Abstract
Electron transport and the electrochemical proton gradient across the thylakoid membrane are two fundamental parameters of photosynthesis. A combination of the electron acceptor, ferricyanide and the ΔpH indicator, 9-aminoacridine, was used to measure simultaneously electron transport rates and ΔpH solely by changes in the fluorescence of 9-aminoacridine. This method yields values for the rate of electron transport that are comparable with those obtained by established methods. Using this method a relationship between the rate of electron transport and ΔpH at various uncoupler concentrations or light intensities was obtained. In addition, the method was used to study the effect of reducing the disulfide bridge in the γ-subunit of the chloroplast ATP synthase on the relation of electron transport to ΔpH. When the ATP synthase is reduced and alkylated, the threshold ΔpH at which the ATP synthase becomes leaky to protons is lower compared with the oxidized enzyme. Proton flow through the enzyme at a lower ΔpH may be a key step in initiation of ATP synthesis in the reduced enzyme and may be the way by which reduction of the disulfide bridge in the γ-subunit enables high rates of ATP synthesis at low ΔpH values.
Electron transport in thylakoids and the electrochemical potential of the proton across the thylakoid membrane are two parameters that describe the integrity of the photosynthetic apparatus. Both parameters can be measured in various ways (Hormann et al., 1994). Electron transport in isolated thylakoids can be measured directly by dyes that change their color upon oxidation/reduction (Saha et al., 1971) or by measuring oxygen evolution in either thylakoids, intact chloroplasts, or whole tissues (Ewy and Dilley, 2000). Activity of the photosystems in intact cells or tissues can be deduced from the measurement of modulated chlorophyll (Chl) fluorescence (Schreiber et al., 1993). ΔpH between the stroma and the thylakoid lumen is measured indirectly because the small volume of the lumen does not enable the insertion of a pH electrode. A commonly used method for transthylakoid ΔpH measurement is the determination of amine distribution between the medium and the thylakoid lumen during illumination (Gaensslen and McCarty, 1971; Rottenberg and Grünwald, 1972; Rottenberg et al., 1972; Schuldiner et al., 1972). Changes in the absorbance of a pH indicator, such as neutral red trapped inside the thylakoid lumen, (Forster and Junge, 1981) can also be followed. Proton movement through the chloroplast ATP synthase can be detected by the use of external pH indicators (Junge, 1987). Usually ΔpH and electron transport are measured separately. Here we describe a method to simultaneously measure electron transport and ΔpH. This method requires only a spectrofluorimeter and a combination of an electron acceptor and a fluorescent dye in the reaction medium.
It was previously shown (Dekouchkovsky et al., 1982) that 9-aminoacridine (9-AA) fluorescence increases in a mixture containing thylakoids, 9-AA, and ferricyanide (FeCy), as FeCy is reduced during illumination. In this report we use the increase in 9-AA fluorescence when FeCy is reduced to the colorless ferrocyanide as a measure of electron transport. The 9-AA fluorescence change was shown to be a reliable quantitative tool to measure electron transport. This method was used to follow the relationship between electron transport and ΔpH under continuous light. 9-AA fluorescence quenching is routinely used for the determination of ΔpH across the thylakoid membrane (Strotmann et al., 1990; Ivanov and Edwards, 1997; Achnine et al., 1999). Modifications of the chloroplast ATP synthase were performed and their effects on both electron transport and ΔpH were detected and discussed in the context of conformational changes in the ATP synthase.
It was previously shown that a proton leak is induced above a threshold ΔpH in the chloroplast ATP synthase (Portis et al., 1975; Davenport and McCarty, 1984). Similar ΔpH dependence was shown for the onset of ATP synthesis in illuminated chloroplasts (Davenport and McCarty, 1980). It is shown here that when the disulfide bridge in the γ-subunit of the chloroplast ATP synthase is reduced, the threshold ΔpH for initiating proton movement through the ATP synthase is greatly diminished. This observation is discussed in the context of ATP synthesis mechanism and activation conditions.
RESULTS
Electron transport was measured in two ways. The first (Evron and Avron, 1990) measured the change in absorbance of FeCy using a dual wavelength spectrophotometer. The reaction mixture (RM; final volume of 2 mL) contained 50 mm Tricine-NaOH (pH 8.0), 50 mm NaCl, 0.3 mm FeCy, 1 μm 9-AA, and thylakoids containing 10 μg Chl/mL. Electron transport was measured by the difference in absorption of FeCy (ε420 nm = 1.0 mm −1 cm−1; Lee et al., 1967) at 420 nm before and after illumination. The absorbance was measured in a dual wavelength spectrophotometer (DW, Aminco, Lake Forest, CA) set to the dual wavelength mode with a reference wavelength of 470 nm.
