Somatosensory impairment was found to be associated with reduced participation in activities poststroke.
Abstract
OBJECTIVE. Our objective was to determine the effect of loss of body sensation on activity participation in stroke survivors.
METHOD. Participants (N = 268) were assessed at hospital admission for somatosensory and motor impairment using the National Institutes of Health Stroke Scale. Participation was assessed using the Activity Card Sort (ACS) in the postacute phase. Between-group differences in activity participation were analyzed for participants with and without somatosensory impairment and with or without paresis.
RESULTS. Somatosensory impairment was experienced in 33.6% of the sample and paresis in 42.9%. ACS profiles were obtained at a median of 222 days poststroke. Somatosensory loss alone (z = 1.96, p = .048) and paresis in upper and lower limbs without sensory loss (z = 4.62, p < .001) influenced activity participation.
CONCLUSION. Somatosensory impairment is associated with reduced activity participation; however, paresis of upper and lower limbs can mask the contribution of sensory loss.
One in 2 people experience loss of body sensation, or somatosensation, after stroke (Carey, 2012; Carey & Matyas, 2011; Winward, Halligan, & Wade, 2002). They typically have difficulty sensing touch, pressure, and temperature; perceiving own limb position; discriminating textures; and recognizing objects through the sense of touch (Carey, 1995). They may also experience reduced or altered pain sensation (Klit, Finnerup, Andersen, & Jensen, 2011). People who experience somatosensory loss after stroke report problems such as communicating through a handshake, using utensils, dressing, and holding objects without crushing or dropping them. They also report problems with personal, family, and work roles. Despite the high prevalence and apparent importance of somatosensation in daily activities, there is limited empirical evidence to quantify the presence and nature of a relationship between impaired somatosensation and participation in daily activities, as highlighted in two recent reviews (Carey, Lamp, & Turville, 2016; Meyer, Karttunen, Thijs, Feys, & Verheyden, 2014). The impact of somatosensory loss on participation is therefore critical to address to understand the factors that affect participation and to improve client-centered care planning.
Somatosensory impairment after stroke has been associated with impaired daily actions and activities, according to clinical and laboratory studies (Borstad & Nichols-Larsen, 2014; Carey et al., 2016; Kessner, Bingel, & Thomalla, 2016; Meyer et al., 2014). Somatosensory loss reduces control of hand movements (Jeannerod, Michel, & Prablanc, 1984) and of the fundamental pinch-grip-lift-and-hold task (Blennerhassett, Matyas, & Carey, 2007) and limits grip force control during object manipulation (Nowak & Hermsdörfer, 2005). A relationship has also been demonstrated between clinical measures of hand function and dexterity (Blennerhassett, Carey, & Matyas, 2008; Kong, Chua, & Lee, 2011; Park, Wolf, Blanton, Winstein, & Nichols-Larsen, 2008).
Studies have demonstrated that the affected limb may not be used spontaneously, despite adequate movement abilities. This lack of use may contribute to a learned nonuse of the limb and to further deterioration of motor function after stroke (Carey, 1995; Dannenbaum & Dykes, 1988). Moreover, stroke survivors have expressed uncertainty about whether to use their sensory-impaired arm for task performance (Connell, McMahon, & Adams, 2013). A predictive relationship between somatosensory loss and independence in activities of daily living (ADLs) and mobility has also been demonstrated, for example, in a prospective sample of 102 stroke survivors (Tyson, Hanley, Chillala, Selley, & Tallis, 2008) and using a life table analysis (N = 95; Reding & Potes, 1988). It is therefore plausible that sensory impairment may also be associated with reduced participation in daily activities and life roles; yet our understanding of its impact on participation is limited.
The International Classification of Functioning, Disability and Health (ICF) defines participation as “a person’s involvement in a life situation,” for example, in leisure, social, and work activities (World Health Organization, 2001, p. 10). A major knowledge gap about the impact of somatosensory impairment after stroke on participation in life activities has been recently identified in a scoping review (Carey et al., 2016) and in a systematic review (Meyer et al., 2014). Hill, Fisher, Schmid, Crabtree, and Page (2014) reported a good to excellent relationship between performance of and satisfaction with valued activities, assessed using the Canadian Occupational Performance Measure (Law et al., 2014), and intact sensation of the hand in 50 stroke survivors. Desrosiers, Noreau, Rochette, Bravo, and Boutin (2002) found a low but significant association between light touch of the upper limb and proprioception assessed at discharge and ADLs and social roles assessed 6 mo later (N = 102).
Somatosensory impairment is commonly experienced in association with motor impairment, and degree of weakness has been significantly associated with sensory impairment (Tyson et al., 2008). Upper limb motor impairment is reported in 77% of stroke survivors (Lawrence et al., 2001) and is likely to have an impact on participation. Paresis may affect participation in its own right and in addition to somatosensory impairment. We therefore investigated the impact of somatosensory impairment on participation in stroke survivors with and without motor impairment. This approach is important because rehabilitation traditionally targets motor problems, often without attending to somatosensory deficits known to affect performance (Carey, 2015; Kalra, 2010), despite the availability of interventions to remediate somatosensory deficits (Carey et al., 2016; Carey, Macdonell, & Matyas, 2011).
