Introduction
As identified in previously published articles, including those published in this issue, the detection of early cognitive impairment among older adult populations is worthy of diagnostic and clinical recognition. Several definitions and classifications have been applied to this form of cognitive impairment over time1–4 including mild cognitive impairment (MCI),4–6 cognitive impairment no dementia,7,8 malignant senescent forgetfulness,9 and age-associated cognitive decline.10 Consistent with current usage this review uses the term MCI, and focuses on progression from MCI to dementia.
Prevalence estimates of MCI among populations of community-dwelling older adults are as high as 22% of those aged 71 years and older,11 with prevalence rates among older adults cared for in memory care practices estimated at nearly 40%.12 The likelihood of progression from MCI to any form of dementia has been suggested to occur at a rate 3 to 5 times higher than those with normal cognition,4,13–15 with an annual rate of progression of 12% in the general population and up to 20% in populations at higher risk.11 As a transitional stage of early cognitive impairment, much attention has been focused on the identification of modifiable and nonmodifiable risk factors to prevent or delay the progression of MCI to dementia.
Several known risk factors for the development of dementias have been identified, including age, genetic characteristics, lower educational attainment, and various clinical characteristics.16–20 Among those with MCI, several risk factors influencing the progression to dementia have been identified and are discussed in detail in this clinical review. Early studies aiming to prevent the onset of Alzheimer-type dementia among older adults with amnestic MCI have been reported, including a study using the only approved pharmacologic class in dementia, acetylcholinesterase inhibitors, or vitamin E. The investigators reported that acetylcholinesterase inhibitors did not delay the progression to Alzheimer-type dementia over 3 years, except in those who were carriers of an apolipoprotein ε4 (APOE ε4) allele.21 A second study evaluating donepezil in reducing the progression of MCI did not show significant improvement in cognition with higher adverse events and dropouts, but did not evaluate for the presence of APOE alleles or the diagnosis of dementia as an outcome.22 A second prevention study found that cognitive training did not delay the progression from MCI to dementia.23 Additional studies evaluating potential methods to delay or reduce the likelihood of progression from MCI to dementia have generally found no evidence of support for nonsteroidal anti-inflammatory drugs (NSAIDs) or ginkgo biloba,24–26 further limiting the potential therapeutic options.
To assist clinicians in understanding factors associated with the progression from MCI to dementia, this article reviews both markers of disease activity and clinical risk factors influencing the progression of MCI to dementia. Biomarkers and imaging characteristics have been extensively studied as pathologic indicators of neurologic disease. The authors first review the validity of biomarkers in monitoring the progression from MCI to dementia. Risk factors for the progression of MCI to dementia are then reviewed, and categorized as modifiable and nonmodifiable. Modifiable risk factors, such as cardiovascular risk factors, depression, or adverse drug effects, are defined as characteristics that, if manipulated (such as improved success at treatment goals), may modify the risk or rate of progression to dementia. Nonmodifiable risk factors are defined as risk factors that cannot be manipulated, such as demographic or genetic characteristics. Such categorization may provide a platform for future clinical interventions targeting modifiable risk factors to reduce the progression of MCI to dementia.
Markers of Disease Process
Imaging
Progressive atrophy of the brain can be detected by structural magnetic resonance imaging (MRI), and has been shown to monitor the disease process in those developing Alzheimer-type dementia.27–30 Structural MRI has proven sensitivity in monitoring changes with aging,31–33 and is also sensitive to the detection of a more rapid rate of change as seen when cognitive symptoms progress to dementia.34 The patterns of change within various regions of the cortex have been shown to have predictive value for progressive cognitive impairment and Alzheimer-type dementia among those with MCI.35–37 A small study of Italian older adults with MCI found that atrophy of the medial temporal lobe identified on MRI was a better predictor of the progression from MCI to any form of dementia than were baseline neuropsychiatric assessments and demographic characteristics.38
White matter lesions (WML) are recognized as areas of higher signal intensity on MRI and are common in older adults, with higher frequencies among those with vascular risk factors.39–41 Compared with healthy controls, these lesions are progressively more extensive among those with MCI and both vascular and Alzheimer-type dementia.42,43 A higher severity of lesions has also been identified in those with worse cognitive impairment reported by neuropsychiatric assessment in comparison with healthy controls.41,44 A recent study by Devine and colleagues45 evaluated the WML severity and the time to conversion to any form of dementia among a small group of older adults with MCI receiving care in a memory clinic in the United Kingdom. The investigators found no relationship between WML severity and the time to progression to dementia. Age and subtype of dementia were the only independent variables associated with a shorter time to conversion to dementia, noting that more advanced age and the amnestic subtype of MCI progressed to dementia sooner. As Alzheimer-type dementia was the primary type of dementia diagnosed in this group (62%), this result appears to be consistent with a recent review by Debette and Markus46 suggesting that WML reflect cardiovascular disease burden and are more consistently associated with an increased risk of the progression to vascular or mixed dementias, but not Alzheimer-type dementia. However, a recent article by Brickman and colleagues47 shows an association between white matter hyperintensities and incident Alzheimer-type dementia in a population without MCI at baseline. The conflicting evidence underscores a lack of accepted opinion in this matter. In addition, a study by Bombois and colleagues48 also supports the association of imaging with vascular and mixed dementia, noting that increasing amounts of subcortical hyperintensities were only associated with vascular or mixed dementias.
Central and Peripheral Biomarkers
Cerebrospinal fluid (CSF) biomarkers, focusing on detection of Tau proteins and amyloid-β (Aβ) proteins, have also been studied as predictors of the progression of MCI to Alzheimer-type dementia.49–52 Because of their predictive value, structural MRI and CSF biomarkers have been used as outcome measures to increase trial power and reduce the sample-size burden.53,54 In addition, biomarkers have been incorporated into recently released recommendations from the National Institute on Aging and the Alzheimer's Association for the recognition of MCI and stratification into diagnostic subtypes of MCI that better describe the risk of progression to Alzheimer-type dementias.55
Time to progression of MCI has been evaluated by CSF biomarkers and MRI results in 91 Dutch older adults with MCI who progressed to Alzheimer-type dementia.56 Predictors of time to progression of MCI to Alzheimer-type dementia included only atrophy of medial temporal lobe (hazard ratio [HR] 2.6, 95% confidence interval [CI] 1.1–6.1; P = .03), whereas APOE ε4 genotype and Aβ1–42 were not associated with time to progression to Alzheimer-type dementia. This result suggests that markers of brain injury (such as Tau proteins or imaging results suggesting progressive neurologic damage), but not pathologic markers (such as Aβ1–42), may better predict the time to progression to Alzheimer-type dementia among older adults with MCI.56
Peripheral biomarkers have also been considered, given the challenges in acquiring CSF samples and translating CSF studies to the general population. To date the evaluation of peripheral markers, including various species of cytokines,57–59 Aβ and other serum proteins, 60–68 and APOE ε isoforms,69 have not identified clear and reproducible peripheral biomarkers associated with an increased risk of Alzheimer-type dementia. Llano and colleagues70 recently published on the use of data from the Alzheimer's Disease Neuroimaging (ADNI) project to identify 5 plasma analytes with modest predictive value in differentiating subjects with Alzheimer-type dementia from normal controls (range of sensitivity and specificity 74%–85%). No signature panel was able to adequately predict the progression from MCI to Alzheimer-type dementia within this ADNI population. The investigators recognize that the signature panels have insufficient predictive value independently, but theorize that these groups of markers may have value in the future as part of a multicomponent predictive tool.
A recent study by Choo and colleagues71 pursued the hypothesis that combinations of biomarkers and imaging results would improve the prediction of progression to Alzheimer-type dementia among Swedish older adults with a diagnosis of MCI. After performing a stepwise multivariate logistic regression analysis for the outcome of Alzheimer-type dementia only, the investigators reported parietal glucose metabolic rate and total Tau proteins predicted the progression from MCI to Alzheimer-type dementia. Although APOE genotype, a known predictor for dementia, did predict progression in univariate models, it did not add further predictive capability to the 2-predictor model in this cohort.
