Abstract
Purpose: Endoscopic management of upper tract urothelial carcinoma (UTUC) is associated with higher recurrences, which could be reduced by application of topical therapy. Adjuvant induction Bacillus Calmette–Guerin has shown inferior outcomes for UTUC compared to bladder cancer, and maintenance regimens for UTUC are unexplored. We report on the efficacy, safety, and tolerability of Mitomycin C (MMC) induction and maintenance adjuvant topical therapy for UTUC.
Materials and Methods: Patients with UTUC who received adjuvant topical therapy after complete endoscopic control of Ta/T1 tumors were retrospectively reviewed. Patients were treated using percutaneous nephrostomy tube (NT) or cystoscopically placed weekly ureteral catheters, per patient preference, and all patients were offered induction and maintenance. Standardized follow-up of every 3 months in the first year, then at a minimum every 6 months, with ureteroscopy and at least annual CT, was performed. Primary outcomes were recurrence-free, progression-free, nephroureterectomy-free rate and cancer-specific and overall survival. Secondary outcomes were safety and treatment tolerability.
Results: Twenty-seven patients with 28 renal units received adjuvant topical therapy from January 2008 to March 2015. Median follow-up was 19 months (range 7–92). Three year recurrence-free, progression-free, and nephroureterectomy-free survival rates were 60% [confidence interval (95% CI): 42, 86%], 80% [95% CI: 64, 100%], and 76% [95% CI: 60, 97%]. Cancer-specific mortality rate was 0%, and 3-year overall survival was 92.9%. Nine patients experienced adverse outcomes, all related to interventions and none related to systemic toxicity.
Conclusions: Induction and maintenance adjuvant topical MMC for endoscopically resected UTUC is feasible, well tolerated and shows promising intermediate term data on recurrence, progression, and nephroureterectomy-free survival.
Keywords: : ureteral cancer, renal pelvis cancer, chemotherapy, urothelial cancer, outcomes
Introduction
Although the standard of care for upper tract urothelial carcinoma (UTUC) is considered to be radical nephroureterectomy with bladder cuff excision,1,2 there are indications for kidney-preserving approaches. These include patients with bilateral tumors, tumors in a solitary kidney, chronic renal failure, and more electively, those with select small low-grade tumors. Kidney-preserving treatments may be delivered surgically or endoscopically, through either antegrade or retrograde access, and are well described.1,2
Unfortunately, reported recurrence rates after endoscopic treatment of UTUC have been high, from 30% to 90% (Table 1).3–12 Based on the effectiveness of adjuvant intravesical instillation therapy for UC of the bladder,13,14 the role of topical therapy for properly selected cases of UTUC has been described. Several agents have been proposed for topical treatment.15–20 But due to the rarity of the disease the sample sizes are small, and the optimal route and true benefit of adjuvant instillation therapy for UTUC remain unknown.
Table 1.
Recurrence Rates Demonstrated in Studies for Patients Undergoing Endoscopic Ablation Alone or with Either Adjuvant Mitomycin C or Bacillus Calmette–Guerin
| Intervention | Author (reference) | Date of publication | Number of patients | Recurrence rate, % |
|---|---|---|---|---|
| Endoscopic ablation | ||||
| Daneshmand et al.5 | 2003 | 30 | 90 | |
| Chen and Bagley6 | 2000 | 23 | 65 | |
| Raymundo et al.10 | 2011 | 22 | 48 | |
| Grasso et al.7 | 1999 | 14 | 50 | |
| Cutress et al.3 | 2012 | 73 | 69 | |
| Roupret et al.8 | 2006 | 27 | 22 | |
| Thompson et al.9 | 2008 | 83 | 55 | |
| Gadzinski et al.11 | 2010 | 34 | 84 | |
| Pak et al.12 | 2009 | 57 | 90 | |
| Endoscopic ablation with adjuvant BCG | ||||
| Patel and Fuchs20 | 1998 | 12 | 13 | |
| Rastinehad et al.16 | 2009 | 89 | 36 | |
| Giannarini et al.15 | 2011 | 22 | 59 | |
| Endoscopic ablation with single dose adjuvant MMC | ||||
| Aboumarzouk et al.19 | 2013 | 19 | 35 | |
| Keeley and Bagley18 | 1997 | 19 | 54 | |
| Cutress et al.3 | 2012 | 17 | 68 | |
BCG = Bacillus Calmette–Guerin; MMC = Mitomycin C.
Published data show a recurrence-free survival (RFS) of 41% and progression free-survival (PFS) of 59% in patients with Ta or T1 UTUC treated with antegrade perfusion of bacillus Calmette–Guerin (BCG) as an induction regimen.15 In that series, 23% of the treated units underwent subsequent nephroureterectomy.15 These results are inferior to what is seen in adjuvant treatment of bladder cancer,13 possibly due to higher rates of residual disease, greater difficulty in definitive delivery to the upper tract, and the absence of a reservoir allowing long dwell times. The results of BCG for treatment of upper tract carcinoma in situ (CIS) appear to be more promising.4,15
The results of adjuvant Mitomycin C (MMC) as induction have also been reported in several small series.3,18,19,21 These results demonstrate the safety of upper tract instillation of MMC. However, neither the agent nor its method of delivery has been standardized. To our knowledge the feasibility, tolerability, and efficacy of induction and maintenance therapy have not been systematically assessed. Given the inferior outcomes of BCG as adjuvant treatment of endoscopically treated UTUC, we made a clinical decision in 2008 to offer MMC adjuvant therapy as induction, as well as maintenance therapy, in the hopes of achieving more optimal outcomes. We now report intermediate term data on the efficacy, safety, and tolerability of MMC topical adjuvant therapy given as an induction and maintenance regimen.
