Abstract
Polarized one- and two-dimensional infrared spectra were obtained from the epidermis of onion (Allium cepa) under hydrated and mechanically stressed conditions. By Fourier-transform infrared microspectroscopy, the orientation of macromolecules in single cell walls was determined. Cellulose and pectin exhibited little orientation in native epidermal cell walls, but when a mechanical stress was placed on the tissue these molecules showed distinct reorientation as the cells were elongated. When the stress was removed the tissue recovered slightly, but a relatively large plastic deformation remained. The plastic deformation was confirmed in microscopic images by retention of some elongation of cells within the tissue and by residual molecular orientation in the infrared spectra of the cell wall. Two-dimensional infrared spectroscopy was used to determine the nature of the interaction between the polysaccharide networks during deformation. The results provide evidence that cellulose and xyloglucan associate while pectin creates an independent network that exhibits different reorientation rates in the wet onion cell walls. The pectin chains respond faster to oscillation than the more rigid cellulose.
The cell wall is an elaborate extracellular matrix that encloses each cell in a plant. It consists of a microfibrillar cellulose phase and a matrix phase that contains a variety of polymers such as poly-GalUA (PGA), hemicelluloses, proteins, and phenolics, including lignin (Carpita and Gibeaut, 1993; Brett and Waldron, 1996). The passive reorientation of microfibrils forms an important part of the “multinet” hypothesis (Roelofsen and Houwink, 1953) but so far, little is known about interactions that control the mechanical properties of the cell wall (Cosgrove, 1993). The largest volume of the cell wall space is occupied by water, which is crucial for the rheological properties and modifies the interactions between components (Foster et al., 1996). Hydrated macromolecules show a wider variation in mobility than in their dehydrated form (Ha et al., 1997).
The common onion (Allium cepa) has been used as a model system for investigating the architecture of plant cell walls (McCann et al., 1992, 1993; Chen et al., 1997). It contains unicellular epidermal layers that are directionally ordered during growth, it has high tissue strength, and it is of ideal thickness to allow acquisition of infrared (IR) transmission spectra. The IR spectra of onion epidermis are dominated by absorption bands of cellulose and pectin, while minor constituents such as protein, ferulic acid, lignin, and hemicelluloses can also be detected.
With polarized light, the orientation of particular functional groups can be determined with respect to the long axis of cells. Based on the crystal structure analysis and polarized IR spectra, the tentative assignment of native cellulose bands has been carried out (Cael et al., 1975; Marchessault and Sundararajan, 1983). From the observed bands and their dichroism, it has been possible to determine the preferred orientation of functional groups, either parallel or perpendicular to the chain direction.
Fourier-transformed IR (FT-IR) microspectroscopy has been used to determine the presence and orientation of functional groups in cellulose and pectin molecules within an individual plant cell wall (McCann et al., 1993; Séné et al., 1994; Chen et al., 1997). IR bands associated with the cellulose and the PGA chain backbone are more intense with the polarization parallel to the direction of cell elongation, whereas bands associated with pectin ester and carboxylate groups are stronger in polarization perpendicular to the direction of cell elongation (Chen et al., 1997).
To study the mechanical properties of the wall we have developed a mechanical creep apparatus, which allows the displacement of the tissue to be measured using a constant load applied axially to one end. The tissue is mounted horizontally and the apparatus attached either to a bright field microscope stage or to the stage of an IR microscope. The hydration of the tissue can also be controlled using a specially constructed cell (Chen et al., 1997). A tissue sample can be examined before, during, and after an application of stress to obtain both optical images and spectral information so that we can investigate how cell wall biopolymer orientation and cell shape change when a stress is applied and removed.
To investigate the nature, if any, of the interaction between the macromolecular networks in stressed systems, we have used a new technique called two-dimensional IR spectroscopy (2D FT-IR; Noda 1990; Noda et al., 1999; Hinterstoisser and Salmen, 2000). We have adapted this technique for hydrated plant tissues and, in the experiments reported here, prestretched onion epidermis is subjected to unidirectional mechanical oscillation that induces molecular reorientation. Changes in the intensity of bands during oscillation, measured using polarized light, can provide direct information on the reorientation rates of individual molecules or parts of individual molecules.
RESULTS AND DISCUSSION
Effect of Hydration
Cell walls are naturally composed of a hydrated polymer network, and a knowledge of the influence of water and humidity is very important in understanding the structure and role of particular cell wall components. Edelmann (1995) found that the extension state of cell wall material depended on the water content. Therefore we found it necessary to keep the sample sufficiently wet to avoid inducing irreversible molecular orientations.
