Abstract
Background:
Ambient air pollution and tuberculosis (TB) have an impact on public health worldwide, yet associations between the two remain uncertain.
Objective:
We determined the impact of residential traffic on mortality during treatment of active TB.
Methods:
From 2000–2012, we enrolled 32,875 patients in California with active TB and followed them throughout treatment. We obtained patient data from the California Tuberculosis Registry and calculated traffic volumes and traffic densities in to radius buffers around residential addresses. We used Cox models to determine mortality hazard ratios, controlling for demographic, socioeconomic, and clinical potential confounders. We categorized traffic exposures as quintiles and determined trends using Wald tests.
Results:
Participants contributed 22,576 person-years at risk. There were 2,305 deaths during treatment for a crude mortality rate of 1,021 deaths per 10,000 person-years. Traffic volumes and traffic densities in all buffers around patient residences were associated with increased mortality during TB treatment, although the findings were not statistically significant in all buffers. As the buffer size decreased, fifth-quintile mortality hazards increased, and trends across quintiles of traffic exposure became more statistically significant. Increasing quintiles of nearest-road traffic volumes in the buffer were associated with 3%, 14%, 19%, and 28% increased risk of death during TB treatment [first quintile, referent; second quintile hazard ratio [95% confidence interval (CI): 0.86, 1.25]; third quintile (95% CI: 0.95, 1.37); fourth quintile (95% CI: 0.99, 1.43); fifth quintile (95% CI: 1.07, 1.53), respectively; ].
Conclusions:
Residential proximity to road traffic volumes and traffic density were associated with increased all-cause mortality in patients undergoing treatment for active tuberculosis even after adjusting for multiple demographic, socioeconomic, and clinical factors, suggesting that TB patients are susceptible to the adverse health effects of traffic-related air pollution. https://doi.org/10.1289/EHP1699
Introduction
Tuberculosis (TB) has an impact on health worldwide, with an estimated 9.6 million people developing active TB and 1.5 million people dying from the disease in 2014 (WHO 2015). TB mortality rates remain high despite adequate treatment (Fielder et al. 2002; Pascopella et al. 2014), and the effects of environmental factors such as ambient air pollution on TB outcomes remain uncertain. The majority of the world’s population is exposed to unhealthy levels of ambient air pollution, with approximately 89% living in areas where fine particulate mat-ter in diameter () exceeds World Health Organization (WHO) air quality standards (Brauer et al. 2012), and it is estimated that ambient air pollution contributes to approximately 3.3 million premature deaths each year (Lelieveld et al. 2015).
It has long been observed that certain inhaled toxicants are associated with pulmonary infection: Published studies as far back as 100 y ago reported associations between tobacco smoking and TB (Webb 1918). There is now ample evidence to suggest that active tobacco smokers are at increased risk for TB infection, progression to active TB, and worse treatment outcomes including mortality (Bates et al. 2007; Horne et al. 2012; Jee et al. 2009; Lin et al. 2007; Maciel et al. 2013; Slama et al. 2007). This evidence has led the WHO to affirm tobacco smoking as a significant TB risk factor (WHO 2016).
Tobacco smoke is composed of a number of chemical compounds that are also found in traffic emissions (CDC 2010; Gentner et al. 2013; Karjalainen et al. 2014), raising concerns that traffic-related air pollution (TRAP) could also be associated with adverse TB outcomes. Ambient air pollution is an established risk factor for community-acquired pneumonia (Chiu et al. 2009; Neupane et al. 2010; Zanobetti et al. 2000), yet studies linking ambient air pollution to other pulmonary infections such as TB are limited. The effects of ambient air pollution on TB incidence have been investigated in a few studies with mixed findings. In a nested case–control study using a northern California managed-care database, nitrogen dioxide () was associated with increased odds of active TB (Smith et al. 2016); in a community-based cohort study in Taipei, Taiwan, both and were associated with increased risk of active TB (Lai et al. 2016); and in Beijing and Hong Kong, China, outdoor was associated with seasonal changes in TB incidence (You et al. 2016). However, in a time-series study in Seoul, South Korea, spikes in sulfur dioxide () but not in particulate matter in diameter (), , or ozone were associated with temporally related increases in TB incidence (Hwang et al. 2014). The effects of ambient air pollution on TB treatment outcomes remain uncertain, and there has been only one published study to date that has examined the association of ambient air pollution with mortality during TB treatment; this study found that annual mean estimates of were associated with increased mortality (Peng et al. 2017). Additionally, there has been only one published study to evaluate traffic proximity as a TB risk factor. This study did not find a statistically significant association between distance to the nearest major road or freeway and smear positivity among patients with pulmonary TB in Los Angeles, California (Jassal et al. 2013). However, the study’s small sample size and cross-sectional design preclude firm conclusions linking these traffic metrics to TB outcomes, thereby warranting a larger study with a prospective cohort study design from which causal inferences can be made.
We used proximity to road traffic volumes and proximity to traffic density as measures of traffic-related air pollution instead of individual air pollutant models because our traffic proximity models capture the mix of pollutants emitted from motor vehicles as opposed to models for individual pollutants that might provide a less-complete representation of traffic exposure. For instance, in a study evaluating the association of traffic exposures with childhood incident asthma, the effect of on incident asthma, which had a hazard ratio (HR) of 2.17 [95% confidence interval (CI): 1.18, 4.00], was attenuated to 1.37 (95% CI: 0.69, 2.71) when adjusting for traffic exposure using a line source dispersion model that included distance to roadways and vehicle counts (McConnell et al. 2010), suggesting that traffic proximity was a key factor for determining TRAP health effects. Furthermore, in another study comparing different TRAP exposure metrics, traffic density was found to be “reasonably consistent with the more sophisticated metrics” (Batterman et al. 2014).
Both TB and ambient air pollution are significant public health issues in California. Nearly 25% of all U.S. TB cases occur in California (Salinas et al. 2016), and of Californians live in counties with unhealthy ambient air concentrations of , ozone, or both (ALA 2016). Additionally, the predominant source of air pollution in California is from traffic emissions (ARB 2017). We hypothesized that proximity to road traffic volumes and traffic density would be associated with increased mortality in patients undergoing TB treatment and that the effects would be mediated through clinical markers of TB severity. To test these hypotheses, we designed and implemented a large cohort study of TB patients reported to the State of California and followed longitudinally by California Department of Public Health TB clinics throughout their TB treatment.
Methods
Study Cohort
All reported pediatric and adult TB cases in California between 1 January 2000 and 31 December 2012 were eligible for inclusion. A TB case was specified as a person of any age with clinically diagnosed or microbiologically confirmed (or both) active TB as defined by the Centers for Disease Control and Prevention (CDC). Clinical diagnostic criteria included symptoms, physical exam, and radiographic findings consistent with TB along with an appropriate response to treatment. Acceptable microbiologic confirmation included culture or nucleic acid amplification testing (or both) positive for Mycobacterium tuberculosis (Mtb). Patients were excluded from survival analyses if traffic data were unavailable at residential addresses, if residential addresses were either not available or not geocodable to street-level resolution, if patients died or moved out of California before treatment was initiated, or if treatment dates were unavailable.
Traffic Exposure Assessment
Residential street addresses were obtained at the time of TB diagnosis and were later transformed into geocoded coordinates using browser-based geocoding software developed by the California Environmental Health Tracking Program (CEHTP) at the California Department of Public Health (CDPH). This software matched addresses to geographic coordinates using Tele Atlas® (TomTom Telematics), Navteq® (Nokia Here), and TIGER® (U.S. Census Bureau) reference data sets for 2010 and 2011. For homeless patients, we used the shelter address or the street intersection of the patient’s most recent sleeping location. Geocoding accuracy was quantified using a score based on how well the input address text elements matched the same elements of the geocoding reference database. The score ranged from 0 to 100, with 100 representing an exact match.
Traffic data from 2004 were obtained from the California Department of Transportation (Caltrans) Highway Performance Monitoring System, with data available for roads functionally classified as collectors, arterials, freeways, expressways, and interstates, collectively referred to as “major” roads in this study. Traffic data were not available for small local roads. Because the distance from traffic source for maximum health impact was uncertain (Puett et al. 2014), we calculated traffic exposures within four circular zones (buffers) of radius around each participant’s residential address using CEHTP Traffic Spatial Linkage Service software (CDPH). Within each of these buffers, we evaluated traffic volume [average number of vehicles (vh) traversing a road segment per 24 h] at the nearest major road and at the highest-trafficked road, and traffic density was calculated as the sum of length-adjusted major road segment traffic counts within each buffer per hour (vehicle·kilometers/hour). We selected these traffic indicators to best characterize distance to exposure source as well as peak and overall exposure concentrations. Left-censored traffic data due to the absence of major roads in a buffer were considered missing.