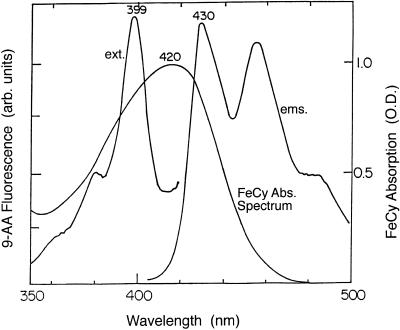
FeCy quenches 9-AA fluorescence because its absorption spectrum overlaps the excitation and emission spectra of 9-AA (Fig. 1). Therefore, photons will be absorbed by FeCy and will not be absorbed by 9-AA. The emitted photons from 9-AA can be absorbed by FeCy, further decreasing 9-AA fluorescence. Ferrocyanide, the product of FeCy reduction, does not absorb in this range. When a mixture of thylakoids, FeCy and 9-AA is illuminated, a build-up of ΔpH leads to a rapid quenching of 9-AA fluorescence (Schuldiner et al., 1972). Then a gradual increase in 9-AA fluorescence is observed, resulting from FeCy reduction by the thylakoids. When actinic illumination is turned off, ΔpH collapses leading to the rapid increase in 9-AA fluorescence and its stabilization in a new and higher level (Fig. 2).
Figure 1.
FeCy absorption and 9-AA excitation and emission spectra. FeCy solution contained, in a final volume of 3 mL, 50 mm Tricine-NaOH (pH 8.0), 50 mm NaCl, and 0.2 mm FeCy. 9-AA excitation (ext) and emission (ems) spectra were recorded in spectrofluorimeter (SLM 8000c, Aminco). RM was similar except that instead of FeCy it contained 1 μm 9-AA.
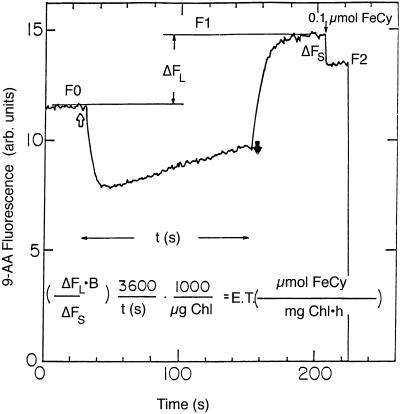
Figure 2.
The increase in 9-AA fluorescence as a measure for FeCy reduction. 9-AA fluorescence was monitored in a spectrofluorimeter (SLM 8000c, Aminco). F0, Initial 9-AA fluorescence; F1, 9-AA fluorescence after illumination; F2, 9-AA fluorescence after addition of FeCy. ΔFL is the relative increase in 9-AA fluorescence during illumination (ΔFL = 1 − F0/F1), ΔFS is the decrease in 9-AA fluorescence due to a standard addition of FeCy (ΔFS = 1 − F2/F1), t is the illumination time in seconds, and B is μmol of FeCy added as standard. The equation gives the conversion of ΔFL into the rate of FeCy reduction, in units of μmol of FeCy reduced mg−1 Chl h−1. In this case 0.1 μmol of FeCy was added as standard.
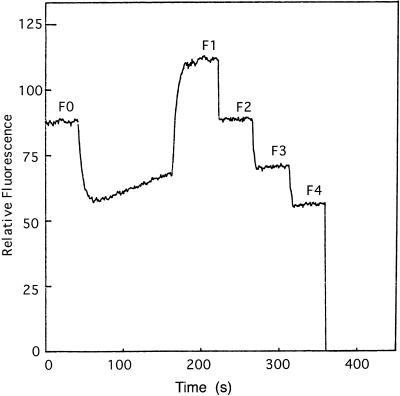
The increase in 9-AA fluorescence after illumination compared with the initial level is a measure for the rate of electron transport. A specific case is described in Figure 2 in which a thylakoid mixture containing FeCy and 9-AA (fluorescence level F0) was illuminated for t seconds. After a short dark period, during which the ΔpH decayed and 9-AA fluorescence stabilized at the level F1, 0.1 μmol of FeCy was added. The addition of the FeCy brings down the 9-AA fluorescence to the level F2. The equation in Figure 2 shows how the electron transport rate is calculated. The method is valid if identical FeCy additions will cause identical fractional decrease in 9-AA fluorescence. FeCy aliquots (0.2 μmol) were added to a thylakoid mixture containing 1 μm 9-AA after the illumination period (Fig. 3). Each decrease in fluorescence due to FeCy addition was the same as the previous one such that:
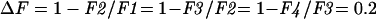 |
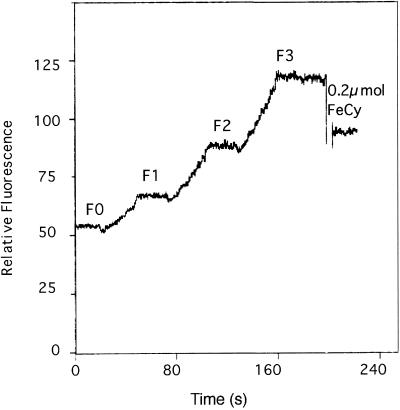
The consistency of electron transport at various FeCy concentrations is shown in Figure 4. Thylakoids were uncoupled by 5 μm gramicidin and illuminated for intervals of 30 s with about 20-s dark periods between illuminations. Identical time intervals give similar ΔF values, where
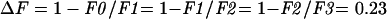 |
This value gives electron transport rate of 690 μmol FeCy mg Chl−1 h−1 when using the equation in Figure 2.
