Abstract
Background:
In 1973–1974, Michigan residents were exposed to polybrominated biphenyls (PBBs) through an accidental contamination of the food supply. Residents were enrolled in a registry assembled after the incident, and they and their children participated in follow-up studies to assess subsequent health outcomes.
Objectives:
We evaluated associations between serum PBBs and polychlorinated biphenyls (PCBs) and markers of thyroid function among Michigan adults.
Methods:
Serum concentrations of four PBB and four PCB congeners were measured at least once in 753 adults, including 79 women who participated in a 2004–2006 study and 683 women and men with follow-up during 2012–2015. Participants completed questionnaires on health conditions (including physician-diagnosed thyroid disease), behaviors, and demographics. Thyroid hormones were measured in a subset without thyroid disease (). In multivariable linear regression models, PBB and PCB congener concentrations, on both the volume (nanogram/milliliter) and lipid (nanogram/gram lipid) basis, were assessed in relation to thyroid hormones. Logistic regression models were used to estimate associations between serum PBBs and PCBs and thyroid disease.
Results:
Thyroid disease was common (18% overall; 25% among women). Among women, all odds ratios (ORs) for PBB-153 and thyroid disease were positive for quintiles above the reference level, but estimates were imprecise and were without a monotonic increase. For an interquartile range (IQR) increase in PBB-153 (), the ; (95% CI: 0.83, 1.52) (); for hypothyroidism, (95% CI: 0.86, 2.13) (). There were 21 cases of thyroid disease in men [ (95% CI: 0.33); 1.44 for an IQR increase () in serum PBB-153]. PCB congeners were statistically significantly associated with greater total and free thyroxine and total triiodothyronine among women and with total and free triiodothyronine among men in lipid-standardized models.
Conclusions:
We found some evidence to support associations of PBBs and PCBs with thyroid disease and thyroid hormone levels. https://doi.org/10.1289/EHP1302
Introduction
Polybrominated biphenyls (PBBs) and polychlorinated biphenyls (PCBs) are classes of persistent organic pollutants that were used as flame retardants in the manufacturing of plastics and electronics (ATSDR 2004) and as coolants in electrical transformers and capacitators (ATSDR 2000), respectively. Although PBBs and PCBs have not been in production in the United States since the 1970s, exposure to these compounds is still widespread owing to their environmental and biological persistence (Xue et al. 2014). In addition, because of their structural similarities and shared toxicological features with other ubiquitous chemical flame retardants, such as polybrominated diphenyl ethers (PBDEs) and others that are currently produced, research on these compounds may shed light on the potential health effects of exposure to their newer derivatives (Birnbaum and Staskal 2004). Experimental evidence suggests that exposure to halogenated compounds such as PBBs and PCBs may interfere with the endocrine system and specifically with thyroid function (Allen-Rowlands et al. 1981; Brouwer et al. 1999; Byrne et al. 1987), but the body of literature among humans has yielded inconsistent results [as reviewed by Hagmar 2003; Salay and Garabrant 2009).
In 1973–1974, millions of people from across the state of Michigan were exposed to PBBs through an accidental contamination of the food supply (Brilliant et al. 1978; Wolff et al. 1982). The Velsicol Chemical Company shipped PBB mixtures instead of livestock feed supplement to the Farm Bureau Services feed mill; these mixtures were then distributed to farms and retail outlets throughout the state and were subsequently incorporated into cattle, chicken, pig, and sheep feed (Carter 1976; Fries 1985). Due to the common practice of eating locally raised livestock and livestock products in this area, people were exposed through direct consumption of the tainted products including meat from the animals, eggs, and dairy products (Kay 1977). In subsequent generations, people were exposed through breastfeeding and in utero transfer (Joseph et al. 2009). Directly following the episode, the Michigan Department of Health enrolled approximately 6,000 individuals in a registry to measure baseline PBB and PCB concentrations and to monitor long-term health outcomes (Landrigan et al. 1979). Persons eligible for inclusion in the registry lived on or received food from farms quarantined by the Michigan Department of Agriculture after the contamination was discovered or were Velsicol workers and members of their households. This cohort of exposed individuals and their children has now been followed for .
Evidence of possible health effects has been noted in these registry participants and in their children, specifically among women of reproductive age: registry members with greater PBB concentrations (compared with those with lower concentrations) were found to have increased odds of miscarriage (Small et al. 2011), shorter menstrual cycles and longer menstrual periods (Davis et al. 2005), earlier menarche in girls (Blanck et al. 2000), and later pubertal development in boys (Small et al. 2009). In conjunction with evidence from studies suggesting that women with thyroid dysfunction are at increased risk of miscarriage (Liu et al. 2014), menstrual function perturbations such as anovulation and menorrhagia (Krassas et al. 1999), and altered timing of pubertal development (Doufas and Mastorakos 2000), the results from previous studies in the Michigan cohort (Blanck et al. 2000; Davis et al. 2005; Small et al. 2009; Small et al. 2011) have motivated the hypothesis that the thyroid may be a target for PBB toxicity in humans. Animal data provide biologic support for this hypothesis, with studies consistently showing decreases in total thyroxine () and triiodothyronine () (Allen-Rowlands et al. 1981; Byrne et al. 1987; Gupta et al. 1983) and increases in thyroid weights (Ringer and Polin 1977; Ringer 1978; Werner and Sleight 1981) associated with PBB exposure. However, to our knowledge, only two epidemiologic investigations of the association between PBBs and thyroid function have been published, and both involved the Michigan livestock feed contamination (Bahn et al. 1980; Yard et al. 2011). Bahn et al. (1980) reported that men who were occupationally exposed to PBBs were more likely to have prevalent hypothyroidism [high thyroid-stimulating hormone (TSH) and low total and free outside of population references] as well as elevated levels of thyroid peroxidase antibodies compared with age-matched controls without occupational exposure to PBBs (4 cases/35 exposed individuals vs. 0 cases/89 controls). A subsequent study conducted among members of the original Michigan PBB Registry who were exposed through consumption of contaminated food examined associations between enrollment PBB and PCB levels and self-reported thyroid disease, which was obtained through follow-up that occurred in intervals between 1991 and 2006 (Yard et al. 2011). This nested case–control study found that among women (212 cases, 844 controls) and men (47 cases, 1,761 controls), thyroid disease was not associated with tertiles of enrollment PBB or PCB concentrations compared with those with concentrations below the limit of detection (LOD). However, these two epidemiologic studies were limited by small sample size, particularly in the case of Bahn et al. (1980); in the Yard et al. (2011) study, limitations included early exposure assessment methodologies that were used to determine baseline PBB and PCB concentrations at the time of the contamination incident and a lack of other biomarker measurements including thyroid hormones.
In the present study, we investigated the association between PBB and PCB exposure and thyroid function among individuals in the Michigan PBB Registry with the addition of key biologic measures assessed using more sensitive methods than those used in previous investigations, including current serum PBB, PCB, and lipid concentrations; circulating thyroid hormone concentrations; and in a subset, urinary iodine concentrations. The purpose of this study was to examine the association between serum PBB and PCB concentrations and measures of thyroid function in adults from the Michigan cohort, including investigation of overt thyroid disease, thyroid hormone concentrations, and in a subset of women, whether these associations were stronger among the iodine deficient.
Methods
Data Collection
The original PBB registry consisted of a heterogeneous group: residents of farms that were classified as quarantined by the Michigan Department of Health based on PBB levels in any sample of meat, eggs, or milk that were above a specified limit; those who had received meat, eggs, or dairy products directly from quarantined premises in 1973 or 1974; workers who had been occupationally exposed to PBBs in a chemical manufacturing plant in Michigan and members of their households; residents on farms with low-level PBB contamination; “self-referred” individuals who had either resided on farms identified as being contaminated by PBBs at levels below quarantine limits or who had eaten food produced on such farms; and “self-referred” volunteers who had no documented connection with contaminated farm premises but likely consumed contaminated farm products that were distributed throughout the state (Landrigan et al. 1979). Offspring of women in the original registry were subsequently enrolled (oral communication, H. Humphrey, environmental epidemiologist, Michigan Department of Public Health, November 1995).
Participants were recruited for this study from the original PBB Registry in two phases (see Figure S1). In the first phase (2004–2006), the Michigan Department of Health recruited women from the PBB registry (from all subgroups) for a blood draw, targeting women who were particularly highly exposed to PBB based on their enrollment levels. These women also completed a computer-assisted telephone interview close to the time of the blood draw that included questions about medical history (particularly with regard to endocrine-sensitive end points, such as thyroid disease), current medication use, demographics, and other lifestyle information. The second phase started in 2012: Members from all subgroups of the original PBB Registry were mailed invitations to attend community meetings held throughout the state, where study recruitment was conducted between 2012 and 2015. Community meetings were also advertised in the local press; thus, individuals from the original registry as well as others attended and subsequently participated in the study. Eligibility criteria to participate in this phase of recruitment of the study were at least one of the following: individuals who lived in the state of Michigan during the time of the contamination (1973–1974) or who were offspring of those who lived in the state during that time. Participation in the study entailed a blood draw that was used to determine PBB and PCB concentrations; completion of online health questionnaires, which included details on medical history (including reporting of physician-diagnosed thyroid disease and age at diagnosis), current medication use, behaviors, and demographics; and anthropometric measurements (height and weight) conducted by study staff, from which body mass index (BMI) was derived. BMI was missing for 34% () because a substantial proportion of individuals did not participate in the physical measurements.
Directly following their overall study participation (blood draw and questionnaire), subsets of women from Phase 1 and Phase 2 were separately recruited to participate in a study measuring menstrual cycle function. Those who were premenopausal, who were not pregnant or lactating, who were not taking hormonal medications, and who were never diagnosed with a reproductive-area cancer or received cancer treatment were eligible. Women collected daily first-morning-urine samples over three menstrual cycles, and the samples were analyzed to obtain iodine concentrations.
A small proportion of individuals (6%) participated in the study more than once (, 2 times; , 3 times) over the course of the two recruitment phases by attending multiple recruitment events; thus, their blood was drawn for exposure assessment each time. Repeat participants consisted of the following: those who participated between 2004 and 2006 and again between 2012 and 2015 (; four of these participated in 2004–2006 and twice between 2012 and 2015), and those who participated multiple times between 2012 and 2015 only (). There were no additional eligibility criteria for participating multiple times. Cluster size was not informative with respect to exposure or outcome. Informed consent was obtained from each individual before participation. Study protocols were approved by the Institutional Review Board at Emory University.
Thyroid Disease
Thyroid disease status and age at diagnosis were derived from self-report of physician-diagnosed disease on the questionnaire (), and we additionally classified individuals who had TSH and total or free outside of clinical reference ranges (; total ; free ) as having thyroid disease () (Braverman and Cooper 2012). Two women who participated in both Phase 1 and Phase 2 developed thyroid disease between these time points and were included as noncases and as cases in the analysis, respectively. Thyroid disease included any thyroid disorder including hypothyroidism; hyperthyroidism; other diagnoses including Hashimoto’s thyroiditis, Graves’ disease, and thyroid nodules; and instances where the participant did not specify the type. Among women, analyses were repeated restricting the outcome to hypothyroidism.
