Abstract
Pain is a common symptom associated with cancer and its treatment. Pain management is an important aspect of oncologic care, and unrelieved pain significantly comprises overall quality of life. These NCCN Guidelines list the principles of management and acknowledge the range of complex decisions faced in the management oncologic pain. In addition to pain assessment techniques, these guidelines provide principles of use, dosing, management of adverse effects, and safe handling procedures of pharmacologic therapies and discuss a multidisciplinary approach for the management of cancer pain.
Overview
Pain is one of the most common symptoms associated with cancer. Pain is defined by the International Association for the Study of Pain as an unpleasant, multidimensional, sensory, and emotional experience associated with actual or potential tissue damage, or is described in relation to such damage.1 Cancer pain or cancer-related pain is distinct from pain experienced by patients without malignancies. Pain occurs in approximately one-quarter of patients with newly diagnosed malignancies, one-third of patients undergoing treatment, and three-quarters of patients with advanced disease,2–4 and is one of the symptoms patients fear most. Unrelieved pain denies patients comfort and greatly affects their activities, motivation, interactions with family and friends, and overall quality of life. Mounting evidence in oncology shows that survival is linked to pain control.5
The importance of relieving pain and the availability of effective therapies make it imperative that physicians and nurses be adept at assessing and treating cancer pain.6–8 This requires familiarity with the pathogenesis of cancer pain; pain assessment techniques; common barriers to the delivery of appropriate analgesia; and pertinent pharmacologic, anesthetic, neurosurgical, behavioral, and complementary approaches to the treatment of cancer pain.
The most widely accepted algorithm for the treatment of cancer pain was developed by the WHO.9,10 It suggests that patients with pain be started on acetaminophen or a nonsteroidal anti-inflammatory drug (NSAID). If this is not sufficient, patients should be escalated to a weak opioid, such as codeine, and then to a strong opioid, such as morphine. Although this algorithm has served as an excellent teaching tool, the management of cancer pain is considerably more complex than this 3-tiered “cancer pain ladder” suggests.
These guidelines are unique in several important ways. First, they list the principles of pain management:
Pain management is essential for maximizing patient outcomes; mounting evidence in oncology shows that survival is linked to effective pain control.5
All patients must be screened for pain at each contact, and a comprehensive pain assessment must be performed if pain exists.
The goal is to improve patient comfort, maximize function, and improve quality of life.
Comprehensive management of pain is needed, because most patients have multiple pathophysiologies of pain.
Analgesic therapy must be administered in conjunction with the management of multiple symptoms or symptom clusters and the complex pharmacologic therapies that patients with cancer are generally prescribed.
Pain intensity must be quantified, and quality must be characterized by the patient (whenever possible). These guidelines base therapeutic decisions on a numerical value assigned to the severity of the pain.
Reassessment of pain intensity must be performed at specified intervals to ensure that the therapy selected is having the maximum benefit with as few adverse effects as possible.
Persistent cancer pain often requires treatment with regularly scheduled analgesics with supplemental doses of analgesics provided as needed to manage breakthrough pain.
A multidisciplinary team may be needed for comprehensive pain management.
Given the multifaceted nature of cancer pain, the use of integrative interventions inclusive of physical and cognitive modalities must be optimized.
Psychosocial support must be made available to patients.
Specific educational material must be provided to patients and family/caregivers.
The experience of pain has been associated with suffering. The multidimensional impact of “suffering” on patients and their families must be considered, and these concerns must be addressed in a culturally respectful manner.





Second, these guidelines acknowledge the range of complex decisions faced in the management of these patients. As a result, they provide dosing guidelines for opioids, nonopioid analgesics, and adjuvant analgesics. They also provide specific suggestions for titrating and rotating opioids, escalation of opioid dosage, management of opioid adverse effects, and when and how to proceed to other techniques/interventions for the management of cancer pain.
Pathophysiologic Classification of Cancer Pain Syndromes
Different types of pain occur in patients with cancer. Several attempts have been made to classify pain according to different criteria. Pain classification includes differentiating between pain associated with tumor, pain associated with treatment, and pain unrelated to either. Acute and chronic pain should also be distinguished when deciding what therapy to use. Therapeutic strategy depends on the pain pathophysiology, which is determined through patient examination and evaluation. Pain has 2 predominant mechanisms of pathophysiology: nociceptive and neuropathic.11,12
Nociceptive pain is the result of injury to somatic and visceral structures and the resulting activation of nociceptors. Nociceptors are present in skin, viscera, muscles, and connective tissues. Nociceptive pain can further be divided into somatic and visceral pain.13 Pain described as sharp, well-localized, throbbing, and pressure-like is probably somatic nociceptive pain, and often occurs after surgical procedures or from bone metastasis. Visceral nociceptive pain is often described as more diffuse, aching, and cramping, and is secondary to compression, infiltration, or distension of abdominal or thoracic viscera.
Neuropathic pain results from injury to the peripheral or central nervous system (CNS). This type of pain might be described as burning, sharp, or shooting. Examples of neuropathic pain include pain from spinal stenosis or diabetic neuropathy, as an adverse effect of chemotherapy (eg, vincristine) or radiation therapy, or from surgical injury to the nerves.
Comprehensive Pain Assessment
A comprehensive evaluation is essential to ensure proper pain management. Failure to adequately assess pain frequently leads to poor pain control. Therefore, it is important to find the cause of the pain and identify optimal therapies.
These guidelines begin with the premise that all patients with cancer should be screened for pain during the initial evaluation, at regular follow-up intervals, and whenever new therapy is initiated. If pain is present on a screening evaluation, the pain intensity must be quantified by the patient (whenever possible). Because pain is inherently subjective, patient self-reports are the current standard of care for assessment. Intensity of pain should be quantified using a 0 to 10 numeric rating scale, a categorical scale, or a pictorial scale (eg, the Faces Pain Rating Scale).14–17 The Faces Pain Rating Scale may be successful for patients who have difficulty with other scales, such as children, elderly patients, and patients with language or cultural differences or other communication barriers. If the patient is unable to verbally report pain, an alternative method must be used to asses and rate the pain.
Patients should also be asked to describe the characteristics of their pain (ie, aching, burning). If the patient has no pain, re-screening should be performed at each subsequent visit or as requested. Identifying the presence of pain through repeated screening is essential to allow implementation of effective pain management.
If the Pain Rating Scale score is above 0, a comprehensive pain assessment is initiated. The comprehensive pain assessment should focus on the type and quality of pain, pain history (eg, onset, duration, course), pain intensity (eg, pain experienced at rest or with movement, or that interference with activities), location, referral pattern, radiation of pain, associated factors that exacerbate or relieve the pain, current pain management plan, patient response to current therapy, prior pain therapies, breakthrough or episodic pain not controlled with existing pain regimen, important psychosocial factors (eg, patient distress, family/caregiver and other support, psychiatric history, risk factors for undertreatment of pain), and other special issues relating to pain (eg, meaning of pain for patient and family/caregiver; cultural beliefs toward pain, pain expression, and treatment; spiritual or religious considerations and existential suffering).18,19 Finally the patient’s goals and expectations of pain management should be discussed, including their level of comfort and function, with family/caregivers included.
In addition, a thorough physical examination and a review of appropriate laboratory and imaging studies are essential for a comprehensive pain assessment. This evaluation should enable caregivers to determine if the pain is related to an underlying cause that requires specific therapy. For example, providing only opioids to a patient experiencing pain from impending spinal cord compression is inappropriate. Without glucocorticoids and local radiation therapy, the pain is unlikely to be well controlled and the patient will remain at high risk for spinal cord injury.
The NCCN Adult Cancer Pain Panel recommends monitoring risk factors for aberrant use or diversion of pain medication, which must be identified during initial screening using tools, such as the SOAPP-R (Screener and Opioid Assessment for Patients with Pain-Revised) or ORT (Opioid Risk Tool). The SOAPP was developed to predict which patients being considered for long-term opioid therapy may exhibit aberrant medications behaviors in the future.20 SOAPP-R is a revised version of the SOAPP.21 Similar to the SOAPP-R, the ORT assesses the risk of aberrant behaviors when patients are prescribed opioid medication for chronic pain, with a high degree of sensitivity and specificity for determining which individuals are at risk for opioid abuse.22 SOAPP-R and ORT discriminate between high-risk and low-risk patients.23 A high-risk score on the SOAPP-R or ORT correlates with an increased likelihood of drug abuse.24
The end point of comprehensive pain assessment is to diagnose the origin and pathophysiology (somatic, visceral, or neuropathic) of the pain. Treatment must be individualized based on clinical circumstances and patient wishes, with the goal of maximizing function and quality of life.
Management of Adult Cancer Pain
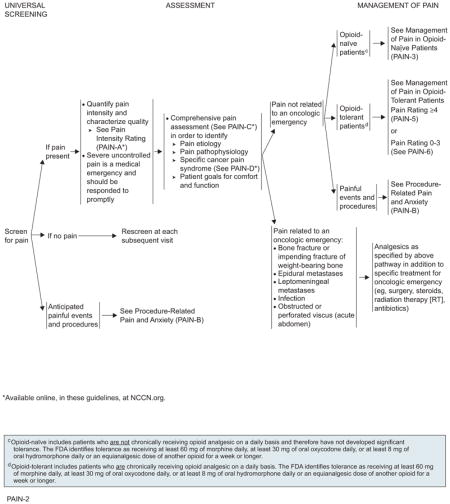
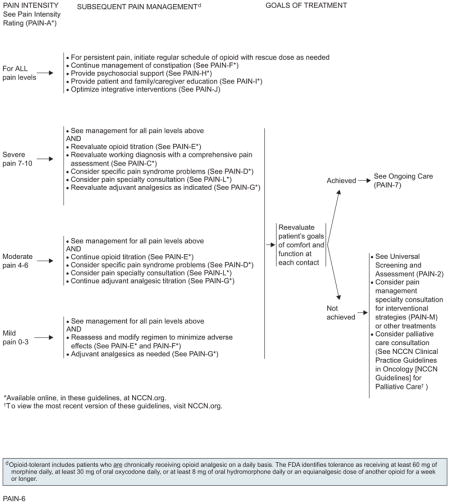
For management of cancer-related pain in adults, the algorithm distinguishes 3 levels of pain intensity, based on a 0 to 10 numerical value obtained using a numerical or the pictorial rating scale (with 0 being no pain and 10 being the worst pain). The 3 levels of pain intensity listed in the algorithm are mild pain (1–3), moderate pain (4–6), and severe pain (7–10).
It is important to separate pain related to an oncologic emergency from pain not related to an oncologic emergency.
The algorithm also distinguishes pain that is unrelated to oncologic emergencies in patients not chronically taking opioids (opioid-naïve) from the pain experienced by those who have previously taken or are chronically taking opioids for cancer pain (opioid-tolerant), and also from anticipated procedure-related pain and anxiety.
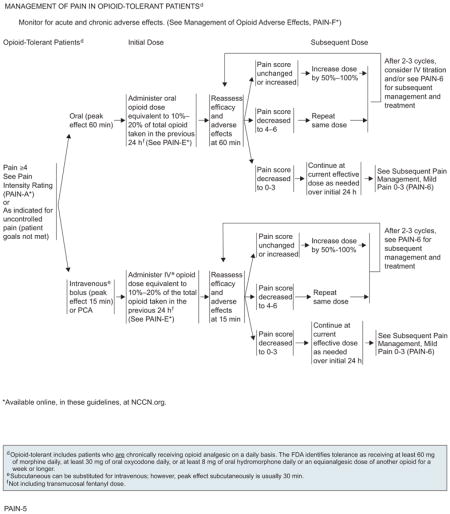
According to the FDA, “patients considered opioid tolerant are those who are taking at least: 60 mg oral morphine/day, 25 mcg transdermal fentanyl/hour, 30 mg oral oxycodone/day, 8 mg oral hydromorphone/day, 25 mg oral oxymorphone/day, or an equianalgesic dose of another opioid for one week or longer.” Therefore, patients who do not meet the definition of opioid-tolerant and who have not had opioid doses at least as much as those stated for a week or more are considered opioid-naïve.
Management of Pain Related to Oncologic Emergency
An oncologic emergency is defined as a life-threatening event directly or indirectly related to a patient’s cancer or its treatment. Pain related to an oncologic emergency includes pain from bone fracture or impending fracture of weight-bearing bone; epidural or leptomeningeal metastases seen in patients with advanced adenocarcinomas; pain related to infection; or obstructed or perforated viscus. Pain associated with oncologic emergency should be treated directly during treatment of the underlying condition.
Management of Pain Not Related to Oncologic Emergency in Opioid-Naïve Patients
For all patients experiencing pain, care providers should provide psychosocial support and begin educational activities. Psychosocial support is needed to ensure that patients encountering common barriers to appropriate pain control (eg, fear of addiction or side effects, inability to obtain opioids) or needing assistance in managing additional problems (eg, depression, rapidly declining functional status) receive appropriate aid. The patient and the family/caregiver must be educated regarding pain management and related issues.25,26 Patients should be reevaluated at each contact and as needed to meet their goals for comfort and function.
Although pharmacologic analgesics, including nonopioids (eg, NSAIDS, acetaminophen), opioids, and adjuvant analgesics (eg, antidepressants, anticonvulsants, topical agents, corticosteroids), are the cornerstone of cancer pain management, they are not always adequate and are associated with many adverse effects. Thus, they often necessitate the implementation of additional therapies or treatments. Optimal use of nonpharmacologic integrative interventions (physical, cognitive modalities, and spiritual) may be a valuable addition to pharmacologic interventions.
