ABSTRACT
The chaperone GRP78 (glucose related protein 78), also called BiP (binding immunoglobulin protein) is a key regulator of endoplasmic reticulum (ER) stress. We recently described that over-expression of GRP78 specifically in the ventromedial nucleus of the hypothalamus (VMH) releases hypothalamic ER stress in rodent obese models leading to weight loss, reduced hepatic steatosis and improved insulin and leptin sensitivity. The action of GRP78 is mediated by a feeding-independent mechanism involving increased sympathetic tone, augmented brown adipose tissue (BAT) thermogenesis and induction browning of white adipose tissue (WAT).
KEYWORDS: BAT, browning, ER stress, GRP78, hypothalamus, obesity, thermogenesis
Obesity is the consequence of elevated energy intake relative to energy expenditure. In the developed world, the prevalence of obesity and its related pathologies has increased over the last 30 years and has now reached pandemic proportions [1,2]. Thus, improving our understanding of the physiological mechanisms that regulate body weight and energy balance has become a great challenge for the scientific community.
Over last years, accumulating evidence has demonstrated that energy balance can be regulated by peripheral signals acting on the central nervous system (CNS), including the hypothalamus [3-5]. The number of studies on this topic is continuously increasing to identify new therapeutic approaches against obesity, but the precise molecular mechanisms involved remain still uncertain. Increased energy expenditure could be a target to reduce body weight [6,7], and in recent years there has been an increasing interest in the activation of the thermogenic process, especially the central control of brown adipose tissue (BAT) activity [8,9], but also in the activation of beige/brite adipocytes in the white adipose tissue (WAT), a process known as browning [7,10-12]. Theoretically, activation BAT and/or browning may represent a therapeutic strategy to combat obesity, therefore both tissues have been widely studied as promising targets against obesity and related disorders.
The hypothalamus in one of the main regulators of BAT and browning. Particularly, the ventromedial nucleus of the hypothalamus (VMH) has been shown to be widely involved in the regulation of both processes in response several peripheral signals [12-21]. Although recent data have demonstrated that AMP-activated protein kinase (AMPK) is a key regulator of thermogenesis in the VMH [5,22], there is still a black hole in the understanding of the molecular mechanism operating in this nucleus.
The endoplasmic reticulum (ER) is a cellular place where proteins are matured, assembled and folded, and any alteration in ER homeostasis disturbs this protein processing, leading to accumulation of unfolded proteins, which triggers the unfolding protein response (UPR) [23-26]. Increasing evidence has shown a strong interaction between ER stress and the pathology of obesity. ER stress is closely related with obesity-associated insulin resistance in peripheral tissues, such as pancreas and liver [27-33]. Current evidence also indicates that obesity and overnutrition-induced inflammation causes ER stress in the hypothalamus, inducing insulin and leptin resistance and, ultimately, weight gain [18,21,34-39]. Of note, improving protein folding (i.e. chemical chaperones) recovers leptin and insulin signaling, normalizing body weight [34-37,39]. Current evidence has also shown that central ceramide-induced lipotoxicity induces ER stress leading to weight gain, glucose intolerance and decreased sympathetic tone and BAT thermogenesis [18,40]. Of note the central action of ceramides can be reversed by decreasing ER stress, specifically into the VMH [18].
Despite the evidence linking hypothalamic ER stress to several metabolic actions, its potential role in the control of white fat browning and its physiological relevance remains unknown. Recent data from our group have helped to better understand the link between hypothalamic ER stress, BAT thermogenesis and WAT browning [21]. Specifically, we show that the chaperone GRP78 (glucose related protein 78), also called BiP (binding immunoglobulin protein), which is located upstream of the UPR pathway [23,25,26], acts within the VMH to exert a beneficial effect on obesity. Notably, this fact was confirmed in several types of models, such as short-term (2 months) and long-term (6 months) high fat diet (HFD)-induced obese rats, as well as in a genetic model, namely obese Zucker rats (OZR) [21]. In all these paradigms, the mechanism was common, showing feeding-independency and sympathetic control. Moreover, the fact that it also operates in OZR suggest independency of leptin signaling [21]. These results reveal that, like BAT activation, white fat browning is regulated by ER stress within the VMH and fit with the idea that stimulation of this process protects from diet induced obesity [21]. The relevance of this result is interesting because, besides decreased body weight, targeting of GRP78 elicited a marked overall improvement of the metabolic phenotype of HFD obese rats, as demonstrated by decreased adiposity, improved leptin signaling and increased insulin sensitivity (Figure 1) [21].