To determine the water content in the samples, the water vapor desorption at discrete relative air humidities (RH) was measured since the equilibrium of hydration is generally described by water activity (Aw). For a spectroscopic measure of water content in the samples, the relative intensity ratio (Ia) of the water deformation band at 1,640 cm−1 versus the cellulose (CH) mode at 1,367 cm−1 was calculated. The correlation of Ia with water content showed that the slope of the curve changed markedly at about 95% RH. Below 95% RH the water content drops below 50% of the original weight of the onion tissue. At humidities above 98% RH the water deformation band becomes very broad, and it is difficult to determine the water content spectroscopically. The optimal humidity conditions for measuring IR spectra of hydrated onion were found to be 96% to 97% RH, (water content between 65% and 82% [w/w]), which corresponds to an intensity ratio (Ia) of 2:3. Therefore water contents around 70% (w/w) were used in all subsequent experiments.
Oriented Cell Wall Polysaccharides
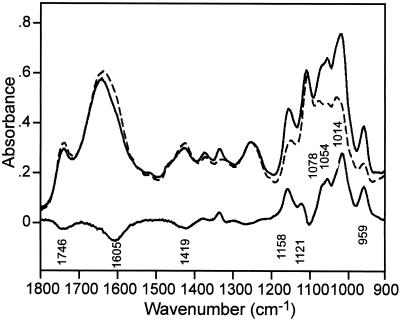
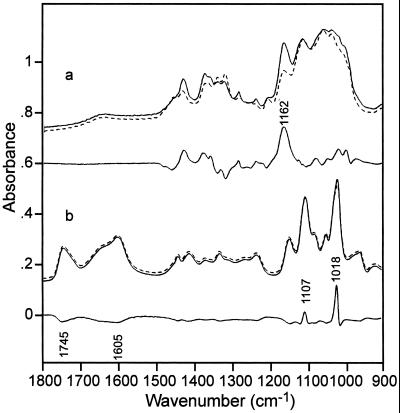
The typical parallel and perpendicular IR transmission spectra of oriented onion epidermis in wet form together with their difference spectrum (∥-⊥) are shown in Figure 1. This difference spectrum indicates the relative orientation of functional groups characterizing particular molecules in the wall. The corresponding parallel, perpendicular, and difference spectra of the two main components of the cell walls, cellulose and PGA, are shown in Figure 2. The IR bands and band assignments of onion cell wall components are listed in Table I. IR band frequencies listed in Table I and discussed in the text are given with 2 cm−1 accuracy. The cellulose and pectin bands in the region of 1,800 to 900 cm−1 are based on literature data (Cael et al., 1975; Marchessault and Sundararajan, 1983; Chen et al., 1997; Wellner et al., 1998). So far, the complete vibrational assignment of oriented molecules is only known for cellulose (Cael et al., 1975; Marchessault and Sundararajan, 1983).
Figure 1.
FT-IR microscope spectra of onion epidermis with parallel (∥, connected) and perpendicular (⊥, dotted) polarization, and subtraction (∥-⊥) spectrum (connected).
Figure 2.
FT-IR transmission spectra of cellulose (a) and pectin (b) measured with parallel (∥, connected) and perpendicular (⊥, dotted) polarization and their difference (∥-⊥) spectrum (connected line below each).
Table I.
Static FT-IR frequencies (cm−1) of oriented cellulose and pectin
| Cellulose | * | Pectin | Band Assignment | Comments | |
|---|---|---|---|---|---|
| 1,745 | (⊥) | ν (C=O) | PGA, ester | ||
| 1,640 | – | 1,640 | δ (HOH) | adsorbed water | |
| 1,605 | (⊥) | νas (COO−) | PGA, carboxylate | ||
| 1,444 | (⊥) | δ (CH) | PGA, ester | ||
| 1,430 | (∥) | δ (CH2) | |||
| 1,419 | (⊥) | νs (COO−) | PGA, carboxylate | ||
| 1,368 | (⊥) | δ (CH2), ν (CC) | |||
| 1,336 | 1,335 | – | δ (CH), ring | ||
| 1,162 | (∥) | 1,150 | (∥) | ν (C-O-C), ring | glycosidic link |
| 1,125;1,110 | (∥) | 1,107 | (∥) | ν (CO), ν (CC), ring | |
| 1,060 | (∥) | 1,055 | (∥) | ν (CO), ν (CC), δ (OCH) | |
| 1,035 | (∥) | 1,033 | (∥) | ν(CO), ν (CC), ν (CCO) | |
| 1,018 | (∥) | ν (CO), ν (CC), δ (OCH), ring | PGA, pectinate | ||
| 1,008 | (∥) | ν (CO), ν (CC), δ (OCH), ring | PGA, pectate | ||
| 985 | (⊥) | 972 | (∥) | OCH3 | |
| CO; Cellulose, ester | |||||
| 963 | (∥) | δ (C=O) | |||
| Other polysaccharides | |||||
| 1,150 | – | ν (C-O-C), glycosidic link | RG I, XG | ||
| 1,078 | – | ν (CO), ν (CC), ring | XG | ||
| 1,070 | – | ν (CO), ν (CC), ring | RG I, galactan | ||
| 1,041 | – | ν (CO), ν (CC), ring | XG |
Orientation: (∥), parallel; (⊥) perpendicular. RG I, Rhamnogalacturonan; XG, xyloglucan; PGA, polygalacturonic acid.
