Abstract
Purpose
The trabecular meshwork (TM) has an important role in the regulation of aqueous humor outflow and IOP. Regulation of the extracellular matrix (ECM) by TGFβ2 has been studied extensively. Bone morphogenetic protein (BMP) and activin membrane-bound inhibitor (BAMBI) has been shown to inhibit or modulate TGFβ2 signaling. We investigate the role of TGFβ2 and BAMBI in the regulation of TM ECM and ocular hypertension.
Methods
Mouse TM (MTM) cells were isolated from B6;129S1-Bambitm1Jian/J flox mice, characterized for TGFβ2 and dexamethasone (DEX)–induced expression of fibronectin, collagen-1, collagen-4, laminin, α-smooth muscle actin, cross-linked actin networks (CLANs) formation, and DEX-induced myocilin (MYOC) expression. MTM cells were transduced with Ad5.GFP to identify transduction efficiency. MTM cells and mouse eyes were transduced with Ad5.Null, Ad5.Cre, Ad5.TGFβ2, or Ad5.TGFβ2 + Ad5.Cre to evaluate the effect on ECM production, IOP, and outflow facility.
Results
MTM cells express TM markers and respond to DEX and TGFβ2. Ad5.GFP at 100 MOI had the highest transduction efficiency. Bambi knockdown by Ad5.Cre and Ad5.TGFβ2 increased fibronectin, collagen-1, and collagen-4 in TM cells in culture and tissue. Ad5.Cre, Ad5.TGFβ2, and Ad5.TGFβ2 + Ad5.Cre each significantly induced ocular hypertension and lowered aqueous humor outflow facility in transduced eyes.
Conclusions
We show for the first time to our knowledge that knockdown of Bambi alters ECM expression in cultured cells and mouse TM, reduces outflow facility, and causes ocular hypertension. These data provide a novel insight into the development of glaucomatous TM damage and identify BAMBI as an important regulator of TM ECM and ocular hypertension.
Keywords: BAMBI, TGFβ2, BMP, trabecular meshwork, ocular hypertension, extracellular matrix
Glaucoma is the second leading cause of blindness worldwide,1 affecting approximately 70 million individuals.2 It is a heterogeneous group of optic neuropathies, characterized by the loss of retinal ganglions cells (RGCs) leading to irreversible vision loss and blindness. Primary open angle glaucoma (POAG) affects the drainage angle of the eye and is associated with elevated IOP. Elevated IOP is due to increased outflow resistance at the trabecular meshwork (TM). Glaucomatous changes at the TM include loss of TM cellularity, thickening of the TM beams, and increased extracellular matrix (ECM) production, deposition, and remodeling. Current treatment options for POAG patients generally involve reducing IOP with pharmacologic drugs that do not target the pathologic processes occurring at the TM. The molecular mechanisms responsible for changes to the TM and aqueous humor outflow resistance needs further investigation.
TGFβ2 is one of the most studied growth factors and the most common isoform in the eye.3–6 Studies have shown that aqueous humor levels of TGFβ2 are elevated in POAG patients.3,7–10 It is well established that TGFβ2 alters the ECM composition and ECM crosslinking of the TM.11–17 We and others have demonstrated previously that TGFβ2 elevates IOP in the anterior segment perfusion organ culture models14,18 and Ad5.TGFβ2 induces ocular hypertension in mice.19–21 It is well known that TM cells express and secrete TGFβ2.22 TGFβ2-induced changes to the TM occur through the canonical SMAD and non-SMAD signaling pathways, and the canonical SMAD pathway is essential for TGFβ2-induced ocular hypertension in mice.11,12,21,23 The canonical signaling pathway is initiated when TGFβ2 binds type II receptors (TGFβRII), which assembles, activates, and phosphorylates type I receptors (TGFβRI). Activated TGFβRI subsequently phosphorylates SMAD2/3 and leads to the association of SMAD4 into a complex. This complex interacts with coactivators or corepressors to regulate gene transcription. To understand the development of ocular hypertension, the homeostatic regulatory molecules of TGFβ2 and signaling molecules must be evaluated.
Bone morphogenetic proteins (BMPs) are a family of growth factors involved in regulation of the ECM. BMPs can suppress TGFβ2-induced ECM deposition,16,24 the BMP antagonist gremlin elevates IOP in perfusion cultured anterior segments,24 and overexpression of gremlin in mouse eyes20 and BMP2 in rats25 causes ocular hypertension, suggesting that BMP signaling is required for regulating outflow. Similar to TGFβ2, BMP signaling requires two types of transmembrane serine/threonine receptors, type I (BMPR-I) and type II (BMPR-II).26 BMPR-II is used most commonly for BMP signaling and is the target of BMP antagonists.27 The activated BMPR-I and BMPR-II complex results in phosphorylation of SMAD1, SMAD5, or SMAD8.26,28–30 The phosphorylated SMAD1/5/8 complex associates with SMAD4, and the complex translocates to the nucleus and associates with coactivators or corepressors to regulate gene transcription.31 We have shown previously that TM cells express BMPs and their receptors; BMP2, BMP4, BMP5, BMP7 BMP-RIA, BMP-RIB, and BMP-RII.32 A better understanding of the regulatory mechanisms involved in BMP and TGFβ2 signaling is necessary for the development of novel disease modifying therapeutic treatments for POAG patients.33
BMP and activin membrane-bound inhibitor (BAMBI) is a transmembrane glycoprotein related to the TGFβ-family type I receptors, but lacks an intracellular kinase domain.34 BAMBI has been shown to be important in embryonic development as it is coexpressed with BMP4 and BAMBI expression is induced by BMP4, suggesting a negative mechanistic feedback loop.35 BAMBI is evolutionarily conserved from fish to humans,34–36 with a mouse and human homology of 85%.34,37,38 BAMBI has been shown to inhibit BMP, activin, and TGFβ signaling.34 BAMBI can interact with BMP receptors directly and antagonize BMP signaling,39 as well as interact directly with TGFβ receptors and antagonize TGFβ signaling.40 BAMBI also can interact with BMP and TGFβ signaling pathways through other different mechanisms. BAMBI can form a ternary complex with SMAD7/ALK5/TGFβRI and inhibit the interaction between ALK5/TGFβRI and SMAD3; thus, impairing SMAD3 activation.41 BAMBI also has been shown to cotranslocate with SMAD2/3 into the nucleus upon TGFβ treatment and modulate TGFβ signalling.42 The human BAMBI promoter contains the elements that directly associate with SMAD3 and SMAD4, and these elements are critical for the TGFβ responsiveness to the BAMBI promoter.43 BAMBI has been shown to inhibit fibrogenesis through suppression of TGFβ-induced collagen-I expression.44 Knockdown of Bambi expression enhances canonical41 and noncanonical TGFβ signaling.45 Recently, we have shown that BAMBI is expressed in human TM cells and is downregulated by the presence of TGFβ2 (5 ng/mL) at 24 hours.46 These data suggest that BAMBI is an important molecule in TGFβ signaling regulation.
Toll-like receptor 4 (TLR4) signaling has been identified as a regulator of BAMBI expression. TLR4 activation by lipopolysaccharide (LPS) downregulates BAMBI, which enhances TGFβ signaling leading to increased ECM production via a SMAD-dependent pathway.47,48 BAMBI downregulation by TLR4 is regulated by the MyD88-NFκB-dependent pathway.47,49,50 The transcriptional repression of BAMBI by NF-κB p50 enhances TGFβ signaling in hepatic satellite cells.51 In addition, TLR4 enhances TGFβ signaling and hepatic fibrosis by downregulation of BAMBI.52 These data suggest that TLR4 is an important regulator of BAMBI expression in the context of TGFβ2-TLR4 signaling crosstalk. Further experiments are needed to determine the exact mechanistic role of BAMBI in TGFβ2-TLR4 signaling; however, these data suggest that activation of TLR4 downregulates BAMBI leading to TGFβ fibrogenesis.
BAMBI also has been associated with the pathogenesis of several human diseases, including colorectal carcinoma, hepatocellular carcinomas, gastric carcinoma, hepatic fibrosis, and sceleroderma.48,52–54 Bambi knockdown has been shown to increase TGFβ signaling in a model of diabetic globular disease where overexpression of TGFβ has been correlated with the disease pathology.45 We also recently have identified TGFβ2-TLR4 signaling crosstalk as an important regulator of the ECM in the TM and ocular hypertension.55 Here, we demonstrated that TGFβ2 regulates BAMBI expression in TM cells, knockdown of Bambi induces ECM production in TM cells in vitro and in vivo, and knockdown of Bambi in the mouse TM induces ocular hypertension and outflow resistance.
Materials and Methods
Mouse TM Cell Culture
Mouse TM (MTM) cells were isolated, cultured, and maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen-Gibco Life Technologies, Grand Island, NY, USA) containing 15% fetal bovine serum (FBS; Atlas Biologicals Products, Fort Collins, CO, USA) and supplemented with penicillin (100 units/mL), streptomycin (0.1 mg/mL), and L-glutamine (0.292 mg/mL; Gibco BRL Life Technologies). MTM cells were isolated as described below. All experiments were performed on cells within 10 passages.
