ABSTRACT
Irritable Bowel Syndrome (IBS) and Inflammatory Bowel Disease (IBD) are related gastrointestinal disorders characterized by abdominal pain associated with colonic hypersensitivity (CHS). Studies in humans have reported an abnormal colonization of Adherent-Invasive E. coli (AIEC) in the ileum of Crohn's disease (CD) patients associated with overexpression of the bacterial colonizing receptor CEACAM6. The aim of the present study was to investigate whether AIEC reference strain LF82 could induce intestinal impairment during infectious and/or post-infectious periods and subsequently the development of CHS. Transgenic mice overexpressing human CEACAM6 protein (TG) and their wild-type littermates were gavaged by CD-associated AIEC bacteria (reference strain LF82) or PBS for 3 d. Colonic hypersensitivity was assessed by colorectal distension (CRD) test during infectious (D4) and post-infectious periods (D21). Several markers of intestinal inflammation were monitored and the colonic expression of purinergic P2X receptors was quantified. At D4, an increased visceromotor response (VMR) to the CRD test was observed in TG mice infected with CD-associated AIEC LF82 in comparison with non-infected TG mice and persisted in a subgroup of infected animals at D21 after bacteria clearance. Increased VMR was associated with low-grade intestinal inflammation, increased intestinal permeability and expression of P2X 3, 4 and 7. This study shows that certain susceptible hosts infected with CD-associated AIEC bacteria can develop persistent CHS associated with low-grade inflammation and increased P2X receptors expression. Thus, CD-associated AIEC infection in CEACAM6 transgenic mice could be used as a novel post-infectious mouse model mimicking quiescent IBD with IBS-like symptoms such as visceral pain.
KEYWORDS: Adherent invasive E. coli, colonic hypersensitivity, intestinal permeability, P2X receptors, visceral pain
Introduction
Visceral pain, which is frequently related to colonic hypersensitivity (CHS), is a diffuse and stabbing sensation which may be associated with gastrointestinal disorders such as Irritable Bowel Syndrome (IBS) and Inflammatory Bowel Disease (IBD). It is a debilitating complaint because of its impact on patients’ quality of life and the lack of effective therapies.
IBD and IBS are related gastrointestinal disorders. It has been suggested that the development of post-infectious IBS (PI-IBS) could identify individuals at increased risk of IBD.1 Furthermore, 39% of IBD patients exhibit IBS-like symptoms, of which pain and discomfort are the most frequent, even during remission periods.2 Akbar et al. showed that the expression of receptors present on the nerve fibers is modified in the colon of IBD patients with IBS-like symptoms and correlate with pain.3 IBS-like symptoms in quiescent IBD patients are associated with low-grade intestinal inflammation as indicated by high fecal calprotectin levels, increased TNF-α concentration and increased number of intraepithelial lymphocytes in colonic biopsies. IBD patients with IBS-like symptoms also have increased intestinal paracellular permeability that is strongly correlated with IBS severity score in both ulcerative colitis (UC) and Crohn's disease (CD) patients.4
Few animal models reproducing PI-IBS symptoms and etiology are available. The 2 most common are a model of bacterial infection with Campylobacter jejuni, mainly used in rats, and a model of parasitic infection with Trichinella spiralis.5 Mice infection by this intestinal parasite leads to enteric inflammation caused by innate inflammatory response and post-infectious afferent nerve hypersensitivity. Keating et al. have shown that the mechanisms involved in infection with Trichinella spiralis are dependent on P2X7 receptors.6 P2X receptors (P2XRs), in particular P2X3, P2X4, and P2X7, are ATP-gated ion channels involved in the transmission of visceral nociceptive information from the gut to the central nervous system.7 In humans, increased numbers of mucosa-associated E. coli have been observed in CD patients.8 These patients also have an abnormal ileal expression of carcinoembryonic antigen-related cell adhesion molecules (CEACAM) 5 and 6. CEACAM6 acts as a receptor for AIEC LF82.9 Moreover, Dogan et al. reported that E. coli with an IBD-associated AIEC pathotype are common in IBS patients.10
In the present study, we used transgenic mice expressing intestinal CEACAM6 receptor to investigate whether AIEC LF82 infection could lead to the development of colonic hypersensitivity with the aim of developing a novel post-infectious mouse model mimicking quiescent IBD with IBS-like symptoms such as visceral pain. We also studied the involvement of the P2XRs in our model.
Material and methods
Bacterial strain and culture
Ampicillin-erythromycin–resistant E. coli strain LF82, isolated from a chronic ileal lesion of a CD patient, was used as the AIEC reference strain8 and rifampicin-resistant E. coli K12 strain MG1655 was used as the control bacteria. The optical density (620nm) of overnight bacterial culture in Luria-Bertani broth without shaking at 37°C was read. For the experiment with heat-killed bacteria, the bacterial suspension was incubated at 95°C for 5 min. The bacterial culture was then harvested by centrifugation at 5,500 g for 10 min. The bacterial pellet was resuspended in phosphate buffer saline (PBS) to reach a concentration of 5.109 bacteria per milliliter.