, Orientation assignment according to Marchessault and Sundararajan, 1983.
In the spectra of the onion samples, backbone vibrations were assigned for cellulose at 1,158 cm−1, PGA at 1,054; 1,014; and 959 cm−1, and rhamnogalacturonan (RG I) at 1,078 cm−1 (Kačuráková et al., 2000) in positive direction of IR absorptions. The negative band at 1,746 cm−1 came from pectin ester groups, and the 1,605 and 1,419 cm−1 bands came from carboxylate groups (Fig. 1; Table 1). Positive bands can be related to the cellulose and PGA backbone ring vibrations and glycosidic bond vibrational modes in direction of the fiber axis, aligned along the major axis of the cell, whereas the negative peaks in the subtracted spectrum relate to perpendicularly oriented side groups.
In the onion spectra, band shoulders from xyloglucan (XG), RG I, and galactans can be present in the region at about 1,065 to 1,075 cm−1 (Table 1); however, their orientation is so far unknown.
FT-IR Microspectroscopy
During replicate analysis some variability in the IR spectra of the epidermis of different onions was noticed. This variability reflected differences in composition resulting from age, variety, storage conditions, etc. of the onion. However, we found that the compositional variability did not affect the main results of these experiments and that the polarization was not affected by this variability. Consequently, the results we have shown here are typical and illustrate reproducible findings related to the major biopolymer networks.
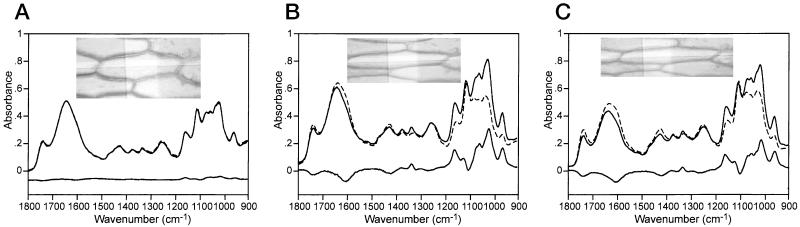
In a typical epidermal cell in hydrated conditions at room temperature before any stress is applied, the length and width of the cell were approximately 125 and 40 μm, respectively (Fig. 3A). The spectra in Figure 3A were taken from the epidermal cell at this initial stage, showing the parallel (∥) and perpendicular (⊥) polarizations, respectively. Initially there was only a small directional alignment in the cell wall macromolecules as shown by the small difference between the parallel and perpendicular spectra. There was a small amount of absorption with positive peaks in the cellulose and PGA backbone vibration region (1,200–950 cm−1) and negative peaks in the carbonyl side group region (1,750–1,600 cm−1).
Figure 3.
Images and FT-IR microscope spectra with parallel (∥, connected) and the perpendicular (⊥, dotted line) polarization and subtraction (∥-⊥) spectrum (connected line below) of initial (A), stressed (B), and relaxed (C) cell in hydrated tissue. The highlighted area in the image represents an area of 10 × 50 μm.
Upon application of a 10-g load for 8 min the cell length increased to 175 μm and width decreased to 22 μm. This cell was chosen as it showed the maximum change in dimensions although six other adjacent cells increased in length by 32 to 50 μm and decreased in width by 7 to 18 μm. In the subtracted spectrum the PGA and cellulose backbone vibrations gave strong positive bands at 1,110 and 1,018 cm−1 and 1,162; 1,058; and 1,000 cm−1, respectively. Negative absorbances at 1,745 and 1,605 cm−1 corresponded to the pectin ester and carboxylate groups. The glycosidic link band positions of non-cellulose polysaccharides at about 1,150 cm−1 (Kačuráková et al., 2000) are overlapped with the strong cellulose band centered at 1,162 cm−1, and therefore we assume that their backbones areco-aligned with the cellulose. The intensity of this difference band can be considered as a measure of orientation of the cell wall polysaccharides. The positive and negative difference IR bands suggest that the preferred orientation of polysaccharides is along the direction of stress. The PGA backbone is also co-aligned with the main chain of microfibrils, whereas the carbonyl side groups are orientated approximately perpendicular. The preferential orientation of polysaccharides along the axis of elongation found in the native onion cell wall (Chen et al., 1997) is thus greatly enhanced under stress. The onion epidermis behaves similarly to the pea stem cell wall, which also has a preferential orientation of microfibrils in the direction of cell elongation (Morikawa et al., 1978). In contrast, the preferred orientation in oat coleoptile (McCann et al., 1993) and carrot cell walls (Morikawa and Senda, 1978) was found perpendicular to the axis of elongation.
Plastic and Elastic Deformation
When the cell is allowed to relax after imposition of stress, the dimensions of the length and width were 161 and 25 μm, respectively (Fig. 3C). Only partial recovery of cell dimensions in the original unstressed state was observed, and again the same six adjacent cells decreased in length by 23 to 37 μm and increased in width by 10 to 15 μm. The subtraction spectrum showed little difference from that of the stressed subtraction spectrum (Fig. 3B), although there was a general small decrease in the absorbance intensity of the subtracted relaxed spectrum in the 1,200 to 950 cm−1 region.