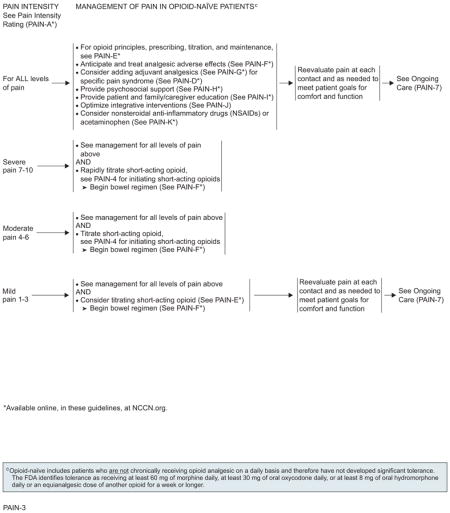
Opioid-naïve patients (those who are not chronically receiving opioids on a daily basis) experiencing severe pain (ie, pain intensity rating 7–10) should receive rapid titration of short-acting opioids (see “Opioid Prescriptions, Titration, and Maintenance,” page 1010). Short-acting formulations have the advantage of rapid onset of analgesic effect. The route of opioid administration (oral vs intravenous) is decided based on what is best suited to the patient’s ongoing analgesic needs.
Several adverse effects are potentially associated with the use of opioid analgesics. Management of these common opioid-induced adverse effects should begin simultaneously with the initiation of opioid therapy. Opioid-induced bowel dysfunction should be anticipated and treated prophylactically with a stimulating laxative to increase bowel motility, with or without stool softeners as indicated.27 Addition of adjuvant analgesics for specific pain syndromes should be considered for all groups of patients. Adjuvant analgesics are drugs used to enhance the effects of opioids or NSAIDs.28
The pathways for opioid-naïve patients, whose pain intensity is moderate with a rating between 4 and 6 at presentation, are similar to those for patients with a pain intensity of 7 to 10. One of the main differences is that treatment begins with slower titration of short-acting opioids.
Opioid-naïve patients experiencing mild pain intensity (pain intensity rating, 1–3) should receive treatment with nonopioid analgesics, such as NSAIDs or acetaminophen, or treatment with consideration of slower titration of short-acting opioids.
Patients with chronic persistent pain controlled by stable doses of short-acting opioids should be provided with round-the-clock extended release or long-acting formulation opioids, with provision of a rescue dose to manage breakthrough or transient exacerbations of pain. The rescue dose is usually equivalent to 10% to 20% of the total daily dose given every hour as needed. Opioids with a rapid onset and short duration are preferred as rescue doses. The repeated need for rescue doses per day may indicate the need to adjust the baseline treatment.
Management of Pain Not Related to Oncologic Emergency in Opioid-Tolerant Patients
Opioid-tolerant patients are those chronically taking opioids for pain relief. To achieve adequate analgesia in opioid-tolerant patients who are experiencing breakthrough pain of intensity rating 4 or greater or a pain intensity less than 4 but whose goals of pain control and function are not met, the previous 24-hour total oral or intravenous opioid requirement must be calculated and the new “rescue” dose must be increased by an opioid dose equivalent to 10% to 20% of the total opioid taken in the previous 24 hours.29,30
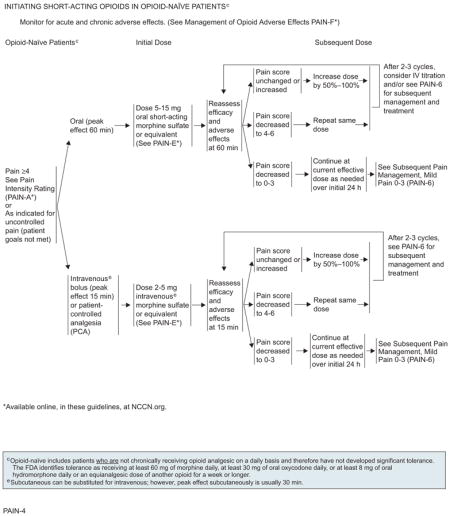
Efficacy and adverse effects should be assessed every 60 minutes for orally administered opioids, and every 15 minutes for intravenous opioids, to determine a subsequent dose. On assessment, if the pain score remains unchanged or is increased, administration of 50% to 100% of the previous rescue dose of opioid is recommended. If the pain score decreases to 4 to 6, the same dose of opioid should be repeated and reassessment performed at 60 minutes for orally administered opioids and every 15 minutes for intravenously administered opioids. If the pain score remains unchanged on reassessment after 2 to 3 cycles of the opioid in patients with moderate to severe pain, changing the route of administration from oral to intravenous or alternate management strategies can be considered. If the pain score decreases to 0 to 3, the current effective dose of either oral or intravenous opioid should be administered as needed over an initial 24 hours before proceeding to subsequent management strategies.
Management of Procedure-Related Pain and Anxiety
Procedure-related pain represents an acute short-lived experience that may be accompanied by a great deal of anxiety. Procedures reported as painful include bone marrow aspirations; wound care; lumbar puncture; skin and bone marrow biopsies; and intravenous, arterial, and central line injections and manipulations. Much of the data available on procedure-related pain are from studies on pediatric patients with cancer, which are then extrapolated to adults.
Interventions to manage procedure-related pain should take into account the type of procedure, the anticipated level of pain, and other individual patient characteristics such as age and physical condition. The interventions may be multimodal and may include pharmacologic and/or nonpharmacologic approaches. Supplemental doses of analgesics should be given in anticipation of procedure-related pain. Anxiolytics are drugs used for the treatment of anxiety and its related psychologic and physical symptoms. Anxiolytics should be given preemptively for control of procedure-related anxiety when feasible.
Local anesthetics can be used to manage procedure-related pain with sufficient time for effectiveness, as per package inserts. Examples of local anesthetics include lidocaine, prilocaine, and tetracaine. Physical approaches such as cutaneous warming, laser or jet injection, and ultrasound may accelerate the onset of cutaneous anesthesia. Sedatives may also be used. However, deep sedation and general anesthesia must be performed only by trained professionals. In addition, use of nonpharmacologic interventions may be valuable in managing procedure-related pain and anxiety. The major goal of nonpharmacologic interventions that include physical and cognitive modalities is to promote a sense of control, thereby increasing hope and reducing the feeling of helplessness experienced by many patients with pain from cancer.
Patients usually tolerate procedures better when they know what to expect. Therefore, patients and family members/caregivers should receive written instructions for managing the pain. Preprocedure patient education on procedure details and pain management strategies is essential. Patients and family members/caregivers should receive written information regarding pain management options.
Subsequent Management of Cancer Pain
Subsequent treatment is based on the patient’s continued pain rating score. Approaches for all pain intensity levels must include administration of regular doses of opioids, with rescue doses as needed and management of constipation, coupled with psychosocial support and education for patients and their families.
If the pain at this time is severe, unchanged, or increased, the working diagnosis must be reevaluated and comprehensive pain assessment performed. For patients unable to tolerate dose escalation of their current opioid because of adverse effects, an alternate opioid must be considered. Addition of adjuvant analgesics should be reevaluated to either enhance the analgesic effect of the opioids or, in some cases, counter the adverse effects associated with the opioids.27 Optimal use of nonpharmacologic integrative interventions (physical, cognitive modalities, and spiritual) may serve as valuable additions to pharmacologic interventions. Given the multifaceted nature of cancer pain, additional interventions for specific cancer pain syndromes and specialty consultation must be considered to provide adequate analgesia. In patients experiencing moderate pain intensity of 4 to 6 and adequate analgesic relief on the current opioid, the current titration of the opioid may be continued or increased. In addition, similar to patients experiencing severe pain, addition of adjuvant analgesics, additional interventions for specific cancer pain syndromes, and specialty consultation must be considered.
In patients experiencing mild pain and adequate analgesic relief but intolerable or unmanageable adverse effects, the analgesic dose may be reduced by 25% of the current opioid dose. Addition of adjuvant analgesics may be considered.
Ongoing Care
Although pain intensity ratings will be obtained frequently to evaluate opioid dose increases, a formal reevaluation to assess patient goals of comfort and function is mandated at each contact.
If an acceptable level of comfort and function has been achieved for the patients and 24-hour opioid requirement is stable, the panel recommends converting to an extended-release oral medication (if feasible) or other extended-release formulation (eg, transdermal fentanyl). Subsequent treatment is based on the patient’s continued pain rating score. Rescue doses of the short-acting formulation of the same long-acting drug may be provided during maintenance therapy to manage pain in patients not experiencing relief with extended-release opioids.
Routine follow-up of inpatients should be performed during each outpatient contact, or at least each day, depending on patient conditions and institutional standards.
System-related barriers include cost of analgesics and a lack of access to/availability of analgesics, particularly in minority neighborhoods or for patients who are poor. Studies have documented the inequalities that persist because those with financial burdens or minorities have less access to pain treatment.19,31 The panel recommends addressing these system barriers.32–35
Patients must be provided with a written follow-up pain plan, including prescribed medications. It is important to ensure that the patient has adequate access to prescribed medications and maintains communication and coordination of care with a pain specialist and relevant providers, especially during transitions between sites of care. Which clinician will be prescribing the patient’s ongoing care should be clarified with the patient. Equally important is monitoring for the use of analgesics as prescribed, especially in patients with risk factors for or history of abuse.
If an acceptable level of comfort and function has not been achieved, universal screening and assessment must be performed and additional strategies for pain relief considered.
Pharmacologic Interventions
Opioids and Miscellaneous Analgesics
Selecting An Appropriate Opioid
When starting therapy, attempts should be made to determine the underlying pain mechanism and diagnose the pain syndrome. Optimal analgesic selection will depend on the patient’s pain intensity, any current analgesic therapy, and concomitant medical illnesses. An individual approach should be used to determine opioid starting dose, frequency, and titration to achieve a balance between pain relief and medication adverse effects.
In a patient who has not been exposed to opioids in the past, morphine is generally considered the standard preferred starting drug.36,37 Oral administration is the preferred route. An initial oral dose of 5 to 15 mg of oral short-acting morphine sulfate or equivalent is recommended for opioid-naïve patients. Patients presenting with severe pain needing urgent relief should be treated with parenteral opioids, usually administered intravenously or subcutaneously. If given parenterally, the equivalent dose is one-third of the oral dose.38 An initial dose of 2 to 5 mg of intravenous morphine sulfate or equivalent is recommended for opioid-naïve patients.
Pure agonists (eg, morphine, oxycodone, oxymorphone, fentanyl) are the most commonly used medications in the management of cancer pain. The short half-life opioid agonists (eg, morphine, hydromorphone, fentanyl, oxycodone) are preferred, because they can be more easily titrated than the long half-life analgesics (methadone and levorphanol).39
Fentanyl is a highly lipid soluble opioid that can be administered via the parenteral, spinal, transdermal, transmucosal, buccal, and intranasal routes. Transdermal fentanyl is not indicated for rapid opioid titration and should only be recommended after pain is controlled by other opioids in opioid-tolerant patients.40 It is usually the preferred treatment for patients who are unable to swallow, those with poor tolerance to morphine, and those with poor compliance. Conversion from intravenous fentanyl to transdermal fentanyl can be accomplished effectively using a 1:1 conversion ratio.41 Transmucosal fentanyl may be considered in opioid-tolerant patients for brief episodes of incident pain not attributed to inadequate dosing of around-the-clock opioid. Data do not support a specific transmucosal fentanyl dose equianalgesic to other opioids or between different transmucosal formulations. Increasing data show that buccal fentanyl is effective in treating breakthrough pain in patients with cancer. 42–44
Hydrocodone may be approximately equipotent with oral morphine; however, its equivalence data are not substantiated. Clinical experience suggests it be used as a mild, initial-use opioid, but the effective dose may vary. It is available only in combination with oral agents, such as acetaminophen or ibuprofen.
Codeine is a prodrug that is metabolized to codeine-6-glucuronide, norcodeine, morphine, morphine-3-glucuronide, morphine-6-glucuronide, and normorphine.45 This process is largely through the action of the cytochrome P450 enzyme, CYP2D6. It is important to note that CYP2D6 exhibits polymorphism among various ethnic groups and among individuals. A significant portion of individuals who are poor metabolizers would experience reduced or no analgesic effects.46
Hydromorphone has properties similar to morphine and is available in oral tablet, liquid, suppository, and parenteral formulations.47 Some evidence suggests that the metabolite of hydromorphone may lead to opioid neurotoxicity, including myoclonus, hyperalgesia, and seizures.48 This metabolite may be more neurotoxic than the morphine metabolite.49
Morphine is available in a wide range of formulations and routes, including oral, parenteral, and rectal delivery.50 Morphine-6-glucoronide, an active metabolite of morphine, contributes to analgesia and may worsen adverse effects as it accumulates in patients with renal insufficiency.51,52
Morphine, hydromorphone, and codeine should be used with caution in patients with fluctuating renal function because of the potential accumulation of renally cleared metabolites that may cause neurologic toxicity.53,54
Oxycodone and oxymorphone are available as immediate- and extended-release formulations.55–59 Oxycodone is also available in combination with acetaminophen; therefore, the dosage must be monitored for safe limits.
