Abstract
Background
The amplitude of the auditory evoked N1 component that can be derived from noninvasive electroencephalographic recordings increases as a function of time between subsequent tones. N1 amplitudes in individuals with schizophrenia saturate at a lower asymptote, thus giving rise to a reduced dynamic range. Reduced N1 dynamic range is a putative electrophysiological biomarker of altered sensory memory function in individuals with the disease. To date, it is not clear what determines N1 dynamic range and what causes reduced N1 dynamic range in individuals with schizophrenia. Here we test the hypothesis that reduced N1 dynamic range results from a shift in excitatory/inhibitory (E/I) balance toward an excitation-deficient or inhibition-dominant state.
Methods
We recorded auditory-evoked potentials (AEPs) while 4 macaque monkeys passively listened to sequences of sounds of random pitch and stimulus-onset asynchrony (SOA). Three independent experiments tested the effect of the N-methyl-d-aspartate receptor channel blockers ketamine and MK-801 as well as the γ-aminobutyric acid (GABA) A receptor–positive allosteric modulator midazolam on the dynamic range of a putative monkey N1 homologue and 4 other AEP components.
Results
Ketamine, MK-801 and midazolam reduced peak N1 amplitudes for the longest SOAs. Other AEP components were also affected, but revealed distinct patterns of susceptibility for the glutamatergic and GABA-ergic drugs. Different patterns of susceptibility point toward differences in the circuitry maintaining E/I balance of individual components.
Limitations
The study used systemic pharmacological interventions that may have acted on targets outside of the auditory cortex.
Conclusion
The N1 dynamic range may be a marker of altered E/I balance. Reduced N1 dynamic range in individuals with schizophrenia may indicate that the auditory cortex is in an excitation-deficient or inhibition-dominant state. This may be the result of an incomplete compensation for a primary deficit in excitatory drive.
Introduction
Individuals with schizophrenia exhibit deficits in simple auditory tasks, such as delayed pitch discrimination.1–6 These deficits have been linked in part to impaired encoding of information into auditory sensory memory.7–9 Auditory sensory memory (i.e., echoic memory) passively maintains a detail-rich, low-level representation (e.g., pitch, loudness) of past sounds for a brief period of time on the order of 8–12 seconds.3,6 Owing to the fleeting nature of auditory information, this memory trace is essential for most auditory functions, such as speech comprehension.9,10 The neural mechanisms of auditory sensory memory are still a matter of debate.11–14 However, it has been noted that the amplitude of the auditory-evoked N1 component, which is reduced immediately after a tone has been processed, an effect referred to as either refractoriness, repetition suppression or short-term adaptation, recovers back to baseline at the same rate at which information decays from auditory sensory memory.15 The gradual recovery of the N1 may thus be a marker of the gradual decay of the auditory sensory memory trace.9,15–18 In individuals with schizophrenia the recovery of the N1 amplitude is blunted, leading to an overall smaller dynamic range.19–23 This reduced dynamic range of the N1 has been suggested to be a putative electrophysiological biomarker of altered auditory sensory memory encoding in individuals with schizophrenia.17,19,24 Note that the N1 dynamic range is distinct from, but potentially associated with, mismatch negativity, which is also believed to depend on the formation of an auditory memory and is also affected in individuals with schizophrenia.
The pathologic process(es) that cause impaired sensory memory encoding and reduced dynamic range of the N1 are still a matter of debate. Postmortem studies have reported structural deficits in auditory cortex pyramidal cells that point toward reduced excitatory function in individuals with schizophrenia. 25–27 In addition, postmortem studies have identified molecular changes in interneurons using γ-aminobutyric acid (GABA) that point toward reduced inhibitory function in individuals with schizophrenia.28 In isolation, these 2 types of deficits are expected to have opposite functional consequences: whereas pyramidal cell deficits alone would lead to an excitation-deficient (inhibition-dominant) state, GABA-ergic deficits alone would lead to an inhibition-deficient (excitation-dominant) state. In combination, the 2 deficits may either cancel out or lead to either outcome, depending on which deficit has a stronger impact on overall circuit function. To date, however, there is no established electrophysiologic marker that indexes the relative strength of the excitatory/inhibitory system (E/I balance). Identifying such a marker would potentially allow us to infer the functional consequences of the observed postmortem deficits (i.e., whether the excitatory or inhibitory deficits observed in postmortem brain are predominant).
Here we hypothesize that the dynamic range of the auditory N1 is a marker of E/I balance in the auditory cortex. It is already known that the N1 reflects postsynaptic currents/potentials in pyramidal cells (i.e., excitatory function). Furthermore, the dynamic range of the N1 can be reduced by pharmacological interventions that decrease excitatory glutamatergic neurotransmission via the N-methyl-d-aspartate receptor (NMDAR).17,29 To date, however, it is not known if the dynamic range of the N1 is also sensitive to alterations of GABA-ergic neurotransmission and therefore affected by changes in both excitation and inhibition. Because GABA-ergic signalling plays a crucial role in regulating pyramidal cell activity, we predict that N1 dynamic range can also be reduced by increasing GABA-ergic transmission. This finding would support the use of N1 dynamic range as a marker of E/I balance and would suggest that the reduced dynamic range of the N1 and impaired encoding of information into auditory sensory memory in individuals with schizophrenia is a consequence of an excitation-deficient/inhibition-dominant state in the disease.
We tested our hypothesis in the rhesus macaque monkey, one of the most established model systems of auditory-evoked EEG potentials (AEP).17,24,30–34 In 3 separate experiments, we used the noncompetitive NMDAR antagonists ketamine and MK-801 to reduce glutamatergic signalling and the GABAA receptor–positive allosteric modulator (PAM) midazolam to increase GABA-ergic transmission. Although the 2 types of manipulations have distinct pharmacological targets, both shift E/I balance toward a state of relative excitation deficiency/inhibition dominance (Fig. 1A) and were thus expected to reduce N1 dynamic range. Although not the immediate focus of our hypothesis, we also measured and quantified the effect of these manipulations on other previously identified auditory-evoked electroencephalography (EEG) components that arise at different latencies and with different topographies. In contrast to earlier work, we used a random auditory environment to evaluate the effect of ketamine on N1 dynamic range independent from the known role of NMDARs in learning and predictive coding.32,35
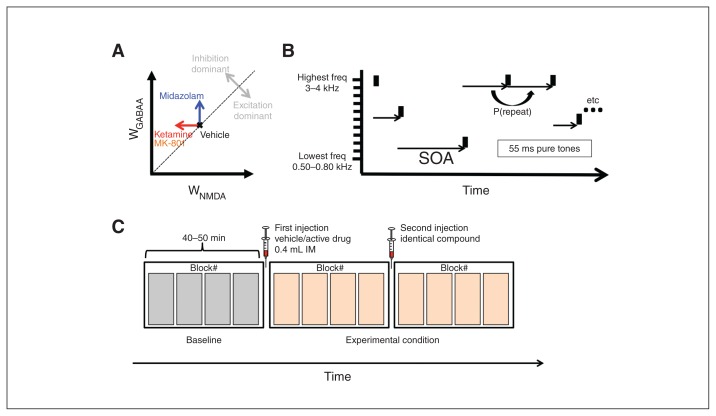
Fig. 1.
