Abstract
Background:
Phenols and phthalates may have immunomodulatory and proinflammatory effects and thereby adversely affect respiratory health.
Objective:
We estimated the associations between gestational exposure to select phthalates and phenols and respiratory health in boys.
Methods:
Among 587 pregnant women from the EDEN (Etude des Déterminants pré et post natals du développement et de la santé de l’Enfant) cohort who delivered a boy, 9 phenols and 11 phthalates metabolites were quantified in spot pregnancy urine samples. Respiratory outcomes were followed up by questionnaires until age 5, when forced expiratory volume in 1 s () was measured by spirometry. Adjusted associations of urinary metabolites log–transformed concentrations with respiratory outcomes and in percent predicted () were estimated by survival and linear regression models, respectively.
Results:
No phenol or phthalate metabolite exhibited clear deleterious associations simultaneously with several respiratory outcomes. Ethyl-paraben was associated with increased asthma rate [; 95% confidence interval (CI): 1.00, 1.21] and tended to be negatively associated with (; 95% CI: , 0.05); bisphenol A tended to be associated with increased rates of asthma diagnosis (; 95% CI: 0.97, 1.55) and bronchiolitis/bronchitis (; 95% CI: 0.99, 1.30). Isolated trends for deleterious associations were also observed between 2,5-dichlorophenol and wheezing, and between monocarboxynonyl phthalate, a metabolite of di-isodecyl phthalate (DIDP), and wheezing.
Conclusion:
Ethyl-paraben, bisphenol A, 2,5-dichlorophenol, and DIDP tended to be associated with altered respiratory health, with ethyl-paraben and bisphenol A exhibiting some consistency across respiratory outcomes. The trends between bisphenol A pregnancy level and increased asthma and bronchiolitis/bronchitis rates in childhood were consistent with a previous cohort study. https://doi.org/10.1289/EHP1015
Introduction
Asthma is now the most frequent chronic childhood disease, affecting of children in Western countries (Baldacci et al. 2015). Changes in the prevalence of exposure to environmental factors in the 20th century, including synthetic chemicals, have been suggested to contribute to the increased asthma prevalence (Bornehag and Nanberg 2010). Concern exists specifically regarding phenols and phthalates, two families of suspected endocrine disruptors.
Phenols and phthalates are produced in large volumes. Bisphenol A is found in food packaging or epoxy resins (such uses were banned in France in 2015). Other phenols, such as parabens, benzophenone-3, and triclosan are found in cosmetics, sunscreens, and antibacterial soaps, while some dichlorophenols are intermediates in the production of herbicides and room deodorizers. Phthalates are mainly used as plasticizers and are present in many plastic products, such as polyvinyl chloride floor covering, toys, and food packaging. Some phthalates are components of solvents and personal care products (e.g., soap, nail polish, lotion, fragrances), and are used as excipients in pharmaceuticals (Koniecki et al. 2011).
Due to immaturity of the lungs, and of the immune system and due to the physiology of development, early-life exposures may have long-term adverse effect on respiratory health (Miller and Marty 2010). Experimental evidence suggests that bisphenol A and phthalates such as di(2-ethylhexyl) phthalate (DEHP), di-isononyl phthalate (DINP), and butylbenzyl phthalate (BBzP) or their monoester metabolites can cross the placenta and may have proallergic properties (Bornehag and Nanberg 2010; Kwak et al. 2009). In mice, prenatal exposure to bisphenol A has been associated with increased allergic sensitization and bronchial inflammation (Nakajima et al. 2012). In humans, few longitudinal studies focused on the prenatal exposure window and have reported increased rates of asthma, wheeze, and respiratory tract infections with bisphenol A, BBzP, and DEHP metabolites (Donohue et al. 2013; Gascon et al. 2015; Ku et al. 2015; Smit et al. 2015; Spanier et al. 2012, 2014b; Whyatt et al. 2014a, 2014b). No prospective study evaluated the impact of phenols other than BPA on respiratory health; regarding phthalates, DINP and di-isodecyl phthalate (DIDP), which are increasingly used as DEHP substitutes, have not been studied. Only one study examined the association between pulmonary function in childhood and prenatal exposure to phenols or phthalates; the study related bisphenol A to spirometric tests, and it suggested an adverse association between bisphenol A level and spirometric tests at 4 y but not at 5 y of age (Spanier et al. 2014b). To our knowledge, no study has investigated the effects of prenatal exposure to other phenols or phthalates on pulmonary function measurements.
Our aim was to characterize associations between prenatal exposure to select phenols and phthalates and the development of respiratory pathologies in the first 5 y of life and pulmonary function in male offspring 5 y old. Compounds with the highest a priori likelihood in favor of an effect were, on the basis of animal studies, DEHP, bisphenol A, and, to a lesser extent, DINP.
Methods
Data Source
This study relied on a subgroup of the EDEN (Etude des Déterminants pré et post natals du développement et de la santé de l’Enfant) mother–child cohort. Briefly, 2,002 pregnant women were recruited before 24 gestational wk in two university hospitals in Nancy and Poitiers, France. Exclusion criteria included prepregnancy diabetes, multiple gestation, inability to read or speak French, and moving outside the region planned within the next 3 y. The detailed study protocol has been described previously (Philippat et al. 2014).
The present study included all male offspring with metabolites of pregnancy phenols and phthalates measured in a maternal spot urine sample and for whom at least one completed respiratory questionnaire or an acceptable pulmonary function test (spirometry) was available. Phenols and phthalates biomarkers were originally quantified in the urine samples of male offspring’s mothers only, as part of previous studies investigating the impact of maternal exposure to endocrine disruptors on male genital anomalies and further on male fetal and postnatal growth (Chevrier et al. 2012; Philippat et al. 2014). All participants provided written informed consent for themselves and their offspring for biological measurements and data collection. The EDEN cohort was approved by the following ethics committees: Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale (CCPPRB) of Kremlin Bicêtre on 12 December 2002 and Commission Nationale Informatique et Liberté, which is the French data privacy institution (Heude et al. 2016). The involvement of the Centers for Disease Control and Prevention (CDC) did not constitute engagement in human subject research.
Exposure Assessment
Between 23 and 29 gestational wk, pregnant women were asked to come for a clinical examination with a sample of their first morning void. If forgotten, the urine sample was collected during the study visit. Polypropylene containers were used to avoid any contamination, and urine samples were stored at before shipments for analyses to the CDC laboratory in Atlanta, Georgia, at two distinct periods.
Urinary concentrations of creatinine, nine phenols, and 11 phthalates metabolites (listed in Tables 1 and S1) were measured. Molar concentrations were summed for four parabens (), 2,4 and 2,5-dichlorophenols (), four DEHP metabolites (), total low-molecular-weight () phthalates (), and high-molecular-weight () phthalates (). Concentrations under the limit of detection were replaced by instrumental reading values or by the compound-specific lowest instrumental reading value divided by the square root of two when the instrumental reading value was missing. Biomarkers and creatinine concentrations were quantified following identical analytical methodology in 2008 () and in 2011 () (Philippat et al. 2014). A two-step standardization approach was applied to reduce the undesirable variability in biomarker urinary concentrations owed to sampling conditions. First, linear regressions were conducted to estimate the effects of sampling conditions (day and hour of sampling, gestational age at urine collection, storage duration at room temperature before freezing, and year of biomarker analysis) and level of creatinine on each ln-transformed biomarker concentration. Second, these regression estimates were used to predict standardized concentrations that would have been observed if all samples had been collected under identical conditions (Mortamais et al. 2012).
Table 1.
Raw and standardized urinary concentrations of phthalate and phenol biomarkers among pregnant women from included population (, EDEN cohort).