Figure 3.
Effect of subsequent additions of FeCy on the fluorescence level of 9-AA. The conditions were the same as in Figure 2. After illumination, several aliquots of 0.2 μmol of FeCy were added to the cuvette. F0, Initial 9-AA fluorescence; F1, 9-AA fluorescence after actinic illumination; F2, F3, and F4, 9-AA fluorescence after subsequent additions of 0.2 μmol of FeCy.
Figure 4.
Consistency of electron transport at various FeCy concentrations. RM contained 50 mm NaCl, 50 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], pH 7.0, 0.4 mm FeCy, 2 μm 9-AA, thylakoids containing 20 μg Chl/mL, and 5 μm gramicidin. Sample was illuminated for three periods of 30 s and the increase in 9-AA fluorescence was recorded. The experiment ended with addition of 0.2 μmol of FeCy. F0, Initial 9-AA fluorescence; F1, F2, and F3, 9-AA fluorescence levels after the first, second, and third illumination periods, respectively.
Electron transport measured by 9-AA fluorescence increase was compared with electron transport measured by FeCy absorbance decrease. 9-AA fluorescence was measured in a spectroflourimeter (SLM 8000, Aminco) at excitation and emission wavelengths of 399 and 430 nm, respectively. The two methods were compared. The initial absorbance was measured in the spectrophotometer and the cuvette was immediately transferred to the spectrofluorimeter for initial fluorescence measurement. After illumination of the sample the final fluorescence and absorbance were measured. Finally, a standard quantity of FeCy was added and again, the new absorbance of FeCy and fluorescence of 9-AA were measured. In all measurements the excitation slit was kept as narrow as possible because high excitation radiation can be actinic and may alter the electron transport. To avoid noise due to a low signal the emission slit was kept at 8 nm, which caused some actinic light to leak into the photomultiplier. This problem was overcome by placing a CuSO4 filter (Corning Glass Works, Corning, NY) in front of the photomultiplier.
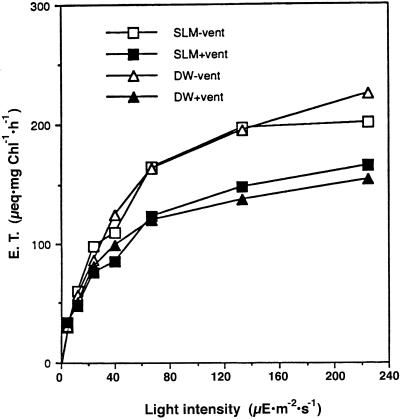
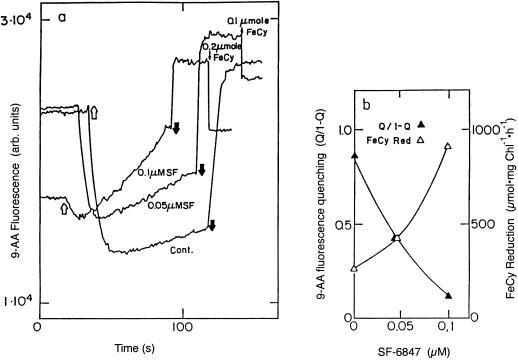
Rates of electron transport measured by the two methods described above are compared in Figure 5. The values obtained for electron transport by the FeCy absorbance and the 9-AA fluorescence methods were very similar. Under steady-state illumination a buildup of ΔpH will occur until the rate of vectorial proton translocation into the thylakoid lumen will be equal to the proton efflux due to membrane conductivity. Since electron transport is coupled to proton translocation into the thylakoid lumen, electron transport rate under continuous illumination is a measure of proton efflux through the thylakoid membrane (Davenport and McCarty, 1984; Hangarter et al., 1987). As expected, venturicidin, an inhibitor of the hydrophobic polypeptide complex of the chloroplast ATP synthase (CFo) (Linnett and Beechey, 1979), decreased the rate of electron transport due to blocking of proton efflux through the ATP synthase (Groth and Junge, 1993). When proton conductivity through the thylakoid membrane is increased, electron transport is much faster at high light intensity. A demonstration of this well-known phenomenon is shown in Figure 6 where electron transport and ΔpH in thylakoids treated with increasing concentrations of the uncoupler SF-6847 were measured simultaneously. The rate of electron transport increases while the magnitude of ΔpH decreases, as expected. Thylakoid membrane energization is presented as the degree of light-induced 9-AA fluorescence quenching, where q is the fraction of fluorescence that is quenched and 1 − q is the fluorescence that remains. ΔpH is equal to log (q/1 − q) + log (Vo/Vi) where Vo is the volume of the RM and Vi is the thylakoid lumen volume (Schuldiner et al., 1972). In our experiments q and 1 − q were measured at the moment the light was turned off, since at that moment electron transport stops and will not induce an error in the measurement of ΔpH.