B6;129S1-Bambitm1Jian/J Mice
All experiments were conducted in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the University of North Texas Health Science Center (UNTHSC; Fort Worth, TX, USA) Institutional Animal Care and Use Committee (IACUC) Guidelines and Regulations. B6;129S1-Bambitm1Jian/J conditional knockout mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and subsequently bred at UNTHSC. All mice were 3 to 5 months old at the start of the experiment. All animals were housed in the UNTHSC vivarium. MTM cells were isolated from B6;129S1-Bambitm1Jian/J as stated. Adenovirus serotype 5 (Ad5) viral vector expressing bioactivated human TGFβ2c226s/c228s (hereafter referred as Ad5.TGFβ2) (University of Iowa, Iowa City, IA, USA) was used to overexpress TGFβ2 as described previously.19–21 Ad5.Cre (Vector Biolabs, Malvern, PA, USA) was used to knockdown Bambi, and Ad5.Null vector (Vector Biolabs) was used as a negative control. Briefly, 2.5 μL of 2.5 × 107 plaque-forming units (pfu) was injected intravitreally into one eye, and the contralateral eyes used as negative controls.
Mouse TM Cells Isolation and Characterization
Intracameral injections, anterior segment dissections, and magnetic bead isolation of MTM cells was performed according to our established protocol with modifications.56 It is well established that TM cells have phagocytic properties.57–70 Briefly, a single culture of TM cells was established from the B6;129S1-Bambitm1Jian/J mouse strain by dissecting TM rings from eight eyes. TM rings were placed in collagenase at 37°C for 2 hours. After digestion, cells were spun down (600g for 10 minutes), resuspended in PBS and passed through a 100 μM cell strainer (Thermo Fisher Scientific, Worcester, MA, USA). The flow through was transferred to Eppendorf tubes and attracted to a magnet on the tube hinge side for 5 minutes. The nonbinding cells were transferred to another Eppendorf tube and attracted cells were resuspended in PBS. This process was repeated at least three times until the Eppendorf tubes containing attracted cells had no visible pigment, after which complete medium was added and cells transferred to a 96-well plate. Isolated MTM cells were cultured in 24-well plates on coverslips and allowed to reach confluency. Cells were treated for 96 hours (protein expression) or 7 days (cross-linked actin networks [CLANs] characterization) with TGFβ2 (5 ng/mL), culture medium (TGFβ2 control), dexamethasone (DEX; 100 nM), or ethanol (ETOH; vehicle control) in serum-free medium. Culture medium was changed every other day. Cells were processed for immunocytochemistry and CLANs counted as described previously.56 CLAN formation rate was expressed as the ratio of CLAN-positive cells/total number of 4′,6-diamidino-2-phenylendole (DAPI)–stained cells. Cells were determined to be CLAN-positive if they contained at least one CLAN. For each treatment, five regions in each coverslip and five to six coverslips were counted.
Adenovirus Transduction
To determine the transduction efficiency of Ad5 in MTM cells isolated from B6;129S1-Bambitm1Jian/J mice, MTM cells were transduced with Ad5.GFP at 25, 50, 100, and 200 multiplicity of infection (MOI) for 12 hours and conditioned medium replaced by serum-free medium for 24 hours. Similarly, MTM cells were transduced with Ad5.Cre at 0, 50, 100, and 200 MOI for 12 hours, conditioned medium replaced, and after 48 hours changes in fibronectin and collagen-1 was evaluated using immunocytochemistry. Additionally, MTM cells were transduced with Ad5.Null, Ad5.Cre, or Ad5.TGFβ2 at 100 MOI for 12 hours, conditioned medium replaced for 48 hours, and the expression of BAMBI, fibronectin, and collagen-1 was evaluated using immunocytochemistry.
Immunocytochemistry
Mouse TM cells were seeded in 24-well plates on coverslips. After completing the treatment time course, cells were washed with PBS, fixed with 4% paraformaldehyde (PFA), permeabilized with 0.05% Triton X-100 in PBS, and blocked using Superblock Blocking Buffer in PBS (Thermo Fisher Scientific) for 60 minutes at room temperature. Cells were labeled overnight at 4°C with rabbit anti-fibronectin (EMD Millipore, Billerica, MA, USA) 1:500 dilution, anti-laminin (Novus Biologicals, LL, Littleton, CO, USA) 1:250 dilution, anti-collagen-1 (Novus Biologicals) 1:250 dilution, anti-collagen-4 (Novus Biologicals) 1:350 dilution, and α-smooth muscle actin (Abcam, Cambridge, MA, USA) 1:500 dilution in Superblock Blocking Buffer in PBS (Thermo Fisher Scientific). Treatment without the primary antibodies was used as negative controls (Supplemental Fig. S1). Coverslips were incubated for 2 hours using Alexa-Fluor-labeled anti-rabbit or anti-mouse antibodies (Life Technologies, Carlsbad, CA, USA) 1:1000 dilution. To label CLANs, MTM cells were probed for filamentous actin (F-actin) using Alexa Fluor 488 phalloidin (Thermo Fisher Scientific) 1:250 dilution. Coverslips were mounted onto slides with Prolong Gold mounting medium containing DAPI (Invitrogen-Molecular Probes, Carlsbad, CA, USA). Image acquisition was performed using the Keyence BZ-X700 fluorescence microscope (Keyence Corporation of America, Itasca, IL, USA). Images were taken either at ×100, ×200, ×400, or ×600 magnification, with each presented Figure containing its corresponding scale bar. Mean fluorescent intensity/area for MTM cells was measured and analyzed using the NIS Elements software (Nikon, Tokyo, Japan).
Immunohistochemistry of Mouse Eyes
To evaluate early changes in the TM, an initial cohort of mice were transduced with viral vectors, Ad5.Null (n = 7), Ad5.Cre (n = 15), and Ad5.TGFβ2 (n = 9), and harvested 11-days after injection. IOP was recorded at 10-days postinjection. Eyes were fixed in 4% PFA overnight, embedded in paraffin, cut into 5 μm sections, and transferred to glass slides. Deparaffinization was performed by washing two times with xylene, 100% ethanol, 95% ethanol, and 50% ethanol for 2 minutes each. Slides then were soaked in PBS for 5 minutes. Tissues were blocked using Superblock Blocking Buffer in PBS (Thermo Fisher Scientific) for 60 minutes. Rabbit anti-fibronectin (EMD Millipore) 1:500 dilution, rabbit anti-collagen-1 (Novus Biologicals, LL) 1:250 dilution, mouse anti-collagen-4 (Sigma-Aldrich Corp., St. Louis, MO, USA) 1:250 dilution, and mouse anti-BAMBI (Abnova; Walnut, CA, USA) 1:250 dilution, were used as primary antibodies, followed by Alexa-Fluor–labeled anti-rabbit or anti-mouse antibodies (Life Technologies, Carlsbad, CA, USA) 1:500 dilution. Prolong Gold mounting medium containing DAPI (Invitrogen-Molecular Probes) was used to mount the slides, and sections were imaged using the Keyence BZ-X700 fluorescence microscope (Keyence Corporation of America, Itasca, IL). All images were taken at ×100 magnification; scale bars represent 100 μm.
Western Blot Analysis
Western blot studies were performed as described previously.55 Briefly, MTM cells were treated as stated above. Conditioned medium samples were prepared as follows: conditioned medium (30 μL) and ×4 Laemmli Buffer (10 μL; Bio-Rad Laboratories, Hercules, CA, USA) were combined for a total volume of 40 μL. Samples were boiled for 10 minutes followed by separation using 12% SDS-PAGE. To verify equal loading for CM samples, gels were stained with Gel Code Blue Stain Reagent (Thermo Fisher Scientific). Proteins were transferred to polyvinylidine fluoride (PVDF) membranes (EMD Millipore), and membranes blocked with Superblock Blocking Buffer in TBS (Thermo Fisher Scientific). Membranes were immunolabeled overnight at 4°C with rabbit anti-fibronectin antibody (EMD Millipore) dilution 1:1000. Blots were incubated for 1 hour with horseradish peroxidase (HRP) conjugated goat anti-rabbit secondary antibody (1:1000; Pierce Biotechnology, Inc., Rockford, IL, USA) diluted in Superblock Blocking Buffer in TBS. Immunolabeled signals were developed using Clarity Western ECL Substrate, and blot images were acquired using ChemiDoc Touch Imaging System (Bio-Rad Laboratories). Each experiment was repeated three times. Densitometry analysis of Western immunoblot images was used to determine changes in protein content after treatment. Band intensity for fibronectin was measured using Image Lab Software (Bio-Rad Laboratories). Fold change was compared to Ad5.Null and represented as the mean ± SEM. Statistical significance was determined by 1-way ANOVA and Tukey post hoc analysis comparing all treatments.
IOP Measurements
After intravitreal injection of 2.5 μL (2.5 × 107 pfu) Ad5.Null (n = 7 mice), Ad5.Cre (n = 9 mice), Ad5.TGFβ2 (n = 10 mice), or Ad5.Cre + Ad5.TGFβ2 (n = 10 mice), IOP was measured as described previously for 56 days after injection.19–21,71 Briefly, IOP was measured under isoflurane anesthesia using the Tonolab tonometer (Colonial Medical Supply, Franconia, NH, USA). All IOP measurements were performed during the same time period of the light-on phase. Statistical significance was determined by Student's paired t-test at each time point comparing the injected and contralateral uninjected control eyes.
Aqueous Humor Outflow Facility
To evaluate the effect of Ad5.Cre and Ad5.TGFβ2 on aqueous humor outflow rate in B6;129S1-Bambitm1Jian/J mice, three to five mice were used for outflow facility after the 56-day IOP time-course as described previously.72,73 Briefly, eyes of anesthetized mice were cannulated intracamerally with a 30-gauge steel needle inserted through the peripheral cornea approximately 1 to 2 mm from the limbus and pushed toward the region of the opposing chamber angle. The needle was connected by tubing to an in-line pressure transducer for continuous determination of pressure within the system. The opposing terminal of the pressure transducer was connected by tubing to a 50 μL glass micro syringe loaded into a microdialysis infusion pump. The pump was switched on and set to a flow rate of 0.1 μL/min. The pump remained running until the pressure in the system was stabilized. Flow rates were then increased sequentially to 0.4, and 0.5 μL/min, and stabilized pressure values at each flow rate were recorded. For each eye, aqueous humor outflow facility (μL/min/mm Hg) was calculated as the reciprocal of the slope of the respective pressure-flow rate curves.