Nonmodifiable Risk Factors
Genetic Characteristics
Long recognized as a risk factor for Alzheimer-type dementia and even MCI, the APOE ε4 allele has emerged as the most consistent genetic risk factor for the progression of MCI to Alzheimer-type dementia.72 A meta-analysis published in 2011 included data from 35 studies with more than 6000 subjects to evaluate this and other genetic risk factors.73 The meta-analysis reports that among those with MCI, carriers of any APOE ε4 allele are more than 2 times as likely to progress to Alzheimer-type dementia (Table 1). Homozygotes for the APOE ε4 allele had a 4-fold higher risk of progressing to Alzheimer-type dementia compared with noncarriers. Of note, this review did not include results from the Nun study, which did not find an association between APOE status and the outcome of any dementia.74 Although the risk of Alzheimer-type dementia was found to be higher, the clinical utility of exclusively using APOE genotype to predict progression to dementia was attenuated by a low sensitivity and specificity (0.53 and 0.67, respectively).73
Table 1. Factors modifying the progression from MCI to any form of dementia reported in at least one published study.
| Authors,Ref. Year | Sample Size and Cohort Definition | Variable | Outcome Definition | Hazard Ratio (95% CI) | Comment |
|---|---|---|---|---|---|
| Genetics | |||||
| Elias-Sonnenschein et al,73 2011 | Meta-analysis of 35 studies including >6000 subjects | APOE ε4 | Alzheimer-type dementia | 2.29 (1.88–2.80) | Progression to Alzheimer-type dementia in homozygotes: 3.94 (2.09–7.33) |
| Rodriguez-Rodriguez et al,75 2012 | 288 Spanish older adults with MCI | APOE ε4, CLU | Alzheimer-type dementia | APOE ε4: 4.56 (2.23–9.38) CLU: 0.25 (0.07–0.84) | Carriers of at least 6 genetic risk factors increased the risk of more rapid progression (HR 1.89, 95% CI 1.01–3.56) |
| Tyas et al,74 2007 | 470 subjects from the Nun study | APOE ε4 | Any dementia | 1.12 (0.60–2.08) | Age was the only predictor for the progression of MCI to dementia |
| MCI Subtype | |||||
| Ravaglia et al,78 2008 | 60 Italian older adults with MCI; 27 with at least memory-domain MCI | MCI with at least memory impairment | Any dementia or Alzheimer-type dementia | Any: 4.78 (2.83–8.07) Alzheimer: 5.92 (3.30–10.91) | No difference in progression to dementia between those with nonamnestic MCI and those without MCI |
| Zhou et al,79 2012 | 397 older adults with MCI from the ADNI cohort | ADAS13 and CDR-sob scores | Alzheimer-type dementia | 6.9 (4.3–11.0) | High-risk groups included those with ADAS13 >15.67 and CDR-sob >1.5 |
| Koepsell and Monsell,80 2012 | 3020 American older adults with MCI | Nonamnestic single domain, MMSE and CDR scores, and FAQ score | Reversion to normal cognition | Nonamnestic single domain: 1.75 (1.29–2.38) MMSE: 1.21 (1.12–1.30) CDR-sob: 0.66 (0.57–0.77) FAQ ≥1: 0.72 (0.56–0.94) | After 1 year of follow-up, 16% reverted to normal cognition and 20% progressed to dementia. Categorical comparison groups were amnestic single-domain, FAQ score of 0, and APOE ε4 noncarriers |
| Yaffe et al,13 2006 | 305 American older adults with MCI | MCI subtype: reference group was amnestic MCI | Any dementia | Single, nonamnestic: 0.60 (0.35–1.05) Multidomain MCI: 0.71 (0.44–1.14) | MCI subtype predicted dementia type: amnestic MCI more likely to develop Alzheimer-type dementia; single nonamnestic MCI predicted FTD |
| Comorbidity | |||||
| Solfrizzi et al,91 2011 | 121 Italian older adults | Metabolic syndrome | Any dementia | 7.80 (1.29–47.20) | Metabolic syndrome did not increase risk of incident MCI |
| Li et al,92 2012 | 257 Chinese older adults with MCI | MRI, CTA, and clinical characteristics | Any dementia | Diabetes: 2.39 (1.07–5.33) WMC: 0.06 (0.02–0.20) Carotid stenosis: 159.06 (4.57–5537.67) | Similar risk of progression to Alzheimer-type dementia |
| Clerici et al,93 2012 | 245 Italian older adults receiving care in a memory disorders clinic | MRI and clinical characteristics | Any or Alzheimer-type dementias | Combination of ≥1 deep WML and HIS ≥4: 3.5 (1.6–7.4) | Similar result for the association with Alzheimer-type dementia |
| Xu et al,98 2010 | 302 Swedish older adults with MCI | Diabetes or prediabetes | Any or Alzheimer-type dementias | 3.89 (1.69–8.32) | Similar risk of Alzheimer-type as with any dementia. Markers of disease control not considered |
| DeCarli et al,95 2004 | 52 American adults with MCI visiting a memory clinic | Vascular risk factors | Alzheimer-type dementia (by CDR-sob score) | Not significantly different, no HR reported | Poor memory and executive function increased the risk of progression to Alzheimer-type dementia |
| Li et al,99 2011 | 837 Chinese older adults with MCI | Vascular risk factors | Alzheimer-type dementia | Treatment reduced Alzheimer dementia | Treatment of more risk factors reduced the risk more than treatment of fewer risk factors |
| Ravaglia et al,94 2006 | 165 Italian older adults with MCI | Vascular risk factors | Any dementia | Diastolic blood pressure: 0.52 (0.32–0.84) Atrial fibrillation: 4.94 (1.89–12.88) | After adjusting for confounders, neither nor hypertension |
| Bettermann et al,100 2012 | American older adults with MCI | Statin use | All-cause and Alzheimer-type dementia | No difference between users and nonusers | Statin use reduced the risk of incident dementia in cognitively normal subjects |
| Neuropsychiatric Symptoms | |||||
| Richard et al,108 2012 | 320 older adults from Manhattan, NY, USA | CES-D ≥4 | Any dementia | 1.8 (1.0–3.1) | Nonsignificant increase in risk of Alzheimer-type dementia |
| Richard et al,104 2012 | 397 older adults from ADNI | GDS-15—3-item Apathy score | Alzheimer-type dementia | 1.85 (1.09–3.15) | Effect only significant in those without depression. A GDS score of ≥6 was an exclusion for the ADNI study |
| Modrego and Ferrandez,105 2004 | 45 Italian older adults with MCI | Depression (GDS and structured interview) | Alzheimer-type dementia | 2.6 (1.8–3.6) | |
| Palmer et al,106 2007 | 47 Swedish older adults with MCI | Comprehensive Psychopathological Rating Scale | Alzheimer-type dementia | 1.8 (1.2–2.7) | Depressed mood increased risk of progression to Alzheimer in cognitively normal subjects |
| Reynolds et al,107 2011 | 57 American older adults with MCI and depression | Antidepressants with or without donepezil | Any dementia | NR; Reduced rate of progression to dementia (10% vs 33%) | Higher risk of recurrent depression (likelihood ratio: 4.91; P = .03) |
Abbreviations: ADAS13, Alzheimer's Disease Assessment Scale with 13 items; ADNI, Alzheimer's Disease Neuroimaging project; APOE, apolipoprotein; CDR-sob, Clinical Dementia Rating scale sum of boxes; CES-D, Center for Epidemiological Studies Depression; CI, confidence interval; CLU, clusterin; CTA, computed tomographic angiography; FAQ, Functional Activities Questionnaire; FTD, frontotemporal dementia; GDS, Global Deterioration Scale; HIS, Hachinski Ischemic Score; MCI, mild cognitive impairment; MMSE, Mini-Mental Status Examination; MRI, magnetic resonance imaging; MTL, medial temporal lobe; NR, not reported; WML, white matter lesions.