Outcome Assessment
Patients were followed longitudinally throughout TB treatment by one of 61 local health department TB programs. Baseline and follow-up demographic, socioeconomic, and clinical data were recorded by trained local health officials into the Report of Verified Case of Tuberculosis (RVCT), a CDC-developed data collection tool. RVCT data were then entered into a CDPH central data repository (the California TB Registry). Our preselected primary outcome was all-cause mortality during active TB treatment. Follow-up time began on the first day of TB treatment and ended on the date of death (event) or was right-censored on the date of adequate completion of TB therapy, cessation of therapy for other reasons, moving out of state, loss to follow-up, or the end of the study, whichever occurred first.
Ethics Approval
The study was approved by the institutional review boards at the University of California, San Francisco and the California Health and Human Services Agency Committee for the Protection of Human Subjects. Informed consent was not required because the study relied on existing public health records rather than on direct patient contact, and all personal identifiers were removed from the database before analysis.
Statistical analysis
We fit Cox proportional hazards models to determine HRs for mortality during TB treatment predicted by each traffic metric in buffers around residential addresses. We transformed traffic volumes and traffic density into quintiles to better adhere to log-linearity of hazard function assumptions, and we tested for linear trends across quintiles using Wald tests (Vittinghoff et al. 2012), reporting calculated p-values as p-trends. Traffic exposure associations with mortality were considered statistically significant for p-trends in at least two of the four buffers tested. In forming quintiles, we chose exposure cut points that would split participants into five equal groups. Individual-level demographic, socioeconomic, and clinical covariates were selected a priori as potential confounders and were included in the final multivariable models if associated with mortality in univariate Cox models with a p-value . In alternative models, we additionally adjusted for two group-level variables: census block group median annual household income from the 2006–2010 American Community Survey (U.S. Census Bureau 2016) and census-tract tobacco smoking prevalence estimates (Ortega Hinojosa et al. 2014). We tested for effect modification by defining interaction terms between traffic density and each of the following: age, sex, race, ethnicity, region of residence, enrollment year, bacteriologic confirmation, directly observed TB treatment (DOT), census-tract tobacco smoking estimates, and HIV; we fitted separate multivariable Cox models with each of these interaction terms and reported effect modification for a p-interaction in at least one buffer and a consistent pattern of effect modification in all buffers. We performed mediation analyses to determine if the effects of traffic on mortality were mediated through TB severity, and we performed multiple sensitivity analyses to test the robustness of our findings across alternative Cox models. Standard diagnostics and assumption checks were performed on all models. Time-varying covariate models were not employed; instead, covariates with time dependency were assigned fixed measurement times and values. The comorbidities diabetes, end-stage renal disease, and non-HIV immunosuppression were reported only from 2010–2012. In subgroup analyses including these comorbidities, we transformed traffic exposures into dichotomous rather than five-level categorical variables as in the other analyses owing to the small number of deaths in each subgroup. We evaluated the effects of nearest-road traffic volumes on secondary outcomes including culture conversion and successful treatment completion using unadjusted Kaplan–Meier survival analyses and testing for statistical significance between fifth- and first-quintile exposures using log-rank tests. Statistical analyses were performed using Stata/SE 13.1 (StataCorp).
Results
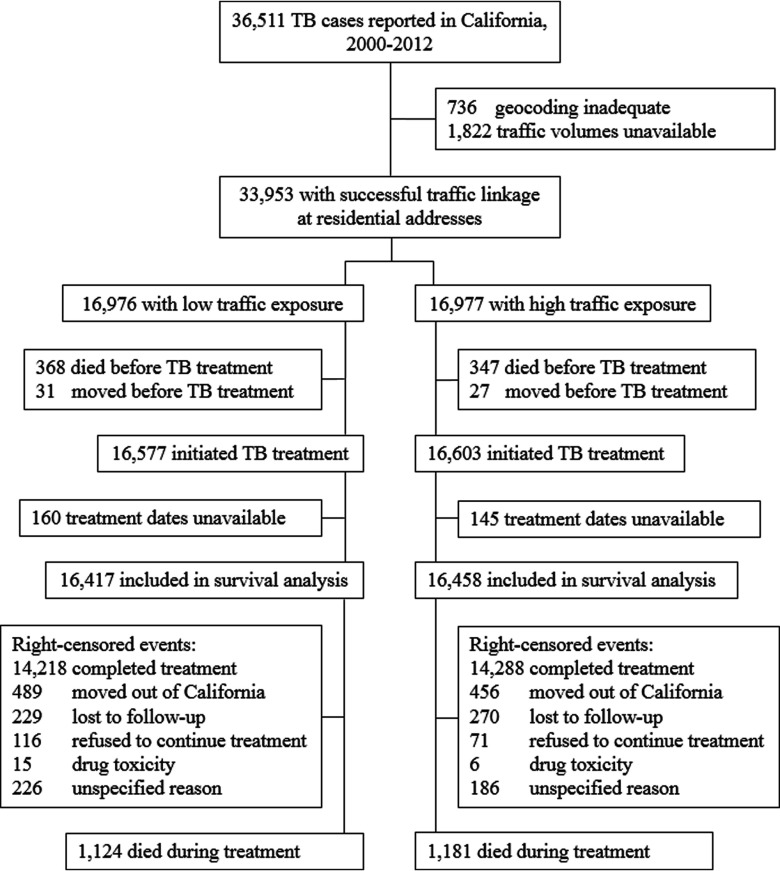
Of 36,511 patients with active TB reported to the State of California, we excluded 2,558 patients (7.0%) for whom we were unable to perform geocoding and traffic linkage with street-level accuracy (Figure 1). We excluded an additional 1,078 patients (3.0%) who moved from California, died before treatment was initiated, or for whom treatment records were incomplete. Our final cohort of 32,875 patients with active TB contributed 22,576 person-years at risk {median follow-up of 224 d [interquartile range (IQR) 184–293] per patient}. There were 2,305 deaths during follow-up for a crude mortality rate of 1,021 deaths per 10,000 person-years. Most right-censored events were due to either treatment completion or moving out of California, and only 2.8% were lost to follow-up or had unspecified outcomes. Sixty percent of included patients were male, 44% were Asian, 38% were Hispanic, 77% were foreign-born, and 5% were HIV-infected (Table 1). Socioeconomic hardship was evident, with 53% unemployed, 5.8% homeless, and 13% using recreational drugs, excess alcohol, or both within the year before enrollment. Pulmonary TB was diagnosed in 80% of participants (24% of these cases were cavitary), extrapulmonary TB was diagnosed in 29% of participants, and 79% of TB cases were microbiologically confirmed. Patients living in high-traffic-density neighborhoods were younger and more likely to be male, Asian, black, foreign-born, a recent immigrant, from southern California or the San Francisco Bay Area, living within the city limits, living in more densely populated and lower-income block groups, unemployed, homeless, a recreational drug/excess alcohol user, and HIV-infected compared with those with low traffic density exposure. A number of covariates were associated with increased mortality during TB treatment in unadjusted analysis, including being unemployed, having both pulmonary and extrapulmonary TB, having TB meningitis, having miliary TB, having microbiologically confirmed TB, and having the following comorbidities: diabetes, HIV, non-HIV immunosuppression, and end-stage renal disease (see Table S1).
Figure 1.
Study flow chart. TB, tuberculosis.
Table 1.
Characteristics by traffic density, .
