ABSTRACT
Background: HOX transcript antisense RNA (HOTAIR) is a long non-coding RNA (lncRNA) widely involved in the progression of numerous malignancies. Whereas, the potential molecular mechanism of HOTAIR involved in cervical cancer progression is still needed to be elaborated.
Methods: The expression of HOTAIR and miR-143-3p were detected in cervical cancer tissues and cells by qRT-PCR. MTT and flow cytometry analysis were performed to measure cell proliferation and apoptosis. Bioinformatics, Dual-Luciferase reporter and RIP were used to analyze the possible correlation between HOTAIR, miR-143-3p and BCL2. The expression of Bax and BCL2 was detected by western blot. Mice xenograft model was established to confirm the role of HOTAIR on tumor growth in vivo.
Results: HOTAIR expression was elevated while miR-143-3p expression was reduced in cervical cancer tissues and cell lines. HOTAIR knockdown suppressed proliferation and enhanced apoptosis in cervical cancer cells. Moreover, HOTAIR could function as a sponge for miR-143-3p. The inhibitory effect of HOTAIR knockdown on cervical cancer cells growth was abolished following decrease of miR-143-3p expression. Furthermore, HOTAIR promoted BCL2 expression by modulating miR-143-3p. BCL2 overexpression attenuated the tumor-suppressive effect of miR-143-3p in cervical cancer. Finally, the carcinogenicity of HOTAIR was validated in mice.
Conclusions: HOTAIR promoted cervical cancer cell growth by modulating BCL2 via miR-143-3p, hinting a novel regulatory mechanism and potential therapeutic target in cervical cancer.
KEYWORDS: BCL2, cervical cancer, HOTAIR, lncRNA, miR-143-3p
Introduction
Cervical cancer has been reported to be one of the most-frequent malignancy in females, with an apparently high morbidity and mortality globally.1 According to relevant statistics, there are more than half of million new cervical cancer cases around the world every year, and the incidence of cervical cancer in less developed countries is approximately 85% of all cases.2 Cervical cancer is difficult to diagnose at the early stage, leading to a delay for effective treatment.3 To date, despite survival time of patients was prolonged via common treatment strategies, such as surgery, radiotherapy or chemotherapy, the prognosis is still dissatisfied.4 Thus, it is extremely momentous to develop novel diagnostic or therapeutic methods for cervical cancer.
Long non-coding RNAs (LncRNAs), a novel type of non-protein coding RANs with larger than 200 nucleotides in length, are involved in the modulation of a wide variety of biological pathways and pathological development in human diseases.5 Recent data have revealed that lncRNAs can respond to external oncogenic stimuli through affecting mRNA stability, RNA splicing, chromatin structure, and miRNA-mediated gene regulation, indicating the important roles of lncRNAs in tumorigenesis.6 HOX transcript antisense RNA (HOTAIR), a well-known lncRNA with about 2158 nt in length, is specifically located between HoxC11 and HoxC12 on chromosome 12q13.13.7 Functionally, HOTAIR has been reported as an oncogenic molecule in many malignancies, including cervical cancer.8 For example, a meta-analysis elucidated that the abnormal expression of HOTAIR was closely associated with cervical cancer development, metastasis, and invasion.9 Also, HOTAIR has been found to exert tumor-promoting effect through activating cell proliferation, migration and invasion by sponging miR-175p and miR-326 in cervical cancer.10,11 However, the precise mechanism of HOTAIR during cervical cancer tumorigenesis is still far from being fully addressed.
MiRNAs, another class of endogenous non-coding RNAs with approximately 20–24 nucleotides in length, can negatively regulate gene expression through binding to complementary sites within the 3'-untranslated region (UTR) of the target mRNAs. MiRNAs actively participate in the pathologic processes of malignancies, such as cell differentiation, apoptosis and proliferation.12 MiR-143-3p has been widely reported as a tumor suppressor in multiple cancers, such as gallbladder cancer,13 breast adenocarcinoma14 and oral squamous cell carcinoma.15 Besides, miR-143-3p was previously documented to inhibit cell proliferation and induced apoptosis in cervical cancer through targeting BCL2.16
Recent studies have revealed a novel regulatory mechanism that lncRNAs can act as competing endogenous RNAs (ceRNAs) modulating cancer-related gene expression through competitive sharing miRNAs.17,18 Here, we aimed to investigate whether HOTAIR could regulate cervical cancer progression by sponging miR-143-3p. As a result, we found a significant elevation of HOTAIR in cervical cancer tissues and cell lines. Moreover, HOTAIR downregulation suppressed proliferation and induced apoptosis in cervical cancer cells. Also, HOTAIR acted as a ceRNA to modulate BCL2 expression via competitively binding to miR-143-3p. Our study elaborated a new HOTAIR-miR-143-3p-BCL2 regulatory pathway in the development of cervical cancer, providing a potential biomarker and therapeutic target for cervical cancer.
Materials and methods
Patients
Cervical cancer tumor tissues and corresponding normal tissues were obtained from 22 patients by surgical resection. The fresh specimens were immediately frozen in liquid nitrogen and stored at -80°C for the extraction of total RNA. This study was approved by the Research Ethic Committee of the first Affiliated Hospital of Zhengzhou University. Informed consents were signed by all patients prior to participating in this study.