Rodriguez-Rodriguez and colleagues75 studied the link between 8 genetic variants with the risk of progression to Alzheimer-type dementia or with the speed of progression to Alzheimer-type dementia. Among 297 patients seen in a Spanish neurology clinic with a diagnosis of MCI, only 2 genetic variants had an impact on the progression to Alzheimer-type dementia. As expected, the APOE ε4 allele more than quadrupled the risk of progression to Alzheimer-type dementia (P<.001). Carriers of the clusterin (CLU) T-allele had a lower risk of conversion to Alzheimer-type dementia than noncarriers of the T-allele. With respect to speed of progression, APOE was the only individual genetic predictor of a more rapid progression (HR 1.77, 95% CI 1.05–2.97; P = .030). In addition, the investigators reported that carriers of at least 6 non-APOE genetic variants had a 2-fold increased risk of more rapid progression to Alzheimer-type dementia.
Subtype of MCI
The National Institute on Aging and Alzheimer's Association recently released recommendations for the stratification of MCI categories in the context of the likelihood of progression to Alzheimer-type dementia.55 Categorization into such groups is based on neuroimaging (such as atrophy of medial temporal lobe) and biomarkers (such as Tau proteins and Aβ1–42). Four categories are proposed: those with high likelihood to progress to Alzheimer-type dementia, intermediate likelihood, unlikely, and a core group with conflicting results (Table 2). Two studies validated these categories using different populations enrolled either in the ADNI study76 or the Alzheimer's Disease Research Center or Mayo Clinic Study on Aging cohorts.77 Guo and colleagues76 found a higher risk of progression to Alzheimer-type dementia when the high-risk group was compared with the core group (HR 2.3, 95% CI 1.4–3.9; P = .002). Jack and colleagues77 used the criteria to classify preclinical disease (among those with normal cognition), to improve the understanding of the disease process and promote the optimal intervention point to minimize future disease progression. However, because categorical definitions for early disease processes and MCI subtypes has been recently released, the majority of categorization schemes have defined MCI subtypes as amnestic or nonamnestic and single-domain or multi-domain characteristics.
Table 2. Risk stratification of MCI subtypes as defined by the NIA-AA.
| Core Criteria: Apply to All | MCI Subtype | Biomarker Indicator | |
|---|---|---|---|
|
| |||
| Neuronal Injury Result | Imaging Result | ||
| 1. Complaint of change in cognitive function | MCI Unlikely | Negative | Negative |
| MCI Core | Untested/indeterminate | Untested/indeterminate | |
| 2. Impairment in more than 1 cognitive domain | MCI Intermediate | Untested/indeterminate | Positive |
| Positive | Untested/indeterminate | ||
| 3. No decline in function | MCI High | Positive | Positive |
| 4. Not dementia | |||
Abbreviations: MCI, mild cognitive impairment; NIA-AA, National Institute on Aging–Alzheimer's Association.
Adapted from Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7(3):270–9.
A subtype of MCI was suggested to identify the possible neurologic abnormality to describe the progression to different dementias in a study of older adults with MCI cared for in a California Alzheimer disease center.13 After a mean follow-up time of 3 years, 65% progressed to any form of dementia; those with amnestic forms of MCI were more likely to progress to Alzheimer-type dementia, whereas nonamnestic forms were more likely to progress to vascular or frontotemporal dementia. Similarly, in a population of 60 Italian older adults followed for a mean of 4 years, any type of MCI increased the risk for any dementia (HR 3.02, 95% CI 1.86–4.89) as well as Alzheimer-type dementia (HR 3.21, 95% CI 1.77–5.81), but not vascular dementia. This risk appeared to be attributable to the risk of amnestic MCI in the progression to any dementia (see Table 1); and, interestingly, the risk of progression to any dementia was no different among those with nonamnestic MCI and those without MCI. However, it must be noted that the sample size with nonamnestic MCI was too small to draw conclusions.78
Zhou and colleagues79 recently highlighted predictors of progression to dementia among the ADNI cohort with MCI by focusing on demographic, genetic, imaging, and cognitive assessments conducted at baseline. The investigators conducted Cox proportional hazard regression models to identify variables increasing the risk of progression to dementia, and further characterized these risks into survival trees. Stepwise Cox regression analysis determined the Alzheimer's Disease Assessment Scale with 13 items (ADAS13) and logical memory with delayed recall as the 2 tests most frequently identifying abnormal results. Alzheimer-type dementia developed in the entire cohort at a rate of 5.6% at 12 months and 58.1% at 48 months. Considering a combination of baseline ADAS13 scores greater than 15.67 and Clinical Dementia Rating scale sum of boxes (CDR-sob) scores greater than 1.5 yielded an incident Alzheimer-type dementia rate of 12.9% at 12 months and 92.7% at 48 months. Using the ADAS13 as the first categorization method, the strength of this predictive risk stratification was similar when either baseline battery tests or imaging measures of cortical thickness of the right inferior temporal lobe were used, all with a similar c-index of 0.68. Although a promising lead, it must be restated that the population included in the ADNI cohort may have limited translation to populations cared for in many clinical settings.
In an alternative and complementary approach, Koepsell and Monsell80 pursued this research question with the objective of identifying predictors of the reversion from MCI to normal cognitive function. The investigators used a cohort of approximately 3000 subjects from the National Alzheimer's Coordinating Center who were at least 65 years old and diagnosed with MCI. After a follow-up period of approximately 1 year, 5 risk factors predicting reversion from MCI to normal cognition were identified: higher baseline Mini-Mental State Examination (MMSE) scores, lower CDR-sob scores, fewer functional impairments, nonamnestic single-domain subtype of MCI, and a lack of APOE ε4 genotype. Of note, these results did not incorporate clinical factors such as burden of vascular disease, and categorized normal cognition as either normal or “impaired but not MCI” as the outcome measure, stating that categorization across multiple centers used each term with variable frequencies. However, the investigators also identified a trend among those patients with MCI who reverted to normal cognitive function that suggests they were more likely to transition back to MCI or to dementia than those cognitively normal at baseline.80
Modifiable Risk Factors
Comorbidity and Other Clinical Characteristics
Chronic comorbid disease such as coronary heart disease and hypertension are common in older adults who develop cognitive impairment,81,82 often requiring the use of several medications to reach therapeutic goals. It has been documented that ambulatory older adults use an average of 11 medications per day and that 74% combine prescription medications and dietary supplements.83,84 Incident cognitive impairment has been suggested to be less likely in those using medications to control for vascular risk factors85–89; however, other medications may result in adverse cognitive outcomes, such as MCI, and represent a potentially reversible risk factor.
A descriptive analysis of a population with cognitive impairment (either MCI or dementia) from the Cache County study suggests that those with cognitive impairment have a more severe burden of comorbid disease than those without cognitive impairment.90 Vascular disease has been considered the most likely comorbidity to cause a more rapid progression of cognitive impairment. One study involving approximately 500 older adults from a Midwestern Alzheimer disease center evaluated risk factors for progression from MCI to dementia. Using a Markov chain model to assess the transition from one cognitive state to another, the investigators did not find an influence of demographic, genetic, or education on the progression to dementia, but rather found that a baseline history of hypertension reduced the likelihood of progression to dementia. This population was largely Caucasian and educated, possibly indicating a higher likelihood of adherence to medications to control hypertension and reduce the burden of vascular disease.
Investigators from the Italian Longitudinal Study in Aging focused an analysis of their population-based database on the relationship of metabolic syndrome with the progression of MCI to dementia.91 Metabolic syndrome is recognized by the presence of at least 3 of the following features: abdominal obesity (measured by waist circumference), elevated plasma triglyceride levels of at least 150 mg/dL, low high-density lipoprotein cholesterol (<40 mg/dL for men and <50 mg/dL for women), high blood pressure (≥130/≥85 mm Hg), or use of antihypertensive treatment. The study observed approximately 2100 patients over a mean of 3.5 years, only 121 of whom had MCI. Multivariate models adjusting for several potential demographic and cardiovascular confounders revealed a higher risk of progression to any dementia among those with metabolic syndrome. Within this same population, interestingly no relationship between metabolic syndrome and incident MCI was identified.
