Summary:
Research involving human subjects after public health emergencies and disasters may pose ethical challenges. These challenges may include concerns about the vulnerability of prospective disaster research participants, increased research burden among disaster survivors approached by multiple research teams, and potentially reduced standards in the ethical review of research by institutional review boards (IRBs) due to the rush to enter the disaster field. The NIEHS Best Practices Working Group for Special IRB Considerations in the Review of Disaster Related Research was formed to identify and address ethical and regulatory challenges associated with the review of disaster research. The working group consists of a diverse collection of disaster research stakeholders across a broad spectrum of disciplines. The working group convened in July 2016 to identify recommendations that are instrumental in preparing IRBs to review protocols related to public health emergencies and disasters. The meeting included formative didactic presentations and facilitated breakout discussions using disaster-related case studies. Major thematic elements from these discussions were collected and documented into 15 working group recommendations, summarized in this article, that address topics such as IRB disaster preparedness activities, informed consent, vulnerable populations, confidentiality, participant burden, disaster research response integration and training, IRB roles/responsibilities, community engagement, and dissemination of disaster research results. https://doi.org/10.1289/EHP2378
Introduction
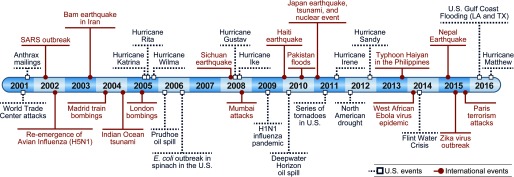
Public health emergencies and disaster events have challenged the world’s preparedness and response capabilities for decades (Figure 1). Since the attack on the World Trade Center in 2001, U.S. government agencies have redoubled their efforts to strengthen national preparedness, response, and recovery. With this national multiagency effort and continued exposure to new public health emergencies and disasters, the field of disaster research has evolved and has become commonplace in the post-disaster setting.
Figure 1.
Timeline of major global public health emergencies and disasters, 2001–2016.
Figure 1 is adapted with permission from Lurie et al. (2013). We modified it significantly by extending the timeline, formatting color, and stratifying disasters by international and domestic.
From a government agency perspective, disaster research is the study of individual, community and organizational preparedness, response, and recovery from a broad range of disaster types. Disaster research is essential to understanding how to prepare for and respond to catastrophic events such as hurricanes, earthquakes, disease outbreaks and pandemics, hazardous material spills, and large-scale acts of terrorism, as well as understanding their impact on human health.
A unique feature of most disaster studies is the urgency of initiating data collection soon after the event to capture ephemeral baseline data that may be lost or subject to recall bias if collected later. Although the value of well-designed research studies in the immediate aftermath of disasters is recognized, there remain significant challenges that must be addressed to facilitate their administration. Some key challenges include time pressures related to the development of protocols and study materials, acquisition of rapid funding to support research work, concerns of the study team interfering with life-saving disaster response activities, and compromising a frail community (Lurie et al. 2013; Miller et al. 2016).
Despite its recognized value, research involving human subjects after disasters may pose ethical concerns (Ferreira et al. 2015; O’Mathúna 2009). For example, the lack of coordination across investigators conducting research after a disaster can result in survivors being approached to join research by multiple research teams asking similar questions and requesting duplicative sample collections. In addition to the burden this may place on survivors, it can lead to unnecessary confusion when representatives from aid organizations offering direct assistance are in the field at the same time (Taylor 2016). Most importantly, concerns about the vulnerability of prospective disaster research participants have been raised and evaluated (Macklin 2014; Levine 2004).
Although the Code of Federal Regulations (45 CFR Part 46—Protection of Human Subjects; DHHS 2009) does establish research protections for certain groups such as children, prisoners, women, and fetuses, there is no explicit protection for potentially vulnerable disaster survivor research participants. These human subject concerns led the National Institute of Environmental Health Sciences (NIEHS) to create an initiative to consider how institutional review boards (IRBs) can play a role in the preservation of ethical standards in the conduct of disaster research.