(A) Two-dimensional reduction of the high-dimensional space that determines excitatory/inhibitory (E/I) balance of a hypothetical neural system. Excitation is represented on the X axis as the strength of glutamatergic neurotransmission at N-methyl-d-aspartate receptors (WNMDA). Inhibition is represented on the Y axis as the strength of γ-aminobutyric acid A (GABAA)-ergic synapses (WGABAA). Points above [below] the diagonal represent states of relative inhibition [excitation] dominance. Experiments 1 and 2 decreased WNMDA (red arrow), whereas experiment 3 increased WGABAA. Both types of intervention shift the system into a state of relative inhibition dominance. (B) Auditory paradigm. Animals passively listened to tones of 11 different auditory frequencies presented at random times. All tones were 55 ms long and were presented at a sound pressure level (SPL) of 80 dB. Stimulus-onset asynchrony (SOA) ranged between 0.25 and 32 seconds and followed a boxcar distribution in log2-space. (C) Tone presentations were structured into blocks, each lasting between 9 and 12 minutes. The first 4 blocks measured baseline response. The following 2 groups of 4 blocks were preceded by an intramuscular (IM) injection of either vehicle or active drug.
Methods
Animals
Experiments were performed on 4 adult male macaque monkeys (macaca mulatta, animals R, J, S and W). The treatment of the monkeys was in accordance with the guidelines set by the U.S. Department of Health and Human Services (National Institutes of Health) for the care and use of laboratory animals. All methods were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. All animals have previously been exposed to similar passive listening paradigms in previous studies.24,36
Cranial EEG recordings
The rhesus EEG recording system was designed to be as similar as possible to human scalp recordings while reducing the setup times and facilitating stable and reproducible long-term recordings over many months. Details of the EEG recording system were reported previously.24,36 Briefly, the different animals had between 21 and 33 cranial electrodes implanted, covering roughly the same anatomy covered by the international 10–20 system.36
Experimental setup
Experiments were performed in 2 small (4 × 4 × 8 feet) sound-attenuating and electrically insulated recording booths (Eckel Noise Control Technology). Animals were positioned and head-fixed in custom-made primate chairs (Scientific Design). Cranial EEG potentials were recorded with a 32-channel digital amplifier system (RHD2000, Intan). Experimental control was handled by a Windows PC running an in-house modified version of the MATLAB monkeylogic software package and presented by routines of the MATLAB Psychtoolbox package. Sounds were presented using a single element 4-inch full-range driver (Tang Band W4–1879) located 8 inches in front of the animals.
Stimuli and experimental design
Details of the auditory paradigm have been reported previously.24,36 Briefly, animals listened to 55 ms–long 80 dB sound pressure level (SPL) pure tones that varied in frequency and time between individual tone onsets (i.e., stimulus-onset asynchrony [SOA]; Fig. 1B). The SOAs were drawn from a boxcar distribution in log2-space between 0.25 and 32 seconds. To increase the number of trials per recording session in follow-up experiments the upper limit of the SOA was reduced to 16 seconds (MK-801 study) and 12.8 seconds (midazolam study). Since animal S entered the experimental pipeline after the SOA had already been reduced, we used an upper limit of 16 seconds in both the ketamine and MK-801 studies.
Tone presentations were structured into blocks with a duration of 9–12 minutes. Each recording session consisted of 12 blocks. After block number 4, the animals were given a 0.4 mL intramuscular injection of either a psychoactive agent or vehicle (either saline or tween, depending on solubility of the corresponding active agent). The same injection was repeated after block number 8 to maintain an approximately level concentration of active drug. The control and experimental conditions occurred on alternating days, with the experimental condition never occurring on more than 2 days per week (Fig. 1C).
Experiment 1 used the NMDAR channel blocker ketamine as the active agent. Experiment 2 used MK-801, which is also a noncompetitive NMDAR channel blocker but has higher affinity and selectivity for the NMDAR than ketamine.37 Experiment 3 used midazolam, a PAM at the GABA receptor.38 A full listing of dose level is outlined in Table 1, Table 2 and Table 3.
Table 1.
Number of recording sessions using a specified concentration of ketamine
| Dose, mg/kg | No. of sessions | |||
|---|---|---|---|---|
|
| ||||
| Animal J | Animal R | Animal S | Animal W | |
| 0.0 | 7 | 9 | 5 | 6 |
| 0.25 | 0 | 0 | 1 | 0 |
| 0.5 | 0 | 0 | 1 | 0 |
| 1 | 4 | 5 | 2 | 5 |
| 4 | 1 | 1 | 0 | 0 |
Table 2.
Number of recording sessions using a specified concentration of MK-801
| Dose, mg/kg | No. of sessions | |||
|---|---|---|---|---|
|
| ||||
| Animal J | Animal R | Animal S | Animal W | |
| 0.0 | 4 | 4 | 5 | 5 |
| 0.03 | 2 | 2 | 1 | 2 |
| 0.06 | 0 | 0 | 1 | 0 |
Table 3.
Number of recording sessions using a specified concentration of midazolam
| Dose, mg/kg | No. of sessions | |||
|---|---|---|---|---|
|
| ||||
| Animal J | Animal R | Animal S | Animal W | |
| 0.0 | 6 | 6 | 3 | 6 |
| 0.02 | 1 | 1 | 0 | 1 |
| 0.04 | 1 | 1 | 0 | 1 |
| 0.08 | 2 | 2 | 2 | 2 |
Tone presentations within each block were either random or regular. In the random condition, tone identity and SOA changed 90% of the time. In the regular condition, tone identity and SOA changed only 10% of the time. The ketamine experiment used only the random condition. Because ketamine had the same effect in the random condition as previously described in the regular condition, experiments 2 and 3 used alternating blocks of the random and the regular condition. Analyses showed no fundamental differences between the regular and random conditions, hence data were pooled across both conditions to increase signal-to-noise ratio.
Auditory-evoked potentials
Raw data were down-sampled from 5000 Hz to 500 Hz and filtered with a 70 Hz low-pass filter. The filtered data were cut into short epochs around the onset of each sound (−150 ms to 750 ms). A subtraction method was used to reduce AEP superposition for tones with short SOAs.24 The data were then exported for use with R statistics software.39 Trials with peak-to-peak amplitudes above 450 μV were excluded to minimize motion artifacts. The remaining trials were sorted into bins of SOA with a width of 1 octave (e.g., 0.25–0.50 s, 0.50–1.00 s) and averaged.
