Abstract
Background:
Food contact articles (FCAs) are manufactured from food contact materials (FCMs) that include plastics, paper, metal, glass, and printing inks. Chemicals can migrate from FCAs into food during storage, processing, and transportation. Food contact materials’ safety is evaluated using chemical risk assessment (RA). Several challenges to the RA of FCAs exist.
Objectives:
We review regulatory requirements for RA of FCMs in the United States and Europe, identify gaps in RA, and highlight opportunities for improving the protection of public health. We intend to initiate a discussion in the wider scientific community to enhance the safety of food contact articles.
Discussion:
Based on our evaluation of the evidence, we conclude that current regulations are insufficient for addressing chemical exposures from FCAs. RA currently focuses on monomers and additives used in the manufacture of products, but it does not cover all substances formed in the production processes. Several factors hamper effective RA for many FCMs, including a lack of information on chemical identity, inadequate assessment of hazardous properties, and missing exposure data. Companies make decisions about the safety of some food contact chemicals (FCCs) without review by public authorities. Some chemical migration limits cannot be enforced because analytical standards are unavailable.
Conclusion:
We think that exposures to hazardous substances migrating from FCAs require more attention. We recommend a) limiting the number and types of chemicals authorized for manufacture and b) developing novel approaches for assessing the safety of chemicals in FCAs, including unidentified chemicals that form during or after production. https://doi.org/10.1289/EHP644
Introduction
Food contact articles (FCAs) are used in production, processing, transport, handling, and storage of food (e.g., food packaging, storage tanks, and conveyor belts). Various food contact materials (FCMs) are used to make FCAs such as plastics, paper and board, metal, glass, adhesives, and printing inks. Food packaging and other FCAs are of high societal importance because they protect food from physical damage, soiling, and microbial spoilage, thereby reducing food waste. However, chemicals can migrate from food packaging and other FCAs into food and thereby potentially affect human health. Food safety can also be compromised by chemical contaminants present in the raw food (e.g., pesticides and heavy metals), produced during processing and cooking (e.g., acrylamide and polycyclic-aromatic hydrocarbons), or introduced during improper handling (e.g., residual cleaning agents) (Figure S1). Although food contact articles are essential for the food supply chain, their benefits need to be balanced with the potential for human-health risks associated with exposure to migrating chemicals, some of which have been classified as endocrine-disrupting chemicals (EDCs) (Geueke et al. 2014). A recent expert panel estimated the economic burden of health conditions (adult diabetes, obesity, IQ loss and associated intellectual disability, cryptorchidism, and male infertility) with a reasonably high probability of causation by EDCs [including bisphenol A (BPA), phthalates, and organophosphate pesticides] to be billion annually in the European Union (Trasande et al. 2015). Therefore, addressing exposure to EDCs and other hazardous chemicals from food packaging and other FCAs is an opportunity for public health intervention and may contribute substantially to reducing health costs.
Here we draw attention to gaps in the current chemical risk assessment (RA) of food contact materials and articles, as practiced in Europe and the United States. Improving risk assessment in this area will lead to better protection of public health by reducing exposure to hazardous chemicals. Presently, the topic of chemical exposures from FCMs and FCAs is discussed mostly by experts on FCMs, but opening this topic for discussion in the broader scientific community will likely benefit public health. The goal of this commentary is to improve understanding of the potential for harmful exposures from FCAs, how they can be identified, and importantly, how they might be minimized.
Chemicals in FCMs and FCAs
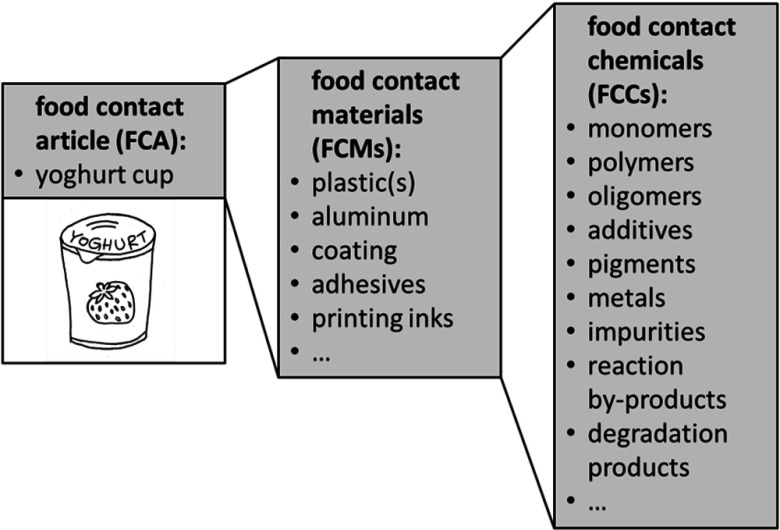
Inventory lists of substances for manufacturing FCMs contain several thousands of chemicals, including starting substances like monomers, or production aids, and additives (Neltner et al. 2013a, Oldring et al. 2014). Risk assessment currently focuses on starting substances and additives. However, these chemicals are often transformed during manufacture, and final food contact articles may therefore contain novel compounds that have not been subjected to RA, even though they may migrate into food (Grob 2014). Here we introduce the term food contact chemicals (FCCs) to capture all chemicals used in FCM manufacture as well as those substances present in the final article (Figure 1). Food contact chemicals include intentionally added substances (IAS), such as monomers, additives, catalysts, and production aids; and impurities and reaction products, such as oligomers, polymers, by-products, and degradation products, which may be referred to as nonintentionally added substances (NIAS) (ILSI Europe 2015).
Figure 1.
Explanation of key terms. Food contact articles (FCAs) are combinations of different FCMs, which consist of food contact chemicals (FCCs) (e.g., a yogurt cup made of polystyrene with printing inks and a coated aluminum cover glued on with adhesives). Food contact materials consist of mixtures of many FCCs. Food contact chemicals are defined as substances used and/or present in the manufacture of FCMs and/or present in FCMs and/or FCAs. Some FCCs are starting substances that no longer exist in the FCM/FCA. Some FCCs are generated during manufacture of an FCM/FCA. Not all FCCs require an authorization, and they are not necessarily subject to risk assessment by an authority.
Regulation for FCMs
Many countries have specific regulations in place addressing FCMs (Magnuson 2013). In Europe, the European Commission’s Framework Regulation states that FCMs and FCAs “shall be manufactured […] so that they do not transfer their constituents to food in quantities which could endanger human health” (EC 2004). In the United States, the U.S. Food and Drug Administration (FDA) classifies FCMs as safe if there is “reasonable certainty in the minds of competent scientists that the substance is not harmful under the intended conditions of use” (Food Additive Amendment 1958).