Methadone is commercially available in multiple-strength oral tablets or oral solution. Individual variations in methadone pharmacokinetics (long half-life ranging from 8 to >120 hours) make its use very difficult in patients with cancer.60 Because of its long half-life, high potency, and interindividual variations in pharmacokinetics, methadone should be started at doses lower than calculated and slowly titrated upward, with provision of adequate short-acting breakthrough pain medications during the titration period. The dosing ratio between methadone and morphine or other opioids, and conversion from another opioid to methadone, is not simple.61,62 Studies show that outpatient initiation and rotation to methadone can be successfully performed in patients with cancer without serious adverse effects.63 The panel cautions and advises practitioners to consult a pain management specialist if they are unfamiliar with methadone prescribing or if individual patient considerations necessitate very rapid switching to or from methadone.
Evidence suggests that high doses of methadone (≥120 mg) may lead to QTc prolongation and torsades de pointes, which if uncorrected may lead to sudden cardiac death.64–66 Oral methadone is commonly used to treat cancer pain, and the average dosing seems to be much lower than is used to treat opioid dependency and chronic nonmalignant pain. A recent study conducted in patients with cancer suggests that QT interval changes exist commonly at baseline and are not changed with the addition of methadone.67 However, physicians initiating methadone should be aware of the drug interactions. The NCCN Adult Cancer Pain Panel recommends a baseline and follow-up echocardiogram for patients treated with methadone doses greater than 100 mg/d, those with cardiac disease, and in those taking other medications also known to prolong QTc (including tricyclic antidepressants). QTc of 450 or greater may indicate the need to reduce or discontinue the methadone dose.
Methadone use should be initiated by physicians with experience and expertise in its use. Patients and their families may need to be educated about analgesic utility of methadone. Some may only be familiar with methadone use for maintenance of addiction and be unaware of its utility as a potent opioid analgesic.
Selecting Miscellaneous Analgesics
Tramadol is a weak opioid receptor agonist with some norepinephrine and serotonin reuptake inhibition used for mild to moderate pain. Tramadol should be avoided in patients receiving selective serotonin reuptake inhibitors (SSRIs) or tricyclic antidepressants. In a double-blind study of cancer patients, tramadol produced more adverse effects, including vomiting, dizziness, and weakness, when compared with hydrocodone and codeine.68 Tramadol is available as immediate-release and extended-release formulations. The panel recommends a maximum daily dose of 400 mg (100 mg 4 times daily) for adults with normal hepatic and renal function. Lower doses are recommended for older adults (age ≥75 years) and those with hepatic and/or renal dysfunction, to reduce the risk of seizures. Even at a maximum dose of 400 mg/d, tramadol is less potent than other opioids and is considered to be approximately one-tenth as potent as morphine.69
Tapentadol is a new opioid that binds to the μ-opioid receptor and inhibits norepinephrine reuptake.70,71 It is available as extended-release and immediate-release formulations and is used to treat moderate to severe pain. Typical doses would start at 50 to 100 mg orally every 4 hours as needed, with a maximal daily dose of 500 mg/d (if using the extended-release formulation) or 600 mg/d (if using the immediate-release formulation only), because of the lack of published data regarding higher doses. Comparative phase II through III studies have demonstrated the efficacy and safety of tapentadol compared with placebo and oxycodone for non–cancer-related pain.72–74 Some data suggest that tapentadol may be associated with a lower incidence of gastrointestinal adverse effects than oxycodone.72 To date, no randomized trial evaluating the efficacy of tapentadol is available in patients with cancer. The first study reporting data from patients with cancer pain was a small, prospective, open-label study with 50 opioid-naïve patients with cancer pain, 39 of whom completed the entire study.75 Results of the study showed that compared with placebo, tapentadol at a dosage of 100 mg/d was well tolerated and effective in decreasing pain intensity from baseline and improving quality of life.75
Transdermal buprenorphine, a partial μ-opioid agonist, has been approved for chronic pain. Although experience with this drug in the management of cancer pain is limited, anecdotal reports, a few small prospective uncontrolled studies, and at least one randomized trial support its use in cancer-related pain.76 Studies of buprenorphine suggest that it exhibits a ceiling to analgesic efficacy, thereby limiting its use in palliative care.77 It may precipitate withdrawal symptoms if administered to individuals currently taking a high-dose opioid. FDA guidelines recommend limiting the dose to a maximum of 20 μg/h because of concern for QT prolongation.
Ketamine is a noncompetitive N-methyl D-aspartate receptor antagonist that blocks glutamate.78 Low (subanesthetic) doses produce analgesia and modulate central sensitization, hyperalgesia, and opioid tolerance. Only limited data are available regarding the use of ketamine as an adjuvant to opioids for the management of cancer pain. A double-blind, randomized, placebo-controlled trial found no significant difference between the outcomes of patients treated with ketamine versus placebo.79
The following agents are not recommended for patients with cancer: 1) mixed agonist-antagonists (eg, butorphanol, pentazocine), 2) meperidine, and 3) placebos. Mixed agonist-antagonists should not be used in combination with opioid agonist drugs for cancer pain management. Converting from an agonist to an agonist-antagonist could precipitate the abstinence syndrome (a withdrawal crisis) in patients who are physically dependent on a pure opioid agonist. Meperidine is contraindicated for chronic pain, especially in patients with impaired renal function or dehydration, because accumulation of metabolites that are cleared renally may result in neurotoxicity (seizures) or cardiac arrhythmias.80 Use of placebo in the treatment of pain is unethical.
Selecting a Route of Administration
The least-invasive, easiest, and safest route of opioid administration should be provided to ensure adequate analgesia.
Oral is the preferred route of administration for chronic opioid therapy.29,80,81 The oral route should be considered first in patients who can take oral medications unless a rapid onset of analgesia is required or the patient experiences adverse effects associated with oral administration. Continuous parenteral infusion, intravenous administration, or subcutaneous administration is recommended for patients who cannot swallow or absorb opioids enterally. Opioids, given parenterally, may produce fast and effective plasma concentrations compared with oral or transdermal opioids. The intravenous route is considered for faster analgesia because of the short lag time between injection and effect (peak, 15 minutes) compared with oral dosing (peak, 60 minutes).82 The subcutaneous route has a slower onset and lower peak (30 minutes) effect compared with the intravenous route.
Opioid Prescription, Titration, and Maintenance
The appropriate dose of opioid is based on the patient’s pain intensity and their goals and avoids causing undesirable and unmanageable adverse drug effects.
The physicians should be aware of potential drug-drug and drug-disease interactions while determining the treatment plan. The patient’s goals and quality of life should also be considered when modifying the treatment plan.
The following methods of ongoing analgesic administration are widely used in clinical practice: “around the clock,” “as needed,” and “patient-controlled analgesia.” For most patients, dosing should be used for continuous pain relief. Additional doses of opioid may be required for pain not relieved by a regular schedule of long-acting (eg, extended-release) opioid.
The panel recommends considering opioid rotation if pain is inadequately controlled or if persistent adverse effects from current therapy occur. Other indications for switching to a different opioid include out-of-pocket costs and limitations based on insurance formularies.
For patients who have intermittent pain with pain-free intervals, opioids are administered on an as-needed basis. The as-needed method is also used when rapid dose titration is required. The patient-controlled analgesia technique allows a patient to control a device that delivers a bolus of analgesic on demand (according to, and limited by, parameters set by a physician).
Breakthrough pain is defined as pain that fails to be controlled or breaks through a regimen of a regularly scheduled opioid. It may be further categorized as incident pain that is associated with specific activities or events, potentially managed with rescue doses of short-acting opioid given in anticipation of those events; end-of-dose failure pain that recurs toward the end of a dosing interval for a regularly scheduled opioid, potentially managed by increasing the dose or frequency of the regularly scheduled opioid; and uncontrolled persistent pain that is routinely uncontrolled by an existing regularly scheduled opioid, potentially managed by adjusting the dose of the regularly scheduled opioid.
The panel also recommends monitoring for aberrant medication drug-related behaviors over the course of treatment using tools such as COMM (Current Opioid Misuse Measure). The COMM tool helps clinicians identify whether a patient, currently on long-term opioid therapy, is exhibiting aberrant behaviors associated with misuse of opioid medications.83 It examines concurrent misuse; in contrast, SOAPP-R or ORT is helpful in predicting which patients being considered for long-term opioid therapy may exhibit aberrant medications behaviors in the future.
Initiating Short-Acting Opioids in Opioid-Naïve Patients
The route of administration of opioid (oral or intravenous) must be selected based on the needs of the patient. These guidelines provide guidance for initiating short-acting opioids in opioid-naïve and opioid-tolerant patients.
For opioid-naïve patients experiencing pain intensity of greater than or equal to 4, or a pain intensity less than 4 but whose goals of pain control and function are not met, an initial dose of 5 to 15 mg of oral morphine sulfate or 2 to 5 mg of intravenous morphine sulfate or equivalent is recommended. Efficacy and adverse effects should be assessed every 60 minutes for orally administered opioids and every 15 minutes for intravenous opioids to determine a subsequent dose. If the pain score remains unchanged or increases, the panel recommends increasing the dose by 50% to 100% of the previous dose of opioid to achieve adequate analgesia. If the pain score decreases to 4 to 6, the same dose of opioid is repeated and the patient is reassessed at 60 minutes for orally administered opioids and every 15 minutes for intravenously administered opioids. If inadequate response is seen in patients with moderate to severe pain on reassessment after 2 to 3 cycles of the opioid, changing the route of administration from oral to intravenous or subsequent management strategies can be considered. If the pain score decreases to 0 to 3, the current effective dose of opioid is administered as needed over an initial 24 hours before proceeding to subsequent management strategies.
Opioid Adverse Effects
Several adverse effects are associated with the use of opioid analgesics. Constipation, nausea and vomiting, pruritus, delirium, respiratory depression, motor and cognitive impairment, and sedation are fairly common, especially when multiple agents are used.84–89 Each adverse effect requires a careful assessment and treatment strategy. Management of opioid-induced adverse effects is integral to opioid pain management.84,90–98
Constipation can almost always be anticipated with opioid treatment, and patients do not develop tolerance to this adverse effect. Therefore, administration of a prophylactic bowel regimen is recommended. However, little evidence exists on which to base the selection of the most appropriate prophylactic bowel regimen. One study has shown that addition of a stool softener, such as docusate, to the laxative, sennosides, was less effective than administering the laxative alone.99 Therefore, for prophylaxis, the panel recommends a stimulant laxative with or without a stool softener or a capful of polyethylene glycol (PEG) with 8 oz of water 2 times daily, along with maintaining adequate fluid intake. Although maintaining adequate dietary fiber intake is recommended, supplemental medicinal fiber, such as psyllium, is ineffective and unlikely to reduce opioid-induced constipation.
Once constipation develops, the cause and severity of constipation must be assessed to rule out obstruction. Stool softeners or laxatives may be titrated as needed, with the goal of achieving one nonforced bowel movement every 1 to 2 days. Adjuvant analgesic may be considered to allow reduction of the opioid dose.
If constipation persists, the cause and severity of constipation must be assessed again to rule out bowel obstruction or impaction. Adding stimulant laxatives, such as magnesium-based products, bisacodyl (available in tablets or suppositories), or osmotic laxatives (eg, sorbitol, lactulose, PEG), may be helpful. Opioid rotation to fentanyl or methadone may be considered. Prokinetic agents such as metoclopramide enhance gastric antral contractility and may be useful in managing persistent constipation. However, chronic use of metoclopramide may be limited because of concern for neurologic complications, including tardive dyskinesia. Enema with fleet, saline, or tap water may be helpful because it dilates the bowel, stimulates peristalsis, and lubricates the stool to encourage a bowel movement. When response to laxative therapy has not been sufficient in patients with advanced illness, methylnaltrexone, an opioid antagonist that works on receptors in the gastrointestinal system and is given subcutaneously, can be used as a rescue when constipation is clearly related to opioid therapy.100–104 Neuraxial analgesics, neuroablative techniques, or other interventions to decrease pain and/or reduce systemic opioid dose may also be considered to reduce the adverse effects.
For patients with a prior history of opioid-induced nausea, prophylactic treatment with antiemetic agents is highly recommended. If nausea develops, other causes of nausea (eg, constipation, CNS pathology, chemotherapy, radiation therapy, hypercalcemia) must be assessed. Effective agents that may be considered include benzodiazepines, such as prochlorperazine or thiethylperazine, or dopamine receptor antagonists, such as metoclopramide or haloperidol. If nausea persists despite an as-needed regimen, antiemetics should be administered around the clock for 1 week, and then dosing changed as needed. When managing opioid-induced persistent nausea, rather than replacing one antiemetic with another, it may be helpful to add therapies that target different mechanisms of action, resulting in a synergistic effect. Adding serotonin receptor antagonists such as granisetron or ondansetron may be helpful. Corticosteroids can also be beneficial for reducing opioid-induced nausea and vomiting, and in particular have been found to be effective in combination with metoclopramide and ondansetron.105 If nausea persists for longer than a week, the cause of nausea must be reassessed and opioid rotation considered.
Pruritus or itchiness is a particularly common and distressing complaint, occurring in 10% to 50% of patients receiving opioids. Even in the presence of attentive skin care, opioids can produce recalcitrant pruritus. If pruritus develops, other causes must be first assessed, such as use of any other medication. Pruritus is more likely to occur early in the course of treatment. Antihistamines such as diphenhydramine or promethazine may be beneficial. If pruritus persists, changing to another opioid should be considered if symptomatic management has failed. Opioid antagonists have also proven useful in the management of patients whose pruritus is not relieved by antihistamines.106 Mixed agonist/antagonists (eg, nalbuphine) can be used to treat opioid-induced pruritus. The μ-opioid receptor antagonists (eg, naloxone) are also used to reverse opioid-induced adverse effects,107 and careful dose titration can produce relief without reversing analgesic efficacy.