Dynamic range of component amplitude
Previous work in the same animals identified 8 distinct middle and long-latency components.36 Based on latency and polarity they were referred to as P14, P21, P31, N43, P55, N85, P135 and N170. Most components could readily be identified in all animals despite interindividual differences in timing and topography. For each animal, each component was associated with a time window and a list of channels. Component amplitudes on each trial were estimated by averaging activity across the corresponding channels and time bins.
Following earlier work, the dependence of component amplitude X as a function of SOA was modelled as an exponential recovery to baseline:40,41
In this framework, a refers to the asymptotic value that will be reached when SOA is infinitely long. The factor b determines the maximal reduction of amplitude for short SOAs. The time-constant λSOA determines how quickly amplitude recovers back to baseline for long SOAs. We fit this function to the data using a gradient descent method for the control and drug conditions separately. The values of the 3 parameters are reported in Appendix 1, Tables S1–S3, available at jpn.ca/170093-a1.
In the context of the present study, we focus on the dynamic range defined here as the difference of component amplitude between 0.250 and 12 seconds. Because there are only a relatively small number of trials with SOA equal to 0.250 or 12 seconds, the dynamic range was estimated based on the model predictions for 0.250 and 12 seconds: x(12.000)–x(0.250). This approach is optimal because it uses an established model and uses all available data. The alternative, nonmodel–based approach would be problematic for several reasons: it would require selecting an arbitrary criterion to bin the data based on SOA; if bin size is large it would require averaging data with substantially different SOAs; and if bin size is small it would require discarding most of the data contained in any bin other than the ones with the shortest and the longest SOAs.
Dynamic range of 3 components (P14, N43, N170) exhibited large between-subject variability in 1 or more of the experiments. To avoid having the variability introduced by these components obscure effects that are present in the other components, they were excluded from the population analysis.
Results
High-density tone-evoked cranial EEG responses were measured in 4 macaque monkeys while they passively listened to sequences of pure tones presented at random SOAs. The present work focuses on 5 previously identified AEP components referred to by polarity and latency as P21, P31, P55, N85 and P135.36 The amplitudes of all 5 components have been shown to increase for longer SOAs.24 The aim of the present experiments was to dissect the pharmacological underpinnings that underlie the scaling of component amplitude with SOA. Specific emphasis was placed on the N85 component, which is one putative homologue of the human N1.17,30,34,36 Note that other authors have suggested an earlier negativity — probably corresponding to our N43 — as a putative N1 homologue.42,43 In addition to the N85, special emphasis was placed on the P21 and P31, which are believed to be generated in the primary auditory cortex and may reflect the depolarization of supragranular pyramidal cells by layer 4 input. Thus, they reflect the earliest stage of cortical processing that can reliably be measured with high signal-to-noise ratio using EEG in the monkey.
Experiment 1: effect of ketamine on AEP dynamic range
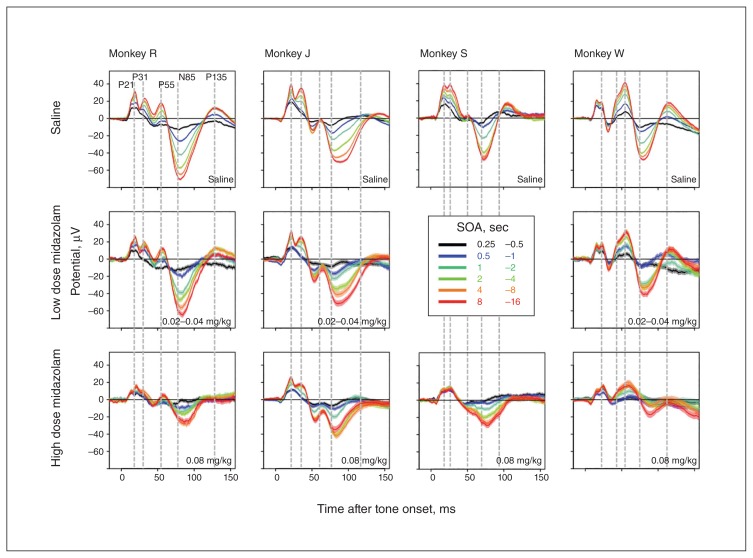
Figure 2 shows the average AEPs at all 32 active electrodes on days following injection of vehicle (black) or 1 mg/kg ketamine (red) for 1 representative animal. Differences can be identified on all electrodes, but are most prominent at frontocentral electrodes around the time of the N85 component. Figure 3 shows AEPs averaged across 6 frontocentral electrodes and split by SOA (colour code) as well as drug condition (panels). Even the low dose of ketamine (≤ 1 mg/kg) has a strong effect on the scaling of AEPs by SOA. These results resemble earlier findings using phencyclidine and long sequences of clicks at fixed SOAs.17
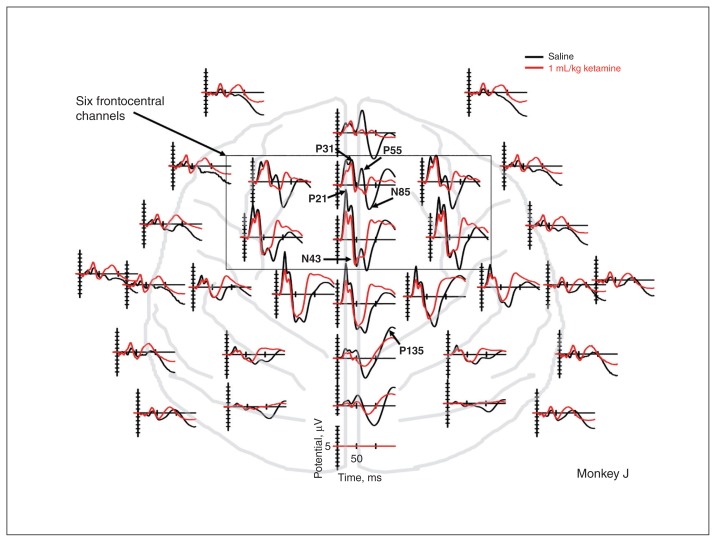
Fig. 2.
Effect of ketamine on evoked potentials in a representative example animal. Tone evoked responses on vehicle (black), and ketamine (red) days is shown across multiple channels as a function of time from tone onset on the X axis. Ketamine has moderate effects on earlier components, such as the P21 and P31, but strongly affects amplitude and peak latency of later components, such as the P55 and N85. This pattern is particularly apparent at frontocentral channels.