Results
Isolation and Characterization of Mouse TM Cells
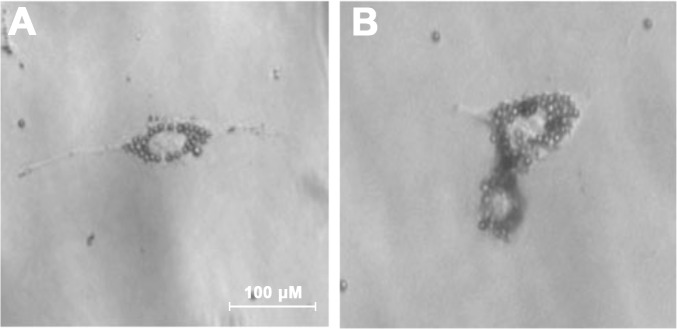
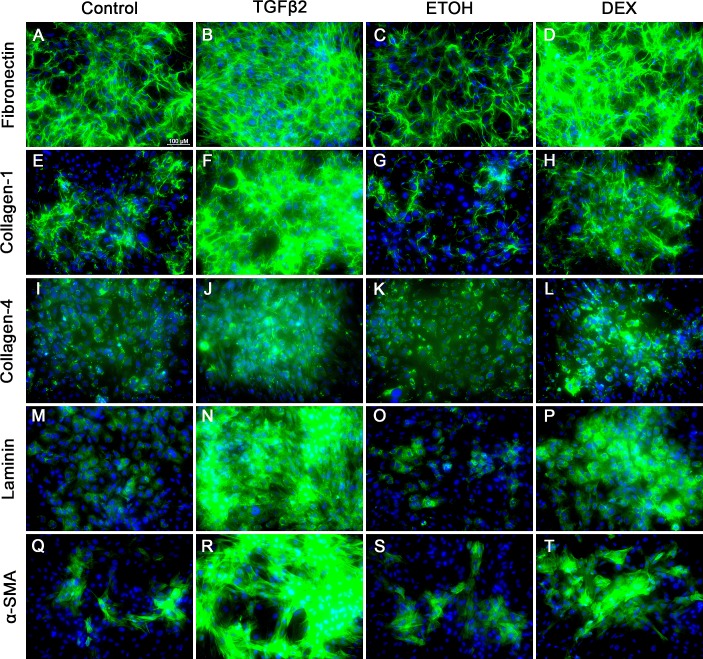
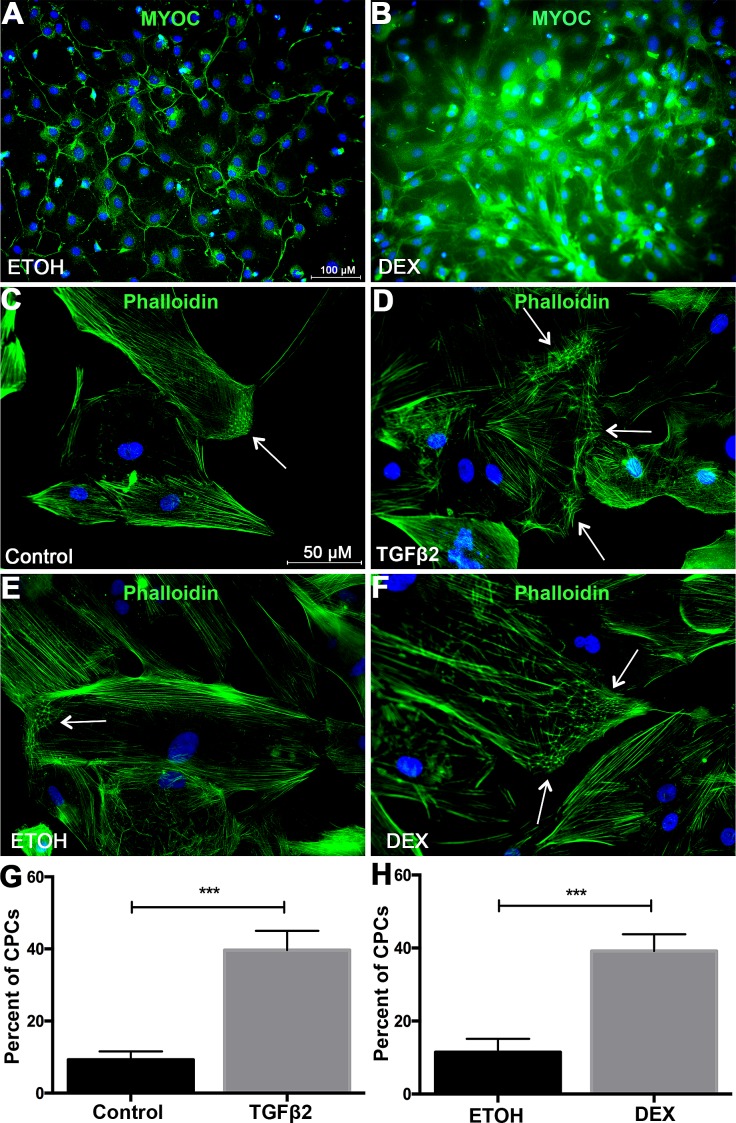
Extracellular matrix deposition at the TM is a major factor involved in the development of ocular hypertension. To better understand the role of BAMBI in ECM deposition in TM cells, MTM cells were isolated from B6;129S1-Bambitm1Jian/J mice using magnetic beads (Figs. 1A, 1B). We used our established protocol56 and removed the pigment during the isolation process (Fig. 1). MTM cells were characterized for the expression of TM markers.74–78 It is well established that TGFβ2 and DEX induce the expression of ECM proteins in the TM. Here, we show that isolated MTM cells express fibronectin, collagen-1, collagen-4, laminin, and α-smooth muscle actin (Fig. 2). In addition, TGFβ2 (ng/mL) and DEX (100 μM) induce an increase in expression of these markers compared to their control treatments (Figs. 2A–T). Recently, we have shown that isolated mouse TM cells in culture respond to DEX treatment by upregulating myocilin expression and inducing the formation of CLANs.56 Here, DEX (100 μM) induced the expression of myocilin at 4 days after treatment compared to vehicle-treated cells (Figs. 3A, 3B). Recombinant human TGFβ2 (5 ng/mL) and DEX (100 μM) each induced the expression of CLANs compared to their controls (Figs. 3C–H). These data demonstrate that mouse TM cells can be isolated and cultured in vitro, express extracellular matrix proteins, and respond to TGFβ2 and DEX.
Figure 1.
Magnetic bead isolation of MTM cells from B6/129S1-Bambitm1Jian/J mice. Mouse TM cells were isolated from B6;129S1-Bambitm1Jian/J mice using our established magnetic bead isolation protocol.56 (A) MTM cells growing in the absence of pigment after 24 hours. (B) MTM cell dividing and containing magnetic beads.
Figure 2.
Characterization of MTM cells for the expression of fibronectin, collagen-1, collagen-4, laminin, and α-smooth muscle actin. MTM cells were grown to confluency, treated for 96 hours with control (serum-free medium; A, E, I, M, Q), TGFβ2 (5 ng/mL; B, F, J, N, R), ETOH (vehicle control for DEX; C, G, K, O, S), or DEX (100 nM; D, H, L, P, T). Control-treated cells each expressed basal levels of fibronectin (A, C), collagen-1 (E, G), collagen-4 (I, K), laminin (M, O), and α-smooth muscle actin (Q, S). TGFβ2 and DEX induced the expression of fibronectin (B, D), collagen-1 (F, H), collagen-4 (J, L), laminin (N, P), and α-smooth muscle actin (R, T) compared to control. N = 3 independent experiments in the B6;129S1-Bambitm1Jian/J mouse TM cell strain.
Figure 3.
Characterization of MTM cells for DEX-induced myocilin expression and formation of CLANs. MTM cells were grown to confluency and treated with (A) ETOH (vehicle control for DEX) or (B) DEX (100 nM) in serum-free medium for 4 days for immunostaining of DEX-induced myocilin expression. To access CLAN formation, MTM cells were grown to confluency and treated with (C) medium, (D) TGFβ2 (5 ng/mL), (E) ETOH, or (F) DEX (100) for 7 days with 1% FBS. (D) TGFβ2 induced the formation of CLANS compared to (C) control treated MTM cells. (F) DEX induced the expression of CLANs compared to (E) ETOH (vehicle control)–treated MTM cells. Quantification of CLAN-positive cells (CPCs) in (G) TGFβ2 and vehicle control, as well as (H) DEX- and ETOH-treated MTM cells. Statistical significance was determined by Student's unpaired t-test (***P < 0.001). Arrows indicate CLANs. N = 3 independent experiments in the B6;129S1-Bambitm1Jian/J mouse TM cell strain.