A large plastic deformation of the cell wall is confirmed in the subtraction spectrum of the relaxed cell (Fig. 3C), which shows a high degree of tissue orientation after the load has been removed. The small elastic deformation is shown by a slight reduction in the absorbance intensities of the subtraction spectrum of the relaxed cell (Fig. 3C) compared with the stressed cell (Fig. 3B). We can conclude that the stress-induced alignment for cellulose and non-cellulosic polysaccharide backbones is in the direction of stress with PGA side groups found orthogonal to this direction in a hydrated system. Under a constant stress, permanent deformation occurs in the walls and consequently in the cells themselves.
2D FT-IR Spectroscopy
The FT-IR polarized microspectroscopy showed that the applied linear stress induces preferred macromolecular orientation in the cell walls parallel to the applied stress and the oriented macromolecules exhibited strong dichroism in certain bands. However, these results do not allow one to conclude whether the networks respond independently to applied stress or whether the PGA is forced into orientation through strong associative links with the cellulose. Therefore we used 2D FT-IR spectroscopy to look for evidence of an interaction between cellulose and non-cellulosic polysaccharides.
The FT-IR spectroscopy revealed the variability of onion epidermis composition where variation in pectic polysaccharides and hemicellulose composition may occur in addition to changing relative amounts of PGA and cellulose. Spectra of bulk samples measured by dynamic 2D FT-IR tended to be very sensitive toward the composition variability. Because of the available transmission IR data for cellulose and PGA we took onion epidermis samples with high cellulose and PGA contents. Three repeat measurements of the onion samples showed that samples with comparable composition reveal good reproducibility of dynamic spectra.
Dynamic 2D FT-IR Spectra
Dynamic in-phase, quadrature, power, and phase spectra of a typical onion epidermis are shown in Figure 4. These spectra do not show the patterns of the normal transmission IR spectrum (Fig. 1); they only show responses of individual spectral bands to the applied strain, and a flat line where IR bands do not respond to the strain. In the wet onion tissue the 1,640 cm−1 band of adsorbed water was not seen in the dynamic spectra, and neither the PGA ester bands (1,740 and 1,444 cm−1) nor the carboxylate vibrations of the pectate form (1,610 and 1,415 cm−1) were observed. Vibrational modes in the 1,800 to 1,200 cm−1 region, mostly arising from side chains or side groups (such as ester and OH), were apparently not very sensitive to the applied perturbation. However, a significant response was found in the bands related to the backbones of the polysaccharides in the 1,200 to 900 cm−1 region. These are cellulose, hemicelluloses, and pectic polysaccharide bands as listed in Table I. However, it is not easy to unravel the XG, rhamnogalacturonan, or galactan bands as they all contain a band or shoulder at about 1,070 cm−1 and between 1,130 and 1,100 cm−1 (Kačuráková et al., 2000).
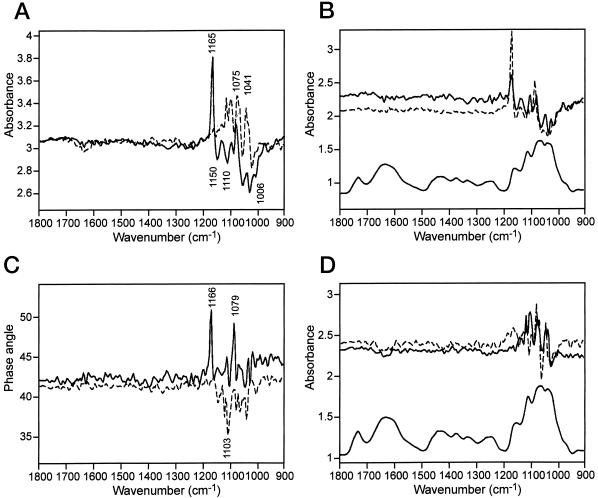
Figure 4.
Dynamic 2D IR spectra A, Power spectra of onion epidermis, parallel (connected) and perpendicular (dotted) polarized. B, Dynamic, in-phase (connected), quadrature (dotted), and static spectra (below; connected), parallel polarized. C, Phase angles of onion epidermis measured with parallel (connected) and perpendicular (dotted) polarization. D, Dynamic, in-phase (connected), quadrature (dotted), and static spectra (below; connected), perpendicular polarized.
Parallel Polarization
From the power spectrum it was apparent that the cellulose glycosidic stretching band at about 1,165 cm−1 had a very high dynamic intensity in parallel polarization (Fig. 4A). The negative band at 1,150 cm−1 may contain some XG and/or PGA contributions that have the same band position (Table I). The PGA bands at 1,150; 1,110; and 1,006 cm−1, attributed to the backbone pyranoid ring vibrational modes, were of medium intensity in positive orientation, whereas other bands at 1,056 and 1,029 cm−1 were from cellulose in negative orientation. The bands at about 1,075 cm−1 can be attributed either to RG I, galactan, or together with a band at 1,041 cm−1 to XG (Kačuráková et al., 2000).
