Abstract
Circulating microvesicles are able to mediate long-distance cell–cell communications. It is essential to understand how microvesicles from pancreatic cancer act on other cells in the body. In this work, serum-derived microvesicles were isolated from 10 patients with locally advanced pancreatic cancer and healthy controls. Using Cell Transwell and WST-1 reagents, we found that microvesicles from pancreatic cancer accelerated migration and proliferation of PANC-1 cells. Meanwhile, the proliferation of these cancer-microvesicle-treated cells (CMTCs) was affected less by 10 μM of gemcitabine relative to healthy microvesicle-treated cells (HMTCs). Next, we optimized the filter-aided sample preparation method to increase the recovery of protein samples and then applied it to the quantification of the proteome of CMTCs and HMTCs. The peptides were labeled and analyzed by liquid chromatography–tandem mass spectrometry. In total, 4102 proteins were identified, where 35 proteins were up-regulated with 27 down-regulated in CMTCs. We verified the quantitative results of three key proteins CD44, PPP2R1A, and TP53 by Western blot. The Ingenuity Pathway Analysis revealed pathways that cancer microvesicles might participate in to promote cell migration and proliferation. These findings may provide novel clues of treatment for tumorigenesis and metastasis.
Keywords: microvesicles, migration, pancreatic cancer, PANC-1 cells, proliferation
Graphical Abstract
INTRODUCTION
Microvesicles originate from the luminal membrane of multivesicular bodies (MVBs) in the late stage of endocytosis in most cells.1 They are membrane-bound nanosize vesicles carrying proteins, metabolites, and nucleic acids (mRNA, miRNA) from their original cells.2 Microvesicles that are secreted and circulate throughout the body in the bloodstream are termed “circulating microvesicles”. Circulating microvesicles can fuse with the plasma membrane of recipient cells and release their cargos.3 In this manner, they are able to mediate nearby or distant cell–cell communications.4–6
Previous studies have shown that tumor-derived micro-vesicles play critical roles in various physiological and pathologic processes including tumorigenesis and metastasis.7–9 For example, gastric cancer-derived microvesicles have been shown to promote peritoneal metastasis by destroying the mesothelial barrier,10 and long noncoding RNA ZFAS1 in circulating microvesicles promotes gastric cancer progression.11 Also, EGFR in microvesicles regulates the liver microenvironment to promote gastric cancer liver metastasis.12 In breast cancer, the miRNAs in microvesicles released by cancer-associated fibroblasts cause an aggressive phenotype.13 Furthermore, microvesicles released by HT29 cells increase the proliferation and motility of colon cancer cells,14 and microvesicles released by mesenchymal nonsmall cell lung cancer cells promote chemoresistance.15
Considering that little improvement has been achieved in outcomes of patients with pancreatic cancer over the past couple of decades, it is essential to understand how microvesicles from pancreatic cancer act on cells to discover novel drug targets and treatment strategies.5,16–18 Because cancer and healthy microvesicles are known to contain different protein cargo, it is important to study the impact of these cancer-related microvesicles on other cells. Several pioneering studies found that many kinds of RNAs are differentially expressed between cancer and healthy microvesicles. For example, certain miRNAs are preferentially expressed in circulating microvesicles derived from esophageal and colorectal cancer.19,20 Proteomic cargo of microvesicles has also been found to be different between osteosarcoma and normal osteoblasts.21
In a previous study from our laboratory, we found dozens of proteins were differentially expressed between pancreatic cancer and healthy microvesicles.22 In the present study, we used a pancreatic cancer cell line to measure the potential of microvesicles derived from patients with pancreatic cancer to promote migration and proliferation. We collected sera from 10 patients with locally advanced pancreatic cancer and used commercial healthy sera as controls. Circulating microvesicles from cancer or healthy sera were isolated and exposed to PANC-1 cells. Also, these effects were evaluated using various concentrations of gemcitabine to treat PANC-1 cells. We have also studied changes in the proteome of PANC-1 cells due to exposure to microvesicles, where differentially expressed proteins were analyzed by the Ingenuity Pathway Analysis (IPA).