Objective and Approach
To address ethical and regulatory challenges in the oversight of post-disaster research, the Office of Human Research Compliance at NIEHS formed the new Best Practices Working Group for Special IRB Considerations in the Review of Disaster Related Research, as part of a larger effort at the National Institutes of Health (NIH) to enhance research oversight capacity after disasters. This effort, called the Disaster Research Response (DR2) program (Lurie et al. 2013; Miller et al. 2016), began as a trans-NIH initiative in 2013 with the aim of developing a national framework to guide and facilitate research on the medical and public health aspects of disasters and public health emergencies. The working group was officially formed in September 2015 with the goals of exploring factors relevant to potential research participation in disasters and preparing IRBs for the review of disaster research protocols.
The multidisciplinary working group consisted of 60 members from 23 U.S. states who were recruited through a nomination process that sought to assemble a diverse group of stakeholders, including academic researchers; bioethicists; disaster responders; local, state, and federal officials; disaster survivors; community advocates; and IRB/regulatory experts, and officials. The diverse nature of the group reflects the fact that disaster research is often a collaborative venture and the recognition that complex disaster issues cannot be adequately addressed through the lens of a single discipline but requires multidisciplinary expertise (NRC 2006). The multi-sector working group was formed with the objective of developing IRB disaster-related research recommendations for the human research protection, IRB/regulatory, and disaster research, and response communities.
At a meeting in July 2016, the working group was charged with addressing four overarching specific aims:
-
1.
Preparing IRBs for the review of disaster research protocols
-
2.
Exploring unique factors or heightened concerns as it relates to potential research participants and communities affected by disasters
-
3.
Identifying participant burden for populations after disasters
-
4.
Outlining duties and considerations of the IRB in the review of research involving disaster-affected communities.
Breakout groups of participants were given a different disaster scenario and disaster research case study, and all groups were asked to react to the same set of discussion questions. The disaster scenarios included earthquake, terrorist attack with detonation of a radiological dispersion device (i.e., a “dirty bomb”), hurricane, pandemic influenza outbreak, and a toxic industrial chemical spill. The disaster scenarios were based on the Planning Scenarios developed by the Homeland Security Council in partnership with the Department of Homeland Security (Homeland Security Council 2006). The case studies that followed the scenarios were hypothetical, condensed disaster research protocols that were designed to be implemented during the immediate response stage of a particular disaster.
Major thematic elements from these discussions were collected and documented as 15 recommendations of the working group. Here we provide the recommendations as a framework and guidance for IRBs engaged in the review of disaster research protocols.
Working Group Recommendations
Recommendation 1: Prior to Consent, Prospective Participants Should Be Asked, to the Extent Feasible, about Unmet Needs and Provided Assistance Including Referrals and Resources to Reduce Risk and Maximize Benefit
In the immediate aftermath of a disaster, survivors are often left behind with acute physical and mental health needs. Additionally, disasters can cause chronic impacts that impair social and economic stability including loss of employment and the dissolution of social networks. It is imperative that the life-sustaining and essential needs of potential research subjects are met for them to have adequate capacity to make a voluntary decision about enrollment in research.
Researchers may be the first outsiders to face a disaster survivor, and they therefore should be trained in this regard and should identify unmet needs created by the disaster—for example, asthmatics and diabetics who no longer have access to their medication, or renal patients who are cut off from their dialysis center. Researchers who encounter urgent concerns among survivors have a responsibility to immediately notify the appropriate response officials. Researchers also should be prepared to provide participants with information on official disaster relief resources that are available (e.g., location of Red Cross tent, FEMA assistance centers) as well as referrals to local medical and/or mental health providers.
Although referrals and resources could provide a benefit to potential participants, research should not interfere with potential research participants’ efforts to meet their survival-related needs. Critical unmet needs must be the priority over enrollment in research.
Recommendation 2: Close Monitoring of the Consent Process Is Key to Address Any Misconceptions about the Research
IRBs should ensure close monitoring of the consenting process during recruitment in disaster studies, especially in the immediate aftermath of a disaster. Research teams must establish a standard plan (e.g., which may include a capacity or competence assessment screening questionnaire) for determining the decision-making ability of disaster-affected research participants to provide informed consent. As a precaution to eliminate confusion concerning the exchange of disaster aid for participating in research (Ahmad and Mahmud 2010), consent forms may include a section requiring the participants to initial for indication they understand that they are participating in research and that their participation in the study is independent of disaster aid administered by local, state, or federal agencies or other entities.
