Abstract
Background:
Drinking water and other sources for lead are the subject of public health concerns around the Flint, Michigan, drinking water and East Chicago, Indiana, lead in soil crises. In 2015, the U.S. Environmental Protection Agency (EPA)’s National Drinking Water Advisory Council (NDWAC) recommended establishment of a “health-based, household action level” for lead in drinking water based on children’s exposure.
Objectives:
The primary objective was to develop a coupled exposure–dose modeling approach that can be used to determine what drinking water lead concentrations keep children’s blood lead levels (BLLs) below specified values, considering exposures from water, soil, dust, food, and air. Related objectives were to evaluate the coupled model estimates using real-world blood lead data, to quantify relative contributions by the various media, and to identify key model inputs.
Methods:
A modeling approach using the EPA’s Stochastic Human Exposure and Dose Simulation (SHEDS)-Multimedia and Integrated Exposure Uptake and Biokinetic (IEUBK) models was developed using available data. This analysis for the U.S. population of young children probabilistically simulated multimedia exposures and estimated relative contributions of media to BLLs across all population percentiles for several age groups.
Results:
Modeled BLLs compared well with nationally representative BLLs (0–23% relative error). Analyses revealed relative importance of soil and dust ingestion exposure pathways and associated Pb intake rates; water ingestion was also a main pathway, especially for infants.
Conclusions:
This methodology advances scientific understanding of the relationship between lead concentrations in drinking water and BLLs in children. It can guide national health-based benchmarks for lead and related community public health decisions. https://doi.org/10.1289/EHP1605
Introduction
Background
The U.S. Environmental Protection Agency (EPA), Centers for Disease Control and Prevention (CDC), and American Academy of Pediatrics agree that there is no known safe level of lead (Pb) in a child's blood; even low levels of Pb in the blood can result in behavior and learning problems, lower IQ and hyperactivity, slowed growth, hearing problems, and anemia (www.epa.gov/lead; http://www.cdc.gov/nceh/lead/; Council on Environmental Health 2016). Triantafyllidou et al. (2014) concluded that low levels of Pb in drinking water could pose a human health concern in sensitive population groups (e.g., young children and particularly formula-fed infants). Drinking water and other exposure sources for Pb have recently been the subject of public health concerns around the Flint, Michigan, drinking water (Hanna-Attisha et al. 2016; Laidlaw et al. 2016) and East Chicago, Indiana, Pb in soil (Goodnough 2016) crises. As part of the EPA’s Safe Drinking Water Act assessment of lead in drinking water, the National Drinking Water Advisory Council (NDWAC)’s Lead and Copper Rule (LCR) Working Group was established to provide advice to EPA in considering potential revisions to the LCR. In December 2015, NDWAC recommended establishment of a “health-based, household action level” for Pb in drinking water based on children’s exposure (NDWAC 2015). The NDWAC working group recommended that “water systems would be required to notify the consumer, state drinking water program, and the local public health agency if this level were exceeded. The expectation is that individuals and local officials would use this information to take prompt actions at the household level to mitigate lead risks. …” While the EPA has not yet determined the specific role of a health-based benchmark for Pb in drinking water in the new rule, the agency sees value in providing states with drinking water systems and the public with a greater understanding of the potential health implications for vulnerable populations of specific levels of Pb in drinking water. The EPA anticipates that a health-based benchmark could also help inform other potential elements of a revised LCR, including public education requirements, prioritization of households for lead service line replacement programs or other risk mitigation actions at the household level, and potential requirements related to schools or other priority locations (U.S. EPA 2016a). To guide a potential health-based benchmark for Pb in drinking water, an approach is needed to advance scientific understanding of the relationship between Pb concentrations in drinking water and blood lead levels (BLLs) in infants and young children.
Objectives
The primary objective was to develop a coupled exposure–dose modeling approach that can be used to determine what drinking water Pb concentrations keep exposed children’s BLL below specified target values, considering exposures from multiple media (water, soil, dust, food, air). There is no acceptable level of Pb in children; selected target values here relate to the CDC blood Pb reference value, currently at the 97.5th percentile of BLLs in U.S. children (cdc.gov/nceh/lead/acclpp/blood_lead_levels.htm). The CDC is considering changing the reference value to (ATSDR 2016). Related objectives of this analysis were to evaluate the coupled model estimates using EPA NHEXAS [National Human Exposure Assessment Survey (Clayton et al. 1999)] and CDC National Health and Nutrition Examination Survey [NHANES (CDC 2013a, 2013b, 2016)] BLL data, to quantify relative contributions by the various media, and to identify key model inputs. Our main hypothesis was that the Stochastic Human Exposure and Dose Simulation (SHEDS)-Multimedia Model (https://www.epa.gov/chemical-research/stochastic-human-exposure-and-dose-simulation-sheds-estimate-human-exposure), the probabilistic exposure model that was previously evaluated and applied for other chemicals, coupled with the Integrated Exposure Uptake and Biokinetic (IEUBK) Model (https://www.epa.gov/superfund/lead-superfund-sites-software-and-users-manuals), can estimate BLLs comparable to observed BLL data, i.e., with a relative error . A second hypothesis was that results from this coupled modeling approach can inform a health-based benchmark for Pb in drinking water considering a multimedia risk cup approach (a conceptual approach for estimating total Pb exposures and risks, aggregated from different sources), and provide a better understanding of the relative importance of exposure pathways and data needs to guide public health decisions for reducing childhood Pb risks.
While this work pertains to the U.S. residential (civilian) population, the same approach could be applied to other populations and countries, depending on available data. This analysis was not designed for specific at-risk populations or households, but some evaluation and contribution analysis results are provided with regional scale (NHEXAS Region 5) data in addition to national scale. The focus of this paper is the modeling and multimedia exposure analysis methodology; results are provided for several selected BLLs and percentiles of the population (based in part on the CDC blood Pb reference value mentioned above).
Methods
Models Used
A probabilistic modeling approach was developed and applied to quantify and analyze children’s Pb exposures and BLLs from drinking water and other environmental media (soil, dust, food, air). The analysis used the EPA’s SHEDS-Multimedia (version 4.1; U.S. EPA) coupled with the IEUBK (version 1.1, build 11; U.S. EPA). The SHEDS-Multimedia model is a physically based probabilistic Monte Carlo exposure model that can simulate aggregate or cumulative exposures over time via dietary and residential routes for a variety of multimedia environmental chemicals using real-world data (i.e., human activity diaries, measured concentration data, exposure factors) for model inputs. SHEDS-Multimedia has been applied for various pesticides, metals, and polychlorinated biphenyls in research applications and to inform EPA regulatory decisions (Xue et al. 2010, 2012, 2014a, 2014b; Zartarian et al. 2006, 2012; Glen et al. 2012). It has been well evaluated against real-world data (e.g., blood biomarker measurements), peer reviewed by multiple EPA external scientific advisory panels (www.epa.gov/sap), and published in over 30 journal articles. These published SHEDS-Multimedia sensitivity analysis and model evaluation analysis methods were used in this Pb application. The IEUBK model for estimation of childhood BLLs has also been externally peer reviewed and used for agency regulatory purposes (U.S. EPA 1994a, 1994b; Hogan et al. 1998; White et al. 1998; NRC 2005). It predicts childhood BLLs resulting from multiple pathways of exposure and supports soil clean-up levels at Superfund sites.