Knockdown of Bambi Increases ECM Expression
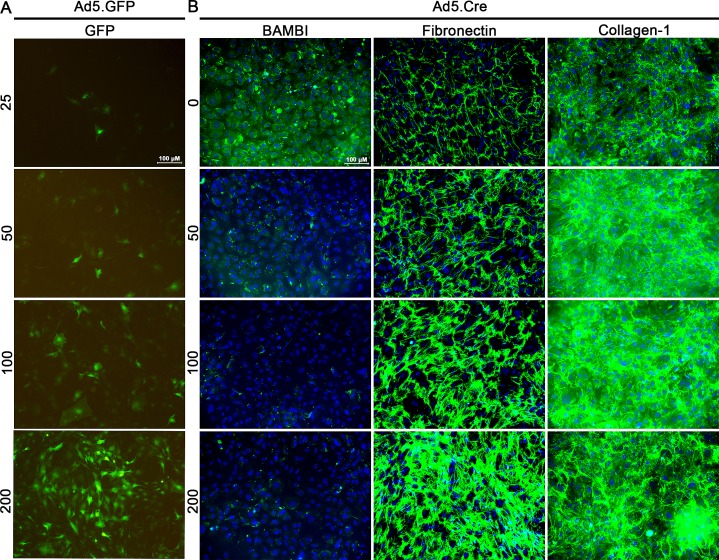
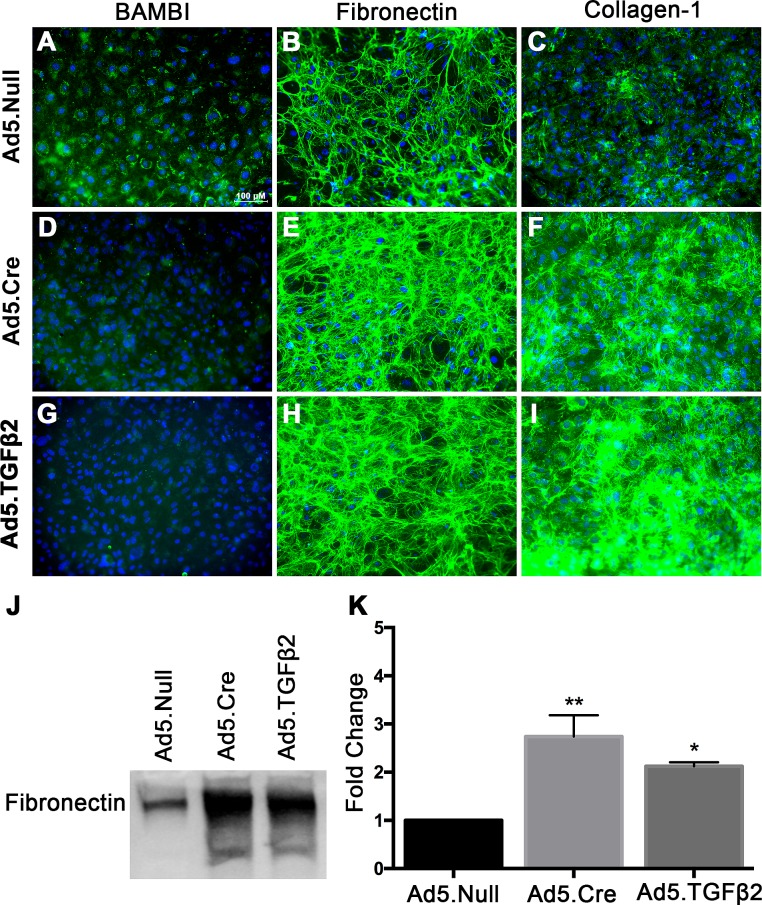
Adenovirus serotype 5 has selective tropism to the TM.79 Therefore, we evaluated the ability of isolated TM cells in culture to be transduced by Ad5.GFP using different MOI's (Fig. 4A). We observed that 200 MOI had the highest transduction efficiency; however, cells were visibly unhealthy and there was evidence of cell death. At 100 MOI, the cells were healthy and the majority of cells were GFP-positive. We also evaluated whether knockdown of Bambi leads to ECM changes in cultured MTM cells. Knockdown of Bambi using Ad5.Cre increased the levels of fibronectin and collagen-1 in an MOI-dependent manner (Fig. 4B, Supplemental Fig. S2). Further, Ad5.TGFβ2 (100 MOI) and Ad5.Cre (100 MOI) decreased BAMBI (Figs. 5D, 5G) expression and increased fibronectin (Figs. 5E, 5H) and collagen-1 (Figs. 5F, 5I) expression compared to Ad5.Null (100 MOI)–treated cells (Figs. 5A–C). Fibronectin expression in the conditioned medium also was quantified by Western blot (Figs. 5J, 5K). Fibronectin expression increased 2.74 ± 0.44-fold in the Ad5.Cre-treated cells and 2.12 ± 0.08-fold in the Ad5.TGFβ2-treated cells compared to the Ad5.Null-treated cells. These data provide evidence that BAMBI is an important regulator of the ECM in mouse TM cells.
Figure 4.
Ad5.GFP transduces primary MTM cells and knockdown of Bambi using Ad5.Cre increases fibronectin and collagen-1 expression. (A) MTM cells were transduced for 12 hours with Ad5.GFP at 25, 50, 100, and 200 MOI followed by incubation for 48 hours in serum-free medium (n = 4 replicates). Ad5.GFP at 200 MOI had the highest transduction efficiency and 100 MOI had the highest cellular health. (B) MTM cells were cultured to confluency and transduced for 12 hours with Ad5.Cre at 50, 100, and 200 MOI. After 12 hours of incubation, cells were cultured for 48 hours in serum-free medium. Ad5.Cre knockdown of Bambi induces the expression of fibronectin and collagen-1 expression at 50, 100, and 200 MOI (n = 3 replicates).
Figure 5.
Ad5.Cre and Ad5.TGFβ2 increases fibronectin and collagen-1 expression in MTM cells. MTM cells (n = 3 replicates) were cultured to confluency and transduced overnight with Ad5.Null, Ad5.Cre, or Ad5.TGFβ2 at 100 MOI. After overnight incubation with the adenovirus, cells were cultured for 48 hours in serum-free medium. Ad5.Null had no effect on (A) BAMBI, (B) collagen-1, and (C) fibronectin expression. Ad5.Cre and Ad5.TGFβ2 decreased (D, G) BAMBI expression and increased (E, H) fibronectin and (F, I) collagen-1 expression. (J, K) Densitometric analysis of conditioned medium showed that Ad5.Cre and Ad5.TGFβ2 increased fibronectin expression compared to Ad5.Null. Statistical significance was determined by 1-way ANOVA and Tukey post hoc analysis, *P < 0.05, **P < 0.01 (* = compared to Ad5.Null).
Conditional Knockdown of Bambi in Mice Increases ECM Expression
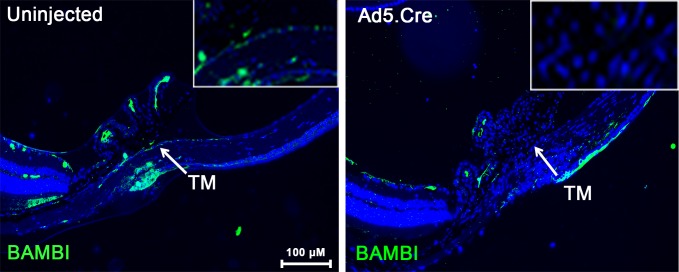
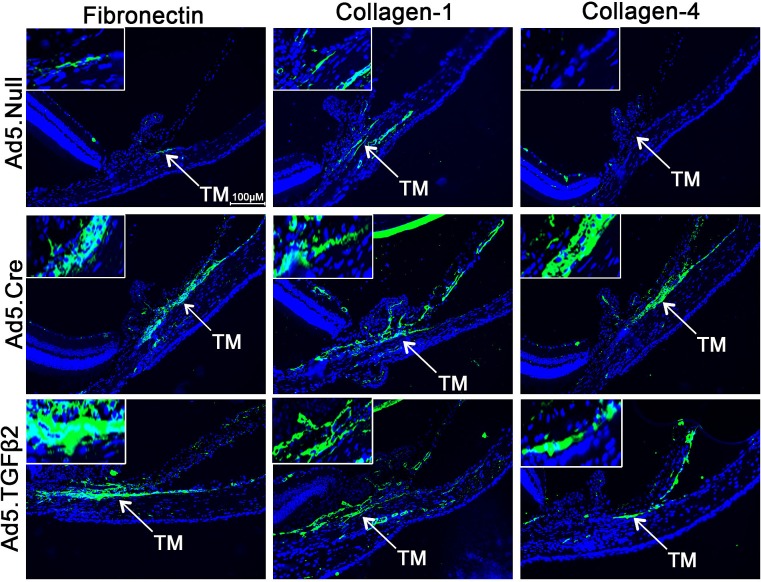
BAMBI has been shown to be a regulator of growth factors involved in ECM remodeling.44,80–85 To evaluate the effects of BAMBI on ECM regulation in vivo, Bambi floxed mice were injected intravitreally with Ad5.Cre in one eye, while the contralateral uninjected eye served as a control. Here, we show for the first time to our knowledge that the mouse TM expresses BAMBI, and Ad5.Cre transduction is sufficient to knockdown Bambi in the TM (n = 5; Fig. 6). We have shown previously that Ad5.TGFβ2 is able to increase the expression of fibronectin mRNA and protein in the TM of mice.20,21,55 We evaluated the importance of BAMBI in the regulation of ECM and used Ad5.TGFβ2 as a positive control. In our study, Ad5.Null (n = 7), Ad5.Cre (n = 15), and Ad5.TGFβ2 (n = 9) were used to transduce the TM of mice. Interestingly, knockdown of Bambi by Ad5.Cre or by Ad5.TGFβ2 increased the expression of fibronectin, collagen-1, and collagen-4 in the TM compared to Ad5.Null (Fig. 7).
Figure 6.
