Abstract
Background:
The prevalence of obesity is increasing in all countries, becoming a substantial public health concern worldwide. Increasing evidence has associated obesity with persistent pollutants such as the pesticide DDT and its metabolite p,p′-DDE.
Objectives:
Our objective was to systematically review the literature on the association between exposure to the pesticide DDT and its metabolites and obesity to develop hazard identification conclusions.
Methods:
We applied a systematic review-based strategy to identify and integrate evidence from epidemiological, in vivo, and in vitro studies. The evidence from prospective epidemiological studies was quantitatively synthesized by meta-analysis. We rated the body of evidence and integrated the streams of evidence to systematically develop hazard identification conclusions.
Results:
We identified seven epidemiological studies reporting prospective associations between exposure to p,p′-DDE and adiposity assessed by body mass index (BMI) z-score. The results from the meta-analysis revealed positive associations between exposure to p,p′-DDE and BMI z-score ( BMI z-score (95% CI: 0.01, 0.25) per log increase of p,p′-DDE). Two studies constituted the primary in vivo evidence. Both studies reported positive associations between exposure to p,p′-DDT and increased adiposity in rodents. We identified 19 in vivo studies and 7 in vitro studies that supported the biological plausibility of the obesogenic effects of p,p′-DDT and p,p′-DDE.
Conclusions:
We classified p,p′-DDT and p,p′-DDE as “presumed” to be obesogenic for humans, based on a moderate level of primary human evidence, a moderate level of primary in vivo evidence, and a moderate level of supporting evidence from in vivo and in vitro studies. https://doi.org/10.1289/EHP527
Introduction
The Obesity Society defines obesity as a disease characterized by an excess of body fat, either total body fat or a particular depot of body fat, which increases the likelihood of comorbidities such as diabetes, hypertension, coronary heart disease, stroke, some cancers, obstructive sleep apnea, or osteoarthritis (Allison et al. 2008; Arnold et al. 2015; Jokinen 2015). Obesity has been increasing in all countries, with prevalence doubling during the past three decades to become a substantial public health concern worldwide (Ogden et al. 2014; WHO 2014). Among children and adolescents, the prevalence of obesity follows similar time trends and those akin comorbidities are also diagnosed at early ages (I’Allemand et al. 2008).
Excess caloric consumption and sedentary behavior are some of the risk factors traditionally identified as the main promoters of obesity and overweight. These risks alone do not explain the increased body weight and odds of obesity have also been observed among primates and rodents serving as experimental controls, feral rodents, and domestic dogs and cats across recent decades in the United States (Klimentidis et al. 2011). Instead the complex etiology of this condition involves multiple interrelated causes, such as genetic, social, and environmental factors (Speakman and O’Rahilly 2012; WHO 2014). Some environmental pollutants, including lipophilic persistent organic pollutants, have been associated with an increased risk of overweight and obesity in epidemiological and experimental studies (Lee et al. 2014; Taylor et al. 2013; Thayer et al. 2012). This evidence supports the “obesogen” hypothesis, which predicts that some xenobiotic chemicals “inappropriately regulate lipid metabolism and adipogenesis to promote obesity” (Grün and Blumberg 2006). Extensive data in support of this hypothesis illustrates that the developmental period is a vulnerable window during which transient environmental exposures may inappropriately regulate energy balance or adiposity over the long term (Grün and Blumberg 2006; La Merrill and Birnbaum 2011).
The body of evidence for obesogenic effects of the pesticide dichlorodiphenyltrichloroethane (DDT) and its metabolite dichlorodiphenyldichloroethylene (DDE) has increased notably in the last decade, with a particular focus on exposure during prenatal development. Technical DDT is a persistent organic pesticide mixture of three isoforms, p,p′-DDT, o,p′-DDT, and p,p′-DDD. In the present paper we use the term DDTs to identify the molecular family including these DDT isoforms and their metabolites (e.g., p,p′-DDE). The commercial formulation was widely used for the control of disease (e.g., malaria, typhus) vectors in most countries from the mid-1940s to the late 20th century. DDT is still manufactured in India for control of malaria primarily in India and Africa, where the quantity used for vector control (71% of total) has not changed substantially since the Stockholm Convention restricted its use (ATSDR 2002; Rogan and Chen 2005; UNEP 2010). Moreover, due to the extremely high persistence and lipophilicity of DDTs, internal exposure to DDTs remains ubiquitous in many countries decades after the ban was enforced (Rogan and Chen 2005; Smith 1999).
DDTs are listed by the California Environmental Protection Agency (CalEPA) as causing developmental and reproductive toxicity and have recently been classified by the International Agency for Research on Cancer (IARC) as probably carcinogenic to humans (Cal/EPA-OEHHA 2016; Loomis et al. 2015), yet no study of its obesogenic effects has attempted to deliver hazard identification conclusions by means of a systematic approach to integrate all the evidence. The advantages of applying a Grading of Recommendations Assessment, Development, and Evaluation (GRADE) working group approach has been extensively demonstrated in fields such as clinical medicine and public health, and some recent studies have depicted the benefits of its implementation in environmental health assessment to increase the transparency, rigor, and reproducibility on the decision-making process (Lam et al. 2014; Morgan et al. 2016; Sheehan and Lam 2015; Woodruff and Sutton 2014). Thus, the main objective of this study was to systematically review and integrate the available literature on the association between exposure to the pesticide DDT and obesity to deliver hazard identification conclusions.
Materials and Methods
We applied a systematic review–based strategy to evaluate and integrate evidence from epidemiological, in vivo, and in vitro studies. The methodological approach is based on the National Toxicology Program Office of Health Assessment and Translation’s (NTP/OHAT) Handbook for Conducting a Literature-Based Health Assessment with support of the Navigation Guide, both of which provide a standardized methodology to implement the GRADE approach to environmental health assessments (OHAT 2015a; Rooney et al. 2014). We followed a pre-specified protocol (see “1. SYSTEMATIC REVIEW PROTOCOL” in the Supplemental Material) that was slightly modified throughout an iterative process to refine the integrated systematic review seeking to answer the study question.
In terms of logistics, the NTP/OHAT Handbook considers two valid approaches depending on the size and complexity of the project: the main review maybe either a) independently conducted by two members of the review team; or b) conducted by one member of the review team, with a second member of the team confirming the exclusion determination of the first reviewer. Accordingly, we implemented the second approach, where the screening, data extraction, and data synthesis process were performed by one reviewer (G.C.-S.) after checking the reproducibility, reliability and validity of outcomes by means of a full-duplicated pilot trial where two reviewers (G.C.-S. and M.A.L.) performed the entire process in a subsample of studies and compared the outcomes. Results from the pilot trial demonstrated that no improvement of accuracy and reliability nor reduction of errors were observed when we compared the results from both reviewers. Discrepancies were discussed with a third reviewer (A.G.S.) and external expert advisors. The confidence on the body of evidence rating was performed through a panel discussion with the presence and final agreement of the reviewers.
Study Question and Eligibility Criteria
We formulated the search question: “Does exposure to DDT increase obesity in humans?” Accordingly, we defined the eligibility criteria for the key elements (population, exposure, comparators, and outcomes; PECO) summarized in the PECO statement (Table 1).
Table 1.
PECO statement.
| Study type | Population | Exposure | Comparators | Outcomes |
|---|---|---|---|---|
| Epidemiological studies | Humans studied prospectively without restrictions on country, race, religion, sex. | Exposure to DDT and derivatives or isoforms based on administered dose or concentrations, environmental measures or indirect measures. The exposure must be measured individually using direct validated biomonitoring methods. We excluded studies to assess the therapeutic use of o,p′-DDD isoform, commercially known as mitotane or lysodren. | Reference groups of population exposed at lower levels of DDTs than the rest of population groups. | Primary outcome: body mass index (BMI) and z-score, overweight, and obesity. |
| All ages and/or life-stage at exposure or outcome assessment were included with exception of newborn (birth outcomes were excluded). | ||||
| In vivo studies | Any animal model, sex, age, lifestage at exposure or outcome assessment. | Exposure to all types of DDT and derivatives or isoforms and their mixtures, including all ranges of concentrations, duration, and routes of exposure. | Experimental animals receiving vehicle-only treatment. | Primary outcome: adiposity (e.g., relative or absolute weight, DXA, EchoMRI). |
| We excluded studies including DDT in mixtures with other pollutants. | Secondary outcomes: dyslipidemia, abnormal lipids, other markers of metabolic homeostasis, energy balance. | |||
| In vitro studies | Any cell lines and/or in vitro procedures. | Exposure to all types of DDT and derivatives or isoforms and mixtures, including all ranges of concentrations, duration, and routes of exposure. | Cells receiving vehicle-only treatment. | Adipogenic differentiation, gene expression of metabolic regulators, adipokines. |
| We excluded studies including DDT in mixtures with other pollutants. |
We initially included human prospective studies reporting associations between DDTs and health outcomes related to increased adiposity, overweight, and obesity, considering continuous body mass index (BMI) and its z-score (BMI-z) as a primary outcome. The preferred choice of BMI-z and BMI among clinicians and their extensive use in epidemiology prompted us to choose these outcomes as primary. Cross-sectional studies were excluded to avoid potential reverse causality that can result from the effect of adiposity on circulating lipophilic chemical levels (La Merrill et al. 2013). The metabolite p,p′-DDE was considered the major biomarker of exposure to DDT given its high occurrence, but we also explored the associations with other isoforms.
We retained in vivo studies reporting associations between DDTs (excluding mixtures with other pollutants) and adiposity as the main stream of evidence. The use of crude body weight has limited applicability to characterize obesity in animal models if adiposity or other related outcomes are not measured, and risk of misclassification has already been demonstrated (Nascimento et al. 2008; Woods et al. 2003).
Based on a preliminary literature search, we anticipated a limited number of studies addressing our primary research question with animals, and expanded the evidence with health outcomes directly related to adiposity. The supporting body of evidence included in vivo studies detailing associations between DDTs and energy imbalance; on the basis that an imbalance between energy intake and energy expenditure is considered the primary etiology for excess fat accumulation (Drenowatz 2015; Martinez 2000), measurements of thermogenesis and energy expenditure were considered directly applicable. We considered abnormal lipids (circulating and hepatic) as additional supporting outcomes because dyslipidemia is a principal metabolic comorbidity associated with obesity (Bays et al. 2013). Adipokines were also considered secondary to adiposity because of the association of adiponectin with adipocyte differentiation and the proportional relationship between circulating levels of leptin and fat mass (Stern et al. 2016).
As depicted in the NTP/OHAT framework, we also considered supporting evidence from in vitro studies that addressed mechanisms underlying the causes of obesity. Among in vitro studies, we considered enhanced adipogenic differentiation of cells, including lipid, protein, and RNA changes associated with this process. Additionally, we considered the adipokines as reliable markers of in vitro adipocyte expansion for their high association with differentiation and regulatory role on lipid homeostasis (Fu et al. 2005; Stern et al. 2016).