| Analyte | LOD () | (%) | Raw concentrations ()a | Standardized concentrations ()b | Spearman correlationc | ||||
|---|---|---|---|---|---|---|---|---|---|
| 5th | 50th | 95th | 5th | 50th | 95th | ||||
| 2,4-Dichlorophenol | 0.2 | 97 | 0.3 | 1.0 | 9.9 | 0.3 | 1.0 | 9.0 | 0.95 |
| 2,5-Dichlorophenol | 0.2 | 100 | 1.6 | 9.8 | 278.0 | 1.8 | 9.4 | 279.4 | 0.97 |
| 0.0 | 0.1 | 1.8 | 0.0 | 0.1 | 1.8 | 0.96 | |||
| Bisphenol A | 0.4 | 99 | 0.6 | 2.6 | 10.7 | 0.8 | 2.4 | 8.9 | 0.86 |
| Benzophenone-3 | 0.4 | 91 | 0.2 | 2.1 | 81.2 | 0.3 | 2.3 | 75.4 | 0.97 |
| Triclosan | 2.3 | 81 | 0.1 | 29.3 | 744.0 | 0.2 | 27.6 | 697.9 | |
| Methyl-paraben | 1.0 | 100 | 7.8 | 118.0 | 1730.0 | 7.9 | 111.4 | 1152.2 | 0.96 |
| Ethyl-paraben | 1.0 | 72 | 0.1 | 4.5 | 74.4 | 0.1 | 3.4 | 68.6 | 0.94 |
| Propyl-paraben | 0.2 | 98 | 0.5 | 16.1 | 289.0 | 0.5 | 14.3 | 258.3 | 0.97 |
| Butyl-paraben | 0.2 | 82 | 0.1 | 1.9 | 59.7 | 0.1 | 1.9 | 57.6 | 0.96 |
| 0.1 | 1.0 | 13.8 | 0.1 | 0.9 | 9.9 | 0.96 | |||
| 0.3 | 1.3 | 8.4 | 0.4 | 1.1 | 6.2 | 0.85 | |||
| MEP | 0.8 | 100 | 20.6 | 113.0 | 1050.0 | 22.0 | 99.0 | 703.2 | 0.90 |
| MnBP | 0.6 | 100 | 8.1 | 52.4 | 515.0 | 12.5 | 44.4 | 444.8 | 0.89 |
| MiBP | 0.3 | 100 | 8.9 | 45.1 | 218.0 | 11.8 | 40.2 | 167.6 | 0.84 |
| 0.1 | 0.5 | 2.2 | 0.2 | 0.5 | 1.7 | 0.84 | |||
| MCPP | 0.2 | 99 | 0.5 | 2.3 | 11.2 | 0.7 | 1.9 | 9.3 | 0.87 |
| MBzP | 0.3 | 100 | 3.0 | 20.0 | 135.0 | 4.6 | 18.2 | 105.5 | 0.85 |
| MCNP | 0.6 | 97 | 0.5 | 1.5 | 12.9 | 0.5 | 1.3 | 10.2 | 0.90 |
| MCOP | 0.7 | 98 | 0.9 | 3.7 | 18.4 | 1.2 | 4.0 | 19.6 | 0.90 |
| MEHHP | 0.7 | 100 | 5.4 | 30.4 | 124.0 | 6.8 | 26.7 | 99.2 | 0.87 |
| MEOHP | 0.7 | 100 | 4.2 | 24.2 | 105.0 | 5.4 | 22.3 | 84.2 | 0.87 |
| MECPP | 0.6 | 100 | 9.9 | 43.0 | 183.0 | 12.3 | 38.2 | 156.9 | 0.88 |
| MEHP | 1.2 | 96 | 0.9 | 8.3 | 40.7 | 1.3 | 7.4 | 34.4 | 0.90 |
| 0.1 | 0.4 | 1.5 | 0.1 | 0.3 | 1.2 | 0.87 | |||
Note: 5th, 50th, 95th, percentiles; , molar sum of di(2-ethylhexyl) phthalate metabolites (MEHHP, MEOHP, MECPP, MEHP); , molar sum of 2-dichlorophenols (2,4-dichlorophenol, 2,5-dichlorophenol); , molar sum of high-molecular-weight phthalates (MCPP, MBzP, MCNP, MCOP, MEHHP, MEOHP, MECPP, MEHP); , molar sum of low-molecular-weight phthalates (MEP, MnBP, MiBP); LOD, limit of detection; MBzP, monobenzyl phthalate; MCNP, monocarboxynonyl phthalate; MCOP, monocarboxy-isooctyl phthalate; MCPP, mono (3-carboxypropyl) phthalate; MECPP, mono(2-ethyl-5-carboxypentyl) phthalate; MEHP, mono(2-ethylhexyl)phthalate; MEHHP, mono-(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP, mono(2-ethyl-5-oxohexyl)phthalate; MEP, monoethyl phthalate; MnBP, mono-n-butyl phthalate; MiBP, mono-isobutyl phthalate; , molar sum of parabens (methyl-, ethyl-, propyl-, butyl-parabens); phet, p-values of heterogeneity. Parent phthalates are detailed in Table S1.
Biomarker concentrations were replaced by instrumental reading values. Machine values equal to 0 were replaced by the lowest machine value divided by square root of two.
Standardized for urine sampling conditions (creatinine level, day and hour of sampling, gestational age, storage duration at room temperature, and year of analysis), as detailed in Mortamais et al. (2012).
Spearman correlation between measured and standardized biomarkers concentrations.
Respiratory Health
The French-enriched version of the International Study of Allergy and Asthma in Children self-report questionnaires was used to assess doctor-diagnosed asthma, wheezing at age 8 months and at each year until age 5 y, and bronchiolitis/bronchitis episode until age 3 y in offspring. Doctor-diagnosed asthma and wheezing were defined from the questions: “Did your child ever have a medical diagnosis of asthma?” and “In the last 12 months (or since birth for the first questionnaire), has your child had wheezing in the chest?” Bronchiolitis/bronchitis was defined from the question: “In the last 12 mo (or since birth for the first questionnaire), has your child had a bronchiolitis or bronchitis?”
Spirometry was performed using SpiroBank® G Spirometer by MIR (Rome, Italy) at about 5 y of age by trained personnel, following the American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines. Boys were seated, wearing nose clips. Between three and eight forced expiratory maneuvers were performed. Results were classified by a pediatric pulmonologist as acceptable or unacceptable in accordance with ATS/ERS recommendations for preschool children (Beydon et al. 2007). Criteria for an acceptable maneuver were: a rapid rise and a smooth or convex descending limb in the flow–volume curve, without artifact(glottic closure, cough, leaks), with forced expiration times larger than 1 s. We considered the forced expiratory volume in 1 s (), a standardized and reproducible test (National Asthma Education and Prevention Program 2007). The highest from any of the satisfactory maneuvers was expressed as a percentage of the age-, height-, sex-, and ethnic-specific predicted value () calculated with the Global Lung Initiative equations (Quanjer et al. 2012).
Statistical Analysis
Associations between each biomarker standardized concentration and the first occurrence of respiratory outcomes were investigated by distinct discrete time survival models with a complementary log–log link function (Jenkins 1995). followed approximately a normal distribution and was analyzed by distinct linear regression models in which biomarker concentrations were considered separately as ln-transformed continuous concentrations. To describe the dose–response relationship, exposure was additionally categorized into tertiles of biomarker concentrations. We calculated for trends using a variable with three categories whose values corresponded to the median value in each tertile, coded in models as a continuous variable (Rothman et al. 2008). Adjustment variables were identified a priori from a review of the literature. Variables were retained in the model if they were associated with the outcome () or if their removal or addition changed the regression coefficients of the associations between phenols or phthalates biomarkers and the outcome by . Selected variables included center of recruitment (Nancy/Poitiers); residence area (city center/urban area/rural area); maternal country of birth (mainland France/others); parental history of asthma, rhinitis, eczema, or food allergies; the highest parental education level (); passive or active maternal smoking during pregnancy (yes/no); presence of older siblings; child care attendance before 1 y; and postnatal passive smoking (yes/no, time-varying covariate in survival models). models were additionally adjusted for offspring’s age (continuous) and height (restricted cubic splines coding) at pulmonary function assessment. Missing data in covariates (between 0.17% and 24.7%) were imputed with multiple imputation methods (White et al. 2011). We did not formally test statistical significance nor correct for multiple comparison, but in interpreting results, we looked for consistency of deleterious associations across several respiratory phenotypes. We conducted further sensitivity analyses to address the robustness of the results a) to the standardization of the concentrations by repeating analyses with nonstandardized concentrations and b) after excluding offspring with the following major risk factors of respiratory symptoms: preterm birth, smoking mother, or parental history of asthma. Analyses were conducted using STATA 12.1 (Stata Corp).
Results
Population
The EDEN cohort included 995 live-born male offspring. From previous studies that investigated the effects of maternal exposure to endocrine disruptors on male genital anomalies and male fetal and postnatal growth, we used urine samples of the mothers of 604 boys in which phenol metabolites had been measured. At least one respiratory questionnaire that had been completed by a parent was available for 587 boys who were included in the analyses of respiratory outcomes. The numbers of boys with complete follow-up data were 428 (73%) for bronchiolitis/bronchitis (until 3 y of age) and 350 (60%) and 447 (76%) for wheezing and asthma diagnosis, respectively (follow-up until 5 y of age). One value for at least one covariate was missing for 277 boys. A spirometric test has been performed in 397 (68%) out of the 587 boys. For 95% of these boys, spirometry occurred between 5.4 and 6.0 y of age, 39% out of 397 boys were excluded due to insufficient forced expiratory times, and 4% did not meet the criteria considered acceptable for at least one of their spirometric tests, so that 228 boys (57%) were included in the present study for analysis. Participants included in the analyses were comparable to the excluded EDEN male offspring with regard to most characteristics, but were more likely to have highly educated parents, to be born from French metropolitan mothers, and to live in a nonsmoking environment (Table 2).
Table 2.