Additionally, research teams should distinguish themselves from responders by wearing vests, shirts, hats, and the like with clear labeling to establish their independence from the official responder community and clearly articulate to potential subjects that they are researchers asking them to engage in an optional research activity.
As with all clinical studies, participants should be reassured throughout the consent process that they may opt out of the research at any time, and the process of opting out should be discussed with them. Consistent with good clinical practice, researchers may consider re-consenting participants weeks to months after enrollment as an additional tool to ensure ongoing maintenance of a robust informed consent process and remind participants of the voluntary nature of study participation, especially for those who enrolled during the initial response phase to the disaster.
Recommendation 3: IRBs Should Guard against Any Reclassification of Minimal Risk Studies Due to the Establishment of New Post-Disaster Norms, and Should Ensure Transparency on Risks and Benefits of Research
When the probability and magnitude of harm anticipated in the research is not greater than those ordinarily encountered in daily life, the research is properly classified as minimal risk. Because disasters can establish new daily norms, one might assume that an IRB could adopt a relative standard for minimal risk studies established in their wake. However, it is inappropriate to tolerate increased research risks even in post-disaster settings where a “new normal” has been established.
There is a strong need for transparency in the research enterprise and clear identification and delineation of all potential risks and benefits of participating in a disaster-related study. Additionally, investigators should make it clear to potential participants, in the consent form and during the consent process, when the research offers no direct benefit. Researchers may want to consider a suitable level of remuneration commensurate with research participant time and effort and pay special attention to avoiding undue inducement under extreme post-disaster circumstances.
Recommendation 4: Research Teams Should Ensure Private Areas to Conduct Study Procedures to Minimize Risk of Confidentiality Breaches
Research procedures conducted in the disaster field may be out in the open because of damage to buildings and the set-up of temporary shelters. The loss of confidentiality may be particularly damaging in disaster studies when the release of personally identifiable information can create a long-lasting stigma of victimhood and potential discrimination experienced by survivors (Harada et al. 2015). Research participants may also be concerned about the disclosure of sensitive medical information to their employers and/or insurance companies (e.g., disaster workers who participate in longitudinal research related to onsite exposures may potentially be banned from current or future work sites because their employer deems them unfit for deployment).
To address privacy and confidentiality issues, research teams should plan in advance how they would assemble private areas to conduct interviews, examinations, or other study procedures. Additionally, researchers may consider applying for a Certificate of Confidentiality issued by the National Institutes of Health, which may serve to protect identifiable research information from forced disclosure and provide additional reassurance to research participants that their research data will be kept confidential. Although there have been rare legal challenges to a Certificate of Confidentiality that have resulted in the loss of confidentiality (Beskow et al. 2008), there is substantial evidence that these certificates fulfill their intended purpose (Wolf et al. 2015).
Recommendation 5: Encourage Research on Groups (as Defined in 45 CFR 46 Such as Pregnant Women and Children) That Require Special Protections per Human Subjects Protection Regulations. Disaster Research Should Also Be Encouraged for Members of Vulnerable Groups That Are Underrepresented in the Disaster Research Literature Such as Women, Racial/Ethnic Minorities, and Elderly and Disabled Populations
Researchers should develop new strategies to overcome the perceived barriers to the conduct of disaster research with groups that require special protections or who may have unique vulnerabilities. Valuable, informative research data may be lost if studies do not include these populations in their disaster studies. This is especially true when conducting research to assess behavioral and mental health outcomes. Indeed, there is mounting evidence that members of vulnerable groups may experience significant long-term mental and physical consequences following disaster events (Lai et al. 2014; King et al. 2012).
Justice demands that research be carried out for the benefit of the population as a whole; therefore, systematic exclusion of protected or vulnerable groups from disaster research studies should be avoided (Mastroianni et al. 1994). Failure to include these groups leaves a knowledge gap in our understanding of the impact of disasters across the entire population.