Concerning the publication type, we only considered reports that contained original data and were peer-reviewed, thus excluding reviews. All publication dates were considered and articles not written in English were excluded. Conference papers were excluded.
Search Strategy
The search string (see “1.3.2. SEARCH” in the Supplemental Material) was applied to three electronic literature databases [MEDLINE (http://www.ncbi.nlm.nih.gov/pubmed), EMBASE (https://www.embase.com), and Scopus (http://www.scopus.com)] on 23 March 2015, and a follow-up search was performed on 8 January 2016. The search strategy was developed to identify human, in vivo, and in vitro studies reporting original data on the associations of DDTs with obesity given that some outcomes of interest here may be indexed under co-morbidities of obesity such as diabetes, dyslipidemia, and metabolic syndrome. We also searched for measures of energy imbalance, and adipogenic differentiation, as well as protein and RNA measures associated with these processes, as indicators of mechanisms underlying the potential causes of obesity. The search was run without filters and without limitation on publication date. The records were pooled in Endnote X7 and screened manually to eliminate duplicates. The resulting library was uploaded to DistillerSR online software (Evidence Partners) to carry out the selection of studies.
Selection of Studies
The selection was performed in DistillerSR (Evidence Partners) software in a two-step process: During the first step, the studies were screened based on the title and abstract. The included and doubtful studies were screened in a second step, using the full-text to conclude if the studies meet the inclusion criteria.
Data Extraction
The data was extracted using data forms specifically designed for human, in vivo, and in vitro studies (see “5. DATA FORMS” in the Supplemental Material) in DistillerSR and exported to Excel. The data was extracted by a main reviewer (G.C.-S.) and checked by an additional external reviewer (M.A.L.) to ensure accuracy. Discrepancies and controversial issues were discussed by the reviewer team, and external advice was requested when it was required. We contacted the authors to request additional data when it was required.
Data Synthesis and Meta-Analysis
We synthesized the data from human epidemiological studies by means of meta-analysis of effect estimates. Data from in vivo and in vitro studies were synthesized and displayed to summarize the direction of the effect while comparing of doses among studies using forest plots adapted from Thayer et al. (2012). The effect estimates initially considered for pooling the data were beta regression coefficients () for continuous outcomes, and risk ratios (RR) and/or odds ratios (OR) for dichotomous outcomes. However, the different methodological approaches, metrics, and outcomes used in the different studies only allowed pooling estimates for continuous models with BMI-z as the dependent variable and p,p′-DDE as the independent variable with the corresponding covariables. The effect sizes were summarized using the inverse variance method for random-effects meta-analysis (DerSimonian and Laird 1986). Studies also provided the measures of variance of the effect size, such as confidence intervals (CI). Between-study variance in a random-effects meta-analysis was represented by tau squared (). Heterogeneity was assessed with the statistic, which quantifies the heterogeneity and degree of inconsistency among studies. The results were interpreted according Cochrane’s criteria: between 0% and 40%: heterogeneity might not be important; between 30% and 60%: may represent moderate heterogeneity; between 50% and 90%: may represent substantial heterogeneity; and between 75% and 100%: considerable heterogeneity (Higgins and Green 2011). Potential small-study bias was evaluated by funnel plots and Egger’s test (Harbord et al. 2006). The influence of each individual study in a meta-analysis was investigated by omitting each study in turn and reestimating the summary estimate.
Rating and Integrating the Evidence for Hazard Identification
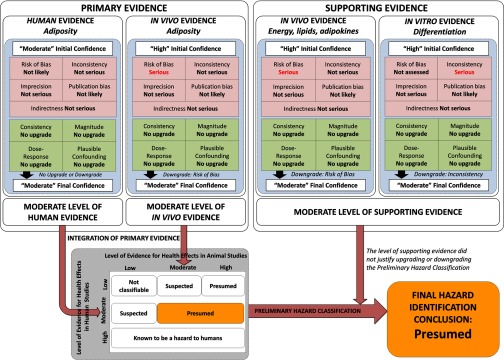
We applied the NTP/OHAT framework (OHAT 2015a), based on the GRADE approach (Guyatt et al. 2011a), to rate the confidence in the body of evidence, translate to a level of evidence, and integrate the different streams of evidence to deliver the hazard identification conclusions. The overall work-flow process is illustrated in Figure 1, considering two primary bodies of evidence from human studies (increased BMI-z) and animal studies (increased adiposity). Two additional bodies of evidence (secondary outcomes from in vivo and in vitro studies) were included as complementary information to support the associations and its biological plausibility. The confidence and level of evidence was evaluated independently for each body of evidence (e.g., human primary outcomes, in vivo primary outcomes, in vivo secondary outcomes, and in vitro secondary outcomes), establishing an initial confidence rating based on key study design features. The body of evidence from in vivo and in vitro studies were initially rated with high confidence because they control the exposure levels, which in turn are prior to the outcome, the outcome measure is collected at the individual level, and a comparison group equal in all conditions save the exposure is always used. In contrast, the body of evidence composed of human prospective studies was initially classified as moderate confidence because observational studies fail to control the exposure levels or provide a comparison group known to be absolutely free of all sources of confounding (compared with randomized controlled trials). Despite the limitations related to the uncontrolled exposure, the prospective observational studies are considered reliable approaches to establish causative associations between pollutant exposures and disease. Moreover, given the ethical limitations on carrying out controlled trials with pollutants in humans, this epidemiological design is considered the most feasible and reliable approach (Johnson et al. 2014).
Figure 1.
Flow chart for rating the quality and integration of evidence from human and animal evidence, and the judgments of primary and supporting evidence for hazard identification conclusions.
Subsequently, these initial ratings were subjected to a sequential process considering those factors that may affect (upgrading or downgrading) the confidence, including the risk of bias, imprecision, publication bias, indirectness, magnitude, dose response, plausible confounding, and consistency across populations and models (Figure 1). The risk of bias was evaluated by means of risk of bias tools specifically designed for human epidemiological studies and animal studies and slightly adapted for DDTs and obesity outcomes (Koustas et al. 2014; OHAT 2015b). The rationale for risk of bias rating and results may be found in the Supplemental Material for humans (see “6. INSTRUCTIONS TO ASSESS THE RISK OF BIAS OF HUMAN EPIDEMIOLOGICAL STUDIES” and Tables S10–S17) and animal studies (see “7. INSTRUCTIONS TO ASSESS THE RISK OF BIAS OF IN VIVO STUDIES” and Tables S24–S32). The extended rationale for rating the confidence and integrating the evidence is reported in the protocol (see “1.4. RATING THE BODY OF EVIDENCE” and “1.5. INTEGRATION OF EVIDENCE AND HAZARD IDENTIFICATION CONCLUSIONS” in the Supplemental Material). We did not assess the risk of bias of in vitro studies because of the lack of risk of bias tools or guidance to assess the internal quality; however, we considered the remaining confidence factors (Figure 1) to rate the confidence in the in vitro body of evidence (Rooney et al. 2016). In brief, the NTP/OHAT’s risk of bias tiered approach considers key elements or risk of bias domains to establish the risk of bias classification for each individual study (Tier 1 to 3). Individual studies are classified in the Tier 1 when the key elements are considered as having “definitely low” or “probably low” risk of bias, and classified in the Tier 3 when the key elements are considered as having “definitely high” or “probably high” risk of bias (see “1.4. RATING THE BODY OF EVIDENCE” in the Supplemental Material). In the second level of risk of bias evaluation, the rating of the overall risk of bias in the body of evidence is classified as “not likely,” “serious” or “very serious,” depending on whether most information is gathered from studies classified as Tier 1, 2 or 3, respectively (see Table S2). The confidence rating process was completed considering the upgrading and downgrading factors and balanced together to deliver a final rating for each of the four bodies of evidence (Figure 1). This final confidence rating for each body of evidence (human, in vivo primary, in vivo supporting, and in vitro supporting) was translated to a level of evidence (low, moderate, or high) for each of the primary evidence streams and for the supporting evidence stream, considering additionally the nature and direction of the effect (“health effect” and “no health effect”).
The two primary bodies of evidence were integrated using the hazard identification scheme to provide a preliminary classification of the obesogen hazard identified for DDTs (“known,” “presumed,” “suspected,” or “not classifiable” hazard for humans; Figure 1). The final level of evidence (low, moderate, or high) from the supporting body of evidence (in vivo secondary outcomes and in vitro secondary outcomes) was considered for its indication that evidence exploring biological plausibility warranted an upgrade or downgrade of the preliminary hazard classification.
Software
The libraries were created in Endnote X7 (Thomson Reuters) and Excel 2010 (Microsoft Windows). The selection and data extraction was managed by the on-line software DistillerSR (Evidence Partners, Ottawa, CAD) and exported to Excel. Statistical meta-analysis was performed with Stata version 14 (StataCorp, College Station, TX, USA).
Results
Study Acquisition
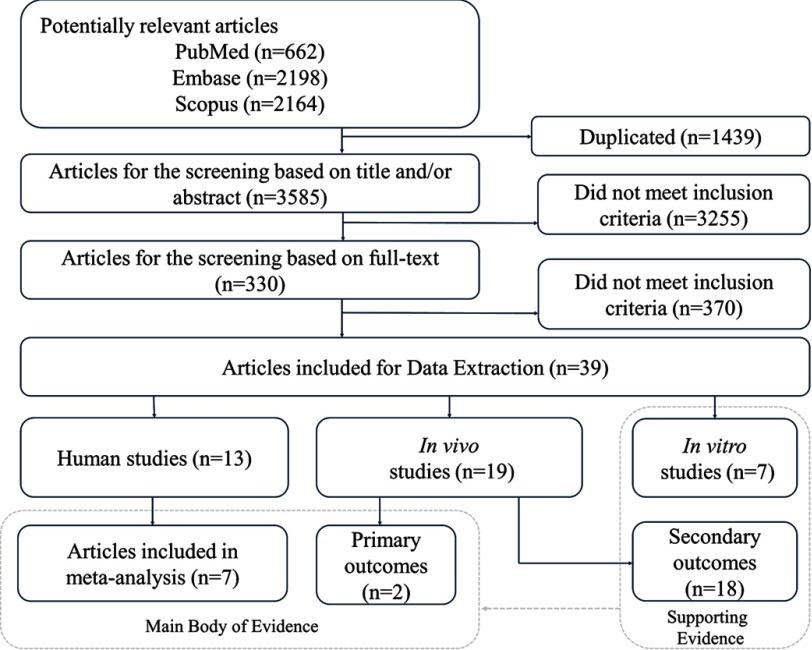
Initially 5,024 articles were identified from PubMed ( articles), Scopus ( articles), and Embase ( articles), which were reduced to 3,585 after manual removal of 1,439 duplicates (Figure 2). After screening the titles and abstracts, we retained 330 records for full-text screening, resulting in 39 full-text peer-reviewed articles retained for data extraction, which comprised 13 human studies, 19 in vivo studies, and 7 in vitro studies. Among the 13 human studies that met the eligibility criteria only 7 studies were retained for quantitative synthesis in the meta-analysis. We were unable to include 6 prospective studies (see Table S18) in the meta-analysis due to heterogeneity introduced by the reported outcomes, for example, beta estimates of BMI, and risk and trend estimates for overweight and obesity. In addition, there was no formal guidance for the qualitative synthesis of those studies in the NTP/OHAT handbook. For space considerations, the study characteristics were placed in the Supplemental Material data for each stream of evidence quantitated here, for example, human studies (see Table S8) and nonhuman studies (see Tables S19–S21).