Given the high prevalence of somatosensory loss in stroke survivors and evidence of its relationship with daily actions and activities, we investigated the effect of somatosensory impairment on activity participation in a longitudinal stroke cohort. We were particularly interested to learn whether the presence of sensory impairment soon after stroke affected the number and type of activities (instrumental, leisure, social) in which survivors retained participation after they had returned home and completed rehabilitation (at least 3 mo after stroke). Because sensory impairment is often experienced concurrently with motor impairment, which may have an effect on participation, we investigated the impact of both motor and somatosensory impairment on activity participation in our analyses. Specifically, our objectives were as follows:
Characterize activity participation outcomes of stroke survivors with and without somatosensory impairment after stroke
Determine whether sensory impairment, identified during the acute stroke phase (at or within a few days of hospital admission after stroke) using the National Institutes of Health Stroke Scale (NIHSS; Brott et al., 1989), predicts activity participation in the postacute phase, that is, after the survivor has returned home and completed rehabilitation
Determine the effects of somatosensory loss on participation in stroke survivors with or without paresis
Explore the effects of somatosensory loss on participation in stroke survivors with and without paresis across the domains of the Activity Card Sort (ACS; Baum & Edwards, 2001).
Method
Participants
The sample comprised a subset of participants from an existing large stroke cohort (Wolf, Baum, & Connor, 2009). Inclusion criteria were first clinical stroke; no history of schizophrenia or dementia; and availability of required data, which included the acute NIHSS individual item scores, obtained on hospital admission, and the ACS construct scores, collected after the participant had returned home and completed rehabilitation.
A sample of 268 cases was obtained from a search sample of 375 after an examination of missing data, aphasia, neglect, pattern of paresis, and latency of the ACS test. Cases with severe aphasia or neglect, defined as scores greater than 1 on the relevant NIHSS scales, were excluded because these conditions have the potential to confound sensory deficit test results. Cases in which the ACS was performed before 92 days poststroke were excluded. Because sensory impairment of the upper limb is most likely associated with activity participation restrictions, based on prior literature (Desrosiers et al., 2002; Hill et al., 2014), and no separate sensory assessment data were available for the lower limb, 36 cases with paresis in only the lower limb were also excluded.
Measures
The primary outcome was percentage of retained activity participation, measured using the ACS. The ACS is a reliable and valid instrument (Edwards, Hahn, Baum, & Dromerick, 2006; Everard, Lach, Fisher, & Baum, 2000; Tse, Douglas, Lentin, & Carey, 2013). A recent review indicated that the ACS covers the most domains of the ICF Activities and Participation domains, relative to other measures, and meets the most psychometric properties (Tse et al., 2013). The version used to collect the data in a telephone interview was the 55-item measure. Participants reported on instrumental activities (e.g., shopping, household maintenance), social activities (e.g., visiting, studying), and high-demand (e.g., swimming, running) and low-demand (e.g., reading, table games) physical leisure activities. For each activity, participants responded to the following descriptors: never done, given up due to stroke, doing less often due to stroke, and doing now. The previous activity score was calculated by adding the doing now, doing less, and given up scores. The current activity score was the total of the doing now and doing less scores. The retained participation score was obtained by dividing the sum total of current activities by the total of previous activities. Higher scores indicate higher retention of prestroke activity. The primary score was the percentage of retained overall activity participation, with additional scores for the domains of Instrumental Activities, Social Activities, High-Demand Leisure Activities, and Low-Demand Leisure Activities.
The presence or absence of somatosensory impairment and motor impairment was assessed by trained nurses within a couple of days of hospital admission using the NIHSS. The NIHSS is widely used early poststroke to quantify the neurological impairment caused by stroke. It has been repeatedly validated as a tool for assessing stroke severity and as an excellent predictor for patient outcomes (Muir, Weir, Murray, Povey, & Lees, 1996). Only somatosensory loss attributed to stroke is scored as abnormal, and the examiner is required to test as many body areas (arms [not hands], legs, trunk, face) as needed to accurately check for hemisensory loss. Sensory loss is scored as 0 = normal; no sensory loss, 1 = mild to moderate sensory loss, and 2 = severe to total sensory loss. Motor impairment is tested separately for the arm and leg and is scored from 0 (no drift; limb holds 90 [or 45] degrees for full 10 s) to 4 (no movement).
Procedure
Participants (N = 268) were assessed at admission for somatosensory and motor impairment using the NIHSS. The activity participation profile was assessed using the ACS at least 3 mo poststroke.
Data Analysis
The NIHSS somatosensory loss variable was dichotomized (unimpaired, impaired). Because paresis is known to have a major impact on functionality and may interact with somatosensation, particularly in upper limb function, the sample was also split according to presence and distribution of paresis. Degree of paresis was dichotomized (unimpaired, impaired). The ACS scores were obtained in the postacute phase, that is, after participants had completed rehabilitation, returned home, and were experiencing the impact of stroke on their activity participation. If participants were tested on the ACS on more than one occasion, we selected the ACS scores closest to 182 days (6 mo) poststroke for analysis. Analyses were performed with IBM SPSS Statistics (Version 23; IBM Corp., Armonk, NY).
Between-group differences in participation were tested for participants with and without somatosensory impairment and with or without paresis. The primary analysis focused on the total retained activity participation scores. A set of five pairwise comparisons was planned to evaluate contrasts of interest across the six independent groups (2 levels of sensory loss × 3 levels of paresis). A primary family of three comparisons investigated the impact of sensory loss in samples with different paresis patterns (none, upper limb only, both upper and lower limbs). To provide an interpretative framework for the somatosensory loss effects, we used a secondary family of two comparisons to investigate the effects of each of the two paresis patterns in samples without sensory loss. The ACS percentage of retained activity score was the outcome variable. Because the total activity score is the total of the four activity domains (Instrumental Activities, Social Activities, High-Demand Leisure Activities, and Low-Demand Leisure Activities), each of the four domains of the ACS was used in exploratory secondary analyses. The same set of comparisons as for the total ACS retained activity score was used for each of these secondary analyses.
