Abstract
Background
There is evidence suggesting neuropsychiatric disorders share genomic, cognitive and clinical features. Here, we ask if autism-spectrum disorders (ASD), attention-deficit/hyperactivity disorder (ADHD) and schizophrenia share neuroanatomical variations.
Methods
First, we used measures of cortical anatomy to estimate spatial overlap of neuroanatomical variation using univariate methods. Next, we developed a novel methodology to determine whether cortical deficits specifically target or are “enriched” within functional resting-state networks.
Results
We found cortical anomalies were preferentially enriched across functional networks rather than clustering spatially. Specifically, cortical thickness showed significant enrichment between patients with ASD and those with ADHD in the default mode network, between patients with ASD and those with schizophrenia in the frontoparietal and limbic networks, and between patients with ADHD and those with schizophrenia in the ventral attention network. Networks enriched in cortical thickness anomalies were also strongly represented in functional MRI results (Neurosynth; r = 0.64, p = 0.032).
Limitations
We did not account for variable symptom dimensions and severity in patient populations, and our cross-sectional design prevented longitudinal analyses of developmental trajectories.
Conclusion
These findings suggest that common deficits across neuropsychiatric disorders cannot simply be characterized as arising out of local changes in cortical grey matter, but rather as entities of both local and systemic alterations targeting brain networks.
Introduction
Neuropsychiatric disorders share common traits along a spectrum of genetic, neurobiological and clinical dimensions.1–3 Mounting evidence for shared risk factors and neurobiological phenotypes has called for improved biological characterization across disorders.4,5 There are shared genetic risk factors across psychiatric disorders, including autism-spectrum disorders (ASD), attention-deficit/hyperactivity disorder (ADHD) and schizophrenia.1,6–8 Well-known epidemiological risk factors, such as perinatal hypoxia, antenatal maternal and postnatal infections, and maternal smoking during pregnancy, have been described across ASD, ADHD, and schizophrenia.9–12 Neurobiologically, there are indications of shared phenotypic traits that cut across diagnoses, with converging evidence of brain connectivity abnormalities across disorders13–18 showing altered default, ventral attention, sensory and executive networks in individuals with ASD; the default mode, frontoparietal and ventral attention networks in those with ADHD; and deficits in all of the mentioned networks in those with schizophrenia. In addition, previous studies have noted shared structural abnormalities across psychiatric disorders, with the anterior cingulate cortex and insula being identified as regional hot spots of structural and functional changes across most psychiatric disorders and corresponding to the salience or ventral attention network.19–21 Collectively, these shared risk factors and neurobiological differences suggest the possibility of common etiologies and susceptibilities to similar environmental exposures between disorders.
The likelihood for shared mechanisms between disorders is also shown by common neurocognitive and clinical deficits across disorders. For example, individuals with ASD, ADHD and schizophrenia share similar deficits in social cognition, language abilities and cognitive function.22,23 Those with ASD and schizophrenia further share somatomotor deficits, while those with ASD and ADHD share impairments in executive function and emotion regulation.24 Finally, there are commonalities in symptom presentation. Patients with ASD and those who have negative symptoms of schizophrenia, for example, show similar patterns of social withdrawal and poor eye contact.25 Up to 75% of indivdiuals with ASD have shown ADHD symptoms, including attention and motor control problems26 and overall hyperactivity. Individuals with ADHD have higher scores than healthy controls on 3 core features of ASD: social impairment, communication impairment, and restricted and repetitive behaviours.27 Symptoms of ADHD and ASD can be observed in patients with schizophrenia at a level that exceeds clinical thresholds; 28,29 conversely a subset of patients with ASD have been reported to exhibit schizophrenia-like positive symptoms,30 although these findings remain controversial.31,32
Given the overlap in risk factors and clinical features, we sought to determine if there is also convergence of structural brain alterations across disorders. We further postulate that these alterations may take 2 different neural topologies. The first, more classical, form would show a convergence of neuroanatomical deficits localized to specific neuroanatomical subregions, indicating “mass-action” homologous deficits across disorders. Here, we use the term “mass action” referring to focal neuroanatomical differences that colocalize within patient groups compared with a control group, thereby resulting in group differences that can be identified using a parametric statistical test. The second representation is a more distributed topology, where subtle alterations may exist at a local level, yet these alterations may be systematically pervasive across specific neural pathways, resulting in preferential alterations (or enrichment) in specific functional networks of the brain. We use the term “distributed topology” to refer to a set of neuroanatomical group differences that may colocalize within a more broadly defined area, such as resting-state networks (RSNs), thereby possibly impacting brain function related to network function. These distributed differences will therefore not necessarily be detectable using standard parametric statistical tests and may require novel methods to elucidate group differences across a network. Thus, if common neuroanatomical deficits indeed exist, understanding their putative involvement within the context of known brain networks is critical to our understanding of these disorders and discovery of cross-disorder phenotypes. We further refine the question by setting criteria that should be required of true overarching phenotypes, including consistent alterations across disorders, replication across studies and convergent findings across neuroimaging modalities. Here, we attempt to fulfill these requirements by examining cortical topography of cortical thickness (CT) and surface area (SA) across patients with ASD, ADHD and schizophrenia and performing 3 different analyses as follows.
To determine if deficits across disorders could be localized to a subset of neuroanatomical structures, we examined neuroanatomical differences between cases and controls across all 3 disorders. We further explored neuroanatomical convergence using conjunction analysis examining shared deficits.
To determine if deficits across disorders specifically impacted functional brain networks, we investigated whether the neuroanatomical anomalies were enriched in canonically defined RSNs involved in cognitive control, affect regulation, and attention and motor control. To perform this analysis, we developed a statistical tool derived from a commonly used method in genomics — gene-set enrichment analysis33 (GSEA) — used to test for enrichment of gene lists with biological pathways. Here, we adapted this methodology for studying the enrichment of neuroanatomical deficits within functional networks; in the present work, we refer to this analysis as “network-set enrichment analysis” (NSEA).
Finally, to determine the validity of our findings, we investigated whether the neuroanatomical anomalies we found align with previous findings from functional neuroimaging studies. We performed this analysis using the Neurosynth database, thereby aiming to build structure–function homology between our structural findings and previous functional MRI (fMRI) results.
Based on previous work, we expected to find shared neuroanatomical differences in the anterior cingulate cortex and insula.19–21 In the NSEA analysis, we hypothesized that the default mode, ventral attention and limbic networks would show common alterations across disorders, based on the aforementioned anterior cingulate cortex and insula being involved in these networks as well as previous studies of individual disorders.13,15–18 Finally, we expected to find correspondence between the NSEA and Neurosynth findings in terms of the networks affected, but not with respect to local differences.
Methods
Data acquisition and processing
We processed T1-weighted MRIs from 32 sites (Appendix 1, Table S1 and S2, available at jpn.ca/170094-a1) and multiple online resources using the CIVET pipeline version 1.1.12 for CT and SA estimation. Individuals were generally free of major medical or neurologic disorders and psychiatric disorders other than the primary diagnosis (Appendix 1, Table S3). About 12.6% were discarded owing to significant movement artifacts, failed registrations and errors in cortical classification, resulting in final case–control sample sizes of 545 controls versus 486 patients with ASD, 334 controls versus 263 patients with ADHD, and 399 controls versus 376 patients with schizophrenia (n = 2403). Demographic and site-wise information is outlined in Appendix 1, Tables S1–S3, and age distributions are shown in Appendix 1, Figure S1.