Animals
All mice were housed under specific pathogen-free conditions (21–22°C, 12:12-h light-dark cycle), with access to food and water ad libitum, in the animal facility at the University of Auvergne (Clermont-Ferrand, France). C57BL/6J WT female mice were purchased from Janvier Laboratories (Le Genest-St-Isle, France) and CEABAC10 transgenic mice (heterozygote)11 were maintained in our animal facility. WT and transgenic CEABAC10 mice were coupled to obtain 50% WT mice (WT) and 50% CEABAC10 mice (TG). Mice from the same generation were used for experimentations. Animal protocols were approved by a local ethics committee (protocol number: 3460-EU0116) and followed the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain.12
Assessment of infection and colonization
Eight-week-old C57BL/6J WT or CEABAC10 transgenic male mice (body weight, ∼24–26 g) were pretreated by administration of the broad-spectrum antibiotic neomycin in drinking water (2 g/l, w/v) for 3 d (day-3 to day-1) to disrupt normal resident bacterial flora in the intestinal tract. They were orally challenged with 109 AIEC LF82 bacteria or 109 AIEC LF82 heat-killed bacteria or E. coli K12 (MG1655) or PBS 24 hours later and for a period of 3 d (day 1 to day 3). At day 4 (D4), day 9 (D9) and day 21 (D21), fresh fecal pellets (100–200 mg) were collected from individual mice and resuspended in PBS (Fig. 1). After appropriate serial dilutions, bacteria were enumerated by plating on Luria-Bertani agar medium containing 50 µg/ml ampicillin and 20 µg/ml erythromycin to isolate AIEC LF82 and incubated at 37°C overnight. Body weight was monitored throughout the experiments. When mice attained 80% of their initial weight or at the end of the experiment (D4 or D21), they were anesthetized with isoflurane and then killed by cervical dislocation. Colon length and colon and spleen weight were measured. Colon specimens were collected to quantify myeloperoxidase activity and to perform quantitative RT-PCR.
Figure 1.
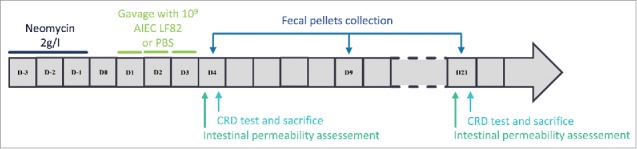
Time course protocol. After 3 d (D-3 to D-1) of pretreatment with neomycin, 8-week-old C57BL/6J WT or CEABAC10 transgenic mice received 109 AIEC LF82 bacteria in PBS or PBS by orogastric gavage for 3 d (D1 to D3). Fresh fecal pellets were collected at D4, D9, and D21. In vivo intestinal permeability was assessed at D4 or D21 and colorectal distension test (CRD test) was performed at D4, D9, and D21 to assess colonic sensitivity. Mice were killed at D4 or D21, after CRD test, for collection of colon specimens.
Tissue myeloperoxidase (MPO) assay
A fragment of colon was homogenized in phosphate buffer (50mM, pH = 6.0°) containing 0.5% hexadecyltrimethylammonium bromide (Sigma-Aldrich, cat. #36932). The homogenate was sonicated, freeze-thawed 3 times and centrifuged at 14,000 g for 15 min. Fifty µl of supernatant was added to 200 µl of reactive buffer (hydrogen peroxide 0.5 × 10−4% (Sigma-Aldrich, cat. #H1009), o-dianisidine hydrochloride 1mg/ml (Sigma-Aldrich, cat. #D3252), phosphate buffer, and the change in absorbance at 450 nm was measured. One unit of MPO activity was defined as the amount of enzyme that consumed 1 µmol of substrate per minute at 25°C. The results are expressed as units of MPO activity per gram of tissue.
Fecal Lipocalin-2 assay
Fecal pellets were collected at D4 and D21, homogenized in PBS and centrifuged at 13,500 g for 10 min. Lipocalin-2 levels were assessed in the supernatant with Mouse Lipocalin-2/NGAL DuoSet Kit (R&D Systems, cat. #DY1857) according to the manufacturer's instructions. Results are expressed as pg/ml/mg of fecal pellets.
In vivo intestinal permeability
In vivo intestinal permeability was assessed using fluorescein dextran (FITC-dextran 3000–5000 Da, Sigma-Aldrich, cat. #FD4). Before neomycin treatment and after infection with AIEC LF82 (D4 and D21), mice were fasted for 6 hours and orally gavaged with 15 mg of FITC-dextran. Blood samples were obtained from the retro-orbital venous plexus 5 hours later under brief anesthesia (2% isoflurane). After centrifugation at 5,500 rpm for 30 minutes, plasma FITC levels were determined by fluorometry at 488 nm with a microplate reader (Tecan, Lyon, France).
Assessment of colonic hypersensitivity
Mice colonic sensitivity was assessed, as described previously,13,14 by measuring electromyographic abdominal contractions induced by colorectal distension (CRD) to quantify visceromotor response (VMR). Briefly, electrodes were implantated into the abdominal oblique muscle at least 5 d before CRD test (D4, D9, and D21). The CRD protocol consists of a set of graded distensions to constant volumes (from 20 to 100 µl). The results are expressed as VMR values (mV/s) or as areas under the VMR curve (AUC) for the distension volumes between 60 and 100 µl computed by Prism7 software (GraphPad, La Jolla, CA, USA).
Analysis of P2XRs mRNA expression
TRIzol reagent (Life Technologies, cat. # 15596026) was used to extract total RNA from mice colons in RNAse free conditions and according to the manufacturer's instructions. After extraction, RNA was purified by a DNAse treatment (RNase free DNase set, Qiagen, cat. # 79254). Reverse transcription was performed with the High Capacity cDNA RT Kit (Applied, cat. #4368814) with 500ng of RNA, followed by a qPCR using LightCycler FastStart DNA Master SYBR Green Kit (Roche, cat. # 03003230001).
The primers used for P2X3, P2X4, P2X7 and HPRT mice genes were as follows:
P2X3 forward: 5’-CTCCTACTTTGTGGGGTGGG-3’
P2X3 reverse: 5’-ACTCTGTTGGCATAGCGTCC-3’
P2X4 forward: 5’-TGGCCGACTATGTGGTCCCA-3’
P2X4 reverse: 5’-GGTTCACGGTGACGATCATG-3’
P2X7 forward: 5’-TGCAGCCATAAATCCGGGAA-3’
P2X7 reverse: 5’-GCTCACCAAAGCAAAGCAGAT-3’
HPRT forward: 5’-TTGCTGACCTGCTGGATTA-3’
HPRT reverse: 5’-AGTTGAGAGATCATGTCCAC-3’
All results were normalized to the HPRT gene. Samples were tested in duplicate, and the average values were used for quantification by the 2−ΔΔCt method.