Results
The 268 cases included yielded a sample with a gender distribution that slightly favored women (53.5%). Age was normally distributed with a mean (M) of 62.8 yr and a standard deviation (SD) of 13.1 yr. There were 239 right dominant cases, 16 left dominant, and 3 ambidextrous, with the remainder unknown. The median NIHSS score for the sample was 3.00, with an interquartile range of 1.00–5.00 (5th percentile = 0.00; 95th percentile = 9.95; range = 0–15). The mean score was 3.71 (SD = 3.11). The distribution of somatosensory impairment and motor impairment in the sample is summarized in Table 1. Activity participation was measured at a median of 222 days poststroke. A skewed distribution (M = 279; median = 222; interquartile range = 184–303 days) with wide dispersion and long tail was noted for the latency of the ACS performed closest to 182 days after admission.
Table 1.
Distribution of Paresis and Somatosensory Loss
| Paresis | Somatosensory Loss | Total | |
| Unimpaired | Impaired | ||
| Unimpaired | 119 | 34 | 153 |
| Upper limb only | 16 | 11 | 27 |
| Upper and lower limbs | 43 | 45 | 88 |
| Total | 178 | 90 | 268 |
Effects of Somatosensory Impairment on Overall Retained Activity Participation
Activity Participation Outcome With and Without Somatosensory Impairment.
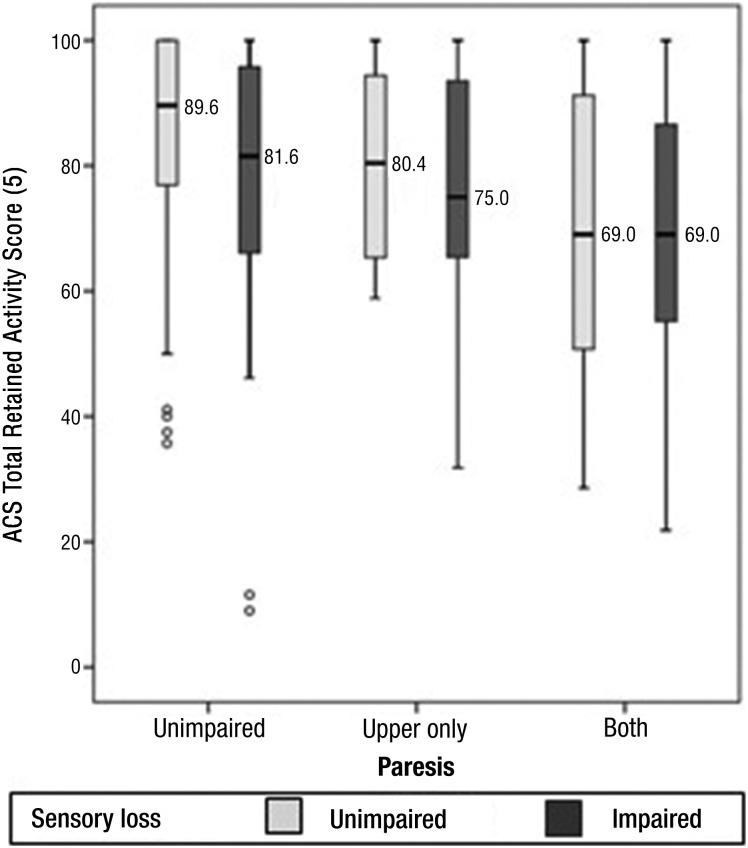
Stroke survivors with somatosensory loss and no paresis showed a significantly reduced percentage of retained activity participation compared with those with no sensory loss (z = 1.96, p = .048, two-tailed). Compared with the median retained activity of 89.6% observed in the comparison group without either paresis or sensory impairments, the group with somatosensory impairment uncomplicated by paresis had a lower median retained activity of 81.6% (Figure 1). Because these samples exhibited significant skew (skew parameters were 5.4 and 3.6 times their respective standard errors), Mann–Whitney U tests were used to compare participation scores. These tests revealed that the 78.0% greater activity loss in the group with sensory impairment (18.4% loss from prestroke) was significantly higher than that of the comparison group (10.4% loss) without either sensory or motor impairment. Thus, somatosensory impairment, identified at hospital admission using the NIHSS, significantly predicts overall percentage of retained activity participation at a median of 222 days poststroke in stroke survivors with somatosensory impairment but no paresis.
Figure 1.
Box plots showing overall percentage of retained activity scores poststroke as measured by the Activity Card Sort (ACS) in six groups of stroke survivors classified according to somatosensory impairment and paresis.
Boxes indicate the 25th and 75th percentiles (i.e., the interquartile range [IQR]). The median (50th percentile) is represented by a line across and within the box. The whiskers extend from the upper and lower edges of the box to the highest and lowest values that are not greater than 1.5 times the IQR above or below the box limits. Outliers, represented by open circles, locate cases with values that deviate more than 1.5 times the IQR above or below the box limits.
Activity Participation Outcome With Paresis.
Both somatosensory loss and paresis appear to be associated with lower total ACS retained activity scores (Figure 1), although all subgroups demonstrated a wide distribution of individual differences in retained activity scores. Despite the wide dispersion, several trends can be identified. Paresis had the typically expected marked effect on reducing activity participation (Figure 1). Paresis of the upper limb alone virtually doubled the median activity loss. Whereas this difference was more than comparable to the effect of sensory loss alone, the paresis effect only approached accepted standards of statistical significance (z = 1.90, p = .057, two-tailed), a result that needs to be interpreted in the context of the very small sample size. Paresis in both upper and lower limbs (without sensory loss) approximately tripled the activity loss in groups without sensory impairment. This effect was both large and statistically significant (z = 4.62, p < .001, two-tailed).