If the inclusion of one or more protected groups introduces unacceptable risks, researchers must justify why they are appropriately excluded from the research. IRBs must be aware of this knowledge gap and question whether such groups are unfairly excluded (e.g., due to perceived regulatory burdens rather than actual increased risks of participation in research procedures) from disaster research proposals. In situations when there is no clear rationale to exclude, IRBs must require research teams to outline a plan for conducting outreach and recruitment of such underrepresented groups into the study.
Recommendation 6: Minimize Participant Burden Associated with Multiple Duplicative Studies in the Field through the Development of a Registry for Disaster Research Projects
Survivors of disasters are often approached by many investigators, all seeking the same or similar information (IOM 2014). This can result in survey and specimen collection fatigue and an overall increase in participant burden (IOM 2014). A coordinated effort among researchers and funders could reduce duplication. One potential solution is the creation of a registry of disaster research projects to centralize and make more transparent the overall disaster research enterprise. Although development of such a registry is not an IRB function, it is consistent with the mission of the IRB to identify potential risks that may act to increase participant burden.
Federal agencies and funders must play a leadership role in organizing such efforts by linking funding decisions to unique disaster research needs. An open and transparent database of disaster research studies, similar to ClinicalTrials.gov, would allow a central point for funders and government agencies to list disaster-related projects and requests for funding opportunities, reducing overall duplication.
Recommendation 7: IRBs That Are Likely to Receive Disaster Research Protocols for Review Should Engage the Disaster Researcher and Responder Community Prior to Disaster Events
Proactive engagement between IRBs, principal investigators (PIs), and the responder community may overcome some barriers to the timely review of disaster research protocols. Examples of engagement provided included inviting first responders and PIs to IRB trainings and meetings, securing responders with disaster expertise as ad hoc consultants to the IRB as a resource in the review of disaster research protocols, and setting up use agreements between IRBs and response agencies to ensure collaborative engagement during a disaster. Additionally, any perception of an antagonistic relationship between PIs and IRBs could be improved by proactive pre-disaster collaborative engagement.
Recommendation 8: Disaster Researchers Should Consider the Development of Pre-event Generic Protocols for Provisional Approval by Their Local IRB. IRBs May Consider the Use of “Contingent Approval” Status for Time-Sensitive Disaster Studies
Development of modular template protocols prior to disasters would facilitate protocol coordination and submission for approval after a disaster. A modular protocol would be one that is sufficiently flexible to fit a range of potential disaster scenarios. Activation of specific modular components that match the type and magnitude of the disaster and research interests could allow researchers to enter into the disaster field faster for time-sensitive disaster studies.
The NIH DR2 program has developed such a protocol (i.e., Rapid Acquisition of Pre- and Post-Incident Disaster Data—RAPIDD) for the study of disaster workers, and the NIEHS IRB provisionally approved it in May 2015 (Miller et al. 2016). The IRB preapproval of RAPIDD as an advancement in disaster research can be emulated in other jurisdictions. Indeed, RAPIDD has already been used as a model to develop such protocols at the University of Iowa and the University of Texas Medical Branch.
Due to the variability that exists with different types and magnitudes of disasters, and depending on when the researcher wants to enter the disaster field, monitoring disaster research implementation in near real-time may help ensure the protection of research participants. IRBs are recommended to contingently approve disaster research protocols with the provision that the research team would report back to the IRB early in the implementation process and follow a fixed time schedule outlined by the IRB regarding any field related concerns or unanticipated issues. Additionally, an IRB may ask for the team to submit a continuing review report more frequently than the once a year required by federal regulations.
Recommendation 9: Outsource Disaster Research Protocols to Specialized IRBs or Designate a Specialized IRB for Review of Disaster-Related Research
IRBs should determine whether they have the appropriate expertise, review experience, training, and resources to properly review time-sensitive disaster-related research protocols. If an IRB determines that it lacks any of these elements, an alternate IRB with more disaster-related review experience should be made available when needed. An expansion of that idea could be the establishment of local or regional IRBs to act as specialized bodies for the review of disaster research protocols; inexperienced IRBs could then set up prepackaged reliance agreements with such entities. An example of such an entity is the Public Health Emergency Research Review Board (PHERRB), which has been put in place by the U.S. Department of Health and Human Services (DHHS) and NIH to serve as a single IRB exclusively for public health emergency research (Lurie et al. 2013). Generally, the PHERRB may only be used for protocols that are conducted, supported, or regulated by HHS; that are subject to 45 CFR 46; and that require multiple IRB review.