Statistical analysis
Statistical analyzes were performed with Prism 7 software (GraphPad, La Jolla, CA, USA). Mann-Withney test (2 groups) or Kruskal-Wallis test or 2-way ANOVA (more than 2 groups) were used for intergroup-comparisons with Dunn's multiple comparisons test or Sidak test for post hoc comparisons. A p-value ≤ 0.05 was considered statistically significant. Data are expressed as means ± SEM.
Results
CD-associated AIEC LF82 bacteria infection induces low-grade intestinal inflammation and increases intestinal permeability.
CEABAC10 transgenic (TG) or wild-type (WT) littermate mice were treated by an antibiotic (neomycin) for 3 d before infection (D-3 to D-1) and then were orally challenged with 109 AIEC LF82 bacteria or PBS for 3 d (D1 to D3). Bacteria were enumerated after feces collection to identify infectious and post-infectious periods. At D4, the day after the last AIEC LF82 administration, the fecal bacterial content was sizable and similar in WT and TG mice with medians, respectively, of 1.3 × 107 and 2.5 × 107 bacteria per gram of feces. From D5 to D8, the fecal bacterial content was higher in CEABAC10 transgenic mice than in WT mice showing a delayed bacterial elimination. At D9, the number of bacteria was significantly reduced in both WT and TG mice (medians: 1.0 × 102 and 1.8 × 102 bacteria per gram of feces respectively) indicating the beginning of the post-infectious period. At D21, all bacteria were cleared in the feces of both WT and TG mice (medians: 1.0 bacteria per gram of feces for each group) (Fig. 2) which characterized the post-infectious period. Body weight was monitored during all experiments. AIEC LF82 bacteria challenge was associated with a slight decrease in body weight in TG mice during the infectious period only (D3 and D4) (Fig. 3A). Otherwise, this infection had no effect on the body weight of WT mice (data not shown). At D4, during the infectious period, AIEC LF82 bacteria did not increase of spleen or colon weight in either WT or TG mice (Fig. 3B and C). Finally, colonic myeloperoxidase activity assessed at D4 was not modified in WT or TG infected mice (Fig. 3D). Plasmatic levels of IL-6 and TNF-α as well as colonic mast cells density at D4 and D21 confirmed the absence of marked intestinal inflammation in WT and TG mice infected with CD-associated AIEC LF82 bacteria (data not shown).
Figure 2.
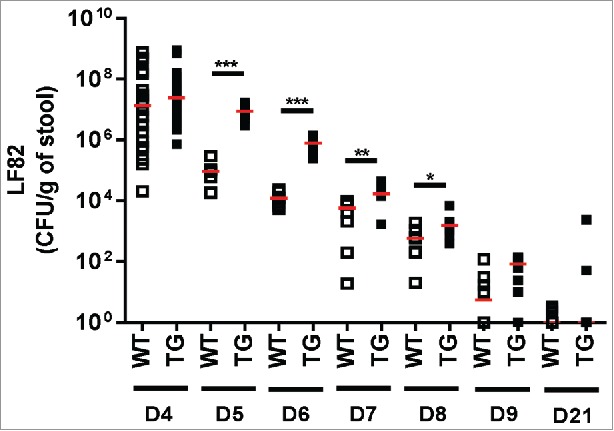
AIEC LF82 intestinal colonization. AIEC LF82 intestinal colonization from day 4 (D4) to day 9 (D9) and at day 21 (D21) in 8-weeks-old WT or CEABAC10 transgenic (TG) mice infected with AIEC LF82 bacteria from day 1 (D1) to day 3 (D3). At D4: WT: n = 22, TG: n = 26; from D5 to D9: WT: n = 8, TG: n = 8; at D21: WT: n = 11, TG: n = 11. Each dot represents one mouse and red lines represent medians. *: p < 0.05, **: p < 0.01, ***: p < 0.001. For each time, significant differences were calculated using the Mann-Whitney test between WT and TG mice.
Figure 3.

CD-associated AIEC LF82 bacteria do not induce severe intestinal inflammation. (A) Body weight monitoring from D-3 to D21 of CEABAC10 transgenic (TG) mice gavaged with PBS or AIEC LF82 bacteria from D1 to D3. TG + PBS: n = 10; TG + LF82: n = 15. Lines represent means and error bars represent SEM. (B) Spleen weight at D4 of WT and CEABAC10 transgenic (TG) mice gavaged with PBS or AIEC LF82 bacteria from D1 to D3. WT + PBS: n = 6; WT + LF82: n = 10; TG + PBS: n = 6; TG + LF82: n = 12. (C) Colon weight at D4 of WT and CEABAC10 transgenic (TG) mice gavaged with PBS or AIEC LF82 bacteria from D1 to D3. Values are expressed as grams per 100 g of body weight. WT + PBS: n = 6; WT + LF82: n = 10; TG + PBS: n = 6; TG + LF82: n = 12. Each dot represents one mouse and red lines represent means. (D) Colonic myeloperoxidase activity at D4 of WT and CEABAC10 transgenic (TG) mice gavaged with PBS or AIEC LF82 bacteria from D1 to D3. Values are expressed as enzymatic units per gram of colon. WT + PBS: n = 6; WT + LF82: n = 10; TG + PBS: n = 6; TG + LF82: n = 12. Bars represent means and error bars represent SEM. **: p < 0.01, 2-way ANOVA test followed by a Sidak post-hoc test.
Fecal lipocalin-2 levels were assessed at D4 and D21. During the infectious period, lipocalin-2 levels were higher in both WT-infected mice (WT + LF82) and TG infected mice (TG + LF82) than in control non-infected mice (WT + PBS and TG + PBS) but not to a level of significance (Fig. 4A). In contrast, at D21, during the post-infectious period, a high level of lipocalin-2 persisted only in CEABAC10 TG mice receiving AIEC LF82 bacteria (TG + LF82: 84.96 ± 35.27 pg/ml/mg of feces vs. TG + PBS: 19.62 ± 5.92 pg/ml/mg of feces, p < 0.05, Dunn's test) (Fig. 4B). Dextran-FITC plasma levels were significantly increased in the TG + LF82 group compared with the WT + PBS, TG + PBS and WT + LF82 groups at D4 (p < 0.05, Tukey test) whereas at D21 they were similar to basal Dextran-FITC plasmatic concentrations (Fig. 4C), indicating that intestinal barrier function at D21 was normal.