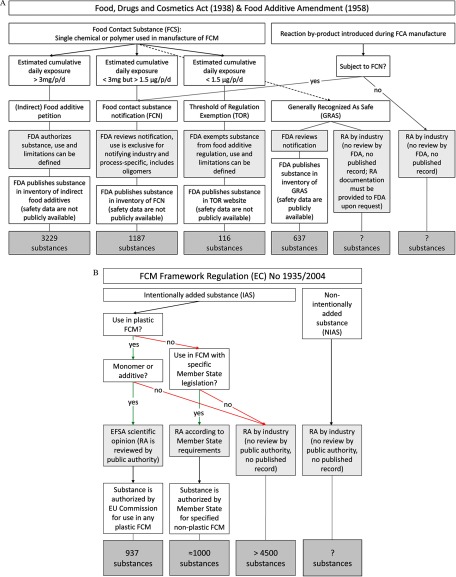
Chemical RA is central to the decision-making process to authorize the use of the FCCs that are subject to review by a public authority. Manufacturers also rely on RA procedures when performing their own safety assessment of an article intended for food contact. For the RA of a chemical, information on toxicological properties, as well as human exposure, is needed. Industry provides information on a substance’s identity, use, migration into food, exposure estimates, and, if required, toxicological data, for FCCs that are subject to authorization. In the European Union, FCCs that are subject to authorization include intentionally added substances (i.e., starting substances and additives used in the manufacture of food contact plastics) (EFSA 2017), and in the United States, these include “indirect food additives,” which are substances that come into contact with and are transferred into food but are not intended to be directly added to food (FDA 2002) (Figure 2; see also Supplemental Material, “Overview of Legislation for Food Contact Materials”). In both the European Union and the United States, the specific requirements for toxicological testing of FCCs requiring authorization depend on estimated consumer exposures (Table 1), which are determined by FCM manufacturers, prior to marketing.
Figure 2.
Risk assessment stakeholder roles for FCCs in United States (A) and European Union (B). (A): In the United States, different rules apply to those FCCs that are food contact substances (FCS) (i.e., single chemicals or polymers used in the manufacture of FCMs), depending on their CEDI per person (p) and day (d) (Food Additive Amendment of 1958). Substances in the FDA’s GRAS inventory may not all be FCSs, as direct food additives can also be notified as GRAS. Further details of U.S. legislation on indirect food additives are provided in the Supplemental Material. (B): In the European Union, starting substances, such as monomers, additives, catalysts and some production aids are considered IAS. Impurities, by-products from manufacturing processes, and degradation products are NIAS (EC 2011). Overall, 17 different FCMs are defined in the European Union (EC 2004); currently, four FCMs are regulated with E.U.-wide specific measures issued by the European Commission, which are binding in all E.U. member states (active and intelligent materials; plastics, including recycled plastic; regenerated cellulose; and ceramics, which do not require FCC authorization). The remaining 13 FCM types may have material-specific European Union member-state regulations in place (Simoneau 2016), which are not issued by the European Commission (adhesives, cork, glass, ion-exchange resins, metals and alloys, paper and board, printing inks, rubbers, silicones, textiles, varnishes and coatings, waxes, and wood). Further details are provided in the Supplemental Material.
Table 1.
Comparison of regulatory frameworks and regulatory requirements for FCM chemical safety assessment in the United States and European Union.
| Regulatory framework | United States of America (U.S.) | European Union (E.U.) |
|---|---|---|
| Regulations | Food Additive Amendment, 1958 (21CFR170) | FCM Framework Regulation (EC 1935/2004) |
| Risk management | Acceptable Daily Intake (ADI) based on Cumulative Estimated Daily Intake (CEDI) | Specific Migration Limit (SML) based on Tolerable Daily Intake (TDI) |
| Toxicity requirements | Tiered toxicity testing, based on estimated daily intake (EDI) | Plastics FCM: Tiered toxicity testing, based on estimated migration |
| Toxicity data (Muncke 2009) | Tiered, based on estimated daily intake: | For plastic FCMs only; tiered, based on estimated migration into food: |
| 1. : includes in vivo tests (two generation, chronica) plus all tests below | 1. food: includes in vivo tests (2 generation, chronicd) plus all tests below | |
| 2. : includes in vivo tests (subchronicb) plus all tests below | 2. food: includes in vivo tests (subchronice) plus all tests below | |
| 3. : includes in vitro tests (genotoxicityc) | 3. food: in vitro tests (genotoxicityf) required for all substances that are expected to migrate (no threshold for exemption) | |
| 4. : no testing, but substance must not have structural alerts for genotoxicity | ||
| Exemptions | Substances used before 1958 and those below 0.5 ppb in food (with no structural alerts for genotoxicity) are exempted from testing requirements | No exemptions specified (all migrating substances require minimum testing for authorization); unauthorized substances may be used in plastic FCMs behind a functional barrier, if migration is below 10 ppb and substances are not in nano form or substances known to be carcinogenic, mutagenic, or toxic to reproduction (CMR). |
| Model assumption: surface area to food ratio | () | |
| Model assumption: food consumption | foodstuff (solids, liquids) | of any foodstuff (solids, liquids) |
| Permissions for use of a substance | — Indirect Food Additive: once authorized, anyone can use according to limitations, intended use | — Monomers, additives in plastics: European Commission authorizes substance for general use, sets limitations of use and SML (Directorate General for Health and Food Safety (DG Santé)) |
| — Food Contact Substance Notification: only for applying industry’s use according to intended use, limitations | ||
| — Threshold of Regulation: anyone can use according to limitations, intended use | ||
| — Generally recognized as Safe (GRAS): general use of GRAS substances under notified uses | ||
| Publicly available information | Applicant, chemical identity, limitations of use (FDA 2017); additional information available upon request | European Food Safety Authority (EFSA) scientific opinions; limitations of use and SML in Annex I (EC 2017a) |
| Enforcement | FDA enforces compliance if violations are found. Notifications to manufacturers are Warning Letters for significant violations, or Untitled Letters for lesser violations (FDA 2016). Industry can report serious violations to the Reportable Food Registry (FDA 2016) | Member States’ national authorities, European Reference Laboratory (EURL) for FCMs. Noncompliance is documented on the Rapid Alert System for Food and Feed (RASFF) portal (EC 2017b) |
Chronic toxicity and carcinogenicity in two rodent species (2 years), one study incl. in utero phase; two-generation reproductive toxicity study (in rats).