Cell culture and transfection
Human cervical cancer cell lines (SiHa, HeLa, Caski, c4-1) and immortalized human epidermal cells (HaCaT) were obtained from American type culture collection (ATCC, Rockefeller, MD, USA). These cells were grown in RPMI-1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, Gibco) and cultured at 37°C in a humidified air with 5% CO2.
HOTAIR-overexpressing plasmid (pcDNA3.1-HOTAIR) and BCL2-overexpressing plasmid (pcDNA3.1-BCL2) were established by inserting the full-length HOTAIR or BCL2 cDNA sequences into pcDNA3.1 vector (Invitrogen, Carlsbad, CA, USA). siRNA for HOTAIR knockdown (si-HOTAIR), siRNA for BCL2 downregulation (si-BCL2) or scramble control (si-NC) were synthesized by GenePharma Co. Ltd. (Shanghai, China). miR-143-3p mimics, miR-143-3p inhibitor (anti-miR-143-3p), miRNA scramble control (miR-NC) and inhibitor control (anti-miR-NC) were also purchased from GenePharma Co. Ltd. (Shanghai, China). SiHa and HeLa cells were plated in 24-well culture plates for 24 h, followed by transfected with different oligonucleotides or plasmids using Lipofectamine 2000 (Invitrogen).
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from cervical cancer tissues and cells by Trizol reagent (Invitrogen). Then HOTAIR was reversely transcribed into cDNA using High Capagity Reverse Transcription System Kit (Takara, Dalian, China), with GAPDH as a house-keeping gene. MiRNA-143-3p was inversely transcribed into cDNA using miRNA First-Stand cDNA Synthesis Kit (GeneCopoeia, Guangzhou, China) with U6 as a house-keeping gene. Samples were quantified using Universal SYBR Green PCR Kit (Takara, Dalian, China) on Applied Biosystems 7500 Real-time PCR Systems (Thermo Fisher Scientific, USA). The primers for miR-143-3p, U6 and GAPDH were purchased from ABM (Peterborough, Canada). HOTAIR primers were displayed as below: 5'- TTT GGA CTG TAA AAT ATG GC -3' (forward) and 5'- TTC TGA CAC TGA ACG GACT -3' (reverse). The relative quantification of gene expression was calculated by the 2−ΔΔCt method. All samples were detected in a triplicate manner.
Cell proliferation and apoptosis assays
The cell proliferation analysis was performed using MTT colorimetric kit (Dojindo, Tokyo, Japan) according to manufacturer's instructions. Cervical cancer cells were diluted into the density of 1 × 105 /well and then transfected with indicated oligonucleotides or plasmids. At 0, 24, 48 and 72 h post-transfection, 0.5 mg/mL of MTT reagent was added for another 4 h incubation, followed by addition of DMSO to resolve the generated formazan. Finally, the absorbance at 490 nm was measured by a microplate reader.
Cell apoptosis assay was performed using Annexin V-FITC/PI Apoptosis Detection Kit (BestBio, Shanghai). About 48 h after transfection, cells were digested with trypsin (without EDTA), washed with PBS and resuspended in binding buffer, then equal amount of Annexin V-FITC and propidium iodide (PI) were introduced. Apoptotic cells were observed by flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA).
Dual-Luciferase Reporter assay
Partial sequences of HOTAIR and BCL2 3′UTR containing wide-type or mutant-type miR-143-3p binding sites were amplified by PCR and then subcloned into pGL3-Basic luciferase vector (Promega, Madison, WI, USA) respectively. Then the generated WT-HOTAIR, Mut-HOTAIR, WT-BCL2-3′UTR or Mut-BCL2-3′UTR reporter plasmids were transfected into cervical cancer cells along with pRL-TK vector (Promega), and miR-NC, miR-143-3p, anti-miR-NC or anti-miR-NC. About 48h after transfection, Dual-Luciferase Reporter assay system (Promega) was used to detect luciferase activity.
RNA immunoprecipitation (RIP)
RIP experiments were performed in SiHa or HeLa cells according to the manufacturer's instructions of Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (Millipore, Bedford, MA, USA). Subsequently, RNA was subjected to qRT-PCR for the detection of HOTAIR and miR-143-3p.
Western-blot assay
Total proteins were extracted from SiHa or HeLa cells by cell lysis buffer (Huashun, Shanghai, China). Equal amounts of proteins were divided by 10% SDS-PAGE, and then transferred to PVDF membranes (Millipore, Billerica, MA, USA). After blocking with 5% non-fat milk powder for 2 h, specific antibodies against BCL2 and Bax (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were added and incubated overnight at 4°C. Subsequently, the membranes were washed by TBST buffer for three times and incubated with HRP-conjugated anti-rabbit secondary antibody (Cell Signaling Technology, Inc, Danvers, MA, USA) for 1 h. Finally, protein bands were visualized by chemiluminescence assay kits (BestBio, Shanghai,China).
Caspase-3 activity assay
Caspase-3 activity was measured using Caspase-3 Colorimetric Assay Kit (Biovision, Shanghai, China) according to manufacturer's instructions. The OD values of samples were detected using a microplate reader (Bio-TekELX800, USA) at a wavelength of 405 nm. Then standard curve was plotted with different concentrations of standards as the abscissa and OD values as the ordinate.