Materials and Methods
Patients
After Institutional Review Board approval, the charts of patients with UTUC who received adjuvant topical therapy after complete endoscopic control of Ta/T1 tumors were retrospectively reviewed. Patients with CIS were excluded as it has been a practice at our center to offer initial BCG to those patients given the more effective published data.15 Patients were classified as being treated on an elective, imperative, or palliative basis. Elective patients were treated with curative intent and had a normal contralateral upper tract and normal renal function. Imperative patients were treated with curative intent and had bilateral tumors, tumor in a solitary kidney, or chronic kidney disease, defined as estimated GFR (eGFR) <60. Palliative patients were treated with the goal of local control for maintenance of renal function.
Additional variables collected included demographic data, smoking history, family history, cytology results, complications, renal function, and follow-up data. Lynch syndrome was considered positive based on Amsterdam criteria II, tissue, or genetic testing.22
Diagnostic methods
Diagnoses were made with ureteroscopic or percutaneous guided biopsy. All pathologic results were reviewed at our institution by genitourinary fellowship trained pathologists.
Treatments
Endoscopic control consisted first of biopsy, then of either ureteroscopic or percutaneous resection, and/or laser ablation of all visible disease burdens. This was done either primarily or in a staged manner, but completed before the initiation of topical therapy. Ureteroscopic management included using a variety of tools to obtain adequate tissue for diagnosis, including use of access sheaths, and a variety of biopsy tools such as a 3F biopsy forceps, steel-wire basket, and 1.7F Nitinol stone extractor basket (Piranha®, Atlas Wire Stone Basket®, and N-Gage®, respectively; Cook Medical, Bloomington, IN). Endoscopic ablation was performed with a holmium laser (200 micron fiber, 10 Hz, 100 mJ initial settings then adjusted as necessary). If staged procedures were necessary due to high volume of disease, or presence of hematuria, they were performed on average 2 weeks apart with a stent left in between the two procedures.
Patients were treated either through a 10F percutaneous nephrostomy tube (NT) or by weekly cystoscopic placement of 5F ureteral catheters (Fig. 1). The method of treatment was per patient preference after being informed of the pros and cons of either modality. Patients with NTs were given 2 weeks to have the tract mature before initiating infusion, with the NT changed every 3 months. Patients treated with a ureteral catheter had office flexible cystoscopy with placement of a ureteral catheter (Beacon® tip; Cook Medical) performed under fluoroscopic guidance using only intraurethral 2% lidocaine jelly for analgesia. A Foley catheter was placed to secure the Beacon tip with a silk tie.
FIG. 1.
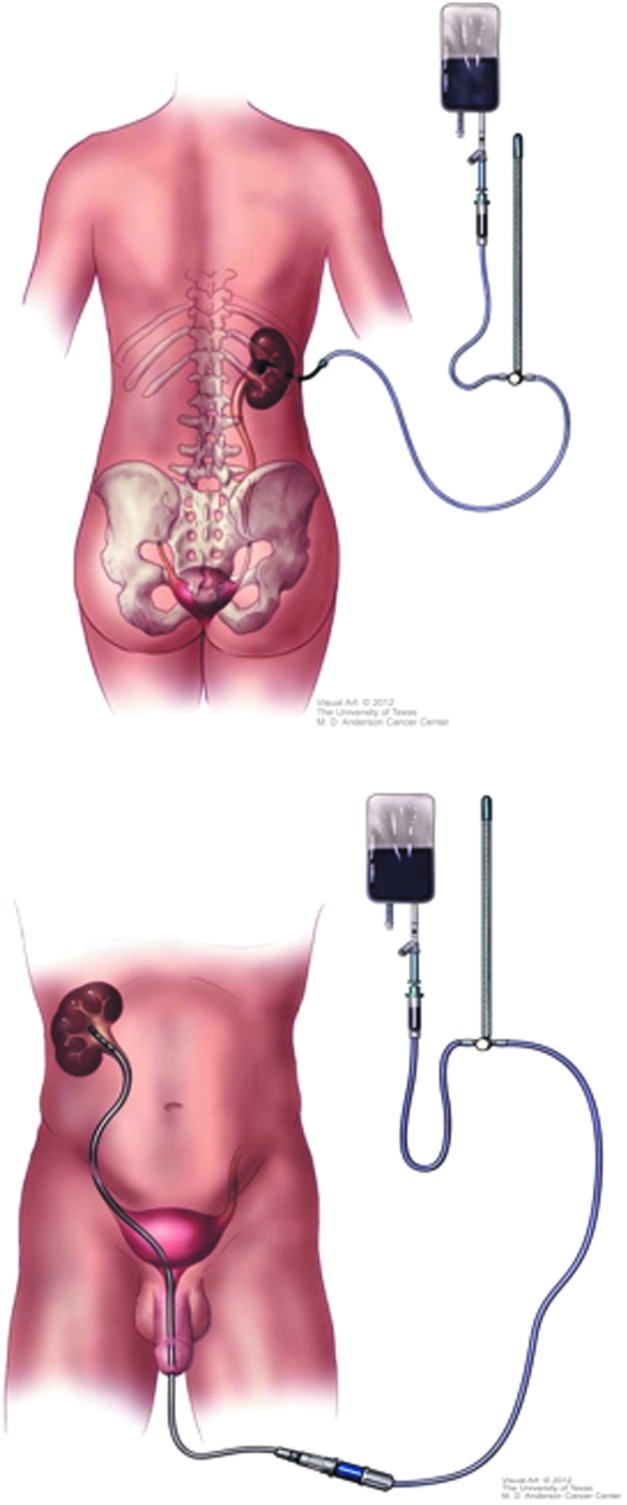
Schema showing how patients received upper tract topical therapy with Mitomycin C either through percutaneous nephrostomy (top) or ureteral catheter (bottom).