Figure 2.
Flow chart of the systematic review process.
Evidence from Human Epidemiological Studies
Study characteristics.
The effect estimates initially considered for pooling the data were regression coefficients for continuous outcomes, and risk ratios and/or odds ratios for dichotomous outcomes. However, the paucity of many possible exposure metrics and outcomes used in the studies led us to conduct the meta-analysis on the most prevalently reported exposure and outcome combination: estimates for continuous models with BMI-z as a dependent variable and p,p′-DDE as an independent variable.
The population size varied among the seven included prospective human studies, ranging from 114 (Delvaux et al. 2014) to 788 participants (Cupul-Uicab et al. 2010). Most studies reported the results pooled for males and females, and only three studies provided stratified or independent results for each gender (Cupul-Uicab et al. 2010; Tang-Péronard et al. 2015; Warner et al. 2014). The rates of participation ranged from 36% (Høyer et al. 2014) to 91% (Cupul-Uicab et al. 2010). The cohorts were from the United States (Warner et al. 2014), Spain (Agay-Shay et al. 2015), Belgium (Delvaux et al. 2014), Greece (Vafeiadi et al. 2015), Greenland (Høyer et al. 2014), Poland (Høyer et al. 2014), Ukraine (Høyer et al. 2014), Mexico (Cupul-Uicab et al. 2010), and Denmark (Tang-Péronard et al. 2015).
Health outcome assessment.
The anthropometric measurements were performed by clinicians, primarily on children between 4 and 9 y of age (Agay-Shay et al. 2015; Delvaux et al. 2014; Høyer et al. 2014; Warner et al. 2014), though two studies focused on the early, up to 2.5 y (Cupul-Uicab et al. 2010), or later, 20 y (Tang-Péronard et al. 2015), stages of life. Given the early age of participants in most studies, obesity and/or overweight were often ascertained by means of standardized anthropometric measurements, such as the body mass index (BMI) z-scores. Due to the lack of unified criteria among clinicians, different reference charts and guidelines were used to calculate BMI-z scores, including standards such as the 2000 Centers for Disease Control and Prevention (CDC) Growth Charts (Cupul-Uicab et al. 2010; Warner et al. 2014), the International Obesity Task Force Growth Charts (Vafeiadi et al. 2015), or the British growth reference data (Delvaux et al. 2014).
Exposure assessment.
Most studies evaluated the association of obesity with prenatal exposure to p,p′-DDE in mothers’ sera, yet one study explored the associations with postnatal (at age 8–10 y) exposure to p,p′-DDE in the index children’s serum (Tang-Péronard et al. 2015). Most studies were performed in the framework of national biomonitoring programs, providing external references on the validity and analytical performance of methodological procedures. These methodologies commonly utilized gas chromatography–high resolution mass spectrometry, which is able to detect nearly 100% of p,p′-DDE. The exposure levels varied largely among studies (see Table S9). Among those studies reporting the exposure levels of p,p′-DDE standardized by lipid content, the median concentrations ranged between (Warner et al. 2014) and (Cupul-Uicab et al. 2010). For those studies reporting exposures by their wet-based values, the median exposure levels of p,p′-DDE presented narrower estimates ranging from (Delvaux et al. 2014) to (Vafeiadi et al. 2015). Beyond the absolute differences in the exposure levels between cohorts, especially large differences were noticed between the arbitrary boundaries of exposure and reference groups when comparing the different studies (see Table S9).
Lipid adjustment.
The complex relationships between the levels of DDTs and other lipophilic pollutants, serum lipids, and obesity are not fully understood (La Merrill et al. 2013), and researchers commonly infer assumptions about these relationships to formulate their causal models. The three most common approaches are to a) model the exposure levels in lipid basis (e.g., ratio of pollutant levels by the triglyceride and cholesterol content), b) include the blood lipid content as a covariate in the regression model, or c) use the unadjusted wet-weight values (Li et al. 2013). Some simulation studies revealed that the first approach (ratio chemical exposure by lipids) may bias the estimates compared with the other approaches (Gaskins and Schisterman 2009; Schisterman et al. 2005). However, there is no consensus on which is the best approach to apply in complex scenarios such as the obesogenic effect of DDTs, where a lipophilic compound is causally related with an obesity outcome and circulating lipid levels (Patel et al. 2012). The two main approaches were present among the studies included in the present meta-analysis, with five studies modeling the exposure concentration levels standardized by lipids (lw) as a ratio of p,p′-DDE/serum lipid levels (Agay-Shay et al. 2015; Cupul-Uicab et al. 2010; Høyer et al. 2014; Tang-Péronard et al. 2015; Warner et al. 2014), and two studies modeling the wet-weight (ww) levels of p,p′-DDE (Delvaux et al. 2014; Vafeiadi et al. 2015), while including the lipid content as a covariate (see Table S8).
Confounding bias.
The studies retained for meta-analysis addressed potential confounding bias by adjusting for known confounders in multivariate regression models (see Table S8). Most studies adjusted for maternal BMI, or occasionally for maternal weight and/or height. Most analyses also included adjustment for maternal age, education, parity, and breastfeeding and an indicator of socioeconomic status (race, education, income, social class, and/or socioeconomic index). Birth weight was also included in the model of two studies (Agay-Shay et al. 2015; Vafeiadi et al. 2015). Physical activity and/or diet were adjusted in models of three studies (Agay-Shay et al. 2015; Høyer et al. 2014; Tang-Péronard et al. 2015). Maternal smoking was modeled as a confounder in the majority of studies retained here (Cupul-Uicab et al. 2010; Delvaux et al. 2014; Høyer et al. 2014; Vafeiadi et al. 2015) with the exception of one study in which maternal smoking did not modify the effect estimate (Warner et al. 2014). One study concluded that risk of obesity associated with DDTs would be exacerbated by maternal smoking (Cupul-Uicab et al. 2010). Maternal alcohol consumption was included as a confounder in the regression models of one study (Høyer et al. 2014).
Sex.
The estrogenic effect of o,p′-DDT and the anti-androgenic effects of p,p-DDT and p,p′-DDE emphasize the potential effect modification of sex and most studies anticipated this by adjusting the model by sex (Agay-Shay et al. 2015; Delvaux et al. 2014; Høyer et al. 2014; Vafeiadi et al. 2015) and/or modeling the data stratified by sex after testing the interaction of sex with p,p′-DDE (Tang-Péronard et al. 2015; Warner et al. 2014; Delvaux et al. 2014). However, the interaction results of individual studies demonstrated no consistent sex-specific trends. Whereas two studies indicated males were more at risk of an association between DDTs and obesity measures (Tang-Péronard et al. 2015; Warner et al. 2014), another study indicated females were more at risk of an association between DDTs and obesity measures (Delvaux et al. 2014).
Results from the meta-analysis.
Seven studies reporting associations between blood p,p′-DDE and continuous BMI-z by means of adjusted coefficients were included in the meta-analysis. The associations of BMI-z with the exposure to the other DDTs were by far less evaluated, and the meta-analysis of such subsamples was not feasible. Similarly, other health outcomes were reported (e.g., BMI, waist circumference, overweight, and obesity) by few studies with heterogeneous methodologies, making the meta-analysis underpowered and inaccurate (see Table S18). The pooled coefficients for males and females were selected for all studies with the exception of Tang-Péronard et al. (2015), where a gender interaction was reported and thus was plotted individually. In the case of Delvaux et al. (2014), we detected a typo in the manuscript and contacted the authors who provided the correct estimate ( instead of 0.95 BMI-z, 95% CI: , 0.51). When the studies provided different values for different percentiles or tertiles of p,p′-DDE instead of continuous trends, we selected the highest estimate (worst-case scenario).
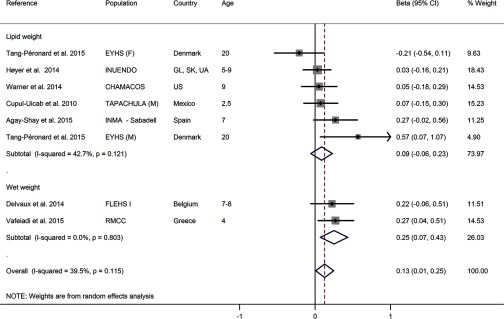
The overall association between exposure to p,p′-DDE and BMI-z was significantly positive with a of 0.13 BMI-z per log increase of p,p′-DDE (95% CI: 0.01, 0.25 BMI-z; studies; Figure 3). The stratified analysis of units (lipid weight vs. wet weight) indicated the associations were on a similar scale across these units and suitable for pooling, supported by the global heterogeneity ( of 39.5%). A sensitivity analysis excluding one study each time showed the confidence intervals overlapped the null in five of the eight possible combinations (see Figure S5). Despite the low number of studies, the funnel plots did not show marked asymmetry and Egger’s test did not reveal statistically significant small-study effects (see Figure S6).
Figure 3.
Forest plot of the association between exposure to p,p′-DDE with BMI-z from human prospective studies, stratified by exposure units (lipid weight and wet weight). The effect size estimate is the adjusted coefficient regression () with 95% confidence intervals (units in BMI z-score per log increase of p,p′-DDE) for combined gender (males and female) unless the strata is specifically reported in the cohort label: (F) females or (M) males. Cohorts: CHAMACOS, Center for the Health Assessment of Mothers and Children of Salinas; EYHS, Danish part of the European Youth Heart Study; FLEHS I, first Flemish Environment and Health Study; RMCC, Rhea Mother–Child Cohort; INMA-Sabadell, Infancia y Medio-Ambiente Child and Environment birth cohort. Countries: GL, Greenland; SK, Warsaw Poland; UA, Ukraine; US, United States. Age: age at outcome assessment.
Confidence in the body of evidence and level of evidence.