| Characteristic | Traffic density, (%)a | ||
|---|---|---|---|
| Low ()b | High ()b | p-Value | |
| Age at diagnosis, median (IQR), y | 47.7 (30.8–64.5) | 46.2 (30.5–62.2) | |
| Male sex | 9,570 (58.3) | 10,012 (60.8) | |
| Race and ethnicity | |||
| Asian | 7,058 (43.0) | 7,252 (44.1) | |
| Hispanic | 6,525 (39.8) | 6,075 (36.9) | |
| White, non-Hispanic | 1,559 (9.50) | 1,452 (8.82) | |
| Black, non-Hispanic | 1,100 (6.70) | 1,508 (9.16) | |
| Native American/Alaskan or Pacific Islander | 144 (0.88) | 137 (0.83) | |
| Unknown | 31 (0.19) | 34 (0.21) | |
| Foreign born | 12,442/16,362 (76.0) | 12,731/16,419 (77.5) | 0.001 |
| Recent immigrant | 3,852/16,362 (23.5) | 4,175/16,419 (25.4) | |
| Region | |||
| Southern California | 8,840 (53.9) | 9,722 (59.1) | |
| Central Valley | 3,106 (18.9) | 1,279 (7.77) | |
| North Coast and Mountain | 393 (2.39) | 172 (1.05) | |
| San Francisco Bay Area | 3,159 (19.2) | 4,760 (28.9) | |
| Central Coast | 919 (5.60) | 525 (3.19) | |
| Residence within the city limits | 15,823/16,376 (96.6) | 16,306/16,440 (99.2) | |
| Population density, , c | |||
| Median annual household income, median (IQR), USDc | 53,832 (38,230–74,836) | 45,722 (31,680–65,214) | |
| Estimated census tract percent smoking prevalence, | |||
| Unemployed | 8,124/15,824 (51.3) | 8,680/16,082 (54.0) | |
| Homeless | 568/16,339 (3.48) | 1,325/16,375 (8.09) | |
| Substance abused | 1,776/16,193 (11.0) | 2,370/16,263 (14.6) | |
| HIV-infected | 648 (3.95) | 985 (5.98) | |
| Microbiologically confirmed TB | 12,868/16,409 (78.4) | 12,995/16,451 (79.0) | 0.21 |
| Pulmonary TB | 13,050 (79.5) | 13,146 (79.9) | 0.39 |
| Extrapulmonary TB | 4,754 (29.0) | 4,886 (29.7) | 0.15 |
| Cavitary TB | 3,221/16,399 (19.6) | 3,145/16,443 (19.1) | 0.24 |
| Miliary TB | 279 (1.70) | 287 (1.74) | 0.76 |
| MDR-TB | 194/12,664 (1.53) | 180/12,891 (1.40) | 0.37 |
| Treatment | |||
| Self-administered for all doses | 2,857 (17.5) | 3,020 (18.5) | |
| DOT for all doses | 8,856 (54.3) | 10,145 (62.0) | |
| DOT and self-administered doses | 4,603 (28.2) | 3,193 (19.5) | |
| Days to culture conversion, median (IQR) | 53 (32–78) | 53 (33–80) | 0.33 |
| Treatment duration,e median (IQR), days | 244 (188–301) | 248 (190–302) | 0.37 |
Note: DOT, directly observed tuberculosis (TB) treatment; IQR, interquartile range; MDR-TB, multidrug-resistant TB, resistant to both isoniazid and rifampicin; SD, standard deviation; USD, U.S. dollars.
Traffic density is defined as the sum of length-adjusted road segment traffic volumes in the buffer around residential addresses, using the median value of as the low-high cutoff. Column values represent “Number (%)” unless indicated otherwise.
The denominator is 16,417 for low traffic density and 16,458 for high traffic density unless otherwise indicated.
Obtained from American Census Survey 2006-2010 census block group data (U.S. Census Bureau 2016).
Excess alcohol and/or recreational drug use (oral, inhaled, or injected) within one year before TB diagnosis.
Treatment duration among those who completed treatment.
Median (IQR) traffic volumes (vehicles/day) were 12,610 (5,000–24,800) vh/d at the nearest major road and 29,000 (17,600–45,000) vh/d at the highest-trafficked road in the buffer (Table 2). Median (IQR) traffic densities (vehicles·kilometer/hour) in the buffers were 106 (39.6–230), 356 (138–746), 820 (337–1,647), and 1,525 (690–3,009) vh·km/h, respectively. In sensitivity analyses, excluded patients were similar to included patients with the following exceptions: Those excluded were more likely to reside outside the city limits [257/3,608 (7.1%) vs. 687/32,816 (2.09%)], in lower population density census block groups [ (SD) vs. ], in less traffic-dense neighborhoods [ in the buffer vs. ], in the Central Valley [800/3,636 (22%) vs. 4,385/32,875 (13%)], and in higher income census block groups [ vs. ]. Geocoding accuracy was slightly higher for patients enrolled between 2010 and 2011 (geocoding score of ) than for those enrolled in other years of the study (geocoding score of ; ). Patients reported with TB between 2010 and 2011 were less likely to be excluded because of an inability to geocode the residential address [ (0.63%)] than those reported with TB in other years of the study [ (2.2%)].
Table 2.
Traffic statistics by buffer and quintile.
| Exposure | Mean | Median | IQR | Minimum | Maximum | |
|---|---|---|---|---|---|---|
| Traffic volumes of nearest road () | ||||||
| buffer | ||||||
| Q1 | 3,866 | 2,077 | 2,082 | 1,210–2,830 | 32 | 3,900 |
| Q2 | 3,815 | 6,087 | 5,960 | 4,940–7,290 | 3,906 | 8,680 |
| Q3 | 3,865 | 12,242 | 12,000 | 10,245–14,100 | 8,686 | 16,500 |
| Q4 | 3,815 | 21,283 | 21,314 | 18,700–23,650 | 16,521 | 26,500 |
| Q5 | 3,830 | 54,292 | 35,056 | 30,000–45,000 | 26,508 | 382,000 |
| All | 19,191 | 19,160 | 12,000 | 4,940–23,600 | 32 | 382,000 |
| buffer | ||||||
| Q1 | 5,621 | 2,110 | 2,110 | 1,210–2,910 | 32 | 3,950 |
| Q2 | 5,573 | 6,212 | 6,060 | 5,000–7,410 | 3,956 | 8,930 |
| Q3 | 5,622 | 12,595 | 12,475 | 10,500–14,450 | 8,938 | 16,827 |
| Q4 | 5,580 | 21,971 | 22,000 | 19,400–24,473 | 16,840 | 27,700 |
| Q5 | 5,573 | 60,158 | 36,929 | 30,795–48,200 | 27,725 | 382,000 |
| All | 27,969 | 20,563 | 12,400 | 5,000–24,400 | 32 | 382,000 |
| buffer | ||||||
| Q1 | 6,212 | 2,119 | 2,130 | 1,210–2,920 | 32 | 3,960 |
| Q2 | 6,246 | 6,249 | 6,100 | 5,000–7,500 | 3,961 | 9,000 |
| Q3 | 6,246 | 12,785 | 12,646 | 10,700–14,700 | 9,010 | 17,000 |
| Q4 | 6,157 | 22,227 | 22,285 | 19,700–24,700 | 17,004 | 27,879 |
| Q5 | 6,201 | 61,321 | 37,208 | 31,000–49,275 | 27,887 | 382,000 |
| All | 31,062 | 20,898 | 12,600 | 5,000–24,700 | 32 | 382,000 |
| buffer | ||||||
| Q1 | 6,523 | 2,134 | 2,130 | 1,210–2,990 | 32 | 4,000 |
| Q2 | 6,434 | 6,283 | 6,126 | 5,000–7,500 | 4,001 | 9,030 |
| Q3 | 6,486 | 12,799 | 12,673 | 10,702–14,700 | 9,035 | 17,000 |
| Q4 | 6,457 | 22,272 | 22,300 | 19,700–14,800 | 17,004 | 27,900 |
| Q5 | 6,463 | 61,578 | 37,300 | 31,246–49,700 | 27,903 | 382,000 |
| All | 32,363 | 20,985 | 12,610 | 5,000–24,800 | 32 | 382,000 |
| Traffic volumes of highest-trafficked road () | ||||||
| buffer | ||||||
| Q1 | 3,912 | 2,682 | 2,600 | 1,481–3,860 | 32 | 5,000 |
| Q2 | 3,821 | 8,080 | 7,960 | 6,470–9,610 | 5,020 | 11,500 |
| Q3 | 3,856 | 15,748 | 15,700 | 13,600–17,700 | 11,530 | 20,300 |
| Q4 | 3,951 | 25,099 | 24,789 | 22,600–27,740 | 20,301 | 30,300 |
| Q5 | 3,758 | 74,191 | 41,853 | 35,000–61,000 | 30,358 | 382,000 |
| All | 19,298 | 24,876 | 15,700 | 6,390–27,500 | 32 | 382,000 |
| buffer | ||||||
| Q1 | 5,684 | 3,956 | 3,958 | 2,290–5,600 | 32 | 7,500 |
| Q2 | 5,701 | 11,842 | 11,900 | 9,500–14,089 | 7,503 | 16,500 |
| Q3 | 5,642 | 20,969 | 21,100 | 18,800–23,300 | 16,504 | 25,200 |
| Q4 | 5,643 | 30,623 | 30,000 | 27,725–33,400 | 25,231 | 37,800 |