Characteristics of included and excluded boys in the two analyses from the EDEN cohort [ (%) or ].
| Characteristic | Spirometry analysis | Respiratory outcomes analysis | ||||
|---|---|---|---|---|---|---|
| Included () | Excluded () | p-Value | Included () | Excluded () | p-Value | |
| Center of recruitment | ||||||
| Nancy | 93 (41) | 371 (48) | 0.04 | 247 (42) | 217 (53) | 0.001 |
| Poitiers | 135 (59) | 396 (52) | 340 (58) | 191 (47) | ||
| Living area | ||||||
| Rural area | 85 (37) | 238 (31) | 0.11 | 200 (34) | 123 (30) | 0.41 |
| Urban area | 100 (44) | 346 (45) | 258 (44) | 188 (46) | ||
| City center | 42 (18) | 183 (24) | 128 (22) | 97 (24) | ||
| Missing | 1 () | ND | 1 () | ND | ||
| Maternal country of birth | ||||||
| Mainland France | 221 (97) | 717 (93) | 0.11 | 563 (96) | 375 (92) | 0.02 |
| All others | 6 (3) | 39 (5) | 19 (3) | 26 (6) | ||
| Missing | 1 () | 11 (1) | 5 () | 7 (2) | ||
| Parental higher education level | ||||||
| 71 (31) | 274 (36) | 0.25 | 193 (33) | 152 (37) | 0.04 | |
| 56 (25) | 161 (21) | 128 (22) | 89 (22) | |||
| 93 (41) | 278 (36) | 241 (41) | 130 (32) | |||
| Missing | 8 (4) | 54 (7) | 8 (4) | 37 (9) | ||
| Pregnancy maternal active smoking (cig/day) | ||||||
| 0 | 173 (76) | 561 (73) | 0.06 | 449 (76) | 285 (70) | |
| 1–5 | 38 (17) | 106 (14) | 89 (15) | 55 (13) | ||
| 17 (7) | 100 (13) | 49 (8) | 68 (17) | |||
| Passive smoking during pregnancy | ||||||
| Yes | 143 (63) | 437 (57) | 0.13 | 372 (63) | 199 (49) | |
| No | 85 (37) | 329 (43) | 215 (37) | 208 (51) | ||
| Missing | ND | 1 () | ND | 1 () | ||
| Parental history of asthma/allergies | ||||||
| Yes | 98 (43) | 339 (44) | 0.68 | 253 (43) | 184 (45) | 0.44 |
| No | 130 (57) | 422 (55) | 333 (57) | 219 (54) | ||
| Missing | ND | 6 () | 1 () | 5 (1) | ||
| Gestational duration (wk) | 0.41 | 0.002 | ||||
| Presence of older siblings | ||||||
| Yes | 104 (46) | 325 (42) | 0.39 | 324 (55) | 242 (59) | 0.20 |
| No | 124 (54) | 442 (58) | 263 (45) | 166 (41) | ||
| Child care attendance before 1 y | ||||||
| Yes | 38 (17) | 124 (16) | 0.39 | 104 (18) | 58 (14) | 0.39 |
| No | 179 (78) | 490 (64) | 453 (77) | 216 (53) | ||
| Missing | 11 (5) | 153 (20) | 30 (5) | 134 (33) | ||
| Postnatal passive smoking | ||||||
| Yes | 112 (49) | 364 (47) | 0.005 | 295 (50) | 181 (44) | |
| No | 109 (48) | 228 (30) | 262 (45) | 75 (18) | ||
| Missing | 7 (3) | 175 (23) | 30 (5) | 152 (37) | ||
| Age at spirometry (years) | 5.6± 0.1 | 0.05 | ||||
| Height at spirometry (cm) | 0.13 | |||||
Note: ND, no data; SD, standard deviation.
Of the compounds measured, those with the highest (raw or standardized) urinary concentrations were for the phenols, triclosan and methyl-paraben, and for the phthalates, monoethyl phthalate (MEP) and mono(2-ethyl-5-carboxypentyl) (MECPP) (Table 1). Within compounds, crude and standardized concentrations were highly correlated (). Standardized concentrations of dichlorophenols, parabens, and DEHP metabolites were highly correlated within each family of compounds (). Strong correlations () existed also between molar sums and associated compounds. The other correlation coefficients between standardized concentrations were below 0.66 (Tables S2–S3).
Asthma was diagnosed in 112 boys by age 5 y [cumulative incidence rate (CIR) at 5 y, 20.4%; 95% confidence interval (CI): 17.2, 24.0%] and parents reported wheezing in 254 boys (; 95% CI: 41.0, 49.3%). Bronchiolitis/bronchitis cumulative incidence was 70.4% at 3 y (95% CI: 66.6, 74.2%; Figure S1). Average was 91.0% (5th–95th percentiles: 72.7–107.5%) and tended to be lower in children with doctor-diagnosed asthma and history of wheezing or bronchiolitis/bronchitis (not detailed).
Phenols and Respiratory Health
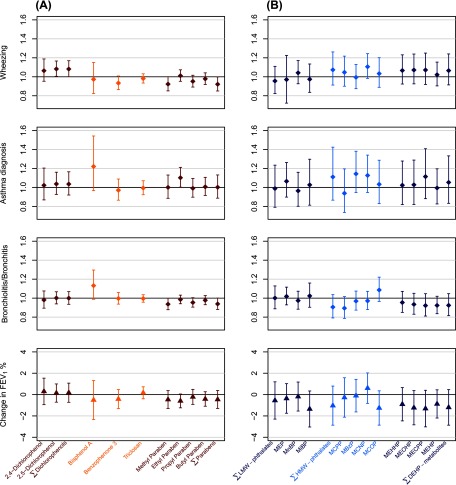
Ln-transformed ethyl-paraben standardized concentration was associated with increased rate of doctor-diagnosed asthma [hazard rate (HR) for 1-unit increase in ln-transformed concentration, 1.10; 95% CI: 1.00, 1.21, ] and reduced mean (beta for 1-unit increase in ln-transformed concentration, ; 95% CI: , 0.05; ). Bisphenol A tended to be associated with increased rates of asthma (HR, 1.23; 95% CI: 0.97, 1.55; ) and of bronchiolitis/bronchitis (HR, 1.13; 95% CI: 0.99, 1.30; ). Ln-transformed 2,5-dichlorophenol concentration was associated with an increased incidence of wheeze (HR, 1.08; 95% CI: 1.00, 1.17; ). Methyl-paraben was associated with reduced rates of bronchiolitis/bronchitis (HR, 0.94; 95% CI: 0.88, 1.00; ) and of wheezing (HR, 0.92; 95% CI: 0.85, 1.00; ). Similar trends were found for propyl-paraben (Table 3, Figure 1A). Benzophenone-3 tended to be associated with reduced rate of wheezing (HR, 0.93; 95% CI: 0.86, 1.01; ). Models with concentrations coded in tertiles showed coherent results (Table 3). No clear association with any respiratory outcome was observed with the other phenols.
Table 3.
Adjusted associations between standardized concentrations of pregnancy phenols and respiratory outcomes () and in boys.