The effect of sensory loss in cases with upper limb paresis was of smaller median magnitude (an additional 4.6% loss) compared with that observed in the group without paresis (additional 7.1% loss) and estimated with lower power from smaller samples. The effect was not significant. The expected strong influence of paresis on activity loss appeared to mask any additional effect from the presence of sensory loss (Figure 1). The median activity losses in the two subgroups with upper limb and lower limb paresis were the strongest of all six subgroups but comparable to each other (Figure 1) and their difference not statistically significant.
Effects of Somatosensory Impairment on Activity Card Sort Domains
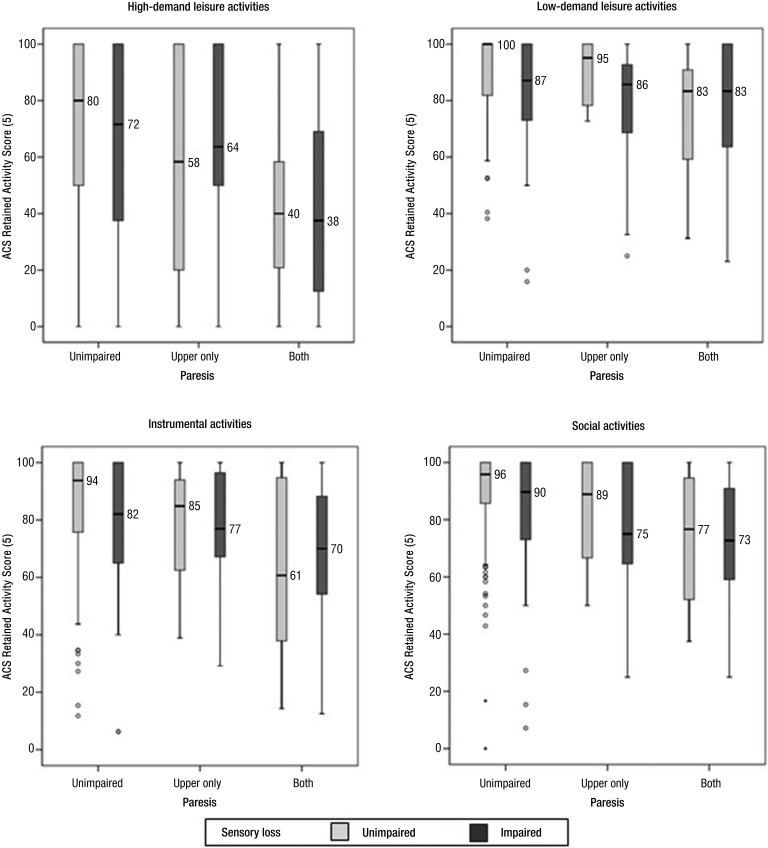
Investigation of the four domains of the total ACS suggested that the pattern revealed in the total score tended to repeat within each domain (Figure 2), the most parsimonious hypothesis given that the four domains sum to the total ACS. In the absence of paresis, the ACS scores generally suggest that somatosensory impairment reduced participation recovery. Paresis tended to reduce recovery of participation, particularly for those with paresis in both upper and lower limbs. The effect of paresis in both limbs masked the effect of somatosensory loss. However, differences in the dispersions within groups for some domains, some variations in the magnitude of the median differences, and the small group sizes for those with upper limb paresis alone resulted in somewhat different results for the formal pairwise comparison sets.
Figure 2.
Box plots illustrating distributions of percentage of retained activity scores of the Activity Card Sort (ACS) domains.
Light gray bars represent groups without somatosensory loss; dark gray bars indicate groups with sensory loss. Median values are rounded to the nearest integer value. Outliers, represented by open circles, indicate cases with values that deviate from the median by more than 1.5 to 3 times the IQR. Extreme outliers (values >3 times the IQR) are indicated by solid circles.
The adverse effect of somatosensory loss on recovery of activity was statistically significant for participants without paresis in low-demand leisure and social activities, but not in the other two domains (Table 2). An effect of somatosensory loss was not detected for samples with upper limb paresis (Table 2) in any of the domains, despite the suggestion of such an effect in Figure 2 for low-demand leisure, instrumental, and social activities. However, the sample sizes for these comparisons were small, resulting in lower statistical power. Paresis of the upper limb alone did not significantly lower activity recovery in any of the domains (Table 2), despite the suggestion of such an effect in Figure 2. The stronger adverse effect on activity caused by paresis of both limbs emerged clearly in all ACS domains, all of which showed statistically significant effects (Table 2).
Table 2.