A prospective study of 257 Chinese older adults with MCI was conducted to identify risk factors for the progression of cognitive decline (evaluated by MMSE score), progression from MCI to any dementia, and progression from MCI to Alzheimer-type dementia.92 The investigators considered vascular risk factors of diabetes, hypertension, hyperlipidemia, and tobacco and alcohol use over a 3-year observation period. MRI and computed tomographic angiography (CTA) were used to identify changes in white matter and degree of cerebral arteriostenosis. Considering all clinical variables and imaging results, diabetes, baseline severity of white matter change, and carotid stenosis were robust predictors for each outcome, including any dementia and Alzheimer-type dementia. The investigators evaluated the impact of antihypertensive use and oral hypoglycemic or insulin use, and found no association with the progression to dementia.92 Such results suggest that vascular risk factors resulting in chronic cerebral hypoperfusion have a negative impact on cognitive function and may advance the progression to any form of dementia.
A similar study conducted in an Italian population of community-dwelling older adults with a diagnosis of MCI followed 245 subjects over approximately 2.5 years to evaluate the impact of vascular diseases on the progression of MCI to dementia.93 Vascular diseases were recognized by the Hachinski Ischemic Score and the Framing-ham Stroke Risk Profile. The Hachinksi score is a marker of existing cerebrovascular disease, whereas the Framingham Stroke Risk Profile is a marker of future cerebrovascular disease risk. The investigators also considered the presence of APOE ε4, smoking status, and the presence of WML as covariates in the analysis. The results suggest that among the 52% of subjects who progressed to dementia during the observation period, no individual clinical factor or vascular summary score was associated with an increased risk of progression to dementia. However, the combination of Hachinski scores greater than 4 with presence of WML near the basal ganglia increased the risk of progression to any form of dementia as well as to Alzheimer-type dementia (HR 3.8, 95% CI 1.2–11.5) after adjusting for age, sex, education, cumulative illness, MMSE, MCI subtype, and APOE genotype. Framingham Stroke Risk Profile was not associated with an increased risk of progression to dementia, either alone or in combination with imaging results.
Other notable studies have evaluated the role of vascular risk factors on the development of dementia, with conflicting results. Results from some observational studies have produced similar results that show no association between individual risk of cardiovascular disease and incident dementia,13,94–96 whereas others have suggested vascular risk factors may independently increase the risk of progression to dementia.97–99 In 2004 DeCarli and colleagues95 reported from a cohort of older adults with MCI and calculated vascular risk through a composite score as the sum of vascular risk factors (up to 6). The investigators also defined Alzheimer-type dementia as a progression from a CDR-sob score of 0.5 to 1.0 or greater. Although limitations in the population as well as the methods are noted, no association between baseline vascular risk factors and progression to Alzheimer-type dementia was identified.
A Swedish longitudinal study on aging reported on the impact of diabetes and pre-diabetes on the progression from MCI to dementia. Approximately 300 subjects with MCI at baseline were followed for a mean of 3 years. After controlling for age, sex, education, body mass index, genotype, and vascular disorders, the risk of progression to any dementia (see Table 1) and Alzheimer-type dementia was increased by a diagnosis of diabetes and prediabetes (HR 4.22, 95% CI1.57–9.01). Kaplan-Meier survival analysis suggested that diabetes accelerated the time to progression to dementia by an average of 3.2 years. Of note, no risk of incident MCI was identified in older adults with diabetes and normal cognition at baseline.98
A recent observational study conducted in a Chinese population over 5 years suggests that vascular risk factors increase the progression of MCI to Alzheimer-type dementia.99 The investigators report that vascular risk factors (including hypertension, diabetes, cerebrovascular disease, and hypercholesterolemia) additively increase the risk of progression to Alzheimer-type dementia, and that treatment of vascular disease was associated with a reduction in the risk of conversion to dementia. Although medication adherence and clinical treatment targets were not assessed, this study gives strong support to interventions aimed at managing vascular risk factors in those with MCI as a method to reduce the progression to dementia. Further support for the treatment of vascular disease in reducing the neuropathology of Alzheimer-type dementia was published in 2009 by Hoffman and colleagues, who used autopsy evidence to show fewer neuritic plaques and neurofibrillary tangles in those with hypertension who received antihypertensive medication than in those not receiving antihypertensive medication.22
Contrasting such results, a secondary analysis of the Ginkgo Evaluation in Memory Study described the role of statins on the progression from MCI to dementia in this observational study.100 Among those with a baseline diagnosis of MCI, statins were found to have no influence on the progression from MCI to all-cause or Alzheimer-type dementia, although statins were shown to reduce incident dementia in the cohort that was cognitively normal at baseline (HR 0.79, 95% CI 0.65–0.96). Though a promising result for incident dementia, this analysis was conducted with self-reported measures of medication exposure, suggesting this association requires further study with more accurate measures of medication exposure as well as control of disease state.
Neuropsychiatric Symptoms
Neuropsychiatric symptoms have been reported in up to 30% of older adults with a diagnosis of MCI.101 Because neuropsychiatric symptoms have been associated with a worse prognosis in dementia,102 the relationship between neuropsychiatric symptoms and the risk for progression of MCI to dementia was assessed in a population enrolled from Cache County, Utah.103 A cohort of 230 older adults diagnosed with MCI was evaluated for predictors of progression to any dementia using a cognitive and neuropsychiatric battery (MMSE, Modified MMSE, CDR, and the Neuropsychiatric Inventory [NPI]). In multivariate regression models controlling for age, education, and APOE status, nighttime behaviors predicted progression to all-cause dementia (HR 1.28, 95% CI 1.08–1.52) and hallucinations predicted progression only to vascular dementia, not all-cause or Alzheimer-type dementia (HR 10.1, 95% CI1.1–91.1). Overall scores on the NPI suggest that even a minor severity of neuropsychiatric symptoms increases the risk of progression to any dementia (HR 1.65, 95% CI 1.01–2.69). Despite the relatively small sample size, this study suggests that further attention to and possibly specific interventions targeting those with neuropsychiatric symptoms may reduce the progression of MCI to dementia.
Other psychiatric symptoms have been evaluated as potential risk factors for the progression of MCI to dementia. Prior work has identified depression and anxiety as 2 risk factors increasing such progression.104–106 Two of these studies present conflicting results on the role of depression in the progression of MCI. Whereas both studies assessed depression and anxiety with an outcome of Alzheimer-type dementia in populations with amnestic or multidomain MCI, Palmer and colleagues106 suggest that depression alone does not increase the risk of progression to dementia except in those with normal cognition, whereas Modrego and Ferrandez105 report that the risk of progression is nearly 2-fold in those with depressive symptoms. The 2 studies used different tools to identify depression symptoms, with Modrego using the Geriatric Depression Scale and Palmer identifying symptoms through the Comprehensive Psychopathological Rating Scale.105,106 Controversially, the recent study by Peters and colleagues103 described earlier does not suggest that either depression or anxiety, assessed as domains in the NPI, influenced the progression of MCI to dementia. It must be borne in mind that depression and anxiety assessments in the NPI are single-item responses, in comparison with other screening tools assessing 15 to 30 items. However, when Peters and colleagues103 grouped mood-related domains of depression, anxiety, apathy, and irritability together, no impact on disease progression was revealed. Of note, a recent study by Richard and colleagues104 found that apathy, but not depressive symptoms, increased the risk of progression from MCI to Alzheimer-type dementia.
The combination of consistent antidepressant treatment (flexible regimen) with or without donepezil in older adults with major depression was recently evaluated. In the cohort with MCI at baseline, adding donepezil to the antidepressant regimen reduced the likelihood of progression to dementia over the 2-year study period; however, this group was also more likely to experience a recurrence of major depression (44% vs 12%, likelihood ratio 4.91; P = .03).107 The investigators appropriately concluded that the potential benefits of adjuvant donepezil and antidepressant therapy require further study, and strong consideration of the risks in the face of the potential benefits.