Patients were treated by nurses in the urology ambulatory office setting with 40 mg of MMC mixed in 20 mL of physiologic saline, with slow drip infusion over 2 hours and controlled by manometry pressures at or below 20 to 30 mmHg. Patients were asked to assume different positions (left side, right side, prone, and supine) every 15 to 20 minutes to ensure adequate contact with the entire upper tract system. After completion, the Beacon tip catheter and Foley catheter were removed. Patients with NTs had the tube capped. Urinary cultures were performed at the initial visit and subsequently only in the presence of symptoms or signs of infection. Prophylactic oral antibiotics were prescribed for one to two doses at the time of each treatment. Induction courses consisted of once weekly instillations for 6 weeks. Maintenance course consisted of either once monthly for at least 3 months or once weekly instillations for 3 weeks, following previously the protocol for BCG therapy13 depending on availability and convenience of the patient.
Follow-up
Patients were followed up every 3 months in the first year and then every 6 months for at least 2 years. Evaluations consisted of ureteroscopy, urine cytology, chemistry panel, complete blood count, and triple-phase CT. In patients with contraindication to contrasted scan, magnetic resonance urogram or retrograde studies were substituted for CT.
Outcome measures and statistical analyses
Primary outcomes measured were RFS, PFS, and nephroureterectomy-free survival (NUxFS) on a per renal unit (RU) basis and cancer- specific and overall survival on a per patient basis. Recurrence was defined as an ipsilateral UTUC tumor. Progression was defined as an ipsilateral UTUC tumor of higher stage and/or grade. Subanalyses were performed to evaluate RFS, PFS, and NUxFS rate based on delivery method and presence or absence of Lynch Syndrome. A secondary outcome measure was treatment tolerability. Adverse events (AEs) were recorded and classified based on the Clavien-Dindo scale.
Statistical analysis
Continuous variables are reported as median values with interquartile range. Comparison was made using Student's t-test for continuous variables and Fisher's exact test for nominal variables. The Kaplan–Meier method was used to estimate probability of RFS, PFS, and NUxFS. The log rank test was applied to compare these survival outcomes between subgroups of patients.
Results
Between January 2008 and January 2015, 140 patients underwent ureteroscopic biopsy for UTUC at our center. Forty-nine patients (35%) were identified for potential topical therapy after complete endoscopic assessment. Of these, 22 (45%) received BCG for CIS. Twenty-seven (55%) patients received MMC for endoscopically-managed papillary Ta/T1 tumors, one of whom had bilateral disease, resulting in 28 total RUs. These 27 patients and 28 RUs comprise the population of this study. Twenty-one RUs had low-grade UTUC (75%) and 7 RUs high-grade UTUC (25%) (Table 2). Median follow-up was 19 months with a median number of three ureteroscopies performed post initial diagnosis.
Table 2.
Baseline Demographic Information for Patients Undergoing Adjuvant Mitomycin C
| Demographics | N = 27 |
|---|---|
| Gender, n (%) | |
| Female | 12 (42.9) |
| Male | 16 (57.1) |
| Age (year) | |
| Median (IQR) | 74 (64–79) |
| Race, n (%) | |
| Asian | 1 (3.6) |
| African American | 3 (10.7) |
| Hispanic | 1 (3.6) |
| Caucasian | 23 (82.1) |
| ASA classification, n (%) | |
| 0–1 | 11 (40.1) |
| 2–3 | 16 (59.3) |
| Biopsy grade, n (%) | |
| High | 7 (25) |
| Low | 21 (75) |
| Tumor focality, n (%) | |
| Solitary | 19 (67.9) |
| Multifocal | 9 (32.1) |
| Renal function at diagnosis, n (%) | |
| Normal | 16 (57.1) |
| Insufficiency | 12 (42.9) |
| Solitary Kidney, n (%) | |
| Solitary | 6 (22.2) |
| Bilateral | 21 (77.8) |
| Treatment indication, n (%) | |
| Elective | 13 (46.4) |
| Imperative | 13 (46.4) |
| Palliative | 2 (7.1) |
| Delivery method, n (%) | |
| Nephrostomy | 9 (32.1) |
| Ureteral catheter | 19 (67.9) |
| Smoking history, n (%) | |
| Smoker | 12 (42.9) |
| Nonsmoker | 16 (57.1) |
| Lynch syndrome, n (%) | |
| Positive | 5 (18.5) |
| Negative | 22 (81.5) |
| Number of ureteroscopies postdiagnosis | |
| Median (IQR) | 3 (2–4) |
ASA = American Society of Anesthesiology; IQR = interquartile range.