The full rationale and results for rating the confidence in the body of evidence are provided in “4.2. RATING THE CONFIDENCE IN THE OF BODY OF EVIDENCE FROM HUMAN STUDIES AND LEVEL OF EVIDENCE FOR HEALTH EFFECT” in the Supplemental Material, and the results of the confidence rating process are summarized in Figure 1 for each stream of evidence considering those upgrading and downgrading factors, as well as the initial and final confidence rating decisions and integration of evidence. We provided a preliminary rating for the confidence with the body of evidence of “moderate” for human studies based on the intrinsic characteristics of observational prospective studies (OHAT 2015a).
We considered risk of bias, unexplained inconsistency, indirectness, imprecision, and publication bias among the downgrading factors (Figure 1). The rationale and results from risk of bias assessment from each individual study are described in Tables S10–S17). The risk of bias domains we most critically considered were confounding bias, performance bias, and detection bias.
We initially considered that confounding bias could be an issue because relevant confounding variables such as physical activity or diet were only included in three of eight models. However, we have not seen any evidence in the experimental literature indicating DDTs cause hyperphagia or sedentary activity (La Merrill 2014; Howell et al. 2015). Indeed, based on a preliminary search of the literature, we generated a directed acyclic graph (DAG) (see Figure S5) to select potential key confounders the maternal BMI, maternal smoking, and sex, which were controlled by all studies. Hence, we did not feel confounding bias was more substantial than allowed for in the penalization of the initial confidence rating to moderate.
We considered that another potential risk of bias was performance bias due to the extended use of single-pollutant models where simultaneous exposure to complex mixtures of xenogenous chemicals was reported by the authors or highly suspected. Only one study addressed this potential performance bias; principal component analysis identified an association of the DDE-containing component with both increased BMI z-score and risk of overweight, but no other components (Agay-Shay et al. 2015). Following the OHAT risk of bias rating tool (2015b), we only penalized studies that did not control for other exposures if the sample population had high exposure; however, only one study population was occupational or acutely contaminated (Warner et al. 2014). From this, we did not find that performance bias was a concerning bias domain.
Detection bias was also discussed because the studies estimated exposure from a single measurement; thus some risk of exposure misclassification could be suspected. Yet given the narrow windows of exposure, we judged the risk of bias to be low due to the high correlation of exposure estimates in biological samples collected serially across the prenatal and neonatal periods (Longnecker et al. 1999). Further, all studies used gas chromatography with mass spectrometry, the gold standard method to assess DDE levels. Overall, we classified most human studies in the Tier 1 of risk bias because we did not suspect bias among the other domains, and the key domains of confounding and detection bias were judged as having definitively or probably low risk of bias, being that the overall risk of bias was considered “not likely.”
The between-studies low heterogeneity ( 39.5%) and variance () were not considered concerning enough to downgrade the confidence for unexplained inconsistency. Some inconsistencies may be explained by the sex-stratified results of Tang-Péronard et al. (2015), the only study that reported a statistically significant interaction of exposure effects with sex effects. We did not penalize the confidence rating concerning indirectness because the human studies prospectively assessed obesity and adiposity outcomes associated with exposures to DDTs. Despite large differences between the exposure groups and reference levels across studies, the narrow confidence intervals of the meta-estimates indicated no evidence for a lack of precision on the meta-estimates; hence we concluded unexplained imprecision was not serious enough to downgrade. The funnel plots did not show asymmetry; however, considering the absence of private funding or conflict of interest, as well as, the lack of potentially unpublished studies (e.g., conference abstracts, gray literature), we determined publication bias was not serious.
Another source of concern was the potential selection bias associated with the exclusion of six studies (see Table S18) from the meta-analysis solely because their outcome metrics differed from the seven included studies. Among the six different studies reporting results from five different cohorts, results from four cohorts indicated some positive associations between p,p′-DDE and measures of adiposity in both children and adults (Michigan fisheaters, Faroe Islands, PIVUS, and AMICS-INMA-Menorca), whereas null associations were reported in one cohort (CPP). Overall we had no reason to suspect that those results could threaten the confidence in the body of evidence included in the present study or reveal new insights on the direction and magnitude of our estimates.
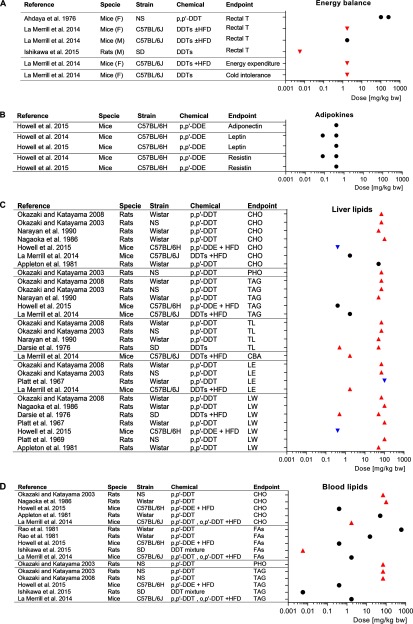
We also considered those factors that may upgrade the confidence, such as the magnitude, dose response, residual confounding, and consistency across populations (Figure 1). We concluded that the magnitude of the effect was too modest to justify upgrading that confidence rating (see Figure S5). The presence and shape of a dose–response trend was inconsistent across studies (see Table S9). This may reflect a nonmonotonic trend and/or wide variability in the levels of DDTs used to define boundaries of reference and exposure groups across studies. We were concerned about potential residual confounding caused by lipid adjustment of exposure levels given that lipid adjusting the levels of contaminants has been demonstrated to provide more biased results than those models using wet values and including the lipid concentration as a covariate in the model (Gaskins and Schisterman 2009; Schisterman et al. 2005). However, if we consider the in vivo results further expanded upon below (Figure 4), we see inconclusive support of the hypothesis that abnormal blood lipids are in the causal pathway between DDTs and obesity (see Figure S7). For instance, only half of the experimental evidence available demonstrated positive relationships between DDTs and blood triglycerides and cholesterol (Figure 4A) in spite of the consistent lipid disruption in liver (Figure 4B); for these reasons, we did not upgrade the residual confounding or consistency.
Figure 4.
Forest plot of the associations between exposure to p,p′-DDT and p,p′-DDE and (A) energy balance, (B) circulating adipokines, (C) abnormal liver lipids, and (D) abnormal blood lipids from in vivo studies. Symbols: upward-pointing triangle, increase effect; downward-pointing triangle, decrease effect; circle, no statistical effect. Upward-pointing triangle means adversity of the health effect. Abbreviations: CBA, conjugated bile acids; CHO, cholesterol; DPC, day post-coitus; F, females; FAs, fatty acids; GD, gestational day; HFD, high-fat diet; LE, lipogenic enzymes; LW, liver weight; M, males; NS, no specified; PG, parental generation; PHO, phospholipids; PND, postnatal day; SD, Sprague-Dawley; T, temperature; TAG, triacylglycerol; TL, total lipids; TnG, transgenerational. Doses were approximated to the daily body weight basis using the conversion factors specified in Table S22.
After balancing the upgrading and downgrading factors, the final rating of the confidence with the body of evidence was finally appraised to be “moderate.” The results supported the direction of the association towards the “health effect,” thus we translated the confidence into a “moderate” level of evidence for the associations between exposure to p,p′-DDE and increased adiposity in humans (Figure 1).
Evidence from Primary in Vivo Studies
We retained two studies evaluating the associations between DDTs and adiposity (La Merrill et al. 2014; Skinner et al. 2013) as a primary body of evidence from in vivo studies (Table S19). Due to the low number of studies, we synthetized the results qualitatively instead of using meta-analysis.
One study evaluated adiposity longitudinally by EchoMRI™ after perinatal exposure to p,p′-DDT and o,p′-DDT ( bw, from gestational day 11.5 to postnatal day 5). Perinatal DDTs caused a transient increase in body and fat mass for several months in young adult female but not male mice, and no differences in female or male body composition when later fed high-fat diet. The effects of perinatal DDTs on adiposity were further explained by reductions in thermogenesis and energy expenditure. The metabolic disruption by perinatal DDTs was accompanied by dyslipidemia, glucose intolerance, hyperinsulinemia, and altered bile acid metabolism (La Merrill et al. 2014).
A transgenerational study performed with adult rats exposed to p,p′-DDT (25 and body weight) followed up obesity status in the subsequent three generations. The classification of obesity was established by an increase of body weight and abdominal adiposity. Among DDT-exposed lineages, only male and female offspring from the generation and male offspring from the generation had an increased prevalence of obesity. The authors concluded that the etiology of obesity in DDT-lineage rats may be in part due to environmentally induced transgenerational inheritance of differential DNA methylation regions in sperm (Skinner et al. 2013).
Confidence in the body of evidence and level of evidence.
The initial rating of the confidence in the body of primary evidence of experimental animal data was considered to be “high” (Figure 1; see also “4.3. RATING THE CONFIDENCE IN THE BODY OF PRIMARY EVIDENCE FROM IN VIVO STUDIES AND LEVEL OF EVIDENCE FOR HEALTH EFFECT” in the Supplemental Material), comparable to human randomized controlled trials where the exposure levels were controlled at individual level prior to the health outcomes, and using suitable control groups. As with the human evidence stream, we evaluated those factors that could modify this preliminary classification. Both studies were rated at low or probably low risk of bias for most of bias domains, which included their proper considerations of litter effects. The exemption was that one study was classified as probably high risk of bias in the sequence generation domain due to the lack of randomization of treatments (see Table S24). We judged the overall risk of bias to be of the “serious” risk of bias rating given that this rating has the criteria that most information was from Tier 1 and 2 studies, although plausible biases raise some doubt about the results (Figure 1; see also Table S2). The results from both experimental studies had some relevant inconsistencies. For instance, Skinner et al. (2013) observed obesity only in the third and fourth generations, whereas La Merrill et al. (2014) reported increased adiposity in the first generation. Inconsistencies in the methodological approaches (e.g., timing, dose, and route of exposure; rodent model) may explain these disparities; however, because there are only two studies, we concluded consistency is unknown in accordance with NTP/OHAT guidance (OHAT 2015a) and thus we did not downgrade due to inconsistency. According GRADE guidelines, downgrading by indirectness may be only justified when there is some compelling reason to suspect the different biology could modify the magnitude of the effect (Guyatt et al. 2011b). Both studies used rodent models (C57BL/6J mice and Hsd:Sprague-Dawley rats), which are considered directly applicable to human obesity, thus we rated indirectness as “not serious.” An acceptable number of animals per treatment and controls were used in both studies (, La Merrill et al. 2014; , Skinner et al. 2013), providing accurate estimates with narrow error bars; accordingly, we decided imprecision was not serious. Despite the limited number of studies, we judged publication bias was not serious enough to downgrade the confidence.