Neuroimaging
The CIVET pipeline performs intensity nonuniformity correction, 34 transformation of all MRI data to Montreal Neurological Institute (MNI) space, classification of grey/white matter voxels,35 and a surface-based reconstruction of white matter and pial surfaces (about 80 000 vertices).36 Cortical thickness is estimated between homologous vertices on both surfaces using the t-link distance.37 Surface area is then estimated as an average of the 3 triangles in the surface mesh connected to each vertex. Reconstructions of the cortical surface were visually inspected by an expert rater (M.T.M.P.), and individuals whose scans failed quality control, including those with motion artifacts, were excluded from further analysis.
We examined 2 different measures of brain structure, namely CT and SA, as they are genetically independent38 and arise through neurobiologically distinct events, show separate developmental trajectories39 and are differentially affected by disorders,40 collectively suggesting they should be considered separately.41
Cross-disorder comparisons using meta-analysis
We used techniques similar to meta-analytic techniques used in recent studies by large consortia (i.e., ENIGMA). However, in this case the critical differences are that the image processing, quality control and summary statistics are not performed by multiple sites;42,43 instead they are all performed by an individual group to maintain homogeneity. Using these data, we performed the following analysis on the effect of diagnosis on CT and SA using multiple linear regression accounting for effects of age and sex as covariates. Sites were generally well-matched across controls and patients for age (Appendix 1, Fig. S1) and sex (Appendix 1, Table S1). For the purposes of this work we chose to examine only the linear associations with age for 2 reasons. The first was that the reasonable age range across sites that extends into adulthood and linear trajectories of cortical thickness have been well-described in adulthood, middle age and beyond.44–46 The second was because of newer work that showed neuroanatomical age-related trajectories, under the influence of proper quality control measures, trend toward being substantially more linear than originally thought.44 Statistical analysis was conducted per site to account for intersite differences in both image acquisitions and age differences across samples. Cohen d effect sizes of diagnosis, representing partial regression coefficients, were calculated per site and pooled in random-effects meta-analysis using the same methods used in previous work,47,48 allowing us to perform meta-analysis over every vertex (81 924 vertices). We conducted meta-analyses for each disorder individually, for pairs of disorders (ASD and ADHD, ASD and schizophrenia, and ADHD and schizophrenia), and across all 3 disorders. Analysis with multiple disorders was carried out by including sites containing the specified disorder groups within a larger meta-analysis. We used the false-discovery rate (FDR)49 to control for multiple comparisons in each hemisphere (q < 0.05).
Based on the results of the meta-analysis, we examined effect size distributions across the cortex for all analyses. We assessed spatial similarity between disorders by correlating −log(p) using Pearson r. We used −log(p) because the meta-analyzed Cohen d measures do not account for standard deviation of the effect sizes, whereas −log(p) provides a more intuitive measure of significance levels. This correlation also provides a measure of the similarity of the topography of our significant findings and shows whether brain regions are similarly affected across the cortex. We compared these correlations across the whole cortex and further within individual RSNs for both CT and SA. The RSNs include the visual, somatomotor, dorsal and ventral attention, limbic, frontoparietal and default mode networks, based on an existing parcellation derived by Yeo and colleagues50 (Appendix 1, Fig. S2). Within-RSN correlations will determine whether structural alterations arise within similar functional networks across disorders. This analysis further motivates our NSEA analysis, as it allows for the determination of whether RSNs are specifically targeted in individual disorders, across pairs of disorders, or across all disorders.
We conducted meta-regression analysis to examine the effect of site demographics, such as mean age, sex distribution and MRI field strength, on effect sizes of diagnosis (case v. control) within each disorder. Similar to the meta-analysis, this analysis was conducted per vertex and corrected for multiple comparisons using FDR. Findings surviving q < 0.05 were deemed significant. This analysis examines whether patient groups with larger age distributions (e.g., the schizophrenia group being older than the ASD and ADHD groups) have substantial effects on the results. By accounting for site-wise demographics, such as mean age and sex distribution, we explored whether differences in brain maturation or sex differences may further drive similarities or differences in effects across disorders.
Cross-disorder conjunction analysis
We conducted conjunction analysis to examine spatial overlap between pairs of disorders and across all 3 disorders by thresholding surface maps at the top 20%, 15%, 10% and 5% of vertices based on ranked p values. We used this analysis to determine if there was a shared topographical patterning across disorders. We did not use absolute p value thresholds, as applying a single threshold to multiple analyses (i.e., from ASD, ADHD and schizophrenia analyses) would result in a differing number of vertices that pass the threshold. Thresholding individual analyses by rank order does not eliminate the need for p value thresholds and multiple comparisons, but simply allows for a method of comparing topographical overlap across disorders despite power differences due to sample size. We estimated the significance of the 3-way overlap using permutation testing with 10 000 iterations, using resampling without replacement.
Automated meta-analysis of fMRI studies and cross-modal convergence
We examined whether the neuroanatomical results derived through the above analyses aligned with functional findings. We used the Neurosynth database (http://neurosynth.org/), as it enables large-scale, automated meta-analysis of previously published fMRI studies.51 We searched the Neurosynth database in March 2016, using the terms “autism,” “schizophrenia” and “ADHD.” We further confined studies, excluding participants at genetic risk (e.g., unaffected siblings), and included results where only functional MRI data were included. This resulted in 148 studies of ASD, 73 studies of ADHD and 249 studies of schizophrenia (supplementary data set). The automated meta-analysis in Neurosynth identifies voxels significant after multiple testing correction using FDR at q = 0.01, presented as a brain map of z scores indicating likelihood of differential activation. These maps were dilated 3-fold using a median dilation approach (as implemented in mincmorph: https://github.com/andrewjanke/mincmorph) to allow reasonable anatomic overlap across disorders without infringing across anatomic boundaries (Appendix 1, Fig. S3). Using these maps, we took the following steps to assess cross-modal convergence. First, dilated maps were binarized and combined to estimate shared functional changes. Second, we estimated the overlap between anatomic differences with these functional maps. We used the hypergeometric distribution to assess significance of overlap.52 This provides a quantitative summary of the degree to which shared anatomic change aligns with shared functional change. Third, binarized maps were projected onto known functional networks based on previous parcellations to facilitate interpretation. We estimated the degree to which each disorder showed atypical activity within each network after adjusting for network size to determine associations between our structural findings and meta-analysis–derived functional findings. These were compared with findings from our NSEA analysis.
Enrichment in the variation of cortical anatomy within RSNs
The previous analyses that were undertaken were to determine whether cortical differences across disorders are the product concordant “mass-action” type of differences that co-localize neuroanatomically. However, normative and abnormal neural development has often been thought to mirror the development of functional RSNs.53,54 Given that our disorders of interest are often thought to be the product of compromised neurodevelopmental processes, we developed a technique that would examine network-level enrichment of neuroanatomical deficits assuming deficits would be pervasive within a functional network, but would not necessarily follow the “mass-action” type of deficit.
In order to achieve this, we adapted statistical methods from GSEA. In its original implementation, GSEA tests whether sets of individual genes are collectively enriched in biological pathways. Its basis rests on the assumption that true biological effects may be only modest in single genes and therefore may not remain significant after correction for multiple testing.33 Such analyses of single genes do not take into account systematic, network-wide alterations in biological pathways, which GSEA aims to address. In our implementation of this work, we specifically ask if the anatomic differences seen in individuals with ASD, ADHD and schizophrenia were more likely to over-represent systematic perturbations within distinct RSNs previously identified50 (Appendix 1, Fig. S2).