Plasmid constructs and in vivo experiments
Sequences of HOTAIR cDNA or sh-RNA targeting HOTAIR (sh-HOTAIR) were subcloned into the pLenti6/V5-D-TOPO vector (Invitrogen). Then the constructed Lenti-HOTAIR or Lenti-sh-HOTAIR vectors were transfected into SiHa cells. A total of 1 × 107 transfected SiHa cells were inoculated into female BALB/c nude mice (6-7 weeks old, HFK bio-Technology, Beijing, China) through subcutaneous infection. Tumor volumes were measured every 4 days. At 31 days after tansplantation, mice were sacrificed, and tumors were collected for weigh calculation and HOTAIR, miR-143-3p or BCL2 expression analysis. The experimental procedures were performed following the Guidelines for Care and Use of Laboratory Animal with approval of Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
Statistical analysis
All data were analyzed using SPSS 20.0 software (SPSS, Chicago, IL, USA) with Student's t-test and one-way ANOVA to estimate the significant group differences. All results were shown as means ± standard deviation (SD). P < 0.05 represented the difference was statistically significant.
Results
HOTAIR expression was increased and miR-143-3p expression was decreased in cervical cancer tissues and cell lines
The expression of HOTAIR and miR-143-3p in cervical cancer tissues and cell lines were firstly detected by qRT-PCR. Results showed that HOTAIR appeared an up-regulated expression in cervical cancer tissues compared with adjacent normal tissues (Fig. 1A), while miR-143-3p expression was suppressed in cervical cancer tissues compared to that in corresponding non-cancerous tissues (Fig. 1B). Subsequently, Pearson correlation analysis displayed an inverse correlativity between HOTAIR and miR-143-3p (Fig. 1C). Moreover, expression levels of HOTAIR and miR-143-3p in cervical cancer cell lines (SiHa, HeLa, Caski, c4-1) were also measured, and results revealed a higher expression of HOTAIR (Fig. 1D) and a lower expression of miR-143-3p (Fig. 1E) in cervical cancer cells compared with those in normal HaCaT cells. Due to the most obvious expression change of HOTAIR and miR-143-3p, SiHa and HeLa cells were selected for subsequent function and mechanism analysis. As a conclusion, HOTAIR and miR-143-3p might be involved in the pathogenesis of cervical cancer.
Figure 1.
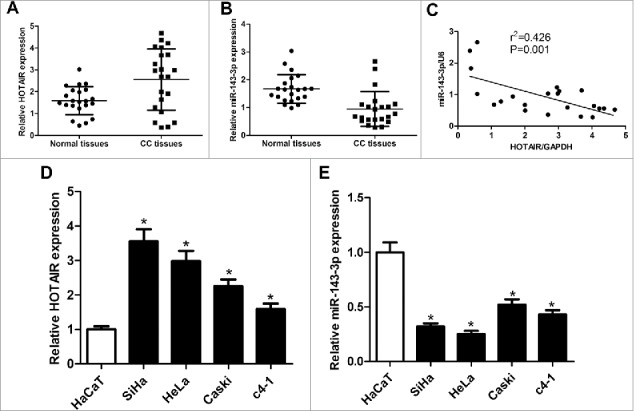
The expression of HOTAIR and miR-143-3p in cervical cancer tissues and cell lines. The expression of HOTAIR (A)and miR-143-3p (B) in 22 cervical cancer tumor tissues and adjacent normal tissues. (C) The correlation analysis of HOTAIR and miR-143-3p levels in 22 cervical cancer tissues. The expression of HOTAIR(D) and miR-143-3p (E) in cervical cancer cell lines (SiHa, HeLa, Caski, c4-1) and immortalized human epidermal cells HaCaT.
HOTAIR overexpression accelerated cervical cancer cell growth
To investigate the function of HOTAIR in cervical cancer, HOTAIR overexpression was performed in SiHa cells and HOTAIR knockdown was executed in and HeLa cells. qRT-PCR analysis revealed a significant increase of HOTAIR expression in pcDNA-HOTAIR-transfected SiHa cells and a significant decrease of HOTAIR expression in si-HOTAIR-transfected HeLa cells (Fig. 2A). Subsequently, the proliferation and apoptosis ability of SiHa and HeLa cells were detected by MTT and flow cytometry assays. Overexpression of HOTAIR markedly promoted the proliferation of SiHa cells, however, suppression of HOTAIR was able to inhibit proliferation of HeLa cells (Fig. 2B). Moreover, apoptotic rate of HeLa cells was obviously increased under the action of HOTAIR inhibitor compared with that in control group (Fig. 2C). As expected, results also showed that HOTAIR knockdown notably enhanced the activity of caspase-3 in HeLa cells (Fig. 2D). All these results indicated the potential carcinogenicity of HOTAIR in cervical cancer.
Figure 2.