| Q5 | 5,651 | 106,348 | 54,600 | 42,500–164,000 | 37,839 | 382,000 |
| All | 28,321 | 34,677 | 21,000 | 9,480–33,300 | 32 | 382,000 |
| buffer | ||||||
| Q1 | 6,286 | 5,633 | 5,500 | 3,260–8,000 | 32 | 10,809 |
| Q2 | 6,322 | 16,253 | 16,400 | 13,666–18,841 | 10,810 | 21,300 |
| Q3 | 6,332 | 25,612 | 25,600 | 23,450–27,700 | 21,303 | 29,865 |
| Q4 | 6,260 | 35,908 | 35,540 | 32,356–39,400 | 29,892 | 44,004 |
| Q5 | 6,308 | 138,578 | 124,000 | 52,718–210,000 | 44,012 | 382,000 |
| All | 31,508 | 44,410 | 25,600 | 13,700–39,400 | 32 | 382,000 |
| buffer | ||||||
| Q1 | 6,566 | 7,929 | 7,890 | 4,530–11,690 | 59 | 14,600 |
| Q2 | 6,607 | 20,247 | 20,499 | 17,600–22,806 | 14,623 | 25,000 |
| Q3 | 6,639 | 29,249 | 29,120 | 27,142–31,269 | 25,009 | 34,000 |
| Q4 | 6,514 | 41,116 | 40,333 | 37,208–45,300 | 34,022 | 52,000 |
| Q5 | 6,549 | 168,797 | 165,000 | 100,000–229,000 | 52,200 | 382,000 |
| All | 32,875 | 53,332 | 29,000 | 17,600–45,000 | 32 | 382,000 |
| Traffic density () | ||||||
| buffer | ||||||
| Q1 | 3,861 | 15.3 | 14.8 | 8.33–14.8 | 0.008 | 30.6 |
| Q2 | 3,860 | 51.1 | 49.8 | 39.6–62.7 | 30.6 | 74.8 |
| Q3 | 3,878 | 107 | 106 | 88.8–125 | 74.8 | 146 |
| Q4 | 3,852 | 204 | 200 | 172–230 | 146 | 276 |
| Q5 | 3,847 | 704 | 419 | 333–633 | 277 | 8,998 |
| All | 19,298 | 216 | 106 | 39.6–230 | 0.008 | 8,998 |
| buffer | ||||||
| Q1 | 5,656 | 51.4 | 50.6 | 26.5–75.4 | 0.004 | 104 |
| Q2 | 5,675 | 175 | 173 | 138–211 | 104 | 256 |
| Q3 | 5,689 | 361 | 356 | 305–413 | 256 | 477 |
| Q4 | 5,659 | 657 | 646 | 553–749 | 477 | 905 |
| Q5 | 5,642 | 2,594 | 1,496 | 1,109–3,335 | 905 | 22,020 |
| All | 28,321 | 766 | 356 | 138–746 | 0.004 | 22,020 |
| buffer | ||||||
| Q1 | 6,266 | 126 | 123 | 61.5–190 | 0.17 | 262 |
| Q2 | 6,316 | 425 | 423 | 335–507 | 262 | 610 |
| Q3 | 6,328 | 829 | 819 | 711–947 | 610 | 1,087 |
| Q4 | 6,318 | 1,453 | 1,416 | 1,241–1,650 | 1,087 | 1,964 |
| Q5 | 6,280 | 5,532 | 3,939 | 2,533–7,614 | 1,964 | 34,642 |
| All | 31,508 | 1,671 | 820 | 337–1,647 | 0.17 | 34,642 |
| buffer | ||||||
| Q1 | 6,547 | 266 | 263 | 127–402 | 0.09 | 542 |
| Q2 | 6,578 | 837 | 827 | 687–988 | 542 | 1,159 |
| Q3 | 6,590 | 1,533 | 1,522 | 1,334–1,729 | 1,160 | 1,954 |
| Q4 | 6,581 | 2,649 | 2,547 | 2,233–3,008 | 1,955 | 3,759 |
| Q5 | 6,579 | 9,590 | 8,226 | 5,098–12,449 | 3,760 | 48,305 |
| All | 32,875 | 2,977 | 1,525 | 690–3,009 | 0.09 | 48,305 |
Note: IQR, interquartile range; Q1–Q5, quintiles 1 through 5, where Q5 is the highest quintile of traffic exposure; vh, vehicle.
Traffic volumes and traffic densities were associated with increased mortality during TB treatment, with statistically significant p-trends for increasing HRs across quintiles for the majority of the 12 scenarios tested (Table 3; see also Table S1). For instance, participants exposed to the second quintile of nearest-road traffic volumes within the buffer had a 3% increased risk of death compared with those exposed to lowest-quintile traffic volumes [ (95% CI: 0.86, 1.25)] (Figure 2). This risk increased incrementally to a 28% risk of death for the highest quintile of traffic volume exposure [ (95% CI: 1.07, 1.53)], a trend that was statistically significant () (Figure 2). Furthermore, as the buffer size decreased, fifth-quintile mortality hazards increased, and trends across quintiles became more statistically significant. For example, although the highest-trafficked road in the buffer was associated with increased mortality, the fifth-quintile HR was only 1.10 (95% CI: 0.95, 1.27), and there was not a significant trend for increasing mortality across quintiles. In contrast, fifth-quintile traffic volumes of the highest-trafficked road in the buffer were associated with a 29% increased hazard of death [ (95% CI: 1.07, 1.55)], with a significant trend across traffic exposure quintiles ().
Table 3.
Adjusted mortality hazard ratios (95% confidence intervals) for traffic metrics in buffers around residential addresses.
| Exposure | buffer | buffer | buffer | buffer |
|---|---|---|---|---|
| Traffic volumes of nearest road () | ||||
| 18,396 | 26,814 | 29,753 | 30,985 | |
| Q1 (HR) | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 [HR (95%CI)] | 1.03 (0.86, 1.25) | 1.02 (0.87, 1.18) | 1.01 (0.87, 1.17) | 0.99 (0.85, 1.14) |
| Q3 [HR (95%CI)] | 1.14 (0.95, 1.37) | 1.13 (0.98, 1.32) | 1.12 (0.97, 1.29) | 1.10 (0.96, 1.26) |
| Q4 [HR (95%CI)] | 1.19 (0.99, 1.43) | 1.19 (1.03, 1.39) | 1.20 (1.04, 1.38) | 1.15 (1.00, 1.33) |
| Q5 [HR (95%CI)] | 1.28 (1.07, 1.53) | 1.19 (1.02, 1.38) | 1.15 (1.00, 1.33) | 1.13 (0.98, 1.30) |
| p-Trend | 0.002 | 0.004 | 0.006 | 0.01 |
| Traffic volumes of highest-trafficked road () | ||||
| 18,500 | 27,156 | 30,185 | 31,480 | |
| Q1 (HR) | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 [HR (95%CI)] | 1.07 (0.88, 1.29) | 1.22 (1.05, 1.42) | 1.22 (1.06, 1.41) | 1.21 (1.05, 1.40) |
| Q3 [HR (95%CI)] | 1.29 (1.08, 1.55) | 1.29 (1.10, 1.50) | 1.30 (1.12, 1.50) | 1.11 (0.96, 1.28) |
| Q4 [HR (95%CI)] | 1.29 (1.07, 1.54) | 1.32 (1.14, 1.54) | 1.22 (1.05, 1.42) | 1.14 (0.98, 1.32) |
| Q5 [HR (95%CI)] | 1.29 (1.07, 1.55) | 1.24 (1.06, 1.45) | 1.15 (0.99, 1.34) | 1.10 (0.95, 1.27) |
| p-Trend | 0.005 | 0.10 | 0.46 | |
| Traffic density () | ||||
| 18,500 | 27,156 | 30,185 | 31,480 | |
| Q1 (HR) | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 [HR (95%CI)] | 1.02 (0.85, 1.23) | 1.25 (1.08, 1.46) | 1.11 (0.96, 1.29) | 1.07 (0.93, 1.24) |
| Q3 [HR (95%CI)] | 1.07 (0.89, 1.29) | 1.25 (1.08, 1.46) | 1.30 (1.12, 1.49) | 1.23 (1.07, 1.42) |
| Q4 [HR (95%CI)] | 1.33 (1.12, 1.59) | 1.31 (1.13, 1.52) | 1.17 (1.01, 1.36) | 1.12 (0.97, 1.30) |
| Q5 [HR (95%CI)] | 1.18 (0.98, 1.41) | 1.17 (1.00, 1.37) | 1.13 (0.97, 1.31) | 1.06 (0.91, 1.23) |
| p-Trend | 0.005 | 0.04 | 0.09 | 0.36 |
Note: CI, confidence interval; HR, hazard ratio; p-trend, the p-value for the trend across quintiles of traffic exposure. Q1-Q5, quintiles 1 through 5, where Q5 is the highest quintile of traffic exposure; vh, vehicle. Adjusted for age, sex, race, ethnicity, foreign birth, recent immigration within 5 y before tuberculosis (TB) diagnosis, population density, region of residence, unemployment within 1 y before TB diagnosis, homeless within one year before TB diagnosis, excess alcohol and/or recreational drug use within one year before TB diagnosis, and HIV infection.
Figure 2.
Cumulative hazards of death by nearest-road traffic volume quintiles in buffers around residential addresses. Adjusted for age, sex, race, ethnicity, foreign birth, recent immigration within 5 y before tuberculosis (TB) diagnosis, population density, unemployment within one year before TB diagnosis, homelessness within one year before TB diagnosis, excess alcohol and/or recreational drug use within one year before TB diagnosis, and HIV infection. CI, confidence interval; HR, hazard ratio.