Network enrichment analysis methods used in this study are directly analogous to those used in GSEA, adopting more recent innovations of GSEA used for interpretation of genome-wide association studies.55,56 The following is an outline of the analytic approach for NSEA (Fig. 1).
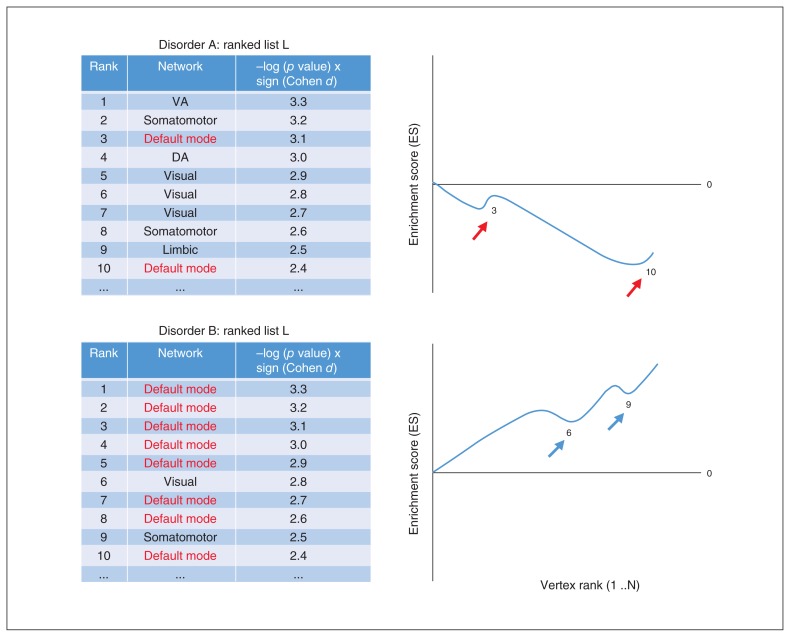
Fig. 1.
Graphical representation of network-set enrichment analysis (NSEA) using 2 hypothetical disorders and corresponding arrays. In both disorders, vertices are ordered using the ranking metric described: −log(p value) × sign(Cohen d), derived from case–control meta-analysis statistics within each disorder. Examining the enrichment score (ES) curves of the default mode network across Disorders A and B: A shows an initially decreasing ES due to default mode network vertices lacking enrichment (clustering) near the top of the ranked list, whereas B shows increasing ES due to highly enriched arrangement in ranked list L near the top.
Similar to GSEA, a ranking metric was devised for ordering vertices based on strength of association to phenotype — in this case, the association between diagnosis and cortical integrity (CT and SA). The −log10(p) was multiplied by the sign of the effect size (Cohen d), and 81 924 vertices across both hemispheres were then sorted in decreasing order to yield list L, where the most positive effects are located at the top and negative effects at the bottom of L (Fig. 1).
The enrichment score (ES) per network is computed by a walk down L. During the walk, for every network running sums are increased if the vertex encountered belongs to the network (Fig. 1, red arrows) and decreased for every other network encountered (Fig. 1, blue arrows). The increment is equal to the absolute value of the ranking metric at the vertex. The decrement is a constant that ensures the running sum at the end of the walk is zero. The ES is then the maximum deviation from zero occurring during the walk, computed individually per network.
Positive enrichment indicates systematic cortical thickening, where vertices are clustered near the top of L. The ES initially increases during the walk, followed by a gradual decrease until the end of the walk.
Negative enrichment indicates systematic cortical thinning, where vertices are clustered near the bottom of L. The ES initially decreases past zero during the walk, and once it reaches the cluster of vertices near the bottom of L, the ES would then increase sharply (assuming there is enrichment of negative effects in the given network) until it reaches the end of the walk.
The significance of the ES is evaluated by permutation testing, where the ranking metric is randomly shuffled and the ES is computed repeatedly across 10 000 permutations. This produces null distributions of ES per network, and nominal p values are calculated based on the following formula:
Here, m refers to the number of values along the null distribution that are greater or less than the actual ES (depending on its direction), and n indicates the number of permutations.57 Normalized ES (NES) is calculated based on the ratio of ES to the mean ES across all permutations.
Multiple testing correction was then applied using FDR across all NSEA analyses, including 7 networks × 2 measures (CT and SA) × 7 analyses (individual, paired, all combined), resulting in 98 tests, where q < 0.05 was deemed significant after correction.
The goal of this analysis was to move away from traditional vertex-based methods in order to better investigate large-scale network-wide neuroanatomical alterations, to better situate structural findings within previous studies examining functional connectivity within these disorders using fMRI, and to obtain an improved understanding of structure–function homologies.
In post hoc analysis, we examined characteristics of networks with high and low NES with differing levels of significance. We additionally examined whether NES, ES or p values were associated with network size. Finally, we examined whether different dilations of the Yeo 7-network parcellation produced inconsistent findings in NSEA by repeating the analysis in the nondilated parcellation and across 1–3 dilations separately (Appendix 1, Fig. S2).
Results
We focussed on cross-disorder comparisons of neuroanatomical alterations and combined effects of all 3 disorders (Fig. 1), and we further examined if structural findings from univariate meta-analyses and NSEA showed cross-modal overlap with Neurosynth-derived maps. Findings within single ( Appendix 1, Fig. S4) and paired-disorder (Appendix 1, Fig. S5) analyses largely replicate findings from previous work. Compared with controls, patients with ASD showed increased CT, those with ADHD showed a thinner cortex, and those with schizophrenia showed marked cortical thinning (Fig. 2A and Appendix 1, Single-disorder effect of diagnosis).
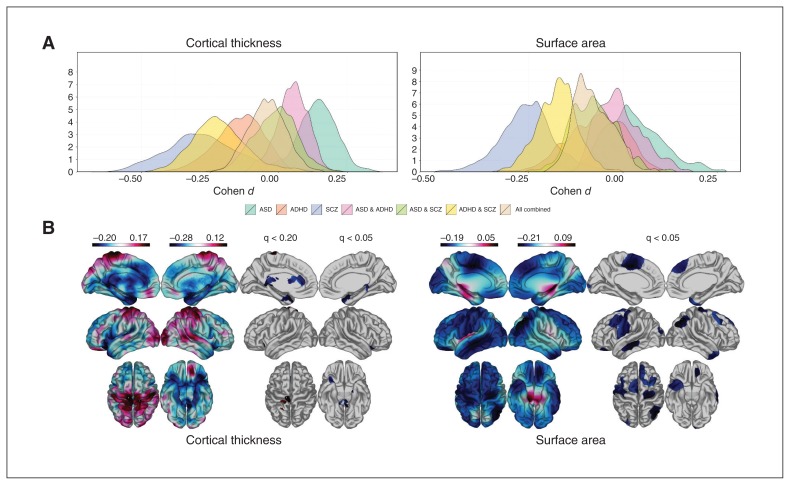
Fig. 2.