EXPERIMENTAL SECTION
Isolation of Microvesicles from Human Serum
Commercially available serum samples (Innovative Research, Novi, MI) were chosen as healthy controls. Serum samples from 10 patients with locally advanced pancreatic cancer were obtained as part of an Institutional Review Board approved protocol. Whole blood samples were centrifuged at 500g for 10 min to isolate serum. All serum samples were stored at −80 °C before use. The initial volume of serum was 1 mL per sample. The serum samples were diluted with 3 mL of PBS (AppliChem, St. Louis, MO) to decrease the viscosity. The diluted serum samples were centrifuged at 2000g for 10 min and 10 000g for 30 min at 4 °C to remove dead cells and cell debris. The supernatant was transferred to Ultra-ClearTM tubes (Beckman Coulter, Indianapolis, IN) and centrifuged at 100 000g using a Beckman Optima XL-70 ultracentrifuge for 70 min at 4 °C. The supernatant was discarded, and 2 mm of supernatant remained above the pellet to avoid the loss of sedimentary microvesicles. The microvesicles were suspended in 4 mL of PBS and centrifuged at 100 000g for 60 min at 4 °C to clean the microvesicles. This clean-up step was repeated three additional cycles to eliminate the serum protein contamination.23
Transmission Electron Microscopy and NanoSight Analysis
The size of microvesicles was measured by transmission electron microscopy (TEM). In brief, a carbon film (Hatfield, PA) was placed in a vacuum environment for 1 min. Glow discharge was then performed by turning on high voltage power to incubate the carbon film with electrons for 2 min. This step was performed to make the surface of the carbon film hydrophilic. Then, 5 μL of microvesicle sample (2.5 × 107) was loaded on the carbon film and incubated for 2 min. Next, the supernatant was removed by a piece of filter paper and 5 μL of 2.5% w/v glutaldehyde in PBS was loaded to fix the microvesicles for 5 min. After removing the supernatant, the carbon film was washed with water three times and then negatively stained with 5 μL of 1% uranyl acetate for 1 min. After removing uranyl acetate, the samples on carbon films were imaged in a Philips CM-100 TEM instrument. Micro-vesicle concentration was measured using the NanoSight NS300 (Malvern, Worcestershire, U.K.) according to the standard protocol. Microvesicles isolated from 1 mL of patient or healthy serum were suspended in 1 mL of PBS and analyzed for 5 min at 25 °C.
Culture of PANC-1 Cells and Microvesicle Exposure
PANC-1 cells were purchased from American Type Culture Collection (ATCC) (Manassas, VA). These cells were cultured in a 96-well plate using Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL of penicillin, and 100 μg/mL of streptomycin (Gibco BRL, San Diego, CA) at 37 °C in a 5% CO2 atmosphere. Microvesicles (1 × 109) isolated from 1 mL of serum (from cancer patients or healthy controls) were added to 200 μL of media and exposed to PANC-1 cells (~50 000) in a well of a 96-well plate for 24 h.
Cell Migration Assay
The migration ability of PANC-1 cells was examined using Transwell chambers (Cell Biolabs, San Diego, CA). Cells were suspended in 200 μL of FBS-free media (DMEM containing 0.5% BSA, 2 mM CaCl2, and 2 mM MgCl2) and transferred to the upper compartment of 24-well Transwell chambers for 2 h. The lower compartment contained culture media (DMEM supplemented with 10% FBS). After 2 h, the medium on the upper compartment was removed carefully. The upper compartment was washed by PBS and swabbed gently by cotton-tipped swabs to remove nonmigratory cells. The upper compartment was then transferred to a clean well containing 400 μL of Cell Stain Solution and incubated for 10 min at room temperature. After staining, the upper compartment was washed and the cells that migrated through the membrane onto the bottom of the upper compartment were viewed under a microscope (SK-Advanced, Kadima Zoran, Israel) and quantified by a microplate reader (BioTek, Winooski, VT) at a wavelength of 560 nm.
Cell Proliferation and Toxicity Assay
The cell proliferation24 and toxicity25,26 of PANC-1 cells were examined using water-soluble tetrazolium (WST)-1 reagent. Cells that had been exposed to 5 × 109/mL of cancer microvesicles or healthy microvesicles for 24 h were treated with various concentrations of gemcitabine for 2 h. To each well was added 100 μL of WST-1 reagent and incubated for 4 h in a cell incubator. The 96-well plate was read by a microplate reader at 450 nm.
Lysis of PANC-1 Cells, Tryptic Digestion, and iTRAQ Labeling
After three times washing with PBS, PANC-1 cells from each well (~50 000) were lysed with 20 μL of lysis buffer composed of 50 mM triethylammonium bicarbonate (TEAB), 4% sodium dodecyl sulfate (SDS), and 100 mM 1,4-dithiothreitol (DTT) at 99 °C for 5 min. We prepared samples using an optimized filter-aided sample preparation (FASP) method. In brief, the sample was cooled and diluted with 800 μL of 8 M urea buffer (containing 50 mM TEAB), to which was added 20 μL of 250 mM iodoacetamide (IAA). Next, the sample solution was transferred to a centrifugal spin YM-30 filter (Millipore, Billerica, MA) and centrifuged at 14 000g until the sample on the filter was <50 μL. Two hundred microliters of 8 M urea was then added to the sample and centrifuged at 14 000g for 20 min. This step was repeated for an additional two times to remove SDS. To remove urea, the sample was washed by 200 μL of 50 mM TEAB for three times under the same centrifugation condition. The samples were then digested by 200 ng sequencing-grade-modified trypsin (Promega, Fitchburg, WI) at 37 °C for 12 h. According to the standard protocol, the tryptic peptides would be filtered through the filter and collected. To efficiently elute the peptides, two cycles of elution with 50 mM ammonium bicarbonate solution and one cycle of elution with 500 mM sodium chloride solution should be performed.27 Here the tryptic peptides on the filter were directly collected, acidified, and cleaned by desalting with C18 tips.28 The eluted samples were dried by SpeedVac (Labconco, Kansas City, MO).