The general consensus of a 1999 workshop was that a fully probabilistic version of the IEUBK model would aid in understanding how exposure variability affects the distribution of BLL (NRC 2005, p. 239). SHEDS-Multimedia complements IEUBK by considering human exposures probabilistically. Coupling these models allows for simulating variability in Pb exposures and doses for different pathways, allowing identification of key model input variables and analysis of relative contribution by media and exposure pathways to BLL for different age groups and population percentiles. While SHEDS-Multimedia is a two-stage Monte Carlo model, we chose to not conduct a quantitative uncertainty analysis for the multimedia Pb analysis at this time, given the major effort involved to characterize level of confidence in each key input and conduct uncertainty simulations and analyses (Xue et al. 2006); thus, we present uncertainties and limitations qualitatively in the “Discussion” section of this paper.
Approach Overview
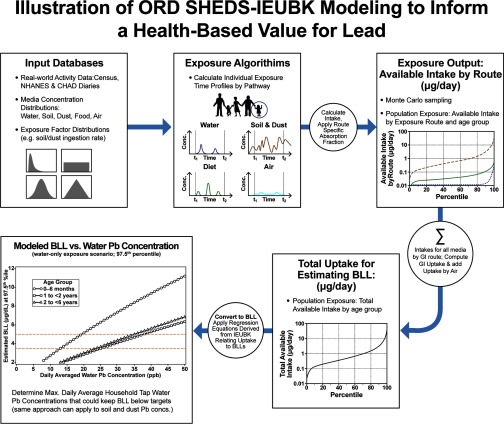
Figure 1 illustrates the general approach for this coupled model analysis. The top three panels illustrate the SHEDS-Multimedia exposure modeling methodology as described in Zartarian et al. (2012). Monte Carlo sampling was applied to obtain population variability distributions of exposures by pathway, from which available intake was determined and summed across pathways to compute uptake. Regression equations derived from IEUBK were applied to convert absorbed dose (uptake) to BLL. See Supplemental Materials Section S1, Table S1, and Figure S1 for details on the SHEDS–IEUBK coupling methodology and model inputs used in the analysis. Modeled BLL was plotted against water Pb concentration for a specified percentile of the population to determine the water Pb concentration that keep BLL below specified values. This process was repeated with different assumed water concentrations to identify the relationship between concentration and resulting BLL at the specified population percentile (Figure 1, bottom left panel). The red horizontal lines in this panel illustrate several target BLL values; the values on the curves intersecting the target BLL values represent the tipping point water Pb concentrations that keep BLL below specified levels.
Figure 1.
Illustration of Stochastic Human Exposure and Dose Simulation (SHEDS)–Integrated Exposure Uptake and Biokinetic (IEUBK) modeling to inform a health-based benchmark for Pb.
Data Used in the Modeling Analysis
Available data from various sources were used for children’s activity patterns, Pb concentrations in different media, exposure factors, and biokinetic dose factors; distributional inputs were based on measurements collected in EPA and other federal agency field studies, or reported in published literature (see Supplemental Materials S2 and Tables S3–S5). Age-specific model inputs were used where available. We simulated infants (0 to 6 mo of age) per NDWAC recommendation, but there is more uncertainty for this age group. We used activity diaries from Consolidated Human Activity Database and NHANES for this age group, but due to lack of exposure factor data, we assumed the same soil/dust ingestion rate as for 1-y-olds. Separate model analysis results were generated for different scenarios. The age groups considered were 0- to 6-mo-olds, 1- to , 2- to , and 0- to 7-y-olds (lifetime average, 0–84 mo). Exposure scenarios considered included Pb in drinking water only and aggregate exposures from Pb from water, soil, food, dust, and air. Several types of SHEDS–IEUBK runs were conducted: a) for model evaluation, a national-scale analysis using NHANES data, and a regional-scale analysis using NHEXAS Region 5 data; b) for analyzing relative contributions by exposure pathway in the United States and NHEXAS Region 5; c) for sensitivity analyses to identify key factors; and d) for national-scale runs with a set of alternative drinking water Pb concentration scenarios to develop the linear relationships between concentration and BLL percentiles, shown in the bottom left of Figure 1.
Model Averaging Time and Addressing Biological Variability in the Coupled Models
Initial analyses were conducted with 2-d model averaging times, given available activity diaries used in SHEDS-Multimedia; we subsequently focused on 30-d averaging time simulations consistent with the IEUBK period, per recommendations of a work-in-progress peer consultation panel (Versar, Inc. 2016). The 30-d analysis results are shown below, and 2-d analysis results are provided in Supplemental Materials for comparison; pros and cons of both are in the Discussion. IEUBK blood Pb estimates do not reflect interindividual behavioral and pharmacokinetic differences; a geometric standard deviation (GSD) of 1.6 is applied to outputs to account for biological variability and measurement error, but does not account for exposure variability (Hogan et al. 1998; White et al. 1998). The SHEDS–IEUBK modeling only represents exposure variability; thus, a variability factor is needed to reflect real-world BLLs that also account for biological variability (this term may also account for other sources of variability, such as measurement and/or model error), and this factor is affected by the model averaging time period.
From the model evaluation results comparing SHEDS–IEUBK BLL estimates vs. NHANES-measured BLLs, the GSDs are 1.64 and 1.62 for 1- to and 2- to groups, respectively, while GSDs for NHANES BLLs are 1.92 and 1.89 for those two age groups, respectively (presented in the “Results” section below). These results indicate that GSDs of the real-world BLL measurements are consistently higher than those of predicted BLLs for both age groups, i.e., GSDs of 1 to and 2 to are almost the same for the two age groups, and the difference between NHANES and SHEDS–IEUBK BLL GSDs is for both age groups. This implies biological variability was missing in our original 30-d averaging time BLL predictions, since only exposure variability is accounted for in coupling SHEDS-Multimedia and IEUBK; the missing variability will affect the distribution of the BLLs and high percentiles. Thus, we used the GSDs of NHANES BLL data as the standard to calculate the missing biological variability as shown in the equations below. We assumed exposure and biological variances are independent, and the distribution is lognormal. In the log–transformed space:
: total variance
: exposure variance
: biological variance
This is the formula that was used to calculate the biological variance by age group:
for 1- to olds and 2- to -olds are 0.185 and 0.176, respectively, which is generally consistent with biological variance, 0.22, specified by IEUBK { and }. We redid our original 30-d model assessment with the above calculated biological variances for model evaluation and prediction of BLLs with daily averaged household tap water Pb concentration.
Results
Results below and in Supplemental Materials demonstrate the SHEDS–IEUBK modeling approach.