Effects of Somatosensory and Motor Impairment on Activity Card Sort Domains Between Comparison Groups
| Comparison Groups | |||||||||||
| Group A | Group B | High-Demand Leisure Activities | Low-Demand Leisure Activities | Instrumental Activities | Social Activities | ||||||
| Paresis | Sensory Loss | Paresis | Sensory Loss | z | p | z | p | z | p | z | p |
| No | No | No | Yes | 0.896 | .371 | 2.567* | .010* | 1.564 | .118 | 2.112* | .035* |
| Upper limb only | No | Upper limb only | Yes | 0.645 | .557 | 1.559 | .134 | 0.901 | .904 | 0.687 | .716 |
| Upper and lower limbs | No | Upper and lower limbs | Yes | 0.143 | .886 | 0.806 | .420 | 0.698 | .485 | 0.205 | .837 |
| No | No | Upper limb only | No | 1.420 | .155 | 0.388 | .698 | 1.615 | .106 | 1.500 | .133 |
| No | No | Upper and lower limbs | No | 4.410* | .000* | 4.600* | .000* | 4.230* | .000* | 4.020* | .000* |
Note. Summary of key statistics obtained from Mann–Whitney pairwise tests conducted for four sets of five comparisons that replicated the primary analysis for each of the four domains of the ACS. The first three comparisons present the effects of somatosensory loss for each paretic status (no paresis, upper limb only, upper and lower limbs). The last two comparisons present the effects of paresis when there is no measured somatosensory loss.
p < .05.
Comparability of Subgroups on Age, Gender, and Handedness
Subgroups, divided according to paresis and somatosensory loss, were compared on age, gender, and hemispheric dominance. No statistically significant differences were noted. In particular, cross-tabulation of gender distribution across sensory status revealed no statistically significant difference for the subsamples without paresis (for those with sensory loss, the female:male ratio was 50%:50%, whereas for those without sensory loss, the ratio was 52.1%:47.9%). The overwhelming preponderance of right handers in the sample made dominance an unlikely potential confounder of the sensory loss effect found for cases without paresis. The obtained distributions were very skewed for both subsamples without paresis (for those without sensory loss, the right:left ratio was 92%:8%, whereas for those with sensory loss, it was 97%:3%). Finally, age was very comparable for these subgroups, with means of 63.8 yr (SD = 12.8) and 61.9 yr (SD = 10.9), a difference that was not significant when evaluated by a t test.
To further verify that age and gender did not confound the observed relationship between sensory loss and recovery of participation, we conducted several multiple regressions using age, gender, latency of ACS tests, and dichotomized sensory impairment scores as conjoint predictors of participation recovery measured by the ACS. Because the ACS distributions were significantly skewed, the IBM SPSS Statistics Generalized Linear Models subroutine was used to fit the data several times, making a variety of distributional assumptions. The regression model demonstrated that in the group without paresis, the association between somatosensory loss and the ACS score was statistically significant (p = .016), even after statistically controlling for the influence of age, gender, and latency of ACS. In contrast, multiple regressions showed that the relationship between sensory loss and ACS scores was not significant (p = .49) in the group with paresis of an upper limb or in the group with paresis of both upper and lower limbs (p = .89). These results support the conclusions reached through nonparametric analyses, reported earlier in this article.
Discussion
Somatosensory impairment at hospital admission after stroke is associated with more activity participation loss compared with survivors without somatosensory loss, when there is no concomitant paresis. This predictive association was identified in a longitudinal cohort (N = 268) of stroke survivors with mild impairment (median NIHSS score = 3.00; interquartile range = 1.00–5.00). It is based on a dichotomous measure of somatosensory impairment (NIHSS) obtained early poststroke, at the time of hospitalization, and on quantitative measurement of retained activity participation (ACS) in instrumental, leisure, and social activities at a median time of 222 days poststroke. This study addresses an identified gap in the literature, highlighted recently in two reviews (Carey et al., 2016; Meyer et al., 2014). Our finding is consistent with and advances the current literature on the effect of somatosensory impairment on activity participation (Desrosiers et al., 2002; Hill et al., 2014; Morris, van Wijck, Joice, & Donaghy, 2013). It demonstrates a predictive relationship between somatosensory loss identified during the acute phase after stroke, using an acute screening measure, and actual retained activities during the postacute phase, when people had completed rehabilitation, returned home, and were experiencing the impact of stroke on their activity participation (median = 7.4 mo poststroke). Previous studies have been cross-sectional (Hill et al., 2014) or have shown a predictive relationship from 2 to 4 wk after stroke or from hospital discharge to 6 mo poststroke (Desrosiers et al., 2002; Morris et al., 2013).
Paresis affecting both upper and lower limbs, as expected, also caused a major reduction in the percentage of retained activity participation, an effect larger than that resulting from somatosensory deficit alone. Paresis with the addition of sensory deficit does not appear to further increase loss in participation when there is paresis of both upper and lower limbs, though a larger sample is needed to clarify whether paresis of the upper limb only has as deleterious an effect on participation as when paresis is accompanied by sensory loss. In other words, the data suggest that motor impairment in both upper and lower limbs is sufficient to cause loss of activity participation over a wide enough range of activities so that the addition of sensory impairment may be insufficient to compromise additional activity participation.
Results suggest that the presence of loss of activity participation associated with paresis of both upper and lower limbs can mask the contribution of somatosensory loss and may promote an impression that somatosensory loss can be left untreated. However, as the group with sensory impairment unaccompanied by paresis shows, sensory impairment alone does significantly affect participation in everyday activities. Although an empirical investigation using interventions to manipulate motor and sensory impairments is ultimately necessary, the results raise the risk that if motor impairment only, and not sensory impairment, is addressed by intervention, the reduction in activity participation as a result of sensory loss will be unmasked. Addressing only motor impairment may lead to suboptimal therapeutic effects in patients whose somatosensory deficit has remained.
Unless motor rehabilitation training addresses sensory deficits (indirectly), or perhaps the survivors’ confidence to engage in activities, outcomes for stroke survivors will likely be diminished. Historically, clinicians and researchers have given precedence to the motor sequelae of stroke, neglecting somatosensation (Kalra, 2010), but despite good movement capacity, survivors with sensory loss learn to not use their limb for task performance (Carey, 1995; Connell et al., 2013; Dannenbaum & Dykes, 1988). Therefore, according to our findings, sensory deficit alone has sufficient impact on retained activity participation to include a somatosensory intervention focus in therapeutic programs.