The cellulose band at 1,165 cm−1 was positive in both in-phase and quadrature spectra (Fig. 4B) with parallel polarized light, as well as the band at 1,079 cm−1. This indicated that the glycosidic link and therefore the chain axis of polysaccharides became more oriented in the direction of the applied strain.
The band at 1,040 cm−1 assigned to XG was found only in the in-phase spectrum. The predominantly negative PGA-related bands at 1,103; 1,054; and 1,021 cm−1 were stronger in the in-phase spectrum than in the quadrature spectrum (Fig. 4B), whereas the cellulose was more intense in the quadrature spectrum. This suggested a difference in the response of the cell wall polymers to the applied stress. Bands in the in-phase spectrum represent elastic response, whereas bands in the quadrature spectrum indicate a more viscous behavior. The phase spectrum of the onion epidermis is shown in Figure 4C. The phase angles of the cellulose glycosidic link (1,165 cm−1) and a ring vibration probably from RG I or XG (1,079 cm−1) were 7 and 8 degrees, respectively, higher than the average. This corresponded to a response 2.0 ms slower than the average phase delay. The phase angles of PGA vibrational modes at 1,152; 1,092; and 1,020 cm−1 were about 2 degrees or 0.6 ms lower than the average. This may reflect the spatially inhomogeneous composition of the bulk tissue.
Perpendicular Polarization
The difference in the response of the polymers to the applied stress was found also in perpendicular polarization (Fig. 4D). No response from the glycosidic bonds at 1,165 to 1,150 cm−1, but bands of higher intensity at 1,075 and 1,041 cm−1 were found in positive orientation. These bands may originate from RG I or XG and they were found in both the in-phase and quadrature spectrum (Fig. 4D) with slightly higher response to the applied strain in parallel orientation. The negative bands (Fig. 4D) are ring vibrations from almost all the discussed polysaccharides and their phase angles showed smaller values than in parallel orientation (Fig. 4C). The 1,103 cm−1 PGA band phase angle corresponded to 2.0 ms faster reaction than XG. No phase angle different from the average was found for cellulose in the perpendicular orientation. Because all the non-cellulosic polysaccharides except for PGA have bands about 1,075 cm−1 (Table 1), further studies will be on model compounds to distinguish them.
Two-Dimensional Correlation
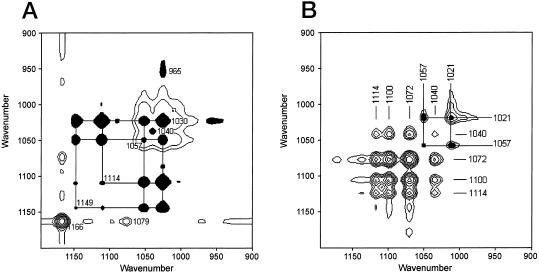
To decide whether the movements of cellulose and PGA are independent we examined the two-dimensional correlation spectra (Noda 1990; Budevska et al., 1994). Positive crosspeaks in the synchronous correlation spectrum showed intensity maxima of cellulose at 1,166 and 1,132 cm−1 and the band at 1,079 cm−1, which indicated that these modes responded together to the applied strain in parallel polarized light. The negative cross peaks corresponded to bands of PGA at 1,149; 1,114; 1,057; 1,030; and 965 cm−1 (Fig. 5A). There were no crosspeaks between PGA and cellulose peaks. Cellulose may interact with a polysaccharide at 1,079 cm−1, however at the present state of knowledge we are not able to prove whether this is also RG I or only XG. The independent reorientational responses of cellulose and PGA chain functional groups suggested that cellulose and PGA are not directly associated.
Figure 5.
Two-dimensional synchronous correlation maps with positive cellulose and XG peaks (contours) and negative pectin peaks (filled in black) from parallel (A), and perpendicular (B) polarized dynamic FT-IR spectra.
In the synchronous correlation spectrum with perpendicular polarized light (Fig. 5B), positive bands arose from XG ring vibrations at 1,114; 1,100; 1,072; and 1,042 cm−1. At 1,057 and 1,021 cm−1, negative PGA bands were found. Again there were no cross-peaks between XG and PGA, excluding direct molecular interactions.
The observation of different rates with which the cellulose and PGA networks react to the mechanical stress demonstrates the potential of 2D FT-IR to give new insight into the molecular interactions within plant cell walls. To understand the significance, we have to consider two findings from different techniques: (a) Mechanical tests performed on whole plant and model tissues have shown that the different network structures provide different mechanical properties. Cellulose dominated the frequency response for small deformation (Whitney et al., 1999) and (b) previous nuclear magnetic resonance measurements (Foster et al., 1996; Ha et al., 1997) have shown a range of different molecular mobilities in cell wall materials, with PGA molecules usually more mobile then cellulose.