Among potential confounders, age and recent immigration augmented mortality hazards, whereas sex, employment status, HIV status, population density, and excess alcohol and/or recreational drug use attenuated mortality hazards. In alternative Cox models, we adjusted for group-level covariates in addition to individual-level covariates (Table 4) and found that the addition of census block group household income did not attenuate HRs. In contrast, adjusting for census-tract smoking estimates augmented mortality hazards such that exposure to the highest quintile (Q5) of nearest-road traffic volumes in the buffer was associated with a 41% increased risk of death during TB treatment [ (95% CI: 1.16, 1.75)] compared with lowest-quintile (Q1) traffic volume exposures. In additional alternative Cox models, the findings did not change significantly with exclusion of cases that were not microbiologically confirmed or with exclusion of patients who lived at multiple addresses during treatment.
Table 4.
Adjusted mortality hazard ratios (95% confidence intervals) for alternative Cox models using nearest-road traffic volume exposures, buffer.
| Exposure: Traffic volumes of nearest road () | Alternative Cox model | |||||
|---|---|---|---|---|---|---|
| Cox model with only individual-level covariatesa | Add group-level household incomeb to adjusted model | Add group-level smoking estimatesc to adjusted model | Exclude non-microbiologically confirmed casesa | Exclude patients who moved within Californiaa | Left-censored values: Assign zero instead of missinga | |
| 18,396 | 18,375 | 14,478 | 14,404 | 17,480 | 32,832 | |
| Q1 (HR) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Q2 [HR (95% CI)] | 1.03 (0.86, 1.25) | 1.03 (0.86, 1.24) | 1.13 (0.91, 1.40) | 1.05 (0.87, 1.28) | 1.05 (0.87, 1.27) | 1.00 |
| Q3 [HR (95% CI)] | 1.14 (0.95, 1.37) | 1.14 (0.95, 1.36) | 1.23 (1.00, 1.52) | 1.16 (0.96, 1.40) | 1.16 (0.96, 1.40) | 0.90 (0.79, 1.02) |
| Q4 [HR (95% CI)] | 1.19 (0.99, 1.43) | 1.19 (0.99, 1.43) | 1.29 (1.05, 1.59) | 1.20 (1.00, 1.46) | 1.21 (1.01, 1.46) | 1.01 (0.90, 1.14) |
| Q5 [HR (95% CI)] | 1.28 (1.07, 1.53) | 1.27 (1.06, 1.52) | 1.41 (1.16, 1.75) | 1.28 (1.06, 1.54) | 1.28 (1.07, 1.54) | 1.10 (0.98, 1.23) |
| p-Trend | 0.002 | 0.003 | 0.004 | 0.002 | 0.04 | |
Note: CI, confidence interval; HR, hazard ratio; p-Trend, the p-value for the trend across quintiles of traffic exposure; Q1–Q5, quintiles 1 through 5, where Q5 is the highest quintile of traffic exposure; vh, vehicle.
Adjusted for individual-level covariates: age, sex, race, ethnicity, foreign birth, recent immigration within 5 y prior to tuberculosis (TB) diagnosis, population density, region of residence, unemployment within one year before TB diagnosis, homeless within one year before TB diagnosis, excess recreational drug and/or alcohol use within one year before TB diagnosis, and HIV infection.
Group-level covariate: census block group median annual household income.
Group-level covariate: census tract smoking prevalence estimates.
Region of residence, directly observed therapy, and census-tract smoking prevalence modified the effects of traffic exposure on mortality (Table 5). For instance, those exposed to the highest quintile of traffic density in the buffer had an 88% increased mortality hazard in the Central Valley and a 19% increased mortality hazard in southern California, but no increased hazard in the San Francisco Bay Area (). Those who self-administered some or all of their TB medication experienced an increased traffic mortality hazard compared with those who received only directly observed therapy, and traffic mortality hazards were smaller for TB patients living in high-smoking census tracts compared with those living in low-smoking census tracts. Additionally, traffic effects were slightly higher in most buffers for patients old, in patients enrolled from 2004–2009, and in patients with diabetes (Table 5; see also Table S2); traffic effects were slightly lower for those with HIV and other forms of immunosuppression, but these interactions did not reach statistical significance except for other forms of immunosuppression in the buffer. We tested multidrug-resistant (MDR) TB and cavitation as possible confounders. Although both types of TB were associated with mortality (see Table S1), adjusting for these variables did not significantly change HRs in multivariable analyses (see Table S4). In addition, we tested both variables as potential effect modifiers in the association between traffic density and mortality and did not find statistically significant interactions in any of the buffers (see Table S5). In mediation analyses, the effects of traffic exposure on mortality did not appear to be mediated through TB severity. Although surrogates for TB severity (smear positivity, having both pulmonary and extrapulmonary TB, TB meningitis, and miliary TB) were all significantly associated with increased mortality (see Table S1), traffic exposures were not statistically significantly associated with TB severity, and the addition of TB severity variables into Cox models did not attenuate mortality HRs. We also evaluated diabetes and end-stage renal disease as intermediaries on the causal pathway from TRAP exposure to mortality during TB treatment and found that although each comorbidity was associated with increased mortality (see Table S1), we did not find a statistically significant association between traffic density and increased risk for diabetes or end-stage renal disease.
Table 5.
Adjusted mortality hazard ratios (95% confidence intervals) for highest quintile traffic density in each buffer, stratified by effect modifier.
| Population | buffer [HR (95% CI)] | buffer [HR (95% CI)] | buffer [HR (95% CI)] | buffer [HR (95% CI)] | |
|---|---|---|---|---|---|
| Entire cohort | 31,480 | 1.18 (0.98, 1.41)* | 1.17 (1.00, 1.37)* | 1.13 (0.97, 1.31) | 1.06 (0.91, 1.23) |
| Region | |||||
| San Francisco Bay Area | 7,731 | 0.88 (0.61, 1.26) | 0.99 (0.73, 1.36) | 1.18 (0.87, 1.61) | 0.93 (0.69, 1.25) |
| Central Valley | 3,902 | 1.88 (1.09, 3.24)* | 1.78 (1.12, 2.84)* | 1.53 (1.02, 2.31) | 1.27 (0.87, 1.87) |
| Southern California | 17,935 | 1.19 (0.94, 1.50)* | 1.13 (0.92, 1.39) | 1.04 (0.85, 1.26) | 1.07 (0.87, 1.30) |
| p-Interaction | — | 0.03 | 0.19 | 0.52 | 0.85 |
| Exclusive DOT | |||||
| No | 13,098 | 1.21 (0.86, 1.70)* | 1.35 (1.00, 1.82)* | 1.47 (1.10, 1.96)* | 1.39 (1.06, 1.83)* |
| Yes | 18,207 | 1.12 (0.91, 1.40) | 1.03 (0.86, 1.24) | 0.93 (0.78, 1.12) | 0.85 (0.71, 1.01) |
| p-Interaction | — | 0.21 | 0.29 | 0.01 | 0.005 |
| Smoking prevalence | |||||
| Low | 12,861 | 1.38 (1.04, 1.84)* | 1.48 (1.16, 1.89)* | 1.41 (1.11, 1.79)* | 1.28 (1.01, 1.62)* |
| High | 12,241 | 1.14 (0.83, 1.55) | 1.12 (0.86, 1.44) | 1.03 (0.81, 1.31) | 0.93 (0.73, 1.17) |
| p-Interaction | — | 0.40 | 0.003 | 0.08 | 0.32 |
| Age, y | |||||
| 0–64 | 24,154 | 1.05 (0.77, 1.41) | 1.11 (0.91, 1.53) | 0.94 (0.73, 1.21) | 0.87 (0.68, 1.11) |
| 7,326 | 1.24 (0.98, 1.55)* | 1.17 (0.96, 1.42) | 1.19 (0.99, 1.43)* | 1.14 (0.96, 1.37) | |
| p-Interaction | — | 0.63 | 0.84 | 0.45 | 0.47 |
| Enrollment Year | |||||
| 2000–2003 | 11,392 | 1.01 (0.77, 1.34) | 1.04 (0.81, 1.33) | 1.05 (0.83, 1.34) | 0.98 (0.77, 1.24) |
| 2004–2006 | 7,140 | 1.68 (1.14, 2.48)* | 1.40 (1.01, 1.95)* | 1.29 (0.94, 1.75) | 1.01 (0.76, 1.36) |
| 2007–2009 | 6,793 | 0.99 (0.67, 1.46) | 1.31 (0.95, 1.82) | 1.37 (0.99, 1.88) | 1.35 (0.99, 1.85) |
| 2010–2012 | 6,155 | 1.33 (0.83, 2.12) | 1.05 (0.73, 1.50) | 0.90 (0.65, 1.25) | 1.00 (0.73, 1.38) |
| p-Interaction | — | 0.07 | 0.69 | 0.84 | 0.44 |
| HIV | |||||
| No | 29,929 | 1.26 (1.04, 1.52)* | 1.19 (1.01, 1.40)* | 1.15 (0.98, 1.34)* | 1.06 (0.91, 1.23) |
| Yes | 1,551 | 0.60 (0.32, 1.14) | 0.95 (0.55, 1.63) | 0.88 (0.51, 1.51) | 1.09 (0.59, 1.99) |
| p-Interaction | — | 0.053 | 0.31 | 0.51 | 0.74 |
Note: CI, confidence interval; DOT, directly observed tuberculosis (TB) treatment; HR, hazard ratio. Adjusted for age, sex, race, ethnicity, foreign birth, recent immigration within 5 y before TB diagnosis, population density, region, unemployment 1 y before TB diagnosis, homeless within one year before TB diagnosis, excess alcohol and/or recreational drug use within one year before TB diagnosis, and HIV infection. The HR represents the highest quintile traffic density compared with the lowest quintile traffic density in each effect modifier level and buffer.