Exposure Analyses
Blood samples were collected via venipuncture, processed to isolate the serum, and stored at until analysis. In batches between March 2013 and June 2015, serum samples were analyzed for target PBB (77, 101, 153, and 180) and PCB (118, 138, 153, 180) congeners using gas chromatography–tandem mass spectrometry (GC-MS/MS; Agilent Technologies; Agilent 7890A gas chromatograph coupled to an Agilent 7000B tandem mass spectrometer) at the Laboratory for Exposure Assessment and Method Development in Environmental Research (LEADER) at Emory University’s Rollins School of Public Health. Detailed laboratory methods have been described previously (Marder et al. 2016). Briefly, samples were subjected to liquid-liquid extraction followed by solid-phase extraction before instrumental analysis using GC-MS/MS in multiple reaction monitoring mode. Quantification was performed using isotope-dilution calibration covering a concentration range of . LODs, which were extrapolated from standard injections, were defined as the lowest standard concentration at which the signal:noise ratio was . Estimated detection limits for all target compounds ranged from . PCB-118 concentrations could not be reported for 99 samples (12.4%) , including all samples collected in Phase 1, due to unstable retention times on the laboratory instrument, which was caused by clipping of the peak such that it was outside the acquisition window.
For analyses, PCB congeners were considered individually as well as summed by the degree of ortho-substitution (Safe 1993) based on the hypothesis that chemical structure is a main determinant of PCB-induced thyroid disruption. The di-ortho chlorinated congeners (PCB-138, 153, and 180) were summed (), and PCB-118, as the only mono-ortho chlorinated congener measured, was considered separately. PBB-153 was the only PBB congener detected in of the samples; therefore, no groupings of PBB congeners were considered.
Lipid Analyses
Total serum triglyceride content was measured using an Abnova Triglyceride Quantification Assay Kit (Abnova Corporation), and total cholesterol content was measured using a Cayman Cholesterol Assay Kit (Cayman Chemical Company) according to the manufacturers’ instructions. Total lipids were calculated using conventional methods based on these individual lipid components (Phillips et al. 1989).
Hormone Analyses
Participants without a history of thyroid disease at the time of blood draw and who reported no current thyroid medication use were tested for thyroid hormones. Thyroid hormones (TSH, total and free , and total and free ) were analyzed at the Emory Clinical Translational Research Laboratory with a Beckman Coulter Access II chemiluminescent immunoassay analyzer (Beckman Coulter). Analyses were conducted as directed by the manufacturer. Daily quality control (QC) samples were run before and after all study samples, and blinded controls were run throughout. If QC samples were more than two standard deviations from the expected values, the assay was repeated.
Urinary Iodine Analyses
A subset of women ( from Phase 1 and from Phase 2) also participated in a study examining menstrual cycle function, which involved daily collection of urine samples for three months directly following their blood draw for this study. Women were eligible for this study if they were premenopausal, were not pregnant or lactating, were not currently taking hormonal medication, and had never had a reproductive cancer or treatment for any cancer. Because it has been shown that 10 to 14 urinary iodine measurements are needed to estimate individual iodine status with 20% precision owing to the high degree of within-person variability in spot urine samples (Andersen et al. 2008; König et al. 2011), we pooled equal volumes from 14 consecutive urine samples for each woman and measured iodine and creatinine concentrations in the pooled sample. The samples selected for pooling were those that were collected closest in time to the blood draw to approximate iodine status at the time thyroid hormones were measured. One woman participated twice such that she had two iodine concentrations (Phase 1 in 2004 and Phase 2 in 2013).
Iodine was determined in diluted samples by inductively coupled plasma–mass spectrometry (ICP-MS) and incorporated an octopole collision cell to reduce interference from polyatomic species. Quantitation was based on the use of internal standards, and a calibration curve was generated using a matched synthetic matrix. Urinary creatinine was measured using a colorimetric method based on the modified Jaffe reaction (Taussky 1954). Creatinine levels were used to adjust urinary iodine concentrations for urine flow rate. The lower limits of quantification were and (NMS Labs).
Statistical Analyses
The distribution of exposure was explored, and geometric means (GMs) were calculated for PBB-153, for each PCB congener (PCB-118, 138, 153, and 180), and for (di-ortho) by thyroid disease status and for each covariate stratum. To estimate the association between serum PBBs and PCBs and thyroid disease prevalence, we fit logistic regression models with PBBs and PCBs considered as natural log–transformed continuous variables (to reduce the influence of extreme outliers), and we report associations for interquartile range (IQR) increases in ln-transformed concentrations as well as for quintiles. IQRs and quintiles were calculated separately for women and men. Models were adjusted for the following covariates that were identified a priori based on the literature and on our conceptualized directed acyclic graph (see Figure S2): lipids, age (18–29, 30–39, 40–49, 50–59, ), study phase (2004–06; 2012–15), and smoking status (current, former, never). Those who reported thyroid disease before the contamination episode were excluded from analyses with PBB-153 because these cases were likely due to other factors; however, they were included in all PCB analyses. In addition, all analyses were stratified by sex owing to the different PBB and PCB exposure distributions and to known differences in thyroid physiology between women and men as well as to greater baseline risks of thyroid dysfunction among women compared with men (Adlersberg and Burrow 2002). We did not control for BMI because BMI may be a consequence of overt thyroid dysfunction (Dale et al. 2001; Tzotzas et al. 2000): Hypothyroid and hyperthyroid patients often present with weight gain or loss, respectively (Fox et al. 2008). In models for hypothyroidism (among women only), those missing thyroid disease type were excluded from the model, and those with other specified disease types (e.g., hyperthyroidism) were considered to be noncases.
Among adults with no history of physician-diagnosed thyroid disease or current thyroid medication use, we fit multivariable linear regression models to estimate the associations between serum PBB and PCB concentrations and thyroid hormones. We fit separate models for each thyroid hormone and for each PBB and PCB congener with a detection frequency (PBB-153 and PCB-118, 138, 153, and 180) as well as for (di-ortho), both as natural log–transformed continuous variables and as deciles. Deciles were chosen a priori because for PBB-153 concentrations, the 10th percentile represents background levels in the general U.S. population per NHANES () (CDC 2004). TSH was natural log–transformed for normalization because there is evidence to suggest that TSH concentrations change on the logarithmic scale as opposed to the additive scale and are lognormally distributed (Demers and Spencer 2003). All regression models with hormone outcomes were controlled for lipids, age (18–29, 30–39, 40–49, 50–59, ), current smoking status (yes, no), and time of blood collection (morning, midday, afternoon/evening). Study phase was not controlled for because it was not associated with the outcome. In the case of missing covariate data, all analyses were conducted using complete case analysis. Associations were evaluated by statistical significance at .
In analyses of PBB-153, measurements below the LOD were imputed using a distribution-based maximum likelihood technique (Chen et al. 2011; Lubin et al. 2004) whose parameters (, ) were estimated assuming a lognormal probability distribution and based on the proportion of measures below the LOD. To incorporate uncertainty in the maximum likelihood estimates, we generated 10 sets of distribution parameters and imputed values below the LOD based on each set of parameters, creating 10 complete data sets (Chen et al. 2011; Lyles et al. 2001). Finally, we used the SAS procedure PROC MIANALYZE to generate summary regression coefficients, standard errors, and 95% confidence intervals (CIs) (Little and Rubin 2002).
Owing to the small proportion of repeat participants, in all analyses (for thyroid hormones as well as for thyroid disease), we used generalized estimating equations with robust standard errors to account for the potential correlated nature of their outcomes. Lastly, in all our analyses, we expressed serum PBBs and PCBs on the wet weight (volume) basis (nanograms/milliliter serum) and controlled for lipids as a covariate based on the potential for serum lipids to act as a confounder because of its association with time of day (likely due to recently eaten meals), which is also associated with thyroid hormones, specifically TSH (see Figure S3); as well as increased statistical flexibility by expressing it as a separate term instead of within the denominator of the PCB or PBB concentration itself (Schisterman et al. 2005). However, we repeated all analyses with PBBs and PCBs expressed on the lipid basis (nanograms/gram lipid).
Finally, among the subset for whom iodine was measured ( individuals, 80 observations), we explored the potential for effect measure modification of the relationship between PBBs and PCBs and thyroid hormones by iodine status by fitting models stratified by iodine status. p-Values were obtained by fitting linear regression models with a cross-product term and assessing the p-value of the interaction term at . Because analysis using the cut-off for deficient iodine [ creatinine (WHO 1994)] was limited by sample size ( iodine deficient), we used the alternative of creatinine, which is considered low iodine (Caldwell et al. 2011).
All statistical analyses were performed using SAS (version 9.4; SAS Institute Inc.).
Results
Population Characteristics
A total of 778 adults had their blood drawn and concurrently completed a health questionnaire during either 2004–2006 or 2012–2015 (or both in some cases). Among them, 3% () were missing information on thyroid disease diagnoses and were subsequently excluded, leaving 753 individuals for this analysis (801 samples). This study sample consisted of 79 individuals (79 samples) from Phase 1 (all women), 683 individuals (722 samples) from Phase 2 (385 women and 298 men); 9 individuals (all women) participated in both phases including 5 women who participated twice (once in 2004–2006 and once again in 2012–2015) and 4 women who participated three times (once in 2004-06 and twice between 2012 and 2015). Among those who participated between 2012 and 2015 only, 21 and 1 women participated 2 and 3 times, respectively, and 10 and 1 men participated 2 and 3 times, respectively.
The study population was white and had a high prevalence of overweight and obesity (69%) and ever smoking (40%) (Table 1). Most of the samples were collected between 2012 and 2015 (91%) and were from women (all samples from 2004–2006 and 60% overall). The majority (72%) were initially exposed to PBBs directly by consuming contaminated food (42% before the age of 16 y), with the remaining 28% likely exposed in utero, through breastfeeding, or both, because they were born after the acute contamination episode (after May 1974).
Table 1.
Population characteristics and serum polybrominated biphenyl (PBB) and polychlorinated biphenyl (PCB) concentrations by thyroid disease status among Michigan adults 2004–2015.
