Abstract
Recent studies showing significant changes in valvular matrix composition with age offer design criteria for age-specific tissue-engineered heart valves. However, knowledge regarding aging-related changes in valvular material properties is limited. Therefore, 6-week, 6-month, and 6-year-old porcine aortic valves (AV) and mitral valves (MV) were subjected to uniaxial tensile testing. In addition to standard material parameters, the radius of transition curvature (RTC) was measured to assess the acuteness of the transition region of the tension–strain curve. Radially, the MV had greater stiffness and a smaller RTC compared with the AV. Circumferentially, the center of the MV anterior leaflet (MVAC) had the highest stiffness (MVAC > AV > MV free edge [MVF]), greater stress relaxation (MVAC > MVF/AV), lowest extensibility (MVAC < AV < MVF), and smaller RTC compared with MVF (AV < MVAC < MVF). AV and MV radial strips had a larger RTC compared with circumferential strips. Aging elevated stiffness for MV and AV radial and circumferential strips, elevated stress relaxation in AV and MVF circumferential strips, and increased RTC for MV radial and MVF circumferential strips. In conclusion, there are significant age-related differences in the material properties of heart valves, which parallel differences in tissue composition and structure, likely impact valve function, and highlight the need for age-specific design goals for tissue-engineered heart valves.
Introduction
Although heart valve composition and microstructure are known to change with aging,1–6 and a number of valve diseases show increased incidence with aging,7,8 the effects of aging on valvular material properties remain largely unknown, with the exception of one study of radial failure strain of aortic valves (AVs).9 It is likely that valve material behavior varies with age given age-related changes in valve composition, including increased collagen content1,2 and crosslinking,2,4 as well as changes in the abundance and turnover of specific extracellular matrix (ECM) components.3,10,11 Potential age-related changes in the material behavior of valves likely contribute to proper physiologic function, as cardiac hemodynamics change during the normal aging process.12 Ultimately, a properly designed tissue-engineered heart valve (TEHV) would similarly need specific material properties that allow it to withstand these different hemodynamics depending on the patient age.
Valves are considered to be quasi-viscoelastic,13,14 highly anisotropic tissues15–20 that demonstrate stress relaxation14,17,20–24 but low hysteresis13,14 and no creep.14,23 Tensile testing of valves results in a characteristic load-elongation curve with a low slope pre-transition region in which the collagen fibers are crimped and the elastic fibers are bearing load, followed by a transition region during which loading is transferred to the collagen fibers, and then a post-transition region whose high slope reflects load bearing by the collagen fibers.21,22,25 Valve anisotropy, in which valves are stiffer and less extensible in the circumferential direction compared with the radial direction,15–20 is attributed to collagen fiber alignment in the circumferential direction.15,26 Stiffness also varies regionally within the mitral valve (MV) (i.e., between the anterior center [MVAC] and the free edge [MVF]),15 as does ECM composition in these regions.27
Therefore, the primary objective of this study was to assess age-related differences in the material properties of porcine MV and AV and to consider these differences in the context of valve microstructure and ECM composition. In addition to standard material parameters, we developed a novel parameter termed “radius of transition curvature” (RTC) to quantify the acuteness of the transition from the pre-transition to post-transition region of the tension–strain curve.
Materials and Methods
Tissue sample procurement
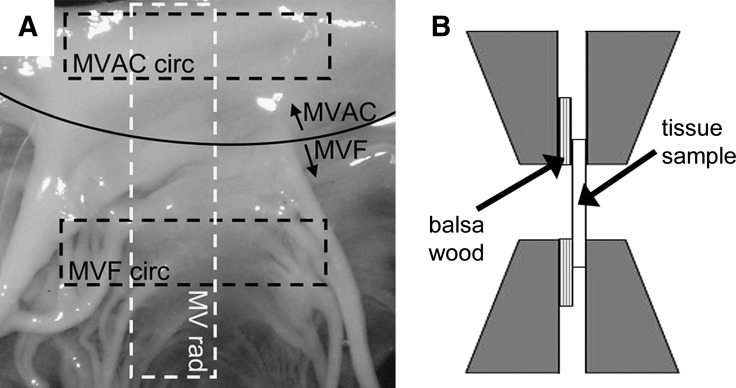
Based on comparable anatomy between porcine and human valves,28–30 valves from 6-week, 6-month, and 6-year-old pigs were used (corresponding to child, young adult, and older adult ages in humans11). Porcine hearts were obtained from abattoirs (from Fisher Ham and Meat [Spring, TX] for 6-week-old and 6-month-old pigs; from Animal Technologies [Tyler, TX] for 6-year-old pigs) and measured to normalize leaflet thickness to heart size (maximum circumference and longitudinal length from the origin of the pulmonary artery to heart apex). MVs and AVs were dissected from the hearts, chordae were removed from the MV, and circumferential and radial strips of 5 mm diameter were cut from both MV and AV (Table 1). Given known differences between the material properties of the MVAC (defined as the central region without chordal attachments) and MVF,15 circumferential strips were taken from both of these regions (Fig. 1A). MV radial strips spanned the entire leaflet height from annulus to free edge. Tissues were stored in phosphate-buffered saline (PBS) at 4°C until testing and tissues were tested within 4 days of harvesting (based on studies showing material properties of valves do not change in that time period31,32).
Table 1.
Sample Set
| Circumferential strips | Radial strips | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MV | AV | AV | |||||||||
| MVAC | MVF | NC | LC | RC | Total | MV | NC | LC | RC | Total | |
| 6-week old | 6 | 5 | 7 | 7 | 6 | 25 | 7 | 8 | 7 | 6 | 28 |
| 6-month old | 5 | 5 | 4 | 5 | 4 | 18 | 6 | 5 | 4 | 3 | 18 |
| 6-year old | 9 | 11 | 6 | 6 | 8 | 31 | 6 | 8 | 9 | 9 | 32 |
MV, mitral valve; AV, aortic valve; NC, noncoronary; MVAC, MV between the anterior center; and MVF, MV between the free edge; LC, left coronary; RC, right coronary.
FIG. 1.
(A) Orientation of tissue strips cut from the mitral valve (MV) (indicated by dashed boxes). Curved solid line indicates the border between the center (MVAC) and free edge (MVF) of the mitral value (MV) anterior leaflet. Rad, radial; circ, circumferential. (B) Diagram illustrating the placement of glued balsa/valve tissue sample construct within grips of material testing system.
Mechanical testing
The thickness of the tissue was measured at four locations using a displacement gauge (Mitutoyo, Kawasaki, Japan). Tissue samples were glued to balsa wood with cyanoacrylate and clamped within an EnduraTec ELF 3200 (Bose, Minnetonka, MN) such that the edge of the balsa wood aligned with the end of the grips (Fig. 1B). The balsa wood improved traction of the tissue within the grips, thereby preventing slippage during testing. Mechanical testing was performed in a 37°C PBS bath as described previously.33 Briefly, mechanical testing began with the tissue in an unloaded state and consisted of 25 cycles of preconditioning at 1 Hz followed by one load-elongation cycle (0.5 Hz). The tissue was then allowed to rest for 15 s before another three cycles of preconditioning and a stress relaxation test (duration 100 s).