A statistically significant trend () exists across quintiles.
In Kaplan–Meier survival analyses, the median time to culture conversion was 54 d (IQR, 32–83), and the median time to successful completion of treatment was 256 d (IQR, 190–309); these times did not differ significantly between lowest- and highest-quintile nearest-road traffic volumes in the buffer (see Table S3, Figure S1, and Figure S2). The 2-month culture conversion rates were 56% overall, 55% for patients exposed to lowest-quintile nearest-traffic volumes (in the buffer), and 57% for those exposed to highest-quintile traffic. The 9-month successful treatment completion rates were 54% overall, 55% for patients exposed to lowest-quintile traffic, and 53% for patients living near the highest-quintile traffic volumes.
Discussion
To our knowledge, this is the first study to investigate the effects of traffic proximity on mortality during active TB treatment in a large, longitudinally followed patient cohort. We found that patients residing in high-traffic neighborhoods in California were at increased risk of death during TB treatment compared with those residing in low-traffic neighborhoods; we also found that there were statistically significant trends of increasing mortality across increasing quintiles of traffic exposure. Our findings were robust across several analytical models and after controlling for multiple demographic, socioeconomic, and clinical variables.
Our findings are consistent with a growing body of evidence linking traffic proximity and traffic-related air pollution with all-cause, cardiopulmonary, and cancer mortality (Beelen et al. 2008b; Cesaroni et al. 2012, 2013; Hart et al. 2011; Jerrett et al. 2005, 2013; Thurston et al. 2015). Our study expands this evidence by focusing on a specific and potentially vulnerable cohort of patients using individual-level exposure and outcome data. Mortality during TB treatment remains high, as was evident in our study cohort and throughout the world (de Meer and van Geuns 1992; Fielder et al. 2002; Sterling et al. 2006). Although many factors have been implicated in these poor TB patient outcomes (Oursler et al. 2002), the effects of environmental factors such as ambient air pollution have remained uncertain. Our findings provide preliminary evidence that residential proximity to traffic could be an important modifiable risk factor for poor TB outcomes, and further studies investigating the effects of specific traffic-related pollutants are needed to confirm these observations.
Traffic effects were greatest and most statistically significant in the smaller buffers around residential addresses. Our observations are consistent with roadside air quality monitoring studies, which have found progressive decay of ambient pollutant concentrations down to background levels within , with the rate of decay over distance from the road dependent on vegetation barriers, wind direction, and type of pollutant (Karner et al. 2010; Nayeb Yazdi et al. 2015; Zhu et al. 2002). However, between-buffer comparisons should be made with caution in our study. Low-traffic neighborhood roads were not measured in this analysis and likely accounted for a larger proportion of missing data in smaller buffers, thus leading to potential differential misclassification of exposure between buffers.
There was a statistically significant dose–response relationship across many of the 12 exposure scenarios tested, as indicated by . However, the traffic effect on mortality typically plateaued in the third or fourth quintile and often dropped slightly in the fifth quintile. It is possible that the traffic effect on mortality is not linear but rather plateaus with higher levels of exposure. The fifth-quintile exposure level also encompassed a much larger exposure range than the other levels (Table 2), and this heterogeneity in exposure in the fifth quintile could have affected the results for this group.
Several interactions were evident from our analyses. Traffic effects were more pronounced in southern California and the Central Valley, where the background air quality is the poorest in the state (ALA 2016), and were also greater in patients living in low-smoking-prevalence census tracts. Evidence of smoking effect modification has been observed in several ambient air pollution health effects cohort studies, with some studies reporting augmented air pollution effects in smokers and others reporting diminished effects (Beelen et al. 2008a, 2008b; Blount et al. 2013; Puett et al. 2014). To our knowledge, there have been no studies examining the effect modification of smoking in the association between air pollution and TB outcomes, and more research is needed in this area using individual-level data because our smoking analysis was limited by aggregate smoking estimates. Traffic mortality hazards were significantly lower in those exclusively receiving DOT for the duration of their treatment than in those who received at least some self-administered dosing, suggesting that substandard adherence to therapy may accentuate the effects of traffic exposures in patients with active TB. We are unaware of other studies that have evaluated medication adherence and air pollution interactions, and further research is needed to corroborate these findings because air pollution and medication adherence may both be important risk factors for poor treatment outcomes among vulnerable groups with respiratory disease (Canino et al. 2006). Traffic effects were slightly higher in those and in those with diabetes, raising concern that advanced age and certain chronic diseases could increase susceptibility to the harmful effects of air pollution. Further research is needed in these vulnerable populations.
Our observational study is hypothesis-generating for future studies. Through what biologic mechanisms did TRAP contribute to mortality in patients undergoing TB treatment? It is possible that exposure to TRAP has deleterious effects on immunologic responses to Mtb, a theory supported by in vitro and animal studies. For instance, TB-infected rats chronically exposed to diesel exhaust particles (DEP) carried a higher mycobacterial burden than nonexposed controls (Hiramatsu et al. 2005), and Mtb-stimulated human peripheral blood monocytes exposed to DEP demonstrated suppression of key host antimycobacterial immune responses (Sarkar et al. 2012).
Our study has several strengths. TB patients were followed longitudinally with demographic, clinical, treatment, and mortality data collected prospectively by trained health care providers at the time of clinic visits, decreasing the likelihood of recall bias and reporting errors. This was a large cohort study focusing on a specific disease process for the entire state of California, where TB notification rates are estimated at (Curtis et al. 2001), limiting the risk for potential selection bias. Medical fees were not a significant barrier to receipt of necessary TB care, which is covered by private health insurance, Medi-Cal, and county Department of Public Health programs, thereby also reducing loss to follow-up secondary to inability to pay for treatment.
Our study also has several limitations. Exposures with time dependency, namely traffic volumes, traffic density, and residential addresses, were assigned fixed measurement times and values, likely leading to exposure misclassification. For instance, we calculated traffic volumes and traffic densities from 2004 Caltrans data, reflecting traffic conditions near the midpoint of our study. This was a reasonable approach because spatial traffic gradients tend to remain stable over time, with a relatively homogenous increase in traffic volumes as population increases (Beelen et al. 2007, 2008a). Any exposure misclassification introduced would likely be nondifferential, biasing results toward the null. Indeed, traffic effects appeared to be smaller in patients enrolled before 2004 and after 2009, although this effect modification did not reach statistical significance. Geocoding was not performed concurrently with patient enrollment, but rather during the analysis, using 2010–2011 geocoding reference data sets. This difference in timing introduced slight inaccuracies in geocoding the addresses of patients enrolled before or after the reference period, but the overall accuracy of the match remained high (score 93/100) with the exclusion of only 2% of TB patients owing to an inability to geocode addresses. Traffic exposure estimates were limited to residential addresses and did not account for microenvironments such as household air pollution, indoor and outdoor pollution at work or school locations, or commuting patterns. We did not estimate exposures to specific traffic-related air pollutants such as , , or ozone. Any misclassifications of exposure were likely randomly distributed, resulting in an underestimate of true adverse effects of TRAP on TB treatment outcomes. We only considered baseline home addresses and did not exclude those who moved to different addresses within California during follow-up. However, exclusion of movers in alternative Cox models did not alter mortality hazards. Determining the hazards of traffic exposures on cause-specific mortality would have been informative, but these data were not available. The differences in baseline characteristics by traffic density are likely a reflection of inherent socioeconomic differences present in those living in highly polluted areas rather than a reflection of selection bias given the similarities in baseline characteristics between those excluded and those included in the final survival analyses. The California TB Registry provided a wealth of covariates but no individual-level direct measures of poverty or tobacco smoking. Poverty could contribute to poor outcomes at multiple stages in the TB management cascade, from late presentation for diagnosis to lack of access to medical care for comorbidities to increased exposure to other risk factors. To address these limitations, in alternative models (Table 4), we adjusted for census block group household income and census-tract smoking prevalence, and we found that adjusting for group-level smoking augmented the effects of traffic exposure on mortality. However, to avoid misinterpretation of group-level data that could give rise to the ecologic fallacy (Haneuse and Bartell 2011), we only employed aggregate data in alternative models. Traffic data were not available for small local roads, which likely led to an underestimation of traffic densities at residential addresses and to the exclusion of patients who did not live near major roads. Excluded patients were similar to those included except for factors that appeared to be related to rural living, and of the included patients, 98% lived within the city limits. As such, our findings are not generalizable to TB patients living outside of towns and cities.