Ad5.Cre knockdown of Bambi in the TM of B6;129S1-Bambitm1Jian/J mice. Mice were injected with Ad5.Cre (2.5 × 107 pfu) intravitreally in one eye of each animal and the contralateral uninjected eyes were used as negative controls (n = 5). Mouse eyes were harvested 11 days after injection and analyzed for the expression of BAMBI by immunohistochemistry. The uninjected eyes express BAMBI in the TM, whereas the eyes injected with Ad5.Cre did not show detectable BAMBI expression.
Figure 7.
Fibronectin, collagen-1, and collagen-4 expression in the TM of B6;129S1-Bambitm1Jian/J mice treated with Ad5.TGFβ2 and Ad5.Cre. Mice were injected with Ad5.Null (2.5 × 107 pfu), Ad5.TGFβ2 (2.5 × 107 pfu), or Ad5.Cre (2.5 × 107 pfu) intravitreally in one eye of each animal and the contralateral uninjected eyes were used as controls. Eyes were harvested 11 days after injection and analyzed for fibronectin, collagen-1, and collagen-4 expression by immunohistochemistry. Ad5.Cre (n = 15) and Ad5.TGFβ2 (n = 9) increased fibronectin, collagen-1, and collagen-4 expression in the TM compared to Ad5.Null (n = 7).
Conditional Knockdown of Bambi Induces Ocular Hypertension in Mice
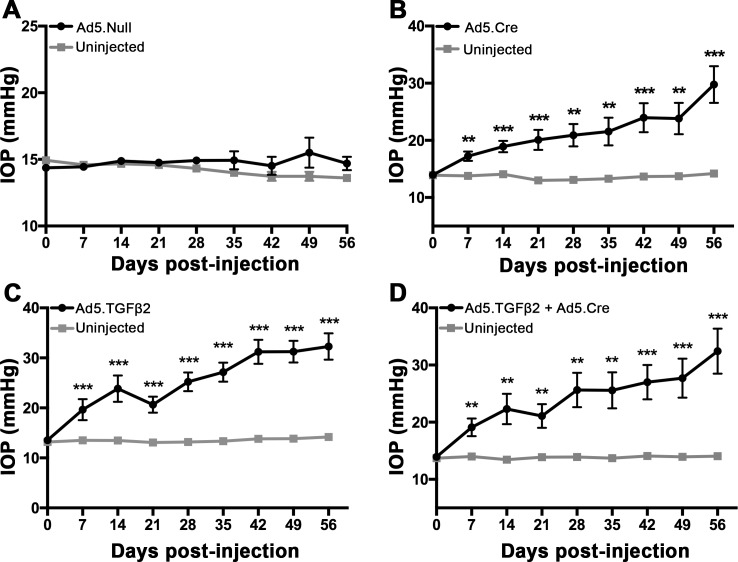
To test the effect of Bambi knockdown on ocular hypertension, Ad5.Null, Ad5.Cre, Ad5.TGFβ2, or Ad5.Cre + Ad5.TGFβ2 was injected intravitreally into B6;129S1-Bambitm1Jian/J mice (Figs. 8A–D). Our established mouse model of ocular hypertension using Ad5.TGFβ2 was used as our positive control19–21 and Ad5.Null was used as a negative control. Ad5.Null, Ad5.Cre, Ad5.TGFβ2, or Ad5.Cre+Ad5.TGFβ2 viral vectors were injected intravitreally into one eye of each animal and the contralateral eye was used as an uninjected control. Ad5.Null did not elevate IOP at any time point (n = 7; Fig. 8A). Interestingly, knockdown of Bambi by Ad5.Cre significantly elevated IOP at all time points after injection compared to the uninjected control eyes (Fig. 8B; P < 0.01, n = 9). At day 56, IOP in the Ad5.Cre-injected eyes reached 29.8 ± 3.2 mm Hg compared to the contralateral uninjected eye, 14.2 ± 0.3 mm Hg (P < 0.001). As expected, Ad5.TGFβ2 had significant IOP elevation at all time points after injection compared to the uninjected control eyes (Fig. 8C; P < 0.001, n = 10). At day 56, IOP in the Ad5.TGFβ2-injected eyes reached 32.3 ± 2.6 mm Hg compared to the contralateral uninjected eye, 14.2 ± 0.3 mm Hg (P < 0.001). Ad5.TGFβ2 + Ad5.Cre also significantly elevated IOP at all time points (Fig. 8D; P < 0.01, n = 10). At day 56, IOP in the Ad5.TGFβ2 + Ad5.Cre injected eyes reached 32.4 ± 3.9 mm Hg compared to the contralateral uninjected eyes, 14.1 ± 0.3 mm Hg (P < 0.001). These data suggest that knockdown of Bambi is sufficient to cause ocular hypertension in mice to the same degree as overexpression of TGFβ2. Cotreatment of Ad5.TGFβ2 + Ad5.Cre did not produce additional IOP elevation above that of Ad5.Cre or Ad5.TGFβ2 alone, suggesting that we may have reached the maximum IOP elevation for this experimental paradigm. Statistical significance was determined by Student's paired t-test at each time point comparing the transduced eye to the contralateral uninjected control eye.
Figure 8.
Effects of Ad5. Null, Ad5.TGFβ2, and Ad5.Cre on IOP in B6;129S1-Bambitm1Jian/J mice. Mice were injected intravitreally with Ad5.Null, Ad5.Cre, Ad5.TGFβ2, or Ad5.TGFβ2 + Ad5.Cre (2.5 × 107 pfu). Day of injection was designated as day 0. The contralateral eye of each mouse was uninjected and served as a paired control. (A) Ad5.Null did not induce ocular hypertension at any time point compared to the contralateral eye (n = 7). (B–D) Injection with Ad5.Cre, Ad5.TGFβ2, or Ad5.TGFβ2 + Ad5.Cre each induced ocular hypertension starting at day 7 after injection and maintained significant IOP elevation throughout the 56-day time course compared to uninjected control eyes (P < 0.01, days 7–56). (B) At day 56 after injection, IOP increased to 29.8 ± 3.2 mm Hg in Ad5.Cre–injected eyes compared to 14.2 ± 0.3 mm Hg in contralateral uninjected eyes (P < 0.001, n = 9). (C) Ad5.TGFβ2 (32.3 ± 2.6 mm Hg) had significant IOP elevation at 56 days after injection compared to 14.2 ± 0.3 mm Hg in uninjected control eyes (P < 0.001, n = 10). (D) Ad5.TGFβ2 + Ad5.Cre (32.4 ± 3.9 mm Hg) had significant IOP elevation at 56 days after injection compared to uninjected control eyes (14.1 ± 0.3 mm Hg; P < 0.001, n = 10). Statistical significance was determined by Student's paired t-test at each time point comparing the transduced eye to the contralateral uninjected control eye, **P < 0.01, ***P < 0.001 (* = compared to uninjected control).
Outflow Facility
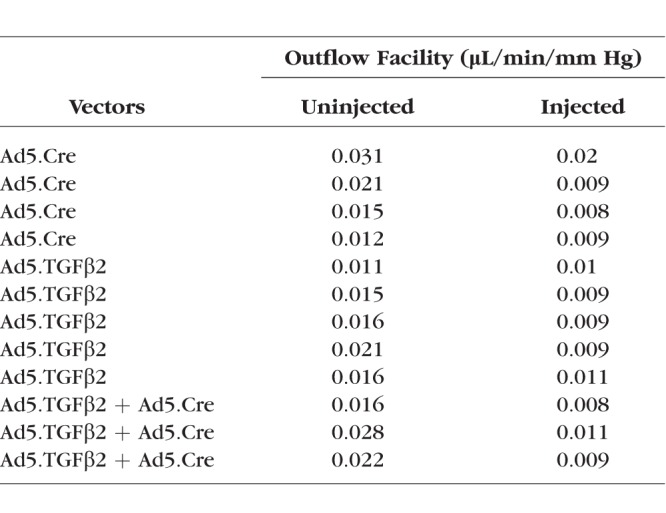
To further understand if the elevated IOP resulted from increased outflow resistance, measurement of outflow facility was performed on transduced (Ad5.Cre, Ad5.TGFβ2, or Ad5.Cre+Ad5.TGFβ2) mouse eyes and control uninjected eyes after the 56-day time point (Fig. 9, Table). Aqueous humor outflow facility was significantly lower in Ad5.Cre (0.0115 ± 0.0028 μL/min/mm Hg, P = 0.028, n = 4), Ad5.TGFβ2 (0.0096 ± 0.0004 μL/min/mm Hg, P = 0.025, n = 5), and Ad5.TGFβ2 + Ad5.Cre (0.0093 ± 0.0009 μL/min/mm Hg, P = 0.04, n = 3) transduced eyes compared to their contralateral uninjected eyes (Ad5.Cre, 0.0198 ± 0.0042; Ad5.TGFβ2, 0.0158 ± 0.0016; Ad5.Cre + Ad5.TGFβ2, 0.0220 ± 0.0035 μL/min/mm Hg). Further, the reduction in outflow facility correlated closely with the observed increase in IOP. Statistical significance was determined by Student's paired t-test at each time point comparing the transduced and contralateral uninjected control eyes.
Figure 9.