| Characteristic | Total study sample | Thyroid diseasea (17.9%)b,c | No thyroid disease (82.3%)c,d | p-Valuee |
|---|---|---|---|---|
| Exposures [GM; ng/mL (ng/g lipid)] | ||||
| PBB-153f | 0.17 (26.3) | 0.22 (33.2) | 0.16 (25.0) | 0.07 (0.09) |
| PCB-118 | 0.05 (7.9) | 0.08 (11.5) | 0.05 (7.3) | () |
| PCB-138 | 0.17 (25.8) | 0.22 (33.3) | 0.16 (24.4) | () |
| PCB-153 | 0.19 (29.0) | 0.24 (36.8) | 0.18 (27.5) | () |
| PCB-180 | 0.14 (20.8) | 0.18 (26.5) | 0.13 (19.7) | () |
| (di-ortho)g | 0.51 (76.9) | 0.65 (97.7) | 0.48 (73.0) | () |
| Sex [n (%)] | ||||
| Female | 455 (60.4)g | 113 (24.8) | 344 (75.6) | |
| Male | 298 (39.6)g | 22 (7.4) | 276 (92.6) | |
| Age (y) at blood draw [n (%)]h | ||||
| 18–29 | 100 (13.3) | 3 (3.0) | 97 (97.0) | |
| 30–39 | 176 (23.4) | 19 (10.8) | 157 (89.2) | |
| 40–49 | 153 (20.3) | 32 (20.9) | 121 (79.1) | |
| 50–59 | 150 (19.9) | 33 (22.0) | 117 (78.0) | |
| 186 (24.7) | 49 (26.3) | 137 (73.7) | ||
| Age (y) at PBB contamination [n (%)] | ||||
| at contamination | 226 (30.0) | 58 (25.7) | 168 (74.3) | |
| 0–15 at contamination | 315 (41.8) | 61 (19.4) | 256 (81.3) | |
| Born after contamination | 212 (28.2) | 16 (7.5) | 196 (92.5) | |
| Study years [n (%)] | ||||
| 2004–2006 onlyi | 70 (9.3) | 4 (5.7) | 66 (94.3) | |
| 2012–2015 only | 674 (89.5) | 128 (19.0) | 546 (81.0) | |
| 2004–2006 and 2012–2015c | 9 (1.2) | 3 (33.3) | 8 (88.9) | |
| Race/ethnicity [n (%)] | ||||
| Non-Hispanic white | 712 (96.1) | 128 (18.0) | 586 (82.3) | 0.92 |
| Hispanic white | 22 (3.0) | 3 (13.6) | 19 (86.4) | |
| Other | 7 (0.9) | 1 (14.3) | 6 (85.7) | |
| Missing | 12 | 3 | 9 | |
| Body mass indexh,j [n (%)] | ||||
| 155 (31.1) | 16 (10.3) | 140 (90.3) | 0.02 | |
| Overweight | 156 (31.3) | 20 (12.8) | 137 (87.8) | |
| Obese | 190 (38.2) | 42 (22.1) | 148 (77.9) | |
| Missing | 255 | 57 | 198 | |
| Smoking statusj [n (%)] | ||||
| Current smokers | 120 (16.1) | 14 (11.7) | 106 (88.3) | 0.11 |
| Former smokers | 182 (24.4) | 35 (19.2) | 147 (80.8) | |
| Never smokers | 444 (59.5) | 86 (19.4) | 360 (81.1) | |
| Missing | 7 | 0 | 7 |
Note: GM, Geometric mean. Total study sample: individuals, samples.
Includes those who specified physician-diagnosed thyroid disease [ (107 women; 21 men)] and those who had thyroid hormone concentrations outside clinically normal ranges [ (6 women; 1 man)]; includes 8 women and 1 man who reported thyroid disease before PBB contamination; includes 13 (9.1%) hyperthyroid (13 individuals), 66 (46.2%) hypothyroid (60 individuals), 17 (11.9%) other types (15 individuals), and 47 (32.9%) not specified (47 individuals).
143 samples.
women participated in 2004–2006 and again 2012–2015 and developed thyroid disease during that time; thus, these participants are counted in both categories, and percents sum to .
658 samples.
For comparison between thyroid disease versus no thyroid disease (statistical tests: Student’s t-test using natural log–transformed PBB and PCB levels; chi-squared test for categorical variables; Fisher’s exact test for categorical variables with sparse cells (expected value of cell is ).
women and 17 men with PBB-153 concentrations below the limit of detection (LOD); GMs for PBB-153 computed after multiple imputation for observations .
.
individuals participated twice, and individuals participated three times over time; thus, cell counts sum to , and percents sum to .
Only women participated between 2004 and 2006.
Assessed at time of blood draw.
Table 2 shows the distribution of serum PBB and PCB congener concentrations. PCBs were detected in all participants, and PBB-153 was detected in the vast majority (93%). PCB congeners were highly correlated (across all pairs of congeners, range of ; ) but were less correlated with PBB-153 (range of ; ). PBB-77, 101, and 180 were detected in of samples and were excluded from further analyses.
Table 2.
Distribution of serum polybrominated biphenyl (PBB) and polychlorinated biphenyl (PCB) congener concentrations among Michigan women, 2004–2015.
| PBB/PCB congener | LODa (ng/mL) | Percent detection | Minimum ng/mL (ng/g lipid) | 25th percentile ng/mL (ng/g lipid) | Median ng/mL (ng/g lipid) | 75th percentile ng/mL (ng/g lipid) | Maximum ng/mL (ng/g lipid) | Meanb ng/mL (ng/g lipid) | |
|---|---|---|---|---|---|---|---|---|---|
| PBB-77 | 801 | 0.005 | 5.9 | 0.34 (43.2) | — | ||||
| Women | 491 | 2.2 | 0.07 (16.1) | — | |||||
| Men | 298 | 11.6 | 0.34 (43.2) | — | |||||
| PBB-101 | 801 | 0.004 | 7.4 | 0.60 (77.8) | — | ||||
| Women | 491 | 3.9 | 0.11 (19.5) | — | |||||
| Men | 298 | 12.9 | 0.60 (77.8) | — | |||||
| PBB-153 | 801 | 0.001 | 93.3 | 0.07 (9.3) | 0.26 (38.2) | 0.63 (91.6) | 221.4 (27466) | 1.73 (279.4) | |
| Women | 491 | 92.5 | 0.06 (8.8) | 0.23 (32.6) | 0.48 (65.7) | 128.0 (22504) | 1.25 (215.1) | ||
| Men | 298 | 94.5 | 0.07 (12.1) | 0.33 (54.3) | 0.82 (138.3) | 221.4 (27466) | 2.50 (381.5) | ||
| PBB-180 | 801 | 0.006 | 0.3 | 0.31 (38.5) | — | ||||
| Women | 491 | 0.0 | — | ||||||
| Men | 298 | 0.7 | 0.31 (38.5) | — | |||||
| PCB-118c | 702 | 0.001 | 99.7 | 0.03 (4.0) | 0.06 (8.0) | 0.10 (14.8) | 0.89 (199.9) | 0.08 (13.2) | |
| Women | 402 | 99.5 | 0.03 (4.1) | 0.06 (7.9) | 0.11 (16.3) | 0.89 (199.9) | 0.10 (14.2) | ||
| Men | 300 | 100.0 | 0.003 (0.41) | 0.03 (4.0) | 0.05 (8.1) | 0.09 (13.5) | 0.72 (134.7) | 0.07 (11.8) | |
| PCB-138 | 801 | 0.001 | 100.0 | 0.01 (1.3) | 0.08 (12.3) | 0.19 (26.3) | 0.35 (53.5) | 2.58 (635.8) | 0.27 (42.4) |
| Women | 491 | 100.0 | 0.01 (1.3) | 0.08 (11.7) | 0.18 (24.7) | 0.35 (48.6) | 2.58 (635.8) | 0.26 (40.2) | |
| Men | 298 | 100.0 | 0.02 (2.6) | 0.10 (13.9) | 0.20 (29.0) | 0.36 (61.6) | 2.00 (295.7) | 0.27 (45.8) | |
| PCB-153 | 801 | 0.002 | 100.0 | 0.01 (1.59) | 0.10 (15.2) | 0.22 (31.3) | 0.39 (60.2) | 2.22 (486.3) | 0.29 (45.1) |
| Women | 491 | 100.0 | 0.01 (1.59) | 0.09 (13.7) | 0.20 (28.4) | 0.36 (53.6) | 1.97 (486.3) | 0.28 (41.2) | |
| Men | 298 | 100.0 | 0.02 (3.0) | 0.12 (17.2) | 0.24 (35.8) | 0.42 (72.0) | 2.22 (336.9) | 0.31 (51.3) | |
| PCB-180 | 801 | 0.001 | 100.0 | 0.01 (0.62) | 0.07 (10.5) | 0.16 (22.6) | 0.28 (41.6) | 4.92 (1076.3) | 0.21 (34.0) |
| Women | 491 | 100.0 | 0.01 (0.62) | 0.06 (9.28) | 0.14 (19.9) | 0.25 (34.7) | 1.40 (344.0) | 0.18 (27.3) | |
| Men | 298 | 100.0 | 0.01 (0.91) | 0.11 (14.7) | 0.20 (31.4) | 0.35 (59.5) | 4.92 (1076.3) | 0.26 (44.7) | |
| (di-ortho)d | 801 | — | — | 0.03 (3.7) | 0.27 (39.4) | 0.59 (82.7) | 1.03 (156.8) | 5.95 (1466.2) | 0.77 (121.5) |
| Women | 491 | 0.03 (3.7) | 0.22 (35.8) | 0.51 (74.7) | 0.97 (138.2) | 5.95 (1466.2) | 0.73 (108.8) | ||
| Men | 298 | 0.05 (7.0) | 0.31 (48.0) | 0.64 (97.3) | 1.13 (188.9) | 5.49 (1201.2) | 0.84 (141.7) |
Note: —, Data not calculable; LOD, limit of detection. Cohort of women ( samples, individuals) and men ( samples, individuals).
LOD ranges (nanogram/gram lipid): PBB-77: 0.3–5.7; PBB-101: 0.2–4.6; PBB-153: 0.1–1.1; PBB-180: 0.3–6.9; PCB-118: 0.1–1.1; PCB-138: 0.1–1.1; PCB-153: 0.1–2.3; PCB-180: 0.1–1.1.
Means only calculated for congeners where the majority of samples were .
PCB-118 was not reportable (missing) for 99 samples owing to unstable retention time on laboratory instrument; including all samples from 2004–2006.
.
Among the 753 participants, 17% () reported ever being diagnosed with thyroid disease (including 8 women and one man who reported a diagnosis before the contamination ceased in May 1974), and an additional 7 people (6 women, 1 man) had TSH and total or free outside of normal ranges, meeting the clinical definitions of hypothyroidism or hyperthyroidism (18% total) (Table 1). Two women who participated in 2004–06 and again in 2012–15 developed thyroid disease in the interval between phases and thus were included as both noncases and cases, respectively, in analyses. Women (vs. men); those older at interview (vs. younger); obese (vs. overweight or normal); and never or former smokers (vs. current smokers) were more likely to report thyroid disease or to be classified as having thyroid disease based on laboratory parameters. Those with thyroid disease had higher GM serum PBB-153 and PCB-118, 138, 153, and 180 concentrations (wet weight and nanograms/gram lipid) than those without thyroid disease.
Geometric mean serum PBB and PCB concentrations increased monotonically with age at the time of blood draw in the cohort as a whole (Table 3; see also Table S1) and in samples from those without thyroid disease who were tested for thyroid hormones (see Table S2). This correlation between age and exposure was also clear when comparing GM serum PBB-153 and PCB congener levels across strata of age at PBB contamination. However, although those born after the contamination incident had lower PBB-153 concentrations than those born before 1973, 77% were still above the LOD (compared with 99% and 100% of those ages 0–15 y and at the contamination, respectively). Men were more highly exposed to PBBs and to PCB-138, 153, and 180 than women, and this was particularly true for PBB-153 ( vs. ). There was no consistent pattern between PBB-153 and PCB congener concentrations and BMI categories.
Table 3.