Sedation may hinder the achievement of dose titration of opioids to levels that provide adequate analgesia.27 If opioid-induced sedation develops and persists for more than a week, it may be managed by administration of psychostimulants such as or methylphenidate, dextroamphetamine, or modafinil, or by adding caffeine. When using CNS stimulants for sedation, the dosing should be limited to morning and early afternoon to avoid nighttime insomnia.
Delirium is a pathophysiologic condition characterized by altered consciousness and inattention, cognitive dysfunction, and disturbed psychomotor behavior. Delirium may be treated with various interventions, such as adding a neuroleptic drug such as haloperidol, olanzapine, or risperidone, or switching to another opioid.108
Studies have shown that stable doses of opioids (>2 weeks) are not likely to interfere with psycho-motor and cognitive function, but these functions should be monitored during analgesic administration and titration.109
Respiratory depression is another adverse effect feared by physicians and patients. Physicians should be aware that patients with limited cardiopulmonary reserve are more susceptible and that hypercarbia occurs before hypoxia. Naloxone remains a useful antidote for the reversal of opioid-induced respiratory and CNS depression, but it should be administered cautiously so as not to precipitate acute opioid withdrawal syndrome in opioid-tolerant patients.
The details of prophylactic regimens and other measures to prevent opioid-induced adverse effects are provided in “Management of Opioid Adverse Effects,” available online, in these guidelines, at NCCN.org (PAIN-F).
Opioid Rotation
No single opioid is optimal for all patients.110 If opioid adverse effects are significant, an improved balance between analgesia and adverse effects might be achieved through changing to an equivalent dose of an alternative opioid. This approach is known as opioid rotation.84,111 Relative effectiveness is important to consider when switching between oral and parenteral routes to avoid subsequent overdosing or underdosing. Equianalgesic dose ratios, opioid titration and maintenance, and clinical examples of converting from one opioid to another are listed in “Opioid Principles, Prescribing, Titration, Maintenance, and Safety,” available online, in these guidelines, at NCCN.org (PAIN-E).
Opioids and Risk Evaluation and Mitigation Strategy
Although opioids are the principal analgesics for managing moderate to severe pain, they pose risks to patients and society. Opioid abuse is an increasing concern. In the United Sates, poisoning is now the leading cause of death from injuries, and 89% of poisonings are related to drugs. In 2008, of the 36,500 drug poisoning deaths, 14,800 (40%) involved opioid analgesics, compared with 5100 cocaine-related deaths and 3000 heroin-related deaths.112 Although it is important to ensure that opioids continue to be prescribed for patients for whom they are appropriate, it is also essential to ensure that these drugs are prescribed carefully. To reduce addiction, misuse, abuse, overdose, and death, the FDA is establishing Risk Evaluation and Mitigation Strategy (REMS) programs for all potent opioid products.113 The principal recommendations of opioid REMS programs are to educate the provider, patient, and family/caregiver.
The highlights of provider responsibilities included in the REMS are:
Establishing goals of opioid analgesic therapy for each patient and regularly evaluating therapeutic opioid response to guide further therapy
Evaluating each patient for risk factors associated with opioid misuse or abuse
Educating each patient on safe use, storage, and disposal of opioid
Routinely monitoring patients for opioid misuse or abuse
The REMS programs are currently in place for all transmucosal fentanyl and transdermal buprenorphine.114,115 The REMS for fentanyl products require a patient-prescriber agreement that involves patient education. In July 2012, the FDA mandated the development of REMS for all extended-release and long-acting opioids. The complete list of currently approved REMS is available on the FDA Web site.91 It is expected that drug manufacturers of all extended-release and long-acting opioids will meet the REMS requirement by providing educational grants for accredited entities to provide continuing education programs to prescribers. All prescribers are encouraged to discuss the risks and benefits of these products with their patients. A patient counseling document approved with the REMS will be made available by the manufacturers to assist the prescribers in these discussions.
Additional Pharmacologic Therapies for Cancer Pain Syndromes
Opioids alone may not provide optimal therapy, but when used in conjunction with nonopioid analgesics (such as NSAIDs) or adjuvant analgesics (antidepressants, anticonvulsants, topical agents, and corticosteroids) along with psychological and physical approaches, they can help improve patient outcomes.27
Adjuvant Analgesics for Neuropathic Pain
The term adjuvant refers to medications that are coad-ministered to manage an adverse effect of an opioid or to analgesics that are added to enhance analgesia. These drugs can be helpful for patients whose pain is only partially responsive to opioids.
Clinically, adjuvant analgesics consist of a diverse range of drug classes, including anticonvulsants116 (eg, gabapentin, pregabalin), antidepressants (eg, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants), corticosteroids, and local anesthetics/topical agents (eg, topical lidocaine patch).
Adjuvant analgesics are commonly used to help manage bone, neuropathic, and visceral pain and to reduce systemic opioid requirement. They are particularly important in treating neuropathic pain.117 Extrapolating from studies conducted in neuropathic pain, tricyclic antidepressants are believed to provide relief from neuropathic pain in patients with noncancer conditions.118–120 Several antidepressants are known inhibitors of hepatic drug metabolism via inhibition of cytochrome P450 enzymes, especially CYP2D6. Tamoxifen is an estrogen receptor blocker commonly used in patients with hormone receptor–positive breast cancer. Tamoxifen undergoes extensive hepatic metabolism, and inhibition of CYP2D6 decreases production of tamoxifen active metabolites, potentially limiting tamoxifen efficacy. Clinical studies indicate increased risk of breast cancer recurrence in tamoxifen-treated patients with breast cancer also treated with SSRI antidepressants versus those receiving tamoxifen alone.121,122 If concomitant use of an SSRI is required in a patient receiving tamoxifen, use of a mild CYP2D6 inhibitor (sertraline, citalopram, venlafaxine, escitalopram) may be preferred over a moderate-to-potent inhibitor (paroxetine, fluoxetine, fluvoxamine, bupropion, duloxetine).123
The most commonly used anticonvulsant drugs for the treatment of cancer pain are gabapentin and pre-gabalin.124 They have been studied primarily in non-cancer neuropathy syndromes.125 Gabapentin has been reported to reduce mucositis pain in patients receiving concomitant radiotherapy and chemotherapy.126
A review of cancer trials found that adjuvant analgesics (antidepressants and antiepileptics) added to opioids provide additional neuropathic pain relief.127
Topical local anesthetic agents are useful in preventing procedural pain and relieving neuropathic pain. They act locally and are also thought to have some central inhibitory effect on pain. They may be used as an analgesic in combination with an opioid, antidepressant, and/or anticonvulsant. Topical agents include lidocaine or diclofenac patch. Both the gel and patch forms of lidocaine have been shown to reduce the pain of postherpetic neuropathy and cancer-related pain.128,129
Corticosteroids have long been used to relieve neuropathic pain syndromes. Corticosteroids have also been effective for treating bone pain because of their anti-inflammatory effects, and for relieving malignant intestinal obstruction.28,130
Nonopioid Analgesics
The nonopioid analgesics include NSAIDs and acetaminophen.
Acetaminophen is analgesic and antipyretic but not anti-inflammatory.131 Recently, attention has been drawn towards the relative limited efficacy and significant adverse effects of acetaminophen, particularly hepatic and renal toxicity.132,133 This concern is compounded by the inclusion of acetaminophen in a variety of prescription opioid preparations (eg, hydrocodone, codeine) as well as in a wide selection of over-the-counter products. Because of concerns about liver toxicity, the panel advises that acetaminophen should be used with caution or not used at all with combination opioid-acetaminophen products to prevent excess acetaminophen dosing.
The FDA believes that limiting the amount of acetaminophen per tablet, capsule, or other dosage unit in prescription products will reduce the risk of severe liver injury from acetaminophen overdosing, or an adverse event that could lead to liver failure, liver transplant, or death. To reduce the risk of severe liver injury from acetaminophen overdosing, the FDA recently announced that it is asking “manufacturers of prescription acetaminophen combination products to limit the maximum amount of acetaminophen in these products to 325 mg per tablet, capsule, or other dosage unit.” The drug companies will have 3 years from the date of publication of the Federal Register Notice (January 14, 2011) to limit the amount of acetaminophen in their prescription drug products to 325 mg per dosage unit. The FDA is requiring a new boxed warning to communicate the risk of severe liver injury associated with acetaminophen to health care professionals. In addition, the companies are required to add a new warning about the risk of allergic reactions, including anaphylaxis, to the label of all prescription acetaminophen-containing products.
NSAIDs produce analgesia by blocking the biosynthesis of prostaglandins, inflammatory mediators that initiate, cause, intensify, or maintain pain. History of peptic ulcer disease, advanced age (>60 years), male sex, and concurrent corticosteroid therapy should be considered before NSAID administration to prevent upper gastrointestinal tract bleeding and perforation. Well-tolerated proton pump inhibitors are recommended to reduce gastrointestinal adverse effects induced by NSAIDs.
NSAIDs should be prescribed with caution in patients older than 60 years or in the presence of compromised fluid status, renal insufficiency, concomitant administration of other nephrotoxic drugs, and renally excreted chemotherapy to prevent renal toxicities. The addition of NSAIDs to opioids has the potential benefit of reducing the opioid dose when sedation, cognitive function, or other CNS effects of opioid analgesic therapy become burdensome.
In patients at high risk for cardiac toxicities, such as those with a history of cardiovascular disease or at risk for cardiovascular disease or complications, NSAIDs taken with prescribed anticoagulants, such as warfarin or heparin, may significantly increase the risk of bleeding complications. NSAIDs should be discontinued if congestive heart failure or hypertension develops or worsens. Naproxen and ibuprofen are preferred NSAIDS for individuals at high risk for cardiac toxicities.
The NSAID and acetaminophen prescribing guidelines are listed in the algorithms under Non-Opioid Analgesic (Nonsteroidal Anti-Inflammatory Drugs [NSAIDS] and Acetaminophen) Prescribing, available online, in these guidelines, at NCCN.org (PAIN-K).
Management of Bone Pain Without an Oncologic Emergency
The clinical complications of bone metastases include debilitating bone pain, which tends to be most prominent with movement, pathologic fractures, spinal cord compression, neurologic complications, and hypercalcemia of malignancy. The term skeletal related events (SREs) refers to a constellation of skeletal complications, including fracture, need for surgery to bone, need for radiation to bone, and spinal cord compression, and, in some situations, includes hypercalcemia of malignancy. Although bone-modifying agents (bisphosphonates and denosumab) are primarily used to reduce overall SREs, clinical trials have established that bisphosphonates have an analgesic effect on patients with metastatic bone pain from a variety of tumors.134–138 Because of differences in patient populations and the methods for assessing bone pain, direct comparison of bisphosphonates to determine their relative effects on bone pain across studies is difficult.
Surgical and radiation treatment for bone metastases is performed to relieve local bone pain, provide stabilization, and prevent impending fracture or spinal cord compression.139 In some situations, surgery (eg, vertebroplasty/kyphoplasty) provides a greater likelihood of return to ambulatory status than radiation alone. Identification of patients who have impending fractures and are referred to an orthopedic specialist for stabilization before fracture is important for optimal surgical pain management.
Consultation with an interventional pain specialist is recommended to determine the optimal management strategy for vertebral augmentation.
Management of Pain From Bowel Obstruction
Malignant bowel obstruction is a common complication in patients with abdominal or pelvic cancers. The initial management of patients presenting with bowel obstruction includes evaluation of the cause of the obstruction. Although surgery, radiation, and chemotherapy are the primary palliative treatments for malignant bowel obstruction, patients with advanced disease or poor general condition who are unfit to undergo these therapies may require other palliative measures to relieve distressing symptoms, such as bowel rest, nasogastric suction, corticosteroids, and/or octreotide (see NCCN Clinical Practice Guidelines in Oncology for Palliative Care; to view the most recent version of these guidelines, visit NCCN.org).
Specialty Consultations
Continued pain ratings should be obtained and documented in the medical record to ensure that the patient’s pain remains under good control and goals of treatment are achieved. Specialty consultations can be helpful in providing interventions to assist with difficult cancer pain problems. The major indication for referral to a specialty service provider is when the pain is likely to be relieved with the consultation or if an intervention will help patients become functional in their daily activities. These interventions are delivered by a specialty service provider, and pain management is accomplished by establishing individualized goals and then providing specific treatment and education for patients. The specialties include physical/occupational therapy; psychosocial supportive services; psychiatric consultation; pain and palliative care services; substance abuse consultation if questions/concerns about medication misuse or diversion exist; depression/distress consultation; spiritual care consultation; or social work services.
Nonpharmacologic Interventions for Cancer Pain Management
Integrative Interventions
Because pain encompasses physical, psychosocial, and spiritual dimensions, the treatment of cancer pain inherently requires integration of therapies inclusive of cognitive-behavioral interventions.