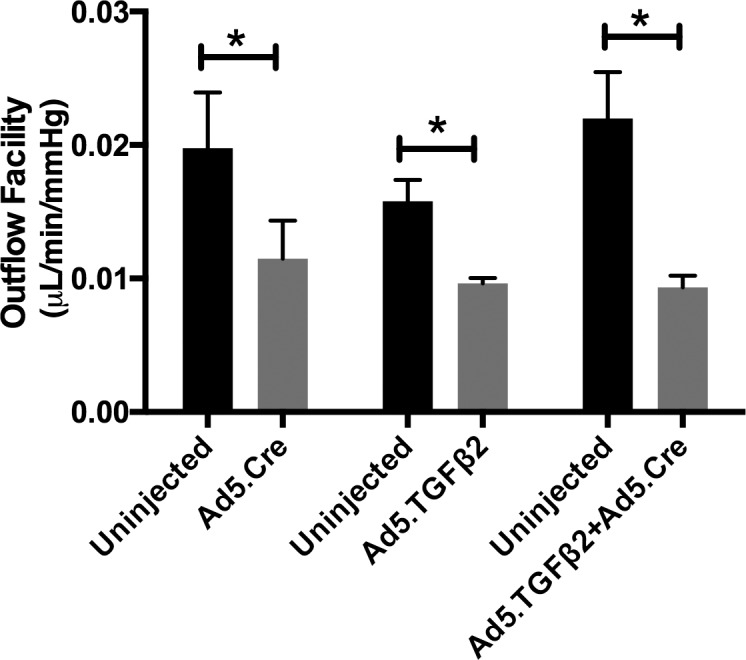
Effects of Ad5.TGFβ2 and Ad5.Cre on outflow facility. At 56-days after injection of Ad5.Cre, Ad5.TGFβ2, or Ad5.TGFβ2 + Ad5.Cre, 3 to 5 mice were randomly selected for outflow facility. Aqueous humor outflow facility was significantly lower in transduced eyes compared to control uninjected eyes: Ad5.Cre injected (P = 0.028, n = 4), Ad5.TGFβ2 injected (P = 0.025, n = 5), and Ad5.TGFβ2 + Ad5.Cre (P = 0.04, n = 3). Statistical significance was determined by Student's paired t-test comparing the transduced eye to the contralateral uninjected control eye.
Table.
Individual Outflow Facility (μL/min/mm Hg) Values for Uninjected and Vector-Treated Eyes
Discussion
In this study, we demonstrate that BAMBI is a novel regular of the ECM and fibrosis in the TM. Isolated MTM cells from B6;129S1-Bambitm1Jian/J mice express TM markers and knockdown of Bambi in these cells increased ECM protein expression. Interestingly, knockdown of Bambi in the TM of mice elevated IOP, reduced outflow facility, and increased ECM proteins in the TM. These findings suggest that BAMBI has an important role in TM ECM and IOP regulation.
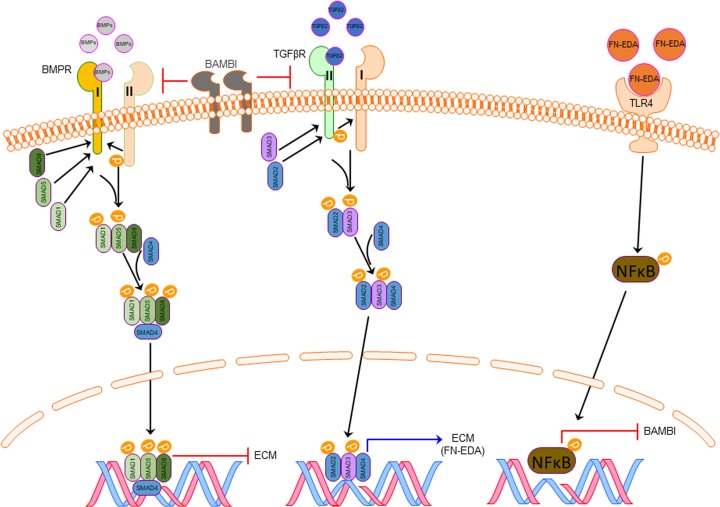
Regulatory mechanisms to control TGFβ signaling have been shown to affect directly the TGFβ receptors and downstream signaling molecules. BAMBI has been identified as an important regulator of TGFβ signaling. The extracellular domain of BAMBI is related closely to type-I TGFβ and BMP receptors.34 BAMBI can incorporate into complexes with TGFβR I and II,34 thereby preventing their dimerization (Fig. 10). The intracellular domain of BAMBI does not encode a serine/threonine-kinase domain, thus inhibiting TGFβ and BMP signaling. TGFβ can directly increase BAMBI protein expression through SMAD3/4 binding to the SMAD binding element of the hBAMBI promoter.43 We reported previously that TGFβ2 decreases BAMBI mRNA and protein expression in human TM cells.46 In addition, TLR4 signaling has been shown to downregulate BAMBI expression via a NF-kB dependent pathway.51 TGFβ2-TLR4 signaling crosstalk has been implicated in several fibrosis diseases, including scleroderma, liver cirrhosis, and kidney disease.48,49,50,52
Figure 10.
Proposed pathway of ECM regulation by BAMBI, TLR4, and TGFβ2 signaling pathways in the glaucomatous TM. Increased TGFβ2 leads to an increase in DAMP and ECM production such as fibronectin-EDA (FN-EDA), which can further activate TLR4. TLR4 activation by FN-EDA downregulates the expression of BAMBI. Downregulation of BAMBI produces uninhibited TGFβ2 and BMP signaling. Uninhibited TGFβ2 and BMP alters ECM synthesis. The feed-forward loop produced by TGFβ2 -TLR4 signaling leads to progressive fibrosis in the TM.
Recently, we have shown TGFβ2-TLR4 signaling in the development of glaucomatous TM damage.55 TLR4 can be activated by damage-associated molecular patterns (DAMPs), such as cellular fibronectin containing the EDA isoform (cFN-EDA). We demonstrated that activation of TLR4 in TM cells by cFN-EDA induces ECM production.55 Inhibition of TLR4 signaling by a selective TLR4 inhibitor (TAK-242) blocked ECM production. In addition, Tlr4 mutant mice were resistant to TGFβ2-induced ocular hypertension. These data suggest that in the TM, TGFβ2-TLR4 signaling crosstalk is important in regulating ECM production. Recent evidence suggests that upon TLR4 activation, NF-kB translocates into the nucleus and serves as a transcription factor to suppress BAMBI expression.52 It has been shown previously that knockdown of Bambi enhances TGFβ2 signaling,86 and downregulation of Bambi by TLR4 activation results in enhanced TGFβ signaling and increased ECM production.47,48 Downregulation of BAMBI in the TM could contribute to uninhibited TGFβ2 signaling, resulting in the increase in TM ECM production and ocular hypertension (Fig. 10).
Our studies suggest that regulation of the TGFβ2 signaling pathway is important for TM and IOP homeostasis. In addition, TLR4 activation and Bambi knockdown has been shown to effect TGFβ signaling.87 Overexpression of BAMBI suppresses the effect of TGFβ.42,88,89 We show for the first time to our knowledge that knockdown of Bambi in the TM is sufficient to induce ocular hypertension and glaucoma-like changes to the TM. Future studies are necessary to understand the effect of BAMBI on endogenous levels of TGFβ2, BMPs, activin, and DAMPs in the TM.
Conclusions
In summary, we demonstrate that BAMBI is involved in the production and regulation of the ECM in the TM. These data provide evidence that BAMBI is a critical link molecule in TGFβ2-TLR4 signaling crosstalk. Our data further provide a new insight into the molecular mechanism involved in the development of glaucomatous TM damage.
Supplementary Material
Acknowledgments
Supported by the Bright Focus Foundation G2014063 (CMM), National Institutes of Health R01EY026529 (CMM), and Neurobiology of Aging Training Grant T32AG020494 (HH).