Cross-disorder comparisons and combined meta-analysis. (A) Distribution of Cohen d effects across single- and combined-disorder analyses. (B) Meta-analysis of combined-disorder effects (all 3) relative to healthy controls. Colour bars indicate the direction of effect (Cohen d), with warmer colours (red) indicating increased cortical thickness/surface area (CT/SA) and cooler colours (blue) indicating decreased CT/SA compared with controls. Significance levels after false-discovery rate (FDR) correction (or lack thereof) are noted in the second panels. ADHD = attention-deficit/hyperactivity disorder; ASD = autism-spectrum disorder; SCZ = schizophrenia.
Cross-disorder analyses
We then compared between-disorder similarities using correlations between −log(p) values from single-disorder meta-analyses. Global correlations for CT and SA indicated that patients with ADHD and those with schizophrenia showed the greatest similarity in terms of brain regions affected ( Appendix 1, Fig. S6). Patients with ASD had negative correlations for CT and SA with both schizophrenia and ADHD, indicating that ASD affects different brain regions than ADHD and schizophrenia and in opposite directions (thickening in patients with ASD v. thinning in those with ADHD and schizophrenia).
Combining all 3 disorders in a single case–control meta-analysis to determine possible convergence in cortical alterations yielded sine regions that survived FDR correction (Fig. 2B). For CT, we found nominally significant decreases in the left (q < 0.20) and right (q < 0.05) hemispheres, respectively, in the anterior and isthmus cingulate, entorhinal and parahippocampal gyri, and increased CT in the left pre- and postcentral gyri (Fig. 2B). We found significantly decreased SA (q < 0.05) in the left paracentral, precentral, postcentral, medial temporal and lateral occipital cortices and in the right superior frontal, angular and middle frontal cortices and temporal poles (Fig. 2B). Overall, there was more convergence between disorders for SA than CT. However, forest plots of site-wise effect size distributions show inconsistencies across diagnoses that suggest opposite disorder-specific effect size directions and may be biased, particularly in the left precentral and right parahippocampal regions for CT and in the right angular and superior frontal regions for SA (Appendix 1, Fig. S7).
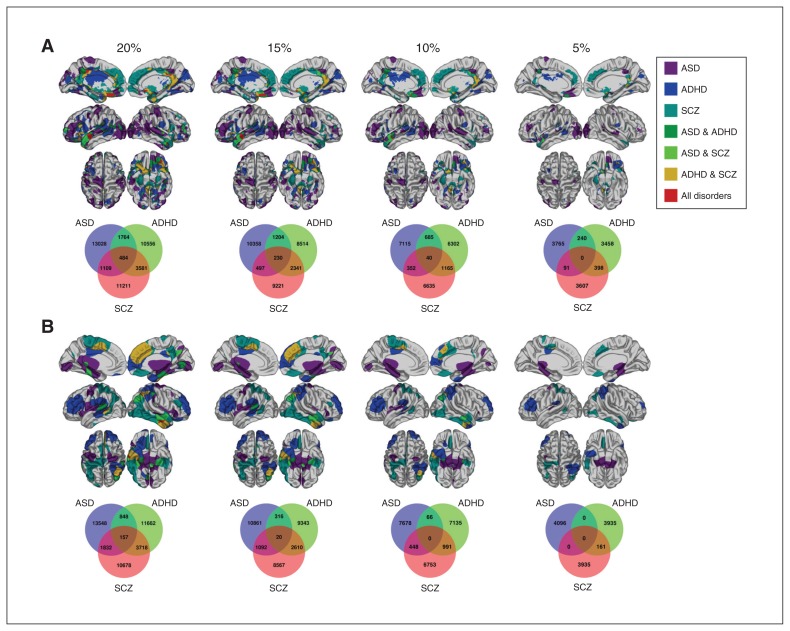
We examined overlap of significant vertices between disorders via conjunction analysis to better understand the possibility of overlapping cortical topographies across patients. In general, pairs of disorders and all 3 disorders show minimal overlap between vertices, even with liberal p value thresholds (Fig. 3). For CT, we found that at a generous threshold of top 20% significant p values (regardless of effect direction), there was minor overlap among all disorders in the left orbitofrontal, bilateral medial frontal, posterior cingulate and superior temporal gyri (Fig. 3A). Much of this overlap persisted until the 10% threshold, with the only remaining overlap in the left superior temporal gyrus. For SA, there was minor overlap among all disorders in the right angular and inferior temporal gyri, which persisted until the 15% threshold (Fig. 3B). Between pairs of disorders, the overlap between ADHD and schizophrenia was the greatest for both CT and SA. The 3-way overlap for CT and SA at all thresholds was not significant after permutation testing with 10 000 iterations (all p > 0.05).
Fig. 3.
Conjunction analysis examining overlap between disorders by thresholding p values to the top 20%, 15%, 10% and 5% significant vertices within each disorder for (A) cortical thickness, with p value thresholds as follows: p = 0.004 at 20%, p = 0.002 at 15%, and p < 0.001 at both the 10% and 5% thresholds for patients with autism-spectrum disorders (ASD); p = 0.07 at 20%, p = 0.06 at 15%, p = 0.040 at 10% and p = 0.023 at the 5% threshold for patients with attention-deficit/hyperactivity disorder (ADHD); and p < 0.001 at all thresholds for patients with schizophrenia (SCZ), and (B) surface area, with p value thresholds as follows: ASD p = 0.20 at 20%, p = 0.14 at 15%, p = 0.09 at 10% and p = 0.032 at the 5% threshold for patients with ASD; p = 0.12 at 20%, p = 0.09 at 15%, p = 0.05 at 10% and p = 0.028 at the 5% threshold for patients with ADHD; and p < 0.001 at all thresholds for patients with schizophrenia.
Moderator analysis showed no significant effect of mean age, sex distribution, or field strength on diagnostic effect sizes after correction for multiple comparisons (up to q < 0.20; Appendix 1, Figs. S8–10). A few regions were significant at an uncorrected level of p < 0.05, but only within a few scattered regions, suggesting a random distribution that is not significantly affected by demographics (Appendix 1, Figs. S8–10).
Finally, examining overlap between structural findings and functional findings from Neurosynth automated meta-analysis showed minimal cross-modal convergence between structural and functional neuroimaging (Appendix 1, Searching for local convergence between imaging modalities and Fig. S11).
Characterizing network-wide effects of disorders
We applied NSEA to structural differences to assess network enrichment within and across disorders. With regards to CT, patients with ASD showed significant (q < 0.05) positive enrichment (i.e., greater CT) across the default, frontoparietal, limbic and somatomotor networks (Fig. 4A). In contrast to patients with ASD, those with ADHD presented with significant negative enrichment (decreased CT) across the default and ventral attention networks (Fig. 4A). Patients with schizophrenia presented with significant negative enrichment in the dorsal attention, frontoparietal, limbic and ventral attention networks (Fig. 4A).