Most patients received all 6 doses of induction MMC (24/28, 85.7%), 1 (3.7%) RU received 5 doses, and 4 (14.8%) patients received 3 doses. One maintenance course was completed by 60.7% (17/28), 35.7% (10/28) completed 2 maintenance courses 17.8% (5/28) completed courses 3, 4, and 5, and 7.1% (2/28) patients completed 6 courses. Two of the patients that received three doses of induction therapy went on to receive monthly maintenance. The other three patients who stopped induction early did not have any further instillation due to recurrent urinary tract infections1; one patient did not want to continue traveling to our center and one patient had bothersome bladder spasms.
Upper tract recurrence occurred in 11 (39%) patients, with a 3-year recurrence-free survival of 60% [confidence interval (95% CI): 42, 86%]. Of these recurrences, 8 (38%) patients had low-grade disease and 3 (43%) had high-grade disease. Bladder recurrence was seen in 8 (29%) patients, 7 (33%) low grade and 1 (14%) high grade. The 3-year RFS rate was 62% [95% CI: 42, 91%] for low-grade patients and 60% [95% CI: 29, 100%] for high-grade patients (Table 3). Fifty-three percent (9.17) of patients had no evidence of recurrence on maintenance.
Table 3.
Recurrence-Free Survival for Patients Undergoing Adjuvant Mitomycin C
| Variable | N | Recurrence | RFS rate at 3 years (95% CI) | RFS rate at 5 years(95% CI) |
|---|---|---|---|---|
| Overall | 28 | 11 | 0.6 (0.42, 0.86) | 0.36 (0.16, 0.80) |
| Tumor grade | ||||
| Low | 21 | 9 | 0.62 (0.42, 0.91) | 0.31 (0.11, 0.89) |
| High | 7 | 2 | 0.6 (0.29, 1) | |
| Focality | ||||
| Solitary | 9 | 6 | 0.73 (0.52, 1) | |
| Multifocal | 9 | 5 | 0.4 (0.17, 0.94) | 0.4 (0.17, 0.94) |
| Renal function at diagnosis | ||||
| eGFR ≥60 | 15 | 4 | 0.67 (0.43, 1) | 0.67 (0.43, 1) |
| eGFR <60 | 13 | 7 | 0.53 (0.30, 0.95) | |
| Treatment indication | ||||
| Elective | 13 | 3 | ||
| Imperative | 13 | 7 | 0.50 (0.27, 0.89) | 0.37 (0.16, 0.84) |
| Palliative | 2 | 1 | 1 (1, 1) | |
| Tobacco history | ||||
| Nonsmoker | 12 | 5 | 0.509 (0.27, 0.95) | |
| Smoker | 16 | 6 | 0.69 (0.44, 1) | 0.33 (0.12, 0.97) |
| Delivery method | ||||
| Ureteral catheter | 19 | 9 | 50% (0.25,1) | 0.26 (0.08, 0.76) |
| Nephrostomy tube | 9 | 2 | 63% (0.410, 0.99) | 0.40 (0.23, 0.90) |
| Lynch syndrome | ||||
| Negative | 23 | 8 | 0.62 (0.42, 0.93) | 0.45 (0.09, 0.91) |
| Positive | 5 | 3 | 0.56 (0.28, 1) | 0.2 (0.10, 0.78) |
CI = confidence interval; eGFR = estimated GFR; RFS = recurrence-free survival.
Local progression was seen in 5 (18%) patients
The 3-year progression-free survival rate was 80% [95% CI: 64, 100%], 87% [95% CI: 72, 100%] for low-grade patients and 67% [95% CI: 38, 100%] for high grade (Table 4).
Table 4.
Progression-Free Survival for Patients Undergoing Adjuvant Mitomycin C
| Variable | n | Progression | PFS rate at 3 years (95% CI) | PFS rate at 5 years (95% CI) |
|---|---|---|---|---|
| Overall | 28 | 5 | 0.80 (0.64, 1) | 0.64 (0.39, 1) |
| Tumor grade | ||||
| Low | 21 | 3 | 0.87 (0.72, 1) | 0.65 (0.36, 1) |
| High | 7 | 2 | 0.67 (0.38, 1) | |
| Focality | ||||
| Solitary | 19 | 4 | 0.8 (0.62, 1) | 0.53 (0.23, 1) |
| Multifocal | 9 | 1 | 0.83 (0.58, 1) | 0.83 (0.58, 1) |
| Renal function at diagnosis | ||||
| eGFR ≥60 | 16 | 2 | 0.83 (0.63, 1) | 0.83 (0.63, 1) |
| eGFR <60 | 12 | 3 | 0.79 (0.56, 1) | 0.39 (0.10, 1) |
| Treatment indication | ||||
| Elective | 13 | 2 | ||
| Imperative | 13 | 3 | 0.78 (0.55, 1) | 0.58 (0.3, 1) |
| Palliative | 2 | 0 | 1 (1, 1) | 1 (1, 1) |
| Tobacco history | ||||
| Nonsmoker | 12 | 2 | 0.78 (0.549, 1) | |
| Smoker | 16 | 3 | 0.8 (0.611, 1) | 0.61 (0.32, 1) |
| Delivery method | ||||
| Ureteral Catheter | 19 | 4 | 0.86 (0.69, 1) | 0.56 (0.32, 0.1) |
| Nephrostomy tube | 9 | 1 | 0.71 (0.45, 1) | 0.83 (0.583,1) |
| Lynch syndrome | ||||
| Negative | 23 | 4 | 0.77 (0.57, 1) | 0.56 (0.32, 1) |
| Positive | 5 | 1 | 0.86 (0.63, 1) | 0.67 (0.41, 1) |
PFS = progression-free survival.