We further considered factors dictating an upgrade the initial rating of the confidence, but we concluded that the limited magnitude of the effect, the absence of dose-response analysis, and the absence of plausible residual confounding would not justify a decision to upgrade the confidence (see “4.3. RATING THE CONFIDENCE IN THE BODY OF PRIMARY EVIDENCE FROM IN VIVO STUDIES AND LEVEL OF EVIDENCE FOR HEALTH EFFECT” in the Supplemental Material). Despite the consistent direction of results in two mammalian species, and in turn with the human epidemiological results, we did not upgrade for consistency because of the limited number of available studies to conclude such a relationship.
Overall, we considered the main body of evidence from animal studies, assembled by only two studies, as having “serious” risk of bias. Thus, we downgraded the initial confidence, finally rating the confidence in the body of evidence as “moderate.” The nature or direction of the effect was to a health effect, thus the confidence was translated to a “moderate” level of evidence of obesogenic effects of DDTs in in vivo studies (Figure 1).
Evaluating the Support for Biological Plausibility: in Vivo Studies
We considered measures of energy imbalance such us body temperature and cold intolerance, as well as associated protein and RNA measures, as indicators of mechanisms underlying the potential causes of obesity. We considered tissue lipid levels as secondary outcomes in the supporting body of evidence as they are merely correlated with an obese state. Six studies were retained because of their reported associations with end points closely related to metabolic homeostasis such as energy imbalance and adipokines (Figure 4A,B). We retained 15 in vivo studies reporting associations between DDTs and abnormal lipids (Figure 4C,D), which is one of the main metabolic comorbidities associated with excessive body fat (Bays et al. 2013; Klop et al. 2013). Some studies simultaneously reported evidence of different outcomes giving a total count of 19 studies (Tables S20 and S21).
Markers of metabolic disruption.
We considered impaired energy expenditure and changes in circulating adipokines as markers of metabolic disruption associated with obesity (Figure 4A,B). Two of three studies found that exposure to DDTs decreased rectal temperature, a surrogate marker of thermogenesis that contributes 60–90% to total energy expenditure (Landsberg 2012), in two rodent species. Perinatal exposure to DDTs ( bw) decreased body temperature, energy expenditure and cold tolerance of female mice (La Merrill et al. 2014), consistent with risk of obesity. Those findings where mechanistically supported by the reduction of brown adipose mRNA expression of Ppargc1a, master regulator of thermogenesis, and Dio2, an upstream mediator of thermogenesis. In agreement with these results, obese Sprague-Dawley rats exposed to DDT ( body weight/d) exhibited lower core body temperature compared with the control during HFD feeding and subsequent 60% caloric restriction periods (Ishikawa et al. 2015). However in the third study, mice with acutely toxic DDT exposure () had no change in rectal temperature (Ahdaya et al. 1976). The exposure of C57BL/6H mice to p,p′-DDE at 0.4 and for 5 or 13 wk had no effect on the serum levels of adipokines closely related with energy balance such as leptin, adiponectin, or resistin (Howell et al. 2014; Howell et al. 2015).
Abnormal lipids.
We defined abnormal lipids as elevated lipids (cholesterol, triglycerides, or fatty acids) in blood or liver, increased liver weight (as surrogate measurement of hepatic steatosis), and increased activity of liver lipogenic enzymes. An overall positive association was seen between a wide range of DDTs and abnormal lipids consistently in the livers of rats and mice, where DDTs increased hepatic lipids, total liver weights, and lipogenic enzymes (Figure 4C). The majority of conflicting findings were clustered in blood (9 null of 16 data points in blood; Figure 4D), suggesting weaker evidence associating DDTs with dyslipidemia, particularly blood fatty acids. However, fatty acid composition and distribution in adipose tissue from Wistar rats was disrupted after oral exposure to p,p′-DDE () for 12 wk (Rodríguez-Alcalá et al. 2015). Lipid homeostasis was also disrupted by experimental treatment of DDTs in two nonmammalian systems, sailfin mollies and Japanese quail. For instance, the whole-body levels of total lipids and triglycerides in sailfin mollies were reduced at the highest exposure levels of o,p′-DDT (Benton et al. 1994). The DDT isoform 1,1-di-p-chlorophenyl-2 chloroethylene (DDMU) increased the liver weights and hepatic triglycerides of Japanese quail (Westlake et al. 1979).
Confidence in the body of evidence and level of evidence.
We established a preliminary rating of “high” confidence with the body of evidence based on the features of animal study design (Figure 1; see also “4.4. RATING THE CONFIDENCE IN THE BODY OF SUPPORTING EVIDENCE FROM IN VIVO STUDIES”). Considering most studies have “probably high” risk of bias for randomization, concealment, and blinding when these methods are not reported, we downgraded the confidence based on risk of bias (Figure 1). The central role of energy imbalance in causing obesity and the close relationship of lipid abnormalities with adiposity were the main rationale to judge the directness sufficient. We did not consider imprecision serious and had no reason to suspect publication bias.
The available evidence for the increased hepatic lipids and impaired thermogenesis by DDTs was consistent across two mammalian species (Figure 4) and with the expectations from the positive meta-estimates of the associations of p,p′-DDE with adiposity in human studies (Figure 3). However, because of the lack of consistency of blood lipid disruption and absence of effects on adipokine levels, we did not upgrade due to consistency. There was no justification for upgrading confidence based on the modest magnitude of effects in most studies, the infrequent assessment of dose response, and unlikelihood of residual confounding. After assessing the different factors that may affect the confidence, we modified the initial confidence rating of “high” to “moderate” as the final rating of confidence and level of evidence for in vivo supporting evidence considering the direction of the effect to the presence of “health effects” (Figure 1).
Evaluating the Support for Biological Plausibility: in Vitro Studies
Typical phenotypic changes during pathological fat expansion may include increased adipogenesis (number of differentiated cells and/or quantity of fat accumulation), disruption of lipid metabolism (lipolytic and lipogenic processes) and disruption of adipokines involved in energy balance (e.g., leptin and adiponectin) (Bays et al. 2013). The most reported mechanism of action for obesogen compounds involve the disruption of , which is considered a master regulator of adipogenesis and lipid homeostasis (Gore et al. 2015). Reflecting on these prior observations, we considered measures of enhanced adipogenic differentiation—as well as lipid, protein, and RNA measures associated with this process—as those mechanisms evidencing a potential cause of obesity.
Adipocyte differentiation and lipogenesis.
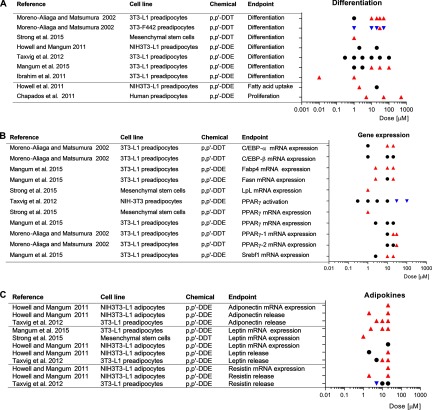
We retained seven references reporting associations between exposure to DDTs and outcomes related to adiposity using in vitro models (see Table S23). Exposure to p,p′-DDT consistently increased the adipogenic differentiation (Figure 5A) of 3T3-L1 preadipocytes (Moreno-Aliaga and Matsumura 2002) and mesenchymal stem cells (MSCs) (Strong et al. 2015). The presence of p,p′-DDT also initially accelerated the differentiation process of 3T3-F442A cells; however, their differentiation was not complete (Moreno-Aliaga and Matsumura 2002). The adipogenic effect of p,p′-DDT may relate to its estrogen receptor agonism (Nelson 1974), given that adipogenic differentiation of MSCs was strongly inhibited by the estrogen receptor inhibitor fulvestrant (Strong et al. 2015).
Figure 5.
Forest plot of the association between exposure to p,p′-DDT and p,p′-DDE and (A) adipogenic differentiation, (B) expression of metabolic regulators, and (C) adipokines from in vitro studies. Symbols: upward-pointing triangle, increase effect; downward-pointing triangle, decrease effect; circle, no statistical effect. Upward-pointing triangle means adversity of the health effect. Abbreviations: ATGL, adipose triglyceride lipase; CEBP enhancer-binding protein; Fabp4, Fatty acid binding protein 4; Fasn, fatty acid synthase; Insig1, Insulin-induced gene-1; LpL, lipoprotein lipase; PPAR, peroxisome proliferator-activated receptor; Srebf1, sterol regulatory element-binding protein 1c.
Unlike p,p′-DDT, exposure to p,p′-DDE showed inconsistent effects on adipogenesis (Figure 5A). Although one study found insignificant effects on adipogenic differentiation up to p,p′-DDE (Taxvig et al. 2012), two studies reported significant effects at low () and high () concentrations (Ibrahim et al. 2011; Mangum et al. 2015). Mangum et al. (2015) argued that some methodological limitations could justify the null results in their previous study (Howell and Mangum 2011). Furthermore, p,p′-DDE increased the fatty acid uptake in NIH3T3-L1 and increased the proliferation of human preadipocytes (Chapados et al. 2011; Howell and Mangum 2011).
Expression of metabolic regulators.
Overall, positive associations between exposure to either p,p′-DDT or p,p′-DDE and both adipokines and master regulators of adipogenesis were reported in mice preadipocytes and human MSCs (Figure 5B,C).
Consistent with increased adipogenesis, the master regulator of adipocyte differentiation , was more highly expressed in p,p′-DDT treated cells than the controls in differentiated 3T3-L1 cells (Moreno-Aliaga and Matsumura 2002) and mesenchymal stem cells (Strong et al. 2015). The effect of p,p′-DDE was not consistent; whereas one study did not show statistically significant effects (Mangum et al. 2015), the other showed decreased activation at the highest doses (Taxvig et al. 2012). Srebf1 RNA, encoding the downstream target of and mediator of adipogenic differentiation SREBP1C, was also overexpressed in cells treated with p,p′-DDE (Mangum et al. 2015). Protein , considered with the key transcription regulation factors in adipogenesis and lipogenesis, was also increased after incubation with p,p′-DDT, but the expression of was unaffected (Moreno-Aliaga and Matsumura 2002). Above these doses of DDTs, the activation of was reduced in NIH-3T3 cells (Taxvig et al. 2012).
The majority of studies found increased adipokine parameters in pre- and differentiated adipocytes by DDTs (Howell and Mangum 2011; Mangum et al. 2015; Taxvig et al. 2012), with the exception of one study whose authors found a decrease of resistin at the lowest concentration tested () (Taxvig et al. 2012).
Confidence in the body of evidence and level of evidence.