The resulting tryptic peptide samples of cells were labeled by 4-plex iTRAQ reagent according to the instructions enclosed in the commercial kit. The labeled samples were acidified, mixed, and then desalted using C18 tips. The eluted samples were dried by SpeedVac for mass spectrometry analysis.
nanoLC–MS/MS and Data Analysis
The tryptic digests of PANC-1 cells were separated on an EASY-nLC 1000 liquid chromatograph system (Thermo Fisher Scientific, San Jose, CA) with a 250 mm and 75 μm ID reverse-phase (RP) C18 column. The samples were eluted under a 120 min linear gradient from 2 to 35% acetonitrile in 0.1% formic acid at a constant flow rate of 300 nL/min.29
Samples were analyzed by an Orbitrap Fusion mass spectrometer (Thermo Fisher Scientific, San Jose, CA) in the positive ion mode. The capillary temperature and the spray voltage were set as 200 °C and 2.5 kV. The data were acquired in a data-dependent mode; the 15 strongest MS1 peaks were selected for subsequent MS2 analysis. For every selected peak, higher energy collisional dissociation (HCD) was performed. The MS1 spectra (m/z 350–1650) and the MS2 spectra were both acquired in the Orbitrap.
The raw data were searched against the protein database by Proteome Discoverer 1.4 software (Thermo Fisher Scientific) with SEQUEST as the search engine. The parameters were set as follows: database, human UniProt; enzyme, trypsin; fixed modifications, carbamidomethyl (C) and 4-plex iTRAQ (N-term and K); variable modification, oxidation (M); up to two missed cleavages allowed; mass tolerance, 10 ppm for MS1 and mass tag, 0.05 Da for MS2; 1% false discovery rate allowed for peptides.
We normalized the quantification results to eliminate the difference of protein amounts from different samples using Perseus software. The database search results were saved as .txt files, which were then loaded into Perseus. There were three columns of ratios 115/114, 116/114, and 117/114. All of these ratio values were log2-transformed. The log2 ratio values in each column were normalized by subtracting “Tukey’s biweight”. We performed a t test to filter out proteins with a large variation of expression level among different patients, such as very low-abundance proteins, which were only identified in a couple of patients. A protein was considered significantly changed if it had a normalized ratio >2 (or <0.5), where a normalized log2 ratio was greater than 1 or less than −1, with p value <0.05.
Western Blot
Proteins from PANC-1 were separated on a 4–15% SDS-PAGE gradient gel (Bio-Rad, Berkeley, CA) and transferred to a PVDF membrane (Bio-Rad). After blocking, the membrane was incubated overnight with anti-PPP2R1A antibody, anti-TP53 antibody, or anti-CD44 antibody (Abcam, San Francisco, CA), followed by incubation with HRP-conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA) and was visualized using a chemiluminescent method kit (Merck Millipore, Billerica, MA).
RESULTS
Isolation of Circulating Microvesicles from Serum and Exposure to PANC-1 Cells
In this study, circulating microvesicles were isolated from the serum of cancer patients and healthy controls. To obtain microvesicles with a minimum contamination of serum proteins, we performed five cycles of ultracentrifugation in the microvesicle isolation step (Figure 1). To evaluate the loss of microvesicles by multiple cycles of ultracentrifugation, we used Nanosight to compare the number of microvesicles acquired by one cycle and five cycles and found that the microvesicle amounts from 1 mL of serum were 1.51 × 109 and 1.00 × 109, respectively. Although there was 33% loss compared with one cycle, five cycles removed almost all of the contaminant serum proteins. We could acquire ~0.2 μg of microvesicle protein from 4 mL of serum after five cycles of ultracentrifugation. Thus the serum microvesicles were ~2 × 1010/μg. The TEM images showed that the circulating microvesicles isolated by the ultracentrifugation from pancreatic cancer patients and healthy controls had similar morphology (Figure 2a,b) and size distribution (Figure 2c). We further used a negative stain EM where we used antibody-conjugated gold beads (5 nm diameter) to capture microvesicles from both pancreatic cancer patients and healthy controls, where anti-CD9, anti-CD63, and anti-CD81 antibodies were employed, respectively. The microvesicles captured by each of the antibody-conjugated gold beads also had similar morphology and size distribution between pancreatic cancer patients and healthy controls (Supplemental Figure S-1).
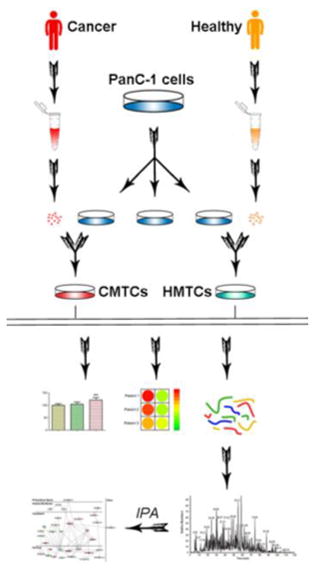
Figure 1.