Figure 5.
Comparison of FeCy reduction rates obtained by measuring FeCy absorbance changes or 9-AA fluorescence changes. RM contained in a final volume of 2 mL of 20 mm MOPS [3-(N-morpholino)propanesulfonic acid], 20 mm TAPS (N-tris[hydroxymethyl]methyl-3-aminopropanesulfonic acid, pH 8.0), 50 mm NaCl, 0.2 mm FeCy, and 1 μm 9-AA. After one reading, venturicidin (vent) was added to a final 0.5 μm and the measurement was repeated. SLM and DW correspond to measurement of electron transport via 9-AA fluorescence or FeCy absorbance, respectively. + or − vent indicates presence or absence of venturicidin.
Figure 6.
Simultaneous measurement of energization and electron transport at various uncoupler concentrations. The uncoupler SF-6847 was added dissolved in ethanol, which did not exceed 1% in the RM. The data in 6b was calculated from the 9-AA fluorescence traces in 6a. Q/1 – Q was calculated from the increase in 9-AA fluorescence when the light was turned off. Light or dark arrows correspond to actinic light on and off, respectively.
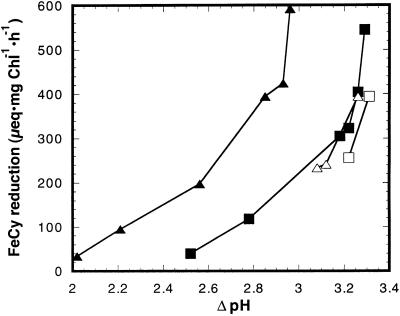
The simultaneous method was used to study the effect of reduction and alkylation of the disulfide Cys in the γ-subunit of chloroplast coupling factor 1 (CF1) on proton flux through the ATP synthase. As was previously demonstrated, chloroplast ATP synthase becomes leaky to protons under illumination at alkaline pH (Underwood and Gould, 1980; Evron and Avron, 1990) when a threshold ΔpH is obtained (Davenport and McCarty, 1984). A threshold ΔpH was also observed with regard to the onset of ATP synthesis in the light (Portis and McCarty, 1974; Ort et al., 1976) and was shown to be attributed to the magnitude of the electrochemical proton gradient (Junesch and Gräber, 1991). A unique feature of the chloroplast ATP synthase is its activation by a reduction of a disulfide bridge in the γ-subunit (Cys-199–Cys-205; Ketcham et al., 1984). This activation is manifested by a lower threshold for ATP synthesis under illumination of the thylakoid membrane (Mills and Mitchell, 1984), and activation of ATP hydrolysis of the membrane bound enzyme (Davenport and McCarty, 1981) or isolated CF1, the catalytic portion of the ATP synthase (Ketcham et al., 1984). When the disulfide bridge is formed, the ATP synthase becomes a latent ATPase and the ΔpH threshold for ATP synthesis increases (Mills and Mitchell, 1982). The relation between enzyme activity and proton movement mediated by the reduced enzyme when it is not catalyzing ATP synthesis was not addressed. The relationship between electron transport and ΔpH in reduced versus oxidized thylakoids was studied under steady-state illumination. Since FeCy is a strong oxidizing agent, γCys-199 and γCys-205 were protected from reoxidation by alkylation with N-ethylmaleimide (NEM; Ketcham et al., 1984). The curve that relates the rate of electron transport to ΔpH was shifted to the lower ΔpH values by the reduction and alkylation by about 0.5 ΔpH unit (Fig. 7). Previously it was shown that reduction shifts the curve that relates the rate of ATP synthesis to ΔpH to lower values (Ketcham et al., 1984; Junesch and Gräber, 1987).
Figure 7.
Dependence of electron transport on ΔpH in reduced and alkylated thylakoids. Reduction and alkylation of the γ-subunit disulfide were carried out as described in “Materials and Methods.” ATP (10 μm) was added where indicated just prior to illumination. ▪, Oxidized thylakoids; ▴, reduced and alkylated; □, oxidized + ATP; ▵, reduced and alkylated + ATP.
Proton influx rates are directly proportional to the rate of electron flow. Thus, the fact that higher rates of electron transport are required to generate a given value of ΔpH in reduced and alkylated thylakoids than in oxidized thylakoids is a clear indication that proton efflux rates are increased by reduction and alkylation. This proton efflux is partially blocked by adenine nucleotides, in both reduced and oxidized thylakoid preparations (Fig. 7), indicating that much of the efflux is through the ATP synthase. The increase in ΔpH by Mg-ATP is much greater in reduced and alkylated thylakoids. A similar increase in the proton efflux by reduction was observed when iodoacetic acid (I-Ac), rather than NEM, was used as the alkylating agent. Thus, reduction of the disulfide bond of the γ-subunit of CF1 enhances the ΔpH dependent flux of protons through the ATP synthase not only during ATP synthesis, but also under non-phosphorylating conditions.