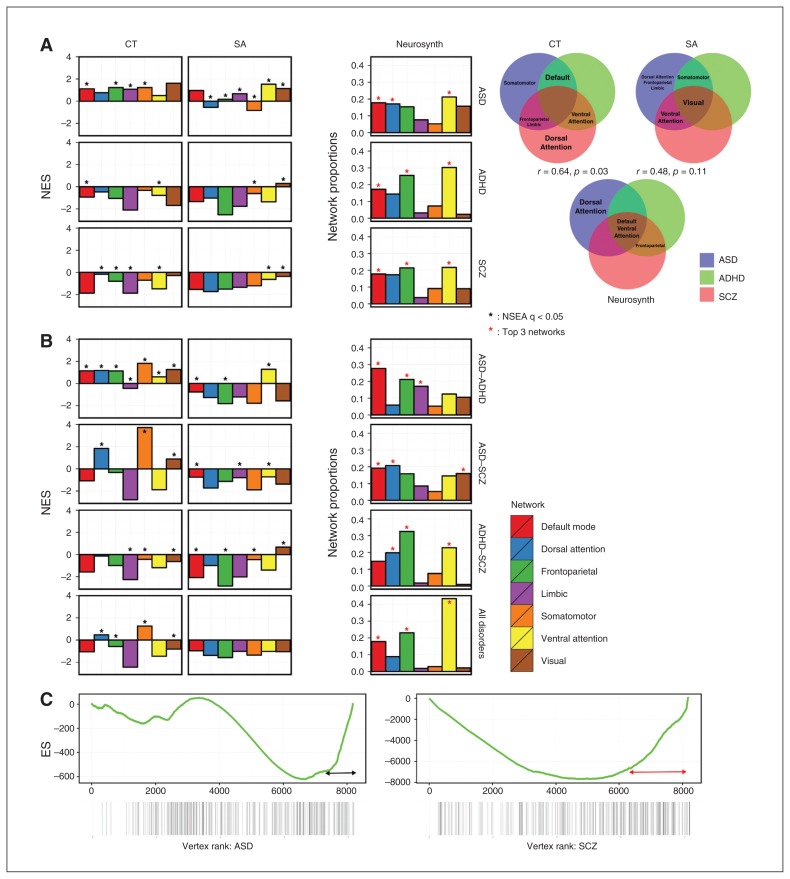
Fig. 4.
Assessing cross-modal homology comparing structural MRI to Neurosynth functional MRI (fMRI) findings at the network level. (A) Network-set enrichment analysis (NSEA) applied to single-disorder analyses for cortical thickness (CT) and surface area (SA), with Neurosynth comparisons. The Y axis indicates the normalized enrichment score (NES) for all meta-analysis results. Venn diagrams show networks that were significantly enriched across disorders. Correlations of Venn diagrams between CT and Neurosynth was significant (r = 0.64, p = 0.032), whereas the correlation between SA and Neurosynth was not significant (r = −0.48, p = 0.11). (B) The NSEA applied to combined disorder analyses and was compared with Neurosynth results. (C) Examining the dorsal attention SA network ES curves between patients with autism-spectrum disorder (ASD) and schizophrenia (SCZ). The Y axis indicates ES, and the X axis indicates ranked vertices from both hemispheres.
Across disorders, patients with ASD and those with ADHD presented with significant enrichment in the default mode network (ASD: NES = 1.12; ADHD: NES = −0.94), but those with ASD showed positive and those with ADHD showed negative enrichment (Fig. 4A). Thus, although the direction of local change differed (increased CT in patients with ASD v. decreased CT in those with ADHD; Fig. 2A), both showed convergence at the network level. Patients with ASD and those with schizophrenia both showed significant enrichment in the frontoparietal (ASD: NES = 1.22; schizophrenia: NES = −0.80) and limbic (ASD: NES = 1.07; schizophrenia: NES = −1.87) networks, but those with schizophrenia showed negative and those with ASD showed positive enrichment (Fig. 4A). Patients with ADHD and those with schizophrenia both showed significant negative enrichment for the ventral attention network (ADHD: NES = −0.78; schizophrenia: NES = −1.48). Common network-wide alterations across disorders are further indicated in Venn diagram format (Fig. 4A); no single network was commonly enriched for effects across all 3 disorders for CT.
In examining SA enrichment, we found greater convergence across disorders than in CT. In patients with ASD, significant positive enrichment was seen for all networks except the default mode network (Fig. 4A), suggesting network-specific patterns of cortical overgrowth. Patients with ADHD presented network-specific directions of enrichment; specifically, they showed significant negative enrichment in the somatomotor network (NES = −0.61) and positive enrichment in the visual network (NES = 0.30). Conversely, in comparison to patients with ASD and ADHD, those with schizophrenia presented with network-specific negative enrichment for the ventral attention (NES = −0.64) and visual networks (NES = −0.36). Patients with ASD and those with ADHD showed significant negative enrichment in the somatomotor network; those with schizophrenia also showed negative enrichment, but this finding was not significant after permutation testing. Interestingly, although SA in the visual network was significantly enriched across all 3 disorders (Fig. 4), the within-network correlations across disorders were positive only for the ASD–ADHD pairing and negative for the ASD–schizophrenia and the ADHD–schizophrenia pairings (Fig. 4B). The visual network, as defined in the present study, is broad and covers almost the entire occipital lobe (Appendix 1, Fig. S2) and divides into smaller networks in the 17-network cluster definition.50 This indicates that although all 3 disorders do not affect a specific region within the visual network, different subcomponents of the extended visual network are affected in patients with schizophrenia compared with those with ASD and ADHD.
Searching for network-based convergence between imaging modalities
We compared the single-disorder NSEA to Neurosynth results. By denoting the top 3 network proportions within each analysis (Fig. 4A), we estimated highly represented networks per disorder and built a Venn diagram to show overlap. Overall, networks found to be significantly enriched in NSEA were also highly represented in Neurosynth (Fig. 4A). Networks with shared enrichment between disorders for CT and SA, such as the default mode, ventral attention and frontoparietal networks, were also highly represented in the Neurosynth Venn diagram (Fig. 4A). We tested for a possible correlation between Venn diagrams by assigning a numerical score to each network (0 = not significantly enriched in any disorder, 1 = significantly enriched in 1 disorder, 2 = significantly enriched in 2 disorders, and 3 = significantly enriched across all disorders). We calculated Pearson correlations and estimated p values using permutation testing with 1000 permutations for CT and SA to void biases or a “lever effect.” The correlation between CT and Neurosynth was significant (r = 0.64, p = 0.032), whereas the correlation between SA and Neurosynth was not (r = −0.48, p = 0.11). This indicates that networks that are commonly affected across disorders in structural MRI (CT) are also likely to be affected in fMRI (Neurosynth), more so than SA. The NSEA applied to the paired and combined disorder analyses showed less similar patterns of network enrichment than the Neurosynth results (Fig. 4B).
Post hoc analyses examining NSEA behaviour, specifically that of the enrichment curve (Fig. 4C), ruled out effects of network size or dilations on NSEA significance (Appendix 1, post hoc analyses).
Discussion
Our understanding, or at least the approach to analysis, of altered neuroanatomy in psychiatric disorders has traditionally focused on finding reductions of grey matter in distinct regions of the brain. However, we showed that by studying multiple psychiatric disorders simultaneously, patterns of altered brain structure may extend beyond simple reductions or increases of MRI-derived metrics of cortical topology. Instead, we found that deficits across disorders may arise through coordinated, widespread alterations within functional RSNs responsible for dynamic cortical processing of complex human traits.
In our first assessment of local alterations in CT and SA across ASD, ADHD and schizophrenia, we replicated previously reported patterns in individual disorders as well as potentially noteworthy regions of convergence when analyzing disorders in combination. The right entorhinal, and parrahippocampal gyri showed significantly (q < 0.05) reduced CT in the combined analysis (Fig. 2B). For example, previous work links the anterior cingulate to symptom severity across ASD,58,59 ADHD60,61 and schizophrenia;62,63 with results spanning multiple imaging studies, many identify differences in structure, connectivity, activation and metabolism associated with changes in executive functioning across disorders — all indicating that common disruptions may support common deficits across the neurocognitive–motor spectrum. Although some of our results were significant in the combined univariate analysis of all 3 disorders, there still remain 3 important caveats: divergent effect size directions (positive in ASD and negative in ADHD and schizophrenia; Fig. 2 and Appendix 1, Fig. S7), low or negative correlations between disorders ( Appendix 1, Fig. S6) and lack of overlap between disorders (i.e., these disorders affect different brain regions; Fig. 3), and lack of overlap between structural and functional MRI findings (Appendix 1, Fig. S11).