| Phenolc | Wheezing (until age 5 y)a | Asthma diagnosis (until age 5 y)a | Bronchiolitis/Bronchitis (until age 3 y)a | b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | phetd | p-Value | HR (95% CI) | phetd | p-Value | HR (95% CI) | phetd | p-Value | Beta (95% CI) | phetd | p-Value | |
| 2,4-Dichlorophenol | ||||||||||||
| Continuouse | 1.06 (0.95, 1.19) | 0.27 | 1.02 (0.87, 1.20) | 0.79 | 0.98 (0.89, 1.08) | 0.68 | 0.29 (, 1.54) | 0.64 | ||||
| T1 | 1.00 | 0.64 | 0.74f | 1.00 | 0.49 | 0.96f | 1.00 | 0.50 | 0.40f | 0.00 | 0.50 | 0.93f |
| T2 | 1.16 (0.85, 1.58) | 1.30 (0.83, 2.05) | 1.08 (0.84, 1.38) | 2.01 (, 5.60) | ||||||||
| T3 | 1.09 (0.81, 1.49) | 1.07 (0.66, 1.72) | 0.93 (0.73, 1.19) | 0.45 (, 3.94) | ||||||||
| 2,5-Dichlorophenol | ||||||||||||
| Continuouse | 1.08 (1.00, 1.17) | 0.04 | 1.04 (0.93, 1.16) | 0.52 | 1.00 (0.94, 1.07) | 0.97 | 0.14 (, 1.00) | 0.74 | ||||
| T1 | 1.00 | 0.01 | 0.13f | 1.00 | 0.24 | 0.35f | 1.00 | 0.82 | 0.54f | 0.00 | 0.93 | 0.71f |
| T2 | 1.66 (1.20, 2.28) | 1.46 (0.90, 2.34) | 1.03 (0.81, 1.33) | 0.03 (, 3.64) | ||||||||
| T3 | 1.51 (1.10, 2.08) | 1.41 (0.88, 2.25) | 1.08 (0.85, 1.38) | 0.59 (, 4.11) | ||||||||
| Continuouse | 1.08 (1.00, 1.17) | 0.048 | 1.04 (0.92, 1.17) | 0.55 | 1.00 (0.93, 1.07) | 0.99 | 0.17 (, 1.07) | 0.70 | ||||
| T1 | 1.00 | 0.03 | 0.08f | 1.00 | 0.24 | 0.24f | 1.00 | 0.76 | 0.47f | 0.00 | 0.95 | 0.78f |
| T2 | 1.45 (1.05, 1.99) | 1.41 (0.87, 2.27) | 1.00 (0.78, 1.29) | (, 3.48) | ||||||||
| T3 | 1.47 (1.07, 2.01) | 1.45 (0.91, 2.32) | 1.08 (0.85, 1.39) | 0.38 (, 3.91) | ||||||||
| Bisphenol A | ||||||||||||
| Continuouse | 0.97 (0.82, 1.15) | 0.75 | 1.23 (0.97, 1.55) | 0.09 | 1.13 (0.99, 1.30) | 0.08 | (, 1.32) | 0.58 | ||||
| T1 | 1.00 | 0.99 | 0.90f | 1.00 | 0.28 | 0.12f | 1.00 | 0.52 | 0.26f | 0.00 | 0.46 | 0.62f |
| T2 | 0.98 (0.72, 1.33) | 1.04 (0.63, 1.71) | 1.06 (0.83, 1.36) | (, 1.27) | ||||||||
| T3 | 0.98 (0.72, 1.34) | 1.39 (0.87, 2.22) | 1.15 (0.90, 1.48) | (, 2.23) | ||||||||
| Benzophenone-3 | ||||||||||||
| Continuouse | 0.93 (0.86, 1.01) | 0.08 | 0.97 (0.87, 1.09) | 0.60 | 1.00 (0.94, 1.06) | 0.91 | (, 0.47) | 0.36 | ||||
| T1 | 1.00 | 0.03 | 0.01f | 1.00 | 0.26 | 0.16f | 1.00 | 0.65 | 0.85f | 0.00 | 0.83 | 0.56f |
| T2 | 0.87 (0.65, 1.17) | 0.81 (0.52, 1.26) | 0.89 (0.70, 1.14) | (, 2.90) | ||||||||
| T3 | 0.66 (0.48, 0.91) | 0.68 (0.42, 1.09) | 0.93 (0.73, 1.19) | (, 2.40) | ||||||||
| Triclosan | ||||||||||||
| Continuouse | 0.98 (0.93, 1.03) | 0.46 | 0.99 (0.92, 1.07) | 0.86 | 1.00 (0.96, 1.04) | 0.83 | 0.17 (, 0.73) | 0.56 | ||||
| T1 | 1.00 | 0.79 | 0.57f | 1.00 | 0.91 | 0.75f | 1.00 | 0.80 | 0.62f | 0.00 | 0.96 | 0.91f |
| T2 | 0.94 (0.69, 1.27) | 1.06 (0.67, 1.66) | 1.05 (0.82, 1.34) | 0.48 (, 3.94) | ||||||||
| T3 | 0.90 (0.67, 1.22) | 0.96 (0.60, 1.52) | 0.97 (0.76, 1.23) | 0.69 (, 3.84) | ||||||||
| Methyl-paraben | ||||||||||||
| Continuouse | 0.92 (0.85, 1.00) | 0.051 | 1.00 (0.89, 1.13) | 0.99 | 0.94 (0.88, 1.00) | 0.046 | (, 0.37) | 0.28 | ||||
| T1 | 1.00 | 0.03 | 0.17f | 1.00 | 0.90 | 0.94f | 1.00 | 0.02 | 0.01f | 0.00 | 0.19 | 0.18f |
| T2 | 0.68 (0.50, 0.92) | 0.90 (0.57, 1.43) | 0.80 (0.64, 1.03) | 1.66 (, 5.21) | ||||||||
| T3 | 0.74 (0.55, 1.00) | 0.99 (0.62, 1.58) | 0.70 (0.54, 0.89) | (, 1.78) | ||||||||
| Ethyl-paraben | ||||||||||||
| Continuouse | 1.01 (0.95, 1.07) | 0.72 | 1.10 (1.00, 1.21) | 0.04 | 0.99 (0.94, 1.03) | 0.53 | (, 0.05) | 0.07 | ||||
| T1 | 1.00 | 0.63 | 0.65f | 1.00 | 0.047 | 0.08f | 1.00 | 0.004 | 0.15f | 0.00 | 0.52 | 0.31f |
| T2 | 1.15 (0.85, 1.57) | 1.66 (1.02, 2.71) | 1.40 (1.10, 1.79) | (, 2.36) | ||||||||
| T3 | 1.13 (0.83, 1.55) | 1.81 (1.10, 2.98) | 0.97 (0.76, 1.26) | (, 1.47) | ||||||||
| Propyl-paraben | ||||||||||||
| Continuouse | 0.95 (0.89, 1.02) | 0.14 | 0.99 (0.90, 1.09) | 0.85 | 0.95 (0.91, 1.00) | 0.08 | (, 0.49) | 0.55 | ||||
| T1 | 1.00 | 0.58 | 0.44f | 1.00 | 0.91 | 0.89f | 1.00 | 0.13 | 0.12f | 0.00 | 0.63 | 0.38f |
| T2 | 0.88 (0.66, 1.19) | 0.91 (0.58, 1.45) | 0.83 (0.65, 1.06) | (, 3.91) | ||||||||
| T3 | 0.86 (0.63, 1.17) | 1.00 (0.63, 1.59) | 0.79 (0.61, 1.00) | (, 2.25) | ||||||||
| Butyl-paraben | ||||||||||||
| Continuouse | 0.98 (0.92, 1.04) | 0.49 | 1.01 (0.92, 1.11) | 0.89 | 0.98 (0.93, 1.03) | 0.36 | (, 0.27) | 0.23 | ||||
| T1 | 1.00 | 0.24 | 0.15f | 1.00 | 0.59 | 0.68f | 1.00 | 0.02 | 0.01f | 0.00 | 0.30 | 0.15f |
| T2 | 1.13 (0.84, 1.53) | 1.26 (0.79, 2.01) | 1.13 (0.89, 1.44) | (, 2.36) | ||||||||
| T3 | 0.86 (0.62, 1.20) | 1.22 (0.75, 2.01) | 0.79 (0.61, 1.03) | (, 0.76) | ||||||||
| Continuouse | 0.92 (0.85, 1.00) | 0.05 | 1.00 (0.89, 1.13) | 0.98 | 0.94 (0.88, 1.00) | 0.054 | (, 0.38) | 0.29 | ||||
| T1 | 1.00 | 0.06 | 0.11f | 1.00 | 0.94 | 0.75f | 1.00 | 0.05 | 0.10f | 0.00 | 0.06 | 0.13f |
| T2 | 0.73 (0.54, 0.99) | 0.95 (0.61, 1.50) | 0.77 (0.61, 0.98) | 2.58 (, 6.09) | ||||||||
| T3 | 0.73 (0.54, 0.99) | 0.92 (0.57, 1.48) | 0.77 (0.60, 0.98) | (, 1.82) | ||||||||
Note: CI, confidence interval; ; ; HR, hazard rate; (methyl-, ethyl-, propyl-, butyl-parabens); phet, p-values of heterogeneity; T, tertile.
Models adjusted for center, residence area, parental history of asthma or allergies, maternal ethnicity, maximal parental education level, maternal or passive smoking during pregnancy, postnatal passive smoking, older siblings, and child care. Missing values in covariates were imputed for at least one covariate in 277 boys, using the multiple imputation by chained equations multiple imputation method (100 imputations were performed).
Additionally adjusted for child’s height and age.
Standardized for urine sampling conditions (creatinine level, day and hour of sampling, gestational age, storage duration at room temperature and year of analysis), as detailed in Mortamais et al. (2012).
of heterogeneity test.
Estimates for 1-unit increase in ln-transformed standardized concentration.
of monotonic trend test.
Figure 1.
Adjusted associations of (A) phenols and (B) phthalates metabolites ln-transformed standardized concentrations with respiratory outcomes (HR, ) and in boys (beta, , EDEN cohort). Effect estimates for 1-unit increase in ln-transformed standardized concentrations. Adjusted for center, residence area, parental history of asthma/allergies, maternal ethnicity, maximal parental education level, passive or active smoking during pregnancy, postnatal passive smoking, older siblings, child care (and additionally adjusted for boy’s height and age in spirometry analysis). Multiple imputation was used to handle missing values in covariates (100 imputations were performed). Phenols and phthalates metabolites concentrations were standardized for urine sampling conditions (see Methods section). Diamond and triangle markers represent HR and beta values, respectively, with error bars for 95% CI.
In sensitivity analyses, very small variations in regression estimates were observed when standardized biomarker concentrations were replaced by raw concentrations. Analyses restricted to full-term boys ( and 217 for survival analyses and analyses, respectively), to boys from nonsmoking mothers ( and 171), or nonasthmatic parents ( and 185) led to similar trends of deleterious associations between ethyl-paraben and both asthma diagnosis and , between bisphenol A and asthma, as well as bronchiolitis/bronchitis and between 2,5-dichlorophenol and wheezing. Similarly, trends for protective associations between methyl-paraben and wheezing or bronchiolitis/bronchitis remained (Tables S4-S10).