Model Evaluation
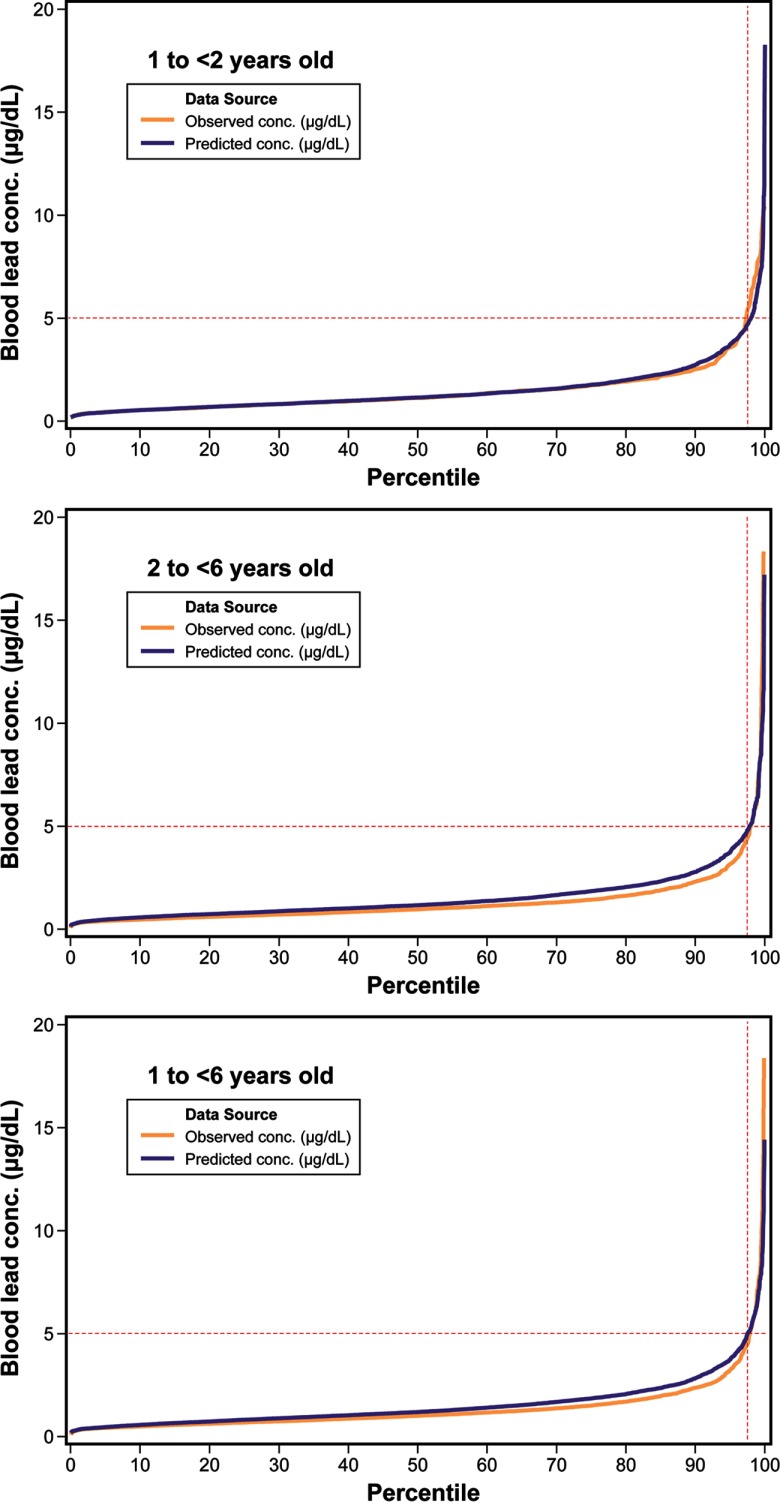
For evaluating or ground-truthing national scale estimates of BLLs using the SHEDS–IEUBK coupled model approach, we used NHANES 2009–2014 BLL data. Performance of the coupled models at the national scale was evaluated by the relative error between estimated BLLs and observed BLLs (i.e., the difference between the estimated and observed BLL divided by the observed BLL value). Available representative Pb concentrations for food (U.S. FDA 2014), soil and dust (HUD 2011), and water (U.S. EPA 2010) were used for model inputs. For 30-d exposure time frame analyses, we used correlated inputs for soil, dust, and water Pb concentrations (see Table S5). The relative error in BLL was 0–23%, depending on age and percentile (see Figure 2 and Table 1; note that for 2-d analyses not considering biological variability and possibly overestimating exposure variability, relative error was for all percentiles and age groups as shown in Table S6, Figure S2). SHEDS–IEUBK modeling underestimated BLL for NHANES 2009–2010 sampling period data, overpredicted for 2013–2014, and better predicted for 2011–2012 (Figure S3 and Figure S4). Additional model evaluation results (e.g., with NHEXAS data) are presented in Table S7; for the regional-scale analysis, relative error was 35–42%, depending on age and percentile.
Figure 2.
Evaluation of Stochastic Human Exposure and Dose Simulation (SHEDS)–Integrated Exposure Uptake and Biokinetic (IEUBK) modeled blood lead levels (BLL) vs. National Health and Nutrition Examination Survey (NHANES) 2009–2014 BLL for different age groups. conc., concentration.
Table 1.
Stochastic Human Exposure and Dose Simulation (SHEDS)–Integrated Exposure Uptake and Biokinetic (IEUBK) modeling blood lead level (BLL) evaluation with 2009–2014 National Health and Nutrition Examination Survey (NHANES) blood data, longitudinal (30 d) with correlated key inputs.
| Age group | Source | Mean | SD | 50th | GM | GSD | 95th | 97.5th | 99th | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 to old | Observed | 475 | 1.47 | 1.30 | 1.12 | 1.16 | 1.92 | 3.60 | 5.54 | 7.90 | 6.95 |
| Predicted | 3,000 | 1.46 | 1.27 | 1.13 | 1.16 | 1.92a | 3.58 | 4.60 | 6.41 | 7.70 | |
| Relative error | 0% | 0% | |||||||||
| 2 to old | Observed | 1,892 | 1.33 | 1.60 | 0.98 | 1.03 | 1.89 | 3.13 | 4.39 | 7.15 | 5.44 |
| Predicted | 3,000 | 1.55 | 1.28 | 1.20 | 1.25 | 1.88a | 3.84 | 4.94 | 6.67 | 8.60 | |
| Relative error | 17% | 23% | 21% | 23% | 12% | 7% |
Note: Relative error here is absolute value of predicted minus observed, divided by observed, multiplied by 100. GM, geometric mean; GSD, geometric standard deviation; ; SD, standard deviation.
This GSD reflects the effect of exposure and biological variability on BLL.