Chromosomal damage in rodent hematopoietic cells in vivo; two subchronic oral toxicity tests in vivo (rodent and non-rodent species) (90 days); further testing (chronic exposure) with further endpoints can be recommended (e.g., metabolism studies, teratogenicity, reproductive toxicity, neurotoxicity, immunotoxicity studies).
Mammalian in vitro cytogenicity assay or assay; gene mutations (bacteria, e.g., Ames).
Reproduction study (one species), developmental toxicity (in two species); chronic toxicity and carcinogenicity in two species (2 y); ADME study (absorption, distribution, metabolism and excretion) in vivo.
Two subchronic oral toxicity tests in vivo (rodent and nonrodent species) (90 d) plus study on ADME (adsorption, distribution, metabolism and excretion) if log .
Chromosomal aberrations in mammalian cells in vitro; gene mutations in mammalian cells in vitro ( assay); gene mutations (bacteria).
In the United States, the Threshold of Regulation (TOR) Exemption applies if estimated human exposure is below , and if a substance is neither generally recognized as safe (GRAS) nor “prior sanctioned” (i.e., has been in use since 1958 or before): Such FCCs are exempted from regulation as food additives, and no toxicological data are required if structural alerts for genotoxicity are absent (Shanklin and Cahill 2008). For substances with a higher exposure estimate, some toxicity tests are recommended (Nelson et al. 2011; FDA 2002). The specific testing protocols are similar to those required by the European Union, as described below, and an overview is provided in Table 1.
In Europe, three in vitro mutagenicity and genotoxicity tests are mandatory for all starting IAS and additives authorized in plastics, regardless of the estimated level of exposure (Table 1), but there is no E.U.-wide testing requirement for NIAS formed during plastic manufacture and for substances used in most other FCMs (e.g., paper and board). Additional tests (e.g., subchronic, chronic, two-generation studies) are necessary when chemical migration from plastic FCMs is above food in the European Union, or in the United States, respectively. Testing for developmental and reproductive toxicity is triggered only if the estimated exposure exceeds (US) or (Europe).
Some intentionally added FCCs may be used by industry in Europe and the United States without notification or review by a public authority, based on a determination by the manufacturer that the FCC is GRAS (in the United States), though documentation of the safety of such FCCs must be provided to authorities on request. In Europe, this applies to IAS that are not used in plastic FCM, or are neither plastic monomers nor plastic additives, and that are not covered by any national or E.U.-wide regulation; this also applies to NIAS (Figure 2B). In the United States, additional information on impurities and oligomers is required for Food Contact Substance Notifications (FCNs) of polymers, whereas NIAS that are introduced during later manufacturing steps, and that are not included explicitly in the FCN, are not subject to authorization.
Threshold of Regulation and Threshold of Toxicological Concern
In the absence of toxicological data, generic thresholds for safe human exposure to chemicals are used: In the European Union, the Threshold of Toxicological Concern (TTC) is recommended for RA of NIAS for which there are no toxicological data (EFSA 2012, 2016a). Specifically, the TTC for untested substances are extrapolated from the normal distribution of no-observed-effect level (NOEL) data for tested FCCs and other chemicals with a similar two-dimensional chemical structure, based on the assumption that structurally similar chemicals will have similar toxicological properties. Specifically, the threshold is set by assuming a 5% probability that the NOEL for the untested substance is in the lowest fifth percentile of the NOEL distribution of known chemicals in the same Cramer Class (Cramer et al. 1978), with an additional safety factor of 100 applied for extrapolating from animal to human data (Munro et al. 1996). Three different TTCs have been set by grouping structurally related chemicals into one of three Cramer Classes: low (Cramer Class I): ; intermediate (Cramer Class II): ; high (Cramer Class III): (Munro et al. 1996). However, the TTC uses a lower threshold for genotoxicants (), and the TTC concept cannot be applied to substances belonging to the classes of exempted compounds that include aflatoxin-like, azoxy- or N-nitroso-compounds and benzidines, inorganic chemicals, metals, organometallics, proteins, steroids, and organo-silicon compounds (Kroes et al. 2004).
The TTC concept has been questioned regarding its limitations and uncertainties in the derivation of its thresholds (Nordic Council of Ministers 2005; Bschir 2016). For example, the validity of a given TTC level strongly depends on the validity and accuracy of the underlying NOEL data used to derive the threshold (Falk-Filipsson et al. 2007). On the other hand, when tested with a dataset of authorized FCCs, the TTC was shown to be predictive (Pinalli et al. 2011). Specifically, the NOELs of 232 compounds authorized in Europe for use in food contact plastics were compared with their respective TTCs (according to their chemical structures). For 96% of analyzed compounds, the ratio of NOEL/TTC was above 1, indicating that TTCs were lower than NOELs that were based on subchronic or chronic toxicity data. Because these findings support its usefulness, an update of the TTC is being carried out by the European Food Safety Authority (EFSA) (EFSA 2016b).
Data Gaps in the RA of FCCs: Exposure
The most basic requirement for estimating exposure to an FCC is that its chemical structure, i.e., its chemical identity, is known. Identification of chemical structure information, however, is not always the case because many NIAS in finished FCAs are uncharacterized impurities and reaction by-products (Hoppe et al. 2016; Nerin et al. 2013). For example, Bradley and Coulier (2007) investigated five commodity plastics for food contact use with respect to their composition of NIAS. Plastics were manufactured under controlled conditions, extracted using organic solvents, and analyzed for all their reaction and breakdown products. For all plastic samples, unidentified compounds were reported that could not be predicted from the known composition (including impurities) of the starting substances. This aspect indicates that comprehensive qualitative and quantitative chemical analyses of plastic FCAs are currently impossible (Hoppe et al. 2016, Pieke et al. 2017), and that, consequently, there is a likely ongoing exposure of the general population to unknown and untested chemicals through migration from FCAs into food.
For known FCCs—both intentionally added to and nonintentionally present in food contact articles—migration into food can be quantified only if the chemical standard (i.e., the pure substance, nontechnical grade), required for calibrating the analytical method, is available. An alternative to quantifying migration (e.g., in the absence of a chemical standard) is estimating migration by means of partitioning models; however, this estimation is possible only for FCCs in polymeric FCAs and if an FCC’s level in the article is known (i.e., for substances added intentionally) (Begley et al. 2005).