Nonpharmacologic integrative interventions (physical, cognitive, and spiritual) may serve as valuable additions to pharmacologic interventions. Physical measures include massage, use of heat or cold, acupuncture, and acupressure. Cognitive interventions are aimed at enhancing a sense of control over the pain or underlying disease. Breathing exercises, relaxation, imagery/hypnosis, and other behavioral therapies can be very useful.140–146 Attention should also be focused on psychosocial support and providing education to patients and families.147 All of these can greatly enhance patients’ sense of control and greatly reduce the family/caregivers’ feeling of helplessness.145 A meta-analysis of the effect of psychosocial interventions on cancer pain highlights the importance of a multimodal approach to the management of cancer pain.148 The integration of physical, psychosocial, and spiritual modalities should also be based on assessment of cultural factors. In cancer care, increasing attention has been given to spiritual needs and the existential concerns often associated with pain. Many patients hold cultural beliefs about these treatments, and home remedies, rituals, prayer, and other spiritual practices may be most helpful in relieving or coping with pain for some. Involvement of chaplains and other spiritual care providers is essential.149 Spiritual needs should be routinely assessed and spiritual care should be incorporated as a component of comprehensive pain management.
Patient-based educational interventions have a significant impact in providing pain relief.150 Skills training helps modify the patient’s experience of pain and helps them acquire techniques for pain management, such as deep muscle relaxation. Education teaches patients and family/caregivers how to use analgesics correctly and how to address side effects or unrelieved pain.
Interventional Strategies
Some patients experience inadequate pain control despite pharmacologic therapy or may not tolerate an opioid titration program because of side effects. Some patients may prefer interventional therapies instead of a chronic medication regimen. Interventional techniques have been shown in some cases to eliminate or significantly reduce the level of pain, and/or may allow a significant decrease in systemic analgesics.
Interventional therapies, including nerve blocks, vertebroplasty, kyphoplasty, and other techniques, can be useful in the relief of cancer pain.27,151–155 The major indications for referral for interventional therapies include pain that is likely to be relieved with nerve block (eg, pancreas/upper abdomen with celiac plexus block, lower abdomen with superior hypogastric plexus block, intercostal nerve, peripheral/plexus nerve) and/or inability to achieve adequate analgesia and/or the presence of intolerable side effects. For example, a patient with pancreatic cancer who was not tolerating opioids or not receiving adequate analgesia could be offered a neurolytic celiac plexus block. Neurolytic celiac plexus block may offer some improvement in pain control over systemic analgesics, but is generally associated with a reduction in adverse effects.156,157
Several interventional strategies are available if a patient does not experience adequate analgesia. Regional infusion of analgesics (epidural, intrathecal, and regional plexus) is one of the approaches. This approach minimizes the distribution of drugs to receptors in the brain, potentially avoiding adverse effects of systemic administration. The intrathecal route of opioid administration should be considered in patients with intolerable sedation, confusion, and/or inadequate pain control with systemic opioid administration. This approach is a valuable tool to improve analgesia for patients who have pain from a variety of anatomic locations (eg, head and neck, upper and lower extremities, trunk).158,159
Percutaneous kyphoplasty and vertebroplasty might be useful for the treatment of lytic osteoclastic spinal metastases or in cases of vertebral compression fractures or spinal instability for which surgery is not feasible or indicated. Vertebroplasty/kyphoplasty helps restore mechanical stability while reducing pain and neurologic symptoms.160–165
Neurodestructive procedures may be used for well-localized pain syndromes (eg, back pain from facet or sacroiliac joint arthropathy; visceral pain from abdominal or pelvic malignancy).
Neurostimulation procedures have been suggested to be useful for painful chemotherapy-induced peripheral neuropathies, neuralgias, and complex regional pain syndrome.166
Radiofrequency ablation for bone lesions has proven successful in pain management, especially in those not achieving adequate analgesia without intolerable effects.167,168
Interventional strategies listed earlier are not appropriate if patients are unwilling or in those with infections, coagulopathy, or very short life expectancies. Furthermore, the experts performing the interventions must be made aware of any medications that the patient is taking that might increase bleeding risk (eg, anticoagulants [warfarin, heparin], antiplatelet agents [clopidogrel, dipyridamole], or antiangiogenesis agents [bevacizumab]). If this occurs, the patient may need to be off the medication for an appropriate amount of time before the pain intervention and may need to stay off the medication for a specified amount of time after the procedure. Interventions are not appropriate if technical expertise is not available.
Summary
In most patients, cancer pain can be successfully controlled with appropriate techniques and safe drugs. The overall approach to pain management encompassed in these guidelines is multimodal and comprehensive. It is based on routine pain assessments, uses both pharmacologic and nonpharmacologic interventions, and requires ongoing reevaluation of the patient. The NCCN Adult Cancer Pain Panel advises that cancer pain can be well controlled in most patients if the algorithms presented are systematically applied, carefully monitored, and tailored to the needs of the individual patient.
NCCN Categories of Evidence and Consensus.
Category 1
Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2A
Based upon lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2B
Based upon lower-level evidence, there is NCCN consensus that the intervention is appropriate.
Category 3
Based upon any level of evidence, there is major NCCN disagreement that the intervention is appropriate.
NCCN Adult Cancer Pain Panel Members
-
*Robert A. Swarm, MD/Chairφ£
Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine
-
Amy Pickar Abernethy, MD†£
Duke Cancer Institute
-
Doralina L. Anghelescu, MDφ
St. Jude Children’s Research Hospital/The University of Tennessee Health Science Center
-
Costantino Benedetti, MDφ£
The Ohio State University Comprehensive Cancer Center – James Cancer Hospital and Solove Research Institute
-
Sorin Buga, MD£
Moffitt Cancer Center
-
Charles Cleeland, PhDθ
The University of Texas MD Anderson Cancer Center
-
Oscar A. deLeon-Casasola, MDφ£
Roswell Park Cancer Institute
-
June G. Eilers, PhD, APRN#
UNMC Eppley Cancer Center at The Nebraska Medical Center
-
Betty Ferrell, RN, PhD£#
City of Hope Comprehensive Cancer Center
-
Mark Green, MEd¥
Patient Advocate
-
Nora A. Janjan, MD, MPSA, MBA§
The University of Texas MD Anderson Cancer Center
-
Mihir M. Kamdar, MDÞ£
Massachusetts General Hospital Cancer Center
-
Michael H. Levy, MD, PhD£†
Fox Chase Cancer Center
-
Maureen Lynch, MS, APRN£#
Dana-Farber/Brigham and Women’s Cancer Center
-
Rachel M. McDowell, ACNP-BC#
Vanderbilt-Ingram Cancer Center
-
Natalie Moryl, MDÞ£
Memorial Sloan-Kettering Cancer Center
-
Suzanne A. Nesbit, PharmD, BCPSΣ
The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins
-
*Judith A. Paice, PhD, RN£#
Robert H. Lurie Comprehensive Cancer Center of Northwestern University
-
Michael W. Rabow, MDÞ£
UCSF Helen Diller Family Comprehensive Cancer Center
-
Karen L. Syrjala, PhDθ
Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance
-
Susan G. Urba, MD£†
University of Michigan Comprehensive Cancer Center
-
*Sharon M. Weinstein, MD£Ψ
Huntsman Cancer Institute at the University of Utah
NCCN Staff: Mary Dwyer, MS, and Rashmi Kumar, PhD
KEY:
*Writing Committee Member
Specialties: φAnesthesiology; £Supportive Care, Including Palliative, Pain Management, Pastoral Care, and Oncology Social Work; †Medical Oncology; ÞInternal Medicine; θPsychiatry, Psychology, Including Health Behavior; #Nursing; §Radiotherapy/Radiation Oncology; ΣPharmacology; ΨNeurology/Neuro-Oncology; ¥Patient Advocacy
Individual Disclosures for the NCCN Adult Cancer Pain Panel
| Panel Member | Clinical Research Support | Advisory Boards, Speakers Bureau, Expert Witness, or Consultant | Patent, Equity, or Royalty | Other | Date Completed |
|---|---|---|---|---|---|
| Amy Pickar Abernethy, MD | Alexion Pharmaceuticals, Inc.; Amgen Inc.; BioVex Group, Inc.; Bristol-Myers Squibb Company; DARA BioSciences, Inc.; Helsinn Therapeutics, Inc.; KangLaiTe USA; MiCo; and Pfizer Inc. | Amgen Inc.; Helsinn Therapeutics, Inc.; Proventys; and Pfizer Inc. | None | None | 6/11/12 |
| Doralina L. Anghelescu, MD | None | None | None | None | 9/28/12 |
| Costantino Benedetti, MD | None | None | None | None | 12/5/11 |
| Sorin Buga, MD | None | None | None | None | 10/10/12 |
| Charles Cleeland, PhD | None | None | None | None | 9/13/12 |
| Oscar A. deLeon-Casasola, MD | None | None | None | None | 8/20/12 |
| June G. Eilers, PhD, APRN | None | Novartis Pharmaceuticals Corporation; and EUSA Pharmaceuticals | None | None | 7/6/11 |
| Betty Ferrell, RN, PhD | None | None | None | None | 8/7/12 |
| Mark Green, MEd | None | None | None | None | 5/3/12 |
| Nora A. Janjan, MD, MPSA, MBA | ASCO; Dunes LLC; Harborside Press; MediSend; National Center for Policy Analysis; RSNA Foundation; Texas Radiation Advisory Board; and UBM Medica Publishing | ASCO; ASTRO; BP; Dunes LLC; Epix; National Center for Policy Analysis; RSNA; Texas Medical Association; Texas Radiation Advisory Board; and UBM Medica Publishing | None | ASCO | 8/14/12 |
| Mihir M. Kamdar, MD | None | None | None | None | 5/24/13 |
| Michael H. Levy, MD, PhD | None | Cephalon, Inc. | None | None | 4/30/13 |
| Maureen Lynch, MS, APRN | None | None | None | None | 2/19/13 |
| Rachel McDowell, ACNP-BC | None | None | None | None | 12/10/12 |
| Natalie Moryl, MD | None | Archimedes Pharma Ltd. | None | None | 8/6/12 |
| Suzanne A. Nesbit, PharmD, BCPS | None | Incline Therapeutics exp 12/12 | None | None | 2/15/13 |
| Judith A. Paice, PhD, RN | None | None | None | None | 8/6/12 |
| Michael W. Rabow, MD | None | None | None | None | 8/10/12 |
| Robert A. Swarm, MD | None | None | None | None | 2/21/13 |
| Karen L. Syrjala, PhD | None | None | None | None | 8/20/12 |
| Susan G. Urba, MD | Eisai Inc. | Amgen Inc. | None | None | 6/20/12 |
| Sharon M. Weinstein, MD | None | None | None | None | 1/18/12 |
The NCCN guidelines staff have no conflicts to disclose.
Footnotes
Please Note
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) are a statement of consensus of the authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult the NCCN Guidelines® is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The National Comprehensive Cancer Network® (NCCN®) makes no representation or warranties of any kind regarding their content, use, or application and disclaims any responsibility for their applications or use in any way. The full NCCN Guidelines for Adult Cancer Pain are not printed in this issue of JNCCN but can be accessed online at NCCN.org.
Disclosures for the NCCN Adult Cancer Pain Oncology Panel
At the beginning of each NCCN Guidelines panel meeting, panel members review all potential conflicts of interest. NCCN, in keeping with its commitment to public transparency, publishes these disclosures for panel members, staff, and NCCN itself.
Individual disclosures for the NCCN Adult Cancer Pain Panel members can be found on page 1022. (The most recent version of these guidelines and accompanying disclosures are available on the NCCN Web site at NCCN.org.)
These guidelines are also available on the Internet. For the latest update, visit NCCN.org.