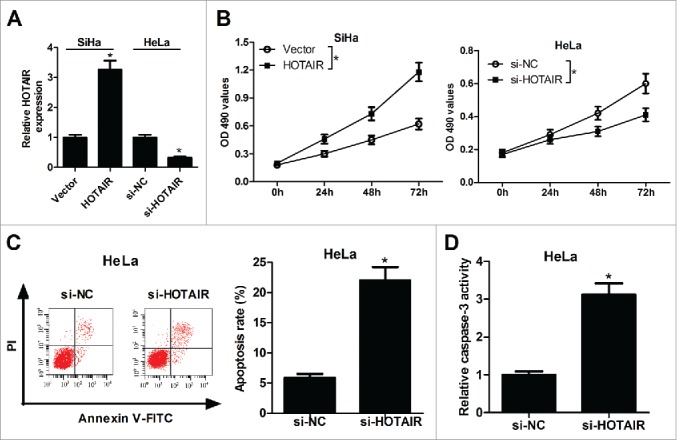
HOTAIR stimulated the growth of cervical cancer cells. (A) HOTAIR expression levels were detected by qRT-PCR in pcDNA-HOTAIR-transfected SiHa cells and si-HOTAIR-transfected HeLa cells. (B) The effects of HOTAIR overexpression or knockdown on the proliferation activity of SiHa and HeLa cells were assessed by MTT assay. (C) The effects of HOTAIR knockdown on apoptosis of HeLa cells were detected by flow cytometry analysis. (D) The effects of HOTAIR deficiency on caspase-3 activity were measured by colorimetric assay in SiHa and HeLa cells.
HOTAIR acted as a molecular sponge for miR-143-3p
For the further investigation of carcinogenic mechanism of HOTAIR on cervical cancer, miRcode online website was used to assay the identifiable miRNA sequences on HOTAIR. The prediction results displayed the existence of recognition sites between miR-143-3p and HOTAIR (Fig. 3A). To further demonstrate the functional interaction between miR-143-3p and HOTAIR, dual-luciferase reporter, RIP and qRT-PCR analysis were performed respectively. As shown in Fig. 3B, the luciferase activity of wild-type HOTAIR reporter was suppressed with the transfection of miR-143-3p in SiHa cells, and it was enhanced following transfection of miR-143-3p inhibitor inHeLa cells. However, there was no significant change of luciferase activity in mutant-type HOTAIR reporter after overexpressing or inhibiting miR-143-3p. RIP analysis was conducted to analyze the potentially endogenous interaction between HOTAIR and miR-143-3p. As our expected, both miR-143-3p and HOTAIR were greatly enriched by Ago2 antibody in SiHa cells (Fig. 3C). Moreover, qRT-PCR assay showed that HOTAIR upregualation notably suppressed the expression of miR-143-3p, inversely, HOTAIR knockdown induced miR-143-3p expression in SiHa and HeLa cells (Fig. 3D). These results indicated that HOTAIR negatively regulated miR-143-3p expression via direct interaction.
Figure 3.
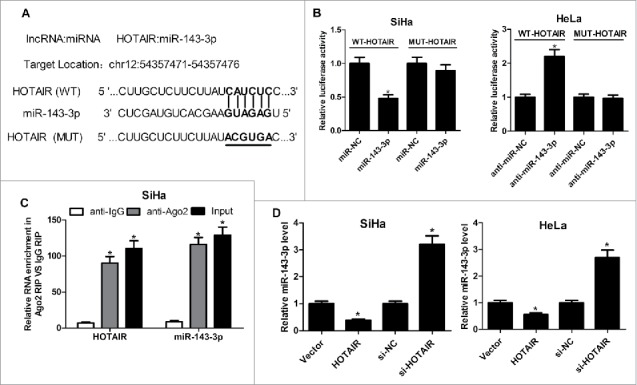
HOTAIR acted as a molecular sponge for miR-143-3p. (A) The predicted binding sites between miR-143-3p and HOTAIR, as well as the mutant sites in the mutant-HOTAIR luciferase vector. (B) Luciferase reporter assays were used to detect luciferase activity of SiHa or HeLa cells transfected with WT-HOTAIR or MUT-HOTAIR and (miR-NC or miR-143-3p, anti-miR-NC or anti-miR-143-3p). (C) RIP analysis was performed to assess the enrichment degree of HOTAIR and miR-143-3p by Ago2 antibody in SiHa cells. (D) qRT-PCR assay were utilized to measure miR-143-3p levels in SiHa or HeLa cells after transfected with HOTAIR or si-HOTAIR.
miR-143-3p upregulation reversed the promotive effect of HOTAIR on cervical cancer cells growth
To understand whether the carcinogenic role of HOTAIR was mediated by miR-143-3p, further restoration assays were performed in SiHa and HeLa cells. MTT analysis results showed that miR-143-3p overexpression markedly reversed the pro-proliferation activity of HOTAIR in SiHa cells, conversely, miR-143-3p downregulation weakened the anti-proliferation ability of si-HOTAIR in HeLa cells (Fig. 4A). Flow cytomerty analysis demonstrated that HOTAIR-knockdown-induced apoptosis was evidently reduced after inhibiting miR-143-3p expression in HeLa cells (Fig. 4B). Similarly, si-HOTAIR-mediated increase in Bax expression (Fig. 4C) and caspase-3 activity (Fig. 4D), as well as si-HOTAIR-elicited decrease in BCL2 expression (Fig. 4C), was remarkably attenuated after downregulating miR-143-3p in HeLa cells. These results indicated that HOTAIR accelerated cell proliferation and inhibited apoptosis by lowering miR-143-3p expressionin cervical cancer.
Figure 4.