Relative Exposure Pathway Contributions
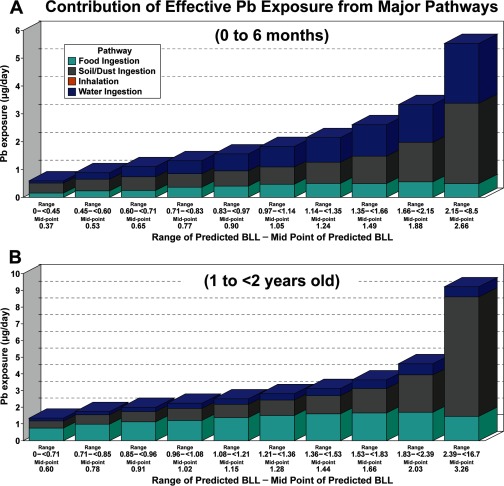
The modeled exposure pathway contribution analyses revealed that for children older than 1 y, the soil/dust ingestion and food ingestion pathways contributed more to BLL than drinking water, and the air pathway contributed the least with a small amount. For higher percentiles of the BLL distribution, soil/dust ingestion is the major pathway. Water ingestion is also an important contributor, especially for infants. For context, the CDC currently has a reference BLL for 1- to 5-y-olds of based on the distribution of BLLs in the United States; this is the reference level at which CDC recommends public health actions be initiated. As shown in Figure 3, for the national analyses:
-
•
For 0- to 6-mo-olds, soil/dust and water ingestion pathways predominate at the highest BLL percentiles. At the 90th to 100th percentiles with median predicted BLL (range: 2.15 to ), soil/dust and water account for and , respectively. At the 70th–80th percentile or median predicted BLL (1.35 to ), soil/dust and water ingestion together account for of Pb exposure. Soil/dust, food, and water ingestion have similar contributions up to the percentile of the population at predicted median BLL (0.83 to ). Food intake is a background exposure accounting for , depending on the BLL percentile, and food intake accounts for of BLL.
-
•
For 1- to , soil/dust ingestion was the dominant pathway above the BLL percentile. Above the 90th BLL percentile or predicted median BLL (2.39 to ), soil/dust, food intake, and water account for 77%, 16%, and 7%, respectively. Food intake was a major contributor below the percentile BLL, and contributed , on average, across all percentiles. Water accounted for of the BLL, depending on the percentile, and contributed on average.
-
•
Not illustrated in Figure 3, the pathway contributions for 2- to -olds were essentially the same as for 1- to -olds (see Figure S5).
Figure 3.
Estimated contribution of exposure pathways to BLL, for national scale. Bar charts provide Pb daily exposure contributions from diet, soil and dust ingestion, water, and inhalation from air for percentiles of the BLL distribution. The bars are 10% increments in the BLL distribution. The median BLL for each increment is indicated under each bar. Exposure in the figure is adjusted for bioavailability of Pb in each exposure pathway. Panel (A), national scale for 0- to 6-mo-olds; Panel (B), national scale for 1- to -olds.
Additional contribution analysis results with NHEXAS Region 5 data are presented in Figure S6.
Key Model Inputs Identified by Sensitivity Analyses
Model results were most sensitive to dietary inputs for lower percentiles and soil/dust ingestion inputs for higher percentiles of BLL distributions, as illustrated in Figure 3. Sensitivity analyses showed soil/dust ingestion rate, soil Pb concentration, food Pb intake, and bioavailability are key inputs. Food Pb intake was highly sensitive to methods for handling nondetects (see Table S8). For soil/dust ingestion rates, the most influential input considered for the coupled model outputs, we did an additional sensitivity analysis using the central tendency value of suggested by U.S. EPA (2011) and also , and found the BLL targets at the 97.5th percentile were exceeded without drinking water Pb. Similarly, targets were exceeded with a sensitivity analysis using von Lindern et al. (2016) soil/dust ingestion rates for 1 to (see Table S9 and Figure S7); this analysis also found SHEDS–IEUBK overestimated NHANES BLLs. The sensitivity analyses show that the blood Pb prediction for 0- to 7-y-olds is very sensitive to soil/dust ingestion rate when it was scaled from the input based on Özkaynak et al. (2011) to ; for example, at the 97.5th percentile, the daily averaged tap water Pb concentration that could keep BLL below was reduced from 5 ppb to 1 ppb (see Table S10). Details on these inputs and sensitivity analyses are provided in Supplemental Materials. There are three current approaches for estimating soil/dust ingestion rate as described in U.S. EPA (2011); this variable is highly uncertain for children under age 2 y.
Drinking Water Lead Concentrations at Example Target Blood Lead Levels
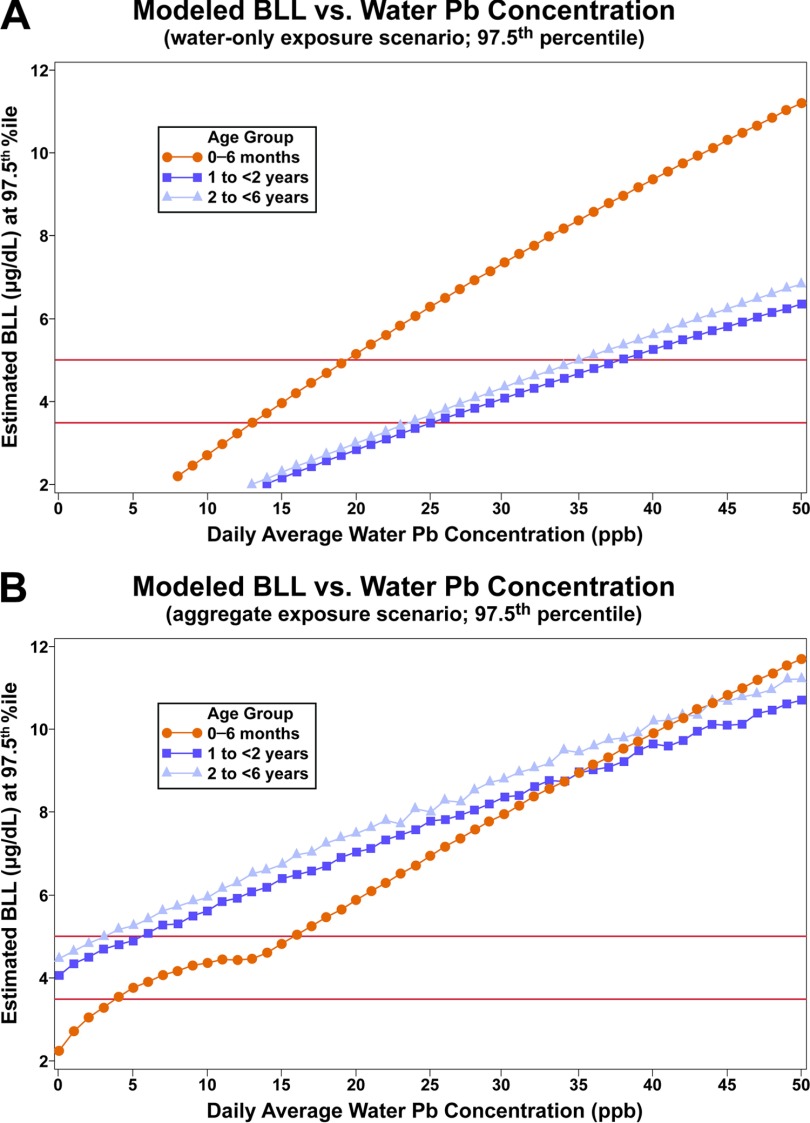
Figure 4 and Table 2 show SHEDS–IEUBK results for estimated maximum daily average household tap water Pb concentrations that could keep BLL below specified targets (30-d averaging time); these were derived as described with Figure 1. Figure 4 illustrates the predicted BLL at the 97.5th percentile of the U.S. population as a function of daily average household tap Pb water concentration for the different age and exposure scenarios. These plots allowed us to extract the daily average water Pb concentration that could keep BLLs below the specified targets of 3.5 and at the 97.5th percentile BLL of the U.S. population of each age range, as shown in Table 2. The dashes in Table 2 for three aggregate scenarios indicate that even with no Pb in water, this target would be exceeded. The robustness of this modeling approach allows consideration of household tap water Pb concentrations for other percentiles. However, the numbers in Table 2 could be conservatively low because key model input values based on the available older data (e.g., soil and dust concentrations, and soil/dust ingestion rate) may be higher than they are currently.