Similar to the in vivo studies, we classified the body of evidence from in vitro studies with an initial high level of confidence based on the features of routine in vitro study designs (Figure 1; see also “4.5. RATING THE CONFIDENCE IN THE BODY OF SUPPORTING EVIDENCE FROM IN VITRO STUDIES” in the Supplemental Material). Among downgrading and upgrading factors, we noted a lack of consistency among the results of adipogenic differentiation caused by p,p′-DDE. Only half of the results showed statistically significant increases, and the positive results were not consistent acoss overlapping dosing concentrations (Ibrahim et al. 2011; Mangum et al. 2015; Taxvig et al. 2012). Similarly, lack of consistency extended to the effects of p,p′-DDE on mRNA expression of the main master regulator of adipogenic differentiation (Figure 5B). We contrasted these p,p′-DDE inconsistencies with the generally consistent increased differentiation and Pparg expression with p,p′-DDT exposure that supported the main in vivo evidence. We decided to downgrade due to the inconsistency observed in p,p′-DDE (Figure 1) given that its relevance to the human stream of evidence and that risk of bias could not be assessed here but was deemed serious in all other experimental streams of evidence evaluated. The remaining downgrading and upgrading factors were not considered compelling enough to modify the overall evaluation (see “4.5. RATING THE CONFIDENCE IN THE BODY OF SUPPORTING EVIDENCE FROM IN VITRO STUDIES” in the Supplemental Material), including the applicability of the tested doses (see Figure S9). For example, although adipokine parameters were consistent across all in vitro studies, their lack of consistency with in vivo secondary outcomes precluded upgrade on this basis. The balance led to “moderate” as the final rating of confidence and level of evidence, accounting for the presence of “health effects” as the nature of the associations (Figure 1).
Integration of the Body Evidence and Hazard Identification
We first integrated the two streams of primary evidence—moderate level of human evidence and moderate level of in vivo evidence—and thus we classified p,p′-DDT and p,p′-DDE as “presumed” obesogens to humans (Figure 1). We applied a systematic approach to integrate the supporting evidence with the preliminary classification of the human and in vivo primary evidence. According our conceptual framework, a high or low level of supporting evidence of biological plausibility from in vitro and/or in vivo studies may justify upgrading or downgrading the preliminary classification, respectively. In this regard, the moderate supporting evidence from in vivo and in vitro studies did not justify any modification of the preliminary hazard classification (Figure 1). Thus, the final hazard identification conclusion was that p,p′-DDT and p,p′-DDE are “presumed” to be obesogenic in humans, based on a moderate level of primary human evidence, a moderate level of primary in vivo evidence, and a moderate level of evidence from in vivo and in vitro studies that supported the biological plausibility of the association.
Conclusions
Obesity is characterized by the expansion of adipose tissue mass, which is often accompanied by metabolic dysfunctions. Results from this meta-analysis, limited to prospective epidemiological studies, demonstrated a significant positive association between exposure to p,p′-DDE and adiposity. These epidemiological observations were integrated with experimental evidence of increased rodent adiposity, impaired energy expenditure, fatty liver, and adipogenic expansion that were estimated to fall within the range of the human exposures from prospective studies (see Figures S8 and S9).
The risk of obesity was observed among human populations exposed to p,p′-DDE, mainly during the prenatal period although one study also provided estimates from postnatal exposure. The increased risk of human obesity due to prenatal exposure to p,p′-DDE was also in agreement with in vivo primary evidence, demonstrating prenatal exposure to DDTs increases the adiposity of subsequent generations of rodents. In accordance with the primary in vivo evidence, the limited in vitro studies available reported higher adipogenic differentiation among preadipocytes exposed to p,p′-DDT but inconsistently so when exposed to p,p′-DDE.
Developmental exposure is one of the main pillars of the obesogenic hypothesis because the vulnerability of developing tissues to adult metabolic disease (Barker 1990; La Merrill and Birnbaum 2011). The latency of developmental exposure effects on obesity is postulated to result from impaired adipocyte differentiation (La Merrill et al. 2013) and/or epigenetic changes (Heindel et al. 2015), both evidenced here. For example, in the rats whose obesity was associated with the exposure of their ancestors to DDT, DNA methylation of sperm differed according to that trans-generational DDT exposure (Skinner et al. 2013). The potential human relevance of that provocative finding is suggested by the recent demonstration that both obesity and surgical weight loss also cause dramatic changes in the DNA methylation of sperm collected from men (Donkin et al. 2016).
Obesity is ultimately the result of energy imbalance, and energy expenditure via thermogenesis was impaired in two mammalian species exposed to DDTs. Although the mechanism(s) underlying this physiological phenomenon were sparsely studied, one in vivo study further supported biological plausibility by demonstrating a decrease in brown adipose RNA responsible for regulating thermogenesis (La Merrill et al. 2014).
Epidemiological Research Needs
The research on DDTs has focused on the associations between obesity outcomes and the major metabolite p,p′-DDE however comparatively little is known about the role of the primary commercially important parent compound p,p′-DDT. Given that p,p′-DDT exposure is primarily due to its manufacture or use, whereas p,p′-DDE exposure can be attributed to contamination of the environment and food supply, distinguishing the causal obesogen among them would have substantial implications for public health policies. The meta-analysis of dose–response profiles across populations remains analytically prohibitive with respect to the variable background exposure levels and increments between exposure categories. Improvements in both the quantitation and statistical analysis tools for the exposome can address these needs.
Several potential residual confounding factors should be considered in future models because of their relevant role in obesity etiology. For example, few epidemiological studies controlled for poor diet or sedentary lifestyle, two substantial obesity risk factors. However, we found no evidence that DDTs reduce exercise or cause hyperphagia (Howell et al. 2015; La Merrill et al. 2014). Future epidemiological studies should evaluate measures of energy intake and expenditure as potential confounders of the association between DDTs and obesity given the current paucity of this investigation.
Experimental Research Needs
There is a substantial need for further in vivo and in vitro mechanistic studies to demonstrate biological plausibility of the association between DDTs and obesity. For instance, the causal role of the endocrine system (e.g., insulin, thyroid, estrogen, and androgen axes (Chen et al. 1997; Kelce et al. 1995; La Merrill et al. 2014; Nelson 1974) on obesity associated with exposure to DDTs is uncharacterized despite evidence that both exposure and outcome are associated with these endocrine systems. Indeed mechanistic questions involving DDTs and obesity related outcomes should be conducted at numerous doses relevant to the human condition in multiple species. For example, in vitro studies of human cells are urged to demonstrate that mechanistic findings exhibited in mouse cells can operate in humans. Such efforts will allow for more rigorous dose–response analyses, and for stronger evidence of consistency.
Future experimental obesogen studies with animals must measure adiposity directly. The use of crude body weight has very limited applicability to characterize obesity in animal models if adiposity or other related outcomes are not measured and greatly limited the number of primary in vivo studies analyzed here (Nascimento et al. 2008; Woods et al. 2003). The body of evidence from animal studies addressing primary outcomes also needs to be strengthened by corroborating existing results, especially considering dose relevance and transgenerational outcomes. Better mechanistic characterization of the effects of DDTs on diseases often comorbid with obesity, for example, type 2 diabetes, dyslipidemia, and hepatic steatosis, will also be critical in informing causal models underlying epidemiological analyses.
Primary Limitations and Strengths of This Study
Limitations
-
•
This study relied on a search strategy designed to address multiple outcomes from multiple streams of evidence, focusing on high sensitivity rather specificity. More specific search strategies may better characterize mechanistic pathways; nonetheless, we did not suspect we missed relevant publications.
-
•
The paucity of prospective epidemiological data on DDTs and adiposity outcomes did not allow us to parse out robust stratified meta-analysis. For instance, comparing effect modification between DDTs, lipid adjustment, and exposure and outcome windows.
-
•
The variable and narrow range of exposure to DDTs across studied human populations is likely a poor reflection of the entire dose-response relationship. The impact this has on variability in the defined range of reference groups, the absence of p,p′-DDT data in humans, and the possibility of non-monotonic dose–response among populations could underestimate the meta-estimate effect size.
-
•
Judgmental inference was required to rate the confidence and integrate evidence. Potential subjective influence was minimized by critical review of multiple coauthors iteratively until consensus was reached.
-
•
Risk of bias tools and guidelines were unavailable for in vitro studies. They are needed for applying the evidence-based framework to in vitro data here and to the growing body of evidence from high-throughput screening programs.
Strengths
-
•
The meta-analysis of human evidence, limited to prospective studies determined with quantitative biomonitoring techniques, exhibited moderate heterogeneity and statistically significant positive associations.
-
•
Experimental evidence from in vivo studies, limited to adiposity as primary outcome, was consistent with impaired thermogenesis, a secondary outcome relevant to obesity etiology.
-
•
In this study, we applied a systematic and structured approach to data collection, data analysis, evidence rating, and integration using the GRADE-based NTP/OHAT protocol to draw hazard identification conclusions on the obesogenic effects of DDTs; that increases the rigor, transparency, and reproducibility.
To the best of our knowledge, this is the first study to systematically integrate evidence about the obesogenic effects of the pesticide DDT and its metabolite DDE. We integrated different streams of evidence from human, primary in vivo, and secondary in vivo and in vitro studies, and determined that each provides a moderate level of evidence supporting our conclusion that DDT and DDE exposures during the developmental period can be classified as “presumed” human obesogens. This is essential to inform decisions in the ongoing cost–benefit debate of the continued use of DDT as an insecticide (Conis 2010). Further, this study also highlights metabolic disruption triggered by environmental pollutants as a novel end point to be considered in risk assessment frameworks. Finally, it has been estimated that the annual economic impact of obesity as a consequence of exposure to DDT and DDE is 62 million USD in the European Union and United States (Attina et al. 2016). Thus, policy makers should also consider the preventive strategies reducing the exposure to obesogen compounds in overall disease and budget management.
Supplemental Material
Acknowledgments
We are grateful for the technical support of K. Thayer on the implementation of the National Toxicology Program/Ongoing Methods Development Activities (NTP/OHAT) framework and for the feedback of the anonymous reviewers.
This work is supported by the U.S. Department of Agriculture’s National Institute of Food and Agriculture (Hatch project 1002182), the California Office of Environmental Health Hazard Assessment (award 13-E0014-1), and the National Institutes of Health (grant ES019919).