Serum polybrominated biphenyl (PBB) and polychlorinated biphenyl (PCB) (nanograms/milliliter serum and nanograms/gram lipid) concentrations by population characteristics in Michigan adults, 2004–2015.
| Characteristics | PBB-153 (GM)a ng/mL (ng/g lipid) | PCB-118b (GM) ng/mL (ng/g lipid) | (di-ortho)c (GM) ng/mL (ng/g lipid) |
|---|---|---|---|
| Sex | |||
| Female | 0.14* (21.0)* | 0.06** (8.1) | 0.47* (68.6)* |
| Male | 0.24 (38.0) | 0.05 (7.8) | 0.58 (92.9) |
| Age (y) at blood drawd | |||
| 18–29 | 0.01* (1.9)* | 0.03* (4.7)* | 0.27* (44.2)* |
| 30–39 | 0.08 (13.3) | 0.03 (4.7) | 0.27 (43.9) |
| 40–49 | 0.25 (35.6) | 0.05 (6.9) | 0.46 (67.2) |
| 50–59 | 0.44 (62.1) | 0.06 (9.2) | 0.74 (106.2) |
| 0.53 (81.9) | 0.09 (14.2) | 1.03 (157.2) | |
| Age (y) at PBB contamination | |||
| at contamination | 0.51* (78.4)* | 0.09* (13.3)* | 0.97* (147.8)* |
| 0–15 at contamination | 0.40 (58.9) | 0.05 (7.8) | 0.49 (73.7) |
| Born after contamination | 0.02 (2.5) | 0.03 (4.7) | 0.27 (42.2) |
| Study yearsd | |||
| 2004–2006 | 0.99* (174.3)* | — | 0.29* (50.4)* |
| 2012–2015 | 0.14 (21.4) | 0.05 (7.9) | 0.54 (80.8) |
| Body mass indexd,e,f | |||
| 0.15 (21.8) | 0.04 (5.6)*** | 0.40 (60.3) | |
| Overweight | 0.12 (18.0) | 0.04 (6.2) | 0.42 (62.2) |
| Obese | 0.11 (17.6) | 0.04 (7.1) | 0.40 (62.6) |
| Smoking statuse,f | |||
| Current smokers | 0.09* (13.6)* | 0.04* (5.6)* | 0.43* (66.1)* |
| Former smokers | 0.28 (42.8) | 0.06 (9.0) | 0.64 (97.4) |
| Never smokers | 0.17 (26.4) | 0.06 (8.3) | 0.48 (73.1) |
Note: —, Data not applicable; GM, Geometric mean. Total: individuals, samples. [See Table S1 for information on individual PCB congeners (PCB-138, 153, and 180).] *; **; ***; p-values computed using Student’s t-tests or analysis of variance (ANOVA) using natural log–transformed PBB and PCB levels for comparison between categories.
GMs for PBB-153 computed after multiple imputation for observations .
PCB-118 not reportable for 99 (12.4%) of samples owing to unstable retention time on laboratory instrument; includes all samples from 2004–2006 (all women; ) and 20 samples from 2012–2015 (10 women, 10 men).
.
individuals participated twice, and individuals participated three times over time; thus, cell counts sum to and percents sum to .
missing BMI; missing smoking status.
Assessed at time of blood draw.
Associations between PBBs/PCBs and Thyroid Disease
Among those with thyroid disease (), the following types were specified [or were apparent based on thyroid assays ()]: 13 (9.6%) hyperthyroid (10 women, 3 men), 60 (44.4%) hypothyroid (52 women, 8 men), 15 (11.1%) other types (14 women, 1 man), and 47 (34.8%) not specified (37 women, 10 men). The average age at thyroid disease diagnosis was 35 (, ) for women and 42 (, ) for men. Among women, estimated adjusted odds ratios (aORs) for thyroid disease in association with IQR increases in ln-transformed serum PBB-153, PCB-118, and (di-ortho) concentrations were above the null [e.g., (95% CI: 0.83, 1.52) for a (IQR) increase in PBB-153 (Table 4), and in lipid-standardized models, (95% CI: 0.88, 1.59) for a lipid (IQR) increase in PBB-153 (see Table S3)]. Individual PCB congeners had similar estimates (see Tables S4 and S5) to that of their sum, (di-ortho) [e.g., for an IQR increase in PCB-153 (), (95% CI: 0.75, 1.64) (see Table S4); for an IQR increase in (di-ortho) (), (95% CI: 0.77, 1.68) (Table 4)]. When exposures were modeled as quintiles, aORs for PBB-153 quintiles 2 through 5 were all , although all estimates were imprecise (Table 4). The aOR for the fourth versus first PBB-153 quintile was statistically significant [ (95% CI: 1.1, 7.5)], but the OR for the fifth quintile [ (95% CI: 0.53, 3.94)] was closer to the null than the aOR for the second quintile. Corresponding aORs for lipid-adjusted PBB-153 (nanograms/gram lipid) were farther from the null but also imprecise (see Table S3). Thyroid disease in women did not appear to be associated with PCB congeners modeled as quintiles. Estimates for PBB-153 and hypothyroidism in women followed a pattern similar to those for all thyroid disease but were further from the null for the highest quintiles [ (95% CI: 1.08, 10.9) for the fourth quintile (14 cases) compared with the first (8 cases); (95% CI: 0.67, 7.23) for the fifth quintile (11 cases)] (Table 4). ORs for hypothyroidism in association with lipid-adjusted PBB-153 (nanograms/gram lipid) were consistent with those for wet-weight concentrations (see Table S3).
Table 4.
Adjusted odds ratios (aORs) and 95% confidence intervals (95% CIs) for the association between polybrominated biphenyl (PBB)-153, polychlorinated biphenyl (PCB)-118 and (nanograms/milliliter) and thyroid disease among Michigan females, 2004–2015.
| Exposure | Totalb | All thyroid diseasea | Hypothyroidism | ||
|---|---|---|---|---|---|
| (%)c | aOR (95% CI) | (%)d | aOR (95% CI) | ||
| PBB-153 | |||||
| PBB-153 ln-transformed ()e,f | 447 | 105 (23.5) | 1.12 (0.83, 1.52) | 49 (11.8) | 1.35 (0.86, 2.13) |
| PBB-153 Q1 () | 100 | 12 (12.0) | 1.00 (Reference) | 8 (8.0) | 1.00 (Reference) |
| PBB-153 Q2 () | 91 | 22 (24.2) | 1.53 (0.63, 3.76) | 9 (10.6) | 1.22 (0.37, 4.03) |
| PBB-153 Q3 () | 88 | 23 (26.1) | 1.30 (0.49, 3.45) | 8 (10.3) | 1.08 (0.32, 3.64) |
| PBB-153 Q4 () | 92 | 31 (33.7) | 2.89 (1.11, 7.49) | 14 (17.1) | 3.43 (1.08, 10.90) |
| PBB-153 Q5 () | 87 | 19 (21.8) | 1.44 (0.53, 3.94) | 11 (13.6) | 2.19 (0.67, 7.23) |
| PCB-118 | |||||
| PCB-118 ln-transformed ()e | 378 | 107 (28.3) | 1.20 (0.89, 1.63) | 48 (14.1) | 1.39 (0.90, 2.13) |
| PCB-118 Q1 () | 80 | 15 (18.8) | 1.00 (Reference) | 10 (12.7) | 1.00 (Reference) |
| PCB-118 Q2 () | 79 | 20 (25.3) | 1.13 (0.48, 2.66) | 9 (11.8) | 0.72 (0.25, 2.05) |
| PCB-118 Q3 () | 79 | 19 (24.1) | 0.95 (0.40, 2.25) | 9 (12.5) | 0.69 (0.26, 1.87) |
| PCB-118 Q4 ) | 80 | 25 (31.3) | 1.19 (0.52, 2.73) | 11 (15.9) | 0.98 (0.37, 2.62) |
| PCB-118 Q5 () | 75 | 31 (41.3) | 1.39 (0.59, 3.27) | 11 (18.3) | 1.59 (0.57, 4.43) |
| (di-ortho)g | |||||
| ln-transformed ()e | 455 | 113 (24.8) | 1.13 (0.77, 1.68) | 52 (12.4) | 1.46 (0.89, 2.40) |
| Q1 () | 96 | 16 (16.7) | 1.00 (Reference) | 10 (10.4) | 1.00 (Reference) |
| Q2 () | 94 | 15 (16.0) | 0.77 (0.33, 1.80) | 5 (5.5) | 0.37 (0.11, 1.32) |
| Q3 () | 97 | 26 (26.8) | 1.02 (0.46, 2.25) | 15 (16.9) | 1.20 (0.49, 2.94) |
| Q4 () | 96 | 25 (26.0) | 0.80 (0.36, 1.80) | 9 (10.6) | 0.59 (0.22, 1.57) |
| Q5 () | 95 | 36 (37.9) | 1.22 (0.56, 2.68) | 16 (20.0) | 1.70 (0.69, 4.15) |
Note: IQR, interquartile range; LOD, limit of detection; Q1–Q5, quintiles 1 through 5. Models for PBB-153 and (di-ortho) control for lipids, age, study phase, and smoking status; models for PCB-118 do not control for study phase because all samples from Phase 1 were not reportable owing to instrumentation error [see Tables S4 and S5 for information on individual PCB congeners (PCB-138, 153, and 180).]
Includes those who specified physician-diagnosed thyroid disease () and those who had thyroid hormone concentrations outside clinically normal ranges (); includes 10 hyperthyroid, 52 hypothyroid, 14 other types, and 37 type not specified.
PBB-153 analyses exclude 8 women who were diagnosed with thyroid disease before the contamination event; PCB-118 analyses exclude 88 women (89 samples) who had missing values owing to unstable retention time on laboratory instrument; (di-ortho) analyses include all observations.
30 women participated multiple times, so cell counts may not sum to total.
Hypothyroidism analyses exclude 37 observations owing to lack of information on thyroid disease type (32 in PBB-153 model); these are excluded from denominator of percent calculations.
All IQRs and quintiles calculated among women only.
PBB-153 concentrations below the LOD were imputed using a distribution-based multiple imputation approach.
.
Men were much less likely to report being diagnosed with thyroid disease (, 7.4%) including 1 case diagnosed before the PBB contamination incident and 1 case classified based on thyroid hormone levels versus a self-reported diagnosis. Estimated aORs for thyroid disease in association with IQR increases in wet-weight serum PBB and PCB concentrations were imprecise, with inverse associations estimated for PBB-153 [for an IQR increase (), (95% CI: 0.3, 1.4)] and (di-ortho) [for an IQR increase (), (95% CI: 0.4, 2.1)], and a positive association with PCB-118 [for an IQR increase (), (95% CI: 0.9, 2.5)] (Table 5).
Table 5.
Adjusted odds ratios (aORs) and 95% confidence intervals (95% CIs) for the association between polybrominated biphenyl (PBB)-153, polychlorinated biphenyl (PCB)-118, PCB-138, PCB-153, PCB-180, and (nanograms/milliliter) and thyroid disease among Michigan males, 2004–2015.
| Exposure | All thyroid diseasea | ||
|---|---|---|---|
| Totalb | (%)c | aOR (95% CI) | |
| PBB-153d | |||
| PBB-153 ln-transformed () | 297 | 21 (7.1%) | 0.69 (0.33, 1.44) |
| PCB-118 | |||
| PCB-118 ln-transformed () | 291 | 21 (7.2%) | 1.50 (0.89, 2.53) |
| PCB-138 | |||
| PCB-138 ln-transformed () | 298 | 22 (7.4%) | 1.07 (0.52, 2.20) |
| PCB-153 | |||
| PCB-153 ln-transformed () | 298 | 22 (7.4%) | 0.98 (0.44, 2.20) |
| PCB-180 | |||
| PCB-180 ln-transformed () | 298 | 22 (7.4%) | 0.81 (0.36, 1.83) |
| (di-ortho)e | |||
| ln-transformed () | 298 | 22 (7.4%) | 0.93 (0.41, 2.11) |
Note: IQR, interquartile range. All models control for lipids, age, and smoking status. Estimates are reported for natural log–transformed continuous exposures only owing to small numbers of cases in men.
