle-income countries: a critical review of the "third delay". Acta Obstet Gynecol Scand 2013;92:496–507. 10.1111/aogs.12071 [DOI] [PubMed] [Google Scholar]
- 10. Ghana Health Services. Annual reproductive and child health report. 2013. http://bit.ly/2GvRSCl (accessed 16 Aug 2017).
- 11. Adu-Bonsaffoh K, Oppong SA, Samuel OA, et al. Maternal deaths attributable to hypertensive disorders in a tertiary hospital in Ghana. Int J Gynaecol Obstet 2013;123:110–3. 10.1016/j.ijgo.2013.05.017 [DOI] [PubMed] [Google Scholar]
- 12. Ganyaglo GY, Hill WC. A 6-year (2004-2009) review of maternal mortality at the Eastern Regional Hospital, Koforidua, Ghana. Semin Perinatol 2012;36:79–83. 10.1053/j.semperi.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 13. Gumanga SK, Kolbila DZ, Gandau BB, et al. Trends in maternal mortality in Tamale Teaching Hospital, Ghana. Ghana Med J 2011;45:105–10. [PMC free article] [PubMed] [Google Scholar]
- 14. Lee QY, Odoi AT, Opare-Addo H, et al. Maternal mortality in Ghana: a hospital-based review. Acta Obstet Gynecol Scand 2012;91:87–92. 10.1111/j.1600-0412.2011.01249.x [DOI] [PubMed] [Google Scholar]
- 15. Ansong-Tornui J, Armar-Klemesu M, Arhinful D, et al. Hospital based maternity care in Ghana – findings of a confidential enquiry into maternal deaths. Ghana Med J 2007;41:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ramaswamy R, Iracane S, Srofenyoh E, et al. Transforming maternal and neonatal outcomes in tertiary hospitals in Ghana: an integrated approach for systems change. J Obstet Gynaecol Can 2015;37:905–14. 10.1016/S1701-2163(16)30029-9 [DOI] [PubMed] [Google Scholar]
- 17. Srofenyoh EK, Kassebaum NJ, Goodman DM, et al. Measuring the impact of a quality improvement collaboration to decrease maternal mortality in a Ghanaian regional hospital. Int J Gynaecol Obstet 2016;134:181–5. 10.1016/j.ijgo.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 18. Das JK, Kumar R, Salam RA, et al. Evidence from facility level inputs to improve quality of care for maternal and newborn health: interventions and findings. Reprod Health 2014;11(Suppl 2):S4 10.1186/1742-4755-11-S2-S4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Association of Women’s Health, Obstetric and Neo-natal Nurses (AWHONN): Washington, DC. Women’s health perinatal nursing care quality measures specifications 2013. 2013. https://www.awhonn.org/resource/resmgr/Downloadables/perinatalqualitymeasures.pdf (accessed 16 Aug 2017).
- 20. Goodman DM, Ramaswamy R, Jeuland M, et al. The cost effectiveness of a quality improvement program to reduce maternal and fetal mortality in a regional referral hospital in Accra, Ghana. PLoS One 2017;12:e0180929 10.1371/journal.pone.0180929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ridde V. Need for more and better implementation science in global health. BMJ Glob Health 2016;1:e000115 10.1136/bmjgh-2016-000115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nilsen P. Making sense of implementation theories, models and frameworks. Implement Sci 2015;10:53 10.1186/s13012-015-0242-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Library of Medicine, National Institutes of Health, Bethesda, MD. Dissemination and implementation science. Health services research information central. https://www.nlm.nih.gov/hsrinfo/implementation_science.html (accessed 16 Aug 2017).
- 24. Metz A, Bartley L. Active implementation frameworks for program success. Zero to Three 2012;32:11–18. [Google Scholar]
- 25. Floyd L, Bryce F, Ramaswamy R, et al. The introduction of a midwife-led obstetric triage system into a regional referral hospital in Ghana. Midwifery 2018;61:45–52. 10.1016/j.midw.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 26. Langley GL, Moen RD, Nolan KM, et al. The improvement guide: a practical approach to enhancing organizational performance. 2nd edn San Francisco: Jossey-Bass, 2009. [Google Scholar]
- 27. Wandersman A, Chien VH, Katz J. Toward an evidence-based system for innovation support for implementing innovations with quality: tools, training, technical assistance, and quality assurance/quality improvement. Am J Community Psychol 2012;50:445–59. 10.1007/s10464-012-9509-7 [DOI] [PubMed] [Google Scholar]
- 28. Angelini D, Howard E. Obstetric triage: a systematic review of the past fifteen years: 1998–2013. MCN Am J Matern Child Nurs 2014;39:284–97. 10.1097/NMC.0000000000000069 [DOI] [PubMed] [Google Scholar]