Figure 4.

CD-associated AIEC LF82 bacteria induce low-grade intestinal inflammation and increase intestinal permeability. (A) Fecal lipocalin-2 concentration of WT and transgenic (TG) mice gavaged with PBS or AIEC LF82 bacteria from D1 to D3 at D4. WT + PBS: n = 11; WT + LF82: n = 12; TG + PBS: n = 12; TG + LF82: n = 21. (B) Fecal lipocalin-2 concentration of WT and transgenic (TG) mice gavaged with PBS or AIEC LF82 bacteria from D1 to D3 at D21. Values are expressed as pg/ml of lipocalin-2 per grams of feces. WT + PBS: n = 5; WT + LF82: n = 5; TG+PBS: n = 5; TG + LF82: n = 8. Bars represent means and error bars represent SEM. (C) FITC-Dextran 4kDa plasmatic concentration, 5 hours after oral gavage with 15 mg of FITC-Dextran, at D0, D4 and D21, of WT and CEACBAC10 transgenic (TG) mice gavaged with PBS or AIEC LF82 bacteria from D1 to D3. Values are expressed as µg of FITC-Dextran per ml of plasma. At D0: WT + PBS: n = 4, WT + LF82: n = 6, TG + PBS: n = 4, TG + LF82: n = 5; at D4: WT + PBS: n = 4, WT + LF82: n = 6, TG + PBS: n = 4, TG + LF82: n = 5; at D21: WT + PBS: n = 4, WT + LF82: n = 5, TG + PBS: n = 4, TG + LF82: n = 5. Bars represent means and error bars represent SEM. *: p < 0.05. The 2-way ANOVA test was used to calculate significant differences and the Tukey test for post-hoc analysis.
CD-associated AIEC LF82 bacteria infection induces colonic hypersensitivity in CEABAC10 transgenic mice.
The visceromotor response (VMR) to the CRD test in WT AIEC LF82 infected mice (WT + LF82) was similar to WT non-infected mice (WT + PBS) during infectious (Fig. S1A and B) and post-infectious periods (Figs. S1C to S1F).
At D4, there was a significant increase in the VMR to the CRD test in transgenic mice receiving AIEC LF82 bacteria (TG + LF82) for the 2 highest distension volumes (80 and 100 µl), in comparison with TG non-infected mice (Fig. 5A). Areas under the curve for the highest distension volumes were 16.98 ± 2.20 AU in TG non-infected mice (TG + PBS) and 30.63 ± 3.07 AU in TG infected mice (TG + LF82) (p < 0.01, Mann-Whitney test) (Fig. 5B).
Figure 5.

CD-associated AIEC LF82 bacteria induce colonic hypersensitivity in CEABAC10 transgenic mice. (A) Visceromotor response (VMR) to colorectal distension test at D4 and (B) corresponding AUC of the visceromotor response, between 60 and 100 µl in CEABAC10 transgenic (TG) mice gavaged with PBS or AIEC LF82 bacteria from D1 to D3. TG + PBS: n = 7; TG + LF82: n = 7. (C) Visceromotor response (VMR) to colorectal distension test at D21 and (D) corresponding AUC of the visceromotor response, between 60 and 100 µl in CEABAC10 transgenic (TG) mice gavaged with PBS or AIEC LF82 bacteria from D1 to D3. TG + PBS: n = 16; TG + LF82: n = 13. (E) Visceromotor response (VMR) to colorectal distension test at D21 in sensitized and non-sensitized CEABAC10 transgenic (TG) mice gavaged with PBS or AIEC LF82 bacteria from D1 to D3. TG + PBS: n = 16; TG + LF82 sensitized: n = 6; TG + LF82 non-sensitized: n = 7. For VMR to CRD test, dots represent means and error bars represent SEM. For AUC, each dot represents one mouse and red lines represent means. (F) FITC-Dextran 4kDa plasmatic concentration, 5 hours after oral gavage with 15mg of FITC-Dextran, at D4, of CEACBAC10 transgenic (TG) mice gavaged with PBS or AIEC LF82 bacteria from D1 to D3. Values are expressed as µg of FITC-Dextran per ml of plasma. TG + PBS: n = 16; TG + LF82 sensitized: n = 6; TG + LF82 non-sensitized: n = 7. Bars represent means and error bars represent SEM. * or $: p < 0.05; ** or $$: p < 0.01; ***: p < 0.001; **** or $$$$: p < 0.0001. Statistical analyses were performed by the Mann-Whitney test or by the one –way ANOVA and Dunn's test for post-hoc analysis or by the 2-way ANOVA test and by the Sidak test for post-hoc analysis.
This increased VMR persisted at D9, at the beginning of the post-infectious period, for the 2 highest distension volumes in the TG + LF82 group (Fig. S2A). Areas under the curve analysis showed that the response to CRD was homogeneously increased in all the mice of the group (Fig. S2B).
At D21, after bacteria clearance, there was a slightly significant increase in VMR in TG mice receiving AIEC LF82 bacteria but only for the highest distension volume of 100 µl (Fig. 5C). Areas under the curve analysis showed the presence of 2 mice subpopulations, one with an increase in their VMR to colonic distension (sensitized mice, n = 6) and one with VMR comparable to that of the control TG mice group treated with PBS (non-sensitized mice, n = 7) (Fig. 5D). Thus, analysis of the 2 subgroups showed that the VMR to CRD test was significantly increased for the 60, 80 and 100 µl distension volumes only in sensitized mice (Fig. 5E). Comparison of non-sensitized or sensitized mice at D21 based on FITC-dextran plasma levels measured at D4 in the TG + LF82 group showed that increased intestinal permeability during infectious period seems to be determinant for the upholding of colonic hypersensitivity during post-infectious period (Fig. 5F).