The γ-subunit contains two additional Cys residues. Modification of γCys-322 with NEM or I-Ac has no effect on catalysis. γCys-89 is exposed to alkylation only when the membrane is energized and in the absence of nucleotides. Alkylation of γCys-89 strongly inhibits ATP synthesis (McCarty et al., 1972) and ATP hydrolysis (Soteropoulos et al., 1994). In addition, γCys-89 alkylation causes a large increase of the alkaline pH-dependent proton leak through the ATP synthase (Evron and Pick, 1997). To ensure that γCys-89 did not react during the alkylation of γCys-199 and γCys-205, ATP hydrolysis and ATP synthesis in reduced and alkylated thylakoids were tested. Sulfite-activated Mg-ATPase activity (Larson and Jagendorf, 1989) was 7- to 8-fold higher in reduced and alkylated thylakoids than in control thylakoids, indicating significant reduction of the γ-disulfide (Table I). There was no inhibition of ATP synthesis due to the alkylation, indicating that γCys-89 did not react with either NEM or I-Ac.
Table I.
Effect of NEM and I-Ac modification on ATP hydrolysis and synthesis
| Treatment | Rate of ATP Hydrolysis | Rate of ATP Synthesis |
|---|---|---|
| μmol Pi × mg Chl−1 h−1 | μmol ATP × mg Chl−1 h−1 | |
| None | 27 | 1,390 |
| 2 mm NEM | 202 | 1,625 |
| 10 mm I-Ac | 218 | 1,525 |
Thylakoids were first reduced by incubation with 50 mm diothiothreitot (DTT) and then alkylated by either NEM or I-Ac as described in “Materials and Methods.” “None” refers to thylakoids that had not been incubated with DTT or an alkylating reagent.
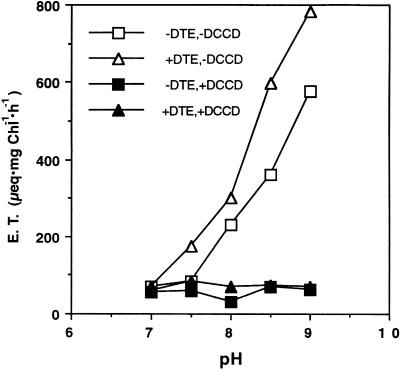
The pH dependence of electron transport from water to methyl viologen in oxidized and reduced thylakoids was compared. As shown in Figure 8, both oxidized and reduced thylakoids show high rates of electron flow at alkaline pH values. At pH values of 7.5 and above, the rates of electron transport by reduced thylakoids are higher than those of oxidized thylakoids. Electron transport in both thylakoid preparations at alkaline pH was strongly inhibited by N,N′-dicyclohexylcarbodiimide, an inhibitor of proton transport through CFo. These results support the conclusion that the increase in electron transport rates in reduced thylakoids is a consequence of increased proton leak rates through the ATP synthase.
Figure 8.
Electron transport dependence on external pH in control and reduced thylakoids. Electron transport was measured as oxygen consumption in the presence of methyl viologen. Where indicated, thylakoids were pretreated with N,N′-dicyclohexylcarbodidimide (DCCD) to block proton movement through CFo. Measurement started after 20 s of illumination to allow reduction by dithioerythritol (DTE). + or − indicates presence or absence of DCCD and DTE.
DISCUSSION
This work presents a simple, simultaneous method for the measurement of both electron transport and ΔpH. The method was verified by comparison with an established method, namely, the change in the absorbance of FeCy at 420 nm (Evron and Avron, 1990). The method was used to study the effect of ATP synthase modification on a change in the enzyme that increases proton efflux through it. We show that reduction of the disulfide bridge in the γ-subunit leads to a lower energy threshold, a change that leads to an increased proton flux through the ATP synthase under continuous illumination. This observation correlates well with the observation that the onset of ATP synthesis takes place at lower ΔpH values in thylakoids containing the reduced enzyme compared with those containing the oxidized enzyme (Ketcham et al., 1984; Junesch and Gräber, 1987). This correlation may indicate that the key difference between oxidized and reduced ATP synthase is the ability to translocate protons at lower ΔpH values. Proton transport through the ATP synthase is gated and there is an energy cost to open this gate. This cost is significantly lower in reduced thylakoids.
The observation that Mg-ATP reverses the increase in electron transport by reduction and alkylation (Fig. 7) is in accordance with previous reports on the inhibitory effect of Mg-ATP on proton efflux through the ATP synthase (McCarty et al., 1971; Yagi and Mukohata, 1977; Evron and Avron, 1990; Groth and Junge, 1993). This inhibition may arise from locking the enzyme in one conformation as compared with free movement, which may occur in the absence of nucleotides (Groth and Junge, 1993). This locking is superimposed on the enzyme whether oxidized or reduced, but the effect on the reduced enzyme (Fig. 7) is much greater, as the leak in the absence of nucleotides is much greater. The lower ΔpH required for the onset of proton flux through the ATP synthase in the reduced thylakoids may suggest that the key step in the activation of ATP synthesis at low ΔpH values is the beginning of proton movement through the ATP synthase. The recent models of the structure and function of F1Fo ATPases suggest a stationary part composed of subunits α, β, and δ of F1 and I, II, and IV of Fo, and a moving part composed of subunits γ and ε of F1 and maybe subunit III of Fo (Dimroth et al., 2000). It could be that allowing proton flux at lower ΔpH values indicates a lower resistance to movement due to a weaker interaction between the moving subunits and the stationary subunits.