Recommendation 10: IRBs Should Develop Disaster and Community Profile Templates to Be Used by Research Teams to Gather Contextual Information to Guide IRB Review and Decision Making
Disaster and community context is essential for IRBs to make informed decisions on disaster research protocols. IRBs should develop templates that would be populated by disaster researchers to provide the board with essential information about the disaster context. This template should include information on affected neighborhoods, morbidity and mortality associated with the event, post-disaster hazards and risks, and evacuation patterns among other variables. The template could also include detailed information on the community targeted for research (e.g., demographics, influential community groups, functional public health or medical infrastructure).
Recommendation 11: Researchers Must Be Aware of a Disaster’s Contextual Factors to Determine How They Impact Their Studies and to Optimize Timing of the Research Activities to Minimize Any Additional Stressors on Potential Research Participants while Maximizing Data Acquisition
Optimal timing of research in the post-disaster setting is of paramount importance. IRBs need to have access to near real-time data on the nature and impact of the disaster, as it unfolds, on the affected community targeted for research. Depending upon the type, timing, and magnitude of a disaster, there may be certain time periods after a disaster when prospective research participants may have multiple unmet needs and lack specific survival-related resources. During this time, research would be inappropriate, especially when it does not offer goods or services needed to meet survivors’ needs.
Disaster events that result in mass casualties and/or cause long-term disruptions in critical infrastructure (e.g., utilities, health care systems) are more likely to lead to periods of acute stress and uncertainty among survivors. When post-disaster settings become normalized, a window of opportunity for research may present itself. Conversely, periods of stress and uncertainty may increase over time, especially when social and economic systems continue to erode after a disaster or when the disaster evolves slowly (e.g., the Flint water crisis).
Recommendation 12: Encourage Mechanisms to Provide Pre-Disaster Local Community Knowledge to IRBs to Provide Context Specific to a Local Community
IRBs based in localities at risk for disaster should, in the pre-disaster phase, identify community advisory groups and stakeholders that represent the broader community and who can serve as ad hoc consultants. The engagement of existing community advisory groups is an effective avenue to understanding community concerns and pre-disaster context so that post-disaster context can be accurately assessed. IRBs should be sure to give adequate attention to disadvantaged socioeconomic populations that may be at risk for undue inducement or exploitation. Although it is recognized that community knowledge on IRBs has value for the review of all types of research, it is especially true in disaster studies when affected communities may be particularly challenged.
Because disasters are unpredictable in the communities they impact, preparedness efforts may only go so far. In the post-disaster setting, IRBs should make a concerted effort to contact community advisory groups in close proximity to the disaster to provide assistance in the review of a disaster research protocol. National organizations such as the Community-Campus Partnerships for Health provide access to community groups and academic institutions that can assist IRBs in their efforts. Disaster researchers can provide additional context by ensuring that their protocols include current information on the community to be studied and define strategies for gathering input from and ensuring participation by members of the community.
Recommendation 13: IRBs That Wish to Establish Competency in the Review of Disaster Research Protocols Should Create and Adopt a Disaster Research Training Program and Resource Guide; Disaster Research Teams Would Also Benefit from Emergency Response Training
Few IRBs have significant experience reviewing disaster protocols. IRB members should receive training on the basics of disaster management and specific human subject protection issues that can arise during the phases of disaster response and recovery as well as critical elements of IRB review for disaster-related research. PIs and their research teams could, in turn, be targeted for training on the regulatory aspects of the IRB review of disaster-related research.