Includes those who specified physician-diagnosed thyroid disease () and those who had thyroid hormone concentrations outside clinically normal ranges (); includes 3 hyperthyroid, 8 hypothyroid, 1 other type and 10 type not specified.
PBB-153 analyses excludes 1 man who was diagnosed with hypothyroidism before the contamination event; PCB-118 analyses exclude 10 men that had missing values owing to unstable retention time on laboratory instrument, (di-ortho) analyses include all observations.
11 men participated multiple times, so cell counts may not sum to total.
PBB-153 concentrations below the LOD were imputed using a distribution-based multiple imputation approach.
.
Thyroid Hormones
Among 551 participants who did not report thyroid disease at the time of their blood draw and were not classified as having thyroid disease based on thyroid hormone levels, the mean total and free decreased with age, and GM TSH concentrations were higher in samples collected in the morning versus afternoon or evening (see Table S6). In addition, among those for whom urinary iodine was measured, TSH and total concentrations were greater among those who were iodine deficient ( creatinine), although differences were minimal across strata of low iodine ( creatinine). Lastly, among women, those who reported hormonal medication use and the few who were pregnant at the time of the blood draw had greater total and concentrations.
Associations between PBBs/PCBs and Thyroid Hormones
In linear regression models with the exposures expressed as natural log–transformed continuous variables, PCB congeners (and not PBB-153) were associated with modest differences in various hormones (Table 6). Among women, PCBs (modeled on either the volume or the lipid basis) were associated with higher total and free and total concentrations (Table 6; see also Table S7) [i.e., a natural log–unit increase in (di-ortho) was associated with greater total (95% CI: , 0.35), greater free (95% CI: 0.01, 0.04), and greater total (95% CI: 0.53, 7.40)]. The estimates for PCB-118 were greater than those for the other measured PCB congeners in relation to differences in total and concentrations [a natural log–unit increase in PCB-118 was associated with (95% CI: 0.04, 0.43) greater total and (95% CI: 2.05, 7.83) greater total ]. Among men, PCB congeners were associated with greater total and free , although estimated differences were larger and were only statistically significant on the lipid basis (Table 6; see also Table S7). Overall, all estimates corresponded to very small relative differences (1–5%) when considered in conjunction with the distribution of circulating levels of each thyroid hormone (estimated for total and free and from Table S8 by dividing each beta coefficient by its corresponding median thyroid hormone value).
Table 6.
and 95% confidence intervals (95% CI) from regression models for associations of ln-transformed polybrominated biphenyl (PBB) and polychlorinated biphenyl (PCB) congeners (nanograms/milliliter) with thyroid hormones among Michigan adults without thyroid disease ( individuals, 571 samples), 2004–2015.
| Exposure | ln TSH ()a | Total ()b | Total (ng/dL)b | Free (ng/dL)b | Free (pg/mL)b | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Women (95% CI) | Men (95% CI) | Women (95% CI) | Men (95% CI) | Women (95% CI) | Men (95% CI) | Women (95% CI) | Men (95% CI) | Women (95% CI) | Men (95% CI) | |
| PBB-153c | 0.00 (, 0.02) | (, 0.05) | 0.02 (, 0.10) | (, 0.15) | 1.08 (, 2.41) | (, 1.89) | 0.00 (, 0.00) | 0.00 (, 0.01) | 0.04 (0.01, 0.06) | (, 0.04) |
| PCB-118 | 0.03 (, 0.09) | (, 0.08) | 0.23 (0.04, 0.43) | (, 0.08) | 4.94 (2.05, 7.83) | 0.54 (, 4.89) | 0.02 (0.01, 0.04) | 0.00 (, 0.02) | 0.03 (, 0.07) | 0.03 (, 0.12) |
| PCB-138 | (, 0.04) | 0.00 (, 0.10) | 0.16 (, 0.33) | (, 0.10) | 3.61 (0.41, 6.81) | 1.17 (, 5.26) | 0.03 (0.01, 0.04) | 0.00 (, 0.02) | 0.00 (, 0.05) | 0.01 (, 0.09) |
| PCB-153 | (, 0.04) | (, 0.10) | 0.16 (, 0.35) | (, 0.13) | 4.21 (0.78, 7.63) | 1.15 (, 5.65) | 0.03 (0.01, 0.04) | 0.00 (, 0.03) | 0.01 (, 0.06) | 0.02 (, 0.12) |
| PCB-180 | (, 0.04) | 0.03 (, 0.15) | 0.14 (, 0.34) | (, 0.17) | 3.65 (0.23, 7.07) | 1.41 (, 6.39) | 0.02 (0.01, 0.04) | 0.00 (, 0.02) | 0.02 (, 0.07) | 0.03 (, 0.13) |
| (di-ortho)d | (, 0.04) | 0.00 (, 0.11) | 0.16 (, 0.35) | (, 0.13) | 3.97 (0.53, 7.40) | 1.53 (, 6.14) | 0.03 (0.01, 0.04) | 0.00 (, 0.03) | 0.01 (, 0.06) | 0.02 (, 0.12) |
Note: , triiodothyronine; , thyroxine; TSH, thyroid-stimulating hormone; x is a variable. Models fit among 343 women (363 samples) for all hormones except total , for which one woman was missing owing to insufficient serum volume [total models fit among 342 women (362 samples)] and among 208 men (208 samples). All models controlled for lipids, age at blood draw (indicator variables for each decade), time of blood collection (indicator variables for morning, midday, afternoon/evening), current smoking status (yes/no).
TSH is ln-transformed; thus, should be interpreted as follows: a natural log–unit increase in a given congener is associated with a multiplicative change in TSH of . In terms of changes in PBB and PCB levels (not logged), an x% change in a given congener is associated with a multiplicative change in TSH of .
Other thyroid hormones are not transformed; thus, should be interpreted as follows: a natural log–unit increase in a given PBB or PCB congener is associated with an additive change in each hormone of . In terms of changes in PBB and PCB levels (not logged), a x% change in a given congener is associated with an additive change in each hormone of .
PBB-153 measures below the LOD were multiply imputed () based on a lognormal probability distribution whose parameters were determined by maximum likelihood estimation.
.
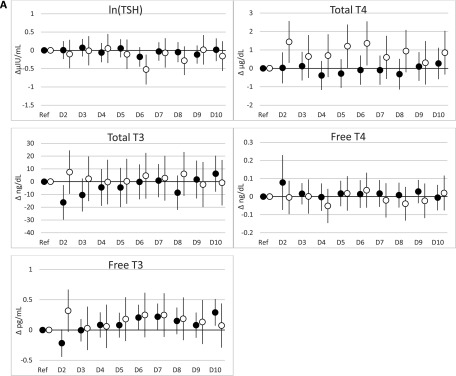
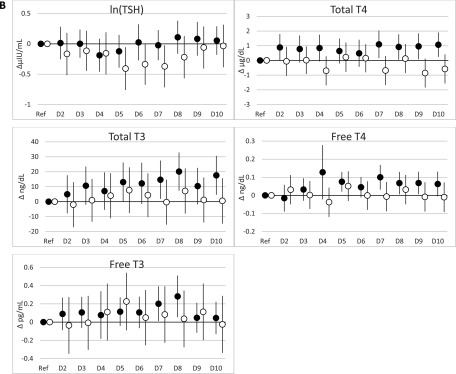
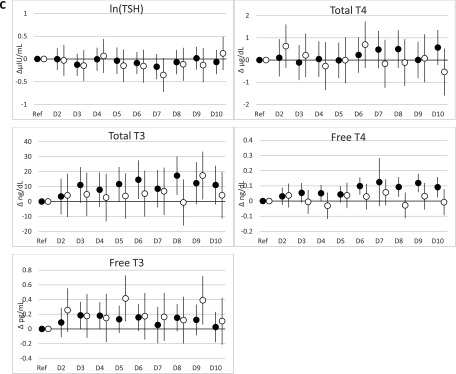
When PBB-153 and PCBs were expressed as deciles, the results were largely consistent with the results of the continuous natural log–transformed analyses (Figure 1; see also Tables S9–S12). Among women, the positive associations between PCBs and total and free and largely remained; again, estimates were largest for PCB-118 (see Tables S9 and S11). For PCB-118 and total among women, estimates increased from the first to the second decile and subsequently leveled off without further increases in the upper deciles, whereas estimates for total continued to increase throughout the upper deciles. However, among men, there was some evidence for a positive association between PBB-153 and total concentrations, which was not observed in analyses with PBB-153 expressed as a natural log–transformed continuous variable, although estimates for the highest deciles were close to the null (see Table S10).
Figure 1.
and 95% confidence intervals (95% CIs) from regression models for associations of PBB-153, PCB-118, and (di-ortho) deciles (nanograms/milliliter) with thyroid hormones stratified by sex. (A) PBB-153; (B) PCB-118; (C) (di-ortho). Note: D2–10, deciles 2–10; Ref, reference (decile 1); T4, thyroxine; T3, triiodothyronine; TSH: thyroid-stimulating hormone. All models adjusted for lipids, age, time of blood collection, and current smoking status (See S9 and S10 for and 95% CIs.) Sum of PCB 138, 153, and 180. (See the footnotes of Tables S9 and S10 for interpretations of .) Black circles denote women, and white circles denote men.
Associations between PBBs/PCBs and Thyroid Hormones Stratified by Iodine Status
Finally, in the women with urinary iodine measurements (), 18% () were iodine deficient ( creatinine), and 54% () had low iodine ( creatinine) (see Table S4). Heterogeneity in the estimates was detected based on urinary iodine concentrations creatinine (Table 7). (di-ortho) was positively associated with TSH concentrations among 43 women classified as having low urinary iodine ( creatinine): a 24.6% increase in TSH associated with a natural log–unit increase in (95% CI: 3.0, 49.2%); in contrast, among the 37 women classified as having sufficient iodine ( creatinine), was inversely associated with TSH [ (95% CI: , ) for a natural log–unit increase in ; interaction )]. The pattern of associations was similar for PCB-118, but stratum-specific estimates were closer to the null and were not significantly different from each other (interaction ).
Table 7.
Associations between ln-transformed polybrominated biphenyl (PBB)-153, polychlorinated biphenyl (PCB)-118, and (di-ortho) (nanograms/milliliter) and ln[thyroid stimulating hormone(TSH)] stratified by iodine sufficiency status
| Exposure | Cr () (95% CI) | Cr () (95% CI) | p-Valuea |
|---|---|---|---|
| PBB-153 | 0.01 (, 0.06) | 0.04 (, 0.15) | 0.90 |
| PCB-118b | 0.16 (, 0.32) | (, 0.08) | 0.32 |
| (di-ortho)c | 0.22 (0.03, 0.40) | (, ) |
Note: 95% CI, 95% confidence interval; x is a variable. One woman provided urinary data at two time points such that this model was fit among 79 women and 80 observations. All models control for lipids, age, time of blood collection, and smoking status. TSH is ln-transformed; thus, should be interpreted as follows: a natural log–unit increase in a given congener is associated with a multiplicative change in TSH of . In terms of changes in PBB and PCB levels (not logged), a x% change in a given congener is associated with a multiplicative change in TSH of .
p-Value for the interaction from cross-product term in linear model.
observations missing for PCB-118 owing to instrument error [model fit among 50 women: (64%) with iodine concentrations creatinine and (36%) with creatinine].
.