Figure 4.
Illustrative graphs for determining household tap water Pb concentrations were calculated for different scenarios.
y-Axis is modeled blood Pb level at 97.5th percentile of simulated population; x-axis is daily average water Pb concentration. The different colored lines represent different ages: orange is infants age 0–6 mo, dark blue is 1- to -olds, and light blue is 2- to -olds.
Table 2.
Stochastic Human Exposure and Dose Simulation (SHEDS)–Integrated Exposure Uptake and Biokinetic (IEUBK) results for maximum daily average household tap water Pb concentrations that could keep BLL below specified values (30-d averaging time; accounting for correlations and other external peer consult input described in Supplemental Material).
| Age group | Exposure scenario | BLL: 97.5th percentile | BLL: 97.5th percentile | BLL: 95th percentile | BLL: 95th percentile |
|---|---|---|---|---|---|
| 0 to 6 mo old | Water only | 13 ppb | 19 ppb | 14 ppb | 21 ppb |
| Aggregate | 4 ppb | 16 ppb | 7 ppb | 17 ppb | |
| 1 to -old | Water only | 25 ppb | 38 ppb | 31 ppb | 46 ppb |
| Aggregate | — | 5 ppb | 3 ppb | 14 ppb | |
| 2 to -old | Water only | 24 ppb | 35 ppb | 29 ppb | 44 ppb |
| Aggregate | — | 3 ppb | 1 ppb | 12 ppb | |
| 0 to 7 y old | Water only | 20 ppb | 30 ppb | 27 ppb | 41 ppb |
| Aggregate | — | 5 ppb | 2 ppb | 13 ppb |
Note: Daily average of a distribution reflecting real-world monitoring scheme to be determined. —, BLL will not be below targets even with 0 ppb Pb in water.
Discussion
This paper presents a state-of-the-science methodology that can guide a health-based benchmark for Pb in drinking water and can also be applied to other media. The well-reviewed, published, evaluated models allowed for contribution and sensitivity analyses, and identification of key factors, media, and exposure pathways. The coupled SHEDS–IEUBK estimates compared well against BLL data from NHANES and NHEXAS (0–23% and relative error, respectively), despite compiling different input data sets not originally intended for this purpose. The ability to probabilistically simulate multimedia exposures for the U.S. population and provide blood lead predictions consistent with NHANES BLLs represents an advance in science and a potential to guide public health decisions. Human exposures and public health outcomes are considered in the EPA’s Pb policies, such as the LCR. For example, revisions underway to strengthen the LCR include a potential health-based benchmark for Pb in drinking water and assessment of the benefits of lead service line replacement programs. Recent surveys [conducted in 2011 and 2013 and discussed in Cornwell et al. (2016)] conducted by the American Water Works Association indicate that between 15 to 22 million people of the 293 million served by U.S. community water systems have either full or partial Pb-containing lines servicing their home (7%) (Cornwell et al. 2016). The SHEDS–IEUBK multimedia exposure modeling analysis approach presented in this paper could inform national rulemaking efforts that translate to the local scale through state and local drinking water programs. If communities with water Pb issues are aware that soil and dust Pb can also be important contributors to children’s BLLs and understand the limits of drinking water program efforts, community-level education and outreach efforts can be targeted to maximize multimedia exposure reduction efforts for minimizing children’s Pb risks.
Another strength of this SHEDS–IEUBK analysis is that it uniquely reports percent contribution to children’s BLL by pathway, population percentile, and age group. The EPA’s 2007 Risk and Exposure Assessment for Lead did provide an urban case study of Pb pathway contributions with estimates of 20.5% of Pb from diet, 11.9% from drinking water, 43.7% from outdoor soil/dust, 23.7% from indoor dust, and 0.1% from air by inhalation (based on average annual uptake from each media until a child is 7-y-old and assuming a maximum monthly average airborne Pb) (U.S. EPA 2007). There are a number of papers reporting on the importance of the soil/dust pathway to BLL of children as described in U.S. EPA (2013) and references therein (e.g., Mielke et al. 2011). The relative media contributions at the upper percentiles of SHEDS–IEUBK estimates for -olds are consistent with the U.S. EPA (2007) results; we estimate dietary contribution greater in lower percentiles, and water contribution higher for infants 0–6 mo of age. However, contributions from pathways are highly dependent on scenarios being considered (e.g., Elwood et al. 1984; Mielke et al. 2011; Zahran et al. 2013). Isolated events and widespread occurrences of drinking water contaminated with Pb have been associated with and thought to be the dominant contributor to elevated BLLs in North Carolina, Maine, Michigan, and Washington, DC (Edwards et al. 2009; Hanna-Attisha et al. 2016). Additionally, underestimates of the contribution to BLL from Pb-contaminated water may occur due to potential indirect exposure from food preparation (Triantafyllidou and Edwards 2012). Other studies have also shown indoor dust sources from both Pb-based paint (Blette 2008) and legacy soil Pb concentrations (Mielke and Reagan 1998) to be major contributors to elevated BLL, and in some cases to be a dominant source of exposure (Gasana et al. 2006).
The SHEDS–IEUBK model evaluation was stronger (lower relative error between observed and modeled values) for earlier NHANES time frames. BLLs have been decreasing over decades and have continued to decrease since 2010 (U.S. EPA 2016b, 2016c; Laidlaw et al. 2016). Whether this recent change can be explained by changing media concentrations, human activity patterns (two main components of human exposure), or both, remains unclear. Certainly, due to federal regulations, the removal or reduction of Pb in gasoline, paint, and plumbing has contributed (Council on Environmental Health 2016). The apparent decline in time spent outdoors by children in the United States (Roberts and Foehr 2008) may also have contributed by reducing Pb soil ingestion. There is also seasonal variation in BLL, with BLLs tending to be increased in the fall (e.g., see Laidlaw et al. 2016), which we could not model using IEUBK.