The 2D FT-IR data link these two above observations. When the tissue was stretched, changes in cellulose IR bands dominated the power spectrum. However, the phase angles showed that the cellulose response was slower than the matrix, whereas some PGA chain reorientation occurred faster. We conclude that the mechanical strain was taken up first by the more mobile PGA matrix (including the middle lamella between cells) and then by the cellulose microfibrils in the individual cell walls. The role of XG and RG I remains open because of the assignment difficulties.
MATERIALS AND METHODS
FT-IR Microspectroscopy
Biological Material
For mechanical studies, strips of onion (Allium cepa) lower epidermis (15 mm long, 2 mm wide) from the equatorial section of the bulb scale were used. The first outer scale of the onion bulb was removed and discarded and subsequent scales were used. The lower epidermal strip was carefully removed from the onion scale with a forceps, then floated on distilled water for 30 min to allow any induced stress from the removal step to relax.
Oriented Model Compounds
Model compounds were used for assignment of polarization bands. Cotton cellulose fibers were manually co-aligned. A potassium pectinate film with 59% degree of esterification was prepared from aqueous solution at ambient temperature. The film was partially rehydrated and molecular orientation was introduced by stretching to about 10% of relative deformation (Δl/l).
Mechanical Stress Studies
The ends of the epidermal strip were glued onto stainless steel sample holders between the carriages in the creep apparatus, leaving an IR transparent sample area 8 mm long by 2 mm wide (Fig. 6). To control the humidity the sample was enclosed in a hydration cell fitted with two barium fluoride windows (1 mm thick, 13 mm in diameter), of which the upper one was held in a removable lid for sample loading (Chen et al., 1997). The sample holders entered the cell through two small holes in the sides, allowing free movement. Additional inlets and outlets allowed moist air to be circulated using a peristaltic pump. Wet filter paper strips inserted around the inner edge of the cell helped maintain a constant humidity level around the tissue sample.
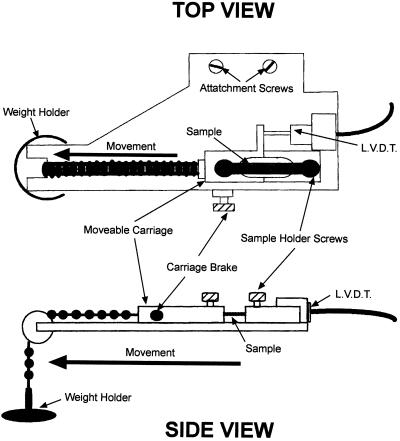
Figure 6.
Stretching apparatus (top and side view).
To determine the optimum degree of hydration for the experiments, onion epidermis samples in the hydration cell were exposed to an atmosphere of controlled RH above water (100% RH) or saturated solutions (Greenspan, 1977) of K2SO4 (97% RH), Na2SO3 (95% RH), KBr (84% RH), and NaCl (76% RH). After 48 h the samples were weighed and their IR spectra collected. The samples were then air-dried at 35°C to obtain the dry material content, and the water content was calculated from the difference. The samples were measured only at high humidities because onion shrinks and softens at 64% RH (Jones and Mann, 1963).
The creep apparatus allows the epidermal tissue sample to be stressed while in a horizontal position. This is achieved by using a weight attached to a moveable low friction carriage that is attached to one end of the sample with the other end of the sample being fixed (Fig. 6).
FT-IR Microspectroscopy of Stressed Samples
The IR spectra of stressed samples were measured with a spectrometer (FTS 175C, Bio-Rad, Cambridge, MA) with an external microscope (UMA 500, Bio-Rad) attachment that allows detailed measurements of a single cell down to an area of 10 × 10 μm, with a polarizer in the optical port and an adjustable aperture at the remote focal plane. A KRS5 (thallium bromide and iodide mixed crystal) wire grid polarizer (Graseby Specac Ltd., Orpington, Kent, UK) was used to polarize the IR beam.
Spectra were measured with the incident light polarized parallel (∥) and perpendicular (⊥) to the stretching direction. Subtraction spectra were calculated as parallel minus perpendicular polarized spectrum (∥-⊥).
In preliminary experiments we found that a water content of the sample of about 70% (w/w) was optimal for spectroscopic measurements and this was used in all subsequent experiments.
A sample area in the center of one of the lower epidermal cells (typically 10 × 50 μm) was selected for collecting the IR spectra. A pair of single-beam spectra in parallel and perpendicular polarizations were collected from the same spot on the cell being sampled. For each sample a background spectrum without sample in place was obtained with the same polarization and humidity conditions. The spectral resolution used was 8 cm−1 and 128 interferograms were co-added to improve spectral quality. This procedure was carried out for the same cell with the initial, stressed, and relaxed conditions as described below.