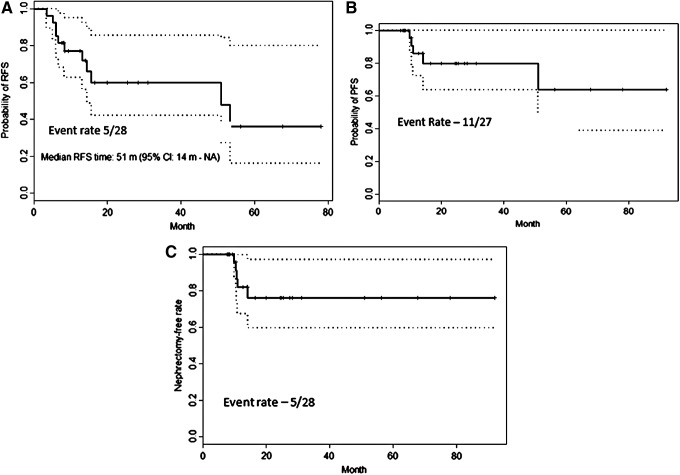
Five (18%) of the 28 RUs proceeded to nephroureterectomy with a 3-year nephroureterectomy-free rate of 76% [95% CI: 60, 97%], 82% [95% CI: 65, 100%] for low-grade patients and 67% [95% CI: 38, 100%] for high grade (Table 5). Four patients who had a nephroureterectomy had tumor progression, two of whom were volume progression and two of whom were grade progression. One patient had multiple low-grade recurrences on MMC and elected for nephroureterectomy. Cancer-specific mortality rate was 0%, and overall 3-year survival rate was 92.9% (5% CI: 0.803, 1). Figure 2 shows Kaplan–Meir estimates for cumulative RFS, PFS, and NUxFS.
Table 5.
Nephroureterectomy-Free Survival for Patients Undergoing Adjuvant Mitomycin C
| Variable | n | Nephroureterectomy | NUxFS rate at 3 years (95% CI) | NUxFS rate at 5 years (95% CI) |
|---|---|---|---|---|
| Overall | 28 | 5 | 0.76 (0.60, 0.97) | 0.76 (0.60, 0.97) |
| Tumor grade | ||||
| Low | 21 | 3 | 0.82 (0.65, 1) | 0.82 (0.65, 1) |
| High | 7 | 2 | 0.67 (0.38, 1) | |
| Focality | ||||
| Solitary | 9 | 3 | 0.8 (0.62, 1) | 0.8 (0.62, 1) |
| Multifocal | 9 | 2 | 0.71 (0.45, 1) | 0.71 (0.45, 1) |
| Renal function at diagnosis | ||||
| eGFR ≥60 | 15 | 3 | 0.76 (0.55, 1) | 0.76 (0.55, 1) |
| eGFR <60 | 13 | 2 | 0.79 (0.56, 1) | 0.79 (0.56, 1) |
| Treatment indication | ||||
| Elective | 13 | 2 | ||
| Imperative | 13 | 3 | 0.7 (0.47, 1) | 0.7 (0.467, 1) |
| Palliative | 2 | 0 | 1 (1, 1) | 1 (1, 1) |
| Tobacco history | ||||
| Nonsmoker | 12 | 3 | 0.7 (0.47, 1) | |
| Smoker | 16 | 2 | 0.82 (0.61, 1) | 0.82 (0.61, 1) |
| Delivery method | ||||
| Ureteral catheter | 19 | 4 | 0.86 (0.69, 1) | 0.56 (0.32, 0.1) |
| Nephrostomy tube | 9 | 1 | 0.63 (0.37, 1) | 0.833 (0.58,1) |
| Lynch syndrome | ||||
| Negative | 23 | 4 | 0.72 (0.52, 1) | 0.56 (0.32, 1) |
| Positive | 5 | 1 | 0.86 (0.63,1) | 0.67 (0.41, 1) |
NUxFS = nephroureterectomy-free survival.
FIG. 2.
Kaplan–Meier estimates for cumulative recurrence-free (A), progression-free (B), and nephroureterectomy-free (C) survival.
Ten AEs were seen in nine patients of which four (14%) were detected after induction and six (12%) after maintenance (Table 6). No events related to MMC absorption such as bone marrow depression, anemia, or leukopenia were identified. Four patients had ureteral strictures, all treated with ureteral catheter, three had ureteral tumors treated with strictures occurring at the site of endoscopic resection, and one patient had a past history of prostate radiation therapy.
Table 6.