MATERIALS AND METHODS
Chloroplast Preparation
Thylakoids were prepared from market spinach (Spinacia oleracea) as described previously (McCarty and Racker, 1967) and stored at 2 mg/mL Chl, 20 mm Tricine [N-tris(hydroxymethyl)methylglycine]-NaOH (pH 8.0), 10 mm NaCl, and 0.4 m Suc. Thylakoids were used within 4 h of preparation in which time the loss of activity was insignificant.
Alkylation of the Disulfide Cys in the γ-Subunit of CF1
The reduction of the disulfide bond in the CF1 γ-subunit and alkylation of the thiols generated by reduction with NEM (Ketcham et al., 1984) or I-Ac were performed with the thylakoid membranes. Thylakoids (0.2 mg Chl/mL) in an RM containing 50 mm Tricine-NaOH (pH 8.0), 50 mm NaCl, and 5 mm MgCl2 were incubated with 50 mm DTT for 2 h at 4°C in the dark. They were collected by a 10-min centrifugation at 4°C and 9,800g, resuspended in RM, and incubated for 10 min with 2 mm NEM. The NEM was removed by incubation with 1 mm DTT and another wash with RM. Alternatively, 10 mm I-Ac for 30 min was used instead of NEM. I-Ac was removed by two washes with RM and resuspension in the same buffer.
ATP Synthesis Measurements
Thylakoids containing 50 μg of Chl were added to ATP synthesis buffer containing 50 mm Tricine-NaOH (pH 8.0), 50 mm NaCl, 4 mm ADP, 2 mm inorganic phosphate, and 50 μm phenazine methosulfate in a total volume of 1 mL (Soteropoulos et al., 1994). After 90 s of illumination (2,200 μE m−2 s−1) in a water-cooled chamber, the reaction was stopped by the addition of 50 μL of 50% (w/v) trichloroacetic acid and dark. After a 2-min centrifugation in a microfuge, aliquots of 200 μL were taken for colorimetric phosphate determination (Taussky and Shorr, 1953).
ATP Hydrolysis Measurements
Sulfite-activated ATP hydrolysis (Du and Boyer, 1990) was measured in an RM containing 50 mm Tris [tris(hydroxymethyl)aminomethane]-HCl, pH 8.0, 4 mm ATP, 2 mm MgCl2, 100 mm sodium sulfite, and thylakoids containing 15 μg of Chl in a total volume of 1 mL. The reaction was initiated by addition of RM to thylakoids at 37°C and stopped after 5 min by adding 50 μL of 50% (w/v) trichloroacetic acid and placing on ice. Phosphate determination was carried out after 2 min of centrifugation in a microfuge.
Electron Transport Measurement in Oxygen Electrode
Oxygen uptake in the presence of methyl viologen (the Mehler reaction) was measured in an illuminated, water-cooled Clark type oxygen electrode (Rank Brothers, Bottisham, Cambridge, UK) attached to a chart recorder. RM contained 50 mm NaCl, 20 mm MOPS plus 20 mm TAPS at the indicated pH values, 0.1 mm methyl viologen, and thylakoids containing 10 μg Chl/mL in a final volume of 3 mL. Electron transport rates were calculated from the recorder traces assuming that air-saturated water at 25°C contains 250 μm O2.
ACKNOWLEDGMENT
We would like to thank the McCarty laboratory members for fruitful discussions.
Footnotes
Part of this work was performed while Y.E. was at the Weizmann Institute of Science, Israel in the laboratory of the late Mordhay Avron, and later with Uri Pick. This work was supported by the National Science Foundation (grant no. MCB974–23945).
This work is dedicated to the late Prof. Mordhay Avron of the Weizmann Institute of Science, Israel.