Schematic overview of migration and proliferation assays, the sample preparation and LC–MS/MS analysis of HMTCs and CMTCs. Microvesicles were isolated from serum of patients with pancreatic cancer or commercial healthy serum using several steps of differential centrifugation. Then, cancer microvesicles or healthy microvesicles were exposed to PANC-1 cells and migration and proliferation assays were performed. Next, proteins from HMTCs and CMTCs were extracted, alkylated, and digested in ultrafiltration tubes using the filter-aided sample preparation (FASP) method. After iTRAQ labeling, peptides were mixed and run on a mass spectrometer. Database search of raw data and statistical analysis identified the differentially expressed proteins. Finally, IPA database revealed the network of directly interacting proteins and possible pathways involved in cell migration and proliferation.
Figure 2.
TEM images and the size distribution of the microvesicles. TEM images showed that the circulating microvesicles from pancreatic cancer patients and healthy controls had similar morphology (a,b) and size distribution (c).
PANC-1 cells were then exposed to the cancer and healthy microvesicles. Cell assays and mass spectrometry analysis were performed on the PANC-1 cells to demonstrate the effects of microvesicles derived from pancreatic cancer on other cells.
Optimization of FASP Method
FASP is a routine method to prepare proteomic samples.30–32 In brief, the lysate was added on the top of the 30 kDa filter in a centrifugal spin YM-30 filter. After centrifugation, small molecules were filtered out while proteins were trapped. Proteins were cleaned by several cycles of buffer replenishing and centrifugation and then digested by trypsin. The tryptic peptides are filtered through the filter by several cycles of elution under the centrifugation at the last step. However, peptides are lost due to the absorption by the filter. When the sample amount is small, as in this case, the peptide loss is substantial. In this study, the original protein yield of the PANC-1 cells (~50 000 cells) in a well of a 96-well plate was ~2.5 μg. The rate of sample loss would be significant after many steps. According to the standard protocol, the elution by 500 mM NaCl will help to elute more peptides at the last step. Our results indicate that there is no significant difference between 500 mM NaCl and water when eluting 0.1, 1, or 10 μg of bovine serum albumin (BSA) digests (Figure 3a). In addition, we found BSA could be detected even after six rounds of elution, although only three rounds of elution are suggested by the standard protocol (Figure 3b). We then digested 2 μg of protein from PANC-1 cells in each of two centrifugal spin YM-30 filter tubes. We collected and combined the eluent of six rounds from a tube (Sample A) while we directly collected the digested sample from the top of the filter from the other tube (Sample B). We then desalted the two samples before the analysis by mass spectrometry. It should be noted that the desalting step is very crucial to remove contaminants that hamper the mass spectrometry analysis. We found that 6–8% more peptides were identified in the Sample B (Figure 3c). The mass spectrometry analyses of peptides directly collected from the top of the filter are found not to be affected by potential contaminants such as nucleic acids. The results indicate that it is not necessary to filter the peptide sample. We used this optimized FASP method to prepare PANC-1 cells in the next set of experiments.
Figure 3.
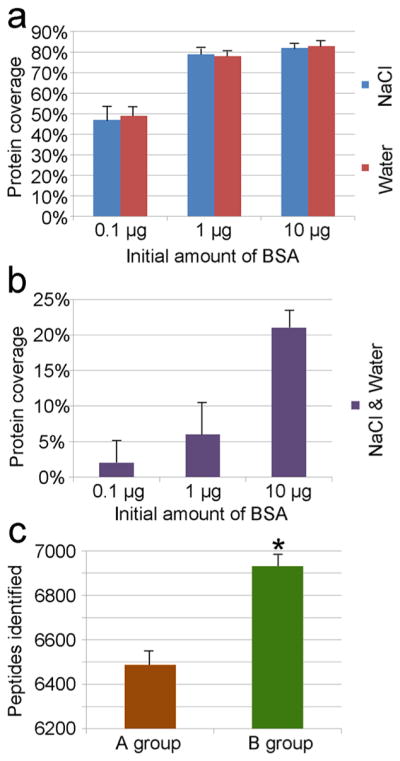
Comparison of protein coverage and identified peptide number by histograms. (a) Elution efficiency comparison of 500 mM NaCl and water on eluting 0.1, 1, or 10 μg of BSA digests. There is no significant difference between NaCl and water on elution efficiency. (b) Different amounts of BSA (0.1, 1, or 10 μg) were eluted six rounds; peptides still could be identified by mass spectrometry in the seventh eluent. (c) The former six eluents of digest (Sample A) and digest on the top of filter (Sample B) were collected and identified. We found 6–8% more peptides were identified in Sample B. There were three replicates for each assay.