Future Considerations
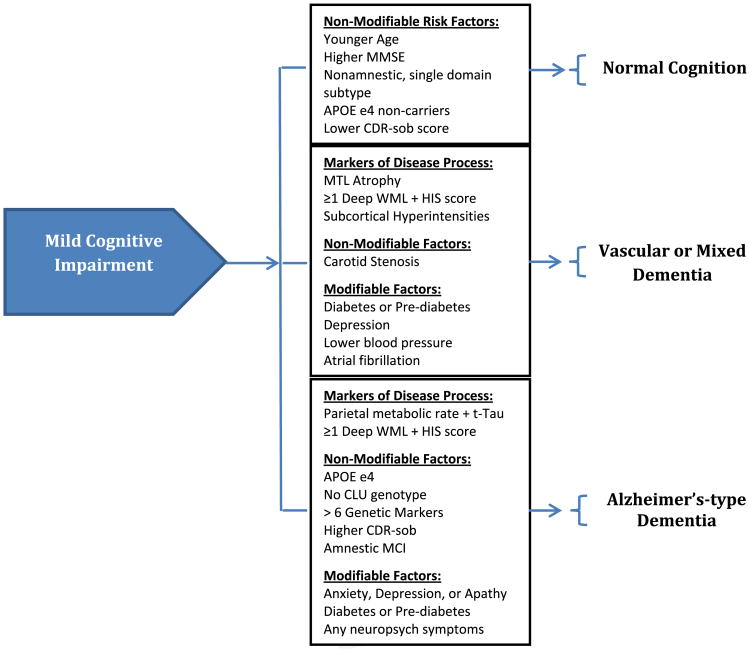
Risk stratification based on factors identified in observational studies may offer an opportunity to focus interventions on reducing the risk of incident dementia in those patients at highest risk. As described earlier,79 existing databases of cohorts with MCI have been used to predict the transition to dementia. Fig. 1 summarizes the risk factors previously identified to modify the progression from MCI to normal cognition, mixed or vascular dementia, or Alzheimer-type dementia. As a starting point, these results represent promising methods of identifying those populations at highest risk; however, the limitations of such cohorts from the existing literature, often composed of participants involved in research activity, may not represent the population encountered in many clinical environments and reduces the clinical applicability of such results. Similarly, no study has yet focused on interventions such as control of cardiovascular risk factors specifically in those with MCI, making risk-stratification methods applicable to only research environments. To date, studies evaluating the use of acetylcholinesterase inhibitors, NSAIDs, and ginkgo biloba have not yielded results promoting their routine use in clinical practice among those with MCI.
Fig. 1.
Risk factors for progression of MCI to normal cognition, vascular or mixed dementia, or Alzheimer-type dementia. APOE, apolipoprotein; CDR-sob, Clinical Dementia Rating scale sum of boxes; CLU, clusterin; HIS, Hachinski Ischemic Score; MMSE, Mini-Mental Status Examination; MCI, mild cognitive impairment; MTL, medial temporal lobe; t-Tau, total Tau protein from cerebrospinal fluid; WML, white matter lesions.
Further evidence is necessary to clarify the impact of comorbid disease, specifically related to the level of disease control, on the progression of MCI to dementia. As noted earlier, there has recently been a significant amount of activity toward understanding the role of comorbid disease on the progression of cognitive impairment. Findings that the treatment of comorbid vascular disease may reduce the likelihood of progression to dementia are promising22,99; however, no consideration of the intensity of treatment such as target blood pressure or hemoglobin A1c has been considered. Considering the complexity of medication management and adherence in this population, especially among those experiencing difficulty in executive function, this issue of disease-state control should be pursued with careful attention to risks and benefits of medications. Appropriate methods for assessing medication adherence and monitoring plans that frequently address potential adverse events should be established by clinicians in attempting to control multiple comorbidities in this frail older adult population.
Lastly, although associations between modifiable risk factors, such as hypertension and diabetes, have been suggested, little evidence in the identification of potentially relevant, nonvascular disease states has been pursued, such as oxygen deprivation states of asthma/chronic obstructive pulmonary disease, and severe heart failure. Similarly, certain medications thought to temporarily or permanently impair cognitive function, including anticholinergics, benzodiazepines, and possibly statins, are poorly represented in the existing literature.
Summary
Several risk factors for the progression of cognitive impairment in those diagnosed with MCI have been considered. The variability in results found in the existing literature arises in part from the heterogeneity in populations studied, subtypes of MCI considered, and dementia outcomes evaluated as study end points. Risk factors identified to increase the risk of Alzheimer disease pathology include the presence of deep WML combined with Hachinski Ischemic Scores greater than 4, the presence of APOE ε4 and absence of CLU genotypes, presence of more than 6 genetic markers for Alzheimer-type dementia, amnestic subtype of MCI, psychiatric symptoms of anxiety, depression, or apathy, and presence of diabetes or prediabetes. Risk factors increasing the risk of mixed or vascular dementias include carotid stenosis, diabetes (or prediabetes), depression, low blood pressure, and atrial fibrillation.
From a researcher's perspective, this information is of value as it may improve population selection, design of the intervention, and definition of the outcomes of interest in the quest to minimize the progression of MCI to dementia. Clinicians, patients, and the health care system value this information because it permits a more informed conversation about disease prevention, clinical treatment, and advanced care planning. The risk factors identified thus far represent modifiable and nonmodifiable variables to be incorporated into both research and clinical practice as possible targets for prevention and treatment of progressive cognitive impairment. Given the recognition of common comorbid conditions such as hypertension and diabetes as potentially modifiable risk factors, future management of prevention strategies for the progression of MCI to dementia is likely to fall into the hands of primary care and general practice physicians and mid-level providers.
Key Points.
Medial temporal lobe atrophy, total Tau proteins, and Aβ1–42 have been used as markers of disease processes that improve the identification of preclinical cognitive impairment.
Modifiable risk factors, such as hypertension, diabetes, and depression, represent potential areas for therapeutic interventions to minimize the progression of mild cognitive impairment (MCI).
Future interventions targeting cardiovascular and other clinical interventions must consider the complexities of medication management in attempts to reduce the progression of MCI to dementia.
Acknowledgments
Funding Support: This work was supported by grant R01 HS019818-01 from the Agency for Healthcare Research and Quality. The sponsor had no role in the article development.
Footnotes
The authors report no conflicts of interest for this article.