The parallel and perpendicular spectra of the epidermal layer were obtained from the onion lower epidermis in the relaxed condition. A load of 10 g was then applied for a fixed time period, which stressed the sample, and the displacement was measured as a function of time. Finally the weight was removed and the sample was allowed to relax for a fixed time accompanied by logging of the displacement. The cell images and spectra in both polarization directions were acquired for each of these steps.
The cell images were acquired with a camera (CCD-IRIS/GGB, Sony, Tokyo) attached to the microscope and processed with a video capture card in a PC using Shadow II software (Bio-Rad).
FT-IR Spectroscopy of Model Compounds
Polarised FT-IR spectra of oriented models were measured on a spectrometer (FTS 6000, Bio-Rad) with a KRS5 wire grid polarizer. The dry cotton cellulose fibers were co-aligned and pressed in a diamond anvil cell (Diacell, Leicester, UK). The potassium pectinate film was prestretched while kept in the humid hydration compartment and measured in the polymer stretcher sample holder.
2D FT-IR Spectroscopy
For dynamic 2D FT-IR spectral measurements an epidermal strip (1 × 1.5 cm) was placed in a polymer stretcher (Bio-Rad), which was modified to enclose the sample in a hydration cell with barium fluoride windows based on the design of Chen et al. (1997). The air humidity in the cell was kept constant with sponges soaked in water and inserted into the hydration cell cavities. The sponges created an atmosphere that allowed the onion samples to maintain the water content of about 70% of the total weight during the measurements (2–3 h). The onion samples were prestretched to about 30% of relative deformation to partially align cellulose and PGA, and were then subjected to a small periodic strain with frequency ω = 20 Hz and a sample modulation amplitude of 200 μm.
The 2D FT-IR measurements were carried out on a spectrometer (FTS 6000, Bio-Rad) in step scan mode using a narrow band nitrogen cooled MCT (HgCdTe) detector. A KRS5 wire grid polarizer was used to obtain radiation polarized parallel and perpendicular to the stretching direction, which was chosen to coincide with the axis of the cell wall elongation. Four scans at 8 cm−1 resolution were averaged. The spectrometer was controlled by a computer running Win-IR Pro Version 2.4 (Bio-Rad).
Two-dimensional spectra were obtained from the modulated step-scan spectra using Digital Signal Processing. The phase modulation frequency was 400 Hz. The in-phase static spectra were normalized against a single beam background spectrum and the in-phase and quadrature dynamic spectra were normalized against the in-phase static spectrum. The phase spectra were calculated with the spectrometer software (Bio-Rad) and the power spectra were calculated in Matlab (Mathworks, Inc., Natick, MA).
2D FT-IR Spectra
Dynamic IR spectroscopy can be used to examine individual submolecular constituents (e.g. backbone segments, side chains, and various functional groups) in a polymer system where the variation of IR dichroism is measured as function of time. Dichroisim is the property of molecules such that the IR absorption bands measured with light polarized parallel and perpendicular to any reference direction are not identical and infers the presence of orientation at the molecular level. The response of side chain functional groups may not be fully synchronized with polymer backbone motions (Noda et al., 1999). In the 2D FT-IR experiment a sinusoidal mechanical oscillation was applied to the onion epidermis, which induces small changes in the spatial arrangement of the macromolecules. As a result we obtain a time-dependent variation of the spectral intensity superimposed onto the static (normal) IR spectrum. This is then broken down into two dynamic spectra: The in-phase spectrum represents the reorientational motions that occur simultaneously with the periodic strain, and the quadrature spectrum characterizes the motions that are 90° out of phase with the periodic strain. Two-dimensional plots, phase, and power spectra can all be calculated from these two dynamic spectra (Budevska et al., 1994; Noda et al., 1999).
Two-dimensional cross-correlation of the dynamic spectral response at each wavelength is used to infer the existence of connectivity and interactions of functional groups. In synchronous plots the autocorrelation bands lie on the diagonal and off-diagonal crosspeaks exist between bands with similar time response (Noda, 1990; Noda et al., 1996).
A phase spectrum provides for each absorption band the phase delay to the stretching frequency ω, directly showing relative reorientation rates of each functional group. The power spectrum shows the amount of spectral absorption change induced by the applied external perturbation (Noda, 1990; Budevska et al., 1993, 1994; Noda et al., 1999).
ACKNOWLEDGMENTS
The authors thank Dr. M.C. Jarvis and Dr. M.C. McCann for comments on the manuscript.
Footnotes
M.K. (on leave from SAS, Bratislava, SK) was supported by a NATO Royal Society fellowship and R.H.W., A.C.S., P.K.S., N.W., and K.W.W. were suported by a Biotechnology and Biological Science Research Council Competitive Strategy Grant.