Exploration of the effect of somatosensory impairment on the ACS domains revealed a significant impact on low-demand leisure and social activities but not on high-demand leisure and instrumental activities. The association with social and low-demand leisure activities is consistent with findings for social roles, as assessed with the Assessment of Life Habits (Desrosiers et al., 2002) and the social isolation and perceived physical activity subscales of the Nottingham Health Profile (Morris et al., 2013). Although the reduced statistical sensitivity resulting from small sample sizes must be noted, we did not find a significant association with instrumental activities, potentially at variance with the low but significant association between somatosensation at discharge and ADLs (Desrosiers et al., 2002).
The data confirm clinical impressions that the range of individual differences in retained activity participation is large after stroke, even when there is neither sensory nor paretic impairment nor neglect or aphasia. Clearly, much remains to be added to predict activity participation poststroke beyond the measures used here. However, it is possible to anticipate that these large individual differences cause strategic decisions about approach to therapy (e.g., ignore somatosensory impairment) difficult to make on the basis of clinical impressions and individual assessment alone. Population-wide, epidemiologically informed reasoning using empirical measurement of impairment is indicated by these results.
Limitations and Recommendations for Future Research
The sensory subscale of the NIHSS was used to identify the presence of somatosensory loss. Although this tool is commonly used to assess the presence and severity of acute neurological impairment and involves standardized administration, testing of somatosensation is not quantitative or supported by psychometric data. Studies using quantitative somatosensory measures with strong psychometric properties to better assess the nature and extent of the predictive relationship are indicated. A further benefit would be assessment of the ability of this commonly used clinical measure to predict quantitative somatosensory impairment tests with evaluated reliability and validity.
The NIHSS was administered only once, at or within a few days of hospital admission after stroke. Therefore, when sensory loss, aphasia, neglect, or other NIHSS variables were involved, the analyses indicate a predictive relationship based on the patient status on these variables at admission. The level of sensory loss, neglect, aphasia, and so forth at the time of ACS administration is unknown.
To protect against the possibility that an earlier ACS may have influenced the data, we conducted a post hoc analysis excluding participants from the sample with ACS scores obtained in the 92–155 days range. Findings from this trimmed sample (N = 240) essentially remained the same: There was a somatosensory loss effect in the absence of paresis (total retained activity participation: z = 2.50, p = .012; low-demand leisure: z = 2.90, p = .004; social: z = 2.75, p = .006; instrumental z = 2.04, p = .042) and a highly significant effect of paresis in both limbs for total retained activities and all subcategories (z = 3.88–4.26, p < .001).
Finally, use of existing data from the prospective cohort limited choice of measures and the demographic and organismic covariates that might be studied. Our initial findings of a predictive association does however encourage future studies to verify the observation. A design is recommended that uses superior measurement tools for somatosensory deficit and paresis and considers demographic or organismic variables that might arguably influence participation recovery or the strength of the relationship between somatosensory impairment and participation in a sufficiently large sample.
Implications for Occupational Therapy Practice
Our findings have the following implications for occupational therapy practice:
Practitioners should assess for the presence of somatosensory impairment in stroke survivors, given its impact on participation, especially in those without concomitant paresis.
Rehabilitation directed at upper limb function and return to participation in previous life activities should consider investigation of the effect of interventions with a specific focus on somatosensory phenomena.
Therapeutic program planning should consider that the impact of somatosensory impairment may be unmasked when rehabilitation has been directed only at motor impairment recovery and the result has been below expectation.
Clinical evaluation and treatment in stroke have traditionally focused on addressing motor impairment without considering the impact of somatosensory functions. Our findings suggest that additional gain may be obtained in participation outcomes by augmenting motor-based interventions with a somatosensory rehabilitation component. Investigations of this possibility are supported by the present findings.
Conclusion
Somatosensory impairment is associated with reduced activity participation. On the basis of evidence that somatosensory improvements are obtained, a focus on somatosensory intervention in rehabilitation is recommended for people with somatosensory impairment (Carey et al., 2011). Monitoring participation outcomes may yield evidence of additional improvements in participation associated with somatosensory intervention. Such investigation will be important in furthering evidence not only of the relationship between somatosensory impairment and participation but also the potential impact of somatosensory-focused interventions on participation outcomes. Our findings also indicate that paresis of the upper and lower limbs can mask the contribution of sensory loss. This finding suggests that the potential impact of somatosensation on participation when movement returns should not be ignored.
Acknowledgments
We acknowledge financial support for this research from the James S. McDonnell Foundation (JSMF Grants 21002032 and 220020087, awarded to CB) and National Health and Medical Research Council (NHMRC) Grants 307902 and 1022694 (awarded to LMC) and support for analysis, write-up, and researchers from the James S. McDonnell Foundation 21st Century Science Initiative in Cognitive Rehabilitation Collaborative Award (JSMF Grant 220020413), the Victorian Government’s Operational Infrastructure Support Program, an Australian Research Council Future Fellowship (FT0992299), and an NHMRC Career Development Award (307905) awarded to LMC. We acknowledge the contribution of Lisa Tabor Connor to an earlier presentation of preliminary data related to this study and to Tamara Tse for assistance with data compilation and checking.