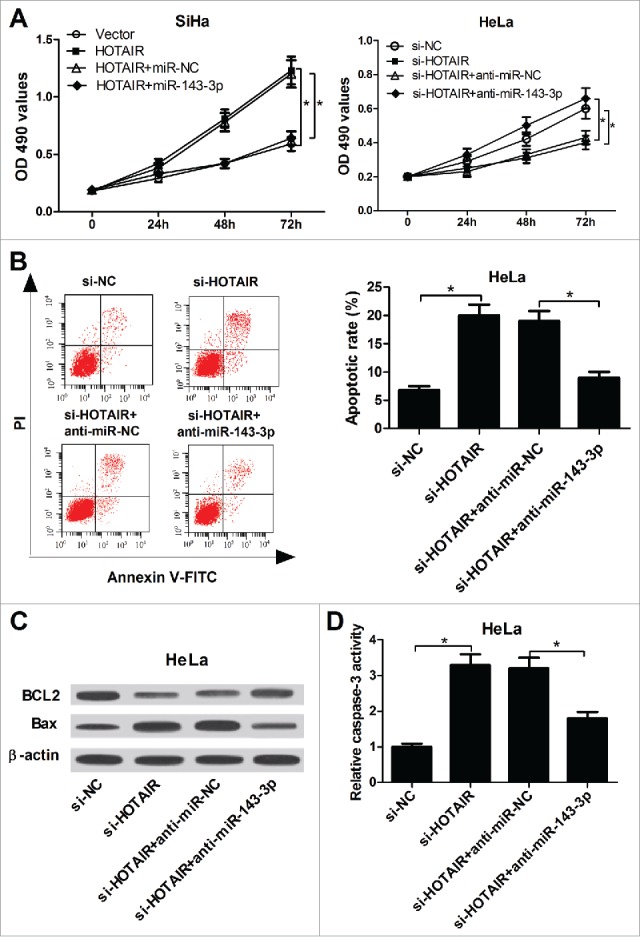
miR-143-3p reversed the promotion of HOTAIR on the growth of cervical cancer cells. (A) The impacts of miR-143-3p on HOTAIR-mediated pro-proliferation, as well as the effects of anti-miR-143-3p on si-HOTAIR-mediated anti-proliferation in SiHa and HeLa cells were determined by MTT assay. (B) The effect of anti-miR-143-3p on si-HOTAIR-mediated pro-apoptosis in HeLa cells was displayed by flow cytometry. (C and D) The regulatory effects of anti-miR-143-3p on BCL2 and Bax expression, as well as caspase-3 activity in si-HOTAIR-transfected HeLa cells.
HOTAIR regulates BCL2 expression via competitively interaction with miR-143-3p
To further identify the mechanism of miR-143-3p influencing cervical cancer behavior, TargetScan software was employed to predict the potential candidate target mRNAs of miR-143-3p. The result indicated the possibility of a specific interaction between miR-143-3p and BCL2 through some complementary sites (Fig. 5A). To further verify the true binding between miR-143-3p and BCL2, luciferase assay was carried out in SiHa and HeLa cells. As expected, miR-143-3p overexpression suppressed the luciferase activity of WT-BCL2-3’UTR in SiHa cells, and miR-143-3p inhibition improved the luciferase activity of WT-BCL2-3’UTR in HeLa cells (Fig. 5B). However, these regulatory effects were disappeared in MUT-BCL2-3’UTR, of which miR-143-3p binding sites were mutated. Western blot analysis result showed that BCL2 expression was decreased in SiHa cell after transfection with miR-143-3p, while BCL2 level was increased in HeLa cells after transfection with miR-143-3p inhibitor (Fig. 5C). These data suggested that BCL2 was a direct target of miR-143-3p.
Figure 5.
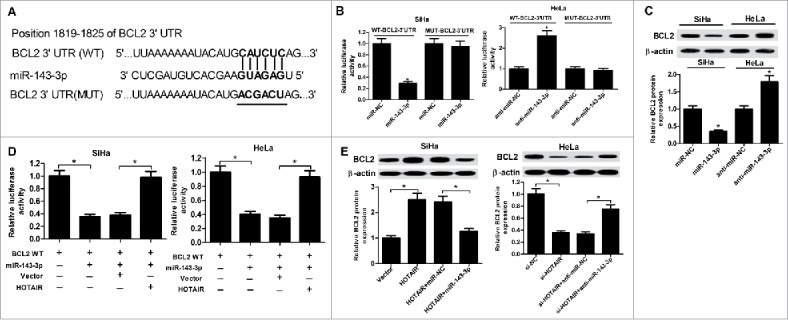
BCL2 was a target gene of miR-143-3p. (A) The putative binding sites between BCL2 3′UTR and miR-143-3p, and the mutation in 3′UTR of BCL2. (B) Luciferase activity analysis was performed to measure luciferase activity in SiHa and HeLa cells transfected with WT-BCL2-3′UTR or MUT-BCL2-3′UTR and (miR-NC or miR-143-3p, anti-miR-NC or anti-miR-143-3p). (C) Western blot analysis was utilized to measure BCL2 expression at protein level in miR-143-3p-introduced SiHa cells and anti-miR-143-3p-introduced HeLa cells. (D) The regulation of HOTAIR on the luciferase activity of BCL-WT reporter co-transfected with miR-143-3p in SiHa and HeLa. (E) The suppression of miR-143-3p on HOTAIR-triggered enhancement of BCL2 expression in SiHa cells and the promotion of anti-miR143-3p on si-HOTAIR-induced decrease of BCL2 expression in HeLa cells were determined by western blot assay.