References
- 1.Ganguli M, Petersen RC. Mild cognitive impairment: challenging issues. Am J Geriatr Psychiatry. 2008;16(5):339–42. doi: 10.1097/JGP.0b013e31816c3fdb. [DOI] [PubMed] [Google Scholar]
- 2.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–6. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 5.Petersen RC, Smith GE, Ivnik RJ, et al. Apolipoprotein E status as a predictor of the development of Alzheimer's disease in memory-impaired individuals. JAMA. 1995;273(16):1274–8. [PubMed] [Google Scholar]
- 6.Smith G, Petersen RC, Parisi JE, et al. Definition, course, and outcome of mild cognitive impairment. Aging Neuropsychol Cognit. 1996;3:131–47. [Google Scholar]
- 7.Graham JE, Rockwood K, Beattie BL, et al. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349(9068):1793–6. doi: 10.1016/S0140-6736(97)01007-6. [DOI] [PubMed] [Google Scholar]
- 8.Ebly EM, Hogan DB, Parhad IM. Cognitive impairment in the nondemented elderly Results from the Canadian Study of Health and Aging. Arch Neurol. 1995;52(6):612–9. doi: 10.1001/archneur.1995.00540300086018. [DOI] [PubMed] [Google Scholar]
- 9.Kral VA. Senescent forgetfulness: benign and malignant. Can Med Assoc J. 1962;86:257–60. [PMC free article] [PubMed] [Google Scholar]
- 10.Levy R. Aging-associated cognitive decline Working Party of the International Psychogeriatric Association in collaboration with the World Health Organization. Int Psychogeriatr. 1994;6(1):63–8. [PubMed] [Google Scholar]
- 11.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148(6):427–34. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boustani MA, Sachs GA, Alder CA, et al. Implementing innovative models of dementia care: The Healthy Aging Brain Center. Aging Ment Health. 2011;15(1):13–22. doi: 10.1080/13607863.2010.496445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaffe K, Petersen RC, Lindquist K, et al. Subtype of mild cognitive impairment and progression to dementia and death. Dement Geriatr Cogn Disord. 2006;22(4):312–9. doi: 10.1159/000095427. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell AJ, Shiri-Feshki M. Temporal trends in the long term risk of progression of mild cognitive impairment: a pooled analysis. J Neurol Neurosurg Psychiatry. 2008;79(12):1386–91. doi: 10.1136/jnnp.2007.142679. [DOI] [PubMed] [Google Scholar]
- 15.Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66(12):1447–55. doi: 10.1001/archneurol.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raffaitin C, Gin H, Empana JP, et al. Metabolic syndrome and risk for incident Alzheimer's disease or vascular dementia: the Three-City Study. Diabetes Care. 2009;32(1):169–74. doi: 10.2337/dc08-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solfrizzi V, Scafato E, Capurso C, et al. Metabolic syndrome and the risk of vascular dementia: the Italian Longitudinal Study on Ageing. J Neurol Neurosurg Psychiatry. 2010;81(4):433–40. doi: 10.1136/jnnp.2009.181743. [DOI] [PubMed] [Google Scholar]
- 18.Vanhanen M, Koivisto K, Moilanen L, et al. Association of metabolic syndrome with Alzheimer disease: a population-based study. Neurology. 2006;67(5):843–7. doi: 10.1212/01.wnl.0000234037.91185.99. [DOI] [PubMed] [Google Scholar]
- 19.Razay G, Vreugdenhil A, Wilcock G. The metabolic syndrome and Alzheimer disease. Arch Neurol. 2007;64(1):93–6. doi: 10.1001/archneur.64.1.93. [DOI] [PubMed] [Google Scholar]
- 20.Bowler JV. Vascular cognitive impairment. J Neurol Neurosurg Psychiatry. 2005;76(Suppl 5):v35–44. doi: 10.1136/jnnp.2005.082313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352(23):2379–88. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 22.Doody RS, Ferris SH, Salloway S, et al. Donepezil treatment of patients with MCI: a 48-week randomized, placebo-controlled trial. Neurology. 2009;72(18):1555–61. doi: 10.1212/01.wnl.0000344650.95823.03. [DOI] [PubMed] [Google Scholar]
- 23.Unverzagt FW, Guey LT, Jones RN, et al. ACTIVE cognitive training and rates of incident dementia. J Int Neuropsychol Soc. 2012;18(4):669–77. doi: 10.1017/S1355617711001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aisen PS, Thal LJ, Ferris SH, et al. Rofecoxib in patients with mild cognitive impairment: further analyses of data from a randomized, double-blind, trial. Curr Alzheimer Res. 2008;5(1):73–82. doi: 10.2174/156720508783884602. [DOI] [PubMed] [Google Scholar]
- 25.Snitz BE, O'Meara ES, Carlson MC, et al. Ginkgo biloba for preventing cognitive decline in older adults: a randomized trial. JAMA. 2009;302(24):2663–70. doi: 10.1001/jama.2009.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeKosky ST, Williamson JD, Fitzpatrick AL, et al. Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA. 2008;300(19):2253–62. doi: 10.1001/jama.2008.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jack CR, Jr, Shiung MM, Weigand SD, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65(8):1227–31. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitwell JL, Shiung MM, Przybelski SA, et al. MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology. 2008;70(7):512–20. doi: 10.1212/01.wnl.0000280575.77437.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McEvoy LK, Fennema-Notestine C, Roddey JC, et al. Alzheimer disease: quantitative structural neuroimaging for detection and prediction of clinical and structural changes in mild cognitive impairment. Radiology. 2009;251(1):195–205. doi: 10.1148/radiol.2511080924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakkour A, Morris JC, Dickerson BC. The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology. 2009;72(12):1048–55. doi: 10.1212/01.wnl.0000340981.97664.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy EA, Holland D, Donohue M, et al. Six-month atrophy in MTL structures is associated with subsequent memory decline in elderly controls. Neuroimage. 2010;53(4):1310–7. doi: 10.1016/j.neuroimage.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker JA, Hedden T, Carmasin J, et al. Amyloid-beta associated cortical thinning in clinically normal elderly. Ann Neurol. 2011;69(6):1032–42. doi: 10.1002/ana.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fjell AM, Walhovd KB, Fennema-Notestine C, et al. One-year brain atrophy evident in healthy aging. J Neurosci. 2009;29(48):15223–31. doi: 10.1523/JNEUROSCI.3252-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald CR, McEvoy LK, Gharapetian L, et al. Regional rates of neocortical atrophy from normal aging to early Alzheimer disease. Neurology. 2009;73(6):457–65. doi: 10.1212/WNL.0b013e3181b16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67(5):834–42. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Leon MJ, Mosconi L, Blennow K, et al. Imaging and CSF studies in the preclinical diagnosis of Alzheimer's disease. Ann N Y Acad Sci. 2007;1097:114–45. doi: 10.1196/annals.1379.012. [DOI] [PubMed] [Google Scholar]
- 37.Tondelli M, Wilcock GK, Nichelli P, et al. Structural MRI changes detectable up to ten years before clinical Alzheimer's disease. Neurobiol Aging. 2012;33(4):825. doi: 10.1016/j.neurobiolaging.2011.05.018. e25-36. [DOI] [PubMed] [Google Scholar]
- 38.Geroldi C, Rossi R, Calvagna C, et al. Medial temporal atrophy but not memory deficit predicts progression to dementia in patients with mild cognitive impairment. J Neurol Neurosurg Psychiatry. 2006;77(11):1219–22. doi: 10.1136/jnnp.2005.082651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Breteler MM, van Swieten JC, Bots ML, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology. 1994;44(7):1246–52. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- 40.Scarpelli M, Salvolini U, Diamanti L, et al. MRI and pathological examination of post-mortem brains: the problem of white matter high signal areas. Neuroradiology. 1994;36(5):393–8. doi: 10.1007/BF00612126. [DOI] [PubMed] [Google Scholar]