Phthalates and Respiratory Health
No phthalate metabolite was clearly associated with several respiratory outcomes, but monocarboxynonyl phthalate (MCNP) tended to be associated with increased rate of wheezing (HR for 1-unit increase in ln-transformed concentration, 1.11; 95% CI: 0.98, 1.24; ) and asthma (; 95% CI: 0.95, 1.35; ), and monocarboxyoctyl phthalate (MCOP) tended to be associated with increased bronchiolitis/bronchitis rate (; 95% CI: 0.97, 1.22; ) and decreased (; 95% CI: , 0.35; ). Mono-isobutyl phthalate (MiBP) and DEHP metabolites tended to be associated with reduced (p between 0.12 and 0.26, Table 4, Figure 1B). Mono(3-carboxypropyl) phthalate (MCPP) tended to be associated with a reduced rate of bronchiolitis/bronchitis (; 95% CI: 0.79, 1.02). When biomarkers’ concentrations were categorized into tertiles, we observed the same trends of associations (Table 4).
Table 4.
Adjusted associations between pregnancy phthalate metabolites standardized concentrations and respiratory outcomes () and in boys.
| Phthalatec | Wheezing (until age 5 y)a | Asthma diagnosis (until age 5 y)a | Bronchiolitis/bronchitis (until age 3 y)a | b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | phete | p-Value | HR (95% CI) | phete | p-Value | HR (95% CI) | phete | p-Value | Beta (95% CI) | phete | p-Value | |
| Continuousd | 0.96 (0.82, 1.11) | 0.55 | 0.99 (0.80, 1.24) | 0.96 | 1.00 (0.89, 1.13) | 0.99 | (, 1.19) | 0.54 | ||||
| T1 | 1.00 | 0.92 | 0.77f | 1.00 | 0.53 | 0.91f | 1.00 | 0.86 | 0.75f | 0.00 | 0.17 | 0.06f |
| T2 | 1.03 (0.76, 1.40) | 1.29 (0.82, 2.02) | 1.07 (0.84, 1.37) | (, 3.09) | ||||||||
| T3 | 0.97 (0.71, 1.33) | 1.09 (0.68, 1.76) | 1.05 (0.82, 1.35) | (, 0.42) | ||||||||
| MEP | ||||||||||||
| Continuousd | 0.97 (0.86, 1.09) | 0.62 | 1.07 (0.90, 1.27) | 0.44 | 1.02 (0.93, 1.12) | 0.70 | (, 1.02) | 0.61 | ||||
| T1 | 1.00 | 0.87 | 0.97f | 1.00 | 0.56 | 0.38f | 1.00 | 0.84 | 0.63f | 0.00 | 0.74 | 0.44f |
| T2 | 0.92 (0.68, 1.25) | 0.90 (0.56, 1.46) | 1.06 (0.83, 1.35) | (, 3.09) | ||||||||
| T3 | 0.97 (0.72, 1.32) | 1.16 (0.74, 1.83) | 1.07 (0.84, 1.37) | (, 2.19) | ||||||||
| MnBP | ||||||||||||
| Continuousd | 1.04 (0.93, 1.17) | 0.49 | 0.97 (0.80, 1.16) | 0.72 | 0.97 (0.88, 1.07) | 0.59 | (, 1.17) | 0.79 | ||||
| T1 | 1.00 | 0.53 | 0.33f | 1.00 | 0.75 | 0.57f | 1.00 | 0.52 | 0.51f | 0.00 | 0.92 | 0.83f |
| T2 | 0.95 (0.69, 1.29) | 0.87 (0.55, 1.36) | 0.88 (0.69, 1.12) | 0.43 (, 3.92) | ||||||||
| T3 | 1.12 (0.83, 1.52) | 0.85 (0.54, 1.35) | 0.90 (0.70, 1.14) | (, 3.29) | ||||||||
| MiBP | ||||||||||||
| Continuousd | 0.97 (0.84, 1.13) | 0.74 | 1.03 (0.82, 1.30) | 0.79 | 1.02 (0.90, 1.16) | 0.71 | (, 0.34) | 0.12 | ||||
| T1 | 1.00 | 0.76 | 0.94f | 1.00 | 0.83 | 0.74f | 1.00 | 0.99 | 0.99f | 0.00 | 0.14 | 0.05f |
| T2 | 0.89 (0.65, 1.22) | 1.09 (0.70, 1.71) | 1.02 (0.79, 1.30) | (, 3.10) | ||||||||
| T3 | 0.96 (0.72, 1.31) | 0.95 (0.59, 1.52) | 1.00 (0.78, 1.28) | (, 0.21) | ||||||||
| Continuousd | 1.07 (0.91, 1.26) | 0.39 | 1.11 (0.87, 1.43) | 0.40 | 0.91 (0.79, 1.04) | 0.15 | (, 0.80) | 0.27 | ||||
| T1 | 1.00 | 0.25 | 0.89f | 1.00 | 0.97 | 0.98f | 1.00 | 0.50 | 0.29f | 0.00 | 0.26 | 0.47f |
| T2 | 1.29 (0.95, 1.75) | 1.06 (0.67, 1.69) | 0.91 (0.71, 1.16) | (, 0.61) | ||||||||
| T3 | 1.09 (0.79, 1.49) | 1.02 (0.64, 1.62) | 0.87 (0.68, 1.11) | (, 1.62) | ||||||||
| MCPP | ||||||||||||
| Continuousd | 1.05 (0.90, 1.22) | 0.55 | 0.94 (0.74, 1.20) | 0.62 | 0.89 (0.79, 1.02) | 0.09 | (, 1.58) | 0.77 | ||||
| T1 | 1.00 | 0.66 | 0.37f | 1.00 | 0.78 | 0.58f | 1.00 | 0.37 | 0.23f | 0.00 | 0.31 | 0.73f |
| T2 | 1.06 (0.77, 1.44) | 0.88 (0.56, 1.38) | 1.05 (0.83, 1.33) | (, 1.21) | ||||||||
| T3 | 1.15 (0.85, 1.57) | 0.86 (0.54, 1.37) | 0.88 (0.69, 1.13) | 0.18 (, 3.64) | ||||||||
| MBzP | ||||||||||||
| Continuousd | 1.00 (0.87, 1.13) | 0.93 | 1.15 (0.95, 1.39) | 0.15 | 0.97 (0.87, 1.07) | 0.55 | (, 1.42) | 0.90 | ||||
| T1 | 1.00 | 0.32 | 0.45f | 1.00 | 0.39 | 0.97f | 1.00 | 0.46 | 0.21f | 0.00 | 0.20 | 0.25f |
| T2 | 1.18 (0.87, 1.60) | 1.37 (0.86, 2.19) | 0.99 (0.77, 1.26) | (, 0.66) | ||||||||
| T3 | 0.94 (0.69, 1.29) | 1.11 (0.69, 1.79) | 0.86 (0.68, 1.11) | (, 0.84) | ||||||||
| MCNP | ||||||||||||
| Continuousd | 1.11 (0.98, 1.24) | 0.09 | 1.13 (0.95, 1.35) | 0.16 | 0.97 (0.88, 1.07) | 0.56 | 0.60 (, 2.03) | 0.41 | ||||
| T1 | 1.00 | 0.22 | 0.18f | 1.00 | 0.19 | 0.26f | 1.00 | 0.69 | 0.88f | 0.00 | 0.72 | 0.84f |
| T2 | 1.24 (0.91, 1.70) | 1.51 (0.93, 2.46) | 1.10 (0.87, 1.41) | (, 2.15) | ||||||||
| T3 | 1.29 (0.95, 1.76) | 1.46 (0.91, 2.35) | 1.01 (0.79, 1.30) | (, 3.32) | ||||||||
| MCOP | ||||||||||||
| Continuousd | 1.03 (0.89, 1.20) | 0.67 | 1.03 (0.83, 1.29) | 0.76 | 1.09 (0.97, 1.22) | 0.17 | (, 0.35) | 0.13 | ||||
| T1 | 1.00 | 0.81 | 0.52f | 1.00 | 0.52 | 0.77f | 1.00 | 0.90 | 0.92f | 0.00 | 0.42 | 0.22f |
| T2 | 0.96 (0.71, 1.31) | 0.80 (0.50, 1.27) | 1.05 (0.82, 1.34) | 0.02 (, 3.59) | ||||||||
| T3 | 0.90 (0.67, 1.23) | 1.01 (0.65, 1.58) | 1.00 (0.78, 1.28) | (, 1.43) | ||||||||
| MEHHP | ||||||||||||
| Continuousd | 1.07 (0.92, 1.23) | 0.38 | 1.02 (0.82, 1.28) | 0.83 | 0.95 (0.85, 1.07) | 0.43 | (, 0.66) | 0.26 | ||||
| T1 | 1.00 | 0.70 | 0.71f | 1.00 | 0.41 | 0.62f | 1.00 | 0.71 | 0.54f | 0.00 | 0.33 | 0.35f |
| T2 | 1.14 (0.84, 1.56) | 1.26 (0.80, 2.00) | 1.05 (0.82, 1.33) | (, 0.87) | ||||||||
| T3 | 1.09 (0.80, 1.48) | 0.95 (0.59, 1.53) | 0.94 (0.73, 1.20) | (, 1.83) | ||||||||
| MEOHP | ||||||||||||
| Continuousd | 1.07 (0.93, 1.24) | 0.36 | 1.03 (0.82, 1.29) | 0.80 | 0.93 (0.83, 1.05) | 0.26 | (, 0.37) | 0.13 | ||||
| T1 | 1.00 | 0.39 | 0.70f | 1.00 | 0.89 | 0.68f | 1.00 | 0.38 | 0.45f | 0.00 | 0.10 | 0.10f |
| T2 | 1.24 (0.91, 1.69) | 1.02 (0.65, 1.62) | 1.11 (0.87, 1.41) | (, 0.09) | ||||||||
| T3 | 1.11 (0.81, 1.51) | 0.92 (0.58, 1.46) | 0.94 (0.73, 1.20) | (, 0.35) | ||||||||
| MECPP | ||||||||||||
| Continuousd | 1.07 (0.92, 1.25) | 0.38 | 1.11 (0.88, 1.41) | 0.36 | 0.92 (0.81, 1.05) | 0.21 | (, 0.40) | 0.13 | ||||
| T1 | 1.00 | 0.18 | 0.52f | 1.00 | 0.42 | 0.81f | 1.00 | 0.88 | 0.62f | 0.00 | 0.12 | 0.15f |
| T2 | 1.34 (0.98, 1.82) | 1.36 (0.85, 2.15) | 0.98 (0.77, 1.24) | (, 0.14) | ||||||||
| T3 | 1.17 (0.85, 1.61) | 1.13 (0.70, 1.83) | 0.94 (0.74, 1.20) | (, 0.57) | ||||||||
| MEHP | ||||||||||||
| Continuousd | 1.02 (0.90, 1.15) | 0.73 | 0.99 (0.83, 1.20) | 0.95 | 0.92 (0.84, 1.02) | 0.12 | (, 0.45) | 0.19 | ||||
| T1 | 1.00 | 0.59 | 0.36f | 1.00 | 0.69 | 0.44f | 1.00 | 0.27 | 0.48f | 0.00 | 0.35 | 0.15f |