IRBs also should strongly encourage PIs and research teams to receive emergency response training (e.g., Incident Command System, National Incident Management System) before entering the disaster field, particularly during the immediate aftermath and especially when the research requires formal integration with the emergency response structure, in part to avoid impeding disaster response operations. In the pre-disaster phase, collaboratively training PIs, IRBs, and disaster responders together would be beneficial for the entire disaster research enterprise. An excellent example of such preparedness training could be the development of tabletop and field exercises that simulate the planning and implementation of disaster studies in the midst of a disaster response.
Recommendation 14: IRBs Can Play an Important Role in Assessing the Feasibility of Disaster Research and Identifying Research That Might Not Lead to Generalizable Knowledge Due to the Disaster Context
The IRB review process includes an assessment of the feasibility of the research. If the research is unlikely to be successful in testing its hypotheses due to logistical constraints in the disaster field (e.g., lack of stable utilities, difficulty of ingress and egress to disaster sites), IRBs should require research teams to establish contingency plans and modify their research protocols. IRBs can also play a role in identifying and rejecting disaster research that may pose unacceptable risks to the study participants or the research team itself, or that clearly interfere with the life and property-saving work of disaster responders.
Recommendation 15: IRBs Should Assure That All Approved Disaster Research Specify and Confirm a Plan for the Timely Dissemination of Actionable Research Results Back to Key Stakeholders
One of the principles of ethical research is to provide results and feedback to stakeholders, and disaster research is no different in that regard (Emanuel et al. 2008). IRBs should require researchers to develop a dissemination plan for the results that clearly describes how the data will be reported back to participants and the community throughout the life cycle of the study. The plan must ensure a timely report back and should consider specific entities such as community groups and health educators that can help translate scientific findings into lay language. Methods of dissemination should be carefully considered to optimize information exchange with the community and may include town hall forums, newsletters, and use of social media.
Conclusion
The burgeoning field of disaster research has placed greater demands on IRBs to ensure that the welfare and rights of human research subjects are protected during disaster studies. The review of disaster research protocols requires new tools and training for IRBs to assure the protection of disaster survivors from research-related harms. These recommendations are currently being evaluated and prioritized by NIH officials to determine the process for moving forward with implementation. Although disaster research conducted during response may be challenging, IRBs can play useful roles in achieving careful, balanced, thoughtful procedures that both consider the value of the research to advance science and reduce suffering—and that also consider the potential for harm based on the unique vulnerabilities of disaster survivors in a disaster aftermath.
Acknowledgments
The authors would like to acknowledge their fellow members of the NIEHS Best Practices Working Group Steering Committee: B. Clark, J. “Chip” Hughes, S. Phillips, C. Philput, D. Resnik, and D. Wendler. They also acknowledge the contributions of the following individuals who provided assistance to the project: D. Abramson, L. Baker, C. Bebelle, P. Cacioppo, M. Chien-Hale, L. Close, F. Daniels, C. Edwards, B. Elmore, C. Garrard, M. Hanna-Attisha, B. Hoffman, K. James, M. Justice, M. Kudumu, J. Lambert, E. Lee, E. O’Connell, R. Stephens, M. Stewart, V. Timmons, E. Walter, P. Windsor, C. Wladyka, and F. Yucel.
This research was supported by the Intramural Research Program and the Office of the Director of the National Institute of Environmental Health Sciences, National Institutes of Health.
References
- Ahmad A, Mahmud SM. 2010. Philanthropic misconception. Asian Bioethics Rev 2(2):154–161. [Google Scholar]
- Beskow LM, Dame L, Costello EJ. 2008. Certificates of confidentiality and the compelled disclosure of research data. Science 322(5904):1054–1055, PMID: 19008431, 10.1126/science.1164100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHHS (U.S. Department of Health and Human Services). 2009. “Code of Federal Regulations – Title 45 Public Welfare CFR 46, Protection of Human Subjects.” https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/index.html [accessed 1 August 2017]. [PubMed]
- Emanuel EJ, Wendler D, Grady C. 2008. An ethical framework for biomedical research. In: The Oxford Textbook of Clinical Research Ethics. Emanuel EJ, Grady CC, Crouch RA, Lie RK, Miller FG, Wendler DD, eds. New York, NY:Oxford University Press, 123–135. [Google Scholar]
- Ferreira RJ, Buttell F, Ferreira SB. 2015. Ethical considerations for conducting disaster research with vulnerable populations. J Soc Work Values Ethics 12(1):29–40. [Google Scholar]
- Harada N, Shigemura J, Tanichi M, Kawaida K, Takahashi S, Yasukata F. 2015. Mental health and psychological impacts from the 2011 Great East Japan Earthquake Disaster: a systematic literature review. Disaster Mil Med 1(1):17, PMID: 28265432, 10.1186/s40696-015-0008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homeland Security Council. 2006. National Planning Scenarios: Created for Use in National, Federal, State, and Local Homeland Security Preparedness Activities. Version 21.3, https://info.publicintelligence.net/DHS%20-%20National%20Planning%20Scenarios%20March%202006.pdf [accessed 27 July 2017].