Figure 1.
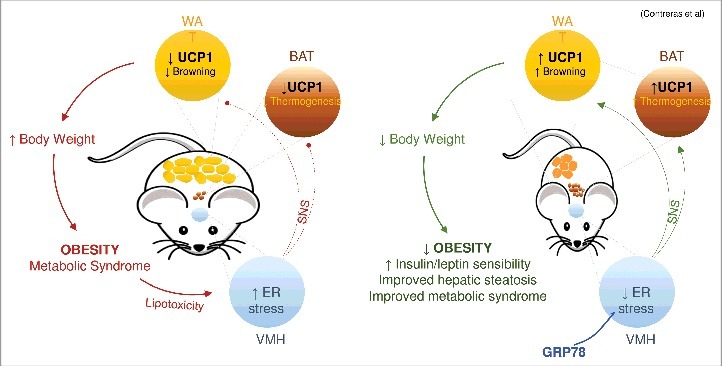
GRP78 in the VMH modulates BAT and WAT. Over-expression of GRP78 specifically in the VMH ameliorates hypothalamic ER stress in rodent obese models leading to weight loss, reduced hepatic steatosis and improved insulin and leptin sensitivity. The action of GRP78 in the VMH is mediated by a feeding-independent mechanism involving increased tone of the sympathetic nervous system (SNS), increased BAT thermogenesis and stimulation browning of WAT.
Despite the initial enthusiasm following the identification of BAT in adult humans [41-45], further data demonstrated that human BAT is mainly composed by beige/brite adipocytes cells rather than brown cells [46,47]. Browning of white fat has therapeutic potential to promote body fat reduction. Although several mechanisms have been proposed [12,48,49], the neuronal pathways within the CNS controlling WAT browning have remained largely unknown. Our study provides novel evidence that amelioration of ER stress in the VMH by GRP78 is a central mechanism regulating WAT browning. Overall, these data suggest that targeting the hypothalamic control of WAT browning may be a potential strategy against obesity and associated comorbidities. In this sense, chemical chaperones, which are a common agent for mitigating ER stress [21], have the potential to improve leptin resistance in overnutrition and overweight. For example, tauroursodeoxycholic acid (TUDCA) or 4-phenyl butyric acid (4-PBA), which ameliorate ER stress and enhances leptin sensitivity in vitro and in vivo [21,35,36], can strengthen weight loss and anorectic effects when co-administered with exogenous leptin [36]. Furthermore, 4-PBA and TUDCA have been approved by the U.S. Food and Drug Administration (FDA) and have high safety profiles in humans [50,51], thus providing an emerging therapeutic approach for metabolic diseases. Considering that our data also demonstrate that TUDCA induces BAT and browning in our HFD obese rats [21], therefore it is tempting to speculate that similar effects could be found in humans, a hypothesis that deserves further investigation.
Funding Statement
Xunta de Galicia (2015–CP079), Ministry of Economy and Competitiveness (SAF2015–71026–R) and AtresMedia. CIBER de Fisiopatología de la Obesidad y Nutrición is an initiative of ISCIII. The funders had no role in decision to publish, or preparation of the manuscript.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The author thanks Dr. Johan Fernø (University of Bergen, Norway) for his comments.