| T2 | 0.97 (0.71, 1.33) | 0.97 (0.61, 1.55) | 0.82 (0.64, 1.04) | (, 2.70) | ||||||||
| T3 | 1.13 (0.83, 1.53) | 1.17 (0.74, 1.83) | 0.89 (0.69, 1.13) | (, 0.97) | ||||||||
| Continuousd | 1.06 (0.91, 1.24) | 0.42 | 1.05 (0.83, 1.33) | 0.66 | 0.92 (0.82, 1.05) | 0.22 | (, 0.47) | 0.16 | ||||
| T1 | 1.00 | 0.19 | 0.86f | 1.00 | 0.61 | 0.77f | 1.00 | 0.70 | 0.44f | 0.00 | 0.27 | 0.49f |
| T2 | 1.32 (0.97, 1.79) | 1.20 (0.76, 1.90) | 1.01 (0.79, 1.28) | (, 0.61) | ||||||||
| T3 | 1.09 (0.80, 1.50) | 0.98 (0.61, 1.57) | 0.92 (0.72, 1.17) | (, 1.97) | ||||||||
Note: CI, confidence interval; , molar sum of di(2-ethylhexyl) phthalate metabolites (MEHHP, MEOHP, MECPP, MEHP); , forced expiratory volume in 1 is expressed in percent predicted; HR, hazard rate; , molar sum of high-molecular-weight phthalates (MCPP, MBzP, MCNP, MCOP, MEHHP, MEOHP, MECPP, MEHP); , molar sum of low-molecular-weight phthalates (MEP, MnBP, MiBP); phet, p-values of heterogeneity; T, trimester. Parent compounds and associated metabolites are detailed in Table S1 (Supplemental material).
Models adjusted for center, residence area, parental history of asthma or allergies, maternal ethnicity, maximal parental education level, maternal or passive smoking during pregnancy, postnatal passive smoking, older siblings, and child care. Missing values in covariates were imputed for at least one covariate in 277 boys, using the multiple imputation by chained equations multiple imputation method (100 imputations were performed).
Additionally adjusted for child’s height and age.
Standardized for urine sampling conditions (creatinine level, day and hour of sampling, gestational age, storage duration at room temperature, and year of analysis), as detailed in Mortamais et al. (2012).
Estimates for 1-unit increase in ln-transformed standardized concentration.
of heterogeneity test.
of monotonic trend test.
Sensitivity analyses led to similar results for the phthalates metabolites, with consistent HRs and beta coefficients (Tables S5-S11).
Discussion
This study evaluated possible deleterious effects of prenatal exposure to phthalates and phenols on respiratory health in childhood. In our male population, associations were not in the same direction across chemicals. First, increased levels of ethyl-paraben, bisphenol A, 2,5-dichlorophenol, and DIDP tended to be associated with altered respiratory health, with ethyl-paraben and bisphenol A exhibiting some consistency across respiratory outcomes. Inversely, and contrary to our a priori hypothesis, we observed reduced rates of bronchiolitis/bronchitis and wheezing with increased exposure to methyl- and propyl-parabens and benzophenone-3. MCPP, a metabolite of di-n-octylphthalate, di-n-butyl phthalate (DnBP), and several HMW phthalates tended to reduce rates of bronchiolitis/bronchitis.
Regarding bisphenol A, a study of 208 children reported a strong inverse association between prenatal bisphenol A concentration and at age 4 y (; 95% CI: , for an increase by one unit), which totally disappeared at age 5 y (; 95% CI: , 9) (Spanier et al. 2014b). The latter wide CI is consistent with our results at age 5 y estimated for a unit (, 95% CI: , 3). Regarding associations between prenatal bisphenol A exposure and questionnaire-based respiratory outcomes, Donohue et al. (2013) reported no association with asthma status evaluated once between ages 5 and 12 y, whereas Gascon et al. (2015) reported trends of deleterious association with asthma, bronchitis, and chest infections until age 7 y (point estimates for relative risks for 1-unit increase in –transformed concentration varied from 1.15 to 1.21 across phenotypes). In a larger population with a similar bisphenol A concentration range, our results also suggested elevated rates of doctor-diagnosed asthma and bronchiolitis/bronchitis of similar effect sizes to Gascon et al. (2015). Regarding wheezing, our study did not evidence any association, and results from the previous studies are inconsistent, with studies reporting either protective association (Donohue et al. 2013) or trend for deleterious association (Gascon et al. 2015; Spanier et al. 2012, 2014b). Spanier et al. (2014b) showed that the association was stronger considering concentrations from urine samples collected at 16 gestational wk compared with 26 gestational wk, suggesting that the exposure window may play a role in the association with wheezing. In mice, prenatal exposure to bisphenol A through drinking water promoted the development of allergic asthma in offspring (Nakajima et al. 2012). Bisphenol A may affect the immune functions and increase IgE serum levels or the production of proallergic mediators, such as cytokine interleukin 4 (Kwak et al. 2009; Rogers et al. 2013). Such immune effects might be mediated by interactions with oestrogen receptors or the family of peroxisome proliferator–activated receptors (PPARs) (Rogers et al. 2013).
The literature regarding other phenols is limited to cross-sectional studies. In our study, 2,5-dichlorophenol tended to be associated with wheezing rate, an association never considered, to our knowledge, in a prospective setting. The protective associations we observed between methyl-paraben or propyl-paraben and wheezing or bronchiolitis/bronchitis are in line with those previously reported with nonatopic wheezing (Savage et al. 2012; Spanier et al. 2014a). One proposed hypothesis might be an effect through their antimicrobial properties (Savage et al. 2012). To our knowledge, no previous experimental or human study considered association between benzophenone-3 and risk of wheezing.
Only one cross-sectional study has investigated associations of several phthalates and pulmonary function, reporting deleterious associations of spirometric measurements with MCPP, mono-n-butyl phthalate (MnBP), and DEHP metabolites (Cakmak et al. 2014). In a population somewhat older than ours (boys were 6–16 y old), decreased with urinary concentrations of DEHP metabolites, consistently with the trend observed in our study; associations were stronger with the forced vital capacity (FVC) and the ratio , outcomes that we could not consider. We also observed suggestive associations between MCOP and MiBP concentrations and decreased ( and , respectively, for the log-transformed concentrations), associations which have, to our knowledge, never been considered so far.