- IOM (Institute of Medicine). 2014. Enabling Rapid and Sustainable Public Health Research during Disasters: Summary of a Joint Workshop by the Institute of Medicine and the U.S. Department of Health and Human Services. Washington, DC:National Academies Press. [PubMed] [Google Scholar]
- King S, Dancause K, Turcotte-Tremblay AM, Veru F, Laplante DP. 2012. Using natural disasters to study the effects of prenatal maternal stress on child health and development. Birth Defects Res C Embryo Today 96(4):273–288, PMID: 24203917, 10.1002/bdrc.21026. [DOI] [PubMed] [Google Scholar]
- Lai BS, Auslander BA, Fitzpatrick SL, Podkowirow V. 2014. Disasters and depressive symptoms in children: a review. Child Youth Care Forum 43(4):489–504, PMID: 25067897, 10.1007/s10566-014-9249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine C. 2004. The concept of vulnerability in disaster research. J Trauma Stress 17(5):395–402, PMID: 15633918, 10.1023/B:JOTS.0000048952.81894.f3. [DOI] [PubMed] [Google Scholar]
- Lurie N, Manolio T, Patterson AP, Collins F, Frieden T. 2013. Research as a part of public health emergency response. N Engl J Med 368(13):1251–1255, PMID: 23534565, 10.1056/NEJMsb1209510. [DOI] [PubMed] [Google Scholar]
- Macklin R. 2014. Studying vulnerable populations in the context of enhanced vulnerability. In: Disaster Bioethics: Normative Issues When Nothing is Normal.O’Mathúna DP, Gordijn B, Clarke M, eds. New York, NY:Springer Science+Business Media Dordrecht, 159–173. [Google Scholar]
- Mastroianni AC, Faden RR, Federman DD. 1994. Women and Health Research: Ethical and Legal Issues of Including Women in Clinical Studies. Washington, DC:National Academies Press. [PubMed] [Google Scholar]
- Miller A, Yeskey K, Garantziotis S, Arnesen S, Bennett A, O’Fallon L, et al. 2016. Integrating health research into disaster response: the new NIH Disaster Research Response Program. Int J Environ Res Public Health 13(7):676, PMID: 27384574, 10.3390/ijerph13070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC [National Research Council (U.S.) Committee on Disaster Research in the Social Sciences: Future Challenges and Opportunities; National Research Council (U.S.) Division on Earth and Life Studies]. 2006. Facing Hazards and Disasters: Understanding Human Dimensions. Washington, DC:National Academies Press. [Google Scholar]
- O’Mathúna DP. 2009. Conducting research in the aftermath of disasters: ethical considerations. J Evid Based Med 3:65–75, PMID: 21349047, 10.1111/j.1756-5391.2010.01076.x. [DOI] [PubMed] [Google Scholar]
- Taylor HA. 2016. Review and conduct of human subjects research after a natural or man–made disaster: findings from a pilot study. Narrat Inq Bioeth 6(3):211–222, 10.1353/nib.2016.0061. [DOI] [Google Scholar]
- Wolf LE, Patel MJ, Williams Tarver BA, Austin JL, Dame LA, Beskow LM. 2015. Certificates of confidentiality: protecting human subject research data in law and practice. J Law Med Ethics 43(3):594–609, PMID: 26479569, 10.1111/jlme.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]