This increase in the VMR was specific to AIEC LF82 bacteria. Indeed, VMR to the CRD test in WT or transgenic mice receiving E. coli K12 (MG1655) non-pathogenic bacteria (WT+MG1655 and TG+MG1655) were similar to WT or TG non-infected mice (WT+PBS and TG+PBS) respectively at D21 (Figs. S3A to S3D). Furthermore, AIEC LF82 heat-killed bacteria did not increase the VMR to the CRD test in CEABAC10 transgenic mice (Fig. S4).
AIEC LF82 bacteria induced colonic hypersensitivity is associated with increased colonic purinergic receptors expression.
To identify the mechanisms involved in the AIEC LF82 bacteria induced increased VMR to the CRD test, the mRNA expression of colonic purinergic receptors (P2XRs) was quantified during infectious (D4) and post-infectious periods (D21). P2X3R mRNA expression was non-significantly increased in the TG + LF82 group at D4 (Fig. 6A), whereas this expression was significantly increased at D21 compared with that in TG non-infected mice (1.188 ± 0.749 vs. 0.142 ± 0.011, p < 0.01, Dunn's test) (Fig. 6B). The mRNA expression of P2X4 (Fig. 6C and D) and P2X7 (Fig. 6E and F) receptors was greater during the post-infectious period in the TG + LF82 group in comparison with TG non-infected mice. This increase was significant for P2X4 receptors.
Figure 6.

AIEC LF82 bacteria induced colonic hypersensitivity is associated with increased colonic purinergic receptors expression. (A) mRNA colonic expression of P2X3 at D4, WT + PBS: n = 5; WT + LF82: n = 6; TG + PBS: n = 4; TG + LF82: n = 4, (B) mRNA colonic expression of P2X3 at D21, WT + PBS: n = 7; WT + LF82: n = 8; TG + PBS: n = 6; TG + LF82: n = 8, (C) mRNA colonic expression of P2X4 at D4, WT + PBS: n = 5; WT + LF82: n = 6; TG + PBS: n = 4; TG + LF82: n = 4, (D) mRNA colonic expression of P2X4 at D21, WT + PBS: n = 7; WT + LF82: n = 8; TG + PBS: n = 6; TG + LF82: n = 8, (E) mRNA colonic expression of P2X7 at D4, WT + PBS: n = 5; WT + LF82: n = 6; TG + PBS: n = 4; TG + LF82: n = 4, (F) mRNA colonic expression of P2X7 at D21, WT + PBS: n = 7; WT + LF82: n = 8; TG + PBS: n = 6; TG + LF82: n = 8, in WT or CEABAC10 transgenic mice gavaged with PBS or AIEC LF82 from D1 to D3. Values are expressed as relative expression of P2XRs compared with HPRT expression. Bars represent means and error bars represent SEM. *: p < 0.05; **: p < 0.01. The Kruskal-Wallis test was used to calculate significant differences and Dunns’ test was used for post-hoc analysis.
Discussion
Numerous publications suggest that gastrointestinal pathologies such as IBS and IBD are associated with CHS .15-19 IBS is characterized by recurrent abdominal pain related to defecation and associated with a change in frequency or form of stool, in the absence of any measurable change in intestinal structure and function.20 IBD are chronic disorders of the gastrointestinal tract that manifest as 2 distinct conditions, Crohn's Disease (CD) and Ulcerative Colitis (UC). These conditions are characterized by acute intestinal inflammatory phases interspersed by periods of remission. CHS may have an infectious etiology. Prospective studies have shown that IBS may occur as a result of acute gastroenteritis caused by various pathogens such as Shigella spp., E. coli, Salmonella, C. jejuni and G. duodenalis21 in 3% to 36% of IBS patients.22 These patients have neuronal alterations, low-grade intestinal inflammation, and an increased intestinal permeability. IBD, and CD in particular, may also have an infectious etiology. Analysis of a UK primary care database has suggested that the incidence of CD rises after acute gastroenteritis.23 In addition, an increased number of mucosa-associated E. coli referred to as AIEC has been observed in CD patients.8 Like PtdIns-IBS, CD is thought to result from a hyperactive immune response to luminal antigens and has been associated with changes in intestinal permeability.24 The aim of the present work was to develop a novel post-infectious mouse model of visceral pain by determining if AIEC LF82 could lead to the development of CHS.