Microvesicles from Patients with Pancreatic Cancer Accelerate the Migration of PANC-1 Cells
The effect of cancer-derived microvesicles on the migration ability of PANC-1 cells was evaluated using Transwell chambers. In a preliminary experiment, we isolated micro-vesicles from 1 mL of cancer serum or healthy control and added them to 5 mL of media to culture cells in a 10 cm plate, respectively. No difference in migration was observed between them. We then increased the concentration of microvesicles significantly. We added microvesicles isolated from 1 mL of serum (1 × 109) into 200 μL of media to culture cells in a well of a 96-well plate. We quantified the migrating cells by staining and measuring the absorbance at 560 nm and found that the migration ability of cancer-microvesicle-treated cells (CMTCs) was 21.5% higher than healthy microvesicle-treated cells (HMTCs) or cells without microvesicle exposure (Figure 4a). It should be noted that the majority of circulating microvesicles from cancer patients were not from carcinoma tissues.33 We speculate that this was why the migration ability of CMTCs did not increase substantially.
Figure 4.
Microvesicles from pancreatic cancer patients increase the migration and proliferation of PANC-1 cells. PANC-1 cells were exposed to microvesicles isolated from patients with pancreatic cancer or healthy control for 24 h before the migration and proliferation assay. We used pooled microvesicles in this assay due to limited amount of serum from each patient. (a) CMTCs migrated faster than HMTCs or PANC-1 cells without exposure. Error bars represent SD (n = 3). (b) CMTCs have higher proliferation activity. (c) Compared with HMTCs, CMTCs were more resistant to treatment with gemcitabine.
Effect of Cancer Microvesicles and Gemcitabine on the Proliferation of PANC-1 Cells
The WST-1 reagent has been broadly applied to assessing cell metabolic activity. Here we used this reagent to further evaluate the effect of cancer-derived microvesicles on the proliferation ability of PANC-1 cells. We thus treated cells with various concentrations of gemcitabine, a widely used chemotherapy drug for pancreatic cancer. The results showed that CMTCs had higher proliferation activity than HMTCs independent of the gemcitabine concentration (Figure 4b). Compared with HMTCs, the proliferation of CMTCs was inhibited more significantly by low concentration (0.1 μM) of gemcitabine but less significantly by high concentration (10 μM) (Figure 4c).
HMTCs versus PANC-1 Cells without Microvesicle Exposure
With the iTRAQ-based mass spectrometry technique, we compared HMTCs with PANC-1 cells without exposure to microvesicles. In the quantitative proteomics results, few proteins were found to be changed between the above two groups, but 23 proteins (Supplemental Table S-1) were identified only in cells without exposure to serum-derived microvesicles. We used the IPA database to analyze these “unique” proteins. The results did not show any interactions among these proteins, and no canonical pathways related to these proteins were found. Next, we checked the signals of these proteins manually and found that they were all low abundance proteins. Even in the group where they were identified, they were usually in only one or two samples. Thus we speculated that these “unique” proteins most likely exist in both groups but were not identified in one of the groups due to their low-abundance. On the basis of the results above, one should be very careful to consider proteins only identified in one group as unique proteins unless the protein signals are high in one group and not detected in the other.
HMTCs versus CMTCs
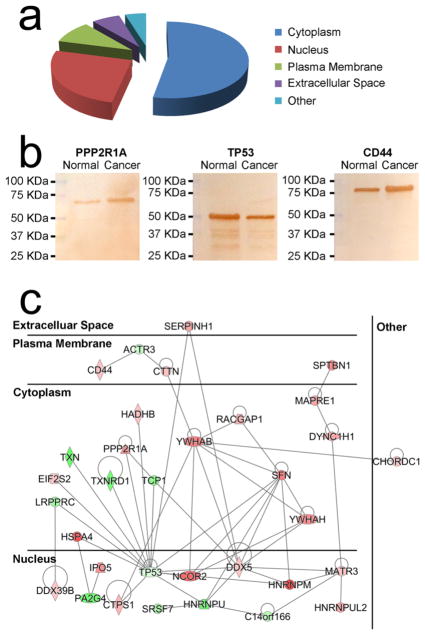
We compared HMTCs with CMTCs exposed to microvesicles from 10 patients with locally advanced pancreatic cancer. We estimated the amount of peptides injected into the mass spectrometer was <1 μg according to the signal intensity in mass spectrometry. In total, 4102 proteins were identified (Supplemental Table S-2), where 35 proteins were up-regulated with 27 were down-regulated in CMTCs (see Table 1) based on the consistent results of all samples. Because a Venn diagram cannot accommodate 10 groups, we have included a Venn diagram of five groups (Supplemental Figure S-2), where every group consists of two different patients. More than 80% of total proteins were identified in all five of these groups. The differentially expressed proteins from the cytoplasm, nucleus, plasma membrane, and extracellular space account for 53.23, 25.81, 9.68, and 6.45%, respectively (Figure 5a). There were three key proteins CD44, PPP2R1A, and TP53 (see Table 1) that attracted our attention. The Western blot results of two samples looked similar and verified the quantitative results of mass spectrometry (Figure 5b). Among these differentially expressed proteins, the IPA database indicated that 31 proteins had direct interactions (Figure 5c). Among the differentially expressed proteins, PL53, NCOR2, PPP2R1A, MAPRE1, CTTN, CD44, TP53, PA2G4, and TXN were found to be involved in cell proliferation. In addition, the canonical pathway of integrin linked kinase (ILK) was found to be involved in the cell proliferation process (Supplemental Figure S-3).