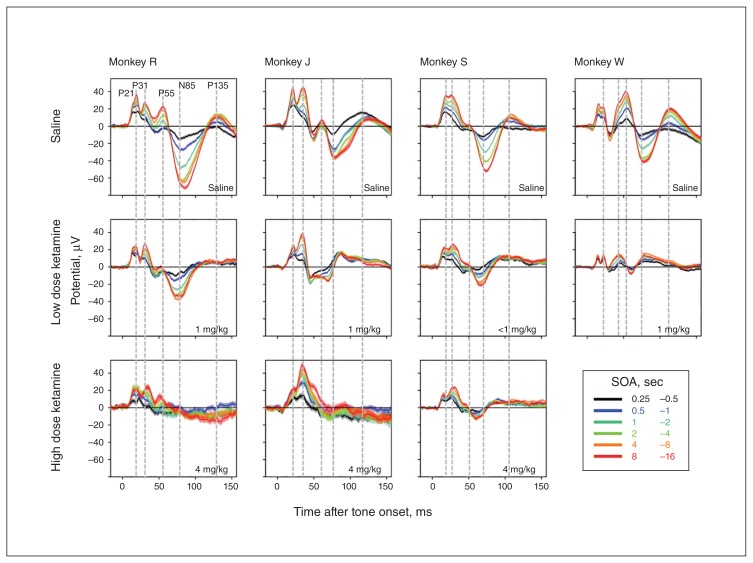
Fig. 3.
Effect of ketamine on auditory-evoked potentials (AEPs) split by stimulus-onset asynchrony (SOA). Evoked potentials averaged across 6 frontocentral electrodes are plotted as a function of time from tone onset on the X axis. Colours correspond to different ranges of SOA, and line thickness corresponds to the standard error of the mean across trials. All 5 highlighted components show robust scaling of amplitude with SOA. This effect is less evident for P135 because it peaks on more posterior electrodes not represented in the 6 prefrontal electrodes averaged here. After administration of ketamine, this scaling with SOA is blunted, and in some cases completely abolished. Blunting is particularly pronounced for the N85 component. In addition, ketamine also reduces the duration of the N85 component and, as a result, its peak latency. The peak latencies of earlier components are not affected. Of note, the scaling of the P31 with SOA is largely resilient even to the highest dose (0.4 mg/kg) of ketamine.
Closer inspection of Figure 3 reveals that neither peak latencies nor overall duration of mid-latency components (P21, P31 and P55) seem to be affected by ketamine (see vertical lines in Fig. 3). In contrast, duration of the N85 is shortened by ketamine, and as a result, peak latency is reduced (e.g., animals J and S; Fig. 3). In addition, mid-latency component amplitude and dynamic range seem to exhibit more resilience to ketamine. This is most apparent at the higher dose of ketamine (4 mg/kg), where the N85 is practically absent and the mid-latency components, particularly the P31, continue to scale with SOA (e.g., animal J; Fig. 3, third row).
Figure 4A–E plots average normalized amplitude as a function of SOA for all 5 AEP components. In vehicle sessions (black) all components show a steady increase in amplitude before reaching an asymptotic value for the longest SOAs. Following ketamine administration (red), peak amplitudes fail to recover with SOA, leading to lower AEP amplitudes, especially for the longest SOAs. As noted previously, scaling of the P21 and P31 following ketamine administration appears to be more preserved relative to scaling of the P55 and N85. The “Quantification and comparison of different active compounds” section provides a quantitative analysis of these findings and a comparison between different drugs.
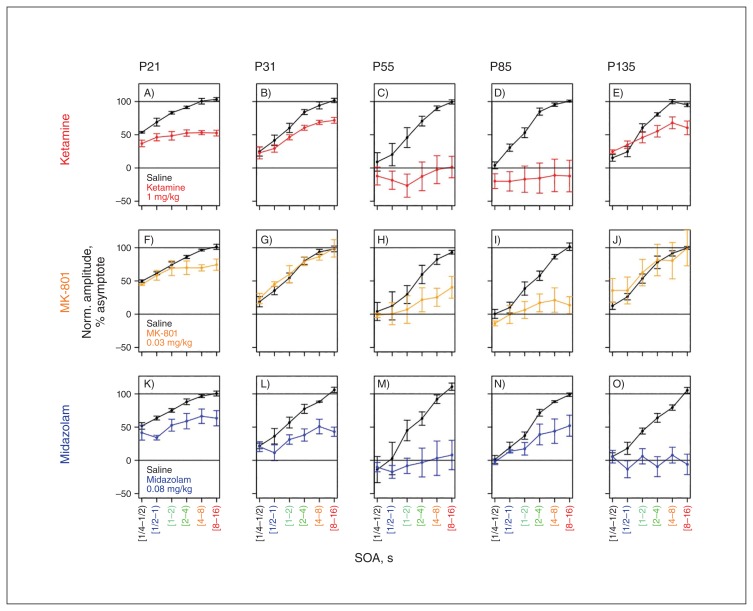
Fig. 4.
Effect of ketamine, MK-801 and midazolam on refractoriness. Individual panels show normalized component amplitude as a function of stimulus-onset asynchrony (SOA) averaged across all 4 animals for vehicle sessions (black) and drug trials (colour code). Error bars represent standard error of the mean across animals. At larger SOAs, component amplitude saturates, typically between 8 and 12 seconds. (A–E) Ketamine: blunted scaling of amplitude with SOA is apparent across all components. The effect of ketamine is most pronounced on components P55 and N85. Scaling of amplitude with SOA is more resilient to ketamine for components P31 and P135. (F–J) MK-801: blunted scaling is apparent for the P55 and N85 components. The effect of MK-801 is limited for P21 and seems completely absent for P31 and P135. (K–O) Midazolam: blunted scaling of amplitude with SOA is apparent across all components. In contrast to ketamine, N85 retains amplitude scaling, whereas P135 does not.
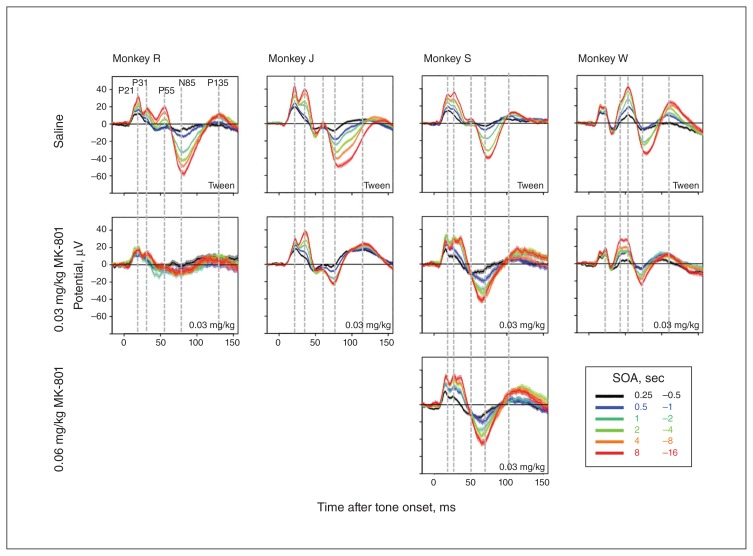
Experiment 2: effect of MK-801 on AEP dynamic range
Figure 5 shows AEPs in the MK-801 experiment. As for ketamine, we observed blunted responses for the longest SOAs. However, this attenuation seemed less pronounced and more restricted to the N85 component. This impression is further supported by comparing the first and second rows of panels in Figure 4. Anecdotally, it is interesting to note that in contrast to ketamine, MK-801 had noticeable motor and motivational adverse effects, even at the lowest dose (0.03 mg/kg), that were evident when animals returned to their home cages.