Discussion
In this study of 753 adults from a cohort historically exposed to PBBs, thyroid disease was prevalent (18.0%), particularly among women (24.8%). Among women, there was some evidence of a positive association between serum PBB-153 concentrations and thyroid disease, specifically hypothyroidism, although the association did not increase with increasing exposure, and estimates were imprecise. Serum levels of PCBs but not of PBB-153 were associated with subtle differences in thyroid hormone concentrations, which were generally stronger among women.
Compared with NHANES data from 2003–2004, this cohort had substantially higher exposure to PBB-153, with 59% having levels above the national 95th percentile (; lipid) (CDC 2017). In contrast, the distribution of PCB exposure was more similar to national levels (median PCB-153 and and lipid, respectively) (CDC 2017). Theoretically, this observation could be consistent with the observed burden of thyroid disease among women in this population (24.8%), which was somewhat greater than expected (18.2%) based on NHANES data from 2013–2014 (CDC 2014). Age-adjusting the prevalence estimate in our study population using 2014 Census data (U.S. Census Bureau Population Division 2014) did not result in a meaningful change (25.7%).
A substantial body of literature supports an association between PCB exposure and decreased total concentrations in experimental animal models (Brouwer et al. 1998, 1999). In addition, many epidemiologic studies among adults exposed to PCBs have reported lower levels of total in relation to exposure, although the literature is inconsistent overall (Hagmar 2003; Salay and Garabrant 2009). However, we found evidence of a positive association between PCB congeners 118, 138, 153, and 180 and several thyroid hormone concentrations (total and free and ) among women and increases in total and free (when analyzed on the lipid basis) among men. This finding could be caused by random error given the intraindividual variability in thyroid hormones (Andersen et al. 2002), by uncontrolled bias from several sources; or by the selection of a less susceptible subpopulation owing to the substantial burden of thyroid disease in this population and because we could only assess potential impacts on thyroid hormones among those without a history of thyroid disease. Another possibility is that the relationships between PCBs and thyroid hormones may vary by PBB levels. One study among 2,046 adults from a polluted area in eastern Slovakia found negative associations between serum PCBs and total and free at lower PCB exposure levels ( lipid), but positive associations were observed at greater exposure levels (Langer et al. 2007b). In our study, we did not have strong evidence that this was the case by PCB or PBB levels, although our high PBB levels overall may have impeded our ability to detect this.
We found evidence of a positive association between serum PBB-153 and thyroid disease that is consistent with several recent studies on the similarly structured PCBs and PBDEs (Allen et al. 2016; Langer et al. 2007a, 2007c; Oulhote et al. 2016; Turyk et al. 2008). However, two previous studies, which both included selected members from the Michigan registry, investigated PBB exposure in relation to thyroid disease (Bahn et al. 1980; Yard et al. 2011) and had mixed results. Bahn et al. (1980) studied men who were occupationally exposed to PBBs and found an increased prevalence of hypothyroidism and elevated antibodies (4 cases out of 35) compared with unexposed workers (0 cases out of 89). However, PBBs were not measured; instead, the authors compared workers from a plant that produced PBBs with the control group, which was made up of unexposed workers from other industries (e.g., steelworkers and wiremen) and community volunteers. In the other study, Yard et al. (2011) examined PBB and PCB levels in relation to thyroid disease incidence among women and men from the original Michigan registry and reported no significant associations or suggestive patterns, although they did observe an elevated OR for thyroid disease among men in the highest tertile only [ (95% CI: 0.50, 6.15) compared with those )] (Yard et al. 2011). That study was conducted as a nested case–control study among members of the original Michigan registry who were born before 1 July 1973 and thus exposed to PBBs through consumption of contaminated foods. In contrast to these two studies, we found some evidence to suggest increased odds of thyroid disease in relation to current serum levels of PBB-153 among women. There are many possible reasons for these discrepant findings. First, the continued follow-up on this cohort allows for increased opportunity for manifestation of thyroid disease. In the Yard et al. (2011) study, the average time since the PBB contamination (follow-up time) was 22 y, in contrast to our 39 y. In addition, Yard and colleagues studied women and men who were 25 y old and 27 y old on average at PBB exposure, respectively. In the present study, among those born before the contamination incident, the average age at exposure was 15 (13 for women and 19 for men), and we included those born after the incident as well. Therefore, it may be possible that our study population was more vulnerable to thyroid disruption given the earlier age at initial PBB exposure. Another important difference is the exposure assessment methodology. The Yard et al. (2011) study used enrollment PBB and PCB levels, which were measured in the early 1970s soon after the contamination episode, whereas we were able to quantify individual PBB and PCB congeners with increased sensitivity particularly at low levels owing to a detection limit three orders of magnitude lower than in older methods (Needham et al. 1981). Finally, we also measured thyroid hormones, which allowed us to include those with undiagnosed thyroid diseases () in addition to those identified by self-report, whereas Yard et al. (2011) included self-reported cases only.
One alternative explanation for the observed association between PBBs and thyroid disease among women is that exposure and disease may both be related to participation, thereby potentially inducing an association (Hernán et al. 2004). Because this cohort was historically exposed to PBBs and many had received information about their PBB levels in the past, more highly exposed individuals may have been more motivated to participate in this study. Additionally, individuals with diagnosed health conditions may have been more likely to participate. If both of these factors affected participation, an association between PBBs and disease might be expected.
A second possible explanation for our findings is that thyroid function may influence serum PBB and PCB levels. Although these chemicals are long-lived in human tissue, the most biologically relevant exposure parameter for making causal inference would be at some point before the disease process started. In our study, we measured PBBs and PCBs after disease diagnosis, and because the thyroid regulates metabolism, including actions that metabolize halogenated compounds (Park et al. 2012; Safe 1994; Takahashi et al. 2010), it may be plausible that disease status could affect serum levels.
Our finding that the association between PCBs and TSH varied by iodine status should be interpreted with caution due to the small number of women for whom we had measured urinary iodine. However, similar observations have been reported in several studies with other environmental toxicants (Blount et al. 2006; Jain 2014; Romano et al. 2015). Results from experimental studies have suggested that PBB and iodine may act synergistically on thyroid function, which could be due to inhibition of the incorporation of iodine into thyroidal amino acids (e.g., monoiodotyrosine), which are precursors of thyroid hormones (Allen-Rowlands et al. 1981; Sleight et al. 1978). In humans, the recently proposed “multiple-hit hypothesis” suggests that individuals with multiple thyroid “stressors” may be particularly susceptible to environmental thyroid disruption (Webster et al. 2014, 2015). Although we detected a difference in estimates across strata based on creatinine [rather than the WHO (1994) iodine deficiency cut-off of creatinine], this is still considered to be low iodine status (Caldwell et al. 2011) and may result in thyroidal stress.
In studies involving lipophilic chemicals, there has been considerable debate about how to statistically handle serum lipids (Chevrier 2013; O'Brien et al. 2015; Schisterman et al. 2005). Lipophilic compounds, such as PCBs, expressed on the lipid basis (nanograms/gram lipid) are correlated with the amount stored in body fat (Brown and Lawton 1984; Hirai et al. 2012), and because PCBs accumulate in lipids, this may be the most biologically relevant parameter for adjusting for lipids. However, this method mathematically constrains the form of lipids to be in the denominator of the PCB term (as nanograms/gram lipid) (Schisterman et al. 2005). In contrast, expressing PCBs on the wet weight (volume) basis (nanograms/milliliter serum) and controlling for lipids as a covariate allows for more flexible modeling and still accounts for the variability in serum lipid concentrations (Schisterman et al. 2005). Schisterman et al. (2005) showed through simulations that expressing compounds on the lipid basis induced more bias, consistently in the negative direction, than controlling for lipids as a covariate across various possible causal scenarios. Based on these findings, in our primary analysis, we considered PBBs and PCBs on the volume basis and controlled for lipids as a covariate, although we also performed all analyses on the lipid basis as well. More recently, O’Brien et al. (2015) published a simulation study similar to that of Schisterman et al. (2005) that concluded that modeling PCBs on the lipid basis incurred less bias than other methods and that adjusting for lipids as a covariate tended to bias effect estimates away from the null. In our study, we observed some differences in our results across these two methods. Expressing PBB-153 and PCBs on the lipid basis resulted in associations with thyroid disease that were mostly up and away from the null compared with results on the volume basis. Similarly, among men, we observed larger differences (more positive) in total and free in relation to PCB congeners when expressed on the lipid basis than when expressed on the volume basis. These results are contrary to what may have been expected based on both the Schisterman and O’Brien studies and could be due to chance or to differences between the causal scenarios presented in these papers and the underlying causal relationships in our study. More research is needed to understand whether lipid-standardized PCB levels truly reflect adipose tissue levels or body burden as well as how accounting for lipids may affect epidemiological analyses.
This study benefited from several strengths. First, we were able to measure several important biomarkers, including a full thyroid panel. We observed greater TSH concentrations among blood samples collected in the morning, which is consistent with its known diurnal pattern (Braverman and Cooper 2012). In addition, we also observed greater total and concentrations among women who reported current hormonal medication use or were pregnant, consistent with estrogen-induced increased sialylation of thyroxine-binding globulin (TBG), which leads to greater concentrations of TBG and thyroid hormones (Surks and Sievert 1995). These observations for multiple hormones support the validity of our thyroid hormone measurements. We also measured levels of several PBB and PCB congeners and lipids in serum as well as in a subset; iodine concentrations in longitudinally collected and pooled urinary samples. Although the subset with urinary iodine measurements was small, the design of pooling samples offers a methodological advantage over other studies that have used spot urine samples due to the reduction in the variability in iodine measurements for each individual (König et al. 2011). In addition, we had long-term follow-up on a cohort that was historically exposed to a flame-retardant mixture, which is significant for two reasons. First, it allowed for increased time for health conditions to manifest, including for those in a subsequent generation. Second, environmental PBB exposure occurred for a fixed and limited time, which allows us to infer that external exposure occurred before the outcome(s) of interest even though we assessed blood levels of PBBs and thyroid hormones at the same time, and because of its persistence, we were unable to infer when the potentially biologically relevant exposure may have occurred.
Our study was also subject to several limitations. First, a large proportion of participants (35%) who reported a history of thyroid disease did not specify what type they had been diagnosed with, which led to a loss of power and statistical precision when attempting to analyze specific thyroid disease subtypes such as hypothyroidism. Having access to medical records as opposed to our assessment of self-reported physician-diagnosed thyroid disease would have improved our ability to test associations with these different subtypes and would have allowed us to confirm self-reported diagnoses. Additionally, given that thyroid function comprises a variety of hormonal biomarkers, it is difficult to discern between potentially important hormonal changes and the influence of random error, multiple testing, or uncontrolled bias. Lastly, our study was limited by small sample size, particularly for our assessment of thyroid disease, a heterogeneous outcome, in relation to PBBs and PCBs. Nevertheless, we note that this is still one of the largest studies to date on these exposures and thyroid function.
Although PBBs and PCBs are no longer in production, exposure to PCBs remains widespread in human serum owing to their long biologic half-lives as well as to their persistence in the food chain (Sjödin et al. 2014; Wattigney et al. 2015; Xue et al. 2014). Furthermore, they share structural similarities with their replacements such as PBDEs and with newer flame retardants currently being produced, such as tetrabromobisphenol A and hexabromocyclododecane (Birnbaum and Staskal 2004). This makes the study of these older chemicals still relevant and important, as they may serve as a model for exposure to other persistent lipophilic halogenated compounds.