Data analysis
Gauge length, defined as the length at which the tissue first starts bearing load, was calculated by fitting a cubic function to the pre-transition region of the load-elongation curve and then determining the tissue length corresponding to the local minimum, similar to the method used by Carew and Vesely.34 Recorded displacement values were then divided by gauge length to calculate strains and load converted to tension by dividing by tissue width. The slope of the post-transition portion of the tension–strain curve (post-transition “stiffness”) was calculated by first fitting a straight line to the maximum load and the preceding four points. Additional preceding points were added and the slope of the linear least-squares fit line was recalculated until its value changed by more than 1%. The pre-transition stiffness was determined as the slope of the linear least-squares fit between zero strain to one half of “end-transition” strain (where the end-transition point was the closest point to the origin that was used in the post-transition slope calculation). Extensibility was defined as the intersection of the linear least-squares fit of the post-transition region with the x-axis (strain). The stress relaxation data were fit to the following two-phase decay equation:
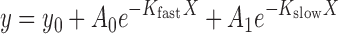 |
The Kfast and Kslow values were calculated iteratively using Graphpad Prism (GraphPad Software, La Jolla, CA). The percentage relaxation (%SR) was calculated as the difference between the initial load and the load remaining at 100 s as a percentage of the initial load. The y0 value refers to the plateau stress in the tissue (where t is approaching 100 s). A0 and A1 are best-fit values from a nonlinear regression referring to the span of the fast and slow portions of the curve. The RTC was calculated by first dividing the y-axis of the tension–strain curve by 100 N/m to make both axes dimensionless and then rotating the tension–strain curve clockwise so that pre-transition and post-transition lines were at equal angles from the vertical (which converted the vertex of the transition region to a global minimum). A hyperbola was then fit to the points in the transition region (from 0 to 1.5 × end-transition strain) and the RTC was calculated as the inverse of the second derivative of this global minimum.
Histology and immunohistochemistry
To evaluate the valvular material behavior in context of the ECM composition, representative tissue strips equivalent to those used for mechanical testing were fixed in 10% formalin overnight, dehydrated, paraffin embedded, and sectioned for histological examination. Movat pentachrome was used to visualize the different ECM components and leaflet layers. Immunohistochemistry was also performed as previously described11,35 to identify and localize the proteoglycans (PGs) decorin, biglycan, and versican (decorin [LF122] and biglycan [LF104] from Dr. Larry Fisher, NIH, Bethesda, MD;36 versican [2B1] from Associates of Cape Cod, East Falmouth, MA), the glycosaminoglycan (GAG) hyaluronan (HA, using HA-binding protein; Associates of Cape Cod), and collagen type III (Col III, which has been shown to change with age,11 LF69; from Dr. Larry Fisher37). Based on studies showing that valve cell contractility can affect valvular material behavior,38 staining was also performed for the myofibroblast marker nonmuscle myosin heavy chain-IIB (NMM; Covance, Berkeley, CA) as previously described.39 Briefly, immunohistochemical staining consisted of antigen retrieval (citrate buffer; Biocare Medical, Concord, CA), except for PGs in which chondroitinase ABC was used35 (Associates of Cape Cod), followed by quenching of endogenous peroxidases. Nonspecific staining was blocked using goat serum buffer, followed by application of primary antibody. Rinses in PBS were performed before application of the appropriate species-specific secondary antibody. Positive staining was visualized using Vectastain Elite ABC and diaminobenzidine kits (Vector Laboratories, Burlingame, CA), followed by hematoxylin counterstaining. Radially oriented strips of the MV and AV, which were previously stained in this same manner as described in a previous publication,10,11 were also used to provide context.
Statistical analysis
Multifactorial analysis of variance (ANOVA) was performed using SigmaStat (SPSS, Chicago, IL). When the data were normally distributed, an ANOVA test was used. When the data set was not normally distributed, a rank transform was performed before the ANOVA. In both cases the level of significance was set at 0.05.
Results
Thickness
All test strips cut from the MV were thicker than in the AV (p < 0.001), whether in the circumferential direction (Fig. 2) or radial direction. For all valve strips, thickness increased with age (each p < 0.001), as reported previously for human valves.40 The normalized thickness of circumferential and radial strips (whether normalized to the circumferential or longitudinal dimension of the heart) of 6-month olds was greater than for other age groups (p ≤ 0.035 for each valve or leaflet region; thickness normalized to circumference).
FIG. 2.
(A) Thickness of circumferential valve strips with age. Results for radial strips were comparable (data not shown). (B) Thickness of circumferential valve strips with age normalized to heart size (maximum circumference of heart). *,^Significantly different between ages within a given valve region. α,βValve regions significantly different for a given direction (radial or circumferential).
Post-transition and pre-transition stiffnesses
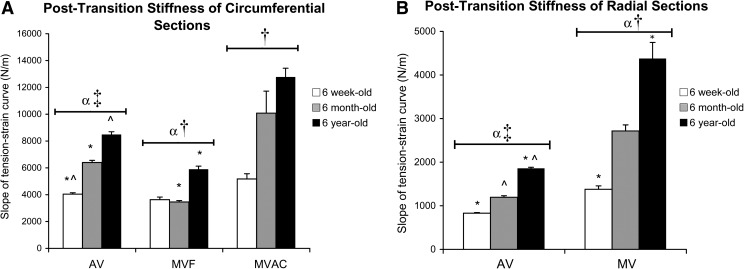
In the post-transition region of the tension–strain curves, MV and AV radial stiffness was less than circumferential (p < 0.001), consistent with previously published studies.15–20 The radial stiffness of MV was greater than for AV (p < 0.001; Fig. 3). Among circumferential strips, the stiffness of MVAC was greatest, followed by AV, and then MVF (p = 0.005). With increasing age, MV and AV radial stiffness increased (each p ≤ 0.003), as did MV and AV circumferential stiffness (each p ≤ 0.010).
FIG. 3.
Slope of post-transition region of tension–strain curves for (A) circumferential strips and (B) radial strips. *,^Significantly different between ages within a given valve region by post hoc ANOVA comparing all ages. αAV and MV significantly different for a given direction (radial or circumferential). ‡AV radial significantly different from AV circumferential; †MVAC circumferential, MVF circumferential, and MV radial strips each significantly different from one another. AV, aortic valve; ANOVA, analysis of variance.