Therefore, we observed limited local anatomic convergence across disorders. This indicates that local measures of CT and SA may not entirely explain deficit overlap between disorders, initially suggesting local measures of CT and SA may not be suitable for cross-disorder analysis of neuroanatomical phenotypes. Another possibility is that a larger sample may be needed to further parse effects at the local level. Furthermore, earlier work showed that local disruptions of neuroanatomy do not necessarily point to dysfunction. Courchesne and colleagues64 found evidence of 2 subgroups in ASD, 1 showing increased cerebellar volume and 1 showing decreases. There exists significant heterogeneity among studies examining brain structure in patients with ASD and other disorders, leading to some conclusions regarding the limited usefulness of MRI in studying ASD.65 As it is impossible to determine the histological and functional underpinnings of altered brain volumes, the characterization and examination of neuropsychiatric disorders as simply local alterations may require improvement. The same concept applies to other neuroimaging modalities as well — for example, with fMRI it is difficult to disentangle the complex circuitry and organization of brain networks.66 It is even more challenging to determine whether directions of changes in activation patterns, connectivity and variable fluctuations between excitation and inhibition truly cause deficits in human brain function.66
Findings at the level of our univariate analyses imply that dimensions of neurocognitive deficits across disorders may not occur simply due to local increases or decreases in grey matter. The wide distribution of effect sizes across disorders (Fig. 2A) and the heterogeneous and low correlations observed between disorders (Appendix 1, Fig. S6) suggest this to be the case. This shows that dimensional analysis of neurocognitive deficits may require novel methods for parsing heterogeneity that accounts for functional networks in addition to local alterations. The findings suggest that systematic, structural alterations to RSNs may underlie findings seen in fMRI studies. In particular, we highlight 2 key points. First, structural anomalies in patients with these disorders arise through clustered, nonrandom and diffuse effects within RSNs. For example, CT in the default mode network was significantly enriched in patients with ASD and ADHD (clustered within the network), but not in those with schizophrenia, whereas the magnitude (effect size) was greatest in patients with schizophrenia, but nonspecifically for the network. Second, networks may show positive or negative enrichment of effects. The CT in the default mode network was significantly enriched in patients with ASD and ADHD, but those with ASD showed positive enrichment whereas those with ADHD showed negative enrichment. This suggests that the underlying pathological processes in respective disorders may result in divergent CT changes observed on MRI, whereas the overlapping neurocognitive deficits observed may arise from common RSN enrichment.
Numerous studies have shown altered RSN connectivity in patients with ASD,13,14 ADHD15,16 and schizophrenia.17,18 Similarly, we found evidence of cross-modal convergence to Neurosynth findings, with significant correlation between the networks enriched for effects in CT and those highly represented in Neurosynth and with differences detected in fMRI seemingly reflecting both alterations in CT (Fig. 4 and Appendix 1, Fig. S11). Neurosynth meta-analysis also showed preferential involvement of the default mode, ventral attention and frontoparietal networks, paralleling our NSEA results. Literature supports these findings, with specific evidence of altered default mode network connectivity across ASD,67 ADHD68 and schizophrenia69 as well as similar findings for the frontoparietal network.70,71 Given recent evidence showing genetic contributions to RSN connectivity,72 there may be overlap in etiology between brain network development and psychiatric disorders. Similar RSNs seem to be affected across disorders, manifesting from changes in both brain structure and function. We speculate this phenotype could be used as a predominant marker of neurocognitive deficits and psychiatric symptoms across disorders. Thus, disentangling the complex etiologies and pathways of how these effects unfold across neurodevelopment and aging is critical.
Limitations
Limitations of this work include the potential for lack of power, as we found minimal local alterations in CT and SA. Additional limitations include the exclusion of information regarding medication status and symptom severity measures from our analysis. This illustrates the drawback of publicly available databases — although we were able to conduct a large-scale study using data from multiple sources, there was a lack of consistency in clinical and demographic information provided across sites. Although we used the meta-analytic framework to account for cross-site differences in imaging protocol, equipment and scan parameters, there could still be residual heterogeneity unaccounted for that may contribute to or mark findings. To the best of our knowledge, most sites excluded patients with neurologic or psychiatric disorders other than their primary diagnosis (Appendix 1, Table S3); however, there are still some limitations owing to differing exclusion criteria across sites, which contribute to intersite heterogeneity. This is a potential limitation as we cannot be absolutely sure of potential secondary or comorbid diagnoses. Furthermore, lack of longitudinal data prevented analysis of normative developmental and altered spatiotemporal trajectories.73 A potential limitation of the Neurosynth analysis is the inability to compare the mean age, sex distribution and diagnostic markers of studies included in the Neurosynth meta-analysis to the sites included in our study. This is because there is no feasible way to extract participant information from the Neurosynth studies, as the Neurosynth analyses draw upon more than 300 studies, each with distinct samples. Although it is difficult to test whether the Neurosynth participant data match our anatomic data, the sheer sample size would most likely capture age ranges and sex distributions covered in the Neurosynth data, which comprise data from multiple previous studies. As such, we deem the anatomic data studied here to be more representative of the underlying population variability. Another limitation of the Neurosynth analysis is the difference between volume-based denotations in Neurosynth versus the surface-based estimations in our data. We aimed to resolve this limitation by expanding the regions in Neurosynth, while cortical gyrification may impede accuracy of mapping between volume- and surface-based regions of interest. Another limiting factor of the study is the issue of within-disorder heterogeneity, as transdiagnostic brain anomalies may have been better detected if within-disorder heterogeneity was resolved first. Transdiagnostic effects might have been better detected if within-disorder heterogeneity could be resolved first. However, limited phenotypic characterization of the data preclude an acceptable heterogeneity or subtyping analysis. We consider this a limitation as well as an avenue for future investigation.
Conclusion
We found cross-modal evidence of cortical convergence and divergence, ranging from divergent local measures of grey matter and poor correlations between disorder-specific effects, toward convergent network-wide alterations across ASD, ADHD and schizophrenia. The heterogeneity of our findings call for revised systemic approaches in studying these disorders. Our conclusions indicate that structural neuroimaging in ASD, ADHD and schizophrenia should move toward investigation of multivariate patterns and establishing multimodal homology using novel statistical methods. Global disruptions in brain structure, rather than strictly localized changes, must be taken into account for greater reproducibility and insight into the association between altered structure and neurocognitive deficits in psychiatric disorders. This is an important consideration for future studies for research methodology and interpretation of results, particularly in our search for biomarkers of dimensional deficits across psychiatric disorders.
Acknowledgements
The authors thank all groups, as noted in Appendix 1, Table S2, for the generous contribution of their data and indirectly supporting this work. M. Chakravarty is funded by the Canadian Institutes of Health Research, National Sciences and Engineering Research Council of Canada, Brain Canada, Alzheimer’s Society, Michael J. Fox Foundation for Parkinson’s Research, and the Weston Brain Institute. M. Chakravarty also received salary and research support from the Fonds du Recherches Santé Québec.