Fig. 5.
Effect of MK-801 on auditory-evoked potentials (AEPs) split by stimulus-onset asynchrony (SOA). Evoked potentials averaged across 6 frontocentral electrodes following the administration of MK-801 or vehicle.
Unexpectedly, we noticed that administration of 0.03 and 0.06 mg/kg of MK-801 led to the emergence of a new AEP component in animal S (Fig. 5). This component occurred approximately 10 ms after the P31 at frontocentral electrodes.
Experiment 3: effect of midazolam on AEP dynamic range
Figure 6 shows AEPs in the midazolam experiment. No obvious effects were observed at the lowest dose (< 0.04 mg/kg). At the high dose (0.08 mg/kg), we observed blunted responses for the longest SOAs. However, in contrast to ketamine, midazolam seemed to have less of an impact on the N85. This impression is further supported by comparing the first and third rows of panels in Figure 4. In contrast to ketamine and MK-801, midazolam seemed to exert a stronger effect on the P135 and a weaker effect on the N85.
Fig. 6.
Effect of midazolam on auditory-evoked potentials (AEPs) split by stimulus-onset asynchrony (SOA). Evoked potentials averaged across 6 frontocentral electrodes following the administration of midazolam or vehicle. Three different levels of dosing were used (0.02, 0.04 and 0.08 mg/kg), and the 2 lowest doses were averaged together.
Quantification and comparison of different active compounds
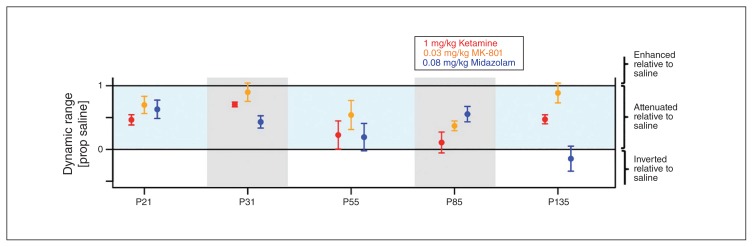
Figure 7 plots normalized dynamic ranges for each component and drug condition separately. A value of 1 corresponds to no change relative to the control condition. Figure 7 conveys the impression that ketamine and MK-801 have similar response profiles, but that compared with MK-801, ketamine is overall more effective at reducing the dynamic range across all 5 AEP components. In contrast, midazolam seems to have a different response profile. Furthermore, Figure 7 conveys the impression that certain components, such as the N85, are particularly susceptible to all pharmacological interventions.
Fig. 7.
Different response profiles of N-methyl-d-aspartate receptor (NMDAR) blockers and γ-aminobutyric acid A (GABAA) positive allosteric modulator (PAM) midazolam. Normalized dynamic range on the Y axis is plotted for all 5 components and all 3 psychoactive agents. A value of 1 indicates no change in dynamic range. Error bars indicate the standard errors of the mean. Ketamine (red) causes widespread attenuation, which is greatest in the later components (P55, N85) and more subtle in the earlier components (P21 and P31). MK-801 (orange) has a similar profile to ketamine, but overall causes less attenuation for all components. Midazolam (blue) exhibits a different profile of attenuation with relatively stronger attenuation for P31 and P135 and relatively weaker attenuation for N85.
To quantify the data described above, we conducted a 2-factorial repeated-measures analysis of variance (ANOVA) with the factors drug (1 mg/kg ketamine, 0.03 mg/kg MK-801, 0.08 mg/kg midazolam) and AEP component (P21, P31, P55, N85, P135). This ANOVA identified no main effect of drug (F2,6 = 3.51, Huynh-Feldt–corrected pHF = 0.10). It did identify a significant main effect of AEP component (F4,12 = 5.53, pHF = 0.019) and a significant drug × component interaction (F8,24 = 3.33, pHF = 0.036).
To determine which components contributed to the main effect of AEP component we performed 10 post hoc ANOVAs, 1 for each of the 10 possible pairs of components. Of the 10 tests, 1 reached significance (P31:P135) and 4 reached a significance level of p < 0.1 (P21:N85, P21:P135, P31:P55, P31:N85). This suggests that the P31 is overall less affected by the pharmacological interventions than the P135. Incorporating the pattern of statistical trends, the data tentatively suggest that the early components P21 and P31 are overall less attenuated by all pharmacological interventions than the later components P55, N85 and P135.
To determine which pairs of drugs contribute to the drug × component interaction, we performed 3 post hoc ANOVAs that included all 3 possible pairs of drugs (MK-801 v. midazolam, ketamine v. midazolam, MK-801 v. ketamine). The drug × component interaction term for the 3 tests were as follows: MK-801 v. midazolam, pHF = 0.028; ketamine v. midazolam, pHF = 0.08; ketamine v. MK-801, pHF = 0.56. These results suggest different response profiles for MK-801 than for midazolam. Although not significant, we observed a similar trend for ketamine compared with midazolam, but not for ketamine compared with MK-801. Taken together, the pattern of results of these post hoc analyses suggests different response profiles for the 2 NMDAR blockers than for the GABAA PAM.
Discussion
Reduced dynamic range of AEP amplitude modulation with SOA is an important noninvasive marker of auditory cortex dysfunction in individuals with schizophrenia that may reflect impaired encoding of auditory information into their auditory sensory memory. Our findings show that this electrophysiological phenotype can be mimicked in the nonhuman primate using 2 types of pharmacological interventions that alter E/I balance either by reducing NMDAR–mediated excitation or by increasing GABAA receptor–mediated inhibition. These findings have important implications for understanding the pharmacological basis of a putative biomarker of auditory sensory memory and its dysfunction in individuals with schizophrenia.
Postmortem schizophrenia studies point toward reduced excitatory function25–27 as well as reduced inhibitory function in people with the disease.28 In isolation, the 2 types of deficits are expected to have opposing functional consequences. In combination, they may cancel out or lead to either outcome, depending on which deficit has a stronger impact on overall circuit function. Our finding in the nonhuman primate that the dynamic range of the N1 is a sensitive marker of both glutamatergic and GABA-ergic neurotransmission establishes it as a putative functional marker of E/I balance. Our results allow us to reinterpret the finding of reduced N1 dynamic range in individuals with schizophrenia as a marker of altered E/I balance. In particular, our findings support the notion of a relative excitation-deficient/inhibition-dominant state of the auditory cortex in individuals with schizophrenia. This interpretation of the results is in line with a recent suggestion that cortical dysfunction in those with schizophrenia results from a pathological state in which reduced pyramidal cell excitation leads to a compensatory reduction of PV basket cell inhibition.44 Importantly, our data suggest that the compensatory reduction of inhibitory function might be incomplete, as evidenced by its failure to re-establish the original dynamic range of the N1.