LITERATURE CITED
- Achnine L, Pereda-Miranda R, Iglesias-Prieto R, Moreno-Sanchez R, Lotina-Hennsen B. Tricolorin A, a potent natural uncoupler and inhibitor of photosystem II acceptor side of spinach chloroplasts. Physiol Plant. 1999;106:246–252. [Google Scholar]
- Davenport JW, McCarty RE. The onset of photophosphorylation correlates with the rise in transmembrane electrochemical proton gradients. Biochim Biophys Acta. 1980;589:353–357. doi: 10.1016/0005-2728(80)90051-1. [DOI] [PubMed] [Google Scholar]
- Davenport JW, McCarty RE. Quantitative aspects of adenosine triphosphate-driven proton translocation in spinach chloroplast thylakoids. J Biol Chem. 1981;256:8947–8954. [PubMed] [Google Scholar]
- Davenport JW, McCarty RE. An analysis of proton fluxes coupled to electron-transport and ATP synthesis in chloroplast thylakoids. Biochim Biophys Acta. 1984;766:363–374. [Google Scholar]
- Dekouchkovsky Y, Haraux F, Sigalat C. Effect of hydrogen-deuterium exchange on energy-coupled processes in thylakoids: a new illustration of the hypothesis of local proton gradients with the energy transducing biomembranes. FEBS Lett. 1982;139:245–249. [Google Scholar]
- Dimroth P, Kaim G, Matthey U. Crucial role of the membrane potential for ATP synthesis by F1Fo ATP synthases. J Biol Chem. 2000;203:51–59. doi: 10.1242/jeb.203.1.51. [DOI] [PubMed] [Google Scholar]
- Du ZY, Boyer PD. On the mechanism of sulfite activation of chloroplast thylakoid ATPase and the relation of ADP tightly bound at a catalytic site to the binding change mechanism. Biochemistry. 1990;29:402–407. doi: 10.1021/bi00454a014. [DOI] [PubMed] [Google Scholar]
- Evron Y, Avron M. Characterization of an alkaline pH-dependent proton slip in the ATP synthase of lettuce thylakoids. Biochim Biophys Acta. 1990;1019:115–120. [Google Scholar]
- Evron Y, Pick U. Modification of sulfhydryl groups in the gamma-subunit of chloroplast-coupling factor 1 affects the proton slip through the ATP synthase. Plant Physiol. 1997;115:1549–1555. doi: 10.1104/pp.115.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewy RG, Dilley RA. Distinguishing between luminal and localized proton buffering pools in thylakoid membranes. Plant Physiol. 2000;122:583–595. doi: 10.1104/pp.122.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster V, Junge W. Flash photometric studies of proton release inside thylakoids in dark-adapted chloroplasts with the pH indicator dye neutral red. Biophys Struct Mechan. 1981;7:296–299. [Google Scholar]
- Gaensslen RE, McCarty RE. Amine uptake in chloroplasts. Arch Biochem Biophys. 1971;147:55–65. doi: 10.1016/0003-9861(71)90309-2. [DOI] [PubMed] [Google Scholar]
- Groth G, Junge W. Proton slip of the chloroplast ATPase: its nucleotide dependence, energetic threshold, and relation to an alternating site mechanism of catalysis. Biochemistry. 1993;32:8103–8111. doi: 10.1021/bi00083a008. [DOI] [PubMed] [Google Scholar]
- Hangarter RP, Jones RW, Ort DR, Whitmarsh J. Stoichiometries and energetics of proton translocation coupled to electron transport in chloroplasts. Biochim Biophys Acta. 1987;890:106–115. [Google Scholar]
- Hormann H, Neubauer C, Schreiber U. An active Mehler-peroxidase reaction sequence can prevent cyclic PS-I electron transport in the presence of dioxygen in intact spinach chloroplasts. Photosyn Res. 1994;41:429–437. doi: 10.1007/BF02183045. [DOI] [PubMed] [Google Scholar]
- Ivanov B, Edwards GE. Electron flow accompanying the ascorbate peroxidase cycle in maize mesophyll chloroplasts and its cooperation with linear electron flow to NADP(+) and cyclic electron flow in thylakoid membrane energization. Photosyn Res. 1997;52:187–198. [Google Scholar]
- Junesch U, Gräber P. Influence of the redox state and the activation of the chloroplast ATP synthase on proton transport-coupled ATP synthesis hydrolysis. Biochim Biophys Acta. 1987;893:275–288. [Google Scholar]
- Junesch U, Gräber P. The rate of ATP-synthesis as a function of delta pH and delta psi catalyzed by the active, reduced H+-ATPase from chloroplasts. FEBS Lett. 1991;294:275–278. doi: 10.1016/0014-5793(91)81447-g. [DOI] [PubMed] [Google Scholar]
- Junge W. Complete tracking of transient proton flow through active chloroplast ATP synthase. Proc Natl Acad Sci USA. 1987;84:7084–7088. doi: 10.1073/pnas.84.20.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketcham SR, Davenport JW, Warncke K, McCarty RE. Role of the gamma-subunit of chloroplast coupling factor-1 in the light-dependent activation of photophosphorylation and ATPase activity by dithiothreitol. J Biol Chem. 1984;259:7286–7293. [PubMed] [Google Scholar]