References
- 1.Merskey H, Bugduk N. Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2. Seattle, WA: IASP Press; 1994. Classification of Chronic Pain. [Google Scholar]
- 2.Cohen MZ, Easley MK, Ellis C, et al. Cancer pain management and the JCAHO’s pain standards: an institutional challenge. J Pain Symptom Manage. 2003;25:519–527. doi: 10.1016/s0885-3924(03)00068-x. [DOI] [PubMed] [Google Scholar]
- 3.Goudas LC, Bloch R, Gialeli-Goudas M, et al. The epidemiology of cancer pain. Cancer Invest. 2005;23:182–190. [PubMed] [Google Scholar]
- 4.Svendsen KB, Andersen S, Arnason S, et al. Breakthrough pain in malignant and non-malignant diseases: a review of prevalence, characteristics and mechanisms. Eur J Pain. 2005;9:195–206. doi: 10.1016/j.ejpain.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 6.Cleeland CS, Gonin R, Hatfield AK, et al. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med. 1994;330:592–596. doi: 10.1056/NEJM199403033300902. [DOI] [PubMed] [Google Scholar]
- 7.Martin LA, Hagen NA. Neuropathic pain in cancer patients: mechanisms, syndromes, and clinical controversies. J Pain Symptom Manage. 1997;14:99–117. doi: 10.1016/s0885-3924(97)00009-2. [DOI] [PubMed] [Google Scholar]
- 8.Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. 1997;69:1–18. doi: 10.1016/s0304-3959(96)03267-8. [DOI] [PubMed] [Google Scholar]
- 9.Stjernsward J. WHO cancer pain relief programme. Cancer Surv. 1988;7:195–208. [PubMed] [Google Scholar]
- 10.Stjernsward J, Colleau SM, Ventafridda V. The World Health Organization Cancer Pain and Palliative Care Program. Past, present, and future. J Pain Symptom Manage. 1996;12:65–72. doi: 10.1016/0885-3924(96)00109-1. [DOI] [PubMed] [Google Scholar]
- 11.Caraceni A, Weinstein SM. Classification of cancer pain syndromes. Oncology (Williston Park) 2001;15:1627–1640. [PubMed] [Google Scholar]
- 12.Hewitt DJ. The management of pain in the oncology patient. Obstet Gynecol Clin North Am. 2001;28:819–846. doi: 10.1016/s0889-8545(05)70238-2. [DOI] [PubMed] [Google Scholar]
- 13.Portenoy RK. Cancer pain. Epidemiology and syndromes. Cancer. 1989;63:2298–2307. doi: 10.1002/1097-0142(19890601)63:11<2298::aid-cncr2820631140>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 14.Hicks CL, von Baeyer CL, Spafford PA, et al. The Faces Pain Scale-Revised: toward a common metric in pediatric pain measurement. Pain. 2001;93:173–183. doi: 10.1016/S0304-3959(01)00314-1. [DOI] [PubMed] [Google Scholar]
- 15.Serlin RC, Mendoza TR, Nakamura Y, et al. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 16.Soetenga D, Frank J, Pellino TA. Assessment of the validity and reliability of the University of Wisconsin Children’s Hospital Pain scale for Preverbal and Nonverbal Children. Pediatr Nurs. 1999;25:670–676. [PubMed] [Google Scholar]
- 17.Ware LJ, Epps CD, Herr K, Packard A. Evaluation of the Revised Faces Pain Scale, Verbal Descriptor Scale, Numeric Rating Scale, and Iowa Pain Thermometer in older minority adults. Pain Manag Nurs. 2006;7:117–125. doi: 10.1016/j.pmn.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Al-Atiyyat HN. Cultural diversity and cancer pain. J Hosp Palliat Nurs. 2009;11:154–164. [Google Scholar]
- 19.Ezenwa MO, Ameringer S, Ward SE, Serlin RC. Racial and ethnic disparities in pain management in the United States. J Nurs Scholarsh. 2006;38:225–233. doi: 10.1111/j.1547-5069.2006.00107.x. [DOI] [PubMed] [Google Scholar]
- 20.Akbik H, Butler SF, Budman SH, et al. Validation and clinical application of the Screener and Opioid Assessment for Patients with Pain (SOAPP) J Pain Symptom Manage. 2006;32:287–293. doi: 10.1016/j.jpainsymman.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Butler SF, Fernandez K, Benoit C, et al. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPP-R) J Pain. 2008;9:360–372. doi: 10.1016/j.jpain.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432–442. doi: 10.1111/j.1526-4637.2005.00072.x. [DOI] [PubMed] [Google Scholar]
- 23.Passik SD, Kirsh KL. The interface between pain and drug abuse and the evolution of strategies to optimize pain management while minimizing drug abuse. Exp Clin Psychopharmacol. 2008;16:400–404. doi: 10.1037/a0013634. [DOI] [PubMed] [Google Scholar]
- 24.Chou R, Fanciullo GJ, Fine PG, et al. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:131–146. doi: 10.1016/j.jpain.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49:796–804. [PubMed] [Google Scholar]
- 26.Syrjala KL, Abrams JR, Polissar NL, et al. Patient training in cancer pain management using integrated print and video materials: a multisite randomized controlled trial. Pain. 2008;135:175–186. doi: 10.1016/j.pain.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Pain Society. Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain. 5. Glenview, IL: American Pain Society; 2003. [Google Scholar]
- 28.Mercadante SL, Berchovich M, Casuccio A, et al. A prospective randomized study of corticosteroids as adjuvant drugs to opioids in advanced cancer patients. Am J Hosp Palliat Care. 2007;24:13–19. doi: 10.1177/1049909106295431. [DOI] [PubMed] [Google Scholar]
- 29.Portenoy RK, Lesage P. Management of cancer pain. Lancet. 1999;353:1695–1700. doi: 10.1016/S0140-6736(99)01310-0. [DOI] [PubMed] [Google Scholar]
- 30.Mercadante S, Arcuri E, Ferrera P, et al. Alternative treatments of breakthrough pain in patients receiving spinal analgesics for cancer pain. J Pain Symptom Manage. 2005;30:485–491. doi: 10.1016/j.jpainsymman.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 31.Green CR, Anderson KO, Baker TA, et al. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Med. 2003;4:277–294. doi: 10.1046/j.1526-4637.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 32.Gordon DB, Pellino TA, Miaskowski C, et al. A 10-year review of quality improvement monitoring in pain management: recommendations for standardized outcome measures. Pain Manag Nurs. 2002;3:116–130. doi: 10.1053/jpmn.2002.127570. [DOI] [PubMed] [Google Scholar]
- 33.Sun VC, Borneman T, Ferrell B, et al. Overcoming barriers to cancer pain management: an institutional change model. J Pain Symptom Manage. 2007;34:359–369. doi: 10.1016/j.jpainsymman.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin CC, Chou PL, Wu SL, et al. Long-term effectiveness of a patient and family pain education program on overcoming barriers to management of cancer pain. Pain. 2006;122:271–281. doi: 10.1016/j.pain.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 35.Chang MC, Chang YC, Chiou JF, et al. Overcoming patient-related barriers to cancer pain management for home care patients. A pilot study. Cancer Nurs. 2002;25:470–476. doi: 10.1097/00002820-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Klepstad P, Kaasa S, Borchgrevink PC. Start of oral morphine to cancer patients: effective serum morphine concentrations and contribution from morphine-6-glucuronide to the analgesia produced by morphine. Eur J Clin Pharmacol. 2000;55:713–719. doi: 10.1007/s002280050003. [DOI] [PubMed] [Google Scholar]
- 37.Klepstad P, Kaasa S, Skauge M, Borchgrevink PC. Pain intensity and side effects during titration of morphine to cancer patients using a fixed schedule dose escalation. Acta Anaesthesiol Scand. 2000;44:656–664. doi: 10.1034/j.1399-6576.2000.440605.x. [DOI] [PubMed] [Google Scholar]
- 38.Foley KM. The treatment of pain in the patient with cancer. CA Cancer J Clin. 1986;36:194–215. doi: 10.3322/canjclin.36.4.194. [DOI] [PubMed] [Google Scholar]
- 39.Cherny NI. The pharmacologic management of cancer pain. Oncology (Williston Park) 2004;18:1499–1515. [PubMed] [Google Scholar]
- 40.Hanks GW, Conno F, Cherny N, et al. Morphine and alternative opioids in cancer pain: the EAPC recommendations. Br J Cancer. 2001;84:587–593. doi: 10.1054/bjoc.2001.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kornick CA, Santiago-Palma J, Khojainova N, et al. A safe and effective method for converting cancer patients from intravenous to transdermal fentanyl. Cancer. 2001;92:3056–3061. doi: 10.1002/1097-0142(20011215)92:12<3056::aid-cncr10166>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 42.Portenoy RK, Taylor D, Messina J, Tremmel L. A randomized, placebo-controlled study of fentanyl buccal tablet for breakthrough pain in opioid-treated patients with cancer. Clin J Pain. 2006;22:805–811. doi: 10.1097/01.ajp.0000210932.27945.4a. [DOI] [PubMed] [Google Scholar]
- 43.Weinstein SM, Messina J, Xie F. Fentanyl buccal tablet for the treatment of breakthrough pain in opioid-tolerant patients with chronic cancer pain: a long-term, open-label safety study. Cancer. 2009;115:2571–2579. doi: 10.1002/cncr.24279. [DOI] [PubMed] [Google Scholar]
- 44.Kleeberg UR, Filbet M, Zeppetella G. Fentanyl buccal tablet for breakthrough cancer pain: why titrate? Pain Pract. 2011;11:185–190. doi: 10.1111/j.1533-2500.2010.00414.x. [DOI] [PubMed] [Google Scholar]
- 45.Srinivasan V, Wielbo D, Tebbett IR. Analgesic effects of codeine-6-glucuronide after intravenous administration. Eur J Pain. 1997;1:185–190. doi: 10.1016/s1090-3801(97)90103-8. [DOI] [PubMed] [Google Scholar]
- 46.Kirchheiner J, Schmidt H, Tzvetkov M, et al. Pharmacokinetics of codeine and its metabolite morphine in ultra-rapid metabolizers due to CYP2D6 duplication. Pharmacogenomics J. 2007;7:257–265. doi: 10.1038/sj.tpj.6500406. [DOI] [PubMed] [Google Scholar]
- 47.Murray A, Hagen NA. Hydromorphone. J Pain Symptom Manage. 2005;29(Suppl 5):S57–66. doi: 10.1016/j.jpainsymman.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 48.Thwaites D, McCann S, Broderick P. Hydromorphone neuroexcitation. J Palliat Med. 2004;7:545–550. doi: 10.1089/jpm.2004.7.545. [DOI] [PubMed] [Google Scholar]
- 49.Wright AW, Mather LE, Smith MT. Hydromorphone-3-glucuronide: a more potent neuro-excitant than its structural analogue, morphine-3-glucuronide. Life Sci. 2001;69:409–420. doi: 10.1016/s0024-3205(01)01133-x. [DOI] [PubMed] [Google Scholar]
- 50.Mercadante S. Intravenous morphine for management of cancer pain. Lancet Oncol. 2010;11:484–489. doi: 10.1016/S1470-2045(09)70350-X. [DOI] [PubMed] [Google Scholar]
- 51.Tiseo PJ, Thaler HT, Lapin J, et al. Morphine-6-glucuronide concentrations and opioid-related side effects: a survey in cancer patients. Pain. 1995;61:47–54. doi: 10.1016/0304-3959(94)00148-8. [DOI] [PubMed] [Google Scholar]
- 52.Portenoy RK, Foley KM, Stulman J, et al. Plasma morphine and morphine-6-glucuronide during chronic morphine therapy for cancer pain: plasma profiles, steady-state concentrations and the consequences of renal failure. Pain. 1991;47:13–19. doi: 10.1016/0304-3959(91)90005-I. [DOI] [PubMed] [Google Scholar]
- 53.Andersen G, Jensen NH, Christrup L, et al. Pain, sedation and morphine metabolism in cancer patients during long-term treatment with sustained-release morphine. Palliat Med. 2002;16:107–114. doi: 10.1191/0269216302pm512oa. [DOI] [PubMed] [Google Scholar]
- 54.Smith MT. Neuroexcitatory effects of morphine and hydromorphone: evidence implicating the 3-glucuronide metabolites. Clin Exp Pharmacol Physiol. 2000;27:524–528. doi: 10.1046/j.1440-1681.2000.03290.x. [DOI] [PubMed] [Google Scholar]
- 55.Davis MP, Varga J, Dickerson D, et al. Normal-release and controlled-release oxycodone: pharmacokinetics, pharmacodynamics, and controversy. Support Care Cancer. 2003;11:84–92. doi: 10.1007/s00520-002-0385-9. [DOI] [PubMed] [Google Scholar]
- 56.Ordonez Gallego A, Gonzalez Baron M, Espinosa Arranz E. Oxycodone: a pharmacological and clinical review. Clin Transl Oncol. 2007;9:298–307. doi: 10.1007/s12094-007-0057-9. [DOI] [PubMed] [Google Scholar]
- 57.Adam Z, Pour L, Krejci M, et al. Treatment of AL-amyloidosis—results from one clinic and review of published experience with new agents (bortezomib, thalidomide and lenalidomide) in AL-amyloidosis. Vnitr Lek. 2010;56:190–209. [PubMed] [Google Scholar]
- 58.Gabrail NY, Dvergsten C, Ahdieh H. Establishing the dosage equivalency of oxymorphone extended release and oxycodone controlled release in patients with cancer pain: a randomized controlled study. Curr Med Res Opin. 2004;20:911–918. doi: 10.1185/030079904125003854. [DOI] [PubMed] [Google Scholar]