Though all 3 compounds reduce the dynamic range of AEPs, it is noteworthy that NMDAR blockers had a different response profile than the GABAA PAM (Fig. 7). In the context of a simple conceptual model (Appendix 1, Fig. S8), we argue that the similarities between drugs can be explained under the umbrella of altered E/I balance, whereas the different response profiles of the drugs reveal interesting quantitative differences in the circuitry maintaining E/I balance of AEP components generated at different times after stimulus onset in different brain regions or cortical layers.
The model focuses on a putative pyramidal cell population whose postsynaptic potentials (PSPs) determine the amplitude of a hypothetical AEP component (Appendix 1, Fig. S1). At baseline (Appendix 1, Fig. S1B and S1C, black lines), both excitatory (EPSP; Appendix Fig. S1B and S1C, left column) and inhibitory (IPSP; Appendix 1, Fig. S1B and S1C, middle column) PSPs of the pyramidal cell population scale with SOA because both are mediated either directly (EPSP) or indirectly (IPSP, via the inhibitory interneurons) by depressing glutamatergic synapses, as suggested previously.24,45–47 Note that key aspects of our model described below do not depend on whether SOA dependence is indeed mediated by depressing glutamatergic synapses or a different upstream mechanism, such as prolonged after-hyperpolarization.48,49 In either case, the summed PSP (EPSP-IPSP, Appendix 1, Fig. S1B and S1C, right column) scales with SOA. For simplicity, we assume that the EPSPs are stronger overall, thus leading to a positive scaling of PSPs with SOA. A more realistic assumption might be that the balance between excitation and inhibition changes as a function of time from stimulus onset with excitation dominating early on, and inhibition later.
The NMDAR channel blockers reduce the scaling of EPSPs with SOA both in the interneurons and the pyramidal cells (Appendix 1, Fig. S1B, red lines). The reduction of EPSPs in the interneurons translates into a reduction of IPSPs to the pyramidal cells. We assume that both IPSPs and EPSPs to the pyramidal cells are attenuated by a similar fraction. Thus, the summed PSPs, and hence AEP amplitude, are also reduced by the same fraction. The GABAA PAMs cause a similar reduction of the dynamic range of AEP amplitude as a function of SOA. However, the phenotype is mediated by a different mechanism. PAMs have no effect on the glutamate receptors and thus leave the EPSPs of both cell populations unaffected. Instead, PAMs exert their effect by strengthening the IPSPs to the pyramidal cells (Appendix 1, Fig. S1C, blue lines). As we assume that the IPSPs are smaller than the EPSPs (see above), PAMs reduce the difference between EPSP and IPSP. This in turn reduces the dynamic range of AEP component amplitude with SOA.
Within the context of our model, we propose 4 mechanisms that could explain the different response profiles of the 2 classes of drugs. The dynamic range of a specific component might be more or less susceptible to NMDAR channel blockers for 3 reasons. First, the brain regions or cortical layers that mediate a component that is more susceptible to NMDAR channel blockers may have a higher ratio of NMDA to AMPA receptors. Second, components that are more susceptible to NMDAR channel blockers may be mediated by cells that have already been partially depolarized by earlier input that removed the voltage-dependent Mg2+ block from the NMDAR channel. Third, components that are more susceptible to NMDAR channel blockers may depend to a higher degree on recurrent activity, owing to slower inactivation of NMDA currents,50 they are deemed especially important to sustain activity in recurrent networks in the absence of external input.51–54 Furthermore, we propose a potential mechanism to explain why a specific component might be more susceptible to GABAA PAMs: components whose amplitude is controlled by strong inhibitory input should be more susceptible to PAMs. Taken together, these 4 mechanisms may explain the different response profiles of the 2 types of drugs.
Our results show that the P31 amplitude is rather resilient to ketamine and MK-801. The monkey P31 has been identified as a putative P1 homologue.17 Hence, our finding that the P31 is largely unaffected by ketamine and MK-801 is consistent with earlier findings that the human P1 is largely unaltered during anesthesia induction with ketamine.55–58 Our results extend these findings and show that the scaling of P31 amplitude with SOA is largely preserved, even for the highest dose of ketamine, which almost completely abolishes the N85 component (Fig. 3, third row). This finding is consistent with the hypothesis that the scaling of component amplitude with SOA is due to a presynaptic mechanism (e.g., vesicle depletion) rather than a postsynaptic mechanism that depends on specific properties of the NMDAR.
N-methyl-d-aspartate receptor function plays an important role in predictive coding (i.e., the ability to anticipate and optimize processing of upcoming sounds). Ketamine disrupts predictive coding regardless of whether sounds can be anticipated based on an individual’s own motor commands35 or based on past stimulus regularities.32 Our ketamine experiment was specifically designed to minimize stimulus regularity and thus predictability of upcoming sounds. Consequently, the paradigm evaluated the effect of ketamine independent of its role in predictive coding. Our finding that ketamine had effects on AEPs even in the absence of predictability led us to conclude that NMDAR function may affect AEP amplitude by 2 distinct mechanisms: a low-level mechanism in which NMDAR function can be simplified as an excitatory drive that complements AMPA-mediated excitation and a high-level mechanism in which unique properties of the NMDAR (i.e., voltage-dependent Mg2+ block, Ca2+ influx and its role that may lead to long-term synaptic plasticity) mediate crucial aspects of predictive coding. The finding that noncompetitive NMDAR antagonists can blunt the dependence of AEP amplitude on SOA in predictable17 as well as unpredictable environments suggests that in both cases the effect depends on the low-level excitatory function of NMDARs.
Limitations
One limitation of our study is the use of systemic pharmacological interventions that may have acted on targets outside the auditory cortex. Future experiments should use local microinjections to confirm that the auditory cortex mediates the effects of both NMDAR blockers and GABAA PAMs. Furthermore, it is important to note that ketamine has many ectopic targets and active metabolites, such as 2R,6R-hydroxynorketamine (HNK), that increase AMPA receptor–mediated EPSPs.59 Thus, it is possible that these non-NMDAR-mediated effects contributed to the observed phenotype. However, it is important to note that MK-801, a drug with higher affinity for the NMDAR as well as fewer ectopic targets and metabolites, yielded a response profile similar to that of ketamine and that 2R,6R-HNK in particular would be expected to increase AMPA receptor–mediated EPSPs, 59 thus countering the EPSP-attenuating effect of ketamine at the NMDAR. This would be hard to reconcile with the notion that ketamine overall was more effective than MK-801 at reducing dynamic range. These observations argue in favour of the notion that ketamine attenuates the dynamic range of AEP components via its action at the NMDAR.
Conclusion
Our study shows that the reduced dynamic range of P1 and N1 amplitudes in individuals with schizophrenia can be mimicked in the nonhuman primate using 2 distinct pharmacological interventions that either decrease NMDAR–mediated excitation or increase GABAA receptor–mediated inhibition. This model system will be valuable to further investigate effects of E/I balance on auditory cortex function at the circuit and single-cell levels. It may shed light on the specific information-processing deficits in early auditory cortex that might go along with E/I imbalance in indivduals with schizophrenia.