References
- Agay-Shay K, Martinez D, Valvi D, Garcia-Esteban R, Basagaña X, Robinson O, et al. 2015. Exposure to endocrine-disrupting chemicals during pregnancy and weight at 7 years of age: a multi-pollutant approach. Environ Health Perspect 123:1030–1037, PMID: 25956007, 10.1289/ehp.1409049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahdaya SM, Shah PV, Guthrie FE. 1976. Thermoregulation in mice treated with parathion, carbaryl, or DDT. Toxicol Appl Pharmacol 35:575–580, PMID: 817420. [DOI] [PubMed] [Google Scholar]
- Allison DB, Downey M, Atkinson RL, Billington CJ, Bray GA, Eckel RH, et al. 2008. Obesity as a disease: a white paper on evidence and arguments commissioned by the Council of the Obesity Society. Obesity (Silver Spring) 16:1161–1177, 10.1038/oby.2008.231. [DOI] [PubMed] [Google Scholar]
- Appleton BS, Shriver CN, Arnrich L, Hathcock JN. 1981. Effects of 3-methylcholanthrene and DDT on cholesterol 7 alpha-hydroxylase in rats. Durg-Nutr Interact 1(1):15–21, PMID: 6926812. [PubMed] [Google Scholar]
- Arnold M, Pandeya N, Byrnes G, Renehan AG, Stevens GA, Ezzati M, et al. 2015. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol 16:36–46, PMID: 25467404, 10.1016/S1470-2045(14)71123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). 2002. Toxicological profile for DDT, DDE, and DDD. Atlanta, GA:ATSDR. [PubMed] [Google Scholar]
- Attina TM, Hauser R, Sathyanarayana S, Hunt PA, Bourguignon JP, Myers JP, et al. 2016. Exposure to endocrine-disrupting chemicals in the USA: a population-based disease burden and cost analysis. Lancet Diabetes Endocrinol 4(12):996–1003, PMID: 27765541, 10.1016/S2213-8587(16)30275-3. [DOI] [PubMed] [Google Scholar]
- Barker DJ. 1990. The fetal and infant origins of adult disease. BMJ 301:1111, PMID: 2252919, 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays HE, Toth PP, Kris-Etherton PM, Abate N, Aronne LJ, Brown WV, et al. 2013. Obesity, adiposity, and dyslipidemia: a consensus statement from the National Lipid Association. J Clin Lipidol 7:304–383, PMID: 23890517, 10.1016/j.jacl.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Benton MJ, Nimrod AC, Benson WH. 1994. Evaluation of growth and energy storage as biological markers of DDT exposure in sailfin mollies. Ecotoxicol Environ Saf 29:1–12, PMID: 7529156. [DOI] [PubMed] [Google Scholar]
- Cal/EPA-OEHHA (California Environmental Protection Agency–Office of Environmental Health Hazard Assessment, Safe Drinking Water and Toxic Enforcement Act of 1986). 2016. Chemicals Known to the State to Cause Cancer or Reproductive Toxicity.
- Chapados NA, Casimiro C, Batal M, Blais JM, Haman F, Robidoux MA, et al. 2011. Increased proliferative effect of organochlorine compounds on human preadipocytes [Abstract]. Can J Diabetes 35:213, 10.1016/S1499-2671(11)52271-3. [DOI] [PubMed] [Google Scholar]
- Chen CW, Hurd C, Vorojeikina DP, Arnold SF, Notides AC. 1997. Transcriptional activation of the human estrogen receptor by DDT isomers and metabolites in yeast and MCF-7 cells. Biochem Pharmacol 53:1161–1172, PMID: 9175721. [DOI] [PubMed] [Google Scholar]
- Conis E. 2010. Debating the health effects of DDT: Thomas Jukes, Charles Wurster, and the fate of an environmental pollutant. Public Health Rep 125(2):337–342, PMID: 20297762, 10.1177/003335491012500224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupul-Uicab LA, Hernández-Avila M, Terrazas-Medina EA, Pennell ML, Longnecker MP. 2010. Prenatal exposure to the major DDT metabolite 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene (DDE) and growth in boys from Mexico. Environ Res 110:595–603, PMID: 20566194, 10.1016/j.envres.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsie J, Gosha SK, Holman RT. 1976. Induction of abnormal fatty acid metabolism and essential fatty acid deficiency in rats by dietary DDT. Arch Biochem Biophys 175(1):262–269, PMID: 952519. [DOI] [PubMed] [Google Scholar]
- Delvaux I, Van Cauwenberghe J, Den Hond E, Schoeters G, Govarts E, Nelen V, et al. 2014. Prenatal exposure to environmental contaminants and body composition at age 7–9 years. Environ Res 132:24–32, PMID: 24742724, 10.1016/j.envres.2014.03.019. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. 1986. Meta-analysis in clinical trials. Control Clin Trials 7:177–188, PMID: 3802833. [DOI] [PubMed] [Google Scholar]
- Donkin I, Versteyhe S, Ingerslev LR, Qian K, Mechta M, Nordkap L, et al. 2016. Obesity and bariatric surgery drive epigenetic variation of spermatozoa in humans. Cell Metab 23:369–378, PMID: 26669700, 10.1016/j.cmet.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Drenowatz C. 2015. Reciprocal compensation to changes in dietary intake and energy expenditure within the concept of energy balance. Adv Nutr 6:592–599, 10.3945/an.115.008615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Luo N, Klein RL, Garvey WT. 2005. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res 46(7):1369–1379, PMID: 15834118, 10.1194/jlr.M400373-JLR200. [DOI] [PubMed] [Google Scholar]
- Gaskins AJ, Schisterman EF. 2009. The effect of lipid adjustment on the analysis of environmental contaminants and the outcome of human health risks. Methods Mol Biol 580:371–381, PMID: 19784610, 10.1007/978-1-60761-325-1_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. 2015. EDC-2: the Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev 36:E1–E150, PMID: 26544531, 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grün F, Blumberg B. 2006. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology 147(6 suppl):S50–S55, PMID: 16690801, 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. 2011a. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 64:380–382, PMID: 21185693, 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso-Coello P, et al. 2011b. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol 64:1311–1316, PMID: 21802902, 10.1016/j.jclinepi.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Harbord RM, Egger M, Sterne JA. 2006. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 25:3443–3457, PMID: 16345038, 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- Heindel JJ, vom Saal FS, Blumberg B, Bovolin P, Calamandrei G, Ceresini G, et al. 2015. Parma consensus statement on metabolic disruptors. Environ Health 14:54, 10.1186/s12940-015-0042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S. 2011. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. http://handbook.cochrane.org/ [accessed 15 August 2015].
- Howell G III, Mangum L. 2011. Exposure to bioaccumulative organochlorine compounds alters adipogenesis, fatty acid uptake, and adipokine production in NIH3T3-L1 cells. Toxicol In Vitro 25:394–402, 10.1016/j.tiv.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GE III, Meek E, Kilic J, Mohns M, Mulligan C, Chambers JE. 2014. Exposure to p,p′-dichlorodiphenyldichloroethylene (DDE) induces fasting hyperglycemia without insulin resistance in male C57BL/6H mice. Toxicology 320:6–14, 10.1016/j.tox.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GE III, Mulligan C, Meek E, Chambers JE. 2015. Effect of chronic p,p′-dichlorodiphenyldichloroethylene (DDE) exposure on high fat diet-induced alterations in glucose and lipid metabolism in male C57BL/6H mice. Toxicology 328:112–122, PMID: 25541407, 10.1016/j.tox.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høyer BB , Ramlau-Hansen CH, Henriksen TB, Pedersen HS, Góralczyk K, Zviezdai V, et al. 2014. Body mass index in young school-age children in relation to organochlorine compounds in early life: a prospective study. Int J Obes (Lond) 38:919–925, PMID: 24718355, 10.1038/ijo.2014.58. [DOI] [PubMed] [Google Scholar]
- I’Allemand D, Wiegand S, Reinehr T, Müller J, Wabitsch M, Widhalm K, et al. 2008. Cardiovascular risk in 26,008 European overweight children as established by a multicenter database. Obesity (Silver Spring) 16(7):1672–1679, PMID: 18451769, 10.1038/oby.2008.259. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Fjære E, Lock EJ, Naville D, Amlund H, Meugnier E, et al. 2011. Chronic consumption of farmed salmon containing persistent organic pollutants causes insulin resistance and obesity in mice. PloS One 6:e25170, PMID: 21966444, 10.1371/journal.pone.0025170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Graham JL, Stanhope KL, Havel PJ, La Merrill MA. 2015. Effect of DDT exposure on lipids and energy balance in obese Sprague-Dawley rats before and after weight loss. Toxicol Rep 2:990–995, 10.1016/j.toxrep.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PI, Sutton P, Atchley DS, Koustas E, Lam J, Sen S, et al. 2014. The Navigation Guide—evidence-based medicine meets environmental health: systematic review of human evidence for PFOA effects on fetal growth. Environ Health Perspect 122:1028–1039, PMID: 24968388, 10.1289/ehp.1307893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen E. 2015. Obesity and cardiovascular disease. Minerva Pediatr 67:25–32, PMID: 25387321. [PubMed] [Google Scholar]
- Kelce WR, Stone CR, Laws SC, Gray LE, Kemppainen JA, Wilson EM. 1995. Persistent DDT metabolite p,p′-DDE is a potent androgen receptor antagonist. Nature 375:581–585, PMID: 7791873, 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- Klimentidis YC, Beasley TM, Lin HY, Murati G, Glass GE, Guyton M, et al. 2011. Canaries in the coal mine: a cross-species analysis of the plurality of obesity epidemics. Proc Biol Sci 278:1626–1632, 10.1098/rspb.2010.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klop B, Elte JW, Cabezas MC. 2013. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients 5:1218–1240, PMID: 23584084, 10.3390/nu5041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koustas E, Lam J, Sutton P, Johnson PI, Atchley DS, Sen S, et al. 2014. The Navigation Guide—evidence-based medicine meets environmental health: systematic review of nonhuman evidence for PFOA effects on fetal growth. Environ Health Perspect 122:1015–1027, PMID: 24968374, 10.1289/ehp.1307177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Merrill M, Birnbaum LS. 2011. Childhood obesity and environmental chemicals. Mt Sinai J Med 78:22–48, PMID: 21259261, 10.1002/msj.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Merrill M, Emond C, Kim MJ, Antignac JP, Le Bizec B, Clément K, et al. 2013. Toxicological function of adipose tissue: focus on persistent organic pollutants. Environ Health Perspect 121:162–169, PMID: 23221922, 10.1289/ehp.1205485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Merrill M, Karey E, Moshier E, Lindtner C, La Frano MR, Newman JW, et al. 2014. Perinatal exposure of mice to the pesticide DDT impairs energy expenditure and metabolism in adult female offspring. PloS One 9:e103337, PMID: 25076055, 10.1371/journal.pone.0103337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J, Koustas E, Sutton P, Johnson PI, Atchley DS, Sen S, et al. 2014. The Navigation Guide—evidence-based medicine meets environmental health: integration of animal and human evidence for PFOA effects on fetal growth. Environ Health Perspect 122:1040–1051, PMID: 24968389, 10.1289/ehp.1307923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberg L. 2012. Core temperature: a forgotten variable in energy expenditure and obesity? Obes Rev 13 (suppl 2):97–104, 10.1111/j.1467-789X.2012.01040.x. [DOI] [PubMed] [Google Scholar]