Conclusions
In this cohort of individuals historically exposed to PBBs, we found continued evidence of elevated levels of exposure to PBB-153 compared with national estimates, as well as a higher than expected prevalence of self-reported thyroid disease, particularly in women. We found some evidence to suggest that PBB-153 concentrations were associated with thyroid disease among women and possibly with hypothyroidism specifically. Lastly, modest thyroid hormone differences were observed in relation to serum PCB concentrations, and there was a suggestion that this may have varied by iodine status. Many decades after an acute exposure to flame retardants, this population remains highly exposed to PBBs, which may have contributed to the prevalence of thyroid disease.
Supplemental Material
Acknowledgments
The authors would like to thank the following parties for their generous support and assistance with laboratory assays: J. Kesner, D. Jones, B. Liang, D. Walker, and D. Cobb. Funding was provided by the National Institute of Environmental Health Sciences/National Institutes of Health (NIEHS/NIH) (grants R01ES012014, P30ES019776, R21ES023927, R01ES024790, and R01ES08341), the U.S. Environmental Protection Agency (EPA) (agreement number R825300), the Eunice Kennedy Shriver National Institute of Child Health and Human Development Reproductive, Perinatal, & Pediatric Training Grant (T32HD052460), and the National Institute for Occupational Safety and Health (NIOSH) Environmental and Occupational Epidemiology Training Grant (5T03OH008609-10). This work was additionally supported by the Laney Graduate School and by the Livingston Fellowship at Emory University.
References
- Adlersberg MA, Burrow GN. 2002. Focus on primary care. Thyroid function and dysfunction in women. Obstet Gynecol Surv 57(3 Suppl):S1–S7, PMID: 12074547, 10.1097/00006254-200203001-00001. [DOI] [PubMed] [Google Scholar]
- Allen JG, Gale S, Zoeller RT, Spengler JD, Birnbaum L, McNeely E. 2016. PBDE flame retardants, thyroid disease, and menopausal status in U.S. women. Environ Health 15(1):60, PMID: 27215290, 10.1186/s12940-016-0141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen-Rowlands CF, Castracane VD, Hamilton MG, Seifter J. 1981. Effect of polybrominated biphenyls (PBB) on the pituitary–thyroid axis of the rat. Proc Soc Exp Biol Med 166(4):506–514, PMID: 6261263, 10.3181/00379727-166-41099. [DOI] [PubMed] [Google Scholar]
- Andersen S, Karmisholt J, Pedersen KM, Laurberg P. 2008. Reliability of studies of iodine intake and recommendations for number of samples in groups and in individuals. Br J Nutr 99(4):813–818, PMID: 17961291, 10.1017/S0007114507842292. [DOI] [PubMed] [Google Scholar]
- Andersen S, Pedersen KM, Bruun NH, Laurberg P. 2002. Narrow individual variations in serum T4 and T3 in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab 87(3):1068–1072, 10.1210/jcem.87.3.8165. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). 2000. Toxicological Profile for Polychlorinated Biphenyls (PCBs). Atlanta, GA:U.S. Department of Health and Human Services, Public Health Service, ATSDR. [PubMed] [Google Scholar]
- ATSDR. 2004. Toxicological Profile for Polybrominated Biphenyls and Polybrominated Diphenyl Ethers (PBBs and PBDEs). Atlanta, GA:U.S. Department of Health and Human Services, Public Health Service, ATSDR. [Google Scholar]
- Bahn AK, Mills JL, Snyder PJ, Gann PH, Houten L, Bialik O, et al. 1980. Hypothyroidism in workers exposed to polybrominated biphenyls. N Engl J Med 302(1):31–33, PMID: 6243165, 10.1056/NEJM198001033020105. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Staskal DF. 2004. Brominated flame retardants: cause for concern? Environ Health Perspect 112(1):9–17, PMID: 14698924, 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanck HM, Marcus M, Tolbert PE, Rubin C, Henderson AK, Hertzberg VS, et al. 2000. Age at menarche and Tanner stage in girls exposed in utero and postnatally to polybrominated biphenyl. Epidemiology 11(6):641–647, PMID: 11055623, 10.1097/00001648-200011000-00005. [DOI] [PubMed] [Google Scholar]
- Blount BC, Pirkle JL, Osterloh JD, Valentin-Blasini L, Caldwell KL. 2006. Urinary perchlorate and thyroid hormone levels in adolescent and adult men and women living in the United States. Environ Health Perspect 114(12):1865–1871, PMID: 17185277, 10.1289/ehp.9466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman LE, Cooper D. 2012. Werner & Ingbar's The Thyroid: A Fundamental and Clinical Text, 10th edition. Philadelphia, PA:Lippincott Williams & Wilkins. [Google Scholar]
- Brilliant L, Van Amburg G, Isbister J, Humphrey H, Wilcox K, Eyster J, et al. 1978. Breast-milk monitoring to measure michigan's contamination with polybrominated biphenyls. Lancet 2(8091):643–646, PMID: 80575, 10.1016/S0140-6736(78)92758-7. [DOI] [PubMed] [Google Scholar]
- Brouwer A, Longnecker MP, Birnbaum LS, Cogliano J, Kostyniak P, Moore J, et al. 1999. Characterization of potential endocrine-related health effects at low-dose levels of exposure to PCBs. Environ Health Perspect 107 (Suppl 4):639–649, PMID: 10421775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer A, Morse DC, Lans MC, Schuur AG, Murk AJ, Klasson-Wehler E, et al. 1998. Interactions of persistent environmental organohalogens with the thyroid hormone system: Mechanisms and possible consequences for animal and human health. Toxicol Ind Health 14(1–2):59–84, PMID: 9460170, 10.1177/074823379801400107. [DOI] [PubMed] [Google Scholar]
- Brown JF Jr, Lawton RW. 1984. Polychlorinated biphenyl (PCB) partitioning between adipose tissue and serum. Bull Environ Contam Toxicol 33(3):277–280, 10.1007/BF01625543. [DOI] [PubMed] [Google Scholar]
- Byrne JJ, Carbone JP, Hanson EA. 1987. Hypothyroidism and abnormalities in the kinetics of thyroid hormone metabolism in rats treated chronically with polychlorinated biphenyl and polybrominated biphenyl. Endocrinology 121(2):520–527, PMID: 3036477, 10.1210/endo-121-2-520. [DOI] [PubMed] [Google Scholar]
- Caldwell KL, Makhmudov A, Ely E, Jones RL, Wang RY. 2011. Iodine status of the U.S. population, National Health and Nutrition Examination Survey, 2005–2006 and 2007–2008. Thyroid 21(4):419–427, PMID: 21323596, 10.1089/thy.2010.0077. [DOI] [PubMed] [Google Scholar]
- Carter LJ. 1976. Michigan's PBB incident: Chemical mix-up leads to disaster. Science 192(4236):240–243, PMID: 17831151, 10.1126/science.192.4236.240. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2004. National Health and Nutrition Examination Survey: NHANES 2003–2004 Laboratory Data. https://wwwn.cdc.gov/nchs/nhanes/search/DataPage.aspx?Component=Laboratory&CycleBeginYear=2003 [accessed 15 August 2016].
- CDC. 2014. National HEALTH and Nutrition Examination Survey: NHANES 2014 Questionnaire Data. https://wwwn.cdc.gov/Nchs/Nhanes/Search/DataPage.aspx?Component=Questionnaire&CycleBeginYear=2013 [accessed 15 August 2016].
- CDC. 2017. Fourth national report on human exposure to environmental chemicals, updated tables (Volume 2). https://www.cdc.gov/exposurereport/index.html [accessed 15 June 2017].
- Chen H, Quandt SA, Grzywacz JG, Arcury TA. 2011. A distribution-based multiple imputation method for handling bivariate pesticide data with values below the limit of detection. Environ Health Perspect 119(3):351–356, PMID: 21097385, 10.1289/ehp.1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier J. 2013. Invited commentary: maternal plasma polybrominated diphenyl ethers and thyroid hormones–challenges and opportunities. Am J Epidemiol 178(5):714–719, PMID: 23924577, 10.1093/aje/kwt138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale J, Daykin J, Holder R, Sheppard MC, Franklyn JA. 2001. Weight gain following treatment of hyperthyroidism. Clin Endocrinol (Oxf) 55(2):233–239, PMID: 11531931, 10.1046/j.1365-2265.2001.01329.x. [DOI] [PubMed] [Google Scholar]
- Davis SI, Blanck HM, Hertzberg VS, Tolbert PE, Rubin C, Cameron LL, et al. 2005. Menstrual function among women exposed to polybrominated biphenyls: A follow-up prevalence study. Environ Health 4:15, PMID: 16091135, 10.1186/1476-069X-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers LM, Spencer CA. 2003. Laboratory medicine practice guidelines: laboratory support for the diagnosis and monitoring of thyroid disease. Clin Endocrinol (Oxf) 58(2):138–140, PMID: 12580927. [DOI] [PubMed] [Google Scholar]
- Doufas AG, Mastorakos G. 2000. The hypothalamic-pituitary-thyroid axis and the female reproductive system. Ann NY Acad Sci 900:65–76, PMID: 10818393, 10.1111/j.1749-6632.2000.tb06217.x. [DOI] [PubMed] [Google Scholar]
- Fox CS, Pencina MJ, D'Agostino RB, Murabito JM, Seely EW, Pearce EN, et al. 2008. Relations of thyroid function to body weight: Cross-sectional and longitudinal observations in a community-based sample. Arch Intern Med 168(6):587–592, PMID: 18362250, 10.1001/archinte.168.6.587. [DOI] [PubMed] [Google Scholar]
- Fries GF. 1985. The PBB episode in Michigan: An overall appraisal. Crit Rev Toxicol 16(2):105–156, PMID: 3002722, 10.3109/10408448509056268. [DOI] [PubMed] [Google Scholar]
- Gupta BN, McConnell EE, Goldstein JA, Harris MW, Moore JA. 1983. Effects of a polybrominated biphenyl mixture in the rat and mouse. I. Six-month exposure. Toxicol Appl Pharmacol 68(1):1–18, PMID: 6302948. [DOI] [PubMed] [Google Scholar]
- Hagmar L. 2003. Polychlorinated biphenyls and thyroid status in humans: a review. Thyroid 13(11):1021–1028, PMID: 14651786, 10.1089/105072503770867192. [DOI] [PubMed] [Google Scholar]
- Hernán MA, Hernández-Díaz S, Robins JM. 2004. A structural approach to selection bias. Epidemiology 15(5):615–625, PMID: 15308962, 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- Hirai T, Fujimine Y, Watanabe S, Nakano T. 2012. Distribution of polybrominated diphenyl ethers in Japanese autopsy tissue and body fluid samples. Environ Sci Pollut Res Int 19(8):3538–3546, PMID: 22544599, 10.1007/s11356-012-0915-z. [DOI] [PubMed] [Google Scholar]
- Jain RB. 2014. Association between thyroid function and selected organochlorine pesticides: Data from NHANES 2001–2002. Sci Total Environ 466-467:706–715, PMID: 23973536, 10.1016/j.scitotenv.2013.07.087. [DOI] [PubMed] [Google Scholar]
- Joseph AD, Terrell ML, Small CM, Cameron LL, Marcus M. 2009. Assessing inter-generational transfer of a brominated flame retardant. J Environ Monit 11(4):802–807, PMID: 19557234, 10.1039/b816867a. [DOI] [PubMed] [Google Scholar]
- Kay K. 1977. Polybrominated biphenyls (PBB) environmental contamination in Michigan, 1973–1976. Environ Res 13(1):74–93, PMID: 191251, 10.1016/0013-9351(77)90006-8. [DOI] [PubMed] [Google Scholar]