LITERATURE CITED
- Brett CT, Waldron KW. Physiology and Biochemistry of Plant Cell Walls. London: Chapman & Hall; 1996. pp. 42–43. [Google Scholar]
- Budevska BO, Manning CJ, Griffiths PR. Step-scan Fourier transform infrared study on the effect of dynamic strain on isotactic polypropylene. Appl Spectrosc. 1993;47:1843–1851. [Google Scholar]
- Budevska BO, Manning CJ, Griffiths PR. Comparison of two-dimensional power and phase spectra generated from sample modulated step-scan FT-IR experiments. Appl Spectrosc. 1994;48:1556–1559. [Google Scholar]
- Cael JJ, Gardner KH, Koenig JL, Blackwell J. Infrared and Raman spectroscopy of carbohydrates: normal coordinate analysis of cellulose I. J Chem Phys. 1975;62:1145–1153. [Google Scholar]
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Chen L, Wilson RH, McCann MC. Infra-red microspectroscopy of hydrated biological systems: design and construction of a new cell with atmospheric control for study of plant cell walls. J Microsc. 1997;188:62–71. [Google Scholar]
- Cosgrove DJ. How do cell walls extend? Plant Physiol. 1993;102:1–6. doi: 10.1104/pp.102.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann HG. Characterization of hydration-dependent wall-extensible properties of rye coleoptiles: evidence for auxin-induced changes of hydrogen bonding. J Plant Physiol. 1995;145:491–497. [Google Scholar]
- Foster TJ, Ablett S, McCann MC, Gidley MJ. Mobility-resolved C-13-NMR spectroscopy of primary plant cell walls. Biopolymers. 1996;39:51–66. [Google Scholar]
- Greenspan L. Humidity fixed points of binary saturated aqueous solutions. J Res Nat Bur Stand. 1977;81A:89–96. [Google Scholar]
- Ha M-A, Apperley DC, Jarvis MC. Molecular rigidity in dry and hydrated onion cell walls. Plant Physiol. 1997;115:593–598. doi: 10.1104/pp.115.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinterstoisser B, Salmen L. Application of dynamic 2D FTIR to cellulose. Vibr Spectrosc. 2000;22:111–118. [Google Scholar]
- Jones HA, Mann LK. Onions and Their Allies. London: Leonard Hill Ltd.; 1963. pp. 147–149. [Google Scholar]
- Kačuráková M, Capek P, Sasinková V, Wellner N, Ebringerová A. FT-IR study of plant cell wall model compounds: pectic polysaccharides and hemicelluloses. Carbohydr Polym. 2000;43:195–203. [Google Scholar]
- Marchessault RG, Sundararajan PR. Cellulose. In: Aspinall GO, editor. The Polysaccharides. Ed 2. New York: Academic Press; 1983. pp. 11–95. [Google Scholar]
- McCann MC, Stacey NJ, Wilson R, Roberts K. Orientation of macromolecules in the walls of elongating carrot cells. J Cell Sci. 1993;106:1347–1356. doi: 10.1242/jcs.106.4.1347. [DOI] [PubMed] [Google Scholar]
- McCann MC, Wells B, Roberts K. Complexity in the spatial localization and length distribution of plant cell-wall matrix polysaccharides. J Microsc. 1992;166:123–136. [Google Scholar]
- Morikawa H, Hayashi R, Senda M. Infrared analysis of pea stem cell walls and oriented structure of matrix polysaccharides in them. Plant Cell Physiol. 1978;19:1151–1159. [Google Scholar]
- Morikawa H, Senda M. Infrared analysis of oat coleoptile cell walls and oriented structure of matrix polysaccharides in the walls. Plant Cell Physiol. 1978;19:1151–1159. [Google Scholar]
- Noda I. Two-dimensional infrared (2D IR) spectroscopy: theory and applications. Appl Spectrosc. 1990;44:550–561. [Google Scholar]
- Noda I, Dowrey AE, Marcot C. Two-dimensional infrared (2D IR) spectroscopy. In: Zerbi G, editor. Modern Polymer Spectroscopy. Weinheim, Germany: Wiley-VCH; 1999. pp. 1–32. [Google Scholar]
- Noda I, Liu YL, Ozaki Y. Two-dimensional correlation spectroscopy study of temperature-dependent spectral variations on N-methylacetamide in the pure liquid state: II. Two-dimensional infrared analysis. J Phys Chem. 1996;100:8665–8673. [Google Scholar]
- Roelofsen PA, Houwink AL. Architecture and growth of the primary cell wall in some plant hairs and in the Phycomyces sporangiophore. Acta Bot Neerland. 1953;2:218–225. [Google Scholar]
- Séné CFB, McCann MC, Wilson RH, Grinter R. Fourier-transform Raman and Fourier-transform infrared spectroscopy: an investigation of five higher plant cell walls and their components. Plant Physiol. 1994;106:1623–1631. doi: 10.1104/pp.106.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellner N, Kačuráková M, Malovíková A, Wilson RH, Belton PS. FT-IR study of pectate and pectinate gels formed by divalent cations. Carbohydr Res. 1998;308:123–131. [Google Scholar]
- Whitney AEC, Gothard MGE, Mitchell JT, Gidley MJ. Roles of cellulose and xyloglucan in determining the mechanical properties of primary plant cell walls. Plant Physiol. 1999;121:657–663. doi: 10.1104/pp.121.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]