Adverse Events for Patients Undergoing Adjuvant Mitomycin C
| Adverse event | n | Grade | Delivery |
|---|---|---|---|
| After induction | 4 | ||
| Recurrent UTI | 1 | 1 | Ureteral catheter |
| Severe bladder spasm | 1 | 1 | Ureteral catheter |
| Ureteral stricture | 1 | 2 | Ureteral catheter |
| Pyelonephritis | 1 | 2 | Nephrostomy tube |
| After maintenance | 5 | ||
| Hematuria | 1 | 1 | Nephrostomy tube |
| Ureteral stricture | 3 | 1,2 | Ureteral catheter |
| Infundibular stenosis | 1 | 3 | Ureteral catheter |
Grade of complication as per Clavien-Dindo Classification.
UTI = urinary tract infection.
Discussion
Adjuvant topical MMC as induction and maintenance for UTUC appears to be a well-tolerated, feasible, and possibly beneficial approach to conservative management of Ta/T1 tumors after complete endoscopic control. Our 3-year RFS and PFS rates of 60% and 80%, respectively, support further consideration of upper tract topical MMC as a reasonable initial therapeutic agent for UTUC when given with a maintenance regimen. This compares favorably to treatment with BCG with RFS and PFS of 41% and 59%, although NUxFS rates were similar.15 The treatment was well tolerated without evidence of systemic absorption, but with potential for local AEs.
Adjuvant topical therapy for UTUC was first described by Herr in 1985 for a patient with positive margins after resection for muscle invasive disease.23 After this, several institutions reported on the use of upper tract BCG perfusion for both primary treatment of CIS and adjuvant therapy of papillary tumors. In a systematic review by Cutress and colleagues, the rate of recurrence of endoscopic resection ranges between 10% and 90%.4 Table 1 provides an outline of the recurrence rates of the larger and more contemporary studies. Adjuvant therapy of BCG and MMC has both been investigated. BCG demonstrated recurrence rates of 13% to 36% in the upper tract.4,16,19,24–26 These data do not mirror the success of intravesical BCG for UC of the bladder that has been extensively published.13 Use of MMC in the upper tract has been performed at times through endoscopic resection.3,18,19,21 And although in small series it is safe, it has demonstrated a recurrence rate of 24% to 68%. Our recurrence-free survival of 62% at 3 years represents one of the more favorable results in the literature. Most recently, much attention has been gained with the potential applicability of MMC using a thermosensitive polymer to increase the dwell time; however, no clinical data have been released.27 We postulated that by utilizing a chemotherapeutic agent that does not rely on prolonged dwell times to incite an immunologic and cytokine response in a maintenance regimen, results could be improved.
Our study shows a noticeable difference in recurrence, progression, and nephroureterectomy rates for patients depending on whether they were treated through NT or ureteral catheter. However, there were more patients with low-grade tumors treated with ureteral catheter (71%) compared to NT (29%). Previous animal studies have shown superior contact with a ureteral catheter than with a NT, suggesting that delivery through a retrograde ureteral catheter may be optimal.28 Our data tend to support that theory, but we cannot discount the possibility of bias given the nature of this retrospective study.
The effect of topical therapy on patients with Lynch Syndrome is still yet to be determined, and our small sample size was not adequately powered to show a difference. The hypermutational status of tumors in patients with Lynch Syndrome is thought to make them more chemosensitive, as is seen with systemic therapy of colorectal cancers.29 Whether this makes Lynch Syndrome patients with noninvasive UTUC more optimally suited to treatment with adjuvant topical chemotherapy is unknown and requires further exploration.
In our study, nine patients experienced AEs. Four of these events occurred during induction therapy, representing an AE rate of 14% as detailed in Table 6. No patients discontinued maintenance therapy due to AEs. The cause of the ureteral strictures is unknown. It could be related to endoscopic resection, multiple ureteroscopies, MMC, delivery method, or the additive effect of all these factors. Our findings are comparable to prior studies such as Aboumarzouk and colleagues who showed that 3/20 (15%) RUs in their cohort developed a ureteral stricture. Again, in their series the cause was unable to be attributed to any single factor.12,19 Other concurrent procedures and treatments such as the initial and subsequent multiple surveillance ureteroscopies may have contributed to the presence of these AEs. For example, the patient with infundibular stenosis had laser ablation of a circumferentially carpeting low-grade papillary tumor around the infundibulum. Regardless of the definitive cause of the ureteral stricture, it is recognized that the ureteral stricture rate in patients receiving upper tract instillation of MMC is high, and observation for onset of hydronephrosis and renal failure is recommended to prevent irreversible renal deterioration. New paradigms that decrease manipulations and improve outcomes are clearly needed.
A criticism of adjuvant and maintenance MMC for UTUC is the potential cost and insurance reimbursement of both weekly cystoscopic procedures and drug costs. We have not had any denials of payment to our knowledge. This may be because we are applying similar principles as for treatment of bladder cancer, the most common organ site for urothelial cancer, which has well accepted protocols.