Combining two cohorts, Smit et al. (2015) did not observe any association between serum oxidative metabolites of DEHP, DINP, and wheeze or asthma in children evaluated at one time point between 5 and 9 y of age. Whyatt et al. (2014a) reported elevated risks of asthma or asthma-like symptoms between 5 and 11 y associated with urinary metabolites of BBzP and DnBP (represented by monobenzyl phthalate (MBzP) and MnBP, respectively), which were not observed in the boys from our study. Gascon et al. (2015) showed deleterious associations of BBzP and DEHP urinary metabolites with asthma, wheeze, chest infections, and bronchitis until age 7 y, which we did not confirm in our male population followed until 5 y. Ku et al. (2015) reported deleterious associations between urinary metabolites of BBzP or DEHP and wheeze at age 8 y in boys, which, again, we could not confirm until age 5. The strongest deleterious association of phthalates metabolites with respiratory outcomes was observed for MCNP (DIDP metabolite), not investigated in the previous longitudinal studies, and for which our study provides only limited evidence in favor of associations with wheezing (p, 0.09 for log–transformed coding) and asthma diagnosis (p, 0.16). Currently, DIDP and DINP are increasingly used as substitutes to DEHP and are the most commonly used plasticizers in Western Europe (INERIS 2012).
Experimental studies suggested that phthalates as DINP and DEHP could release proinflammatory mediators in lung cells and have an adjuvant effect on immune response in mice or rats, following dietary exposure (Chen et al. 2015; Guo et al. 2012; Sadakane et al. 2014), inhalation (Hansen et al. 2007), or subcutaneous injection (Lee et al. 2011). These mechanisms may enhance airway hyper-responsiveness by the infiltration of inflammatory cells in lung tissue (Miller and Marty 2010; Rakkestad et al. 2010). DEHP may also interact with the PPARs nuclear receptors superfamily and lead to abnormal alveolar maturation and reduced surfactant production (Miller and Marty 2010).
We relied on discrete time survival modeling to assess associations with respiratory diseases. This approach was justified by the prospective nature of our study and has the advantage of allowing for efficiently taking into account subjects lost to follow-up and timing of disease occurrence as well as incorporating time-varying adjustment factors, such as postnatal passive smoking. Few previous studies on this topic [e.g., (Gascon et al. 2015)] had relied on survival modeling, to our knowledge.
The present study considered an objective measure of the pulmonary function at an early age. is the most widely used lung function measurement in epidemiology, with strong reproducibility (National Asthma Education and Prevention Program 2007). However, obtaining satisfactory forced expiratory manoeuvers is difficult in children under 6 y of age, as very young children are not always able to produce prolonged expirations (Beydon et al. 2007). We therefore did not analyze the FVC and the ratio , and 39% of the eligible children were not considered in the analysis of because of exhalation times shorter than 1 s. Still, like in a previous study in a similar age range population (Spanier et al. 2014b), mean (90.0%) was lower than expected. In young children, or could be of interest, but were not available in our study. From the questionnaires, we were not able to differentiate bronchiolitis and bronchitis occurrences, since only one question was asked for these diseases. Additionally, our study did not take into account the well-known wheezing phenotypic heterogeneity relying on age at onset and persistency of symptoms (Martinez et al. 1995), as it would require a larger sample size to ensure a satisfactory statistical power.
The initial purpose of phenols and phthalates assays in the EDEN cohort was to investigate the impact of maternal exposure to ubiquitous endocrine disruptors on male genital organogenesis (Chevrier et al. 2012). This was followed by studies on fetal and postnatal growth in male offspring (Philippat et al. 2012, 2014). Hence, data on prenatal exposures were available in boys only. Thus, our study was unable to address the existence of sex-specific effects suggested by previous studies (Cakmak et al. 2014; Gascon et al. 2015; Ku et al. 2015). However, focusing on one sex does not bias results, which only apply to boys and should not be generalized to girls. From a statistical point of view, focusing on a single sex is a way to optimize the study accuracy (limiting variance) in a context of limited total sample size (defined by our budget for chemicals assays) and possible sex-specific effects suggested by previous studies. From a public health point of view, identifying effects in a single sex, or in a specific sensitive subgroup, should be enough to support risk management decisions. Our analyses were conducted in a relatively well-educated population with a majority of nonsmoking mothers during pregnancy, but representativeness is not a condition of validity in etiological studies such as ours (Rothman et al. 2013), and focusing on rather homogeneous populations might actually limit bias due to unmeasured confounders. However, we cannot rule out the possibility of selection bias.
Reliance on a single maternal urine sample generally leads to exposure misclassification in the case of chemicals with high intraindividual temporal variability (Perrier et al. 2016). If we assume that error is of classical type, then the expected impact corresponds to attenuation bias, its amplitude being highest for bisphenol A and DEHP metabolites, the compounds with the largest within-subject variability (Perrier et al. 2016; Rappaport et al. 1995). For this reason, the lack of significant association with most exposure biomarkers, in particular, bisphenol A and DEHP metabolites (those with the lowest intraclass correlations and hence the largest attenuation bias), should not be seen as strong evidence of a lack of effect of these compounds. We had no information on postnatal exposures, which might be correlated to maternal pregnancy levels, so that in theory, any of the associations reported here could be due to postnatal (and not specifically prenatal) exposures. However, it has been shown that phenols and phthalates biomarker concentrations measured postnatally in children were poorly to moderately correlated with those from their mother during pregnancy (Casas et al. 2013; Whyatt et al. 2014a).
Conclusion
In conclusion, our prospective study relying on respiratory outcomes and pulmonary function tests showed possible adverse associations between prenatal urinary concentrations of 2,5-dichlorophenol, ethyl-paraben, bisphenol A, and DIDP biomarkers and respiratory health in boys until age 5 y, with ethyl-paraben and bisphenol A exhibiting some consistency across respiratory outcomes. The associations of bisphenol A pregnancy level with asthma diagnosis and bronchiolitis/bronchitis have been previously reported in a cohort study among boys and girls (Gascon et al. 2015). Our results add to an emerging literature on respiratory health impacts of early exposure to several phenols and phthalates.
Supplemental Material
Acknowledgments
We thank A. Forhan for data management; the participating families; and C. Cyril Schweitzer, F. Marchal, and the midwives at the Nancy and Poitiers obstetrical and pediatrics units; and T. Jia, M. Silva, E. Samandar, Jim J. Preau, X. Zhou, and A. Bishop for technical assistance in measuring phthalates and phenols.
This work was supported by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES) and the European Research Council (consolidator grant , PI, R. Slama). EDEN is supported by the Foundation for Medical Research (FRM), National Agency for Research (ANR), National Institute for Research in Public health (IRESP), French Ministry of Health (DGS), French Ministry of Research, INSERM Bone and Joint Diseases National Research (PRO-A), and Human Nutrition National Research Programs, Paris–Sud University, Nestlé, French National Institute for Population Health Surveillance (InVS), French National Institute for Health Education (INPES), the European Union FP7 programmes (FP7/2007 – 2013, HELIX, ESCAPE, ENRIECO, Medall projects), Diabetes National Research Program [collaboration with the French Association of Diabetic Patients (AFD)], Mutuelle Générale de l’Education Nationale (MGEN), French speaking association for the study of diabetes and metabolism (ALFEDIAM). C.V. benefits from a doctoral grant from University Grenoble-Alpes. Funders had no influence of any kind on analyses or results interpretation. The findings expressed in this article are the opinions of the authors and do not necessarily reflect the official position of the Centers for Disease Control and Prevention.