Footnotes
Competing interests: None declared.
Contributors: M. Park and M. Ckravarty designed the study and acquired the data, which all authors analyzed. All authors wrote and reviewed the article, approved the final version to be published, and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Cross-Disorder Group of the Psychiatric Genomics Consortium. Lee SH, Ripke S, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45:984–94. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rapoport J, Chavez A, Greenstein D, et al. Autism spectrum disorders and childhood-onset schizophrenia: clinical and biological contributions to a relation revisited. J Am Acad Child Adolesc Psychiatry. 2009;48:10–8. doi: 10.1097/CHI.0b013e31818b1c63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barneveld PS, Pieterse J, de Sonneville L, et al. Overlap of autistic and schizotypal traits in adolescents with autism spectrum disorders. Schizophr Res. 2011;126:231–6. doi: 10.1016/j.schres.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 5.Casey BJ, Oliveri ME, Insel T. A neurodevelopmental perspective on the research domain criteria (RDoC) framework. Biol Psychiatry. 2014;76:350–3. doi: 10.1016/j.biopsych.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Hamshere ML, Stergiakouli E, Langley K, et al. Shared polygenic contribution between childhood attention-deficit hyperactivity disorder and adult schizophrenia. Br J Psychiatry. 2013;203:107–11. doi: 10.1192/bjp.bp.112.117432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guilmatre A, Dubourg C, Mosca AL, et al. Recurrent rearrangements in synaptic and neurodevelopmental genes and shared biologic pathways in schizophrenia, autism, and mental retardation. Arch Gen Psychiatry. 2009;66:947–56. doi: 10.1001/archgenpsychiatry.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–9. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva D, Colvin L, Hagemann E, et al. Environmental risk factors by gender associated with attention-deficit/hyperactivity disorder. Pediatrics. 2014;133:e14–22. doi: 10.1542/peds.2013-1434. [DOI] [PubMed] [Google Scholar]
- 10.Waltereit R, Banaschewski T, Meyer-Lindenberg A, et al. Interaction of neurodevelopmental pathways and synaptic plasticity in mental retardation, autism spectrum disorder and schizophrenia: implications for psychiatry. World J Biol Psychiatry. 2014;15:507–16. doi: 10.3109/15622975.2013.838641. [DOI] [PubMed] [Google Scholar]
- 11.Niemela S, Sourander A, Surcel HM, et al. Prenatal nicotine exposure and risk of schizophrenia among offspring in a national birth cohort. Am J Psychiatry. 2016;173:799–806. doi: 10.1176/appi.ajp.2016.15060800. [DOI] [PubMed] [Google Scholar]
- 12.Ornoy A, Weinstein-Fudim L, Ergaz Z. Genetic syndromes, maternal diseases and antenatal factors associated with autism spectrum disorders (ASD) Front Neurosci. 2016;10:316. doi: 10.3389/fnins.2016.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbott AE, Nair A, Keown CL, et al. Patterns of atypical functional connectivity and behavioral links in autism differ between default, salience, and executive networks. Cereb Cortex. 2016;26:4034–45. doi: 10.1093/cercor/bhv191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerliani L, Mennes M, Thomas RM, et al. Increased functional connectivity between subcortical and cortical resting-state networks in autism spectrum disorder. JAMA Psychiatry. 2015;72:767–77. doi: 10.1001/jamapsychiatry.2015.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sripada CS, Kessler D, Angstadt M. Lag in maturation of the brain’s intrinsic functional architecture in attention-deficit/hyperactivity disorder. Proc Natl Acad Sci U S A. 2014;111:14259–64. doi: 10.1073/pnas.1407787111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCarthy H, Skokauskas N, Mulligan A, et al. Attention network hypoconnectivity with default and affective network hyperconnectivity in adults diagnosed with attention-deficit/hyperactivity disorder in childhood. JAMA Psychiatry. 2013;70:1329–37. doi: 10.1001/jamapsychiatry.2013.2174. [DOI] [PubMed] [Google Scholar]
- 17.Berman RA, Gotts SJ, McAdams HM, et al. Disrupted sensorimotor and social-cognitive networks underlie symptoms in childhood-onset schizophrenia. Brain. 2016;139:276–91. doi: 10.1093/brain/awv306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khadka S, Meda SA, Stevens MC, et al. Is aberrant functional connectivity a psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biol Psychiatry. 2013;74:458–66. doi: 10.1016/j.biopsych.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Downar J, Blumberger DM, Daskalakis ZJ. The neural crossroads of psychiatric illness: an emerging target for brain stimulation. Trends Cogn Sci. 2016;20:107–20. doi: 10.1016/j.tics.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–15. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McTeague LM, Huemer J, Carreon DM, et al. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am J Psychiatry. 2017;174:676–85. doi: 10.1176/appi.ajp.2017.16040400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chisholm K, Lin A, Abu-Akel A, et al. The association between autism and schizophrenia spectrum disorders: a review of eight alternate models of co-occurrence. Neurosci Biobehav Rev. 2015;55:173–83. doi: 10.1016/j.neubiorev.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 23.van der Meer JM, Oerlemans AM, van Steijn DJ, et al. Are autism spectrum disorder and attention-deficit/hyperactivity disorder different manifestations of one overarching disorder? Cognitive and symptom evidence from a clinical and population-based sample. J Am Acad Child Adolesc Psychiatry. 2012;51:1160–72.e3. doi: 10.1016/j.jaac.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 24.Taurines R, Schwenck C, Westerwald E, et al. ADHD and autism: differential diagnosis or overlapping traits? A selective review. Atten Defic Hyperact Disord. 2012;4:115–39. doi: 10.1007/s12402-012-0086-2. [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Arlington (VA): APA; 2013. [Google Scholar]
- 26.Sturm H, Fernell E, Gillberg C. Autism spectrum disorders in children with normal intellectual levels: associated impairments and subgroups. Dev Med Child Neurol. 2004;46:444–7. doi: 10.1017/s0012162204000738. [DOI] [PubMed] [Google Scholar]
- 27.Hattori J, Ogino T, Abiru K, et al. Are pervasive developmental disorders and attention-deficit/hyperactivity disorder distinct disorders? Brain Dev. 2006;28:371–4. doi: 10.1016/j.braindev.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Peralta V, de Jalon EG, Campos MS, et al. The meaning of childhood attention-deficit hyperactivity symptoms in patients with a first-episode of schizophrenia-spectrum psychosis. Schizophr Res. 2011;126:28–35. doi: 10.1016/j.schres.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Kern JK, Geier DA, King PG, et al. Shared brain connectivity issues, symptoms, and comorbidities in autism spectrum disorder, attention deficit/hyperactivity disorder, and Tourette syndrome. Brain. 2015;5:321–35. doi: 10.1089/brain.2014.0324. [DOI] [PubMed] [Google Scholar]
- 30.Konstantareas MM, Hewitt T. Autistic disorder and schizophrenia: diagnostic overlaps. J Autism Dev Disord. 2001;31:19–28. doi: 10.1023/a:1005605528309. [DOI] [PubMed] [Google Scholar]
- 31.Skokauskas N, Gallagher L. Psychosis, affective disorders and anxiety in autistic spectrum disorder: prevalence and nosological considerations. Psychopathology. 2010;43:8–16. doi: 10.1159/000255958. [DOI] [PubMed] [Google Scholar]
- 32.Leyfer OT, Folstein SE, Bacalman S, et al. Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. J Autism Dev Disord. 2006;36:849–61. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- 33.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Nat Acad Sci U S A. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 35.Zijdenbos AP, Forghani R, Evans AC. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 2002;21:1280–91. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]