While this series represents the largest published study on the use of adjuvant topical MMC for UTUC, it is not without significant limitations. It is a retrospective review of a relatively small sample size and there is no available control for this select group of patients. Retrospective bias and clinical heterogeneity may have affected some of the results. Clinical heterogeneity in this series is largely due to patient-focused and shared decision making, such as with the decision for MMC instillation through a ureteral catheter or NT. Given lack of data over the efficacy of either, and the significant impact that the decision may have on the patient's quality of life, we left this decision to the patient. A larger study is needed, preferably prospective and with longer follow-up, to validate these findings. After this study was completed, a phase 3 open label multicenter trial of MitoGel™ (https://clinicaltrials.gov/ct2/show/NCT02793128, accessed May 31, 2017) opened in 2017 for chemoablative and adjuvant treatment of low-grade UTUC. The study design, in fact, emulates our approach of induction and maintenance therapy, but results are likely not forthcoming for a few years. Thus, in the meantime our data may help provide guidance to urologists and their patients. In addition, only after updating clinic processes recently have we systematically treated patients under optimized conditions with relative dehydration and urinary alkalization.30 Thus, these results may be improved in this optimized setting.
Conclusions
We assess intermediate term data on the safety, efficacy, and tolerability of induction and maintenance adjuvant MMC for adjuvant topical therapy of endoscopically resected UTUC. The high degree of tolerability coupled with our intermediate term data showing promising recurrence, progression, and NUxFS is encouraging. While nephroureterectomy clearly remains the most definitive management for UTUC, this study provides support for further investigation of chemotherapeutic agents for topical adjuvant treatment of UTUC. Future research can be directed at testing induction and maintenance therapy in a prospective manner and ideally with methods that not only can improve oncologic outcomes but also minimize instrumentation and risk for AEs.
Abbreviations Used
- AEs
adverse events
- BCG
Bacillus Calmette–Guerin
- CT
computed tomography
- CI
confidence interval
- IQR
interquartile range
- MMC
mitomycin C
- NT
nephrostomy tube
- NUxFS
nephroureterectomy-free survival
- PFS
progression-free survival
- RFS
recurrence-free survival
- RU
renal unit
- UTUC
upper tract urothelial carcinoma
- U
ureteral catheter
Acknowledgments
The authors recognize support from the Monteleone Family Foundation Endowment for Research in Kidney and Bladder Cancer and the Eleanor and Scott Petty Fund for Upper Tract Urothelial Cancer Research. These entities were not involved in any aspect of this study. Kaylynn Brooks assisted with article preparation.
Author Disclosure Statement
Dr. Matin serves as consultant to Urogen Corp. and Taris Biomedical. Neither was involved in any aspect of this study. All other authors have no competing financial interests to disclose.
References
- 1.Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester R, Burger M, et al. European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol 2013;63:1059–1071 [DOI] [PubMed] [Google Scholar]
- 2.Shariat SF, Surena M, Stenzl A. Upper Tract Urothelial Carcinoma (UTUC) A Joint SIU-ICUD International Consultation. Vancouver, Canada, September 8–12, 2013. Société Internationale d'Urologie (SIU), 2013. Available at www.siu-urology.org (accessed May24, 2017) [Google Scholar]
- 3.Cutress ML, Stewart GD, Wells-Cole S, Phipps S, Thomas BG, Tolley DA. Long-term endoscopic management of upper tract urothelial carcinoma: 20-year single-centre experience. BJU Int 2012;110:1608–1617 [DOI] [PubMed] [Google Scholar]
- 4.Cutress ML, Stewart GD, Zakikhani P, Phipps S, Thomas BG, Tolley DA. Ureteroscopic and percutaneous management of upper tract urothelial carcinoma (UTUC): Systematic review. BJU Int 2012;110:614–628 [DOI] [PubMed] [Google Scholar]
- 5.Daneshmand S, Quek ML, Huffman JL. Endoscopic management of upper urinary tract transitional cell carcinoma: Long-term experience. Cancer 2003;98:55–60 [DOI] [PubMed] [Google Scholar]
- 6.Chen GL, Bagley DH. Ureteroscopic management of upper tract transitional cell carcinoma in patients with normal contralateral kidneys. J Urol 2000;164:1173–1176 [PubMed] [Google Scholar]
- 7.Grasso M, Fraiman M, Levine M. Ureteropyeloscopic diagnosis and treatment of upper urinary tract urothelial malignancies. Urology 1999;54:240–246 [DOI] [PubMed] [Google Scholar]
- 8.Roupret M, Hupertan V, Traxer O, Loison G, Chartier-Kastler E, Conort P, et al. Comparison of open nephroureterectomy and ureteroscopic and percutaneous management of upper urinary tract transitional cell carcinoma. Urology 2006;67:1181–1187 [DOI] [PubMed] [Google Scholar]