References
- Baldacci S, Maio S, Cerrai S, Sarno G, Baïz N, Simoni M, et al. 2015. Allergy and asthma: Effects of the exposure to particulate matter and biological allergens. Respir Med 109(9):1089–1104, PMID: 26073963, 10.1016/j.rmed.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Beydon N, Davis SD, Lombardi E, Allen JL, Arets HG, Aurora P, et al. 2007. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med 175(12):1304–1345, PMID: 17545458, 10.1164/rccm.200605-642ST. [DOI] [PubMed] [Google Scholar]
- Bornehag CG, Nanberg E. 2010. Phthalate exposure and asthma in children. Int J Androl 33(2):333–345, PMID: 20059582, 10.1111/j.1365-2605.2009.01023.x. [DOI] [PubMed] [Google Scholar]
- Cakmak S, Dales RE, Hebbern C, Saravanabhavan G. 2014. The association between urinary phthalates and lung function. J Occup Environ Med 56(4):376–381, PMID: 24709763, 10.1097/JOM.0000000000000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas M, Chevrier C, Hond ED, Fernandez MF, Pierik F, Philippat C, et al. 2013. Exposure to brominated flame retardants, perfluorinated compounds, phthalates and phenols in European birth cohorts: ENRIECO evaluation, first human biomonitoring results, and recommendations. Int J Hyg Environ Health 216(3):230–242, PMID: 22795704, 10.1016/j.ijheh.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Chen L, Chen J, Xie CM, Zhao Y, Wang X, Zhang YH. 2015. Maternal disononyl phthalate exposure activates allergic airway inflammation via stimulating the phosphoinositide 3-kinase/Akt pathway in rat pups. Biomed Environ Sci 28(3):190–198, PMID: 25800443, 10.3967/bes2015.025. [DOI] [PubMed] [Google Scholar]
- Chevrier C, Petit C, Philippat C, Mortamais M, Slama R, Rouget F, et al. 2012. Maternal urinary phthalates and phenols and male genital anomalies. Epidemiology 23(2):353–356, PMID: 22317818, 10.1097/EDE.0b013e318246073e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue KM, Miller RL, Perzanowski MS, Just AC, Hoepner LA, Arunajadai S, et al. 2013. Prenatal and postnatal bisphenol A exposure and asthma development among inner-city children. J Allergy Clin Immunol 131(3):736–742, PMID: 23452902, 10.1016/j.jaci.2012.12.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon M, Casas M, Morales E, Valvi D, Ballesteros-Gómez A, Luque N, et al. 2015. Prenatal exposure to bisphenol A and phthalates and childhood respiratory tract infections and allergy. J Allergy Clin Immunol 135(2):370–378, PMID: 25445825, 10.1016/j.jaci.2014.09.030. [DOI] [PubMed] [Google Scholar]
- Guo J, Han B, Qin L, Li B, You H, Yang J, et al. 2012. Pulmonary toxicity and adjuvant effect of di-(2-exylhexyl) phthalate in ovalbumin-immunized BALB/c mice. PLoS One 7(6):e39008, PMID: 22701742, 10.1371/journal.pone.0039008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JS, Larsen ST, Poulsen LK, Nielsen GD. 2007. Adjuvant effects of inhaled mono-2-ethylhexyl phthalate in BALB/cJ mice. Toxicology 232(1-2):79–88, PMID: 17429410, 10.1016/j.tox.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Heude B, Forhan A, Slama R, Douhaud L, Bedel S, Saurel-Cubizolles MJ, et al. 2016. Cohort Profile: the EDEN mother-child cohort on the prenatal and early postnatal determinants of child health and development. Int J Epidemiol 45(2):353–363, PMID: 26283636, 10.1093/ije/dyv151. [DOI] [PubMed] [Google Scholar]
- INERIS (Institut National de l'Environnement Industriel et des Risques). 2012. Données technico-économiques sur les substances chimiques en France: SUBSTITUTS DU DEHP. [in French]. http://www.ineris.fr/substances/fr/substance/getDocument/3231 [accessed 16 June 2016].
- Jenkins SP. 1995. Easy estimation methods for discrete-time duration models. Ox Bull Econ and Stat 57(1):129–138, 10.1111/j.1468-0084.1995.tb00031.x. [DOI] [Google Scholar]
- Koniecki D, Wang R, Moody RP, Zhu JP. 2011. Phthalates in cosmetic and personal care products: concentrations and possible dermal exposure. Environmental Research 111(3):329–336, PMID: 21315328, 10.1016/j.envres.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Ku HY, Su PH, Wen HJ, Sun HL, Wang CJ, Chen HY, et al. 2015. Prenatal and postnatal exposure to phthalate esters and asthma: a 9-year follow-up study of a Taiwanese birth cohort. Plos One 10(4):e0123309, PMID: 25875379, 10.1371/journal.pone.0123309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak ES, Just A, Whyatt R, Miller RL. 2009. Phthalates, pesticides, and bisphenol-A exposure and the development of nonoccupational asthma and allergies: how valid are the links? Open Allergy J 2:45–50, PMID: 20622976, 10.2174/1874838400902010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Oh PS, Lim KT. 2011. Allergy-related cytokines (IL-4 and TNF-α) are induced by Di(2-ethylhexyl) phthalate and attenuated by plant-originated glycoprotein (75 kDa) in HMC-1 cells. Environ Toxicol 26(4):364–372, PMID: 20082445, 10.1002/tox.20563. [DOI] [PubMed] [Google Scholar]
- Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. 1995. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med 332(3):133–138, PMID: 7800004, 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- Miller MD, Marty MA. 2010. Impact of environmental chemicals on lung development. Environ Health Perspect 118(8):1155–1164, PMID: 20444669, 10.1289/ehp.0901856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortamais M, Chevrier C, Philippat C, Petit C, Calafat AM, Ye X, et al. 2012. Correcting for the influence of sampling conditions on biomarkers of exposure to phenols and phthalates: a 2-step standardization method based on regression residuals. Environ Health 11:29, PMID: 22537080, 10.1186/1476-069X-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Goldblum RM, Midoro-Horiuti T. 2012. Fetal exposure to bisphenol A as a risk factor for the development of childhood asthma: an animal model study. Environ Health 11:8, PMID: 22353195, 10.1186/1476-069x-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Asthma Education and Prevention Program. 2007. Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol 120(suppl 5):S94–S138, PMID: 17983880, 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Perrier F, Giorgis-Allemand L, Slama R, Philippat C. 2016. Within-subject pooling of biological samples to reduce exposure misclassification in biomarker-based studies. Epidemiology 27(3):378–388, PMID: 27035688, 10.1097/EDE.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Mortamais M, Chevrier C, Petit C, Calafat AM, Ye X, et al. 2012. Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environ Health Perspect 120(3):464–470, PMID: 21900077, 10.1289/ehp.1103634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Botton J, Calafat AM, Ye X, Charles MA, Slama R, et al. 2014. Prenatal exposure to phenols and growth in boys. Epidemiology 25(5):625–635, PMID: 25061923, 10.1097/EDE.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. 2012. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 40(6):1324–1343, 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakkestad KE, Holme JA, Paulsen RE, Schwarze PE, Becher R. 2010. Mono(2-ethylhexyl) phthalate induces both pro- and anti-inflammatory responses in rat alveolar macrophages through crosstalk between p38, the lipoxygenase pathway and PPAR alpha. Inhal Toxicol 22(2):140–150, PMID: 19938896, 10.3109/08958370903019885. [DOI] [PubMed] [Google Scholar]
- Rappaport SM, Lyles RH, Kupper LL. 1995. An exposure-assessments strategy accounting for within-worker and between-worker sources of variability. Annals of Occupational Hygiene 39(4):469–495, PMID: 7661513, 10.1016/0003-4878(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Rogers JA, Metz L, Yong VW. 2013. Review: Endocrine disrupting chemicals and immune responses: A focus on bisphenol-A and its potential mechanisms. Mol Immunol 53(4):421–430, PMID: 23123408, 10.1016/j.molimm.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Gallacher JE, Hatch EE. 2013. Why representativeness should be avoided. Int J Epidemiol 42(4):1012–1014, 10.1093/ije/dys223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Lash TL. 2008. Modern Epidemiology. 3rd edition Philadelphia:Wolters Kluwer Health/Lippincott Williams & Wilkins, 303–410. [Google Scholar]
- Sadakane K, Ichinose T, Takano H, Yanagisawa R, Koike E. 2014. Effects of oral administration of di-(2-ethylhexyl) and diisononyl phthalates on atopic dermatitis in NC/Nga mice. Immunopharmacol Immunotoxicol 36(1):61–69, PMID: 24328677, 10.3109/08923973.2013.866678. [DOI] [PubMed] [Google Scholar]
- Savage JH, Matsui EC, Wood RA, Keet CA. 2012. Urinary levels of triclosan and parabens are associated with aeroallergen and food sensitization. J Allergy Clin Immunol 130(2):453–460.e7, PMID: 22704536, 10.1016/j.jaci.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit LA, Lenters V, Høyer BB, Lindh CH, Pedersen HS, Liermontova I, et al. 2015. Prenatal exposure to environmental chemical contaminants and asthma and eczema in school-age children. Allergy 70(6):653–660, PMID: 25753462, 10.1111/all.12605. [DOI] [PubMed] [Google Scholar]
- Spanier AJ, Kahn RS, Kunselman AR, Hornung R, Xu Y, Calafat AM, et al. 2012. Prenatal exposure to bisphenol A and child wheeze from birth to 3 years of age. Environ Health Perspect 120(6):916–920, PMID: 22334053, 10.1289/ehp.1104175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanier AJ, Fausnight T, Camacho TF, Braun JM. 2014a. The associations of triclosan and paraben exposure with allergen sensitization and wheeze in children. Allergy Asthma Proc 35(6):475–481, PMID: 25584915, 10.2500/aap.2014.35.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanier AJ, Kahn RS, Kunselman AR, Schaefer EW, Hornung R, Xu Y, et al. 2014b. Bisphenol a exposure and the development of wheeze and lung function in children through age 5 years. JAMA Pediatr 168(12):1131–1137, PMID: 25286153, 10.1001/jamapediatrics.2014.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White IR, Royston P, Wood AM. 2011. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 30(4):377–399, PMID: 21225900, 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Perzanowski MS, Just AC, Rundle AG, Donohue KM, Calafat AM, et al. 2014a. Asthma in inner-city children at 5-11 years of age and prenatal exposure to phthalates: the Columbia Center for Children's Environmental Health Cohort. Environ Health Perspect 122(10):1141–1146, PMID: 25230320, 10.1289/ehp.1307670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, Rundle AG, Perzanowski MS, Just AC, Donohue KM, Calafat AM, et al. 2014b. Prenatal phthalate and early childhood bisphenol A exposures increase asthma risk in inner-city children. J Allergy Clin Immunol 134(5):1195–1197, PMID: 25174861, 10.1016/j.jaci.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.