Acknowledgements
This work was supported by grants MH113041 to T. Teichert, and MH071533 to R. Sweet. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the National Institutes of Health, the Department of Veterans Affairs, or the United States Government.
Footnotes
Competing interests: None declared.
Contributors: W. Holliday, R. Sweet and T. Teichert designed the study. K. Gurnsey and T. Teichert acquired the data, which W. Holliday, R. Sweet and T. Teichert analyzed. W. Holliday and T. Teichert wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Leitman DI, Sehatpour P, Higgins BA, et al. Sensory deficits and distributed hierarchical dysfunction in schizophrenia. Am J Psychiatry. 2010;167:818–27. doi: 10.1176/appi.ajp.2010.09030338. [DOI] [PubMed] [Google Scholar]
- 2.Javitt DC, Sweet RA. Auditory dysfunction in schizophrenia: integrating clinical and basic features. Nat Rev Neurosci. 2015;16:535–50. doi: 10.1038/nrn4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Javitt DC, Strous RD, Grochowski S, et al. Impaired precision, but normal retention, of auditory sensory (“echoic”) memory information in schizophrenia. J Abnorm Psychol. 1997;106:315–24. doi: 10.1037//0021-843x.106.2.315. [DOI] [PubMed] [Google Scholar]
- 4.Rabinowicz EF, Silipo G, Goldman R, et al. Auditory sensory dysfunction in schizophrenia — Imprecision or distractibility? Arch Gen Psychiatry. 2000;57:1149–55. doi: 10.1001/archpsyc.57.12.1149. [DOI] [PubMed] [Google Scholar]
- 5.Strous RD, Cowan N, Ritter W, et al. Auditory sensory (“ echoic”) memory dysfunction in schizophrenia. Am J Psychiatry. 1995;152:1517–9. doi: 10.1176/ajp.152.10.1517. [DOI] [PubMed] [Google Scholar]
- 6.March L, Cienfuegos A, Goldbloom L, et al. Normal time course of auditory recognition in schizophrenia, despite impaired precision of the auditory sensory (“echoic”) memory code. J Abnorm Psychol. 1999;108:69–75. doi: 10.1037//0021-843x.108.1.69. [DOI] [PubMed] [Google Scholar]
- 7.Kubovy M, Howard FP. Persistence of a pitch-segregating echoic memory. J Exp Psychol Hum Percept Perform. 1976;2:531–7. doi: 10.1037//0096-1523.2.4.531. [DOI] [PubMed] [Google Scholar]
- 8.Neisser U. Cognitive Psychology. Hove (UK): Psychology Press; 2014. [Google Scholar]
- 9.Jääskeläinen IP, Ahveninen J, Belliveau JW, et al. Short-term plasticity in auditory cognition. Trends Neurosci. 2007;30:653–61. doi: 10.1016/j.tins.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Scott BH, Mishkin M, Yin P. Effect of acoustic similarity on short-term auditory memory in the monkey. Hear Res. 2013;298:36–48. doi: 10.1016/j.heares.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plakke B, Ng CW, Poremba A. Neural correlates of auditory recognition memory in primate lateral prefrontal cortex. Neuroscience. 2013;244:62–76. doi: 10.1016/j.neuroscience.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Hwang J, Romanski LM. Prefrontal neuronal responses during audiovisual mnemonic processing. J Neurosci. 2015;35:960–71. doi: 10.1523/JNEUROSCI.1328-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plakke B, Hwang J, Romanski LM. Inactivation of primate prefrontal cortex impairs auditory and audiovisual working memory. J Neurosci. 2015;35:9666–75. doi: 10.1523/JNEUROSCI.1218-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott BH, Mishkin M, Yin P. Neural correlates of auditory short-term memory in rostral superior temporal cortex. Curr Biol. 2014;24:2767–75. doi: 10.1016/j.cub.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu ZL, Williamson SJ, Kaufman L. Behavioral lifetime of human auditory sensory memory predicted by physiological measures. Science. 1992;258:1668–70. doi: 10.1126/science.1455246. [DOI] [PubMed] [Google Scholar]
- 16.Ulanovsky N, Las L, Nelken I. Processing of low-probability sounds by cortical neurons. Nat Neurosci. 2003;6:391–8. doi: 10.1038/nn1032. [DOI] [PubMed] [Google Scholar]
- 17.Javitt DC, Jayachandra M, Lindsley RW, et al. Schizophrenia-like deficits in auditory P1 and N1 refractoriness induced by the psychomimetic agent phencyclidine (PCP) Clin Neurophysiol. 2000;111:833–6. doi: 10.1016/s1388-2457(99)00313-2. [DOI] [PubMed] [Google Scholar]
- 18.Näätänen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 19.Shelley AM, Silipo G, Javitt DC. Diminished responsiveness of ERPs in schizophrenic subjects to changes in auditory stimulation parameters: implications for theories of cortical dysfunction. Schizophr Res. 1999;37:65–79. doi: 10.1016/s0920-9964(98)00138-8. [DOI] [PubMed] [Google Scholar]
- 20.Roth WT, Horvath TB, Pfefferbaum A, et al. Event-related potentials in schizophrenics. Electroencephalogr Clin Neurophysiol. 1980;48:127–39. doi: 10.1016/0013-4694(80)90299-0. [DOI] [PubMed] [Google Scholar]
- 21.Roth WT, Goodale J, Pfefferbaum A. Auditory event-related potentials and electrodermal activity in medicated and unmedicated schizophrenics. Biol Psychiatry. 1991;29:585–99. doi: 10.1016/0006-3223(91)90094-3. [DOI] [PubMed] [Google Scholar]
- 22.Erwin RJ, Shtasel D, Gur RE. Effects of medication history on midlatency auditory evoked responses in schizophrenia. Schizophr Res. 1994;11:251–8. doi: 10.1016/0920-9964(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 23.Erwin RJ, Mawhinney-Hee M, Gur RC, et al. Midlatency auditory evoked responses in schizophrenia. Biol Psychiatry. 1991;30:430–42. doi: 10.1016/0006-3223(91)90304-5. [DOI] [PubMed] [Google Scholar]
- 24.Teichert T, Gurnsey K, Salisbury DF, et al. Contextual processing in unpredictable auditory environments: the limited resource model of auditory refractoriness in the rhesus. J Neurophysiol. 2016;116:2125–39. doi: 10.1152/jn.00419.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sweet RA, Bergen SE, Sun Z, et al. Anatomical evidence of impaired feedforward auditory processing in schizophrenia. Biol Psychiatry. 2007;61:854–64. doi: 10.1016/j.biopsych.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 26.Sweet RA, Henteleff RA, Zhang W, et al. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34:374–89. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sweet RA, Bergen SE, Sun Z, et al. Pyramidal cell size reduction in schizophrenia: evidence for involvement of auditory feedforward circuits. Biol Psychiatry. 2004;55:1128–37. doi: 10.1016/j.biopsych.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Moyer CE, Delevich KM, Fish KN, et al. Reduced glutamate decarboxylase 65 protein within primary auditory cortex inhibitory boutons in schizophrenia. Biol Psychiatry. 2012;72:734–43. doi: 10.1016/j.biopsych.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boeijinga PH, Soufflet L, Santoro F, et al. Ketamine effects on CNS responses assessed with MEG/EEG in a passive auditory sensory-gating paradigm: an attempt for modelling some symptoms of psychosis in man. J Psychopharmacol. 2007;21:321–37. doi: 10.1177/0269881107077768. [DOI] [PubMed] [Google Scholar]