References
- [1].Clemmensen C, Muller TD, Woods SC, et al.. Gut-Brain Cross-Talk in Metabolic Control. Cell. 2017;168:758–774. doi: 10.1016/j.cell.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cui H, López M, & Rahmouni K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat. Rev. Endocrinol. 2017;13:338–351. doi: 10.1038/nrendo.2016.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schneeberger M, Gomis R, & Claret M. Hypothalamic and brainstem neurocircuitries controlling homeostatic energy balance. J. Endocrinol. 2014;220:T25-T46. doi: 10.1530/JOE-13-0398. [DOI] [PubMed] [Google Scholar]
- [4].Koch M & Horvath TL. Molecular and cellular regulation of hypothalamic melanocortin neurons controlling food intake and energy metabolism. Mol. Psychiatry. 2014;19:752–761. doi: 10.1038/mp.2014.30. [DOI] [PubMed] [Google Scholar]
- [5].López M, Nogueiras R, Tena-Sempere M, et al.. Hypothalamic AMPK: a canonical regulator of whole-body energy balance. Nat. Rev. Endocrinol. 2016;12:421–432. doi: 10.1038/nrendo.2016.67. [DOI] [PubMed] [Google Scholar]
- [6].Villarroya F & Vidal-Puig A. Beyond the sympathetic tone: the new brown fat activators. Cell Metab. 2013;17:638–643. doi: 10.1016/j.cmet.2013.02.020. [DOI] [PubMed] [Google Scholar]
- [7].Nedergaard J & Cannon B. The browning of white adipose tissue: some burning issues. Cell Metab. 2014;20:396–407. doi: 10.1016/j.cmet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- [8].Morrison SF, Madden CJ, & Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 2014;19:741–756. doi: 10.1016/j.cmet.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Contreras C, González F, Ferno J, et al.. The brain and brown fat. Ann. Med. 2015;47:150–168. doi: 10.3109/07853890.2014.919727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fisher FM, Kleiner S, Douris N, et al.. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shabalina IG, Petrovic N, de Jong JM, et al.. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 2013;5:1196–1203. doi: 10.1016/j.celrep.2013.10.044. [DOI] [PubMed] [Google Scholar]
- [12].Martinez-Sanchez N, Moreno-Navarrete JM, Contreras C, et al.. Thyroid hormones induce browning of white fat. J. Endocrinol. 2017;232:351–362. doi: 10.1530/JOE-16-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].López M, Varela L, Vázquez MJ, et al.. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat. Med. 2010;16:1001–1008. doi: 10.1038/nm.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xu Y, Nedungadi TP, Zhu L, et al.. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Whittle AJ, Carobbio S, Martins L, et al.. Bmp8b increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149:871–885. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Beiroa D, Imbernon M, Gallego R, et al.. GLP-1 Agonism Stimulates Brown Adipose Tissue Thermogenesis and Browning Through Hypothalamic AMPK. Diabetes. 2014;63:3346–3358. doi: 10.2337/db14-0302. [DOI] [PubMed] [Google Scholar]
- [17].Martínez de Morentin PB, González-García I, Martins L, et al.. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 2014;20:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Contreras C, González-García I, Martínez-Sánchez N, et al.. Central Ceramide-Induced Hypothalamic Lipotoxicity and ER Stress Regulate Energy Balance. Cell Rep. 2014;9:366–377. doi: 10.1016/j.celrep.2014.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Martins L, Seoane-Collazo P, Contreras C, et al.. A Functional Link between AMPK and Orexin Mediates the Effect of BMP8B on Energy Balance. Cell Rep. 2016;16:2231–2242. doi: 10.1016/j.celrep.2016.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Martínez-Sánchez N, Seoane-Collazo P, Contreras C, et al.. Hypothalamic AMPK-ER stress-JNK1 axis mediates the central actions of thyroid hormones on energy balance. Cell Metab. 2017;26:212–229. doi: 10.1016/j.cmet.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Contreras C, González-García I, Seoane-Collazo P, et al.. Reduction of Hypothalamic ER Stress Activates Browning of White Fat and Ameliorates Obesity. Diabetes. 2017;66:87–99. doi: 10.2337/db15-1547. [DOI] [PubMed] [Google Scholar]