Conclusion
TB patients living in high-traffic neighborhoods were at increased risk for mortality during treatment even after controlling for demographic, socioeconomic, and clinical factors. Our findings suggest that TB patients are susceptible to the adverse health effects of traffic-related air pollution and that traffic exposure might be an important modifiable risk factor for poor TB treatment outcomes. These findings should be confirmed with additional studies to determine the effects of traffic-related pollutants, and of traffic measures in other settings, on tuberculosis outcomes.
Supplemental Material
Acknowledgments
We thank S. Kanowitz and J. Westenhouse (California Department of Public Health) for TB Registry data management and A. Catanzaro (University of California, San Diego) for assistance with study conception and design. We acknowledge the Berkeley Center for Environmental Public Health Tracking and the Geographic Information Science Health and Exposure Assessment Lab at the University of California, Berkeley for census-tract smoking prevalence estimates.
Supported by the National Institute of Environmental Health Sciences/National Institutes of Health [F32ES022582 (RJB) and K23ES025807 (RJB)] and by the National Institute of Allergy and Infectious Diseases [R01AI104589 (PN)]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The views expressed in this document are solely those of the authors and do not necessarily reflect views of the National Institutes of Health.
References
- ALA (American Lung Association). 2016. State of the air: California overview. http://www.lung.org/local-content/california/documents/state-of-the-air/2016/sota-2016_ca-overview.pdf [accessed 21 April 2016].
- ARB [Air Resources Board (California Environmental Protection Agency)]. 2017. Emission Inventory Data. https://www.arb.ca.gov/ei/emissiondata.htm [accessed 29 May 2017].
- Bates MN, Khalakdina A, Pai M, Chang L, Lessa F, Smith KR. 2007. Risk of tuberculosis from exposure to tobacco smoke: A systematic review and meta-analysis. Arch Intern Med 167:335, PMID: 17325294, 10.1001/archinte.167.4.335. [DOI] [PubMed] [Google Scholar]
- Batterman S, Burke J, Isakov V, Lewis T, Mukherjee B, Robins T. 2014. A comparison of exposure metrics for traffic-related air pollutants: Application to epidemiology studies in Detroit, Michigan. Int J Environ Res Public Health 11(9):9553–9577, PMID: 25226412, 10.3390/ijerph110909553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beelen R, Hoek G, Fischer P, van den Brandt PA, Brunekreef B. 2007. Estimated long-term outdoor air pollution concentrations in a cohort study. Atmos Environ 41(7):1343–1358, 10.1016/j.atmosenv.2006.10.020. [DOI] [Google Scholar]
- Beelen R, Hoek G, van den Brandt PA, Goldbohm RA, Fischer P, Schouten LJ, et al. 2008a. Long-term exposure to traffic-related air pollution and lung cancer risk. Epidemiology 19(5):702–710, PMID: 18633326, 10.1097/EDE.0b013e318181b3ca. [DOI] [PubMed] [Google Scholar]
- Beelen R, Hoek G, van den Brandt PA, Goldbohm RA, Fischer P, Schouten LJ, et al. 2008b. Long-term effects of traffic-related air pollution on mortality in a Dutch cohort (NLCS-AIR study). Environ Health Perspect 116(2):196–202, PMID: 18288318, 10.1289/ehp.10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount RJ, Djawe K, Daly KR, Jarlsberg LG, Fong S, Balmes J, et al. 2013. Ambient air pollution associated with suppressed serologic responses to Pneumocystis jirovecii in a prospective cohort of HIV-infected patients with Pneumocystis pneumonia. PloS One 8(11):e80795, PMID: 24236202, 10.1371/journal.pone.0080795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer M, Amann M, Burnett RT, Cohen A, Dentener F, Ezzati M, et al. 2012. Exposure assessment for estimation of the global burden of disease attributable to outdoor air pollution. Environ Sci Technol 46(2):652–660, PMID: 22148428, 10.1021/es2025752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canino G, Koinis-Mitchell D, Ortega AN, McQuaid EL, Fritz GK, Alegría M. 2006. Asthma disparities in the prevalence, morbidity, and treatment of Latino children. Soc Sci Med 63(11):2926–2937, PMID: 16956704, 10.1016/j.socscimed.2006.07.017. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2010. Chemistry and toxicology of cigarette smoke and biomarkers of exposure and harm. In: How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta, Georgia:Centers for Disease Control and Prevention. [PubMed] [Google Scholar]
- Cesaroni G, Porta D, Badaloni C, Stafoggia M, Eeftens M, Meliefste K, et al. 2012. Nitrogen dioxide levels estimated from land use regression models several years apart and association with mortality in a large cohort study. Environ Health 11:48, PMID: 22808928, 10.1186/1476-069X-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesaroni G, Badaloni C, Gariazzo C, Stafoggia M, Sozzi R, Davoli M, et al. 2013. Long-term exposure to urban air pollution and mortality in a cohort of more than a million adults in Rome. Environ Health Perspect 121(3):324–331, PMID: 23308401, 10.1289/ehp.1205862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu HF, Cheng MH, Yang CY. 2009. Air pollution and hospital admissions for pneumonia in a subtropical city: Taipei, Taiwan. Inhal Toxicol 21(1):32–37, PMID: 18923947, 10.1080/08958370802441198. [DOI] [PubMed] [Google Scholar]
- Curtis AB, McCray E, McKenna M, Onorato IM. 2001. Completeness and timeliness of tuberculosis case reporting: A multistate study. Am J Prev Med 20(2):108–112, PMID: 11165451, 10.1016/S0749-3797(00)00284-1. [DOI] [PubMed] [Google Scholar]
- de Meer G, van Geuns HA. 1992. Rising case fatality of bacteriologically proven pulmonary tuberculosis in the Netherlands. Tuber Lung Dis 73(2):83–86, PMID: 1643302, 10.1016/0962-8479(92)90060-W. [DOI] [PubMed] [Google Scholar]
- Fielder JF, Chaulk CP, Dalvi M, Gachuhi R, Comstock GW, Sterling TR. 2002. A high tuberculosis case-fatality rate in a setting of effective tuberculosis control: Implications for acceptable treatment success rates. Int J Tuberc Lung Dis 6(12):1114–1117, PMID: 12546121. [PubMed] [Google Scholar]
- Gentner DR, Worton DR, Isaacman G, Davis LC, Dallmann TR, Wood EC, et al. 2013. Chemical composition of gas-phase organic carbon emissions from motor vehicles and implications for ozone production. Environ Sci Technol 47(20):11837–11848, PMID: 24011064, 10.1021/es401470e. [DOI] [PubMed] [Google Scholar]
- Haneuse S, Bartell S. 2011. Designs for the combination of group- and individual-level data. Epidemiology 22(3):382–389, PMID: 21490533, 10.1097/EDE.0b013e3182125cff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart JE, Garshick E, Dockery DW, Smith TJ, Ryan L, Laden F. 2011. Long-term ambient multipollutant exposures and mortality. Am J Respir Crit Care Med 183(1):73–78, PMID: 20656944, 10.1164/rccm.200912-1903OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu K, Saito Y, Sakakibara K, Azuma A, Kudoh S, Takizawa H, et al. 2005. The effects of inhalation of diesel exhaust on murine mycobacterial infection. Exp Lung Res 31(4):405–415, PMID: 16025921, 10.1080/01902140590918786. [DOI] [PubMed] [Google Scholar]
- Horne DJ, Campo M, Ortiz JR, Oren E, Arentz M, Crothers K, et al. 2012. Association between smoking and latent tuberculosis in the U.S. population: An analysis of the National Health and Nutrition Examination Survey. PloS One 7(11):e49050, PMID: 23145066, 10.1371/journal.pone.0049050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SS, Kang S, Lee JY, Lee JS, Kim HJ, Han SK, et al. 2014. Impact of outdoor air pollution on the incidence of tuberculosis in the Seoul metropolitan area, South Korea. Korean J Intern Med 29(2):183–190, PMID: 24648801, 10.3904/kjim.2014.29.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassal MS, Bakman I, Jones B. 2013. Correlation of ambient pollution levels and heavily-trafficked roadway proximity on the prevalence of smear-positive tuberculosis. Public health 127(3):268–274, PMID: 23453197, 10.1016/j.puhe.2012.12.030. [DOI] [PubMed] [Google Scholar]