- Lee DH, Porta M, Jacobs DR Jr, Vandenberg LN. 2014. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr Rev 35:557–601, 10.1210/er.2013-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Longnecker MP, Dunson DB. 2013. Lipid adjustment for chemical exposures: accounting for concomitant variables. Epidemiology 24:921–928, PMID: 24051893, 10.1097/EDE.0b013e3182a671e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker MP, Klebanoff MA, Gladen BC, Berendes HW. 1999. Serial levels of serum organochlorines during pregnancy and postpartum. Arch Environ Health 54:110–114, PMID: 10094288, 10.1080/00039899909602244. [DOI] [PubMed] [Google Scholar]
- Loomis D, Guyton K, Grosse Y, El Ghissasi F, Bouvard V, Benbrahim-Tallaa L, et al. 2015. Carcinogenicity of lindane, DDT, and 2,4-dichlorophenoxyacetic acid. Lancet Oncol 16:891–892, PMID: 26111929, 10.1016/S1470-2045(15)00081-9. [DOI] [PubMed] [Google Scholar]
- Mangum LH, Howell GE III, Chambers JE. 2015. Exposure to p,p′-dde enhances differentiation of 3T3-L1 preadipocytes in a model of sub-optimal differentiation. Toxicol Lett 238:65–71, 10.1016/j.toxlet.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JA. 2000. Body-weight regulation: causes of obesity. Proc Nutr Soc 59:337–345, PMID: 10997649. [DOI] [PubMed] [Google Scholar]
- Moreno-Aliaga MJ, Matsumura F. 2002. Effects of 1,1,1-trichloro-2,2-bis(p-chlorophenyl)-ethane (p,p′-DDT) on 3T3-L1 and 3T3-F442A adipocyte differentiation. Biochem Pharmacol 63:997–1007, PMID: 11911853. [DOI] [PubMed] [Google Scholar]
- Morgan RL, Thayer KA, Bero L, Bruce N, Falck-Ytter Y, Ghersi D, et al. 2016. GRADE: assessing the quality of evidence in environmental and occupational health. Environ Int 92-93:611–616, PMID: 26827182, 10.1016/j.envint.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan S, Dani HM, Misra UK. 1990. Changes in lipid profiles of liver microsomes of rats following intratracheal administration of DDT or endosulfan. J Environ Sci Health Part B Pestic Food Contam Agric Wastes 25(2):243–257, PMID: 2380487, 10.1080/10934529009375554. [DOI] [PubMed] [Google Scholar]
- Nascimento AF, Sugizaki MM, Leopoldo AS, Lima-Leopoldo AP, Nogueira CR, Novelli EL, et al. 2008. Misclassification probability as obese or lean in hypercaloric and normocaloric diet. Biol Res 41:253–259, PMID: 19399338, 10.4067//S0716-97602008000300002. [DOI] [PubMed] [Google Scholar]
- Nelson JA. 1974. Effects of dichlorodiphenyltrichloroethane (DDT) analogs and polychlorinated biphenyl (PCB) mixtures on 17β-(3H)estradiol binding to rat uterine receptor. Biochem Pharmacol 23:447–451, PMID: 4360348. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. 2014. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 311:806–814, PMID: 24570244, 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHAT (Office of Health Assessment and Translation). 2015a. Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration. Washington, DC:National Toxicology Program. [Google Scholar]
- OHAT. 2015b. OHAT Risk of Bias Rating Tool for Human and Animal Studies. Washington, DC:National Toxicology Program. [Google Scholar]
- Okazaki Y, Katayama T. 2003. Effects of dietary carbohydrate and myo-inositol on metabolic changes in rats fed 1,1,1-trichloro-2,2-bis (p-chlorophenyl) ethane (DDT). J Nutr Biochem 14(2):81–89, PMID: 12667599. [DOI] [PubMed] [Google Scholar]
- Okazaki Y, Katayama T. 2008. Dietary inositol hexakisphosphate, but not myo-inositol, clearly improves hypercholesterolemia in rats fed casein-type amino acid mixtures and 1,1,1-trichloro-2,2-bis (p-chlorophenyl) ethane. Nutr Res 28(10):714–721, PMID: 19083479, 10.1016/j.nutres.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Patel CJ, Cullen MR, Ioannidis JP, Butte AJ. 2012. Systematic evaluation of environmental factors: persistent pollutants and nutrients correlated with serum lipid levels. Int J Epidemiol 41:828–843, PMID: 22421054, 10.1093/ije/dys003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt DS, Cockrill BL. 1967. Liver enlargement and hepatoxicity: an investigation into the effects of several agents on rat liver enzyme activities. Biochem Pharmacol 16(12):2257–2270, PMID: 4294662. [DOI] [PubMed] [Google Scholar]
- Platt DS, Cockrill BL. 1969. Biochemical changes in rat liver in response to treatment with drugs and other agents. II. Effects of halothane, DDT, other chlorinated hydrocarbons, thioacetamide, dimethylnitrosamine and ethionine. Biochem Pharmacol 18(2):445–457, PMID: 4305095. [DOI] [PubMed] [Google Scholar]
- Rao TN, Sanyal S, Agarwal N, Subrahmanyam D. 1981. Effect of a single oral dose of DDT on lipid metabolism in rat. Toxicol Lett 7(3):263–266, PMID: 7222101. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Alcalá LM, Sá C, Pimentel LL, Pestana D, Teixeira D, Faria A, et al. 2015. Endocrine disruptor DDE associated with a high-fat diet enhances the impairment of liver fatty acid composition in rats. J Agric Food Chem 63:9341–9348, PMID: 26449595, 10.1021/acs.jafc.5b03274. [DOI] [PubMed] [Google Scholar]
- Rogan WJ, Chen A. 2005. Health risks and benefits of bis(4-chlorophenyl)-1,1,1-trichloroethane (DDT). Lancet 366:763–773, PMID: 16125595, 10.1016/S0140-6736(05)67182-6. [DOI] [PubMed] [Google Scholar]
- Rooney AA, Boyles AL, Wolfe MS, Bucher JR, Thayer KA. 2014. Systematic review and evidence integration for literature-based environmental health science assessments. Environ Health Perspect 122:711–718, PMID: 24755067, 10.1289/ehp.1307972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney AA, Cooper GS, Jahnke GD, Lam J, Morgan RL, Boyles AL, et al. 2016. How credible are the study results? Evaluating and applying internal validity tools to literature-based assessments of environmental health hazards. Environ Int 92–93:617–629, PMID: 26857180, 10.1016/j.envint.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schisterman EF, Whitcomb BW, Buck Louis GM, Louis TA. 2005. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ Health Perspect 113:853–857, PMID: 16002372, 10.1289/ehp.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan MC, Lam J. 2015. Use of systematic review and meta-analysis in environmental health epidemiology: a systematic review and comparison with guidelines. Curr Environ Health Rep 2:272–283, PMID: 26231504, 10.1007/s40572-015-0062-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK, Manikkam M, Tracey R, Guerrero-Bosagna C, Haque M, Nilsson EE. 2013. Ancestral dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic transgenerational inheritance of obesity. BMC Med 11:228, 10.1186/1741-7015-11-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. 1999. Worldwide trends in DDT levels in human breast milk. Int J Epidemiol 28:179–188, PMID: 10342677. [DOI] [PubMed] [Google Scholar]
- Speakman JR, O’Rahilly S. 2012. Fat: an evolving issue. Dis Model Mech 5:569–573, PMID: 22915015, 10.1242/dmm.010553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JH, Rutkowski JM, Scherer PE. 2016. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab 23:770–784, PMID: 27166942, 10.1016/j.cmet.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong AL, Shi Z, Strong MJ, Miller DF, Rusch DB, Buechlein AM, et al. 2015. Effects of the endocrine-disrupting chemical DDT on self-renewal and differentiation of human mesenchymal stem cells. Environ Health Perspect 123:42–48, PMID: 25014179, 10.1289/ehp.1408188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang-Péronard JL, Jensen TK, Andersen HR, Ried-Larsen M, Grøntved A, Andersen LB, et al. 2015. Associations between exposure to persistent organic pollutants in childhood and overweight up to 12 years later in a low exposed Danish population. Obes Facts 8:282–292, 10.1159/000438834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taxvig C, Dreisig K, Boberg J, Nellemann C, Schelde AB, Pedersen D, et al. 2012. Differential effects of environmental chemicals and food contaminants on adipogenesis, biomarker release and PPARγ activation. Mol Cell Endocrinol 361:106–115, PMID: 22526026, 10.1016/j.mce.2012.03.021. [DOI] [PubMed] [Google Scholar]
- Taylor KW, Novak RF, Anderson HA, Birnbaum LS, Blystone C, Devito M, et al. 2013. Evaluation of the association between persistent organic pollutants (POPs) and diabetes in epidemiological studies: a National Toxicology Program workshop review. Environ Health Perspect 121:774–783, 10.1289/ehp.1205502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer KA, Heindel JJ, Bucher JR, Gallo MA. 2012. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect 120:779–789, PMID: 22296744, 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNEP (United Nations Environment Programme). 2010. Report of the Expert Group on the Assessment of the Production and Use of DDT and Its Alternatives for Disease Vector Control. Third Meeting. UNEP/POPS/COP.3/24. Geneva, Switzerland: United Nations. [Google Scholar]
- Vafeiadi M, Georgiou V, Chalkiadaki G, Rantakokko P, Kiviranta H, Karachaliou M, et al. 2015. Association of prenatal exposure to persistent organic pollutants with obesity and cardiometabolic traits in early childhood: the Rhea mother–child cohort (Crete, Greece). Environ Health Perspect 123:1015–1021, 10.1289/ehp.1409062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M, Wesselink A, Harley KG, Bradman A, Kogut K, Eskenazi B. 2014. Prenatal exposure to dichlorodiphenyltrichloroethane and obesity at 9 years of age in the CHAMACOS study cohort. Am J Epidemiol 179:1312–1322, PMID: 24722999, 10.1093/aje/kwu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlake GE, Bunyan PJ, Stanley PI, Walker CH. 1979. The effects of 1,1-di(p-chlorophenyl)-2-chloroethylene on plasma enzymes and blood constituents in the Japanese quail. Chem Biol Interact 25:197–210, PMID: 466732. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2014. Global Status Report on Noncommunicable Diseases 2014. Geneva, Switzerland:WHO. [Google Scholar]
- Woodruff TJ, Sutton P. 2014. The Navigation Guide systematic review methodology: a rigorous and transparent method for translating environmental health science into better health outcomes. Environ Health Perspect 122:1007–1014, PMID: 24968373, 10.1289/ehp.1307175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC, Seeley RJ, Rushing PA, D’Alessio D, Tso P. 2003. A controlled high-fat diet induces an obese syndrome in rats. J Nutr 133:1081–1087, PMID: 12672923. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.