Furthermore, to estimate human exposure, information on actual food consumption and on the use of FCAs in contact with certain food types is required. In the United States, the FDA’s Cumulative Estimated Daily Intake (CEDI) database is used to provide the estimated daily intake (EDI) of an FCC from food packaging (FDA 2015). However, details on how estimates are derived are not readily available for public scrutiny (Alger et al. 2013). In Europe, exposure assessment for plastic FCMs is based on default assumptions, with a person daily consuming of food in contact with surface area of FCM (EC 2011), but the use of actual food consumption data has been proposed (EFSA 2016a; Oldring et al. 2014).
In addition, humans are not only exposed to FCCs, but also to chemicals from other sources (Evans et al. 2016). Still, RA is currently performed in separate schemes for different chemicals (e.g., FCCs, pesticides, and industrial chemicals) and not cumulatively. This difference has an implication for a chemical’s estimated exposure: Other sources may contribute to actual human exposure, not only the substance’s migration from food packaging. Consequently, the exposure may be underestimated and safe exposure levels may be exceeded.
Data Gaps in the RA of FCCs: Toxicity
Legally, all migrating FCCs need to be assessed for their risk to human health, regardless of whether they are IAS or NIAS. However, there is no general requirement for toxicity testing of FCCs. For intentionally added FCCs that are subject to authorization (Figure 2), testing is tiered, based on estimated exposure levels from the finished FCA.
As a result of past and current regulatory requirements, toxicological data are available for 27% of the authorized FCCs in the United States, and 73% have not been tested for reproductive or developmental toxicity, nor for subchronic or chronic toxicity (Neltner et al. 2013a). The U.S. FDA recommends specific testing based on a tiered-system of estimated exposures. If a company’s exposure estimate for an FCC prior to its marketing is below a testing threshold, developmental and reproductive toxicity tests are not performed, resulting in data gaps (Figure 2A). This approach of tiered testing is counter to modern scientific understanding that the fetus can be particularly sensitive to exposure to low amounts of certain chemicals (Bern 1992; Colborn et al. 1993; Markey et al. 2003), and a disruption of the processes governing fetal development can lead to disease later in life (Balbus et al. 2013). Moreover, additional windows of sensitivity other than fetal development exist: Female mice are at increased risk for developing diabetes later in life if they were exposed to BPA when pregnant (Alonso-Magdalena et al. 2015).
Toxicological testing requires knowledge of an FCC’s identity and its availability as pure chemical (i.e., nontechnical grade) in sufficient quantity to perform toxicity studies. These requirements are often not fulfilled for NIAS, which presents a major obstacle for safety assessments (Hoppe et al. 2016). In those cases, when, as a minimum requirement, the chemical structure of a NIAS is known and exposure is assumed to be low, use of the TTC is recommended (EFSA 2012, 2016a).
For FCCs with unknown chemical identity, a makeshift approach has been developed based on the TTC concept and utilizing nontargeted chemical analysis (ILSI Europe 2015). Samples of extracts or overall migrates from FCCs or FCAs are spiked with a known concentration of a marker chemical at a level of interest (“quantification marker”), which may be derived from the TTC. The quantification marker’s peak intensity is recorded during chemical analysis and is then used for internal calibration (Pieke et al. 2017). This method of internal calibration, however, results in uncertainties of up to three orders of magnitude for concentration estimates of the unknown substances (Koster et al. 2014). Peaks below the quantification marker’s intensity are assumed to only represent substances below their TTC, i.e., at safe levels. Therefore, this approach assumes no risk for many unknown FCCs, without reliable exposure estimates or actual toxicological information.
Furthermore, FCCs are currently not routinely assessed for endocrine-disrupting properties (Muncke 2009), even though at least 119 known or suspected EDCs are intentionally used in the manufacture of FCAs in the European Union and/or the United States (Geueke et al. 2014). This circumstance is problematic, as it is generally assumed that the safe level for FCCs can be derived from testing at high doses. This assumption disregards evidence that endocrine-active substances can have nonmonotonic dose–response relationships, where adverse effects can occur at concentrations below an apparent safety threshold (Vandenberg et al. 2012). Indeed, a study from 2011 reported that extracts from a majority of tested plastic food packaging samples exhibited estrogenic activity in an in vitro assay (Yang et al. 2011).
Another important data gap concerns heart and metabolic diseases, the most relevant noncommunicable diseases today in terms of premature death (i.e., under the age of 70) (WHO 2015). Although heart and metabolic diseases have been associated with exposure to BPA in epidemiological studies (as reviewed in Rancière et al. 2015), chemical hazard assessment of FCCs does not include endpoints relevant to these outcomes.
Another issue is that toxicity assessment is commonly carried out for individual substances, even though exposure to FCC mixtures occurs, and the overall risk of a mixture can exceed the risk of each individual substance (Grob et al. 2006). In particular, chemicals that affect the same endpoints may act according to the concept of concentration addition, such that toxic effects are possible even if each individual substance is present at an acceptable level (Kortenkamp et al. 2007). Moreover, combined exposures to individual chemicals that affect different physiological endpoints might have mixture effects relevant to carcinogenesis (Goodson et al. 2015) or other pathologic mechanisms (Jacobsen et al. 2012).
Major Challenges for Ensuring the Safety of FCCs and FCAs
In addition to the knowledge gaps and scientific shortcomings in the RA of chemicals migrating from FCAs, several challenges related to current practice exist. First, many intentionally used FCCs are assessed by industry without public oversight (Figure 2). In Europe, no legal requirement to notify the use of an FCC (or its presence in a food contact article, e.g., in the case of NIAS) exists. In the United States, manufacturers and expert panels are allowed to make determinations about whether intentionally added FCCs are GRAS without review by the FDA (Food Additive Amendment 1958, Neltner et al. 2013b). Consequently, public authorities often do not know which FCCs are actually used, and if, or how, their safety has been assessed. For example, since 1997, GRAS substances may be used as direct and/or indirect food additives in the United States (Neltner et al. 2013b), and in 2011, Neltner and colleagues estimated that (publicly unidentified) chemicals had been determined to be GRAS based on manufacturer self-determination, without notification of the FDA (Neltner et al. 2011).