There are some other limitations and uncertainties of this analysis. Daily model average results for Pb in drinking water related to the CDC reference value may be impacted by temporal changes in NHANES in addition to model inputs changing over time. Our approach involves selecting a BLL benchmark (e.g., CDC reference level that may change). The multimedia Pb modeling analysis results are based on inputs for which available data may not reflect recent exposures [e.g., U.S. Department of Housing and Urban Development (HUD) soil Pb data is 2005–2006]. With additional information from future field studies on temporal changes in model inputs in recent years, further evaluation of model predictions against temporal changes in recent NHANES samplings would be possible. Because NHANES sample size is limited to represent the national population, collecting and analyzing states’ blood Pb data may be useful for further model evaluation.
Our modeling indicates that soil and dust ingestion is a dominant exposure pathway. The soil/dust ingestion rate for children is a key input to which model results are highly sensitive, and for which data are limited and uncertain, especially for children ; for older ages, values are similar between Özkaynak et al. (2011) used in this analysis and von Lindern et al. (2016), developed using different methodologies. If higher soil/dust ingestion rate values were used with this analysis, modeled water Pb concentrations would be lower. Although we applied soil and dust ingestion rates, which are among the lowest in the reported literature, our simulations overpredicted blood Pb for the most recent 2013–2014 NHANES cycle. An analysis of U.S. soil Pb studies from 1970 to 2012 reported no association between year and median soil Pb concentration at a national scale, although within single cities, soil Pb generally declined over time (Datko-Williams et al. 2014); thus, we posit that changing human activity patterns, such as soil/dust ingestion rates, may in part explain the BLL declines.
In addition to soil/dust ingestion rate, other uncertainties in this analysis are not accounting for seasonal variations (due to lack of available data and use of IEUBK), model averaging time, and how the coupled models capture biological and other sources of variability in the GSD of BLLs. Because the 30-d exposure period GSD reflects the effect of exposure variability, but not biological variability on BLL, our original results underpredicted the GSD and upper percentiles of BLLs in NHANES, and accordingly, overestimated Pb in water concentrations. Using a 2-d model averaging time does not align with IEUBK, but shows closer comparison to NHANES BLL data and GSD, as shown in Supplemental Materials (Figures S2–S3, and Table S6). The 2-d results may approximate BLL accounting for biologic variability by overestimating exposure variability. With the approach for addressing the biological variability issue described in the “Methods” section above, GSDs between SHEDS–IEUBK estimated and NHANES measured BLLs are very close (1.92 vs. 1.92 for 1- to -olds and 1.89 vs. 1.88 for 2- to -olds; see Table 1), and evaluation with NHANES BLLs has been improved with adding biological variance, especially for higher percentiles (see Table 1 and Figure 2). More BLL data being collected from states (McClure et al. 2016) could help evaluate the biological variance correction factor and which averaging time is more appropriate to guide a health-based benchmark for Pb. State-collected BLL data will also supplement NHANES BLL data, which may not be fully representative of the true distribution of the U.S. population BLLs, particularly at the tails.
While this work pertains to the U.S. population, the same approach could be applied to other populations or countries, but the results might be different. Although we simulated correlations in Pb exposure among dust, soil, and water (using NHEXAS and HUD data), stratified data by housing age, and assessed BLL at upper percentiles of the BLL distribution, our current analyses are not focused on specific at-risk populations, such as Flint, Michigan, and East Chicago, Indiana, or other environmental justice communities or homes with high Pb in soil, dust, or water. The household tap water monitoring scheme is a factor that influences estimated drinking water Pb concentrations and related exposures. Given the spatial and temporal variability of household Pb water concentrations, there are uncertainties in water Pb concentration data collected under the current LCR regulatory sampling that limits the ability to predict Pb exposures from drinking water. Local-scale data for multimedia model inputs and BLLs, preferably collected simultaneously and with geospatial and temporal resolution, would be beneficial for extending the coupled model approach for other applications and specific communities.
Conclusions
This Pb modeling methodology and multimedia analysis advances scientific understanding of the relationship between Pb levels in drinking water and BLLs in infants and young children, and can inform a health-based benchmark for lead in drinking water. The approach can also be applied to soil, dust, food, or other environmental media to guide decision-making, considering exposures aggregated from multiple media. While the focus of this analysis is the national scale, to help inform national rulemaking for Pb policies addressing multimedia exposures and public health outcomes, decisions such as setting a health-based benchmark for Pb in drinking water under the revised LCR would guide local-scale monitoring programs and Pb risk prevention education efforts in communities, and help systematically identify vulnerable communities such as Flint, Michigan, and East Chicago, Indiana, in the United States.
In addition, this modeling approach developed for Pb could apply to other multimedia contaminants for cumulative impact analyses. While model evaluation provides confidence in the results, more up-to-date data and information on key model inputs (e.g., children’s soil/dust ingestion rate and bioavailability) and BLLs would be helpful to refine model estimates for quantifying and reducing uncertainties, and to focus on specific at-risk populations and communities. Modeled estimates of BLL using the SHEDS–IEUBK approach can be extended to quantify health endpoints (e.g., IQ decrements) and to inform benefits analyses for strengthening public health protection (e.g., considering benefits of Pb service line replacement programs under the revised LCR). This modeling approach, together with state-collected BLL data and other data sets, e.g., for environmental justice variables and social determinants of health, could also be applied to help identify the most at-risk communities for Pb exposures and understand key factors for disparities; such analyses could inform decisions for minimizing public health risks from national to local scales.
Supplemental Material
Acknowledgments
We gratefully acknowledge the following individuals: U.S. EPA Office of Research and Development managers and scientists for providing guidance and review, including J. Garland, J. Orme-Zavaleta, P. Price, K. Isaacs, K. Alapaty, A. Gillespie, M. Slimak, A. Geller, R. Kavlock, T. Burke; H. Huang [via Postdoctoral Program administered by the Oak Ridge Institute for Science and Education (ORISE) through Interagency Agreement between DOE and EPA, IA number DW-89-92431601], J. Frank (ORISE), and A. Poulakos (ASRC Federal Vistronix, contract EP-G131-00143) for assisting with inputs and literature review; U.S. EPA Program Office staff for technical input, including E. Helm, A. Hafez, L. Christ, E. Burneson, S. Foster, K. Raffaele, M. Burgess, D. Murphy, and Z. Pekar; Office of Research and Development staff for technical input and assistance with the peer consult, and Quality Assurance Project Plan, including N. Shao, D. Lytle, M. Schock, T. Speth, R. Daniels, B. Stuart, C. Alvarez; P. Ashley of HUD and J. Spungen from FDA for providing data; Versar, Inc. for managing the peer consult and work-in-progress reviewers K. Bogen, D. Hattis, K. Vork, and E. DesHommes; the peer consult occurred with funding under contract EP-C-12-045-91; CSRA for graphics support. The data reported in this paper are presented or available at EPA’s ScienceHub (https://edg.epa.gov/metadata/catalog/main/home.page).