- 9.Thompson RH, Krambeck AE, Lohse CM, Elliott DS, Patterson DE, Blute ML. Endoscopic management of upper tract transitional cell carcinoma in patients with normal contralateral kidneys. Urology 2008;71:713–717 [DOI] [PubMed] [Google Scholar]
- 10.Raymundo EM, Lipkin ME, Banez LB, Mancini JG, Zilberman DE, Preminger GM, et al. Third prize: The role of endoscopic nephron-sparing surgery in the management of upper tract urothelial carcinoma. J Endourol 2011;25:377–384 [DOI] [PubMed] [Google Scholar]
- 11.Gadzinski AJ, Roberts WW, Faerber GJ, Wolf JS., Jr. Long-term outcomes of nephroureterectomy versus endoscopic management for upper tract urothelial carcinoma. J Urol 2010;183:2148–2153 [DOI] [PubMed] [Google Scholar]
- 12.Pak RW, Moskowitz EJ, Bagley DH. What is the cost of maintaining a kidney in upper-tract transitional-cell carcinoma? An objective analysis of cost and survival. J Endourol 2009;23:341–346 [DOI] [PubMed] [Google Scholar]
- 13.Lamm DL. Preventing progression and improving survival with BCG maintenance. Eur Urol 2000;37 Suppl 1:9–15 [DOI] [PubMed] [Google Scholar]
- 14.Malmstrom PU, Sylvester RJ, Crawford DE, Friedrich M, Krege S, Rintala E, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guerin for non-muscle-invasive bladder cancer. Eur Urol 2009;56:247–256 [DOI] [PubMed] [Google Scholar]
- 15.Giannarini G, Kessler TM, Birkhauser FD, Thalmann GN, Studer UE. Antegrade perfusion with bacillus Calmette-Guerin in patients with non-muscle-invasive urothelial carcinoma of the upper urinary tract: Who may benefit? Eur Urol 2011;60:955–960 [DOI] [PubMed] [Google Scholar]
- 16.Rastinehad AR, Ost MC, Vanderbrink BA, Greenberg KL, El-Hakim A, Marcovich R, et al. A 20-year experience with percutaneous resection of upper tract transitional carcinoma: Is there an oncologic benefit with adjuvant bacillus Calmette Guerin therapy? Urology 2009;73:27–31 [DOI] [PubMed] [Google Scholar]
- 17.Motamedinia P, Keheila M, Leavitt DA, Rastinehad AR, Okeke Z, Smith AD. The expanded use of percutaneous resection for upper tract urothelial carcinoma: A 30-year comprehensive experience. J Endourol 2016;30:262–267 [DOI] [PubMed] [Google Scholar]
- 18.Keeley FX, Jr., Bagley DH. Adjuvant mitomycin C following endoscopic treatment of upper tract transitional cell carcinoma. J Urol 1997;158:2074–2077 [DOI] [PubMed] [Google Scholar]
- 19.Aboumarzouk OM, Somani B, Ahmad S, Nabi G, Townell N, Kata SG. Mitomycin C instillation following ureterorenoscopic laser ablation of upper urinary tract carcinoma. Urol Ann 2013;5:184–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel A, Fuchs GJ. New techniques for the administration of topical adjuvant therapy after endoscopic ablation of upper urinary tract transitional cell carcinoma. J Urol 1998;159:71–75 [DOI] [PubMed] [Google Scholar]
- 21.Cornu JN, Roupret M, Carpentier X, Geavlete B, de Medina SG, Cussenot O, et al. Oncologic control obtained after exclusive flexible ureteroscopic management of upper urinary tract urothelial cell carcinoma. World J Urol 2010;28:151–156 [DOI] [PubMed] [Google Scholar]
- 22.Roupret M, Yates DR, Comperat E, Cussenot O. Upper urinary tract urothelial cell carcinomas and other urological malignancies involved in the hereditary nonpolyposis colorectal cancer (lynch syndrome) tumor spectrum. Eur Urol 2008;54:1226–1236 [DOI] [PubMed] [Google Scholar]
- 23.Herr HW. Durable response of a carcinoma in situ of the renal pelvis to topical bacillus Calmette-Guerin. J Urol 1985;134:531–532 [DOI] [PubMed] [Google Scholar]
- 24.Clark PE, Streem SB, Geisinger MA. 13-year experience with percutaneous management of upper tract transitional cell carcinoma. J Urol 1999;161:772–775; discussion 5–6 [PubMed] [Google Scholar]
- 25.Palou J, Piovesan LF, Huguet J, Salvador J, Vicente J, Villavicencio H. Percutaneous nephroscopic management of upper urinary tract transitional cell carcinoma: Recurrence and long-term followup. J Urol 2004;172:66–69 [DOI] [PubMed] [Google Scholar]
- 26.Katz MH, Lee MW, Gupta M. Setting a new standard for topical therapy of upper-tract transitional-cell carcinoma: BCG and interferon-alpha2B. J Endourol 2007;21:374–377; discussion 7 [DOI] [PubMed] [Google Scholar]
- 27.Donin NM, Duarte S, Lenis AT, Caliliw R, Torres C, Smithson A, et al. Sustained-release formulation of mitomycin c to the upper urinary tract using a thermosensitive polymer: A preclinical study. Urology 2017;99:270–277 [DOI] [PubMed] [Google Scholar]
- 28.Pollard ME, Levinson AW, Shapiro EY, Cha DY, Small AC, Mohamed NE, et al. Comparison of 3 upper tract anticarcinogenic agent delivery techniques in an ex vivo porcine model. Urology 2013;82:1451.e1–e6 [DOI] [PubMed] [Google Scholar]
- 29.Carethers JM, Smith EJ, Behling CA, Nguyen L, Tajima A, Doctolero RT, et al. Use of 5-fluorouracil and survival in patients with microsatellite-unstable colorectal cancer. Gastroenterology 2004;126:394–401 [DOI] [PubMed] [Google Scholar]
- 30.Au JL, Badalament RA, Wientjes MG, Young DC, Warner JA, Venema PL, et al. Methods to improve efficacy of intravesical mitomycin C: Results of a randomized phase III trial. J Natl Cancer Inst 2001;93:597–604 [DOI] [PubMed] [Google Scholar]