Second, once an FCC is authorized as an indirect food additive in the United States, listed as exempted from authorization (GRAS, TOR) (FDA 2017), or included in the European regulation for plastic FCMs (EC 2017a), it can be used freely for FCM manufacture, if no patents apply, and with the exception of the U.S. FCN program, where authorization is granted only to the applicant. Consequently, because no systematic postmarket surveillance of authorized FCCs is performed, the extent of actual use is largely unknown, and exposures may exceed the estimated levels indicated by manufacturers during the notification or authorization process.
Third, although the European Commission has set specific migration limits (SMLs) for migration of some FCCs and has established an overall migration limit of food for finished plastic FCAs (EC 2011), the E.U. Reference Laboratory for FCMs recently reported that standards for analytical method calibration are available for only 53% of FCCs currently authorized for use in plastic FCMs (Simoneau 2015). This implies that concentrations of about 440 authorized FCCs cannot be quantified and, consequently, in Europe, legal migration limits cannot be enforced.
Fourth, information about FCCs may not be effectively communicated over the entire food contact material supply chain. For example, Articles 15 and 16 of European Regulation 10/2011 require that a Declaration of Compliance (DoC) be issued at each stage of the plastic-FCM manufacturing process describing work that has been performed to achieve compliance with legal requirements and indicating any additional compliance work that must be performed by downstream users, and demonstrate that supporting documentation for the information reported in the DoC also must be available to authorities on demand (EC 2011). However, it was recently reported that manufacturers of nine plastic FCM products failed to provide supporting documentation for DoCs within six months of a request by Swiss food control authorities (McCombie et al. 2016).
Discussion
Implications: Risk Assessment of FCCs is Ineffective
The protection goals of FCM and FCA legislation require either the quantitative assessment of the risk of harm to human health by FCCs (European Union) (EC 2004) or reasonable certainty that a substance is not harmful under the conditions of use (United States) (Food Additive Amendment 1958). However, compliance with both European and U.S. regulations requires exposure and toxicity data that cannot be generated for many FCCs either because their chemical identities are unknown or because there are no chemical standards for their quantitative assessment. This serious disconnect between legal requirements and the data that are generated, either by manufacturers along the supply chain or by regulatory authorities, leads us to question whether current regulatory approaches and RA practices are sufficient and appropriate for ensuring the safety of FCMs.
Recommendations
We propose that the main knowledge gaps and problems for FCMs (as summarized in Table 2) be addressed by reconsidering key elements of RA, chemical hazard removal and exposure, and by changing the way in which data for RAs are generated.
Table 2.
Knowledge gaps and problems in the RA of FCMs, description of the shortcoming in risk assessment, and recommendation for overcoming the gap.
| No. | Knowledge gap or problem | Description | Recommendation |
|---|---|---|---|
| I | Risk assessments (RAs) focus on starting substances, not on the finished article | Focus is placed on chemicals at the start of the manufacturing process instead of those present in the finished food contact article and migrating into food, i.e., the chemicals people are exposed to. | Ensure adequate toxicological assessment of food contact articles: Assess all food contact chemicals (FCCs) with the potential to migrate from the finished food contact article (including printing inks, labels, closures, etc.). |
| II | Unknown substances migrate from food contact articles (FCAs) into food | The chemical identity of some/many substances in food contact articles is unknown; therefore, no RA using the current approach is possible: neither exposure nor hazard can be assessed. | Ensure adequate toxicological assessment of FCCs, and avoid intentional use of chemicals with unknown toxicity: Assess overall migrate or extract from the finished FCA, e.g., by using in vitro bioassays and subsequent chemical analysis. |
| III | Authorized chemicals are not available as pure analytical standards: exposure cannot be assessed and legal limits cannot be enforced | Legally binding migration limits cannot be controlled and enforced because analytical and calibration methods for many authorized FCCs are unavailable and postmarket exposure assessments are not possible. | Limit intentionally used FCCs to chemicals with analytical standards: Reevaluate authorization status for chemicals based on the availability of chemical standards. Introduce mandatory requirement for users of an intentionally added substance to have a pure analytical standard available for authorities to enforce legal limits. |
| IV | Human exposure estimates are outdated and not publicly accessible | Human exposure estimates are based on premarket data, which is not transparent, and there is no systematic postmarket assessment of authorized substances. | Make all data used for human exposure assessment available to public review. Require mandatory data notification on chemical use to authorities. Perform periodic reviews of human exposure for all authorized FCMs. |
| V | Cumulative exposures are not taken into consideration when exposures are estimated | Exposures from sources other than food contact articles are not generally considered for exposure assessments, because information is often unavailable. | Share chemical use information between authorities to assess actual human exposures. Expand biomonitoring efforts. |
| VI | Generic toxicological thresholds are used in the absence of actual data, and available toxicological data are insufficient for RAs | For many nonintentionally added substances (NIAS), RAs rely on generic toxicological thresholds (TTCs), which may not be sufficiently protective. Endocrine disruptors are not routinely assessed, developmental toxicity data are generated for highest exposure estimates only (based on premarket exposure assumptions). | Avoid chemicals with unknown toxicity. Ensure adequate toxicological assessment of FCCs. Assess validity of TTCs by using recent data from chronic toxicity studies. Use TTCs and in silico data (e.g., from quantitative structure-activity relationship (QSAR) computational models) only intermediately for filling data gaps and prioritization for toxicity testing, and require toxicity data for any food contact chemical migrating into food. |
| VII | Mixture toxicity of the overall migrate is not assessed | Overall migrate into food is not assessed for its mixture effect even though humans are generally exposed to mixtures of chemicals from food packaging. | Ensure adequate toxicological assessment of FCCs. Assess overall migrate from the finished food contact article (including printing inks, labels, closures, etc.) using bioassays. |
| VIII | Hazard characterization does not consider some of the most relevant diseases in the human population | Hazards associated with non-communicable diseases like cardiovascular disease or metabolic syndrome are not routinely assessed, even though these are of very high relevance to public health. | Ensure adequate toxicological assessment of FCCs. In addition to genotoxicity testing, require toxicity data on cardiovascular, metabolic, and endocrine endpoints for any food contact chemical migrating into food at any level. |
| IX | Conflicts of interest are systemic to the current risk assessment approach | Risk assessment may be performed by industry, without public oversight, and data provided to authorities for risk assessment may be from studies commissioned by the applicant that are not publicly available. | FCC risk assessment should be performed by independent third parties. Data should be public. Notification of a safety decision by industry should be mandatory (i.e., authorities are informed about the use/presence of an FCC). |
Avoid hazardous substances and chemicals with unknown toxicity.