In our study, wild-type (WT) or CEABAC10 transgenic mice (mice harboring intestinal CEACAM6 human receptor) were orally gavaged with PBS or CD-associated AIEC LF82 bacteria for 3 d after antibiotic treatment. CRD test showed the colonic hypersensitivity in infected transgenic mice as evidenced by an increase in the VMR. In WT mice, however, infection had no effect. CHS was present during the infectious period (at D4, the day after the last infection) and persisted at the beginning of the post-infectious period (D9) and in certain animals much later post-infection (D21). In contrast, non-pathogenic E. coli K12 bacteria (strain MG1655) did not modify VMR in CEABAC10 transgenic mice, which indicate that CHS was specific to CD-associated AIEC LF82 bacteria. To confirm that this CHS is due to a physical interaction between AIEC LF82 bacteria and CEACAM6 receptors and not only to the intraluminal presence of the bacteria, further experiments using an AIEC LF82 ΔfimH strain cloud be perform. Indeed adhesion of AIEC LF82 to the intestinal epithelial cells, via the CEACAM6 receptor, involves fimH adhesion expressed on type 1 pili.9
Higher FITC-Dextran plasma levels at D4 in TG infected hypersensitive mice than in WT infected mice or in TG infected non-hypersensitive mice were evidence of increased intestinal permeability. Normal barrier function was restored at D21, after bacteria clearance. These results are consistent with those of Denizot et al. who observed increased intestinal permeability in CEABAC10 mice induced by AIEC LF82 gut colonization and in CD patients.25 Drastic increases in intestinal permeability have also been reported in patients with post-infectious IBS.26,27
This increase in intestinal permeability is associated with low-grade intestinal inflammation as indicated by increased fecal lipocalin-2 levels but not by classical markers of inflammation such as an increase in colon and spleen weight and increase in MPO activity. These results are consistent with those of previous studies showing that AIEC LF82 infections are unable to induce gut inflammation but can potentiate inflammation induced by DSS treatment28 or be spontaneously present in susceptible mice (TLR5KO mice).29 Agus et al., showed that a western diet (high fat high sugar diet) induce an increase in fecal lipocalin-2 levels, which reflects a low grade intestinal inflammation.30 High fat diets are also responsible for CHS in mice via a TLR4 dependent mechanism.31 These findings lend weight to the hypothesis that low grade intestinal inflammation results in CHS. Low-grade inflammation could also lead to immune system alterations that might involve immune cells such as mast cells. Barbara et al. suggested that colonic mast cells infiltration and mediators release in proximity to mucosal innervation may contribute to abdominal pain in IBS patients.32 This hypothesis was corroborated by the study of Darbaky et al. using an IBS model induced by TNBS enemas.33
Increased intestinal permeability may also induce CHS via microbiota involvement. It has been demonstrated that AIEC LF82 may alter microbiota composition in susceptible hosts.29 Luminal microbial molecules could be different due to dysbiosis and could also activate the immune system. Microorganisms could also interact directly with the immune system to induce low-grade intestinal inflammation. Microbial metabolites may also activate afferent fibers of the enteric nervous system and induce neuronal modifications.34
In our study, the colonic expression of purinergic receptors colonic expression was modified. AIEC LF82 infection increased P2X3, P2X4, and P2X7 receptor expression in CEABAC10 hypersensitive mice. P2X receptors are ATP-gated ion channels expressed on immune cells and on cells belonging to nociceptive visceral sensory pathways. P2X3 Rs, present on sensory neurons of the central and peripheral nervous systems, are activated by low levels of ATP and can be sensitized after inflammation or nerve damage. P2X4 Rs are located on sensory neurons of the central and peripheral nervous systems but also on immune cells unlike P2X7Rs, which are located predominantly on immune cells such as macrophages and microglia.35 These purinergic receptors are activated in response to cellular danger signals. It could be interesting to investigate the localization of these P2XRs in our model but unfortunately P2XRs antibodies do not work, especially in colon. P2XRs seem to be involved in visceral pain and are directly involved in the pathophysiology of IBD, with regard to intestinal inflammation but also to dysmotility and pain.36 In animals, several studies have also evidenced P2XRs involvement in visceral pain37-39 and in accordance to our findings, in post-infectious CHS,6 even if, in our model, the direct involvement of P2XRs in CHS need to be prove using P2XRs knockout mice or P2XRs antagonists.
Taking together these results show that CD-associated AIEC LF82 bacteria are able to induce CHS in infectious and post-infectious periods. The mechanism responsible for CHS could be the increase in intestinal permeability, which induces a low grade inflammation and thereafter increased P2XRs colonic expression. This overexpression of P2XRs present on neurons of the enteric nervous system may contribute to the processing of pain. CEABAC10 transgenic mice infected with AIEC LF82 could be a novel CHS model combining the etiologies of IBS and IBS and would be of interest in pharmacological studies.
Supplementary Material
Abbreviations
- AIEC
Adherent Invasive Escherichia coli
- BCG
Bacillus
- CD
Crohn's disease
- CEACAM
Carcinoembryonic antigen-related cell adhesion molecules
- CHS
Colonic hypersensitivity
- CRD
Colorectal distension
- DSS
Dextran sulfate sodium
- IBD
Inflammatory Bowel Disease
- IBS
Irritable Bowel Syndrome
- MPO
Myeloperoxidase
- PBS
Phosphate buffer saline
- PI-IBS
Post-Infectious Irritable Bowel Syndrome
- P2XRs
P2X purinergic receptors
- TG
Transgenic
- UC
Ulcerative colitis
- VMR
Visceromotor response
- WT
Wild-type
Disclosure of potential conflicts of interest
The authors have no conflict of interest to declare.
Acknowledgments
Authors would like to thank V. Martin and C. Pourpre for helpful technical assistance and Dr. A. Alloui for animal care (Animal facilities, Clermont-Ferrand, France). The manuscript has been reviewed and approved by a native English speaker, J. Watts.
Funding
This work was supported by the FEDER (Fonds Européen de Développement Régional, Auvergne : 32025), ANR VISCERALGY (ANR-09-MNPS-037–01) and the Conseil Régional d'Auvergne (MHIB grant).
Author contributions
Study concept and design: AL, AG, DA, FAC; acquisition of data: AL, LB, FAC; analysis and interpretation of data / statistical analysis: AL, DA, FAC; drafting/revision of the manuscript: AL, AG, NB, DA, FAC; obtained funding: DA, FAC; technical support: JB, BS. All authors approved the final edited version.