- Jee SH, Golub JE, Jo J, Park IS, Ohrr H, Samet JM. 2009. Smoking and risk of tuberculosis incidence, mortality, and recurrence in South Korean men and women. Am J Epidemiol 170(12):1478–1485, PMID: 19917554, 10.1093/aje/kwp308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrett M, Burnett RT, Ma R, Pope CA 3rd, Krewski D, Newbold KB, et al. 2005. Spatial analysis of air pollution and mortality in Los Angeles. Epidemiology 16(6):727–736, PMID: 16222161, 10.1097/01.ede.0000181630.15826.7d. [DOI] [PubMed] [Google Scholar]
- Jerrett M, Burnett RT, Beckerman BS, Turner MC, Krewski D, Thurston G, et al. 2013. Spatial analysis of air pollution and mortality in California. Am J Respir Crit Care Med 188(5):593–599, PMID: 23805824, 10.1164/rccm.201303-0609OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karjalainen P, Pirjola L, Heikkilä J, Lähde T, Tzamkiozis T, Ntziachristos L, et al. 2014. Exhaust particles of modern gasoline vehicles: A laboratory and an on-road study. Atmos Environ 97:262–270, 10.1016/j.atmosenv.2014.08.025. [DOI] [Google Scholar]
- Karner AA, Eisinger DS, Niemeier DA. 2010. Near-roadway air quality: Synthesizing the findings from real-world data. Environ Sci Technol 44(14):5334–5344, PMID: 20560612, 10.1021/es100008x. [DOI] [PubMed] [Google Scholar]
- Lai TC, Chiang CY, Wu CF, Yang SL, Liu DP, Chan CC, et al. 2016. Ambient air pollution and risk of tuberculosis: A cohort study. Occup Environ Med 73(1):56–61, PMID: 26514394, 10.1136/oemed-2015-102995. [DOI] [PubMed] [Google Scholar]
- Lelieveld J, Evans JS, Fnais M, Giannadaki D, Pozzer A. 2015. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 525(7569):367–371, PMID: 26381985, 10.1038/nature15371. [DOI] [PubMed] [Google Scholar]
- Lin HH, Ezzati M, Murray M. 2007. Tobacco smoke, indoor air pollution and tuberculosis: A systematic review and meta-analysis. PLoS Med 4(1):e20, PMID: 17227135, 10.1371/journal.pmed.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciel EL, Brioschi AP, Peres RL, Guidoni LM, Ribeiro FK, Hadad DJ, et al. 2013. Smoking and 2-month culture conversion during anti-tuberculosis treatment. Int J Tuberc Lung Dis 17(2):225–228, PMID: 23317958, 10.5588/ijtld.12.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R, Islam T, Shankardass K, Jerrett M, Lurmann F, Gilliland F, et al. 2010. Childhood incident asthma and traffic-related air pollution at home and school. Environ Health Perspect 118(7):1021–1026, PMID: 20371422, 10.1289/ehp.0901232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayeb Yazdi M, Delavarrafiee M, Arhami M. 2015. Evaluating near highway air pollutant levels and estimating emission factors: Case study of Tehran, Iran. Sci Total Environ 538:375–384, PMID: 26318222, 10.1016/j.scitotenv.2015.07.141. [DOI] [PubMed] [Google Scholar]
- Neupane B, Jerrett M, Burnett RT, Marrie T, Arain A, Loeb M. 2010. Long-term exposure to ambient air pollution and risk of hospitalization with community-acquired pneumonia in older adults. Am J Respir Crit Care Med 181(1):47–53, PMID: 19797763, 10.1164/rccm.200901-0160OC. [DOI] [PubMed] [Google Scholar]
- Ortega Hinojosa AM, Davies MM, Jarjour S, Burnett RT, Mann JK, Hughes E, et al. 2014. Developing small-area predictions for smoking and obesity prevalence in the United States for use in environmental public health tracking. Environ Res 134:435–452, PMID: 25261951, 10.1016/j.envres.2014.07.029. [DOI] [PubMed] [Google Scholar]
- Oursler KK, Moore RD, Bishai WR, Harrington SM, Pope DS, Chaisson RE. 2002. Survival of patients with pulmonary tuberculosis: Clinical and molecular epidemiologic factors. Clin Infect Dis 34(6):752–759, PMID: 11850859, 10.1086/338784. [DOI] [PubMed] [Google Scholar]
- Pascopella L, Barry PM, Flood J, DeRiemer K. 2014. Death with tuberculosis in California, 1994–2008. Open Forum Infect Dis 1(3):ofu090, PMID: 25734158, 10.1093/ofid/ofu090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Liu C, Xu B, Kan H, Wang W. 2017. Long-term exposure to ambient air pollution and mortality in a Chinese tuberculosis cohort. Sci Total Environ 580:1483–1488, PMID: 28038878, 10.1016/j.scitotenv.2016.12.128. [DOI] [PubMed] [Google Scholar]
- Puett RC, Hart JE, Yanosky JD, Spiegelman D, Wang M, Fisher JA, et al. 2014. Particulate matter air pollution exposure, distance to road, and incident lung cancer in the Nurses' Health Study cohort. Environmental health perspectives 122(9):926–932, PMID: 24911062, 10.1289/ehp.1307490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas JL, Mindra G, Haddad MB, Pratt R, Price SF, Langer AJ. 2016. Leveling of tuberculosis incidence - United States, 2013–2015. MMWR Morb Mortal Wkly Rep 65(11):273–278, PMID: 27010173, 10.15585/mmwr.mm6511a2. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Song Y, Sarkar S, Kipen HM, Laumbach RJ, Zhang J, et al. 2012. Suppression of the NF-κB pathway by diesel exhaust particles impairs human antimycobacterial immunity. J Immunol 188(6):2778–2793, PMID: 22345648, 10.4049/jimmunol.1101380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slama K, Chiang CY, Enarson DA, Hassmiller K, Fanning A, Gupta P, et al. 2007. Tobacco and tuberculosis: A qualitative systematic review and meta-analysis. Int J Tuberc Lung Dis 11(10):1049–1061, PMID: 17945060. [PubMed] [Google Scholar]
- Smith GS, Van Den Eeden SK, Garcia C, Shan J, Baxter R, Herring AH, et al. 2016. Air pollution and pulmonary tuberculosis: A nested case–control study among members of a northern California health plan. Environ Health Perspect 124(6):761–768, PMID: 26859438, 10.1289/ehp.1408166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling TR, Zhao Z, Khan A, Chaisson RE, Schluger N, Mangura B, et al. 2006. Mortality in a large tuberculosis treatment trial: Modifiable and non-modifiable risk factors. Int J Tuberc Lung Dis 10(5):542–549, PMID: 16704037. [PubMed] [Google Scholar]
- Thurston GD, Ahn J, Cromar KR, Shao Y, Reynolds HR, Jerrett M, et al. 2015. Ambient particulate matter air pollution exposure and mortality in the NIH-AARP Diet and Health Cohort. Environ Health Perspect 124(4):484–490, PMID: 26370657, 10.1289/ehp.1509676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. 2016. American Community Survey (ACS). https://www.census.gov/programs-surveys/acs/ [accessed May 3 2016].
- Vittinghoff E, Glidden D, Shiboski SC, McCulloch C. 2012. Regression Methods in Biostatistics. 2nd ed New York:Springer. [Google Scholar]
- Webb GB. 1918. The effect of the inhalation of cigarette smoke on the lungs. A clinical study. Am Rev Tuberc 2(1):25–27. [Google Scholar]
- WHO (World Health Organization). 2015. Global tuberculosis report 2015. http://www.who.int/tb/publications/global_report/gtbr15_main_text.pdf [accessed 8 October 2016].
- WHO. 2016. Tuberculosis fact sheet. http://www.who.int/mediacentre/factsheets/fs104/en/ [accessed 29 April 2016].
- You S, Tong YW, Neoh KG, Dai Y, Wang CH. 2016. On the association between outdoor PM2.5 concentration and the seasonality of tuberculosis for Beijing and Hong Kong. Environ Pollut 218:1170–1179, PMID: 27595179, 10.1016/j.envpol.2016.08.071. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J, Dockery DW. 2000. Airborne particles are a risk factor for hospital admissions for heart and lung disease. Environ Health Perspect 108(11):1071, PMID: 11102299, 10.1289/ehp.001081071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Hinds WC, Kim S, Sioutas C. 2002. Concentration and size distribution of ultrafine particles near a major highway. J Air Waste Manag Assoc 52(9):1032–1042, PMID: 12269664, 10.1080/10473289.2002.10470842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



![Line graph plotting cumulative hazard (y-axis) across days of treatment (x-axis) for quantiles Q1 (referent), Q2 [1.03 (0.86 to 1.25)], Q3 [1.14 (0.95 to 1.37)], Q4 [1.19 (0.99 to 1.43)], and Q5 [1.28 (1.07 to 1.53)]. The p-trend equals 0.002.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/e42c/5915191/02f55d6cd569/EHP1699_f2.jpg)