The first step is to reduce the large number of chemicals in the FCM universe. We previously identified 175 known FCCs as being chemicals of concern based on their inclusion in the SIN list of chemicals that meet the criteria of article 57 of European Commission Regulation 1907/2006 (REACH) or in the TEDX database of endocrine disrupting compounds when our assessment was performed, including 21 Substances of Very High Concern (SVHC), of which six are intended for phase-out under REACH (references for all databases are provided in Geueke et al. 2014). We propose that these FCCs, as well as untested substances and hazardous transformation products of FCCs, be replaced with safe alternatives (i.e., substances that have been tested and shown not to possess hazardous properties) as soon as practically feasible.
Ensure adequate toxicological assessment of all FCCs.
Many FCCs have not been subjected to toxicological testing because they fall below regulatory thresholds (e.g., based on estimated exposures), are substances that have been in use prior to 1958, or do not require regulatory authorization (as for NIAS). In other cases, existing RAs based on generic exposure thresholds (such as TTCs) and other toxicological evaluations may be insufficient (e.g., GRAS when not notified to FDA). In particular, we recommend that the potential for low-dose effects and nonmonotonicity be addressed in light of current scientific understanding; that in vitro toxicity data, including bioassays for endocrine activity, should be requested for all FCCs, regardless of exposure levels; and that toxicological assessments of actual overall migrates (i.e., the mixture of all migrating substances), including FCC mixtures with unknown compounds (e.g., NIAS), should be considered (Bengtström et al. 2016).
Limit intentionally used FCCs to chemicals with analytical standards.
The lack of an analytical standard hampers migration quantification for a given FCC, which in turn makes enforcement of regulatory levels impossible. Consequently, an FCC should be included in a list of authorized chemicals only if compliance with exposure thresholds or migration limits can be assessed.
Risk assessment should be performed by independent third parties.
An obvious conflict of interest exists if the organization performing RAs also has commercial interests in their outcomes (Neltner et al. 2013b). Presently, not all FCCs are subject to an independent third-party RA (e.g., for GRAS in the United States or nonauthorized FCCs in Europe). Existing conflicts of interest could be overcome by (i) introducing mandatory agency review of RAs for all intentionally used FCCs, and (ii) an approach where manufacturers seeking authorization pay into a common fund that is used by authorities to commission migration and toxicological tests by independent third parties that do not have a conflict of interest.
Conclusions
Based on our assessment of the available evidence, we conclude that the current approach of premarket, prospective RA of chemicals in FCMs is insufficient to protect public health. It relies too much on self-assessments by industry and assumptions that do not reflect contemporary scientific knowledge, lacks clear guidance, and cannot be enforced by authorities. Emphasis is on assessing single starting substances whereas consumers are exposed to mixtures of FCCs, including NIAS, from FCAs. Therefore, we think that a novel approach is needed: An amendment of current regulatory frameworks requiring RA of all FCCs migrating from finished FCAs would be more effective, because humans are actually exposed to these chemical mixtures.
Changes are possible if there is an appetite for them. Some food manufacturers and retailers recognize that their own reputation is at risk, and in light of this, they voluntarily apply RA approaches and FCM standards that surpass regulatory requirements (Seltenrich 2015). However, we assume that these few companies currently account for only a small share of the market. Therefore, regulatory or financial mechanisms that address chemical risk along all FCM supply chains (e.g., by internalizing external costs associated with chemical risks, like costs of diseases attributed to chemical exposures) would support the development of widely available, inherently safer products.
In our opinion, raising awareness for the presence of hazardous chemicals in food packaging and other FCAs is important, because it drives research for better alternatives and motivates innovation. We recognize that a functioning and safe food supply chain relies on food packaging and is of high societal importance; therefore, we think that regulatory mechanisms and RA approaches must be updated and strengthened to ensure that hazardous substances do not migrate into food from the materials designed to keep it safe.
Supplemental Material
Acknowledgments
This manuscript was initiated by the Food Packaging Forum’s Scientific Advisory Board. All authors contributed to its outlining and writing.
References
- Alger HM, Maffini MV, Kulkarni NR, Bongard ED, Neltner T. 2013. Perspectives on How FDA Assesses Exposure to Food Additives When Evaluating Their Safety: Workshop Proceedings. Comprehensive Reviews in Food Science and Food Safety 12(1):90–119, 10.1111/j.1541-4337.2012.00216.x. [DOI] [Google Scholar]
- Alonso-Magdalena P, Garcia-Arévalo M, Quesada I, Nadal Á. 2015. Bisphenol-A treatment during pregnancy in mice: a new window of susceptibility for the development of diabetes in mothers later in life. Endocrinology 156(5):1659–1670, PMID: 25830705, 10.1210/en.2014-1952. [DOI] [PubMed] [Google Scholar]
- Balbus JM, Barouki R, Birnbaum LS, Etzel RA, Gluckman PD Sr, Grandjean P, et al. 2013. Early-life prevention of non-communicable diseases. Lancet 381(9860):3–4, PMID: 23290956, 10.1016/S0140-6736(12)61609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley T, Castle L, Feigenbaum A, Franz R, Hinrichs K, Lickly T, et al. 2005. Evaluation of migration models that might be used in support of regulations for ood-contact plastics. Food Addit Contam 22(1):73–90, PMID: 15895614, 10.1080/02652030400028035. [DOI] [PubMed] [Google Scholar]
- Bengtström L, Rosenmai AK, Trier X, Jensen LK, Granby K, Vinggaard AM, et al. 2016. Non-targeted screening for contaminants in paper and board food contact materials using effect directed analysis and accurate mass spectrometry. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 33(6):1080–1093, PMID: 27146477, 10.1080/19440049.2016.1184941. [DOI] [PubMed] [Google Scholar]
- Bern H. 1992. The fragile fetus. In: Chemically-Induced Alterations in Sexual and Functional Development: The Wildlife/Human Connection (Colborn T, Clement C, eds). Princeton:Scientific Publishing Co, 9–15. [Google Scholar]
- Bradley E, Coulier L. 2007. An investigation into the reaction and breakdown products from starting substances used to produce food contact plastics FD 07/01 London:Central Science Laboratory. [Google Scholar]
- Bschir K. 2016. Risk, uncertainty and precaution in science: The Threshold of the Toxicological Concern approach in food toxicology. Sci Eng Ethics 23(2):1–20, PMID: 27192993, 10.1007/s11948-016-9773-2. [DOI] [PubMed] [Google Scholar]
- Colborn T, vom Saal FS, Soto AM. 1993. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect 101(5):378–384, PMID: 8080506, 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer GM, Ford RA, Hall RL. 1978. Estimation of toxic hazard—a decision tree approach. Food Cosmet Toxicol 16(3):255–276, PMID: 357272, 10.1016/S0015-6264(76)80522-6. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority). 2012. Scientific Opinion on exploring options for providing advice about possible human health risks based on the concept of Threshold of Toxicological Concern (TTC). EFSA Journal 10(7):2750–2853, 10.2903/j.efsa.2012.2750. [DOI] [Google Scholar]
- EFSA. 2016a. Recent developments in the risk assessment of chemicals in food and their potential impact on the safety assessment of substances used in food contact materials. EFSA Journal 14(1):4357–4365, 10.2903/j.efsa.2016.4357. [DOI] [Google Scholar]
- EFSA. 2016b. Review of the Threshold of Toxicological Concern (TTC) approach and development of new TTC decision tree. EFSA Supporting Publication 13(3):EN-1006:50, 10.2903/sp.efsa.2016.EN-1006. [DOI] [Google Scholar]
- EFSA. 2017. Administrative guidance for the preparation of applications for the safety assessment of substances to be used in plastic food contact materials. EFSA Supporting Publication 14(5):1224E, 10.2903/sp.efsa.2017.EN-1224. [DOI] [Google Scholar]
- EC (European Commission). 2004. Regulation (EC) No 1935/2004 on materials and articles intended to come into contact with food. http://eur-lex.europa.eu/eli/reg/2004/1935/oj.