In the pre-transition region of the curves, there were no significant differences between circumferential and radial stiffnesses in either the AV or MV. Amongst MV and AV circumferential samples, MVF pre-transition stiffness was greater than MVAC, followed by AV (Fig. 4, p < 0.001). With increasing age, the pre-transition stiffness of MV samples (both circumferential and radial) and AV radial samples increased (each p < 0.001).
FIG. 4.
Slope of the pre-transition region of tension–strain curves for (A) circumferential strips and (B) radial strips. *,^Significantly different between ages within a given valve region by post hoc ANOVA comparing all ages. α,βAV and MV significantly different for a given direction (radial or circumferential).
Stress relaxation
In the AV, radial sections showed a higher Kfast but a lower Kslow than circumferential strips (Table 2, each p < 0.001). In the MV, however, the Kfast and Kslow of MVAC circumferential strips were higher than for MV radial (each p < 0.005). Among radially oriented sections, the AV Kfast and Kslow were both greater than for the MV (each p ≤ 0.002). Among circumferential sections, Kfast of MVAC was greater than AV, followed by MVF (p < 0.001), but Kslow was greatest in AV (AV > MVAC > MVF, p < 0.001). With increasing age, AV circumferential Kfast was reduced (p = 0.006), as was MVF circumferential Kslow (p = 0.046). With respect to the relative magnitudes of relaxation, %SR was greater for AV circumferential than for AV radial (Fig. 5, p < 0.001) and greater for MVAC circumferential, followed by MVF circumferential, then MV radial (p < 0.001). Among circumferential strips, %SR of MVAC was greater than for either AV or MVF (p = 0.014). With increasing age, %SR was increased in AV circumferential strips (p = 0.009) and in MVF circumferential sections (p = 0.005).
Table 2.
Stress Relaxation Time Constants
| Valve | Valve region | Direction | Age | Kfast | Kslow |
|---|---|---|---|---|---|
| AV | Total | Rad1,2 | 6 weeks | 0.545 ± 0.025 | 0.0295 ± 0.0010 |
| 6 months | 0.596 ± 0.030 | 0.0321 ± 0.0013 | |||
| 6 years | 0.543 ± 0.023 | 0.0312 ± 0.0010 | |||
| Circ2,5,6 | 6 weeks | 0.548 ± 0.023a | 0.0361 ± 0.0010 | ||
| 6 months | 0.445 ± 0.030a | 0.0329 ± 0.0013 | |||
| 6 years | 0.485 ± 0.023 | 0.0352 ± 0.0010 | |||
| MV | Total | Rad1,3 | 6 weeks | 0.493 ± 0.046 | 0.0270 ± 0.0013 |
| 6 months | 0.477 ± 0.056 | 0.0264 ± 0.0015 | |||
| 6 years | 0.463 ± 0.056 | 0.0240 ± 0.0015 | |||
| MVF | Circ4,6 | 6 weeks | 0.457 ± 0.069 | 0.0307 ± 0.0019a | |
| 6 months | 0.468 ± 0.069 | 0.0270 ± 0.0019 | |||
| 6 years | 0.499 ± 0.044 | 0.0263 ± 0.0012a | |||
| MVAC | Circ3,4,5 | 6 weeks | 0.545 ± 0.052 | 0.0299 ± 0.0014 | |
| 6 months | 0.566 ± 0.069 | 0.0330 ± 0.0019 | |||
| 6 years | 0.707 ± 0.046 | 0.0285 ± 0.0013 |
Units for Kfast and Kslow are N/s. Data represent mean ± standard error of the mean. Samples with the same numeric superscripts indicate significant differences between Kfast as well as Kslow values for those samples across ages, except four in which Kfast was only significantly different between samples and six in which only Kslow was significantly different between samples. All differences marked represent p < 0.05.
Significant difference between indicated ages within a given valve region/direction.
Rad, radial; circ, circumferential.
FIG. 5.
Total percentage of relaxation (SR) for (A) circumferential strips and (B) radial strips. *,^Significantly different between ages within a given valve region by post hoc ANOVA comparing all ages. αValve regions significantly different for a given direction (radial or circumferential). ‡AV radial significantly different from AV circumferential. †MVAC circumferential, MVF circumferential, and MV radial strips each significantly different from one another.
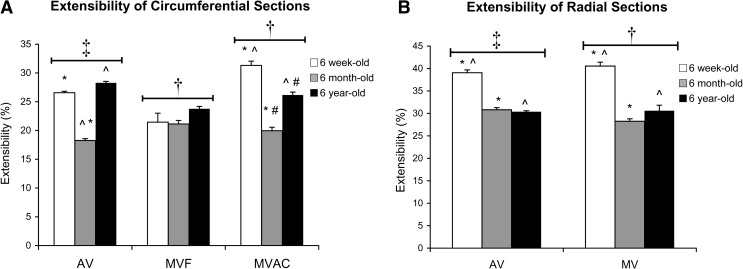
Extensibility
In both the AV and MV, circumferential strips were less extensible than radial strips (p < 0.001; Fig. 6). There were no differences between extensibilities of AV and MV strips. In both MV and AV radial strips, as well as in MVAC circumferential strips, extensibility was reduced with age (each p ≤ 0.010). There was no effect of age on the extensibility of MVF circumferential strips. Among AV circumferential strips, the 6-month-old samples were the least extensible (p < 0.001).
FIG. 6.
Extensibility for (A) circumferential strips and (B) radial strips. *,^,#Significantly different between ages within a given valve region by post hoc ANOVA comparing all ages. ‡AV radial significantly different from AV circumferential. †MV radial strips are significantly different from both MVAC and MVF circumferential strips.
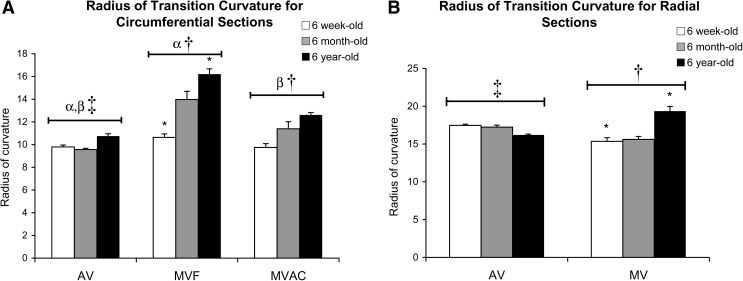
Radius of Transition Curvature (RTC)
AV circumferential RTC was less than AV radial RTC (p < 0.001; Fig. 7). MVAC circumferential RTC was less than MVF circumferential RTC and then MV radial RTC (p < 0.001). Among circumferential strips, AV RTC was less than MVAC RTC, which was less than MVF RTC (p < 0.001). With age, MVF circumferential and MV radial RTC increased (p = 0.003 and p = 0.033), but no age-associated changes were evident in AV strips.
FIG. 7.