- 30.Arezzo J, Pickoff A, Vaughan HG., Jr The sources and intracerebral distribution of auditory evoked potentials in the alert rhesus monkey. Brain Res. 1975;90:57–73. doi: 10.1016/0006-8993(75)90682-4. [DOI] [PubMed] [Google Scholar]
- 31.Javitt DC, Steinschneider M, Schroeder CE, et al. Detection of stimulus deviance within primate primary auditory cortex: intracortical mechanisms of mismatch negativity (MMN) generation. Brain Res. 1994;667:192–200. doi: 10.1016/0006-8993(94)91496-6. [DOI] [PubMed] [Google Scholar]
- 32.Javitt DC, Steinschneider M, Schroeder CE, et al. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc Natl Acad Sci U S A. 1996;93:11962–7. doi: 10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lakatos P, Schroeder CE, Leitman DI, et al. Predictive suppression of cortical excitability and its deficit in schizophrenia. J Neurosci. 2013;33:11692–702. doi: 10.1523/JNEUROSCI.0010-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gil-da-Costa R, Stoner GR, Fung R, et al. Nonhuman primate model of schizophrenia using a noninvasive EEG method. Proc Natl Acad Sci U S A. 2013;110:15425–30. doi: 10.1073/pnas.1312264110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kort NS, Ford JM, Roach BJ, et al. Role of N-methyl-D-aspartate receptors in action-based predictive coding deficits in schizophrenia. Biol Psychiatry. 2017;81:514–24. doi: 10.1016/j.biopsych.2016.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teichert T. Tonal frequency affects amplitude but not topography of rhesus monkey cranial EEG components. Hear Res. 2016;336:29–43. doi: 10.1016/j.heares.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Wong EH, Kemp JA, Priestley T, et al. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 1986;83:7104–8. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nordt SP, Clark RF. Midazolam: a review of therapeutic uses and toxicity. J Emerg Med. 1997;15:357–65. doi: 10.1016/s0736-4679(97)00022-x. [DOI] [PubMed] [Google Scholar]
- 39.R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2009. [Google Scholar]
- 40.Lu ZL, Williamson SJ, Kaufman L. Human auditory primary and association cortex have differing lifetimes for activation traces. Brain Res. 1992;572:236–41. doi: 10.1016/0006-8993(92)90475-o. [DOI] [PubMed] [Google Scholar]
- 41.Briley PM, Krumbholz K. The specificity of stimulus-specific adaptation in human auditory cortex increases with repeated exposure to the adapting stimulus. J Neurophysiol. 2013;110:2679–88. doi: 10.1152/jn.01015.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Itoh K, Nejime M, Konoike N, et al. Noninvasive scalp recording of cortical auditory evoked potentials in the alert macaque monkey. Hear Res. 2015;327:117–25. doi: 10.1016/j.heares.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 43.Fishman YI. The mechanisms and meaning of the mismatch negativity. Brain Topogr Springer US. 2014;27:500–26. doi: 10.1007/s10548-013-0337-3. [DOI] [PubMed] [Google Scholar]
- 44.Lewis DA, Curley AA, Glausier JR, et al. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chung S, Li X, Nelson SB. Short-term depression at thalamocortical synapses contributes to rapid adaptation of cortical sensory responses in vivo. Neuron. 2002;34:437–46. doi: 10.1016/s0896-6273(02)00659-1. [DOI] [PubMed] [Google Scholar]
- 46.Denham SL. Computational models of auditory function computational models of auditory function. 2001. Cortical synaptic depression and auditory perception. [Google Scholar]
- 47.Wehr M, Zador AM. Synaptic mechanisms of forward suppression in rat auditory cortex. Neuron. 2005;47:437–45. doi: 10.1016/j.neuron.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 48.Sanchez-Vives MV, Nowak LG, McCormick DA. Cellular mechanisms of long-lasting adaptation in visual cortical neurons in vitro. J Neurosci. 2000;20:4286–99. doi: 10.1523/JNEUROSCI.20-11-04286.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanchez-Vives MV, Nowak LG, McCormick DA. Membrane mechanisms underlying contrast adaptation in cat area 17 in vivo. J Neurosci. 2000;20:4267–85. doi: 10.1523/JNEUROSCI.20-11-04267.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang XJ. Synaptic basis of cortical persistent activity: the importance of NMDA receptors to working memory. J Neurosci. 1999;19:9587–603. doi: 10.1523/JNEUROSCI.19-21-09587.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arnsten AFT, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–39. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lo C-C, Wang X-J. Cortico-basal ganglia circuit mechanism for a decision threshold in reaction time tasks. Nat Neurosci. 2006;9:956–63. doi: 10.1038/nn1722. [DOI] [PubMed] [Google Scholar]
- 53.Wong K-F, Wang X-J. A recurrent network mechanism of time integration in perceptual decisions. Soc Neurosci. 2006;26:1314–28. doi: 10.1523/JNEUROSCI.3733-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X-J. Probabilistic decision making by slow reverberation in cortical circuits. Neuron. 2002;36:955–68. doi: 10.1016/s0896-6273(02)01092-9. [DOI] [PubMed] [Google Scholar]
- 55.Detsch O, Kochs E. Effects of ketamine on central nervous system function. Anaesthesist. 1997;13:S20–9. doi: 10.1007/pl00002460. [DOI] [PubMed] [Google Scholar]
- 56.Schwender D, Klasing S, Madler C, et al. Mid-latency auditory evoked potentials during ketamine anaesthesia in humans. Br J Anaesth. 1993;71:629–32. doi: 10.1093/bja/71.5.629. [DOI] [PubMed] [Google Scholar]
- 57.Schwender D, Faber-Züllig E, Fett W, et al. Mid-latency auditory evoked potentials in humans during anesthesia with s (+) ketamine — a double-blind, randomized comparison with racemic ketamine. Anesth Analg. 1994;78:267. doi: 10.1213/00000539-199402000-00012. [DOI] [PubMed] [Google Scholar]
- 58.Schwender D, Klasing S, Keller I, et al. Sensorische Informations-verarbeitung während Allgemeinanaesthesie: Der effekt von propofol und ketamin auf die akustisch evozierten potentiale mittlerer latenz (AEPML) Anaesthesist. 1989;38:664–72. [PubMed] [Google Scholar]
- 59.Zanos P, Moaddel R, Morris PJ, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–6. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]