- Larson EM, Jagendorf AT. Sulfite stimulation of chloroplast coupling factor ATPase. Biochim Biophys Acta. 1989;973:67–77. [Google Scholar]
- Lee CP, Sottocasa GL, Ernster L. Use of artificial electron acceptors for abbreviated phosphorylating electron transport: flavin-cytochrome c. In: Eastabrook RW, Pullman ME, editors. Methods in Enzymology. Vol. 10. New York: Academic Press; 1967. pp. 33–37. [Google Scholar]
- Linnett PE, Beechey RB. Inhibitors of the ATP synthethase system. In: Colowick S, Kaplan N, editors. Methods in Enzymology. Vol. 55. New York: Academic Press; 1979. pp. 472–518. [DOI] [PubMed] [Google Scholar]
- McCarty RE, Fuhrman JS, Tsuchiya Y. Effects of adenine nucleotides on hydrogen ion transport in chloroplasts. Proc Natl Acad Sci USA. 1971;68:2522–2526. doi: 10.1073/pnas.68.10.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty RE, Pittman PR, Tsuchiya Y. Light-dependent inhibition of photophosphorylation by N-ethylmaleimide. J Biol Chem. 1972;247:3048–3051. [PubMed] [Google Scholar]
- McCarty RE, Racker E. Partial resolution of the enzymes catalyzing photophosphorylation: II. The inhibition and stimulation of photophosphorylation by N, N′-dicyclohexylcarbodiimide. J Biol Chem. 1967;242:3435–3439. [Google Scholar]
- Mills JD, Mitchell P. Modulation of coupling factor ATPase activity in intact chloroplasts: reversal of thiol modulation in the dark. Biochim Biophys Acta. 1982;679:75–83. [Google Scholar]
- Mills JD, Mitchell P. Thiol modulation of the chloroplast proton motive ATPase and its effect on photophosphorylation. Biochim Biophys Acta. 1984;764:93–104. [Google Scholar]
- Ort DR, Dilley RA, Good NE. Photophosphorylation as a function of illumination time: II. Effects of permeant buffers. Biochim Biophys Acta. 1976;449:108–124. doi: 10.1016/0005-2728(76)90011-6. [DOI] [PubMed] [Google Scholar]
- Portis AR, Magnusson RP, McCarty RE. Conformational changes in coupling factor 1 may control the rate of electron flow in spinach chloroplasts. Biochem Biophys Res Commun. 1975;64:877–884. doi: 10.1016/0006-291x(75)90129-1. [DOI] [PubMed] [Google Scholar]
- Portis AR, McCarty RE. Effects of adenine nucleotides and of photophosphorylation on H+ uptake and the magnitude of the H+ gradient in illuminated chloroplasts. J Biol Chem. 1974;249:6250–6254. [PubMed] [Google Scholar]
- Rottenberg H, Grünwald T. Determination of ΔpH in chloroplasts: III. Ammonium uptake as a measure of pH in chloroplasts and subchloroplast particles. Eur J Biochem. 1972;25:71–74. doi: 10.1111/j.1432-1033.1972.tb01668.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg H, Grünwald T, Avron M. Determination of ΔpH in chloroplasts: I. Distribution of (14C) methylamine. Eur J Biochem. 1972;25:54–63. doi: 10.1111/j.1432-1033.1972.tb01666.x. [DOI] [PubMed] [Google Scholar]
- Saha S, Ouitrakul R, Izawa S, Good NE. Electron transport and photophosphorylation in chloroplasts as a function of the electron acceptor. J Biol Chem. 1971;246:3204–3209. [PubMed] [Google Scholar]
- Schreiber U, Neubauer C, Schliwa U. PAM fluorometer based on medium-frequency pulsed Xe-flash measuring light: a highly sensitive new tool in basic and applied photosynthesis research. Photosyn Res. 1993;36:65–72. doi: 10.1007/BF00018076. [DOI] [PubMed] [Google Scholar]
- Schuldiner S, Rottenberg H, Avron M. Determination of ΔpH in chloroplasts: II. Fluorescent amines as a probe for the determination of ΔpH in chloroplasts. Eur J Biochem. 1972;25:64–70. doi: 10.1111/j.1432-1033.1972.tb01667.x. [DOI] [PubMed] [Google Scholar]
- Soteropoulos P, Ong AM, McCarty RE. Alkylation of Cys 89 of the gamma subunit of chloroplast coupling factor 1 with N-ethylmaleimide alters nucleotide interactions. J Biol Chem. 1994;269:19810–19816. [PubMed] [Google Scholar]
- Strotmann H, Thelen R, Muller W, Baum W. A delta pH clamp method for analysis of steady-state kinetics of photophosphorylation. Eur J Biochem. 1990;193:879–886. doi: 10.1111/j.1432-1033.1990.tb19412.x. [DOI] [PubMed] [Google Scholar]
- Taussky HH, Shorr E. Microcolorimetric method for the determination of inorganic phosphorous. J Biol Chem. 1953;202:675–685. [PubMed] [Google Scholar]
- Underwood C, Gould JM. Modulation of proton efflux from chloroplasts in the light by external pH. Arch Biochem and Biophys. 1980;204:241–246. doi: 10.1016/0003-9861(80)90029-6. [DOI] [PubMed] [Google Scholar]
- Yagi T, Mukohata Y. Effects of purine nucleotides on photosynthetic electron-transport in isolated chloroplasts. J Bioen Biomem. 1977;9:31–40. doi: 10.1007/BF00745041. [DOI] [PubMed] [Google Scholar]