Radius of transition curvature: (A) circumferential strips and (B) radial strips. *Significantly different between ages within a given valve region by post hoc ANOVA comparing all ages. α,βAV and MV significantly different for a given direction (radial or circumferential). ‡AV radial significantly different from AV circumferential. †MVAC circumferential, MVF circumferential, and MV radial strips each significantly different from one another.
Differences between AV leaflets
In 6-year-old AVs, the noncoronary (NC) leaflet was thicker than the left or right coronary leaflets (p = 0.007), as previously reported for human valves.40,41 However, there was no significant difference in thickness between AV leaflets in the other age groups. For both radially and circumferentially oriented strips, Kfast of the NC leaflet was greater than for the other two leaflets (each p ≤ 0.037). However, the circumferential %SR for the NC leaflet was less than for the other two leaflets (p = 0.039). RTC of AV NC leaflets was less than the coronary leaflets (p = 0.031).
Differences in valve matrix composition
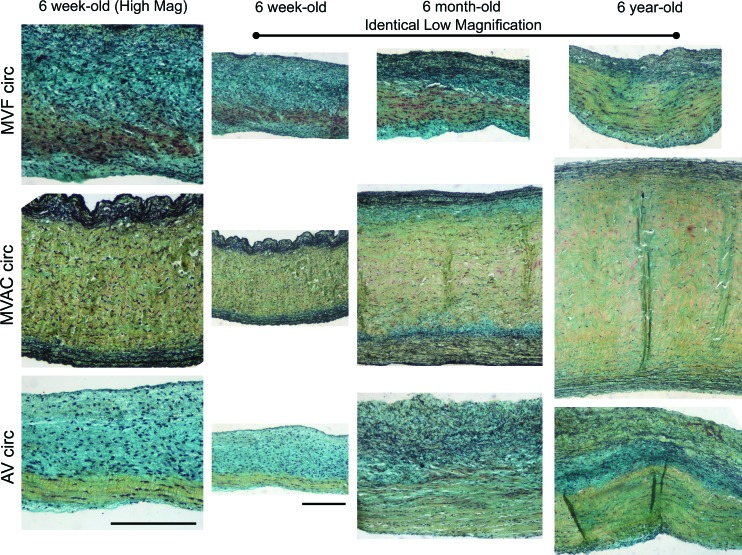
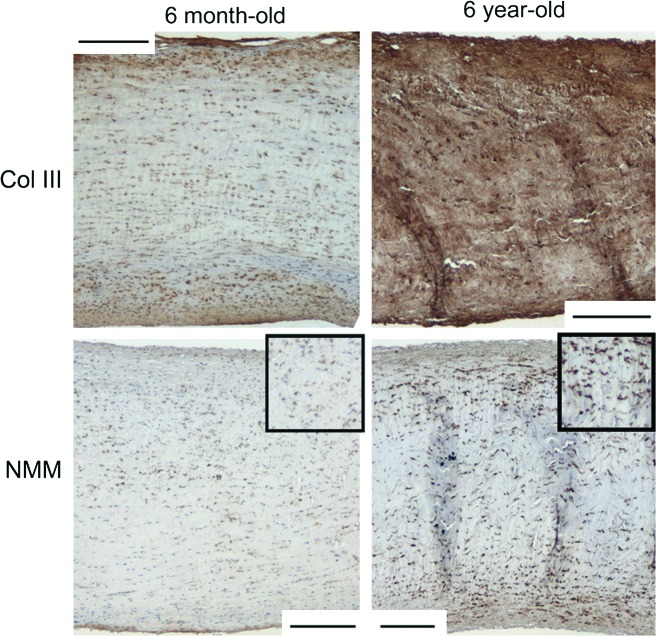
By Movat staining, it was evident that the annulus portion of MV radial strips (corresponding to the MVAC) contained a higher proportion of the collagen-rich fibrosa layer compared with AV radial strips (Fig. 8). MVAC circumferential strips contained a much thicker fibrosa than found in MVF (Fig. 9). With age, the fibrosa thickened and collagen content increased throughout the valve layers, but this increase was particularly evident in the fibrosa layer and in the ventricularis layer, where collagen surrounded elastic fibers. Interestingly, there was also a marbling of PG/GAGs (see inset in Fig. 8) in the fibrosa of the 6-year-old MV radial annulus and MVAC circumferential section, which was not evident in the younger ages. Immunohistochemistry revealed that the PGs versican, biglycan, and decorin, as well as the GAG HA, were present within this marbling. Circumferential strips also contained more Col III with age (Fig. 10), as found previously for radial strips.11 The myofibroblast marker NMM was more strongly expressed in 6-year-old strips of MVAC than in younger valves.
FIG. 8.
Representative Movat-stained tissue sample sections for 6-week-old and 6-year-old radial strips. In Movat pentachrome-stained tissue, yellow = aligned collagen, black = elastic fibers, and green/blue = proteoglycans (PGs)/glycosaminoglycans (GAGs). To facilitate comparison between AV and MV, valves were all oriented with the fibrosa at the bottom of the image and sized to show the complete leaflet thickness allowing visualization of all leaflet layers. Because of differences in leaflet thickness (see Fig. 2), magnifications differ between images. Scale bars equal 200 μm. Note that the MV radial annulus is almost exclusively fibrosa (corresponding to the MVAC), whereas AV radial annulus shows a considerably smaller proportion of the leaflet thickness composed of fibrosa. Also of note is the marbling of PG/GAG with collagen in the fibrosa of 6-year-old MV radial annular strips (see close-up view in inset) and MVAC circumferential strips compared with 6-week-old strips. This marbling may, in part, account for the increase in SR seen with age (see Discussion section). Color images available online at www.liebertonline.com/ten.
FIG. 9.
Representative Movat-stained tissue sample sections for 6-week-old, 6-month-old, and 6-year-old circumferential strips. In Movat pentachrome-stained tissue, yellow = aligned collagen, black = elastic fibers, and green/blue = PGs/GAGs. To facilitate comparison between AV and MV, valves were all oriented with the fibrosa at the bottom of the image. Images in column 1 show high magnification of 6-week-old valves (all images in column 1 are the same magnification). Images in columns 2–4 are lower magnification to demonstrate the differences in leaflet thickness (all images in columns 2–4 are the same magnification). Scale bars equal 200 μm. Compared with MVF, MVAC circumferential sections contain much higher proportion of fibrosa. For all valve regions an increase in collagen was noted throughout the valve layers with age, but most particularly in the fibrosa and ventricularis. Color images available online at www.liebertonline.com/ten.
FIG. 10.
MVAC circumferential sections showing increased Col III and NMM in 6-year-old sections compared with 6-month-old sections. Similar differences were noted for the staining of Col III for other valve regions between the 6-year-old aged sections and the younger ages. Scale bar equals 200 μm. Insets in NMM images are 1.5 × higher magnification for improved visualization of cells. Col III, collagen type III; NMM, nonmuscle myosin heavy chain-IIB. Color images available online at www.liebertonline.com/ten.