Subsequently, the interactions among HOTAIR, miR-143-3p, and BCL2 were further observed in SiHa and HeLa cells. As shown in Fig. 5D, HOTAIR overexpression weakened the inhibition of miR-143-3p on luciferase activity of BCL2-WT reporter plasmid in SiHa and HeLa cells. Western blot results revealed that enforced expression of HOTAIR could positively regulate BCL2 expression, whereas restoration of miR-143-3p expression reversed the promotion of HOTAIR on BCL2 expression in SiHa cells. Besides that, HOTAIR knockdown negatively regulated BCL2 expression, however, this effect was abated in HeLa cells after co-transfected with miR-143-3p inhibitor (Fig. 5E). In conclusion, HOTAIR regulated the expression of BCL2 via competitively binding to miR-143-3p.
BCL2 upregulation reversed the inhibition of miR-143-3p on cervical cancer cells growth
Restoration assays were performed to further investigate the impacts of BCL2 on miR-143-3p-mediated anti-proliferation and pro-apoptotic effect in cervical cancer cells. MTT assay result showed that BCL2 overexpression obviously reversed the inhibition of miR-143-3p on SiHa cells proliferation, while BCL2 knockdown abolished the promotion of anti-miR-143-3p on HeLa cells proliferation (Fig. 6A). Flow cytometry analysis result revealed that restoration expression of BCL2 lowered miR-143-3p-induced apoptosis in SiHa cells (Fig. 6B). Also, miR-143-3p-triggered increase in caspase-3 activity (Fig. 6C) and Bax expression (Fig. 6D), as well as miR-143-3p-triggered decrease in BCL2 expression (Fig. 6D), were attenuated in SiHa cells after co-transfection with BCL2-overexpressing plasmid. These results indicated that miR-143-3p blocked proliferation and enhanced apoptosis in cervical cancer cells by targeting BCL2.
Figure 6.
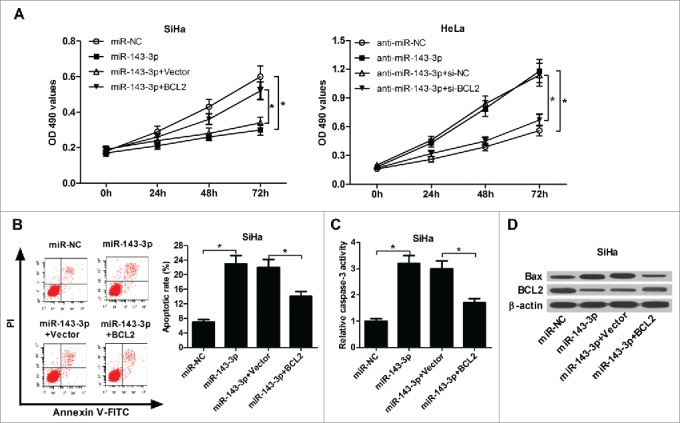
The inhibitory effect of BCL2 on miR-143-3p-modulated anti-proliferation and pro-apoptosis in cervical cancer cells. (A) The impact of BCL2 on miR-143-3p-induced suppression of SiHa cells proliferation, as well as the effects of si-BCL2 on anti-miR-143-3p-mediated promotion of HeLa cells proliferation were determined by MTT analysis. The effects of BCL2 on apoptosis (B), caspase-3 activity (C), as well as Bax and BCL2 expression (D) in miR-143-3p-transfected SiHa cells.
HOTAIR overexpression promoted tumor growth of cervical cancer in vivo
To explore the impact of HOTAIR on tumorigenesis of cervical cancer in vivo, SiHa cells infected with lenti-HOTAIR or lenti-sh-HOTAIR were injected into the BALB/c nude mice to construct xenograft model. A significant increase in tumor volume and weight was observed in the lenti-HOTAIR group and a significant decrease in tumor volume and weight was displayed in the lenti-sh-HOTAIR group compared with those in control group (Fig. 7A-C). Subsequently, the expression of HOTAIR, miR-143-3p and BLC2 in different groups of lumps were measured by qRT-PCR and western-blot. As shown in Fig. 7D-F, HOTAIR and BCL2 expressions were elevated, while miR-143-3p expression was reduced in tumor masses derived from lenti-HOTAIR-transfected SiHa cells. Conversely, it was observed a downregulation of HOTAIR and BCL2 expressions, and an upregulation of miR-143-3p expression in tumor tissues of lenti-sh-HOTAIR group. These results confirmed the carcinogenesis of HOTAIR in cervical cancer progression in vivo.
Figure 7.
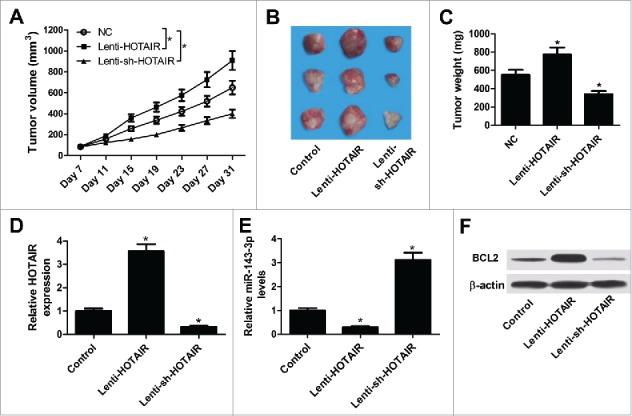
Effects of HOTAIR on tumor growth in vivo. (A) Tumor volumes were calculated every 4 days. (B) Representative images of resected tumor masses in different groups. (C) Tumor weights were evaluated in different groups. The expression levels of HOTAIR (D), miR-143-3p (E) and BCL2 (F) in lumps were detected by qRT-PCR or western-blot.