Table 1.
Differentially Expressed Proteins between CMTCs and HMTCsa
| accession | protein name | abbreviation | p value | log2(CMTCs/HMTCs) |
|---|---|---|---|---|
| Down-regulated | ||||
| P07237 | protein disulfide-isomerase | P4HB | 0.006176 | −5.46123 |
| P31939 | bifunctional purine biosynthesis protein PURH | ATIC | 0.000673 | −5.39358 |
| P10599 | thioredoxin | TXN | 0.001671 | −3.81445 |
| Q9UQ80 | proliferation-associated protein 2G4 | PA2G4 | 0.00191 | −3.78786 |
| Q00839 | heterogeneous nuclear ribonucleoprotein U | HNRNPU | 0.000424 | −3.78086 |
| Q16881 | thioredoxin reductase 1, cytoplasmic | TXNRD1 | 0.002643 | −3.44095 |
| O95994 | anterior gradient protein 2 homologue | AGR2 | 0.000738 | −3.06049 |
| P17987 | T-complex protein 1 subunit alpha | TCP1 | 0.003758 | −2.98979 |
| P49411 | elongation factor Tu, mitochondrial | TUFM | 9.83 × 10−5 | −2.96518 |
| P51571 | translocon-associated protein subunit delta | SSR4 | 0.000953 | −2.95912 |
| P42704 | leucine-rich PPR motif-containing protein, mitochondrial | LRPPRC | 0.000225 | −2.45097 |
| Q16629 | serine/arginine-rich splicing factor 7 | SRSF7 | 1.39 × 10−6 | −2.39883 |
| Q14112 | nidogen-2 | NID2 | 0.000129 | −2.25182 |
| P05187 | alkaline phosphatase, placental type | ALPP | 0.004714 | −2.17463 |
| Q9Y224 | UPF0568 protein C14orf166 | C14orf166 | 0.008743 | −1.83512 |
| Q8IVM0 | coiled-coil domain-containing protein 50 | CCDC50 | 0.005208 | −1.70429 |
| P04114 | apolipoprotein B-100 | APOB | 0.002248 | −1.47771 |
| P61158 | actin-related protein 3 | ACTR3 | 0.007959 | −1.47413 |
| O95202 | LETM1 and EF-hand domain-containing protein 1, mitochondrial | LETM1 | 0.004901 | −1.30823 |
| Q8N715 | coiled-coil domain-containing protein 185 | CCDC185 | 0.009593 | −1.30689 |
| Q9UMZ3 | phosphatidylinositol phosphatase PTPRQ | PTPRQ | 0.006533 | −1.27531 |
| Q8N9N8 | probable RNA-binding protein EIF1AD | EIF1AD | 0.00411 | −1.1958 |
| Q96FQ6 | protein S100-A16 | S100A16 | 0.003489 | −1.11726 |
| Q96I13 | abhydrolase domain-containing protein 8 | ABHD8 | 0.001347 | −1.1119 |
| P05204 | nonhistone chromosomal protein HMG-17 | HMGN2 | 0.000283 | −1.07958 |
| P20340 | Ras-related protein Rab-6A | RAB6A | 0.007328 | −1.0464 |
| P04637 | cellular tumor antigen p53 | TP53 | 9.45 × 10−6 | −1.02266 |
| Up-Regulated | ||||
| P20042 | eukaryotic translation initiation factor 2 subunit 2 | EIF2S2 | 0.003331 | 1.062531 |
| Q13838 | spliceosome RNA helicase DDX39B | DDX39B | 0.00506 | 1.08455 |
| P11586 | C-1-tetrahydrofolate synthase, cytoplasmic | MTHFD1 | 1.64 × 10−5 | 1.101048 |
| P17844 | probable ATP-dependent RNA helicase DDX5 | DDX5 | 0.009201 | 1.10227 |
| Q9UHD1 | cysteine and histidine-rich domain-containing protein 1 | CHORDC1 | 0.001193 | 1.110927 |
| P23526 | adenosylhomocysteinase | AHCY | 0.000506 | 1.1153 |
| P43243 | matrin-3 | MATR3 | 4.21 × 10−5 | 1.119641 |
| P16070 | CD44 antigen | CD44 | 0.000256 | 1.130534 |
| P55084 | trifunctional enzyme subunit beta, mitochondrial | HADHB | 0.000465 | 1.147197 |
| P30044 | peroxiredoxin-5, mitochondrial | PRDX5 | 0.004756 | 1.164929 |
| Q14247 | Src substrate cortactin | CTTN | 0.005807 | 1.177613 |
| P17812 | CTP synthase 1 | CTPS1 | 0.000178 | 1.388005 |
| Q9H0H5 | Rac GTPase-activating protein 1 | RACGAP1 | 0.000123 | 1.522765 |
| O76003 | glutaredoxin-3 | GLRX3 | 3.17 × 10−6 | 1.661114 |
| Q15691 | microtubule-associated protein RP/EB family member 1 | MAPRE1 | 0.003605 | 1.70147 |
| Q14204 | cytoplasmic dynein 1 heavy chain 1 | DYNC1H1 | 0.003087 | 1.701699 |
| Q1KMD3 | heterogeneous nuclear ribonucleoprotein U-like protein 2 | HNRNPUL2 | 0.000377 | 1.712421 |