References
- [1].Ishihara S, Aziz M, Oshima N, Mishima Y, Imaoka H, Moriyama I, Kinoshita Y. Irritable bowel syndrome and inflammatory bowel disease: infectious gastroenteritis-related disorders? Clin J Gastroenterol. 2009;2(1):9−16. doi: 10.1007/s12328-008-0051-y. PMID:26191801 [DOI] [PubMed] [Google Scholar]
- [2].Halpin SJ, Ford AC. Prevalence of Symptoms Meeting Criteria for Irritable Bowel Syndrome in Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. Am J Gastroenterol. 2012;107(10):1474−82. doi: 10.1038/ajg.2012.260. PMID:22929759 [DOI] [PubMed] [Google Scholar]
- [3].Akbar A, Yiangou Y, Facer P, Brydon WG, Walters JR, Anand P, Ghosh S. Expression of the TRPV1 receptor differs in quiescent inflammatory bowel disease with or without abdominal pain. Gut. 2010;59(6):767−74. doi: 10.1136/gut.2009.194449. PMID:20551462 [DOI] [PubMed] [Google Scholar]
- [4].Vivinus-Nébot M, Frin-Mathy G, Bzioueche H, Dainese R, Bernard G, Anty R, Filippi J, Saint-Paul MC, Tulic MK, Verhasselt V, et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: role of epithelial barrier disruption and low-grade inflammation. Gut. 2014;63(5):744−52. doi: 10.1136/gutjnl-2012-304066. PMID:23878165 [DOI] [PubMed] [Google Scholar]
- [5].Qin H-Y, Wu JCY, Tong X-D, Sung JJY, Xu H-X, Bian Z-X. Systematic review of animal models of post-infectious/post-inflammatory irritable bowel syndrome. J Gastroenterol. 2011;46(2):164−74. doi: 10.1007/s00535-010-0321-6. PMID:20848144 [DOI] [PubMed] [Google Scholar]
- [6].Keating C, Pelegrin P, Martínez CM, Grundy D. P2X7 Receptor-Dependent Intestinal Afferent Hypersensitivity in a Mouse Model of Postinfectious Irritable Bowel Syndrome. J Immunol. 2011;187(3):1467−74. doi: 10.4049/jimmunol.1100423. PMID:21697458 [DOI] [PubMed] [Google Scholar]
- [7].Toulme E, Tsuda M, Khakh BS, Inoue K. On the Role of ATP-Gated P2X Receptors in Acute, Inflammatory and Neuropathic Pain. CRC Press/Taylor & Francis; 2010 [PubMed] [Google Scholar]
- [8].Darfeuille-Michaud A, Neut C, Barnich N, Lederman E, Di Martino P, Desreumaux P, Gambiez L, Joly B, Cortot A, Colombel JF.. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology. 1998;115(6):1405−13. doi: 10.1016/S0016-5085(98)70019-8. PMID:9834268 [DOI] [PubMed] [Google Scholar]
- [9].Barnich N, Carvalho FA, Glasser A-L, Darcha C, Jantscheff P, Allez M, Peeters H, Bommelaer G, Desreumaux P, Colombel JF, et al. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J Clin Invest. 2007;117(6):1566−74. doi: 10.1172/JCI30504. PMID:17525800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dogan B, Facey H, Jiang Z, Dogan EI, DuPont HL. SKW. Adherent-invasive E. coli is common in the feces of patients with irritable bowel syndrome. Available from https://elsevier.conference-services.net/reports/template/onetextabstract.xml?xsl=template/onetextabstract.xsl&conferenceID=3869&abstractID=881049 [Google Scholar]
- [11].Chan CHF, Stanners CP. Novel mouse model for carcinoembryonic antigen-based therapy. Mol Ther J Am Soc Gene Ther. 2004;9(6):775−85. doi: 10.1016/j.ymthe.2004.03.009 [DOI] [PubMed] [Google Scholar]
- [12].Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109−10. doi: 10.1016/0304-3959(83)90201-4. PMID:6877845 [DOI] [PubMed] [Google Scholar]
- [13].Scanzi J, Accarie A, Muller E, Pereira B, Aissouni Y, Goutte M, Joubert-Zakeyh J, Picard E, Boudieu L, Mallet C, et al. Colonic overexpression of the T-type calcium channel Ca v 3.2 in a mouse model of visceral hypersensitivity and in irritable bowel syndrome patients. Neurogastroenterol Motil. 2016;28(11):1632−1640. doi: 10.1111/nmo.12860. PMID:27196538 [DOI] [PubMed] [Google Scholar]
- [14].Meleine M, Boudieu L, Gelot A, Muller E, Lashermes A, Matricon J, Silberberg C, Theodorou V, Eschalier A, Ardid D, et al. Comparative effects of α2δ-1 ligands in mouse models of colonic hypersensitivity. World J Gastroenterol. 2016;22(31):7111−23. doi: 10.3748/wjg.v22.i31.7111. PMID:27610021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Major G, Pritchard S, Murray K, Alappadan JP, Hoad CL, Marciani L, Gowland P, Spiller R. Colon Hypersensitivity to Distension, Rather Than Excessive Gas Production, Produces Carbohydrate-Related Symptoms in Individuals With Irritable Bowel Syndrome. Gastroenterology. 2017;152(1):124−33.e2. doi: 10.1053/j.gastro.2016.09.062. PMID:27746233 [DOI] [PubMed] [Google Scholar]
- [16].Qi Q, Chen F, Zhang W, Wang P, Li Y, Zuo X. Colonic N-methyl-D-aspartate receptor contributes to visceral hypersensitivity in irritable bowel syndrome. J Gastroenterol Hepatol. 2017;32(4):828−836. doi: 10.1111/jgh.13588 [DOI] [PubMed] [Google Scholar]
- [17].Yu F-Y, Huang S-G, Zhang H-Y, Ye H, Chi HG, Zou Y, Lv RX, Zheng XB. Comparison of 5-hydroxytryptophan signaling pathway characteristics in diarrhea-predominant irritable bowel syndrome and ulcerative colitis. World J Gastroenterol. 2016;22(12):3451−59. doi: 10.3748/wjg.v22.i12.3451. PMID:27022227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Makharia GK. Understanding and Treating Abdominal Pain and Spasms in Organic Gastrointestinal Diseases. J Clin Gastroenterol. 2011;45:S89-S93. doi: 10.1097/MCG.0b013e31821fbd82. PMID:21666426 [DOI] [PubMed] [Google Scholar]