Contributor Information
Leeanne M. Carey, Leeanne M. Carey, PhD, BAppSc(OT), FAOTA, FOTARA, is Professor of Occupational Therapy, Discipline Lead, Occupational Therapy, School of Allied Health, College of Science, Health, and Engineering, La Trobe University, Melbourne, Victoria, Australia, and Head, Neurorehabilitation and Recovery, Stroke Division, Florey Institute of Neuroscience and Mental Health, Heidelberg, Victoria, Australia; l.carey@latrobe.edu.au
Thomas A. Matyas, Thomas A. Matyas, PhD, is Adjunct Professor, School of Allied Health and School of Psychology and Public Health, College of Science, Health, and Engineering, La Trobe University, Melbourne, Victoria, Australia, and Honorary Professorial Fellow, Neurorehabilitation and Recovery, Stroke Division, Florey Institute of Neuroscience and Mental Health, Heidelberg, Victoria, Australia
Carolyn Baum, Carolyn Baum, PhD, OTR/L, FAOTA, is Professor, Occupational Therapy and Neurology and Social Work, Elias Michael Director, Program in Occupational Therapy, Washington University School of Medicine, St. Louis, MO.
References
- Baum C. M., & Edwards D. F. (2001). Activity Card Sort (ACS): Test manual. St. Louis: Program in Occupational Therapy, Washington University School of Medicine. [Google Scholar]
- Blennerhassett J. M., Carey L. M., & Matyas T. A. (2008). Clinical measures of handgrip limitation relate to impaired pinch grip force control after stroke. Journal of Hand Therapy, 21, 245–253. https://doi.org/10.1197/j.jht.2007.10.021 [DOI] [PubMed] [Google Scholar]
- Blennerhassett J. M., Matyas T. A., & Carey L. M. (2007). Impaired discrimination of surface friction contributes to pinch grip deficit after stroke. Neurorehabilitation and Neural Repair, 21, 263–272. https://doi.org/10.1177/1545968306295560 [DOI] [PubMed] [Google Scholar]
- Borstad A. L., & Nichols-Larsen D. S. (2014). Assessing and treating higher level somatosensory impairments post stroke. Topics in Stroke Rehabilitation, 21, 290–295. https://doi.org/10.1310/tsr2104-290 [DOI] [PubMed] [Google Scholar]
- Brott T., Adams H. P. Jr., Olinger C. P., Marler J. R., Barsan W. G., Biller J., . . . Hertzberg V. (1989). Measurements of acute cerebral infarction: A clinical examination scale. Stroke, 20, 864–870. https://doi.org/10.1161/01.STR.20.7.864 [DOI] [PubMed] [Google Scholar]
- Carey L. M. (1995). Somatosensory loss after stroke. Critical Reviews in Physical and Rehabilitation Medicine, 7, 51–91. https://doi.org/10.1615/CritRevPhysRehabilMed.v7.i1.40 [Google Scholar]
- Carey L. M. (2012). Touch and body sensations. In Carey L. M. (Ed.), Stroke rehabilitation: Insights from neuroscience and imaging (pp. 157–172). New York: Oxford University Press; https://doi.org/10.1093/med/9780199797882.003.0012 [Google Scholar]
- Carey L. M. (2015). Person factors. In Christiansen C. H., Baum C. M., & Bass J. (Eds.), Occupational therapy: Enabling performance, participation and well-being (4th ed., pp. 249–265). Thorofare, NJ: Slack. [Google Scholar]
- Carey L. M., Lamp G., & Turville M. (2016). The state-of-the-science of somatosensory function and its impact on daily life in adults and older adults, and following stroke: A scoping review. OTJR: Occupation, Participation and Health, 36, 27S–41S. https://doi.org/10.1177/1539449216643941 [DOI] [PubMed] [Google Scholar]
- Carey L., Macdonell R., & Matyas T. A. (2011). SENSe: Study of the Effectiveness of Neurorehabilitation on Sensation: A randomized controlled trial. Neurorehabilitation and Neural Repair, 25, 304–313. https://doi.org/10.1177/1545968310397705 [DOI] [PubMed] [Google Scholar]
- Carey L. M., & Matyas T. A. (2011). Frequency of discriminative sensory loss in the hand after stroke in a rehabilitation setting. Journal of Rehabilitation Medicine, 43, 257–263. https://doi.org/10.2340/16501977-0662 [DOI] [PubMed] [Google Scholar]
- Connell L. A., McMahon N. E., & Adams N. (2013). Stroke survivors’ experiences of somatosensory impairment after stroke: An interpretive phenomenological analysis. Physiotherapy, 100, 150–155. https://doi.org/10.1016/j.physio.2013.09.003 [DOI] [PubMed] [Google Scholar]
- Dannenbaum R. M., & Dykes R. W. (1988). Sensory loss in the hand after sensory stroke: Therapeutic rationale. Archives of Physical Medicine and Rehabilitation, 69, 833–839. [PubMed] [Google Scholar]
- Desrosiers J., Noreau L., Rochette A., Bravo G., & Boutin C. (2002). Predictors of handicap situations following post-stroke rehabilitation. Disability and Rehabilitation, 24, 774–785. https://doi.org/10.1080/09638280210125814 [DOI] [PubMed] [Google Scholar]
- Edwards D. F., Hahn M., Baum C., & Dromerick A. W. (2006). The impact of mild stroke on meaningful activity and life satisfaction. Journal of Stroke and Cerebrovascular Diseases, 15, 151–157. https://doi.org/10.1016/j.jstrokecerebrovasdis.2006.04.001 [DOI] [PubMed] [Google Scholar]