Discussion
This study showed for the first time that there are significant age-related differences in numerous material properties of MV and AV. At each age, the valves were more extensible and less stiff radially than circumferentially, as is well known.15–20 All valves, however, demonstrated age-associated increases in post-transition stiffness and most also showed increased pre-transition stiffness. In general, extensibility was reduced with age, especially radially. Stress relaxation parameters also varied with age, particularly circumferentially. The overall shape of the tension–strain curves also changed with age, as demonstrated by the novel parameter RTC, which generally increased with age in the MV and was larger radially than circumferentially. These age-related changes in material properties paralleled an increase in collagen content, particularly in the fibrosa and ventricularis (which is normally rich in elastic fibers), and a marbling appearance of the fibrosa, in which PG/GAGs were interspersed throughout the collagen. Because this study tested MV and AV from the same hearts, it was also possible to demonstrate differences in material properties between MV and AV.
With increasing age, the leaflets showed greater post-transition stiffness both radially and circumferentially. This finding is consistent with age-related stiffening of other connective tissues such as cartilage42 and tendon,43 which are mainly attributed to increased collagen crosslinking.43 Leaflet composition explains much of the differences in stiffness among sample groups. For example, the preponderance of the collagenous fibrosa layer within the MV annulus likely contributes to the greater radial stiffness of MV compared with AV and the greater circumferential stiffness of MVAC compared with MVF. Age-related increases in fibrosa thickness and collagen content shown here for numerous valve regions, as well as previously reported increases in collagen1,2 and in crosslinking2,4 in aging valves, could explain the increased stiffness with age. A greater proportion of cells in the 6-year-old MVAC expressed the activated myofibroblast phenotype, which has been linked with increased leaflet stiffness.38 In addition, collagen crosslinking has been directly correlated with the stiffness of circumferential strips of human AV, although no such correlation was found for radial strips.26 Combined with evidence suggesting that the ventricularis is a key contributor to valvular material behavior in the radial direction,22,44 the age-related increase in radial stiffness could be due to increased collagen in the ventricularis, as observed in the Movat-stained sections.
Interestingly, the pre-transition stiffness also significantly increased with age. This parameter may be due in part to the stiffness of the elastic fibers,45 but is not often reported15,16,18,46 and has never previously been studied with respect to age. The increase in pre-transition stiffness with age could reflect the stiffening of aging elastic fibers,43 as well as increased collagenous reinforcement of the elastic fibers, which was observed in Movat-stained sections. It was also noteworthy that the age-related increases in the thickness of the valve leaflets40 were not proportional to the increase in heart size, possibly due to the ventricular hypertrophy found in older animals.47,48
Accompanying the age-related increase in the post-transition stiffness was a pronounced reduction in radial extensibility of older valves. Extensibility assesses the amount of tissue that can stretch before the collagen fibers are fully uncrimped and able to bear load;21 its magnitude reflects both collagen fiber crimp and alignment. The finding that extensibility is greater radially than circumferentially confirms previous reports;16–18,20 this anisotropy permits the leaflets to stretch radially during valve closure. These findings are also consistent with a previous report on an age-related reduction in maximal radial stretch of human cryopreserved valves.9 A reduction in extensibility with age could be attributed to factors that impact collagen uncrimping, such as increased collagen crosslinking2,4 or elastic fiber fragmentation,43 which can lead to gradual permanent tissue stretch and less-crimped collagen fibers. Indeed, in tendon collagen, crimp amplitude decreases and crimp wavelength increases with age;49 these extensibility changes motivate future study of collagen crimp in aging heart valves. Similarly, heart valves subjected to glutaraldehyde fixation (crosslinking) under pressure demonstrated reduced collagen crimp and extensibility.50
The novel finding of an increase in the percentage of circumferential stress relaxation (in AV and MVF) with age could be due to many factors. First, there is more HA with age10 as well as marbling of PGs/GAGs in the collagenous fibrosa, which would allow more sliding of collagen bundles relative to one another.51 Alternatively, the greater numbers of the PGs decorin and biglycan,10 which bind to the surface of collagen fibrils,52 could be transferring more load from collagen to other matrix components, resulting in greater %SR. Indeed, age-related changes in %SR were most evident in the circumferential direction, the predominant collagen fiber direction.53 Reductions in the functionality43 and content10 of elastic fibers could also increase stress relaxation, according to studies on pericardium.54 Lastly, the greater abundance of Col III, which dissipates more energy than does collagen type I,55 could have contributed to the greater %SR with age. There is some disagreement in the heart valve literature regarding anisotropy in stress relaxation.14,17,20,23,24 Our finding of greater %SR in circumferential strips compared with radial strips corroborates the work by Lee et al., who measured the %SR of fresh leaflets over 1000 s using uniaxial testing.20
The viscoelasticity of valve tissues has been examined using a wide variety of constitutive models,17,19,33,56–59 but the effects of age have not been previously investigated. In particular, the quasi-linear viscoelastic (QLV) model developed by Fung60 has been applied to fresh and fixed valve tissues;19,33,56 analysis using this technique, however, is computationally intense. In this study, a Maxwell–Weichert model with three elements was used to examine biphasic decay behavior of the stress relaxation curve.61,62 The age-related decreases in Kfast of AV circumferential samples and in Kslow of MVF samples both indicate more viscous behavior, which could relate to the increased abundance of PGs and GAGs.10 The Kfast and Kslow values reported in this study also serve as baseline parameters for evaluating mechanical properties of TEHVs. While current TEHV studies have focused primarily on linear material properties, understanding the viscoelastic properties will continue to be of significant value, especially as more advanced materials are developed.
The novel parameter RTC, which quantifies the acuteness of the transition region of the tension–strain curve, can be compared across samples for any soft tissues that display bilinear tension–strain or stress–strain curves. Collagen fibers that are highly aligned and uniformly crimped will have a very acute transfer of load bearing from elastic fibers to the straightened collagen fibers, and hence a very small RTC. If collagen fibers are not uniformly crimped, their transition to load bearing would be more gradual, resulting in a greater RTC. A larger RTC would also result from a random fiber alignment, in which some fibers would be initially aligned with the applied load and begin bearing load at low strains, but the remaining collagen bundles would require varying amounts of additional strain and/or rotational realignment before bearing load.21 Indeed, the angular distribution of collagen fibers significantly changes within this transition region.21 The smaller RTC of MVAC circumferential strips compared with MVF likely indicates greater alignment53 and homogeneity of collagen fiber crimping in MVAC compared with the other valve regions. In the valve, collagen bundles are predominantly oriented circumferentially,53 and therefore, RTC would be lower circumferentially than radially, as found here. The age-related increase in RTC of MVF and MV radial strips could be due to greater collagen crosslinking2,4 and a greater abundance of network-forming Col III. In future, it will be important to relate this parameter to changes in the soft tissue microstructure during this transition region of loading.