- 59.Sloan P. Review of oral oxymorphone in the management of pain. Ther Clin Risk Manag. 2008;4:777–787. doi: 10.2147/tcrm.s1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davis MP, Homsi J. The importance of cytochrome P450 monooxygenase CYP2D6 in palliative medicine. Support Care Cancer. 2001;9:442–451. doi: 10.1007/s005200000222. [DOI] [PubMed] [Google Scholar]
- 61.Mercadante S, Casuccio A, Fulfaro F, et al. Switching from morphine to methadone to improve analgesia and tolerability in cancer patients: a prospective study. J Clin Oncol. 2001;19:2898–2904. doi: 10.1200/JCO.2001.19.11.2898. [DOI] [PubMed] [Google Scholar]
- 62.Moryl N, Santiago-Palma J, Kornick C, et al. Pitfalls of opioid rotation: substituting another opioid for methadone in patients with cancer pain. Pain. 2002;96:325–328. doi: 10.1016/S0304-3959(01)00465-1. [DOI] [PubMed] [Google Scholar]
- 63.Parsons HA, de la Cruz M, El Osta B, et al. Methadone initiation and rotation in the outpatient setting for patients with cancer pain. Cancer. 2010;116:520–528. doi: 10.1002/cncr.24754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krantz MJ, Lewkowiez L, Hays H, et al. Torsade de pointes associated with very-high-dose methadone. Ann Intern Med. 2002;137:501–504. doi: 10.7326/0003-4819-137-6-200209170-00010. [DOI] [PubMed] [Google Scholar]
- 65.Krantz MJ, Kutinsky IB, Robertson AD, Mehler PS. Dose-related effects of methadone on QT prolongation in a series of patients with torsade de pointes. Pharmacotherapy. 2003;23:802–805. doi: 10.1592/phco.23.6.802.32186. [DOI] [PubMed] [Google Scholar]
- 66.Kornick CA, Kilborn MJ, Santiago-Palma J, et al. QTc interval prolongation associated with intravenous methadone. Pain. 2003;105:499–506. doi: 10.1016/S0304-3959(03)00205-7. [DOI] [PubMed] [Google Scholar]
- 67.Reddy S, Hui D, El Osta B, et al. The effect of oral methadone on the QTc interval in advanced cancer patients: a prospective pilot study. J Palliat Med. 2010;13:33–38. doi: 10.1089/jpm.2009.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rodriguez RF, Bravo LE, Castro F, et al. Incidence of weak opioids adverse events in the management of cancer pain: a double-blind comparative trial. J Palliat Med. 2007;10:56–60. doi: 10.1089/jpm.2006.0117. [DOI] [PubMed] [Google Scholar]
- 69.Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43:879–923. doi: 10.2165/00003088-200443130-00004. [DOI] [PubMed] [Google Scholar]
- 70.Wade WE, Spruill WJ. Tapentadol hydrochloride: a centrally acting oral analgesic. Clin Ther. 2009;31:2804–2818. doi: 10.1016/j.clinthera.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 71.Hartrick CT, Rodriguez Hernandez JR. Tapentadol for pain: a treatment evaluation. Expert Opin Pharmacother. 2012;13:283–286. doi: 10.1517/14656566.2012.648616. [DOI] [PubMed] [Google Scholar]
- 72.Afilalo M, Etropolski MS, Kuperwasser B, et al. Efficacy and safety of tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee: a randomized, double-blind, placebo- and active-controlled phase III study. Clin Drug Investig. 2010;30:489–505. doi: 10.2165/11533440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 73.Buynak R, Shapiro DY, Okamoto A, et al. Efficacy and safety of tapentadol extended release for the management of chronic low back pain: results of a prospective, randomized, double-blind, placebo- and active-controlled phase III study. Expert Opin Pharmacother. 2010;11:1787–1804. doi: 10.1517/14656566.2010.497720. [DOI] [PubMed] [Google Scholar]
- 74.Schwartz S, Etropolski M, Shapiro DY, et al. Safety and efficacy of tapentadol ER in patients with painful diabetic peripheral neuropathy: results of a randomized-withdrawal, placebo-controlled trial. Curr Med Res Opin. 2011;27:151–162. doi: 10.1185/03007995.2010.537589. [DOI] [PubMed] [Google Scholar]
- 75.Mercadante S, Porzio G, Ferrera P, et al. Tapentadol in cancer pain management: a prospective open-label study. Curr Med Res Opin. 2012;28:1775–1779. doi: 10.1185/03007995.2012.739151. [DOI] [PubMed] [Google Scholar]
- 76.Pergolizzi JV, Jr, Mercadante S, Echaburu AV, et al. The role of transdermal buprenorphine in the treatment of cancer pain: an expert panel consensus. Curr Med Res Opin. 2009;25:1517–1528. doi: 10.1185/03007990902920731. [DOI] [PubMed] [Google Scholar]
- 77.Johnson RE, Fudala PJ, Payne R. Buprenorphine: considerations for pain management. J Pain Symptom Manage. 2005;29:297–326. doi: 10.1016/j.jpainsymman.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 78.Bell RF, Eccleston C, Kalso EA. Ketamine as an adjuvant to opioids for cancer pain. Cochrane Database Syst Rev. 2012;11:CD003351. doi: 10.1002/14651858.CD003351.pub2. [DOI] [PubMed] [Google Scholar]
- 79.Hardy J, Quinn S, Fazekas B, et al. Randomized, double-blind, placebo-controlled study to assess the efficacy and toxicity of subcutaneous ketamine in the management of cancer pain. J Clin Oncol. 2012;30:3611–3617. doi: 10.1200/JCO.2012.42.1081. [DOI] [PubMed] [Google Scholar]
- 80.Bruera E, Kim HN. Cancer pain. JAMA. 2003;290:2476–2479. doi: 10.1001/jama.290.18.2476. [DOI] [PubMed] [Google Scholar]
- 81.Stevens RA, Ghazi SM. Routes of opioid analgesic therapy in the management of cancer pain. Cancer Control. 2000;7:132–141. doi: 10.1177/107327480000700203. [DOI] [PubMed] [Google Scholar]
- 82.Harris JT, Suresh Kumar K, Rajagopal MR. Intravenous morphine for rapid control of severe cancer pain. Palliat Med. 2003;17:248–256. doi: 10.1191/0269216303pm695oa. [DOI] [PubMed] [Google Scholar]
- 83.Butler SF, Budman SH, Fernandez KC, et al. Development and validation of the Current Opioid Misuse Measure. Pain. 2007;130:144–156. doi: 10.1016/j.pain.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McNicol E, Horowicz-Mehler N, Fisk RA, et al. Management of opioid side effects in cancer-related and chronic noncancer pain: a systematic review. J Pain. 2003;4:231–256. doi: 10.1016/s1526-5900(03)00556-x. [DOI] [PubMed] [Google Scholar]
- 85.Mercadante S. Comments on Wang et al. PAIN, 67 (1996) 407–416. Pain. 1998;74:106–107. [PubMed] [Google Scholar]
- 86.Mercadante S. Pathophysiology and treatment of opioid-related myoclonus in cancer patients. Pain. 1998;74:5–9. [PubMed] [Google Scholar]
- 87.Wilson RK, Weissman DE. Neuroexcitatory effects of opioids: patient assessment #57. J Palliat Med. 2004;7:579. doi: 10.1089/jpm.2004.7.579. [DOI] [PubMed] [Google Scholar]
- 88.Moryl N, Carver A, Foley KM. Pain and palliation. In: Holland JF, Frei E, editors. Cancer Medicine. 7. Hamilton, ON: BC Decker Inc; 2006. pp. 1113–1124. [Google Scholar]
- 89.Moryl N, Obbens EA, Ozigbo OH, Kris MG. Analgesic effect of gefitinib in the treatment of non-small cell lung cancer. J Support Oncol. 2006;4:111–111. [PubMed] [Google Scholar]
- 90.Boettger S, Breitbart W. Atypical antipsychotics in the management of delirium: a review of the empirical literature. Palliat Support Care. 2005;3:227–237. doi: 10.1017/s1478951505050352. [DOI] [PubMed] [Google Scholar]
- 91.Breitbart W, Marotta R, Platt MM, et al. A double-blind trial of haloperidol, chlorpromazine, and lorazepam in the treatment of delirium in hospitalized AIDS patients. Am J Psychiatry. 1996;153:231–237. doi: 10.1176/ajp.153.2.231. [DOI] [PubMed] [Google Scholar]
- 92.Bruera E, Belzile M, Neumann C, et al. A double-blind, crossover study of controlled-release metoclopramide and placebo for the chronic nausea and dyspepsia of advanced cancer. J Pain Symptom Manage. 2000;19:427–435. doi: 10.1016/s0885-3924(00)00138-x. [DOI] [PubMed] [Google Scholar]
- 93.Challoner KR, McCarron MM, Newton EJ. Pentazocine (Talwin) intoxication: report of 57 cases. J Emerg Med. 1990;8:67–74. doi: 10.1016/0736-4679(90)90391-8. [DOI] [PubMed] [Google Scholar]
- 94.Katcher J, Walsh D. Opioid-induced itching: morphine sulfate and hydromorphone hydrochloride. J Pain Symptom Manage. 1999;17:70–72. doi: 10.1016/s0885-3924(98)00115-8. [DOI] [PubMed] [Google Scholar]
- 95.Marinella MA. Acute colonic pseudo-obstruction complicated by cecal perforation in a patient with Parkinson’s disease. South Med J. 1997;90:1023–1026. doi: 10.1097/00007611-199710000-00009. [DOI] [PubMed] [Google Scholar]
- 96.Reissig JE, Rybarczyk AM. Pharmacologic treatment of opioid-induced sedation in chronic pain. Ann Pharmacother. 2005;39:727–731. doi: 10.1345/aph.1E309. [DOI] [PubMed] [Google Scholar]
- 97.Tarcatu D, Tamasdan C, Moryl N, Obbens E. Are we still scratching the surface? A case of intractable pruritus following systemic opioid analgesia. J Opioid Manag. 2007;3:167–170. doi: 10.5055/jom.2007.0055. [DOI] [PubMed] [Google Scholar]
- 98.Prommer E. Modafinil: is it ready for prime time? J Opioid Manag. 2006;2:130–136. doi: 10.5055/jom.2006.0022. [DOI] [PubMed] [Google Scholar]
- 99.Hawley PH, Byeon JJ. A comparison of sennosides-based bowel protocols with and without docusate in hospitalized patients with cancer. J Palliat Med. 2008;11:575–581. doi: 10.1089/jpm.2007.0178. [DOI] [PubMed] [Google Scholar]
- 100.Michna E, Blonsky ER, Schulman S, et al. Subcutaneous methylnaltrexone for treatment of opioid-induced constipation in patients with chronic, nonmalignant pain: a randomized controlled study. J Pain. 2011;12:554–562. doi: 10.1016/j.jpain.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 101.Portenoy RK, Thomas J, Moehl Boatwright ML, et al. Subcutaneous methylnaltrexone for the treatment of opioid-induced constipation in patients with advanced illness: a double-blind, randomized, parallel group, dose-ranging study. J Pain Symptom Manage. 2008;35:458–468. doi: 10.1016/j.jpainsymman.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 102.Chappell D, Rehm M, Conzen P. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med. 2008;359:1071. author reply 1071. [PubMed] [Google Scholar]
- 103.Sanz Rubiales A, del Valle Rivero ML. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med. 2008;359:1070–1071. doi: 10.1056/NEJMc081373. author reply 1071. [DOI] [PubMed] [Google Scholar]
- 104.Thomas J, Karver S, Cooney GA, et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med. 2008;358:2332–2343. doi: 10.1056/NEJMoa0707377. [DOI] [PubMed] [Google Scholar]
- 105.Bruera E, Seifert L, Watanabe S, et al. Chronic nausea in advanced cancer patients: a retrospective assessment of a metoclopramide-based antiemetic regimen. J Pain Symptom Manage. 1996;11:147–153. doi: 10.1016/0885-3924(95)00161-1. [DOI] [PubMed] [Google Scholar]
- 106.Friedman JD, Dello Buono FA. Opioid antagonists in the treatment of opioid-induced constipation and pruritus. Ann Pharmacother. 2001;35:85–91. doi: 10.1345/aph.10121. [DOI] [PubMed] [Google Scholar]
- 107.Chamberlain JM, Klein BL. A comprehensive review of naloxone for the emergency physician. Am J Emerg Med. 1994;12:650–660. doi: 10.1016/0735-6757(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 108.Gagnon P, Allard P, Masse B, DeSerres M. Delirium in terminal cancer: a prospective study using daily screening, early diagnosis, and continuous monitoring. J Pain Symptom Manage. 2000;19:412–426. doi: 10.1016/s0885-3924(00)00143-3. [DOI] [PubMed] [Google Scholar]
- 109.Bruera E, Macmillan K, Hanson J, MacDonald RN. The cognitive effects of the administration of narcotic analgesics in patients with cancer pain. Pain. 1989;39:13–16. doi: 10.1016/0304-3959(89)90169-3. [DOI] [PubMed] [Google Scholar]
- 110.Slatkin NE. Opioid switching and rotation in primary care: implementation and clinical utility. Curr Med Res Opin. 2009;25:2133–2150. doi: 10.1185/03007990903120158. [DOI] [PubMed] [Google Scholar]
- 111.Vissers KC, Besse K, Hans G, et al. Opioid rotation in the management of chronic pain: where is the evidence? Pain Pract. 2010;10:85–93. doi: 10.1111/j.1533-2500.2009.00335.x. [DOI] [PubMed] [Google Scholar]
- 112.NCHS Data Brief. [Accessed July 17, 2013];Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/nchs/data/databriefs/db81.htm.
- 113.Risk Evaluation and Mitigation Strategy (REMS) for Extended-Release and Long-Acting Opioids. [Accessed July 17, 2013];US Food and Drug Administration Web site. Available at: http://www.fda.gov/drugs/drugsafety/informationbydrugclass/ucm163647.htm.
- 114.Approved Risk Evaluation and Mitigation Strategies (REMS) [Accessed July 17, 2013];US Food and Drug Administration Web site. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm111350.htm.
- 115.NCCN Resource Tool: Risk Evaluation & Mitigation Strategies (REMS) [Accessed July 17, 2013];National Comprehensive Cancer Network Web site. Available at: http://www.nccn.org/rems/default.asp.