- EC. 2011. Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food. http://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1498596278669&uri=CELEX:32012R0528.
- EC. 2017a. Food contact materials database. https://webgate.ec.europa.eu/foods_system/main/?event=display [accessed 8 June 2017].
- EC. 2017b. RASFF: Food and feed safety alerts. http://ec.europa.eu/food/safety/rasff_en [accessed 8 June 2017].
- Evans RM, Martin OV, Faust M, Kortenkamp A. 2016. Should the scope of human mixture risk assessment span legislative/regulatory silos for chemicals? Sci Total Environ 543(Pt A):757–764. PMID: 26573369, 10.1016/j.scitotenv.2015.10.162. [DOI] [PubMed] [Google Scholar]
- Falk-Filipsson A, Hanberg A, Victorin K, Warholm M, Wallén M. 2007. Assessment factors—applications in health risk assessment of chemicals. Environ Res 104(1):108–127, PMID: 17166493, 10.1016/j.envres.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Food Additive Amendment of 1958. Food for human consumption. U.S. Code of Federal Regulations. 21 CFR 170.3.
- FDA (U.S. Food and Drug Administration). 2002. Guidance for Industry: Preparation of Food Contact Notifications for Food Contact Substances: Toxicology Recommendations. http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/IngredientsAdditivesGRASPackaging/ucm081825.htm [accessed 8 June 2017].
- FDA. 2015. Cumulative Estimated Daily Intakes (CEDI) database. http://www.fda.gov/Food/IngredientsPackagingLabeling/PackagingFCS/CEDI/default.htm [accessed 8 June 2017].
- FDA. 2016. Compliance & enforcement. https://www.fda.gov/Food/ComplianceEnforcement/default.htm [accessed 8 June 2017].
- FDA. 2017. List of available datasets. https://www.accessdata.fda.gov/scripts/fdcc/ [accessed 8 June 2017].
- Geueke B, Wagner C, Muncke J. 2014. Food contact substances and chemicals of concern: a comparison of inventories. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 31(1):1438–1450, PMID: 24999917, 10.1080/19440049.2014.931600. [DOI] [PubMed] [Google Scholar]
- Goodson WH 3rd, Lowe L, Carpenter DO, Gilbertson M, Manaf Ali A, Lopez de Cerain Salsamendi A, et al. 2015. Assessing the carcinogenic potential of low-dose exposures to chemical mixtures in the environment: the challenge ahead. Carcinogensis 36(suppl 1):S254–S296, PMID: 26106142, 10.1093/carcin/bgv039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grob K. 2014. Work plans to get out of the deadlock for the safety assurance of migration from food contact materials? A proposal. Food Control 46:312–318, 10.1016/j.foodcont.2014.05.044. [DOI] [Google Scholar]
- Grob K, Biedermann M, Scherbaum E, Roth M, Rieger K. 2006. Food contamination with organic materials in perspective: packaging materials as the largest and least controlled source? A view focusing on the European situation. Crit Rev Food Sci Nutr 46(7):529–535, PMID: 16954061, 10.1080/10408390500295490. [DOI] [PubMed] [Google Scholar]
- Hoppe M, de Voogt P, Franz R. 2016. Identification and quantification of oligomers as potential migrants in plastics food contact materials with a focus in polycondensates – a review. Trends Food Sci Tech 50:118–130, 10.1016/j.tifs.2016.01.018. [DOI] [Google Scholar]
- ILSI Europe 2015. Guidance on best practices on the risk assessment of non-intentionally added substances (NIAS) in food contact materials and articles. ILSI Europe Report Series. 1–70.