- 41.Longstreth WT, Jr, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people The Cardiovascular Health Study. Stroke. 1996;27(8):1274–82. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 42.van der Flier WM, van Straaten EC, Barkhof F, et al. Medial temporal lobe atrophy and white matter hyperintensities are associated with mild cognitive deficits in non-disabled elderly people: the LADIS study. J Neurol Neurosurg Psychiatry. 2005;76(11):1497–500. doi: 10.1136/jnnp.2005.064998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshita M, Fletcher E, Harvey D, et al. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67(12):2192–8. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and subjective cognitive dysfunction: the Rotterdam Scan Study. Neurology. 2001;56(11):1539–45. doi: 10.1212/wnl.56.11.1539. [DOI] [PubMed] [Google Scholar]
- 45.Devine ME, Fonseca JA, Walker Z. Do cerebral white matter lesions influence the rate of progression from mild cognitive impairment to dementia? Int Psychogeriatr. 2013;25(1):120–7. doi: 10.1017/S1041610212000932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brickman AM, Provenzano FA, Muraskin J, et al. Regional white matter hyperintensity volume, not hippocampal atrophy, predicts incident Alzheimer disease in the community. Arch Neurol. 2012;69(12):1621–7. doi: 10.1001/archneurol.2012.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bombois S, Debette S, Bruandet A, et al. Vascular subcortical hyperintensities predict conversion to vascular and mixed dementia in MCI patients. Stroke. 2008;39(7):2046–51. doi: 10.1161/STROKEAHA.107.505206. [DOI] [PubMed] [Google Scholar]
- 49.Hansson O, Zetterberg H, Buchhave P, et al. Association between CSF bio-markers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5(3):228–34. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 50.Mattsson N, Zetterberg H, Hansson O, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302(4):385–93. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- 51.Mattsson N, Portelius E, Rolstad S, et al. Longitudinal cerebrospinal fluid biomarkers over four years in mild cognitive impairment. J Alzheimers Dis. 2012;30(4):767–78. doi: 10.3233/JAD-2012-120019. [DOI] [PubMed] [Google Scholar]
- 52.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59(3):512–9. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 53.Heister D, Brewer JB, Magda S, et al. Alzheimer's Disease Neuroimaging I. Predicting MCI outcome with clinically available MRI and CSF biomarkers. Neurology. 2011;77(17):1619–28. doi: 10.1212/WNL.0b013e3182343314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vos S, van Rossum I, Burns L, et al. Test sequence of CSF and MRI biomarkers for prediction of AD in subjects with MCI. Neurobiol Aging. 2012;33(10):2272–81. doi: 10.1016/j.neurobiolaging.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 55.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Rossum IA, Visser PJ, Knol DL, et al. Injury markers but not amyloid markers are associated with rapid progression from mild cognitive impairment to dementia in Alzheimer's disease. J Alzheimers Dis. 2012;29(2):319–27. doi: 10.3233/JAD-2011-111694. [DOI] [PubMed] [Google Scholar]
- 57.Lee KS, Chung JH, Choi TK, et al. Peripheral cytokines and chemokines in Alzheimer's disease. Dement Geriatr Cogn Disord. 2009;28(4):281–7. doi: 10.1159/000245156. [DOI] [PubMed] [Google Scholar]
- 58.Kamer AR, Craig RG, Pirraglia E, et al. TNF-alpha and antibodies to periodontal bacteria discriminate between Alzheimer's disease patients and normal subjects. J Neuroimmunol. 2009;216(1-2):92–7. doi: 10.1016/j.jneuroim.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bermejo P, Martin-Aragon S, Benedi J, et al. Differences of peripheral inflammatory markers between mild cognitive impairment and Alzheimer's disease. Immunol Lett. 2008;117(2):198–202. doi: 10.1016/j.imlet.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 60.Seppala TT, Herukka SK, Hanninen T, et al. Plasma Abeta42 and Abeta40 as markers of cognitive change in follow-up: a prospective, longitudinal, population-based cohort study. J Neurol Neurosurg Psychiatry. 2010;81(10):1123–7. doi: 10.1136/jnnp.2010.205757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Le Bastard N, Leurs J, Blomme W, et al. Plasma amyloid-beta forms in Alzheimer's disease and non-Alzheimer's disease patients. J Alzheimers Dis. 2010;21(1):291–301. doi: 10.3233/JAD-2010-091501. [DOI] [PubMed] [Google Scholar]
- 62.Lui JK, Laws SM, Li QX, et al. Plasma amyloid-beta as a biomarker in Alzheimer's disease: the AIBL study of aging. J Alzheimers Dis. 2010;20(4):1233–42. doi: 10.3233/JAD-2010-090249. [DOI] [PubMed] [Google Scholar]
- 63.Lewczuk P, Kornhuber J, Vanmechelen E, et al. Amyloid beta peptides in plasma in early diagnosis of Alzheimer's disease: a multicenter study with multiplexing. Exp Neurol. 2010;223(2):366–70. doi: 10.1016/j.expneurol.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 64.Blennow K, Zetterberg H. Use of CSF biomarkers in Alzheimer's disease clinical trials. J Nutr Health Aging. 2009;13(4):358–61. doi: 10.1007/s12603-009-0043-8. [DOI] [PubMed] [Google Scholar]
- 65.Kester MI, Verwey NA, van Elk EJ, et al. Evaluation of plasma Abeta40 and Abeta42 as predictors of conversion to Alzheimer's disease in patients with mild cognitive impairment. Neurobiol Aging. 2010;31(4):539–40. doi: 10.1016/j.neurobiolaging.2008.07.024. [author reply: 541] [DOI] [PubMed] [Google Scholar]
- 66.Schupf N, Tang MX, Fukuyama H, et al. Peripheral Abeta subspecies as risk biomarkers of Alzheimer's disease. Proc Natl Acad Sci U S A. 2008;105(37):14052–7. doi: 10.1073/pnas.0805902105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thambisetty M, Lovestone S. Blood-based biomarkers of Alzheimer's disease: challenging but feasible. Biomark Med. 2010;4(1):65–79. doi: 10.2217/bmm.09.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guntert A, Campbell J, Saleem M, et al. Plasma gelsolin is decreased and correlates with rate of decline in Alzheimer's disease. J Alzheimers Dis. 2010;21(2):585–96. doi: 10.3233/JAD-2010-100279. [DOI] [PubMed] [Google Scholar]
- 69.Gupta VB, Laws SM, Villemagne VL, et al. Plasma apolipoprotein E and Alzheimer disease risk: the AIBL study of aging. Neurology. 2011;76(12):1091–8. doi: 10.1212/WNL.0b013e318211c352. [DOI] [PubMed] [Google Scholar]
- 70.Llano DA, Devanarayan V, Simon AJ, et al. The Alzheimer's Disease Neuroimaging Initiative. Evaluation of plasma proteomic data for Alzheimer disease state classification and for the prediction of progression from mild cognitive impairment to Alzheimer disease. Alzheimer Dis Assoc Disord. 2012 doi: 10.1097/WAD.0b013e31826d597a. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 71.Choo IH, Ni R, Scholl M, et al. Combination of 18F-FDG PET and cerebrospinal fluid biomarkers as a better predictor of the progression to Alzheimer's disease in mild cognitive impairment patients. J Alzheimers Dis. 2013;33(4):929–39. doi: 10.3233/JAD-2012-121489. [DOI] [PubMed] [Google Scholar]
- 72.Tschanz JT, Welsh-Bohmer KA, Lyketsos CG, et al. Conversion to dementia from mild cognitive disorder: the Cache County Study. Neurology. 2006;67(2):229–34. doi: 10.1212/01.wnl.0000224748.48011.84. [DOI] [PubMed] [Google Scholar]
- 73.Elias-Sonnenschein LS, Viechtbauer W, Ramakers IH, et al. Predictive value of APOE-epsilon4 allele for progression from MCI to AD-type dementia: a metaanalysis. J Neurol Neurosurg Psychiatry. 2011;82(10):1149–56. doi: 10.1136/jnnp.2010.231555. [DOI] [PubMed] [Google Scholar]
- 74.Tyas SL, Salazar JC, Snowdon DA, et al. Transitions to mild cognitive impairments, dementia, and death: Findings from the nun study. Am J Epidemiol. 2007;165(11):1231–8. doi: 10.1093/aje/kwm085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rodriguez-Rodriguez E, Sanchez-Juan P, Vazquez-Higuera JL, et al. Genetic risk score predicting accelerated progression from mild cognitive impairment to Alzheimer's disease. J Neural Transm. 2013;120(5):807–12. doi: 10.1007/s00702-012-0920-x. [DOI] [PubMed] [Google Scholar]