| P50454 | serpin H1 | SERPINH1 | 0.000736 | 1.726279 |
| O00410 | importin-5 | IPO5 | 0.007701 | 2.071047 |
| P62158 | calmodulin | CALM1 | 0.004363 | 2.12305 |
| P30153 | serine/threonine-protein phosphatase 2A 65 kDa regulatory subunit A alpha isoform | PPP2R1A | 7.52 × 10−5 | 2.129178 |
| Q16891 | mitochondrial inner membrane protein | IMMT | 0.002204 | 2.174018 |
| Q04917 | 14–3–3 protein eta | YWHAH | 0.000902 | 2.192749 |
| Q9UBQ7 | glyoxylate reductase/hydroxypyruvate reductase | GRHPR | 0.003872 | 2.199724 |
| P14868 | aspartate–tRNA ligase, cytoplasmic | DARS | 0.00541 | 2.218161 |
| Q6PIU2 | neutral cholesterol ester hydrolase 1 | NCEH1 | 0.008315 | 2.237665 |
| Q01082 | spectrin beta chain, nonerythrocytic 1 | SPTBN1 | 0.001524 | 2.244113 |
| P31946 | 14–3–3 protein beta/alpha | YWHAB | 0.001868 | 2.411062 |
| P31947 | 14–3–3 protein sigma | SFN | 2.44 × 10−7 | 2.485867 |
| P60660 | myosin light polypeptide 6 | MYL6 | 0.003148 | 2.707021 |
| P13667 | protein disulfide-isomerase A4 | PDIA4 | 0.006502 | 2.844721 |
| Q9Y618 | nuclear receptor corepressor 2 | NCOR2 | 0.002516 | 2.887301 |
| P34932 | heat shock 70 kDa protein 4 | HSPA4 | 0.007072 | 3.339028 |
| P13797 | plastin-3 | PLS3 | 0.000567 | 3.353014 |
| P52272 | heterogeneous nuclear ribonucleoprotein M | HNRNPM | 0.005324 | 3.824637 |
Compared with HMTCs, 35 proteins were up-regulated with 27 down-regulated in CMTCs.
Figure 5.
Western blot verification and subcellular and functional analysis of differentially expressed proteins between CMTCs and HMTCs. Among 4102 quantified proteins, 62 proteins had GO information. (a) The subcellular distribution shows that proteins from cytoplasm, nucleus, plasma membrane, and extracellular space account for 53.23, 25.81, 9.68, and 6.45%, respectively. (b) Western blot verified the quantification results of CD44, TP53, and PPP2R1A by mass spectrometry. (c) The IPA analysis shows that 31 proteins have direct interactions. Red and green represent up- and down-regulation in CMTCs, respectively.
Interaction of Differentially Expressed Proteins in PANC-1 Cells and Those in Microvesicles
In our previous study, circulating exosomal proteomes from 10 patients with locally advanced pancreatic cancer were compared with those from healthy controls. In total, 99 differentially expressed proteins were identified between cancer micro-vesicles and healthy microvesicles (Supplemental Table S-3) in all patients.22 On the basis of the fusion of microvesicles and recipient cells, these differentially expressed proteins entered PANC-1 cells and remodeled them. As a result, cell migration and proliferation of PANC-1 cells were accelerated by cancer microvesicles. We found 62 differentially expressed proteins (35 up-regulated and 27 down-regulated proteins) in CMTCs compared with HMTCs. The 62 differentially expressed proteins are different from those 99 proteins except for one protein, namely, Apolipoprotein B-100 (APOB), which is down-regulated in both cancer groups. The question is how the exosomal protein cargos influenced the recipient cells. We investigated the relationship of the differentially expressed proteins in different microvesicles and those in their recipient cells. We combined the two groups of proteins (99 that were in microvesicles and 62 that were in recipient cells) and analyzed using the IPA database to reveal the possible pathways in which these proteins participated. In addition to the ILK pathway, we also found that the Remodeling of Epithelial Adherens Junctions (REAJ) pathway (Supplemental Figure S-4) was likely involved in cell migration.