References
- ATSDR (Agency for Toxics Substances and Disease Registry). 2016. Meeting of the Lead Poisoning Prevention Subcommittee of the NCEH/ATSDR Board of Scientific Counselors. 19 September 2016, Atlanta, GA, Record of the Proceedings https://www.atsdr.cdc.gov/science/lpp/docs/lead_subcommittee_minutes_9_19_2016_508.pdf [accessed 13 July 2017].
- Blette V. 2008. Drinking water public right-to-know requirements in the United States. J Water Health 6(suppl 1):43–51, 10.2166/wh.2008.031. [DOI] [PubMed] [Google Scholar]
- CDC. 2013a. (Centers for Disease Control and Prevention). National Health and Nutrition Examination Survey (NHANES 2009–2010). https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Laboratory&CycleBeginYear=2009 [accessed 13 July 2017].
- CDC. 2013b. National Health and Nutrition Examination Survey (NHANES 2011–2012). https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Laboratory&CycleBeginYear=2011 [accessed 13 July 2017].
- CDC. 2016. National Health and Nutrition Examination Survey (NHANES 2013–2014). https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Laboratory&CycleBeginYear=2013 [accessed 13 July 2017].
- Clayton CA, Pellizzari ED, Whitmore RW, Perritt RL, Quackenboss JJ. 1999. National Human Exposure Assessment Survey (NHEXAS): distributions and associations of lead, arsenic and volatile organic compounds in EPA region 5. J Expo Anal Environ Epidemiol 9(5):381–392, PMID: 10554141, 10.1038/sj.jea.7500055. [DOI] [PubMed] [Google Scholar]
- Cornwell DA, Brown RA, Via SH. 2016. National survey of lead service line occurrence. J Am Water Works Assoc 108(4):E182–E191, 10.5942/jawwa.2016.108.0086. [DOI] [Google Scholar]
- Council on Environmental Health. 2016. Prevention of Childhood Lead Toxicity. Pediatrics 138(1):e20161493, PMID: 27325637, 10.1542/peds.2016-1493. [DOI] [PubMed] [Google Scholar]
- Datko-Williams L, Wilkie A, Richmond-Bryant J. 2014. Analysis of U.S. soil lead (Pb) studies from 1970 to 2012. Sci Tot Env 468–469:854–863, 10.1016/j.scitotenv.2013.08.089. [DOI] [PubMed] [Google Scholar]
- Edwards M, Triantafyllidou S, Best D. 2009. D. Elevated blood lead in young children due to lead-contaminated drinking water: Washington, DC, 2001–2004. Environ Sci Technol 43(5):1618–1623, 10.1021/es802789w. [DOI] [PubMed] [Google Scholar]
- Elwood PC, Gallacher JEJ, Phillips KM, Davies BE, Toothill C. 1984. Greater contribution to blood lead from water than from air. Nature 310:138–140, 10.1038/310138a0. [DOI] [PubMed] [Google Scholar]
- Gasana J, Hlaing WM, Siegel KA, Chamorro A, Niyonsenga T. 2006. Blood lead levels in children and environmental lead contamination in Miami inner city, Florida. Int J Environ Res Public Health 3(3):228–234, PMID: 16968968, 10.3390/ijerph2006030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glen G, Zartarian V, Smith L, Xue J. 2012. The Stochastic Human Exposure and Dose Simulation Model for Multimedia, Multipathway Chemical: Residential Module (SHEDS-Residential version 4, Technical Manual). Washington, DC:U.S. Environmental Protection Agency, Office of Research and Development. [Google Scholar]
- Goodnough A. 2016. Their soil toxic, 1,100 Indiana residents scramble to find new homes. The New York Times (news story). U.S. section, online edition. 31 August 2016. https://www.nytimes.com/2016/08/31/us/lead-contamination-public-housing-east-chicago-indiana.html [accessed 6 July 2017].
- Hanna-Attisha M, LaChance J, Sadler RC, Champney Schnepp A. 2016. Elevated blood lead levels in children associated with the Flint drinking water crisis: a spatial analysis of risk and public health response. Am J Public Health 106(2):283–290, PMID: 26691115, 10.2105/AJPH.2015.303003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan K, Marcus A, Smith R, White P. 1998. Integrated exposure uptake biokinetic model for lead in children: empirical comparisons with epidemiologic data. Environ Health Perspect 106(suppl 6):1557–1567, PMID: 9860915, 10.1289/ehp.98106s61557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUD (U.S. Department of Housing and Urban Development). 2011. American Healthy Homes Survey, American Healthy Homes Survey Lead and Arsenic Findings (Lead concentration data provided in 2016 from Policy and Standards Division, Office of Lead Hazard Control and Healthy Homes, U.S. Department of Housing and Urban Development). http://portal.hud.gov/hudportal/documents/huddoc?id=AHHS_Report.pdf [accessed 6 July 2017].
- Laidlaw MAS, Filippelli GM, Sadler RC, Gonzales CR, Ball AS, Mielke HW. 2016. Children's Blood Lead Seasonality in Flint, Michigan (USA), and Soil-Sourced Lead Hazard Risks. Int J Environ Res Public Health 13(4):358, PMID: 27023578, 10.3390/ijerph13040358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure LF, Niles JK, Kaufman HW. 2016. Blood lead levels in young children: US, 2009–2015. J Pediatr 75:173–81, PMID: 27297207, 10.1016/j.jpeds.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Mielke HW, Gonzales CR, Mielke PW. 2011. The continuing impact of lead dust on children’s blood lead: comparison of public and private properties in New Orleans. Environ Res 111(8):1164–1172, PMID: 21764050, 10.1016/j.envres.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Mielke HW, Reagan PL. 1998. Soil is an important pathway of human lead exposure. Environ Health Perspect 106(suppl 1):217–229, PMID: 9539015, 10.1289/ehp.98106s1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NDWAC (National Drinking Water Advisory). 2015. “Report of the Lead and Copper Rule Working Group to the National Drinking Water Advisory Council. Final. August 24, 2015.” 36–37. https://www.epa.gov/sites/production/files/2016-01/documents/ndwaclcrwgfinalreportaug2015.pdf [accessed 6 July 2017].