Discussion
Due to the delay-diagnosis and poor therapeutic efficacy in cervical cancer, it is urgent to illuminate the underlying molecular mechanisms of cervical cancer progression, with a purpose to lay a foundation of developing a novel targeted-therapeutic strategy. As the research moves along, lncRNAs have attracted extensive attention owing to the involvement in multiple cancers, including cervical cancer.19,20 Although a small number of lncRNA appeared functional characteristic, it is also needful to identify a novel lncRNA associated with the progression of cervical cancer.
LncRNA HOTAIR has been found to be highly expressed and is considered to be a tumor activator in many human malignancies.21 A previous report stated that the lack of HOTAIR effectively suppresses tumor growth in cervical cancer by attenuating p21 expression.22 HOTAIR was also revealed to promote cervical cell growth and invasion through regulating the Notch-Wnt signal pathway and epithelial-mesenchymal transition (EMT).23 Moreover, HOTAIR overexpression facilitated the proliferation, migration and invasion of cervical cancer cells by modulating human leucocyte antigen-G (HLA-G) expression via competitively binding to miR-148a.24 In present study, we observed the apparent increase of HOTAIR level both in cervical cancer tissue and cell lines. Just as we expected, further gain- and loss-of-function research illuminated the pro-proliferative and anti-apoptotic effect of HOTAIR in cervical cells. Xenograft model experiment also indicated the prominent acceleration of HOTAIR on tumor growth in BALB/c nude mice. These results indicated that HOTAIR might participate in the progression of cervical cancer as a tumor-activator.
Accumulated studies have confirmed that lncRNAs could act as molecular sponges of miRNAs to regulate the expression of interrelated miRNA. In this research, to better expound the inter-mechanism of HOTAIR in cervical cancer, a series of related analysis were conducted and the results certified the complementary binding of HOTAIR and miR143-3p. MiR-143-3p expression was found to be downregulated in cervical cancer tissues and cells. Restoration assay further revealed the inhibiting effect of miR-143-3p on HOTAIR-modulated pro-proliferation and anti-apoptosis in cervical cells. The tumor-suppressive effects of miR-143-3p in cervical cancer have been elucidated in previous documents.14,25 Previously, miR-143 was reported to promote the pathogenesis of cervical cancer via direct modulation of BCL2.16 microRNA-143/Bcl-2-Bax signaling pathway was found to be implicated in 5-Aminolevulinic acid photodynamic therapy in human cervical cancer.26 Therefore, we further explored whether the HOTAIR could regulate cervical cancer progression by miR-143-3p/BCL2 pathway. Mechanistic analysis demonstrated that HOTAIR upregulated BCL2 expression by sponging miR-143-3p. Moreover, BCL2 overexpression abolished the inhibitory effect of miR-143-3p on cervical cancer growth. In vivo experiments also discovered a decrease of miR-143-3p expression and an increase of BCL2 expression in tumor tissues of Lenti-HOTAIR group. All these data suggested that HOTAIR promoted the progression of cervical cancer through regulating miR-143-3p/BCL2 axis.
In conclusion, our studies revealed the stimulative effect of HOTAIR and the inhibitory effect of miR-143-3p on cervical cancer progression. HOTAIR could function as a ceRNA to modulate miR-143-3p expression. Besides that, we also certified that HOTAIR competed with BCL2 to share miR-143-3p and then affected cervical cancer progression. These findings demonstrated a novel HOTAIR/miR-143-3p/BCL2 regulatory pathway in cervical cancer, indicating that HOTAIR might be a potential therapeutic target for cervical cancer.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Ventriglia J, Paciolla I, Pisano C, Cecere SC, Di NM, Tambaro R, Califano D, Losito S, Scognamiglio G, Setola SV, et al.. Immunotherapy in ovarian, endometrial and cervical cancer: State of the art and future perspectives. Cancer Treat Rev. 2017;59:109–16. doi: 10.1016/j.ctrv.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Manaf RA, Ismail S, Cecilia NC. Global Burden of Cervical Cancer: A Literature Review. Int J Pub Health Clin Sci. 2017;4:10–18. [Google Scholar]
- 3.Noordhuis MG, Fehrmann RS, Wisman GB, Nijhuis ER, van Zanden JJ, Moerland PD, Ver Loren van Themaat E, Volders HH, Kok M, ten Hoor KA, et al.. Involvement of the TGF-beta and beta-catenin pathways in pelvic lymph node metastasis in early-stage cervical cancer. Clin Cancer Res. 2011;17:1317–30. doi: 10.1158/1078-0432.CCR-10-2320. [DOI] [PubMed] [Google Scholar]
- 4.Junichi K, Noriko S, Satoko M, Tomoyuki K, Keiichiro N, Atsushi H, Yuji H. Prognostic factors in stage IB-IIB cervical adenocarcinoma patients treated with radical hysterectomy and pelvic lymphadenectomy. J Surg Oncol. 2010;101:413–17. doi: 10.1002/jso.21499. [DOI] [PubMed] [Google Scholar]