- 36.Kim JS, Singh V, Lee JK, et al. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005;27:210–21. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 37.Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005;24:163–73. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 38.Panizzon MS, Fennema-Notestine C, Eyler LT, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–35. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wierenga LM, Langen M, Oranje B, et al. Unique developmental trajectories of cortical thickness and surface area. Neuroimage. 2014;87:120–6. doi: 10.1016/j.neuroimage.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Dickerson BC, Feczko E, Augustinack JC, et al. Differential effects of aging and Alzheimer’s disease on medial temporal lobe cortical thickness and surface area. Neurobiol Aging. 2009;30:432–40. doi: 10.1016/j.neurobiolaging.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winkler AM, Kochunov P, Blangero J, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–46. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson PM, Stein JL, Medland SE, et al. The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain. 2014;8:153–82. doi: 10.1007/s11682-013-9269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson PM, Andreassen OA, Arias-Vasquez A, et al. ENIGMA and the individual: predicting factors that affect the brain in 35 countries worldwide. Neuroimage. 2017;145:389–408. doi: 10.1016/j.neuroimage.2015.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ducharme S, Albaugh MD, Nguyen TV, et al. Trajectories of cortical thickness maturation in normal brain development — the importance of quality control procedures. Neuroimage. 2016;125:267–79. doi: 10.1016/j.neuroimage.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee S, Habeck C, Razlighi Q, et al. Selective association between cortical thickness and reference abilities in normal aging. Neuroimage. 2016;142:293–300. doi: 10.1016/j.neuroimage.2016.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raznahan A, Shaw P, Lalonde F, et al. How does your cortex grow? J Neurosci. 2011;31:7174–7. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Erp TG, Hibar DP, Rasmussen JM, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21:547–53. doi: 10.1038/mp.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82:591–605. doi: 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- 49.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 50.Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yarkoni T, Poldrack RA, Nichols TE, et al. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–70. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson NL, Kemp AW, Kotz S. Univariate Discrete Distributions. Hoboken (NJ): John Wiley & Sons, Inc; 2005. Hypergeometric distributions; pp. 251–301. [Google Scholar]
- 53.Raznahan A, Lerch JP, Lee N, et al. Patterns of coordinated anatomical change in human cortical development: a longitudinal neuroimaging study of maturational coupling. Neuron. 2011;72:873–84. doi: 10.1016/j.neuron.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alexander-Bloch A, Raznahan A, Bullmore E, et al. The convergence of maturational change and structural covariance in human cortical networks. J Neurosci. 2013;33:2889–99. doi: 10.1523/JNEUROSCI.3554-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holden M, Deng S, Wojnowski L, et al. GSEA-SNP: applying gene set enrichment analysis to SNP data from genome-wide association studies. Bioinformatics. 2008;24:2784–5. doi: 10.1093/bioinformatics/btn516. [DOI] [PubMed] [Google Scholar]
- 56.Zhang K, Cui S, Chang S, et al. i-GSEA4GWAS: a web server for identification of pathways/gene sets associated with traits by applying an improved gene set enrichment analysis to genome-wide association study. Nucleic Acids Res. 2010;38:W90–5. doi: 10.1093/nar/gkq324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phipson B, Smyth GK. Permutation P-values should never be zero: calculating exact P-values when permutations are randomly drawn. Stat Appl Genet Mol Biol. 2010;9 doi: 10.2202/1544-6115.1585. Article39. [DOI] [PubMed] [Google Scholar]
- 58.Tebartz van Elst L, Maier S, Fangmeier T, et al. Disturbed cingulate glutamate metabolism in adults with high-functioning autism spectrum disorder: evidence in support of the excitatory/inhibitory imbalance hypothesis. Mol Psychiatry. 2014;19:1314–25. doi: 10.1038/mp.2014.62. [DOI] [PubMed] [Google Scholar]
- 59.Solomon M, Yoon JH, Ragland JD, et al. The development of the neural substrates of cognitive control in adolescents with autism spectrum disorders. Biol Psychiatry. 2014;76:412–21. doi: 10.1016/j.biopsych.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Francx W, Oldehinkel M, Oosterlaan J, et al. The executive control network and symptomatic improvement in attention-deficit/hyperactivity disorder. Cortex. 2015;73:62–72. doi: 10.1016/j.cortex.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 61.Van der Meer D, Hoekstra PJ, Zwiers M, et al. Brain correlates of the interaction between 5-HTTLPR and psychosocial stress mediating attention deficit hyperactivity disorder severity. Am J Psychiatry. 2015;172:768–75. doi: 10.1176/appi.ajp.2015.14081035. [DOI] [PubMed] [Google Scholar]
- 62.Rowland LM, Krause BW, Wijtenburg SA, et al. Medial frontal GABA is lower in older schizophrenia: a MEGA-PRESS with macromolecule suppression study. Mol Psychiatry. 2016;21:198–204. doi: 10.1038/mp.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fryer SL, Roach BJ, Ford JM, et al. Relating intrinsic low-frequency BOLD cortical oscillations to cognition in schizophrenia. Neuropsychopharmacol. 2015;40:2705–14. doi: 10.1038/npp.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Courchesne E, Saitoh O, Yeung-Courchesne R, et al. Abnormality of cerebellar vermian lobules VI and VII in patients with infantile autism: identification of hypoplastic and hyperplastic subgroups with MR imaging. AJR Am J Roentgenol. 1994;162:123–30. doi: 10.2214/ajr.162.1.8273650. [DOI] [PubMed] [Google Scholar]
- 65.Haar S, Berman S, Behrmann M, et al. Anatomical abnormalities in autism? Cereb Cortex. 2016;26:1440–52. doi: 10.1093/cercor/bhu242. [DOI] [PubMed] [Google Scholar]
- 66.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–78. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 67.Doyle-Thomas KA, Lee W, Foster NE, et al. Atypical functional brain connectivity during rest in autism spectrum disorders. Ann Neurol. 2015;77:866–76. doi: 10.1002/ana.24391. [DOI] [PubMed] [Google Scholar]
- 68.Christakou A, Murphy CM, Chantiluke K, et al. Disorder-specific functional abnormalities during sustained attention in youth with attention deficit hyperactivity disorder (ADHD) and with autism. Mol Psychiatry. 2013;18:236–44. doi: 10.1038/mp.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meda SA, Ruano G, Windemuth A, et al. Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizoph. Proc Natl Acad Sci U S A. 2014;111:E2066–75. doi: 10.1073/pnas.1313093111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baker JT, Holmes AJ, Masters GA, et al. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. 2014;71:109–18. doi: 10.1001/jamapsychiatry.2013.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cortese S, Kelly C, Chabernaud C, et al. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry. 2012;169:1038–55. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Richiardi J, Altmann A, Milazzo AC, et al. Brain networks. Correlated gene expression supports synchronous activity in brain networks. Science. 2015;348:1241–4. doi: 10.1126/science.1255905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shaw P, Malek M, Watson B, et al. Trajectories of cerebral cortical development in childhood and adolescence and adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2013;74:599–606. doi: 10.1016/j.biopsych.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]