Disclosure: H. Hernandez, None; J.C. Millar, None; S.M. Curry, None; A.F. Clark, None; C.M. McDowell, None
References
- 1. Kingman S. . Glaucoma is second leading cause of blindness globally. Bull World Health Organ. 2004; 82: 887– 888. [PMC free article] [PubMed] [Google Scholar]
- 2. Quigley HA. . Number of people with glaucoma worldwide. Br J Ophthalmol. 1996; 80: 389– 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Min SH, Lee TI, Chung YS, Kim HK. . Transforming growth factor-beta levels in human aqueous humor of glaucomatous, diabetic and uveitic eyes. Korean J Ophthalmol. 2006; 20: 162– 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cousins SW, McCabe MM, Danielpour D, Streilein JW. . Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Invest Ophthalmol Vis Sci. 1991; 32: 2201– 2211. [PubMed] [Google Scholar]
- 5. Jampel HD, Roche N, Stark WJ, Roberts AB. . Transforming growth factor-beta in human aqueous humor. Curr Eye Res. 1990; 9: 963– 969. [DOI] [PubMed] [Google Scholar]
- 6. Granstein RD, Staszewski R, Knisely TL,et al. . Aqueous humor contains transforming growth factor-beta and a small (less than 3500 daltons) inhibitor of thymocyte proliferation. J Immunol. 1990; 144: 3021– 3027. [PubMed] [Google Scholar]
- 7. Inatani M, Tanihara H, Katsuta H, Honjo M, Kido N, Honda Y. . Transforming growth factor-beta 2 levels in aqueous humor of glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol. 2001; 239: 109– 113. [DOI] [PubMed] [Google Scholar]
- 8. Ochiai Y, Ochiai H. . Higher concentration of transforming growth factor-beta in aqueous humor of glaucomatous eyes and diabetic eyes. Jpn J Ophthalmol. 2002; 46: 249– 253. [DOI] [PubMed] [Google Scholar]
- 9. Ozcan AA, Ozdemir N, Canataroglu A. . The aqueous levels of TGF-beta2 in patients with glaucoma. Int Ophthalmol. 2004; 25: 19– 22. [DOI] [PubMed] [Google Scholar]
- 10. Tripathi RC, Li J, Chan WF, Tripathi BJ. . Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp Eye Res. 1994; 59: 723– 727. [DOI] [PubMed] [Google Scholar]
- 11. Sethi A, Jain A, Zode GS, Wordinger RJ, Clark AF. . Role of TGFbeta/Smad signaling in gremlin induction of human trabecular meshwork extracellular matrix proteins. Invest Ophthalmol Vis Sci. 2011; 52: 5251– 5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tovar-Vidales T, Clark AF, Wordinger RJ. . Transforming growth factor-beta2 utilizes the canonical smad-signaling pathway to regulate tissue transglutaminase expression in human trabecular meshwork cells. Exp Eye Res. 2011; 93: 442– 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Welge-Lussen U, May CA, Lutjen-Drecoll E. . Induction of tissue transglutaminase in the trabecular meshwork by TGF-beta1 and TGF-beta2. Invest Ophthalmol Vis Sci. 2000; 41: 2229– 2238. [PubMed] [Google Scholar]
- 14. Fleenor DL, Shepard AR, Hellberg PE, Jacobson N, Pang IH, Clark AF. . TGFbeta2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest Ophthalmol Vis Sci. 2006; 47: 226– 234. [DOI] [PubMed] [Google Scholar]
- 15. Wordinger RJ, Fleenor DL, Hellberg PE,et al. . Effects of TGF-beta2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Invest Ophthalmol Vis Sci. 2007; 48: 1191– 1200. [DOI] [PubMed] [Google Scholar]
- 16. Fuchshofer R, Yu AH, Welge-Lussen U, Tamm ER. . Bone morphogenetic protein-7 is an antagonist of transforming growth factor-beta2 in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007; 48: 715– 726. [DOI] [PubMed] [Google Scholar]
- 17. Welge-Lüssen U, May CA, Eichhorn M, Bloemendal H, Lütjen-Drecoll E. . AlphaB-crystallin in the trabecular meshwork is inducible by transforming growth factor-beta. Invest Ophthalmol Vis Sci. 1999; 40: 2235– 2241. [PubMed] [Google Scholar]
- 18. Gottanka J, Chan D, Eichhorn M, Lütjen-Drecoll E, Ethier CR. . Effects of TGF-beta2 in perfused human eyes. Invest Ophthalmol Vis Sci. 2004; 45: 153– 158. [DOI] [PubMed] [Google Scholar]
- 19. Shepard AR, Millar JC, Pang I-H, Jacobson N, Wang W-H, Clark AF. . Adenoviral gene transfer of active human transforming growth factor-beta2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Invest Ophthalmol Vis Sci. 2010; 51: 2067– 2076. [DOI] [PubMed] [Google Scholar]
- 20. McDowell CM, Hernandez H, Mao W, Clark AF. . Gremlin induces ocular hypertension in mice through smad3-dependent signaling. Invest Ophthalmol Vis Sci. 2015; 56: 5485– 5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McDowell CM, Tebow HE, Wordinger RJ, Clark AF. . Smad3 is necessary for transforming growth factor-beta2 induced ocular hypertension in mice. Exp Eye Res. 2013; 116: 419– 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tripathi RC, Chan WF, Li J, Tripathi BJ. . Trabecular cells express the TGF-beta 2 gene and secrete the cytokine. Exp Eye Res. 1994; 58: 523– 528. [DOI] [PubMed] [Google Scholar]
- 23. Zode GS, Sethi A, Brun-Zinkernagel A-M, Chang IF, Clark AF, Wordinger RJ. . Transforming growth factor-β2 increases extracellular matrix proteins in optic nerve head cells via activation of the Smad signaling pathway. Mol Vis. 2011; 17: 1745– 1758. [PMC free article] [PubMed] [Google Scholar]
- 24. Wordinger RJ, Fleenor DL, Hellberg PE,et al. . Effects of TGF-beta2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Invest Ophthalmol Vis Sci. 2007; 48: 1191– 1200. [DOI] [PubMed] [Google Scholar]
- 25. Buie LK, Karim MZ, Smith MH, Borras T. . Development of a model of elevated intraocular pressure in rats by gene transfer of bone morphogenetic protein 2. Invest Ophthalmol Vis Sci. 2013; 54: 5441– 5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nohe A, Keating E, Knaus P, Petersen NO. . Signal transduction of bone morphogenetic protein receptors. Cell Signal. 2004; 16: 291– 299. [DOI] [PubMed] [Google Scholar]
- 27. Wordinger RJ, Sharma T, Clark AF. . The role of TGF-β2 and bone morphogenetic proteins in the trabecular meshwork and glaucoma. J Ocul Pharmacol Ther. 2014; 30: 154– 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kretzschmar M, Liu F, Hata A, Doody J, Massague J. . The TGF-beta family mediator Smad1 is phosphorylated directly and activated functionally by the BMP receptor kinase. Genes Dev. 1997; 11: 984– 995. [DOI] [PubMed] [Google Scholar]
- 29. Sapkota G, Alarcon C, Spagnoli FM, Brivanlou AH, Massague J. . Balancing BMP signaling through integrated inputs into the Smad1 linker. Mol Cell. 2007; 25: 441– 454. [DOI] [PubMed] [Google Scholar]
- 30. Alarcon C, Zaromytidou AI, Xi Q,et al. . Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell. 2009; 139: 757– 769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang RN, Green J, Wang Z,et al. . Bone morphogenetic protein (BMP) signaling in development and human diseases. Genes Dis. 2014; 1: 87– 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wordinger RJ, Agarwal R, Talati M, Fuller J, Lambert W, Clark AF. . Expression of bone morphogenetic proteins (BMP), BMP receptors, and BMP associated proteins in human trabecular meshwork and optic nerve head cells and tissues. Mol Vis. 2002; 8: 241– 250. [PubMed] [Google Scholar]
- 33. Agarwal R, Agarwal P. . Future target molecules in antiglaucoma therapy: tgf-Beta may have a role to play. Ophthalmic Res. 2010; 43: 1– 10. [DOI] [PubMed] [Google Scholar]
- 34. Onichtchouk D, Chen YG, Dosch R,et al. . Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature. 1999; 401: 480– 485. [DOI] [PubMed] [Google Scholar]
- 35. Grotewold L, Plum M, Dildrop R, Peters T, Ruther U. . Bambi is coexpressed with Bmp-4 during mouse embryogenesis. Mech Dev. 2001; 100: 327– 330. [DOI] [PubMed] [Google Scholar]
- 36. Loveland KL, Bakker M, Meehan T,et al. . Expression of Bambi is widespread in juvenile and adult rat tissues and is regulated in male germ cells. Endocrinology. 2003; 144: 4180– 4186. [DOI] [PubMed] [Google Scholar]
- 37. Knight C, Papagerakis P, Simmons D, Berdal A, MacDougall M. . Genomic organization and localization of mouse Nma/BAMBI: possible implications related to ameloblastoma formation. Connect Tissue Res. 2002; 43: 359– 364. [DOI] [PubMed] [Google Scholar]
- 38. Knight C, Simmons D, Gu TT,et al. . Cloning, characterization, and tissue expression pattern of mouse Nma/BAMBI during odontogenesis. J Dent Res. 2001; 80: 1895– 1902. [DOI] [PubMed] [Google Scholar]
- 39. Borras T, Comes N. . Evidence for a calcification process in the trabecular meshwork. Exp Eye Res. 2009; 88: 738– 746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lin SJ, Lerch TF, Cook RW, Jardetzky TS, Woodruff TK. . The structural basis of TGF-beta, bone morphogenetic protein, and activin ligand binding. Reproduction. 2006; 132: 179– 190. [DOI] [PubMed] [Google Scholar]
- 41. Yan X, Lin Z, Chen F,et al. . Human BAMBI cooperates with smad7 to inhibit transforming growth factor-β signaling. J Biol Chem. 2009; 284: 30097– 30104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pils D, Wittinger M, Petz M,et al. . BAMBI is overexpressed in ovarian cancer and co-translocates with Smads into the nucleus upon TGF-beta treatment. Gynecol Oncol. 2010; 117: 189– 197. [DOI] [PubMed] [Google Scholar]
- 43. Sekiya T, Oda T, Matsuura K, Akiyama T. . Transcriptional regulation of the TGF-β pseudoreceptor BAMBI by TGF-β signaling. Biochem Biophys Res Comm. 2004; 320: 680– 684. [DOI] [PubMed] [Google Scholar]
- 44. Lin L, Wang Y, Liu W, Huang Y. . BAMBI inhibits skin fibrosis in keloid through suppressing TGF-beta1-induced hypernomic fibroblast cell proliferation and excessive accumulation of collagen I. Int J Clin Exp Med. 2015; 8: 13227– 13234. [PMC free article] [PubMed] [Google Scholar]