- [19].Farrell KE, Callister RJ, Keely S. Understanding and targeting centrally mediated visceral pain in inflammatory bowel disease. Front Pharmacol. 2014;5:27. doi: 10.3389/fphar.2014.00027. PMID:24634658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lacy BE, Mearin F, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. . Bowel Disorders. Gastroenterology. 2016;150(6):1393−407.e5. doi: 10.1053/j.gastro.2016.02.031 [DOI] [PubMed] [Google Scholar]
- [21].Beatty JK, Bhargava A, Buret AG. Post-infectious irritable bowel syndrome: mechanistic insights into chronic disturbances following enteric infection. World J Gastroenterol. 2014;20(14):3976−85. doi: 10.3748/wjg.v20.i14.3976. PMID:24744587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Spiller R, Garsed K. Postinfectious Irritable Bowel Syndrome. Gastroenterology. 2009;136(6):1979−88. doi: 10.1053/j.gastro.2009.02.074. PMID:19457422 [DOI] [PubMed] [Google Scholar]
- [23].Rodríguez LAG, Ruigómez A, Panés J. Acute Gastroenteritis Is Followed by an Increased Risk of Inflammatory Bowel Disease. Gastroenterology. 2006;130(6):1588−94. doi: 10.1053/j.gastro.2006.02.004. PMID:16697722 [DOI] [PubMed] [Google Scholar]
- [24].Irvine EJ, Marshall JK. Increased intestinal permeability precedes the onset of Crohn's disease in a subject with familial risk. Gastroenterology. 2000;119(6):1740−44. doi: 10.1053/gast.2000.20231. PMID:11113095 [DOI] [PubMed] [Google Scholar]
- [25].Denizot J, Sivignon A, Barreau F, Darcha C, Chan HF, Stanners CP, Hofman P, Darfeuille-Michaud A, Barnich N. Adherent-invasive Escherichia coli induce claudin-2 expression and barrier defect in CEABAC10 mice and Crohn's disease patients. Inflamm Bowel Dis. 2012;18(2):294−304. doi: 10.1002/ibd.21787. PMID:21688348 [DOI] [PubMed] [Google Scholar]
- [26].Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal Intestinal Permeability in Subgroups of Diarrhea-Predominant Irritable Bowel Syndromes. Am J Gastroenterol. 2006;101(6):1288−94. doi: 10.1111/j.1572-0241.2006.00672.x. PMID:16771951 [DOI] [PubMed] [Google Scholar]
- [27].Marshall JK, Thabane M, Garg AX, Clark W, Meddings J, Investigators Collins SM; WEL. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment Pharmacol Ther. 2004;20(11-12):1317−22. doi: 10.1111/j.1365-2036.2004.02284.x. PMID:15606393 [DOI] [PubMed] [Google Scholar]
- [28].Carvalho FA, Barnich N, Sivignon A, Darcha C, Chan CH, Stanners CP, Darfeuille-Michaud A. Crohn's disease adherent-invasive Escherichia coli colonize and induce strong gut inflammation in transgenic mice expressing human CEACAM. J Exp Med. 2009;206(10):2179−89. doi: 10.1084/jem.20090741. PMID:19737864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chassaing B, Koren O, Carvalho FA, Ley RE, Gewirtz AT. AIEC pathobiont instigates chronic colitis in susceptible hosts by altering microbiota composition. Gut. 2014;63(7):1069−80. doi: 10.1136/gutjnl-2013-304909. PMID:23896971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Agus A, Denizot J, Thévenot J, Martinez-Medina M, Massier S, Sauvanet P, Bernalier-Donadille A, Denis S, Hofman P, Bonnet R, et al. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci Rep. 2016;6:19032. doi: 10.1038/srep19032. PMID:26742586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tramullas M, Finger BC, Dinan TG, Cryan JF. Obesity Takes Its Toll on Visceral Pain: High-Fat Diet Induces Toll-Like Receptor 4-Dependent Visceral Hypersensitivity. PLoS One. 2016;11(5):e0155367. doi: 10.1371/journal.pone.0155367. PMID:27159520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Barbara G, Stanghellini V, De Giorgio R Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126(3):693−702. doi: 10.1053/j.gastro.2003.11.055. PMID:14988823 [DOI] [PubMed] [Google Scholar]
- [33].Darbaky Y, Evrard B, Patrier S, Falenta J, Garcin S, Tridon A, Dapoigny M, Silberberg C, Nivoliez A, Diop L. . Oral probiotic treatment of Lactobacillus rhamnosus Lcr35 ® prevents visceral hypersensitivity to a colonic inflammation and an acute psychological stress. J Appl Microbiol. 2017;122(1):188−200. doi: 10.1111/jam.13320. PMID:26661445 [DOI] [PubMed] [Google Scholar]
- [34].O’ Mahony SM, Dinan TG, Cryan JF. The Gut Microbiota as a Key Regulator of Visceral Pain. Pain. 2017;158(Suppl 1):S19−S28. doi: 10.1097/j.pain.0000000000000779 [DOI] [PubMed] [Google Scholar]
- [35].Burnstock G. Chapter Four – Purinergic Mechanisms and Pain. In: Advances in Pharmacology. 2016;75:91−137. doi: 10.1016/bs.apha.2015.09.001 [DOI] [PubMed] [Google Scholar]
- [36].Ochoa-Cortes F, Liñán-Rico A, Jacobson KA, Christofi FL. Potential for developing purinergic drugs for gastrointestinal diseases. Inflamm Bowel Dis. 2014;20(7):1259−87. doi: 10.1097/MIB.0000000000000047. PMID:24859298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Xu G-Y, Shenoy M, Winston JH, Mittal S, Pasricha PJ. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut. 2008;57(9):1230−37. doi: 10.1136/gut.2007.134221. PMID:18270243 [DOI] [PubMed] [Google Scholar]
- [38].Liu S, Shi Q, Zhu Q, Zou T, Li G, Huang A, Wu B, Peng L, Song M, Wu Q, et al. P2X₇ receptor of rat dorsal root ganglia is involved in the effect of moxibustion on visceral hyperalgesia. Purinergic Signal. 2015;11(2):161-9. doi: 10.1007/s11302-014-9439-y. PMID:25527178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shinoda M, La J-H, Bielefeldt K, Gebhart GF. Altered purinergic signaling in colorectal dorsal root ganglion neurons contributes to colorectal hypersensitivity. J Neurophysiol. 2010;104(6):3113−23. doi: 10.1152/jn.00560.2010. PMID:20861433 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