This study is also the first to show differences in material properties between NC and coronary AV leaflets, such as lower %SR and RTC in the NC leaflet. These differences may be due to the lack of coronary blood flow within the NC sinus; simulations of human AVs have shown that NC experiences higher stress63 and greater diastolic pressure loading than the other leaflets.64 The NC leaflet also contains smaller diameter collagen fiber bundles,65 which may permit easier rotation of collagen fibers as they align in the direction of loading, and hence contribute to a lower RTC. An additional subset of valves was frozen at −20°C for 2 months before testing; as previously demonstrated by Clark,18 significant differences were found between fresh AV and previously frozen AV (data not shown).
Conclusion
In this study, AV and MV from the same porcine hearts were subjected to tensile testing. In the analysis of the tensile testing data, we developed a novel parameter, RTC, which may be useful in quantifying the dynamic stress–strain behavior of biological tissues. Overall, our results showed that between AV and MV, and within each valve, there are profound heterogeneities and age-related changes that reflect the ECM and microstructural composition. In addition, the effects of age on the heart valve tissues differ between valves, valve regions, and material testing orientations. Given the documented similarities between porcine and human valves, these results provide further justification (as well as baseline data) for the development of age-specific TEHVs. These age-related changes in valve material properties likely contribute to proper physiologic function of valves at different ages. For instance, the age-related increase in leaflet stiffness likely allows the AV and MV to withstand the increase in cardiac pressures and concomitant decrease in aortic compliance with age.12 Clearly, considerable work remains in understanding the structural and mechanical complexity of heart valves, their ECM and cell composition, and the contributions of these characteristics to valve function.
Acknowledgments
The authors thank Janet Barzilla, Ph.D., Joyce J. Kuo, B.S., and Daniel Laucirica in the Grande-Allen lab, and Anita Mol, Ph.D., and Martijn Cox, M.Sc., from the Eindhoven University of Technology exchange program. Special thanks also to Scott Baggett, Ph.D., for statistical expertise; Benjamin K. Stephens, Ph.D., for mathematical consultation regarding curve fitting and radius of curvature analysis; Roman M. Natoli, Ph.D., for consultation on constitutive modeling; and Larry Fisher, Ph.D., for the generous gift of multiple antibodies.
Disclosure Statement
No competing financial interests exist.
References
- 1.Keller F. Leutert G. [Age dependence of collagen structures of the human heart] Z Gerontol. 1994;27:186. [PubMed] [Google Scholar]
- 2.Angrist A. Aging heart valves and a unitary pathological hypothesis for sclerosis. J Gerontol. 1964;19:135. doi: 10.1093/geronj/19.2.135. [DOI] [PubMed] [Google Scholar]
- 3.Aikawa E. Whittaker P. Farber M. Mendelson K. Padera R.F. Aikawa M. Schoen F.J. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006;113:1344. doi: 10.1161/CIRCULATIONAHA.105.591768. [DOI] [PubMed] [Google Scholar]
- 4.Bashey R.I. Torii S. Angrist A. Age-related collagen and elastin content of human heart valves. J Gerontol. 1967;9:203. doi: 10.1093/geronj/22.2.203. [DOI] [PubMed] [Google Scholar]
- 5.Sell S. Scully R.E. Aging changes in the aortic and mitral valves. Histologic and histochemical studies, with observations on the pathogenesis of calcific aortic stenosis and calcification of the mitral annulus. Am J Pathol. 1965;46:345. [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald P.C. Wilson J.E. McNeill S. Gao M. Spinelli J.J. Rosenberg F. Wiebe H. McManus B.M. The challenge of defining normality for human mitral and aortic valves: geometrical and compositional analysis. Cardiovasc Pathol. 2002;11:193. doi: 10.1016/s1054-8807(01)00102-8. [DOI] [PubMed] [Google Scholar]
- 7.Goldbarg S.H. Elmariah S. Miller M.A. Fuster V. Insights into degenerative aortic valve disease. J Am Coll Cardiol. 2007;50:1205. doi: 10.1016/j.jacc.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Aronow W.S. Heart disease and aging. Med Clin North Am. 2006;90:849. doi: 10.1016/j.mcna.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Christie G.W. Barratt-Boyes B.G. Age-dependent changes in the radial stretch of human aortic valve leaflets determined by biaxial testing. Ann Thorac Surg. 1995;60:S156. doi: 10.1016/0003-4975(95)00219-b. [DOI] [PubMed] [Google Scholar]
- 10.Stephens E.H. Chu C.-K. Grande-Allen K.J. Valve proteoglycan content and glycosaminoglycan fine structure are unique to microstructure, mechanical load and age: relevance to an age-specific tissue-engineered heart valve. Acta Biomaterialia. 2008;4:1148. doi: 10.1016/j.actbio.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stephens E.H. Grande-Allen K. Age-related changes in collagen synthesis and turnover in porcine heart valves. J Heart Valve Dis. 2007;16:672. [PubMed] [Google Scholar]
- 12.VanAuker M.D. Age-related changes in hemodynamics affecting valve performance. Am J Geriatr Cardiol. 2006;15:277. doi: 10.1111/j.1527-5299.2006.04877.x. [DOI] [PubMed] [Google Scholar]
- 13.Grashow J. Yoganathan A.P. Sacks M.S. Biaxial stress-stretch behavior of the mitral valve anterior leaflet at physiologic strain rates. Ann Biomed Eng. 2006;34:315. doi: 10.1007/s10439-005-9027-y. [DOI] [PubMed] [Google Scholar]
- 14.Stella J. Liao J. Sacks M.S. Time-dependent biaxial mechanical behavior of the aortic heart valve leaflet. J Biomech. 2007;40:3169. doi: 10.1016/j.jbiomech.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunzelman K.S. Cochran R.P. Stress/strain characteristics of porcine mitral valve tissue: parallel versus perpendicular collagen orientation. J Card Surg. 1992;7:71. doi: 10.1111/j.1540-8191.1992.tb00777.x. [DOI] [PubMed] [Google Scholar]