- [22].López M. EJE PRIZE 2017: Hypothalamic AMPK: a golden target against obesity? Eur. J. Endocrinol. 2017;176:R235-R246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schroder M & Kaufman RJ. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- [24].Ron D & Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- [25].de Morentin PB & López M “Mens sana in corpore sano”: exercise and hypothalamic ER stress. PLoS. Biol. 2010;8:e1000464. doi: 10.1371/journal.pbio.1000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gregor MF & Hotamisligil GS. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- [27].Ozcan U, Cao Q, Yilmaz E, et al.. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- [28].Ozcan U, Yilmaz E, Ozcan L, et al.. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lipson KL, Fonseca SG, Ishigaki S, et al.. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 2006;4:245–254. doi: 10.1016/j.cmet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- [30].Kammoun HL, Chabanon H, Hainault I, et al.. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J. Clin. Invest. 2009;119:1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fu S, Watkins SM, & Hotamisligil GS. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012;15:623–634. doi: 10.1016/j.cmet.2012.03.007. [DOI] [PubMed] [Google Scholar]
- [32].Imbernon M, Sanchez-Rebordelo E, Romero-Picó A, et al.. Hypothalamic kappa opioid receptor mediates both diet-induced and melanin concentrating hormone-induced liver damage through inflammation and endoplasmic reticulum stress. Hepatology. 2016;64:1086–1104. doi: 10.1002/hep.28716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Porteiro B, Fondevila MF, Delgado TC, et al.. Hepatic p63 regulates steatosis via IKKbeta/ER stress. Nat. Commun. 2017;8:15111. doi: 10.1038/ncomms15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhang X, Zhang G, Zhang H, Karin M, et al.. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hosoi T, Sasaki M, Miyahara T, et al.. Endoplasmic reticulum stress induces leptin resistance. Mol. Pharmacol. 2008;74:1610–1619. doi: 10.1124/mol.108.050070. [DOI] [PubMed] [Google Scholar]
- [36].Ozcan L, Ergin AS, Lu A, Chung J, et al.. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- [37].Won JC, Jang PG, Namkoong C, et al.. Central Administration of an Endoplasmic Reticulum Stress Inducer Inhibits the Anorexigenic Effects of Leptin and Insulin. Obesity. (Silver. Spring). 2009;17:1861–1865. doi: 10.1038/oby.2009.194. [DOI] [PubMed] [Google Scholar]
- [38].Ropelle ER et al.. IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKâ and ER stress inhibition. PLoS. Biol. 2010;8:e1000465. doi: 10.1371/journal.pbio.1000465. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [39].Schneeberger M, Dietrich MO, Sebastián D, et al.. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell. 2013;155:172–187. doi: 10.1016/j.cell.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Turpin SM, Nicholls HT, Willmes DM, et al.. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014;20:678–686. doi: 10.1016/j.cmet.2014.08.002. [DOI] [PubMed] [Google Scholar]
- [41].Nedergaard J, Bengtsson T, & Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol Endocrinol. Metab. 2007;293:E444-E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- [42].Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al.. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- [43].Virtanen KA, Lidell ME, Orava J, et al.. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- [44].Cypess AM, Lehman S, Williams G, et al.. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zingaretti MC, Crosta F, Vitali A, et al.. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23:3113–3120. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]
- [46].Wu J, Boström P, Sparks LM, et al.. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jespersen NZ, Larsen TJ, Peijs L, et al.. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 2013;17:798–805. doi: 10.1016/j.cmet.2013.04.011. [DOI] [PubMed] [Google Scholar]
- [48].Beiroa D, Romero-Pico A, Langa C, et al.. Heterozygous deficiency of endoglin decreases insulin and hepatic triglyceride levels during high fat diet. PLoS. One. 2013;8:e54591. doi: 10.1371/journal.pone.0054591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ruan HB, Dietrich MO, Liu ZW, et al.. O-GlcNAc Transferase Enables AgRP Neurons to Suppress Browning of White Fat. Cell. 2014;159:306–317. doi: 10.1016/j.cell.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Maestri NE, Brusilow SW, Clissold DB, et al.. Long-term treatment of girls with ornithine transcarbamylase deficiency. N. Engl. J. Med. 1996;335:855–859. doi: 10.1056/NEJM199609193351204. [DOI] [PubMed] [Google Scholar]
- [51].Chen WY, Bailey EC, McCune SL, et al.. Reactivation of silenced, virally transduced genes by inhibitors of histone deacetylase. Proc. Natl. Acad. Sci. U. S. A. 1997;94:5798–5803. doi: 10.1073/pnas.94.11.5798. [DOI] [PMC free article] [PubMed] [Google Scholar]