- Jacobsen PR, Axelstad M, Boberg J, Isling LK, Christiansen S, Mandrup KR, et al. 2012. Persistent developmental toxicity in rat offspring after low dose exposure to a mixture of endocrine disrupting pesticides. Reprod Toxicol 34(2):237–250, PMID: 22677472, 10.1016/j.reprotox.2012.05.099. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A, Faust M, Scholze M, Backhaus T. 2007. Low-level exposure to multiple chemicals – reason for human health concern? Environ Health Perspect 115(suppl 1):106–114, PMID: 18174958, 10.1289/ehp.9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster S, Rennen M, Leeman W, Houben G, Muilwijk B, van Acker F, et al. 2014. A novel safety assessment strategy for non-intentionally added substances (NIAS) in carton food contact materials. Food Addit Contam Part A 31(3):422–443, PMID: 24237267, 10.1080/19440049.2013.866718. [DOI] [PubMed] [Google Scholar]
- Kroes R, Renwick AG, Cheeseman M, Kleiner J, Mangelsdorf I, Piersma A, et al. 2004. Structure-based Thresholds of Toxicological Concern (TTC): guidance for application to substances present at low levels in the diet. Food Chem Toxicol 42(1):65–83, PMID: 14630131, 10.1016/j.fct.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Magnuson B, Munro I, Abbot P, Baldwin N, Lopez-Garcia R, Ly K, et al. 2013. Review of the regulation and safety assessment of food substances in various countries and jurisdictions. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 30(7):1147–1220, PMID: 23781843, 10.1080/19440049.2013.795293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markey CM, Coombs MA, Sonnenschein C, Soto AM. 2003. Mammalian development in a changing environment: exposure to endocrine disruptors reveals the developmental plasticity of steroid-hormone target organs. Evol Dev 5(1):67–75, PMID: 12492412, 10.1046/j.1525-142X.2003.03011.x. [DOI] [PubMed] [Google Scholar]
- McCombie G, Hötzer K, Daniel J, Biedermann M, Eicher A, Grob K. 2016. Compliance work for polyolefins in food contact: results of an official control campaign. Food Control 59:793–800, 10.1016/j.foodcont.2015.06.058. [DOI] [Google Scholar]
- Muncke J. 2009. Exposure to endocrine disrupting compounds via the food chain: is packaging a relevant source? Sci Total Environ 407(16):4549–4559, PMID: 19482336, 10.1016/j.scitotenv.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Munro IC, Ford RA, Kennepohl E, Sprenger JG. 1996. Correlation of structural class with no-observed-effect levels: a proposal for establishing a threshold of concern. Food Chem Toxicol 34(9):829–867, PMID: 8972878, 10.1016/S0278-6915(96)00049-X. [DOI] [PubMed] [Google Scholar]
- Nelson CP, Patton GW, Arvidson K, Lee H, Twaroski ML. 2011. Assessing the toxicity of polymeric food-contact substances. Food Chem Toxicol 49(9):1877–1897, PMID: 21723908, 10.1016/j.fct.2011.06.054. [DOI] [PubMed] [Google Scholar]
- Neltner TG, Kulkarni NR, Alger HM, Maffini MV, Bongard ED, Fortin ND, et al. 2011. Navigating the U.S. Food Additive Regulatory Program. Compr Rev Food Sci F 10(6):342–368, 10.1111/j.1541-4337.2011.00166.x. [DOI] [Google Scholar]
- Neltner TG, Alger HM, Leonard JE, Maffini MV. 2013a. Data gaps in toxicity testing of chemicals allowed in food in the United States. Reprod Toxicol 42:85–94, PMID: 23954440, 10.1016/j.reprotox.2013.07.023. [DOI] [PubMed] [Google Scholar]
- Neltner TG, Alger HM, O’Reilly JT, Krimsky S, Bero LA, Maffini MV. 2013b. Conflicts of interest in approvals of additives to food determined to be generally recognized as safe: out of balance. JAMA Intern Med 173(22):2032–2036, PMID: 23925593, 10.1001/jamainternmed.2013.10559. [DOI] [PubMed] [Google Scholar]
- Nerin C, Alfaro P, Aznar M, Domeño C. 2013. The challenge of identifying non-intentionally added substances from food packaging materials: a review. Anal Chim Acta 775:14–24, PMID: 23601971, 10.1016/j.aca.2013.02.028. [DOI] [PubMed] [Google Scholar]
- Nordic Council of Ministers. 2005. Threshold of Toxicological Concern (TTC): Literature Review and Applicability. Copenhagen, Denmark:Nordic Council of Ministers. [Google Scholar]
- Oldring P, O'Mahony C, Dixon J, Vints M, Mehegan J, Dequatre C, et al. 2014. Development of a new modelling tool (FACET) to assess exposure to chemical migrants from food packaging. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 31(3):444–465, PMID: 24215584, 10.1080/19440049.2013.862348. [DOI] [PubMed] [Google Scholar]
- Pieke EN, Granby K, Trier X, Smedsgaard J. 2017. A framework to estimate concentrations of potentially unknown substances by semi-quantification in liquid chromatography electrospray ionization mass spectrometry. Anal Chim Acta 975:30–41, PMID: 28552304, 10.1016/j.aca.2017.03.054. [DOI] [PubMed] [Google Scholar]
- Pinalli R, Croera C, Theobald A, Feigenbaum A. 2011. Threshold of toxicological concern approach for the risk assessment of substances used for the manufacture of plastic food contact materials. Trends Food Sci Tech 22(9):523–534, 10.1016/j.tifs.2011.07.001. [DOI] [Google Scholar]
- Rancière F, Lyons JG, Loh VH, Botton J, Galloway T, Wang T, et al. 2015. Bisphenol A and the risk of cardiometabolic disorders: a systematic review with meta-analysis of the epidemiological evidence. Environ Health 14:46, PMID: 26026606, 10.1186/s12940-015-0036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltenrich N. 2015. A hard nut to crack. Reducing chemical migration in food-contact materials. Environ Health Perspect 123(7):A174–A179, PMID: 26133041, 10.1289/ehp.123-A174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoneau C. 2015. Annual report 2014 of the EURL-FCM on activities carried out for the implementation of Regulation (EC) No 882/2004. Joint Research Centre.
- Simoneau C, Raffael B, Garbin S, Hoekstra E, Mieth A, Lopes JA, et al. 2016. Non-harmonised food contact materials in the EU: Regulatory and market situation. EUR 28357. https://ec.europa.eu/jrc/en/publication/eur-scientific-and-technical-research-reports/non-harmonised-food-contact-materials-eu-regulatory-and-market-situation-baseline-study [accessed 8 June 2017].
- Shanklin AP, Cahill S. 2008. How FDA's Threshold of Regulation program works. http://www.foodsafetymagazine.com/magazine-archive1/december-2008january-2009/how-fdas-threshold-of-regulation-program-works/ [accessed 8 June 2017].
- Trasande L, Zoeller RT, Hass U, Kortenkamp A, Grandjean P, Myers JP, et al. 2015. Estimating burden and disease costs of exposure to endocrine-disrupting chemicals in the European Union. J Clin Endocrinol Metab 100(4):1245–1255, PMID: 25742516, 10.1210/jc.2014-4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee DH, et al. 2012. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 33(3):378–455, PMID: 22419778, 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2015. Noncommunicable diseases. Fact sheet. http://www.who.int/mediacentre/factsheets/fs355/en/ [accessed 8 June 2017].
- Yang CZ, Yaniger SI, Jordan VC, Klein DJ, Bittner GD. 2011. Most plastic products release estrogenic chemicals: a potential health problem that can be solved. Environ Health Perspect 119(7):989–996, PMID: 21367689, 10.1289/ehp.1003220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.