- 45. Fan Y, Li X, Xiao W,et al. . BAMBI elimination enhances alternative TGF-beta signaling and glomerular dysfunction in diabetic mice. Diabetes. 2015; 64: 2220– 2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tovar-Vidales T, Fitzgerald AM, Clark AF. . Human trabecular meshwork cells express BMP antagonist mRNAs and proteins. Exp Eye Res. 2016; 147: 156– 160. [DOI] [PubMed] [Google Scholar]
- 47. Seki E, De Minicis S, Osterreicher CH,et al. . TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007; 13: 1324– 1332. [DOI] [PubMed] [Google Scholar]
- 48. Bhattacharyya S, Kelley K, Melichian DS,et al. . Toll-like receptor 4 signaling augments transforming growth factor-beta responses: a novel mechanism for maintaining and amplifying fibrosis in scleroderma. Am J Pathol. 2013; 182: 192– 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang L, Seki E. . Toll-like receptors in liver fibrosis: cellular crosstalk and mechanisms. Front Physiol. 2012; 3: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Guo J, Friedman SL. . Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis. Fibrogen Tissue Rep. 2010; 3: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu C, Chen X, Yang L, Kisseleva T, Brenner DA, Seki E. . Transcriptional repression of the transforming growth factor beta (TGF-beta) pseudoreceptor BMP and activin membrane-bound inhibitor (BAMBI) by nuclear factor kappaB (NF-kappaB) p50 enhances TGF-beta signaling in hepatic stellate cells. J Biol Chem. 2014; 289: 7082– 7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Seki E, De Minicis S, Osterreicher CH,et al. . TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007; 13: 1324– 1332. [DOI] [PubMed] [Google Scholar]
- 53. Sasaki T, Sasahira T, Shimura H, Ikeda S, Kuniyasu H. . Effect of Nma on growth inhibition by TGF-beta in human gastric carcinoma cell lines. Oncol Rep. 2004; 11: 1219– 1223. [PubMed] [Google Scholar]
- 54. Sekiya T, Adachi S, Kohu K,et al. . Identification of BMP and activin membrane-bound inhibitor (BAMBI), an inhibitor of transforming growth factor-beta signaling, as a target of the beta-catenin pathway in colorectal tumor cells. J Biol Chem. 2004; 279: 6840– 6846. [DOI] [PubMed] [Google Scholar]
- 55. Hernandez H, Medina-Ortiz WE, Luan T, Clark AF, McDowell CM. . Crosstalk between transforming growth factor beta-2 and toll-like receptor 4 in the trabecular meshwork. Invest Ophthalmol Vis Sci. 2017; 58: 1811– 1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mao W, Liu Y, Wordinger RJ, Clark AF. . A magnetic bead-based method for mouse trabecular meshwork cell isolation. Invest Ophthalmol Vis Sci. 2013; 54: 3600– 3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Peotter JL, Phillips J, Tong T, Dimeo K, Gonzalez JM Jr, Peters DM. . Involvement of Tiam1, RhoG and ELMO2/ILK in Rac1-mediated phagocytosis in human trabecular meshwork cells. Exp Cell Res. 2016; 347: 301– 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gagen D, Filla MS, Clark R, Liton P, Peters DM. . Activated alphavbeta3 integrin regulates alphavbeta5 integrin-mediated phagocytosis in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2013; 54: 5000– 5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang X, Ognibene CM, Clark AF, Yorio T. . Dexamethasone inhibition of trabecular meshwork cell phagocytosis and its modulation by glucocorticoid receptor beta. Exp Eye Res. 2007; 84: 275– 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cao Y, Wei H, Da B, Huang Y. . Effect of transforming growth factor-beta 2 on phagocytosis in cultured bovine trabecular meshwork cells. J Tongji Med Univ. 2001; 21: 318– 320. [DOI] [PubMed] [Google Scholar]
- 61. Matsumoto Y, Johnson DH. . Trabecular meshwork phagocytosis in glaucomatous eyes. Ophthalmologica. 1997; 211: 147– 152. [DOI] [PubMed] [Google Scholar]
- 62. Matsumoto Y, Johnson DH. . Dexamethasone decreases phagocytosis by human trabecular meshwork cells in situ. Invest Ophthalmol Vis Sci. 1997; 38: 1902– 1907. [PubMed] [Google Scholar]
- 63. Yang X, Li M. . Establishment of in vitro culture of bovine trabecular meshwork cells and their phagocytosis [in Chinese]. Zhonghua Yan Ke Za Zhi. 1996; 32: 136– 139. [PubMed] [Google Scholar]
- 64. Zhou L, Fukuchi T, Kawa JE, Higginbotham EJ, Yue BY. . Loss of cell-matrix cohesiveness after phagocytosis by trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1995; 36: 787– 795. [PubMed] [Google Scholar]
- 65. Park CH, Latina MA. . Effects of gamma-interferon on human trabecular meshwork cell phagocytosis. Invest Ophthalmol Vis Sci. 1993; 34: 2228– 2236. [PubMed] [Google Scholar]
- 66. Buller C, Johnson DH, Tschumper RC. . Human trabecular meshwork phagocytosis. Observations in an organ culture system. Invest Ophthalmol Vis Sci. 1990; 31: 2156– 2163. [PubMed] [Google Scholar]
- 67. Shirato S, Murphy CG, Bloom E,et al. . Kinetics of phagocytosis in trabecular meshwork cells. Flow cytometry and morphometry. Invest Ophthalmol Vis Sci. 1989; 30: 2499– 2511. [PubMed] [Google Scholar]
- 68. Sherwood ME, Richardson TM. . Phagocytosis by trabecular meshwork cells: sequence of events in cats and monkeys. Exp Eye Res. 1988; 46: 881– 895. [DOI] [PubMed] [Google Scholar]
- 69. Chisholm IA, Grierson I. . Particulate phagocytosis by trabecular meshwork endothelium. Can J Ophthalmol 1977; 12: 293– 299. [PubMed] [Google Scholar]
- 70. Grierson I, Lee WR. . Erythrocyte phagocytosis in the human trabecular meshwork. Br J Ophthalmol. 1973; 57: 400– 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McDowell CM, Luan T, Zhang Z,et al. . Mutant human myocilin induces strain specific differences in ocular hypertension and optic nerve damage in mice. Exp Eye Res. 2012; 100: 65– 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Millar JC, Clark AF, Pang IH. . Assessment of aqueous humor dynamics in the mouse by a novel method of constant-flow infusion. Invest Ophthalmol Vis Sci. 2011; 52: 685– 694. [DOI] [PubMed] [Google Scholar]
- 73. Millar JC, Phan TN, Pang IH, Clark AF. . Strain and age effects on aqueous humor dynamics in the mouse. Invest Ophthalmol Vis Sci. 2015; 56: 5764– 5776. [DOI] [PubMed] [Google Scholar]
- 74. Lin S, Lee OT, Minasi P, Wong J. . Isolation, culture, and characterization of human fetal trabecular meshwork cells. Curr Eye Res. 2007; 32: 43– 50. [DOI] [PubMed] [Google Scholar]
- 75. Knepper PA, Samples JR, Yue BYJT. . Biomarkers of primary open-angle glaucoma. Expert Rev Ophthalmol. 2010; 5: 731– 742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pattabiraman PP, Rao PV. . Hic-5 Regulates actin cytoskeletal reorganization and expression of fibrogenic markers and myocilin in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2015; 56: 5656– 5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dismuke WM, Klingeborn M, Stamer WD. . Mechanism of fibronectin binding to human trabecular meshwork exosomes and its modulation by dexamethasone. PLoS One. 2016; 11: e0165326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mao W, Liu Y, Mody A, Montecchi-Palmer M, Wordinger RJ, Clark AF. . Characterization of a spontaneously immortalized bovine trabecular meshwork cell line. Exp Eye Res. 2012; 105: 53– 59. [DOI] [PubMed] [Google Scholar]
- 79. Ueyama K, Mori K, Shoji T,et al. . Ocular localization and transduction by adenoviral vectors are serotype-dependent and can be modified by inclusion of RGD fiber modifications. PLoS One. 2014; 9: e108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mai Y, Zhang Z, Yang H,et al. . BMP and activin membrane-bound inhibitor (BAMBI) inhibits the adipogenesis of porcine preadipocytes through Wnt/beta-catenin signaling pathway. Biochem Cell Biol 2014; 92: 172– 182. [DOI] [PubMed] [Google Scholar]
- 81. Villar AV, Garcia R, Llano M,et al. . BAMBI (BMP and activin membrane-bound inhibitor) protects the murine heart from pressure-overload biomechanical stress by restraining TGF-beta signaling. Biochim Biophys Acta. 2013; 1832: 323– 335. [DOI] [PubMed] [Google Scholar]
- 82. Luo X, Hutley LJ, Webster JA,et al. . Identification of BMP and activin membrane-bound inhibitor (BAMBI) as a potent negative regulator of adipogenesis and modulator of autocrine/paracrine adipogenic factors. Diabetes. 2012; 61: 124– 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wanninger J, Neumeier M, Bauer S,et al. . Adiponectin induces the transforming growth factor decoy receptor BAMBI in human hepatocytes. FEBS Lett. 2011; 585: 1338– 1344. [DOI] [PubMed] [Google Scholar]
- 84. Tramullas M, Lantero A, Diaz A,et al. . BAMBI (bone morphogenetic protein and activin membrane-bound inhibitor) reveals the involvement of the transforming growth factor-beta family in pain modulation. J Neurosci. 2010; 30: 1502– 1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tsuchida K, Nakatani M, Hitachi K,et al. . Activin signaling as an emerging target for therapeutic interventions. Cell Commun Signal. 2009; 7: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yan X, Lin Z, Chen F,et al. . Human BAMBI cooperates with smad7 to inhibit transforming growth factor-beta signaling. J Biol Chem. 2009; 284: 30097– 30104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. He Y, Ou Z, Chen X,et al. . LPS/TLR4 Signaling Enhances TGF-beta response through downregulating bambi during prostatic hyperplasia. Sci Rep. 2016; 6: 27051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhang Y, Yu Z, Xiao Q,et al. . Expression of BAMBI and its combination with smad7 correlates with tumor invasion and poor prognosis in gastric cancer. Tumour Biol. 2014; 35: 7047– 7056. [DOI] [PubMed] [Google Scholar]
- 89. Zhou L, Park J, Jang KY,et al. . The overexpression of BAMBI and its involvement in the growth and invasion of human osteosarcoma cells. Oncol Rep. 2013; 30: 1315– 1322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.