- 116.Lussier D, Huskey AG, Portenoy RK. Adjuvant analgesics in cancer pain management. Oncologist. 2004;9:571–591. doi: 10.1634/theoncologist.9-5-571. [DOI] [PubMed] [Google Scholar]
- 117.Manfredi PL, Gonzales GR, Sady R, et al. Neuropathic pain in patients with cancer. J Palliat Care. 2003;19:115–118. [PubMed] [Google Scholar]
- 118.Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150:573–581. doi: 10.1016/j.pain.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 119.Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007:CD005454. doi: 10.1002/14651858.CD005454.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saarto T, Wiffen PJ. Antidepressants for neuropathic pain: a Cochrane review. J Neurol Neurosurg Psychiatry. 2010;81:1372–1373. doi: 10.1136/jnnp.2008.144964. [DOI] [PubMed] [Google Scholar]
- 121.Aubert R, Stanek EJ, Yao J, et al. Risk of breast cancer recurrence in women initiating tamoxifen with CYP2D6 inhibitors [abstract] J Clin Oncol. 2009;27(Suppl) Abstract CRA508. [Google Scholar]
- 122.Dezentje V, Van Blijderveen NJ, Gelderblom H, et al. Concomitant CYP2D6 inhibitor use and tamoxifen adherence in early-stage breast cancer: a pharmacoepidemiologic study [abstract] J Clin Oncol. 2009;27(Suppl) doi: 10.1200/JCO.2009.25.0894. Abstract CRA509. [DOI] [PubMed] [Google Scholar]
- 123.Jin Y, Desta Z, Stearns V, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst. 2005;97:30–39. doi: 10.1093/jnci/dji005. [DOI] [PubMed] [Google Scholar]
- 124.Johannessen Landmark C. Antiepileptic drugs in non-epilepsy disorders: relations between mechanisms of action and clinical efficacy. CNS Drugs. 2008;22:27–47. doi: 10.2165/00023210-200822010-00003. [DOI] [PubMed] [Google Scholar]
- 125.Baron R, Brunnmuller U, Brasser M, et al. Efficacy and safety of pregabalin in patients with diabetic peripheral neuropathy or postherpetic neuralgia: open-label, non-comparative, flexible-dose study. Eur J Pain. 2008;12:850–858. doi: 10.1016/j.ejpain.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 126.Bar Ad V, Weinstein G, Dutta PR, et al. Gabapentin for the treatment of pain syndrome related to radiation-induced mucositis in patients with head and neck cancer treated with concurrent chemoradiotherapy. Cancer. 2010;116:4206–4213. doi: 10.1002/cncr.25274. [DOI] [PubMed] [Google Scholar]
- 127.Bennett MI. Effectiveness of antiepileptic or antidepressant drugs when added to opioids for cancer pain: systematic review. Palliat Med. 2011;25:553–559. doi: 10.1177/0269216310378546. [DOI] [PubMed] [Google Scholar]
- 128.Fleming JA, O’Connor BD. Use of lidocaine patches for neuropathic pain in a comprehensive cancer centre. Pain Res Manag. 2009;14:381–388. doi: 10.1155/2009/723179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gammaitoni AR, Alvarez NA, Galer BS. Safety and tolerability of the lidocaine patch 5%, a targeted peripheral analgesic: a review of the literature. J Clin Pharmacol. 2003;43:111–117. doi: 10.1177/0091270002239817. [DOI] [PubMed] [Google Scholar]
- 130.Wooldridge JE, Anderson CM, Perry MC. Corticosteroids in advanced cancer. Oncology (Williston Park) 2001;15:225–234. discussion 234–226. [PubMed] [Google Scholar]
- 131.Stockler M, Vardy J, Pillai A, Warr D. Acetaminophen (paracetamol) improves pain and well-being in people with advanced cancer already receiving a strong opioid regimen: a randomized, double-blind, placebo-controlled cross-over trial. J Clin Oncol. 2004;22:3389–3394. doi: 10.1200/JCO.2004.09.122. [DOI] [PubMed] [Google Scholar]
- 132.Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57:1331–1346. doi: 10.1111/j.1532-5415.2009.02376.x. [DOI] [PubMed] [Google Scholar]
- 133.Israel FJ, Parker G, Charles M, Reymond L. Lack of benefit from paracetamol (acetaminophen) for palliative cancer patients requiring high-dose strong opioids: a randomized, double-blind, placebo-controlled, crossover trial. J Pain Symptom Manage. 2010;39:548–554. doi: 10.1016/j.jpainsymman.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 134.Body JJ, Diel IJ, Lichinitzer M, et al. Oral ibandronate reduces the risk of skeletal complications in breast cancer patients with metastatic bone disease: results from two randomised, placebo-controlled phase III studies. Br J Cancer. 2004;90:1133–1137. doi: 10.1038/sj.bjc.6601663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Body JJ, Diel IJ, Bell R, et al. Oral ibandronate improves bone pain and preserves quality of life in patients with skeletal metastases due to breast cancer. Pain. 2004;111:306–312. doi: 10.1016/j.pain.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 136.Cleeland CS, Body JJ, Stopeck A, et al. Pain outcomes in patients with advanced breast cancer and bone metastases: results from a randomized, double-blind study of denosumab and zoledronic acid. Cancer. 2013;119:832–838. doi: 10.1002/cncr.27789. [DOI] [PubMed] [Google Scholar]
- 137.Rosen LS, Gordon D, Kaminski M, et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J. 2001;7:377–387. [PubMed] [Google Scholar]
- 138.Wardley A, Davidson N, Barrett-Lee P, et al. Zoledronic acid significantly improves pain scores and quality of life in breast cancer patients with bone metastases: a randomised, crossover study of community vs hospital bisphosphonate administration. Br J Cancer. 2005;92:1869–1876. doi: 10.1038/sj.bjc.6602551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Malviya A, Gerrand C. Evidence for orthopaedic surgery in the treatment of metastatic bone disease of the extremities: a review article. Palliat Med. 2012;26:788–796. doi: 10.1177/0269216311419882. [DOI] [PubMed] [Google Scholar]
- 140.Raphael J, Hester J, Ahmedzai S, et al. Cancer pain: part 2: physical, interventional and complimentary therapies; management in the community; acute, treatment-related and complex cancer pain: a perspective from the British Pain Society endorsed by the UK Association of Palliative Medicine and the Royal College of General Practitioners. Pain Med. 2010;11:872–896. doi: 10.1111/j.1526-4637.2010.00841.x. [DOI] [PubMed] [Google Scholar]
- 141.Pfister DG, Cassileth BR, Deng GE, et al. Acupuncture for pain and dysfunction after neck dissection: results of a randomized controlled trial. J Clin Oncol. 2010;28:2565–2570. doi: 10.1200/JCO.2009.26.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stoelb BL, Molton IR, Jensen MP, Patterson DR. The efficacy of hypnotic analgesia in adults: a review of the literature. Contemp Hypn. 2009;26:24–39. doi: 10.1002/ch.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huang ST, Good M, Zauszniewski JA. The effectiveness of music in relieving pain in cancer patients: a randomized controlled trial. Int J Nurs Stud. 2010;47:1354–1362. doi: 10.1016/j.ijnurstu.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 144.Kwekkeboom KL, Cherwin CH, Lee JW, Wanta B. Mind-body treatments for the pain-fatigue-sleep disturbance symptom cluster in persons with cancer. J Pain Symptom Manage. 2010;39:126–138. doi: 10.1016/j.jpainsymman.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cassileth BR, Keefe FJ. Integrative and behavioral approaches to the treatment of cancer-related neuropathic pain. Oncologist. 2010;15(Suppl 2):19–23. doi: 10.1634/theoncologist.2009-S504. [DOI] [PubMed] [Google Scholar]
- 146.Montgomery GH, Weltz CR, Seltz M, Bovbjerg DH. Brief presurgery hypnosis reduces distress and pain in excisional breast biopsy patients. Int J Clin Exp Hypn. 2002;50:17–32. doi: 10.1080/00207140208410088. [DOI] [PubMed] [Google Scholar]
- 147.Keefe FJ, Abernethy AP, Campbell LC. Psychological approaches to understanding and treating disease-related pain. Annu Rev Psychol. 2005;56:601–630. doi: 10.1146/annurev.psych.56.091103.070302. [DOI] [PubMed] [Google Scholar]
- 148.Sheinfeld Gorin S, Krebs P, Badr H, et al. Meta-analysis of psychosocial interventions to reduce pain in patients with cancer. J Clin Oncol. 2012;30:539–547. doi: 10.1200/JCO.2011.37.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Puchalski C, Ferrell B, Virani R, et al. Improving the quality of spiritual care as a dimension of palliative care: the report of the Consensus Conference. J Palliat Med. 2009;12:885–904. doi: 10.1089/jpm.2009.0142. [DOI] [PubMed] [Google Scholar]
- 150.Bennett MI, Bagnall AM, Jose Closs S. How effective are patient-based educational interventions in the management of cancer pain? Systematic review and meta-analysis. Pain. 2009;143:192–199. doi: 10.1016/j.pain.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 151.Brogan S, Junkins S. Interventional therapies for the management of cancer pain. J Support Oncol. 2010;8:52–59. [PubMed] [Google Scholar]
- 152.Eidelman A, White T, Swarm RA. Interventional therapies for cancer pain management: important adjuvants to systemic analgesics. J Natl Compr Canc Netw. 2007;5:753–760. doi: 10.6004/jnccn.2007.0075. [DOI] [PubMed] [Google Scholar]
- 153.Tay W, Ho KY. The role of interventional therapies in cancer pain management. Ann Acad Med Singapore. 2009;38:989–997. [PubMed] [Google Scholar]
- 154.Wong GY, Schroeder DR, Carns PE, et al. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trial. JAMA. 2004;291:1092–1099. doi: 10.1001/jama.291.9.1092. [DOI] [PubMed] [Google Scholar]
- 155.Goetz MP, Callstrom MR, Charboneau JW, et al. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: a multicenter study. J Clin Oncol. 2004;22:300–306. doi: 10.1200/JCO.2004.03.097. [DOI] [PubMed] [Google Scholar]
- 156.Arcidiacono PG, Calori G, Carrara S, et al. Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst Rev. 2011:CD007519. doi: 10.1002/14651858.CD007519.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang CL, Zhang TJ, Guo YN, et al. Effect of neurolytic celiac plexus block guided by computerized tomography on pancreatic cancer pain. Dig Dis Sci. 2008;53:856–860. doi: 10.1007/s10620-007-9905-2. [DOI] [PubMed] [Google Scholar]
- 158.Smith TJ, Staats PS, Deer T, et al. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol. 2002;20:4040–4049. doi: 10.1200/JCO.2002.02.118. [DOI] [PubMed] [Google Scholar]
- 159.Deer TR, Prager J, Levy R, et al. Polyanalgesic Consensus Conference 2012: recommendations for the management of pain by intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panel. Neuromodulation. 2012;15:436–464. doi: 10.1111/j.1525-1403.2012.00476.x. discussion 464–436. [DOI] [PubMed] [Google Scholar]
- 160.Rastogi R, Patel T, Swarm RA. Vertebral augmentation for compression fractures caused by malignant disease. J Natl Compr Canc Netw. 2010;8:1095–1102. doi: 10.6004/jnccn.2010.0078. [DOI] [PubMed] [Google Scholar]
- 161.Tancioni F, Lorenzetti MA, Navarria P, et al. Percutaneous vertebral augmentation in metastatic disease: state of the art. J Support Oncol. 2011;9:4–10. doi: 10.1016/j.suponc.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 162.Gofeld M, Bhatia A, Burton AW. Vertebroplasty in the management of painful bony metastases. Curr Pain Headache Rep. 2009;13:288–294. doi: 10.1007/s11916-009-0046-5. [DOI] [PubMed] [Google Scholar]
- 163.Berenson J, Pflugmacher R, Jarzem P, et al. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol. 2011;12:225–235. doi: 10.1016/S1470-2045(11)70008-0. [DOI] [PubMed] [Google Scholar]
- 164.Eleraky M, Papanastassiou I, Setzer M, et al. Balloon kyphoplasty in the treatment of metastatic tumors of the upper thoracic spine. J Neurosurg Spine. 2011;14:372–376. doi: 10.3171/2010.11.SPINE09909. [DOI] [PubMed] [Google Scholar]
- 165.Zou J, Mei X, Gan M, Yang H. Kyphoplasty for spinal fractures from multiple myeloma. J Surg Oncol. 2010;102:43–47. doi: 10.1002/jso.21574. [DOI] [PubMed] [Google Scholar]
- 166.Flagg A, 2nd, McGreevy K, Williams K. Spinal cord stimulation in the treatment of cancer-related pain: “back to the origins”. Curr Pain Headache Rep. 2012;16:343–349. doi: 10.1007/s11916-012-0276-9. [DOI] [PubMed] [Google Scholar]
- 167.Dupuy DE, Liu D, Hartfeil D, et al. Percutaneous radiofrequency ablation of painful osseous metastases: a multicenter American College of Radiology Imaging Network trial. Cancer. 2010;116:989–997. doi: 10.1002/cncr.24837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79:965–976. doi: 10.1016/j.ijrobp.2010.11.026. [DOI] [PubMed] [Google Scholar]
Recommended Readings
- Levy MH, Chwistek M, Mehta RS. Management of chronic pain in cancer survivors. Cancer J. 2008;14:401–409. doi: 10.1097/PPO.0b013e31818f5aa7. [DOI] [PubMed] [Google Scholar]
- Levy MH, Samuel TA. Management of cancer pain. Semin Oncol. 2005;32:179–193. doi: 10.1053/j.seminoncol.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Kochhar R, Legrand SB, Walsh D, et al. Opioids in cancer pain: common dosing errors. Oncology (Williston Park) 2003;17:571–575. discussion 575–576, 579. [PubMed] [Google Scholar]
- Weinstein SM, Portenoy R, Harrington S. UNIPAC 3: Assessment and Treatment of Physical Pain Associated with Life-Limiting Illness. 4. Glenview, Illinois: American Academy of Hospice and Palliative Medicine; 2012. [Google Scholar]