- 16.May-Newman K. Yin F. Biaxial mechanical behavior of excised porcine mitral valve leaflets. Am J Physiol. 1995;269:H1319. doi: 10.1152/ajpheart.1995.269.4.H1319. [DOI] [PubMed] [Google Scholar]
- 17.Leeson-Dietrich J. Boughner D. Vesely I. Porcine pulmonary and aortic valves: a comparison of their tensile viscoelastic properties at physiological strain rates. J Heart Valve Dis. 1995;4:88. [PubMed] [Google Scholar]
- 18.Clark R. Stress-strain characteristics of fresh and frozen human aortic and mitral leaflets and chordae tendineae. Implications for clinical use. J Thorac Cardiovasc Surg. 1973;66:202. [PubMed] [Google Scholar]
- 19.Sauren A. van Hout M. van Steenhoven A. Veldpaus F. Janssen J. The mechanical properties of porcine aortic valve tissues. J Biomech. 1983;16:327. doi: 10.1016/0021-9290(83)90016-7. [DOI] [PubMed] [Google Scholar]
- 20.Lee J. Courtman D.W. Boughner D. The glutaraldehyde-stabilized porcine aortic valve xenograft. I. Tensile viscoelastic properties of the fresh leaflet material. J Biomed Mater Res. 1984;18:61. doi: 10.1002/jbm.820180108. [DOI] [PubMed] [Google Scholar]
- 21.Liao J. Yang L. Grashow J. Sacks M.S. The relation between collagen fibril kinematics and mechanical properties in the mitral valve anterior leaflet. J Biomech Eng. 2007;129:78. doi: 10.1115/1.2401186. [DOI] [PubMed] [Google Scholar]
- 22.Vesely I. Noseworthy R. Micromechanics of the fibrosa and the ventricularis in aortic valve leaflets. J Biomech. 1992;25:101. doi: 10.1016/0021-9290(92)90249-z. [DOI] [PubMed] [Google Scholar]
- 23.Grashow J. Sacks M.S. Liao J. Yoganathan A.P. Planar biaxial creep and stress relaxation of the mitral valve anterior leaflet. Ann Biomed Eng. 2006;34:1509. doi: 10.1007/s10439-006-9183-8. [DOI] [PubMed] [Google Scholar]
- 24.Vesely I. Boughner D. Leeson-Dietrich J. Bioprosthetic valve tissue viscoelasticity: implications on accelerated pulse duplicator testing. Ann Thorac Surg. 1995;60:S379. doi: 10.1016/0003-4975(95)00261-i. [DOI] [PubMed] [Google Scholar]
- 25.Merryman W.D. Engelmayr G.C., Jr. Liao J. Sacks M.S. Defining biomechanical endpoints for tissue engineered heart valve leaflets from native leaflet properties. Prog Pediatr Cardiol. 2006;21:153. [Google Scholar]
- 26.Balguid A. Rubbens M. Mol A. Bank R. Bogers A. van Kats J. de Mol B. Baaijens F. Bouten C. The role of collagen cross-links in biomechanical behavior of human aortic heart valve leaflets—relevance for tissue engineering. Tissue Eng. 2007;13:1501. doi: 10.1089/ten.2006.0279. [DOI] [PubMed] [Google Scholar]
- 27.Grande-Allen K.J. Calabro A. Gupta V. Wight T.N. Hascall V.C. Vesely I. Glycosaminoglycans and proteoglycans in normal mitral valve leaflets and chordae: association with regions of tensile and compressive loading. Glycobiology. 2004;14:621. doi: 10.1093/glycob/cwh076. [DOI] [PubMed] [Google Scholar]
- 28.Crick S. Sheppard M. Ho S. Gebstein L. Anderson R. Anatomy of the pig heart: comparisons with normal human cardiac structure. J Anat. 1998;193:105. doi: 10.1046/j.1469-7580.1998.19310105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sands M.P. Rittenhouse E.A. Mohri H. Merendino K.A. An anatomical comparison of human pig, calf, and sheep aortic valves. Ann Thorac Surg. 1969;8:407. doi: 10.1016/s0003-4975(10)66071-7. [DOI] [PubMed] [Google Scholar]
- 30.Sim E.K. Muskawad S. Lim C.S. Yeo J.H. Lim K.H. Grignani R.T. Durrani A. Lau G. Duran C. Comparison of human and porcine aortic valves. Clin Anat. 2003;16:193. doi: 10.1002/ca.10149. [DOI] [PubMed] [Google Scholar]
- 31.Patel J. Effect of absolute specimen size on the tensile properties of porcine aortic valve tissues [masters thesis] Case Western Reserve; Cleveland, OH: 2003. [Google Scholar]
- 32.Grande-Allen K. Barber J. Klatka K. Houghtaling P. Vesely I. Moravec C. McCarthy P. Mitral valve stiffening in end-stage heart failure: evidence of an organic contribution to functional mitral regurgitation. J Thorac Cardiovasc Surg. 2005;130:783. doi: 10.1016/j.jtcvs.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 33.Carew E. Barber J. Vesely I. Role of preconditioning and recovery time in repeated testing of aortic valve tissues: validation through quasilinear viscoelastic theory. Ann Biomed Eng. 2000;28:1093. doi: 10.1114/1.1310221. [DOI] [PubMed] [Google Scholar]
- 34.Carew E. Vesely I. A new method of estimating gauge length for porcine aortic valve test specimens. J Biomech. 2003;36:1039. doi: 10.1016/s0021-9290(03)00035-6. [DOI] [PubMed] [Google Scholar]
- 35.Gupta V. Barzilla J.E. Mendez J.S. Stephens E.H. Lee E.L. Collard C.D. Laucirica R. Weigel P.H. Grande-Allen K.J. Abundance and location of proteoglycans and hyaluronan within normal and myxomatous mitral valves. Cardiovasc Pathol. 2009;18:191. doi: 10.1016/j.carpath.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisher L.W. Stubbs J.T., III Young M.F. Antisera and cDNA probes to human and certain animal model bone matrix noncollagenous proteins. Acta Orthop Scand Suppl. 1995;266:61. [PubMed] [Google Scholar]
- 37.Bernstein E.F. Chen Y.Q. Kopp J.B. Fisher L. Brown D.B. Hahn P.J. Robey F.A. Lakkakorpi J. Uitto J. Long-term sun exposure alters the collagen of the papillary dermis. Comparison of sun-protected and photoaged skin by northern analysis, immunohistochemical staining, and confocal laser scanning microscopy. J Am Acad Dermatol. 1996;34:209. doi: 10.1016/s0190-9622(96)80114-9. [DOI] [PubMed] [Google Scholar]