- NRC (National Research Council). 2005. Superfund and Mining Megasites: Lessons from the Coeur d'Alene River Basin. Washington, DC:The National Academies Press; 10.17226/11359 [accessed 6 July 2017]. [DOI] [Google Scholar]
- Özkaynak H, Xue J, Zartarian VG, Glen G, Smith L. 2011. Modeled estimates of soil and dust ingestion rates for children. Risk Anal 31(4):592–608, PMID: 21039709, 10.1111/j.1539-6924.2010.01524.x. [DOI] [PubMed] [Google Scholar]
- Roberts DF, Foehr UG. 2008. Trends in media use. Future Child 18(1):11–37, PMID: 21338004. [DOI] [PubMed] [Google Scholar]
- Triantafyllidou S, Edwards M. 2012. Lead (Pb) in tap water and in blood: implications for lead exposure in the United States. Crit Rev Environ Sci Technol 42(13):1297–1352, 10.1080/10643389.2011.556556. [DOI] [Google Scholar]
- Triantafyllidou S, Gallagher D, Edwards M. 2014. Assessing risk with increasingly stringent public health goals: the case of water lead and blood lead in children. J Water Health 12(1):57–68, PMID: 24642433, 10.2166/wh.2013.067. [DOI] [PubMed] [Google Scholar]
- U.S. EPA. 1994a. Guidance Manual for the IEUBK Model for Lead in Children. OSWER #9285.7-15-1. EPA 540/R-93/081. February 1994. https://www.epa.gov/superfund/lead-superfund-sites-software-and-users-manuals [accessed 6 July 2017].
- U.S. EPA. 1994b. Technical Support Document: Parameters and Equations Used in the Integrated Exposure Uptake Biokinetic Model for Lead in Children (v0.99d). OSWER #9258.7-22. EPA 540/R-94/040. December 1994. https://www.epa.gov/superfund/lead-superfund-sites-software-and-users-manuals [accessed 6 July 2017].
- U.S. EPA. 2007. “Lead: Human Exposure and Health Risk Assessments for Selected Case Studies.” Vol. 2: Appendices. 452/R-07-014b. Research Triangle Park, NC:U.S. Environmental Protection Agency. [Google Scholar]
- U.S. EPA. 2010. “Analysis of Occurrence Data from the Second Six-Year Review of Existing National Primary Drinking Water Regulations.” EPA 815-B-09-006, https://www.epa.gov/sites/production/files/2014-12/documents/815b09006.pdf [accessed 17 July 2017].
- U.S. EPA. 2011. Exposure Factors Handbook: 2011 Edition. National Center for Environmental Assessment, Washington, DC:U.S. EPA Office of Research and Development; EPA/600/R-090/052F, https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=236252 [accessed 13 July 2017]. [Google Scholar]
- U.S. EPA. 2013. “Final Report: Integrated Science Assessment for Lead.” EPA/600/R-10/075F. Washington, DC:U.S. Environmental Protection Agency. [Google Scholar]
- U.S. EPA. 2016a. Lead and Copper Rule Revisions White Paper, USEPA Office of Water, October 2016. https://www.epa.gov/sites/production/files/2016-10/documents/508_lcr_revisions_white_paper_final_10.26.16.pdf [accessed 6 July 2017].
- U.S. EPA. 2016b. Review of the National Ambient Air Quality Standards for Lead, Final Rule. Federal Register 81(201):71906–71943. [Google Scholar]
- U.S. EPA. 2016c. America's Children and the Environment. EPA 240-R-13-001,2013, updated Feb. 2016. Third edition, https://www.epa.gov/sites/production/files/2015-06/documents/ace3_2013.pdf [accessed 12 July 2017].
- U.S. FDA (U.S. Food and Drug Administration). 2014. Total Diet Study Data 2007–2013. FDA-CSFAN data, https://www.fda.gov/downloads/Food/FoodScienceResearch/TotalDietStudy/UCM184301.pdf [accessed 12 July 2017].
- Versar, Inc. 2016. Work in Progress Peer Consult on EPA’s Multimedia Exposure Analysis to Inform a Public Health-Based Value for Lead in Drinking Water. https://cfpub.epa.gov/si/si_public_record_report.cfm?direntryid=335168 [accessed 6 July 2017].
- von Lindern I, Spalinger S, Stifelman ML, Stanek LW, Bartrem C. 2016. Estimating children's soil/dust ingestion rates through retrospective analyses of blood lead biomonitoring from the Bunker Hill superfund site in Idaho. Environ Health Perspect 124(9):1462–1470, PMID: 26745545, 10.1289/ehp.1510144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PD, Van Leeuwen P, Davis BD, Maddaloni M, Hogan KA, Marcus AH, et al. 1998. The conceptual structure of the integrated exposure uptake biokinetic model for lead in children. Environ Health Perspect 106(suppl 6):1513–1530, PMID: 9860910, 10.1289/ehp.98106s61513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Zartarian VG, Ozkaynak H, Dang W, Glen G, Smith L, et al. 2006. A probabilistic arsenic exposure assessment for children who contact chromated copper arsenate (CCA)-treated playsets and decks, Part 2: Sensitivity and uncertainty analyses. Risk Anal 26(2):533–541, 10.1111/j.1539-6924.2006.00748.x. [DOI] [PubMed] [Google Scholar]
- Xue J, Liu SV, Zartarian VG, Geller AM, Schultz BD. 2014a. Analysis of NHANES measured blood PCBs in the general US population and application of SHEDS model to identify key exposure factors. J Expo Sci Environ Epidemiol 24(6):615–621, PMID: 24424407, 10.1038/jes.2013.91. [DOI] [PubMed] [Google Scholar]
- Xue J, Zartarian V, Tornero-Velez R, Tulve NS. 2014b. EPA's SHEDS-multimedia model: Children's cumulative pyrethroid exposure estimates and evaluation against NHANES biomarker data. Environ Int 73:304–311, PMID: 25192887, 10.1016/j.envint.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Xue J, Zartarian V, Wang SW, Liu SV, Georgopoulos P. 2010. Probabilistic modeling of dietary arsenic exposure and dose and evaluation with 2003-2004 NHANES Data. Environ Health Perspect 118(3):345–350, PMID: 20194069, 10.1289/ehp.0901205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Zartarian VG, Liu SV, Geller AM. 2012. Methyl mercury exposure from fish consumption in vulnerable racial/ethnic populations: probabilistic SHEDS-Dietary model analyses using 1999-2006 NHANES and 1990-2002 TDS data. Sci Tot Environ 414:373–379, PMID: 22119327, 10.1016/j.scitotenv.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Zahran S, Laidlaw MAS, McElmurry SP, Filippelli GM, Taylor M. 2013. Linking source and effect: resuspended soil lead, air lead, and children’s blood lead levels in Detroit, Michigan. Environ Sci Technol 47(6):2839–2845, PMID: 23428083, 10.1021/es303854c. [DOI] [PubMed] [Google Scholar]
- Zartarian V, Xue J, Ozkaynak H, Dang W, Glen G, Smith L, et al. 2006. A probabilistic arsenic exposure assessment for children who contact CCA-treated playsets and decks, Part 1: model methodology, variability results, and model evaluation. Risk Anal 26(2):515–531, PMID: 16573637, 10.1111/j.1539-6924.2006.00747.x. [DOI] [PubMed] [Google Scholar]
- Zartarian VG, Xue J, Glen G, Smith L, Tulve NS, Tornero-Velez R. 2012. Quantifying children's aggregate (dietary and residential) exposure and dose to permethrin: application and evaluation of EPA's probabilistic SHEDS-Multimedia model. J Expo Sci Environ Epidemiol 22(3):267–273, PMID: 22434114, 10.1038/jes.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.