- Everard K. M., Lach H. W., Fisher E. B., & Baum M. C. (2000). Relationship of activity and social support to the functional health of older adults. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 55, S208–S212. https://doi.org/10.1093/geronb/55.4.S208 [DOI] [PubMed] [Google Scholar]
- Hill V. A., Fisher T., Schmid A. A., Crabtree J., & Page S. J. (2014). Relationship between touch sensation of the affected hand and performance of valued activities in individuals with chronic stroke. Topics in Stroke Rehabilitation, 21, 339–346. https://doi.org/10.1310/tsr2104-339 [DOI] [PubMed] [Google Scholar]
- Jeannerod M., Michel F., & Prablanc C. (1984). The control of hand movements in a case of hemianaesthesia following a parietal lesion. Brain, 107, 899–920. https://doi.org/10.1093/brain/107.3.899 [DOI] [PubMed] [Google Scholar]
- Kalra L. (2010). Stroke rehabilitation 2009: Old chestnuts and new insights. Stroke, 41, e88–e90. https://doi.org/10.1161/STROKEAHA.109.572297 [DOI] [PubMed] [Google Scholar]
- Kessner S. S., Bingel U., & Thomalla G. (2016). Somatosensory deficits after stroke: A scoping review. Topics in Stroke Rehabilitation, 23, 136–146. https://doi.org/10.1080/10749357.2015.1116822 [DOI] [PubMed] [Google Scholar]
- Klit H., Finnerup N. B., Andersen G., & Jensen T. S. (2011). Central poststroke pain: A population-based study. Pain, 152, 818–824. https://doi.org/10.1016/j.pain.2010.12.030 [DOI] [PubMed] [Google Scholar]
- Kong K. H., Chua K. S., & Lee J. (2011). Recovery of upper limb dexterity in patients more than 1 year after stroke: Frequency, clinical correlates and predictors. NeuroRehabilitation, 28, 105–111. https://doi.org/10.3233/NRE-2011-0639 [DOI] [PubMed] [Google Scholar]
- Law M., Baptiste S., Carswell A., McColl M. A., Polatajko H., & Pollock N. (2014). Canadian Occupational Performance Measure (5th ed.). Ottawa: CAOT Publications. [Google Scholar]
- Lawrence E. S., Coshall C., Dundas R., Stewart J., Rudd A. G., Howard R., & Wolfe C. D. A. (2001). Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke, 32, 1279–1284. https://doi.org/10.1161/01.STR.32.6.1279 [DOI] [PubMed] [Google Scholar]
- Meyer S., Karttunen A. H., Thijs V., Feys H., & Verheyden G. (2014). How do somatosensory deficits in the arm and hand relate to upper limb impairment, activity, and participation problems after stroke? A systematic review. Physical Therapy, 94, 1220–1231. https://doi.org/10.2522/ptj.20130271 [DOI] [PubMed] [Google Scholar]
- Morris J. H., van Wijck F., Joice S., & Donaghy M. (2013). Predicting health related quality of life 6 months after stroke: The role of anxiety and upper limb dysfunction. Disability and Rehabilitation, 35, 291–299. https://doi.org/10.3109/09638288.2012.691942 [DOI] [PubMed] [Google Scholar]
- Muir K. W., Weir C. J., Murray G. D., Povey C., & Lees K. R. (1996). Comparison of neurological scales and scoring systems for acute stroke prognosis. Stroke, 27, 1817–1820. https://doi.org/10.1161/01.STR.27.10.1817 [DOI] [PubMed] [Google Scholar]
- Nowak D. A., & Hermsdörfer J. (2005). Grip force behavior during object manipulation in neurological disorders: Toward an objective evaluation of manual performance deficits. Movement Disorders, 20, 11–25. https://doi.org/10.1002/mds.20299 [DOI] [PubMed] [Google Scholar]
- Park S. W., Wolf S. L., Blanton S., Winstein C., & Nichols-Larsen D. S. (2008). The EXCITE trial: Predicting a clinically meaningful motor activity log outcome. Neurorehabilitation and Neural Repair, 22, 486–493. https://doi.org/10.1177/1545968308316906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reding M. J., & Potes E. (1988). Rehabilitation outcome following initial unilateral hemispheric stroke: Life table analysis approach. Stroke, 19, 1354–1358. https://doi.org/10.1161/01.STR.19.11.1354 [DOI] [PubMed] [Google Scholar]
- Tse T., Douglas J., Lentin P., & Carey L. (2013). Measuring participation after stroke: A review of frequently used tools. Archives of Physical Medicine and Rehabilitation, 94, 177–192. https://doi.org/10.1016/j.apmr.2012.09.002 [DOI] [PubMed] [Google Scholar]
- Tyson S. F., Hanley M., Chillala J., Selley A. B., & Tallis R. C. (2008). Sensory loss in hospital-admitted people with stroke: Characteristics, associated factors, and relationship with function. Neurorehabilitation and Neural Repair, 22, 166–172. https://doi.org/10.1177/1545968307305523 [DOI] [PubMed] [Google Scholar]
- Winward C. E., Halligan P. W., & Wade D. T. (2002). The Rivermead Assessment of Somatosensory Performance (RASP): Standardization and reliability data. Clinical Rehabilitation, 16, 523–533. https://doi.org/10.1191/0269215502cr522oa [DOI] [PubMed] [Google Scholar]
- Wolf T. J., Baum C., & Connor L. T. (2009). Changing face of stroke: Implications for occupational therapy practice. American Journal of Occupational Therapy, 63, 621–625. https://doi.org/10.5014/ajot.63.5.621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2001). International classification of functioning, disability and health. Geneva: Author. [Google Scholar]