- 5.Bunch H. Gene regulation of mammalian long non-coding RNA. Mol Genet Genomics. 2017:1–15. doi: 10.1007/s00438-017-1370-9. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 6.Kondo Y, Shinjo K, Katsushima K. Long non‐coding RNAs as an epigenetic regulator in human cancers. Cancer Sci. 2017;108:1927–33. doi: 10.1111/cas.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough H, Helms JA, Farnham PJ, Segal E, et al.. Functional demarcation of active and silent chromatin domains in human HOX Loci by Non-Coding RNAs. Cell 2007;129:1311–23. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hajjari M, Salavaty A. HOTAIR:an oncogenic long non-coding RNA in different cancers. Cancer Biol Med. 2015;12:1–9. doi: 10.7497/j.issn.2095-3941.2015.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu S, Zhang M, Qu P. Expression level and clinical significance of HOX transcript antisense intergenic RNA in cervical cancer: a meta-analysis. Sci Rep. 2016;6:38047. doi: 10.1038/srep38047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji F, Wuerkenbieke D, He Y, Ding Y. Long noncoding RNA HOTAIR: An oncogene in human cervical cancer interacting with MicroRNA-17-5p. Oncol Res. 2017. doi: 10.3727/096504017X15002869385155. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu X, Cao X, Chen F. LncRNA-HOTAIR activates tumor cell proliferation and migration by suppressing MiR-326 in cervical cancer. Oncol Res. 2017. doi: 10.3727/096504017X15037515496840. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 12.Esquelakerscher A, Slack FJ. Oncomirs – microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–69. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 13.He M, Zhan M, Chen W, Xu S, Long M, Shen H, Shi Y, Liu Q, Mohan M, Wang J. MiR-143-5p deficiency triggers EMT and metastasis by targeting HIF-1α in Gallbladder cancer. Cell Physiol Biochem. 2017;42:2078–92. doi: 10.1159/000479903. [DOI] [PubMed] [Google Scholar]
- 14.Tavanafar F, Safaralizadeh R, Hosseinpourfeizi MA, Mansoori B, Shanehbandi D, Mohammadi A, Baradaran B. Restoration of miR-143 expression could inhibit migration and growth of MDA-MB-468 cells through down-regulating the expression of invasion-related factors. Biomed Pharmacother. 2017;91:920–4. doi: 10.1016/j.biopha.2017.04.119. [DOI] [PubMed] [Google Scholar]
- 15.Sun X, Lei Z. MicroRNA-143 suppresses oral squamous cell carcinoma cell growth, invasion and glucose metabolism through targeting hexokinase 2. Biosci Rep. 2017;37:BSR20160404. doi: 10.1042/BSR20160404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu L, Yu X, Guo X, Tian Z, Su M, Long Y, Huang C, Zhou F, Liu M, Wu X, et al.. miR-143 is downregulated in cervical cancer and promotes apoptosis and inhibits tumor formation by targeting Bcl-2. Mol Med Rep. 2012;5:753–60. doi: 10.3892/mmr.2011.696. [DOI] [PubMed] [Google Scholar]
- 17.Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–52. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan JY, Sirey T, Honti F, Graham B, Piovesan A, Merkenschlager M, Webber C, Ponting CP, Marques AC. Extensive microRNA-mediated crosstalk between lncRNAs and mRNAs in mouse embryonic stem cells. Genome Res. 2015;25:655–66. doi: 10.1101/gr.181974.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong J, Su M, Chang W, Zhang K, Wu S, Xu T. Long non-coding RNAs on the stage of cervical cancer (Review). Oncol Rep. 2017;38:1923–31. doi: 10.3892/or.2017.5905. [DOI] [PubMed] [Google Scholar]
- 20.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–41. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y, Zhang L, Wang Y, Li H, Ren X, Wei F, Yu W, Wang X, Zhang L, Yu J, et al.. Long noncoding RNA HOTAIR involvement in cancer. Tumor Biol. 2014;35:9531–8. doi: 10.1007/s13277-014-2523-7. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Wang Y, Dong R, Yu J, Qiu H. HOTAIR enhanced aggressive biological behaviors and induced radio-resistance via inhibiting p21 in cervical cancer. Tumor Biol. 2015;36:3611–9. doi: 10.1007/s13277-014-2998-2. [DOI] [PubMed] [Google Scholar]
- 23.Lee M, Kim HJ, Sang WK, Park SA, Chun KH, Cho NH, Yong SS, Kim YT. The long non-coding RNA HOTAIR increases tumour growth and invasion in cervical cancer by targeting the Notch pathway. Oncotarget. 2016;7:44558–71. doi: 10.18632/oncotarget.10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun J, Chu H, Ji J, Huo G, Song Q, Zhang X. Long non-coding RNA HOTAIR modulates HLA-G expression by absorbing miR-148a in human cervical cancer. Int J Oncol. 2016;49:943–52. doi: 10.3892/ijo.2016.3589. [DOI] [PubMed] [Google Scholar]
- 25.Zheng F, Zhang J, Luo S, Yi J, Wang P, Zheng Q, Wen Y. miR-143 is associated with proliferation and apoptosis involving ERK5 in HeLa cells. Oncol Lett. 2016;12:3021–7. doi: 10.3892/ol.2016.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo Q, Dong B, Nan F, Guan D, Zhang Y. 5-Aminolevulinic acid photodynamic therapy in human cervical cancer via the activation of microRNA-143 and suppression of the Bcl-2/Bax signaling pathway. Mol Med Rep. 2016;14:544–50. doi: 10.3892/mmr.2016.5248. [DOI] [PubMed] [Google Scholar]