- 38.Merryman W. Huang H. Schoen F. Sacks M. The effects of cellular contraction on aortic valve leaflet flexural stiffness. J Biomech. 2006;39:88. doi: 10.1016/j.jbiomech.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Stephens E.H. Nguyen T.C. Itoh A. Ingels N.B., Jr. Miller D.C. Grande-Allen K.J. The effects of mitral regurgitation alone are sufficient for leaflet remodeling. Circulation. 2008;118:S243. doi: 10.1161/CIRCULATIONAHA.107.757526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sahasakul Y. Edwards W.D. Naessens J. Tajik A. Age-related changes in aortic and mitral valve thickness: implications for two-dimensional echocardiography based on an autopsy study of 200 normal human hearts. Am J Cardiol. 1988;62:424. doi: 10.1016/0002-9149(88)90971-x. [DOI] [PubMed] [Google Scholar]
- 41.Silver M. Roberts W.C. Detailed anatomy of the normally functioning aortic valve in hearts of normal and increased weight. Am J Cardiol. 1985;55:454. doi: 10.1016/0002-9149(85)90393-5. [DOI] [PubMed] [Google Scholar]
- 42.Charlebois M. McKee M.D. Buschmann M.D. Nonlinear tensile properties of bovine articular cartilage and their variation with age and depth. J Biomech Eng. 2004;126:129. doi: 10.1115/1.1688771. [DOI] [PubMed] [Google Scholar]
- 43.Bailey A.J. Molecular mechanisms of ageing in connective tissues. Mech Ageing Dev. 2001;122:735. doi: 10.1016/s0047-6374(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 44.Stella J. Sacks M.S. On the biaxial mechanical properties of the layers of the aortic valve leaflet. J Biomech Eng. 2007;129:757. doi: 10.1115/1.2768111. [DOI] [PubMed] [Google Scholar]
- 45.Vesely I. The role of elastin in aortic valve mechanics. J Biomech. 1998;31:115. doi: 10.1016/s0021-9290(97)00122-x. [DOI] [PubMed] [Google Scholar]
- 46.Ghista D.N. Rao A.P. Mitral-valve mechanics—stress-strain characteristics of excised leaflets, analysis of its functional mechanics and its medical application. Med Biol Eng. 1973;11:691. doi: 10.1007/BF02478657. [DOI] [PubMed] [Google Scholar]
- 47.Safar M. Ageing and its effects on the cardiovascular system. Drugs. 1990;39:1. doi: 10.2165/00003495-199000391-00003. [DOI] [PubMed] [Google Scholar]
- 48.Gardin J. Savage D. Ware J. Henry W. Effect of age, sex, and body surface area on echocardiographic left ventricular wall mass in normal subjects. Hypertension. 1987;9:II36. doi: 10.1161/01.hyp.9.2_pt_2.ii36. [DOI] [PubMed] [Google Scholar]
- 49.Diamant J. Keller A. Baer E. Litt M. Arridge R.G. Collagen; ultrastructure and its relation to mechanical properties as a function of ageing. Proc R Soc Lond B Biol Sci. 1972;180:293. doi: 10.1098/rspb.1972.0019. [DOI] [PubMed] [Google Scholar]
- 50.Broom N.D. Thomson F.J. Influence of fixation conditions on the performance of glutaraldehyde-treated porcine aortic valves: towards a more scientific basis. Thorax. 1979;34:166. doi: 10.1136/thx.34.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scott J. Elasticity in extracellular matrix shape modules of tendon, cartilage, etc. A sliding proteoglycan-filament model. J Physiol. 2003;553:335. doi: 10.1113/jphysiol.2003.050179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kinsella M.G. Bressler S.L. Wight T.N. The regulated synthesis of versican, decorin, and biglycan: extracellular matrix proteoglycans that influence cellular phenotype. Crit Rev Eukaryot Gene Expr. 2004;14:203. doi: 10.1615/critreveukaryotgeneexpr.v14.i3.40. [DOI] [PubMed] [Google Scholar]
- 53.Cochran R.P. Kunzelman K.S. Chuong C.J. Sacks M.S. Eberhart R.C. Nondestructive analysis of mitral valve collagen fiber orientation. ASAIO Trans. 1991;37:M447. [PubMed] [Google Scholar]
- 54.Rabkin S. Berghause D.G. Bauer H.F. Mechanical properties of the isolated canine pericardium. J Appl Physiol. 1974;36:69. doi: 10.1152/jappl.1974.36.1.69. [DOI] [PubMed] [Google Scholar]
- 55.Silver F. Horvath I. Foran D. Mechanical implications of the domain structure of fiber-forming collagens: comparison of the molecular and fibrillar flexibilities of the alpha1-chains found in types I-III collagen. J Theor Biol. 2002;216:243. doi: 10.1006/jtbi.2002.2542. [DOI] [PubMed] [Google Scholar]
- 56.Carew E.O. Talman E.A. Boughner D.R. Vesely I. Quasi-Linear Viscoelastic theory applied to internal shearing of porcine aortic valve leaflets. J Biomech Eng. 1999;121:386. doi: 10.1115/1.2798335. [DOI] [PubMed] [Google Scholar]
- 57.Carew E. Garg A. Barber J. Vesely I. Stress relaxation preconditioning of porcine aortic valves. Ann Biomed Eng. 2004;32:563. doi: 10.1023/b:abme.0000019176.49650.19. [DOI] [PubMed] [Google Scholar]
- 58.Duncan A.C. Boughner D. Vesely I. Dynamic glutaraldehyde fixation of a porcine aortic valve xenograft. I. Effect of fixation conditions on the final tissue viscoelastic properties. Biomaterials. 1996;17:1849. doi: 10.1016/0142-9612(96)00006-3. [DOI] [PubMed] [Google Scholar]
- 59.Duncan A.C. Boughner D. Vesely I. Viscoelasticity of dynamically fixed bioprosthetic valves. II. Effect of glutaraldehyde concentration. J Thorac Cardiovasc Surg. 1997;113:302. doi: 10.1016/S0022-5223(97)70327-1. [DOI] [PubMed] [Google Scholar]
- 60.Fung Y.C. Biomechanics: Mechanical Properties of Living Tissues. 2nd. New York: Springer-Verlag; 1993. pp. 277–292. [Google Scholar]
- 61.Peleg M. Normand M. Comparison of 2 methods for stress-relaxation data presentation of solid foods. Rheol Acta. 1983;22:108. [Google Scholar]
- 62.Mayne A.S. Christie G.W. Smaill B.H. Hunter P.J. Barratt-Boyes B.G. An assessment of the mechanical properties of leaflets from four second-generation porcine bioprostheses with biaxial testing techniques. J Thorac Cardiovasc Surg. 1989;98:170. [PubMed] [Google Scholar]
- 63.Grande K.J. Cochran R.P. Reinhall P.G. Kunzelman K.S. Stress variations in the human aortic root and valve: the role of anatomic asymmetry. Ann Biomed Eng. 1998;26:534. doi: 10.1114/1.122. [DOI] [PubMed] [Google Scholar]
- 64.Lin S. Liu C. Young S. Lin M. Chiou C. Age-related changes in aortic valve with emphasis on the relation between pressure loading and thickened leaflets of the aortic valves. Int J Cardiol. 2005;103:272. doi: 10.1016/j.ijcard.2004.08.079. [DOI] [PubMed] [Google Scholar]
- 65.Doehring T. Kahelin M. Vesely I. Mesostructures of the aortic valve. J Heart Valve Dis. 2005;14:679. [PubMed] [Google Scholar]