- König F, Andersson M, Hotz K, Aeberli I, Zimmermann MB. 2011. Ten repeat collections for urinary iodine from spot samples or 24-hour samples are needed to reliably estimate individual iodine status in women. J Nutr 141(11):2049–2054, PMID: 21918061, 10.3945/jn.111.144071. [DOI] [PubMed] [Google Scholar]
- Krassas GE, Pontikides N, Kaltsas T, Papadopoulou P, Paunkovic J, Paunkovic N, et al. 1999. Disturbances of menstruation in hypothyroidism. Clin Endocrinol (Oxf) 50(5):655–659, PMID: 10468932. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Wilcox KR Jr, Silva J Jr, Humphrey HE, Kauffman C, Heath CW Jr. 1979. Cohort study of Michigan residents exposed to polybrominated biphenyls: Epidemiologic and immunologic findings. Ann NY Acad Sci 320:284–294, PMID: 222186, 10.1111/j.1749-6632.1979.tb13154.x. [DOI] [PubMed] [Google Scholar]
- Langer P, Kočan A, Tajtaková M, Petrík J, Chovancová J, Drobná B, et al. 2007a. Fish from industrially polluted freshwater as the main source of organochlorinated pollutants and increased frequency of thyroid disorders and dysglycemia. Chemosphere 67(9):S379–S385, PMID: 17222442, 10.1016/j.chemosphere.2006.05.132. [DOI] [PubMed] [Google Scholar]
- Langer P, Kočan A, Tajtáková M, Rádiková S, Petrík J, Koska J, et al. 2007b. Possible effects of persistent organochlorinated pollutants cocktail on thyroid hormone levels and pituitary-thyroid interrelations. Chemosphere 70(1):110–118, PMID: 17692893, 10.1016/j.chemosphere.2007.06.046. [DOI] [PubMed] [Google Scholar]
- Langer P, Tajtáková M, Kočan A, Petrík J, Koška J, Kšinantová L, et al. 2007c. Thyroid ultrasound volume, structure and function after long-term high exposure of large population to polychlorinated biphenyls, pesticides and dioxin. Chemosphere 69(1):118–127, PMID: 17537484, 10.1016/j.chemosphere.2007.04.039. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. 2002. Statistical Analysis with Missing Data. 2nd edition Hoboken, NJ:Wiley. [Google Scholar]
- Liu H, Shan Z, Li C, Mao J, Xie X, Wang W, et al. 2014. Maternal subclinical hypothyroidism, thyroid autoimmunity, and the risk of miscarriage: A prospective cohort study. Thyroid 24(11):1642–1649, PMID: 25087688, 10.1089/thy.2014.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, et al. 2004. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect 112(17):1691–1696, PMID: 15579415, 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles RH, Fan D, Chuachoowong R. 2001. Correlation coefficient estimation involving a left censored laboratory assay variable. Stat Med 20(19):2921–2933, PMID: 11568949, 10.1002/sim.901. [DOI] [PubMed] [Google Scholar]
- Marder ME, Panuwet P, Hunter RE, Ryan PB, Marcus M, Barr DB. 2016. Quantification of polybrominated and polychlorinated biphenyls in human matrices by isotope-dilution gas chromatography–tandem mass spectrometry. J Anal Toxicol 40(7):511–518, PMID: 27445313, 10.1093/jat/bkw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham L, Burse V, Price H. 1981. Temperature-programmed gas chromatographic determination of polychlorinated and polybrominated biphenyls in serum. J Assoc Off Anal Chem 64(5):1131–1137, PMID: 6270054. [PubMed] [Google Scholar]
- O'Brien KM, Upson K, Cook NR, Weinberg CR. 2015. Environmental chemicals in urine and blood: Improving methods for creatinine and lipid adjustment. Environ Health Perspect 124(2):220–227, PMID: 26219104, 10.1289/ehp.1509693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulhote Y, Chevrier J, Bouchard MF. 2016. Exposure to polybrominated diphenyl ethers (PBDEs) and hypothyroidism in Canadian women. J Clin Endocrinol Metab 101(2):590–598, PMID: 26606679, 10.1210/jc.2015-2659. [DOI] [PubMed] [Google Scholar]
- Park YJ, Lee EK, Lee YK, Park DJ, Jang HC, Moore DD. 2012. Opposing regulation of cytochrome P450 expression by CAR and PXR in hypothyroid mice. Toxicol Appl Pharmacol 263(2):131–137, PMID: 22503787, 10.1016/j.taap.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DL, Pirkle JL, Burse VW, Bernert JT Jr, Henderson LO, Needham LL. 1989. Chlorinated hydrocarbon levels in human serum: Effects of fasting and feeding. Arch Environ Contam Toxicol 18(4):495–500, PMID: 2505694, 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- Ringer RK. 1978. PBB fed to immature chickens: its effect on organ weights and function and on the cardiovascular system. Environ Health Perspect 23:247–255, PMID: 209983, 10.1289/ehp.7823247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringer RK, Polin D. 1977. The biological effects of polybrominated biphenyls in avian species. Fed Proc 36(6):1894–1898, PMID: 192604. [PubMed] [Google Scholar]
- Romano ME, Webster GM, Vuong AM, Thomas Zoeller R, Chen A, Hoofnagle AN, et al. 2015. Gestational urinary bisphenol A and maternal and newborn thyroid hormone concentrations: The HOME study. Environ Res 138:453–460, PMID: 25794847, 10.1016/j.envres.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S. 1993. Toxicology, structure-function relationship, and human and environmental health impacts of polychlorinated biphenyls: progress and problems. Environ Health Perspect 100:259–268, PMID: 8354174, 10.1289/ehp.93100259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe SH. 1994. Polychlorinated biphenyls (PCBs): Environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol 24(2):87–149, PMID: 8037844, 10.3109/10408449409049308. [DOI] [PubMed] [Google Scholar]
- Salay E, Garabrant D. 2009. Polychlorinated biphenyls and thyroid hormones in adults: A systematic review appraisal of epidemiological studies. Chemosphere 74(11):1413–1419, PMID: 19108870, 10.1016/j.chemosphere.2008.11.031. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Whitcomb BW, Louis GM, Louis TA. 2005. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ Health Perspect 113(7):853–857, PMID: 16002372, 10.1289/ehp.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjödin A, Jones RS, Caudill SP, Wong LY, Turner WE, Calafat AM. 2014. Polybrominated diphenyl ethers, polychlorinated biphenyls, and persistent pesticides in serum from the National Health and Nutrition Examination Survey: 2003–2008. Environ Sci Technol 48(1):753–760, PMID: 24298999, 10.1021/es4037836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleight SD, Mangkoewidjojo S, Akoso BT, Sanger VL. 1978. Polybrominated biphenyl toxicosis in rats fed an iodine-deficient, iodine-adequate, or iodine-excess diet. Environ Health Perspect 23:341–346, PMID: 209997, 10.1289/ehp.7823341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small CM, Murray D, Terrell ML, Marcus M. 2011. Reproductive outcomes among women exposed to a brominated flame retardant in utero. Arch Environ Occup Health 66(4):201–208, PMID: 22014192, 10.1080/19338244.2010.539640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small CM, Terrell ML, Cameron LL, Wirth J, Monteilh CP, Marcus M. 2009. In utero exposure to a brominated flame retardant and male growth and development. Int J Child Adolesc Health 2:371–382. [PMC free article] [PubMed] [Google Scholar]
- Surks MI, Sievert R. 1995. Drugs and thyroid function. N Engl J Med 333(25):1688–1694, PMID: 7477223, 10.1056/NEJM199512213332507. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Inui N, Morita H, Takeuchi K, Uchida S, Watanabe H, et al. 2010. Effect of thyroid hormone on the activity of CYP3A enzyme in humans. J Clin Pharmacol 50(1):88–93, PMID: 19808952, 10.1177/0091270009344336. [DOI] [PubMed] [Google Scholar]
- Taussky HH. 1954. A microcolorimetric determination of creatine in urine by the Jaffe reaction. J Biol Chem 208(2):853–862, PMID: 13174594. [PubMed] [Google Scholar]
- Turyk ME, Persky VW, Imm P, Knobeloch L, Chatterton R, Anderson HA. 2008. Hormone disruption by PBDEs in adult male sport fish consumers. Environ Health Perspect 116(12):1635–1641, PMID: 19079713, 10.1289/ehp.11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzotzas T, Krassas GE, Konstantinidis T, Bougoulia M. 2000. Changes in lipoprotein (a) levels in overt and subclinical hypothyroidism before and during treatment. Thyroid 10(9):803–808, PMID: 11041458, 10.1089/thy.2000.10.803. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau Population Division. 2014. Annual estimates of the resident population by single year of age and sex for the United States: April 1, 2010 to July 1, 2014. https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=bkmk [accessed 15 July 2016].
- Wattigney WA, Irvin-Barnwell E, Pavuk M, Ragin-Wilson A. 2015. Regional variation in human exposure to persistent organic pollutants in the United States, NHANES. J Environ Public Health 2015:571839, PMID: 26839572, 10.1155/2015/571839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GM, Rauch SA, Ste Marie N, Mattman A, Lanphear BP, Venners SA. 2015. Cross-sectional associations of serum perfluoroalkyl acids and thyroid hormones in U.S. adults: Variation according to TPOAb and iodine status (NHANES 2007–2008). Environ Health Perspect 124(7):935–942, PMID: 26517287, 10.1289/ehp.1409589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GM, Venners SA, Mattman A, Martin JW. 2014. Associations between perfluoroalkyl acids (PFASs) and maternal thyroid hormones in early pregnancy: A population-based cohort study. Environ Res 133:338–347, PMID: 25019470, 10.1016/j.envres.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Werner PR, Sleight SD. 1981. Toxicosis in sows and their pigs caused by feeding rations containing polybrominated biphenyls to sows during pregnancy and lactation. Am J Vet Res 42(2):183–188, PMID: 6266287. [PubMed] [Google Scholar]
- WHO (World Health Organization). 1994. Indicators for assessing iodine deficiency disorders and their control through salt iodization. http://apps.who.int/iris/bitstream/10665/70715/1/WHO_NUT_94.6.pdf [accessed 15 July 2016].
- Wolff MS, Anderson HA, Selikoff IJ. 1982. Human tissue burdens of halogenated aromatic chemicals in Michigan. JAMA 247(15):2112–2116, PMID: 6278170, 10.1001/jama.1982.03320400024028. [DOI] [PubMed] [Google Scholar]
- Xue J, Liu SV, Zartarian VG, Geller AM, Schultz BD. 2014. Analysis of NHANES measured blood PCBs in the general US population and application of SHEDS model to identify key exposure factors. J Expo Sci Environ Epidemiol 24(6):615–621, PMID: 24424407, 10.1038/jes.2013.91. [DOI] [PubMed] [Google Scholar]
- Yard EE, Terrell ML, Hunt DR, Cameron LL, Small CM, McGeehin MA, et al. 2011. Incidence of thyroid disease following exposure to polybrominated biphenyls and polychlorinated biphenyls, Michigan, 1974–2006. Chemosphere 84(7):863–868, PMID: 21737118, 10.1016/j.chemosphere.2011.06.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.