- 76.Guo LH, Alexopoulos P, Eisele T, et al. The National Institute on Aging-Alzheimer's Association research criteria for mild cognitive impairment due to Alzheimer's disease: predicting the outcome. Eur Arch Psychiatry Clin Neurosci. 2013;263(4):325–33. doi: 10.1007/s00406-012-0349-0. [DOI] [PubMed] [Google Scholar]
- 77.Jack CR, Jr, Knopman DS, Weigand SD, et al. An operational approach to National Institute on Aging-Alzheimer's Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71(6):765–75. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ravaglia G, Forti P, Montesi F, et al. Mild cognitive impairment: epidemiology and dementia risk in an elderly Italian population. J Am Geriatr Soc. 2008;56(1):51–8. doi: 10.1111/j.1532-5415.2007.01503.x. [DOI] [PubMed] [Google Scholar]
- 79.Zhou B, Nakatani E, Teramukai S, et al. Alzheimer's Disease Neuroimaging. Risk classification in mild cognitive impairment patients for developing Alzheimer's disease. J Alzheimers Dis. 2012;30(2):367–75. doi: 10.3233/JAD-2012-112117. [DOI] [PubMed] [Google Scholar]
- 80.Koepsell TD, Monsell SE. Reversion from mild cognitive impairment to normal or near-normal cognition: risk factors and prognosis. Neurology. 2012;79(15):1591–8. doi: 10.1212/WNL.0b013e31826e26b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Campbell NL, Boustani MA, Lane KA, et al. Use of anticholinergics and the risk of cognitive impairment in an African American population. Neurology. 2010;75(2):152–9. doi: 10.1212/WNL.0b013e3181e7f2ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schubert CC, Boustani M, Callahan CM, et al. Comorbidity profile of dementia patients in primary care: are they sicker? J Am Geriatr Soc. 2006;54(1):104–9. doi: 10.1111/j.1532-5415.2005.00543.x. [DOI] [PubMed] [Google Scholar]
- 83.Murray MD, Young J, Hoke S, et al. Pharmacist intervention to improve medication adherence in heart failure: a randomized trial. Ann Intern Med. 2007;146(10):714–25. doi: 10.7326/0003-4819-146-10-200705150-00005. [DOI] [PubMed] [Google Scholar]
- 84.Nahin RL, Pecha M, Welmerink DB, et al. Concomitant use of prescription drugs and dietary supplements in ambulatory elderly people. J Am Geriatr Soc. 2009;57(7):1197–205. doi: 10.1111/j.1532-5415.2009.02329.x. [DOI] [PubMed] [Google Scholar]
- 85.Murray MD, Lane KA, Gao S, et al. Preservation of cognitive function with antihypertensive medications: a longitudinal analysis of a community-based sample of African Americans. Arch Intern Med. 2002;162(18):2090–6. doi: 10.1001/archinte.162.18.2090. [DOI] [PubMed] [Google Scholar]
- 86.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000;21(1):49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 88.Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ. 2001;322(7300):1447–51. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hanon O, Berrou JP, Negre-Pages L, et al. Effects of hypertension therapy based on eprosartan on systolic arterial blood pressure and cognitive function: primary results of the Observational Study on Cognitive function and Systolic Blood Pressure Reduction open-label study. J Hypertens. 2008;26(8):1642–50. doi: 10.1097/HJH.0b013e328301a280. [DOI] [PubMed] [Google Scholar]
- 90.Lyketsos CG, Toone L, Tschanz J, et al. Population-based study of medical comorbidity in early dementia and “cognitive impairment, no dementia (CIND)”: association with functional and cognitive impairment: The Cache County Study. Am J Geriatr Psychiatry. 2005;13(8):656–64. doi: 10.1176/appi.ajgp.13.8.656. [DOI] [PubMed] [Google Scholar]
- 91.Solfrizzi V, Scafato E, Capurso C, et al. Metabolic syndrome, mild cognitive impairment, and progression to dementia The Italian Longitudinal Study on Aging. Neurobiol Aging. 2011;32(11):1932–41. doi: 10.1016/j.neurobiolaging.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 92.Li L, Wang Y, Yan J, et al. Clinical predictors of cognitive decline in patients with mild cognitive impairment: the Chongqing aging study. J Neurol. 2012;259(7):1303–11. doi: 10.1007/s00415-011-6342-0. [DOI] [PubMed] [Google Scholar]
- 93.Clerici F, Caracciolo B, Cova I, et al. Does vascular burden contribute to the progression of mild cognitive impairment to dementia? Dement Geriatr Cogn Disord. 2012;34(3-4):235–43. doi: 10.1159/000343776. [DOI] [PubMed] [Google Scholar]
- 94.Ravaglia G, Forti P, Maioli F, et al. Conversion of mild cognitive impairment to dementia: predictive role of mild cognitive impairment subtypes and vascular risk factors. Dement Geriatr Cogn Disord. 2006;21(1):51–8. doi: 10.1159/000089515. [DOI] [PubMed] [Google Scholar]
- 95.DeCarli C, Mungas D, Harvey D, et al. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology. 2004;63(2):220–7. doi: 10.1212/01.wnl.0000130531.90205.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wolf H, Ecke GM, Bettin S, et al. Do white matter changes contribute to the subsequent development of dementia in patients with mild cognitive impairment? A longitudinal study. Int J Geriatr Psychiatry. 2000;15(9):803–12. doi: 10.1002/1099-1166(200009)15:9<803::aid-gps190>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 97.Xu WL, Qiu CX, Wahlin A, et al. Diabetes mellitus and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Neurology. 2004;63(7):1181–6. doi: 10.1212/01.wnl.0000140291.86406.d1. [DOI] [PubMed] [Google Scholar]
- 98.Xu W, Caracciolo B, Wang HX, et al. Accelerated progression from mild cognitive impairment to dementia in people with diabetes. Diabetes. 2010;59(11):2928–35. doi: 10.2337/db10-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li J, Wang YJ, Zhang M, et al. Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology. 2011;76(17):1485–91. doi: 10.1212/WNL.0b013e318217e7a4. [DOI] [PubMed] [Google Scholar]
- 100.Bettermann K, Arnold AM, Williamson J, et al. Statins, risk of dementia, and cognitive function: secondary analysis of the ginkgo evaluation of memory study. J Stroke Cerebrovasc Dis. 2012;21(6):436–44. doi: 10.1016/j.jstrokecerebrovasdis.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Peters ME, Rosenberg PB, Steinberg M, et al. Prevalence of neuropsychiatric symptoms in CIND and its subtypes: the Cache County Study. Am J Geriatr Psychiatry. 2012;20(5):416–24. doi: 10.1097/JGP.0b013e318211057d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lyketsos CG, Colenda CC, Beck C, et al. Position statement of the American Association for Geriatric Psychiatry regarding principles of care for patients with dementia resulting from Alzheimer disease. Am J Geriatr Psychiatry. 2006;14(7):561–72. doi: 10.1097/01.JGP.0000221334.65330.55. [DOI] [PubMed] [Google Scholar]
- 103.Peters ME, Rosenberg PB, Steinberg M, et al. Neuropsychiatric symptoms as risk factors for progression from CIND to Dementia: The Cache County Study. Am J Geriatr Psychiatry. 2013 doi: 10.1016/j.jagp.2013.01.049. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Richard E, Schmand B, Eikelenboom P, et al. Symptoms of apathy are associated with progression from mild cognitive impairment to Alzheimer's disease in non-depressed subjects. Dement Geriatr Cogn Disord. 2012;33(2-3):204–9. doi: 10.1159/000338239. [DOI] [PubMed] [Google Scholar]
- 105.Modrego PJ, Ferrandez J. Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type: a prospective cohort study. Arch Neurol. 2004;61(8):1290–3. doi: 10.1001/archneur.61.8.1290. [DOI] [PubMed] [Google Scholar]
- 106.Palmer K, Berger AK, Monastero R, et al. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology. 2007;68(19):1596–602. doi: 10.1212/01.wnl.0000260968.92345.3f. [DOI] [PubMed] [Google Scholar]
- 107.Reynolds CF, 3rd, Butters MA, Lopez O, et al. Maintenance treatment of depression in old age: a randomized, double-blind, placebo-controlled evaluation of the efficacy and safety of donepezil combined with antidepressant pharmacotherapy. Arch Gen Psychiatry. 2011;68(1):51–60. doi: 10.1001/archgenpsychiatry.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Richard E, Reitz C, Honig LH, et al. Late-life depression, mild cognitive impairment, and dementia. Arch Neurol. 2012:1–7. doi: 10.1001/jamaneurol.2013.603. [DOI] [PMC free article] [PubMed] [Google Scholar]