DISCUSSION
Recent studies have shown that microvesicles derived from several types of cancer cells such as colorectal carcinoma, breast carcinoma, and nasopharyngeal carcinoma are able to promote cell migration or proliferation.34–37 In this study, we tested the effects of circulating microvesicles from pancreatic cancer patients on these cellular processes. Circulating microvesicles from pancreatic cancer patients may only contain a small percentage of carcinoma-derived microvesicles. The effects of migration or proliferation were not significant when we exposed PANC-1 cells to a concentration of 2 × 108/mL of circulating microvesicles. However, after we increased the microvesicle concentration to 5 × 109/mL, we found that the migration and proliferation of PANC-1 cells was significantly enhanced.
We performed iTRAQ-based quantitative proteome analysis of HMTCs and CMTCs. We first optimized the FASP procedure to increase the recovery of proteins. Although it was a minor modification, it did increase the number of identified proteins and simplified the procedure. The quantitative proteomics analysis identified 62 differentially expressed proteins between HMTCs and CMTCs, where the IPA database indicated that PL53, NCOR2, PPP2R1A, MAPRE1, CTTN, CD44, TP53, PA2G4, and TXN were involved in cell proliferation. TP53 is a well-known tumor suppressor. It suppresses tumorigenesis mainly through the cell cycle arrest pathway and apoptosis pathway. CD44 is a multifunctional cell surface protein. In pancreatic cancers, a higher level of CD44 has been associated with more aggressive disease. It has been reported as a marker of pancreatic cancer stem cells (CSCs).38 Thus down-regulated TP53 and up-regulated CD44 in CMTCs in the present study are concordant with the increase in cell proliferation. PPP2R1A is a scaffolding subunit of protein phosphatase 2A (PP2A). Inhibiting PP2A makes pancreatic cancer sensitive to radiotherapy both in vitro and in vivo by activation of CDC25C/CDK1 and inhibition of HRR,39 suggesting that the up-regulation of PP2A has a positive relation with the progression of pancreatic cancer. Thus, the up-regulation of PPP2R1A in CMTCs in the present study is consistent with the cell proliferation result.
Senescent cell-derived microvesicles have been shown to promote cancer cell proliferation, where EphA2 activated the Erk pathway through EphA2/ephrin-A1 reverse signaling.40 In the current study, we found that cancer-cell-derived micro-vesicles might promote cell proliferation by the ILK pathway. ILK is a signaling protein, where various domains of ILK interact with different proteins. It connects integrins to the cytoskeleton and transduces signals from the extracellular matrix and growth factors. Here up-regulated PP2A in CMTCs enhanced the activation of ILK. Overexpression of ILK in vivo promotes tumorigenesis.41 Silencing of ILK decreases the abnormal proliferation and migration of human Tenon’s capsule fibroblasts.42 It has important roles in cancer progression and has emerged as a therapeutic target in cancer.43
Gemcitabine is an antimetabolic chemotherapy drug used to treat several types of cancer including pancreatic cancer. It attenuates DNA synthesis in rapidly dividing cells.44 Our cell proliferation assay showed that the effects of cancer micro-vesicles and gemcitabine on PANC-1 cells are antagonistic. Cargos in cancer microvesicles promoted cell proliferation and division by the ILK pathway, while gemcitabine hampered this procedure. However, blocking the creation of new DNA results in unwanted adverse effects.45 Normal tissues, especially bowel, bone marrow, liver, and kidneys, can be adversely affected. In the 99 changed microvesicle proteins, ACTB, ACTC1, and CFL1 might drive target proteins MYL6 and PPP2R1A to change in the ILK pathway in PANC-1 cells. The suppression of the ILK pathway, such as inhibiting PPP2R1A, may be an alternative choice to block cell proliferation with less toxicity.
In the REAJ pathway, cadherins mediate homotypic cell–cell adhesion.46 The E-cadherins in the membrane of adjacent cells are connected together by calcium. In the inner side of the membrane, E-cadherin binds to catenins, providing anchorage to the actin cytoskeleton to form stable cell–cell contacts. It also associates with zyxin, vinculin, and α-actinin, which, in turn, associates with F-actin. The cadherin-catenin-mediated cell–cell adhesion leads to directional cell migration.47 In our results, zyxin and F-actin were both up-regulated in the CMTCs, suggesting that the cell migration was accelerated by the treatment of cancer microvesicles. In the 99 changed microvesicle proteins, ACTB, ARPC3, ARPC4, APRC18, ACTC1, MAPRE2, TUBA4A, and ZYX might drive target proteins ACTR3 and MAPRE1 to change in the REAJ pathway in PANC-1 cells.
CONCLUSIONS
We found that microvesicles derived from patients with pancreatic cancer accelerate cellular processes such as migration and proliferation. Compared with HMTCs, the proliferation of CMTCs was inhibited more significantly by a low concentration (0.1 μM) of gemcitabine but less significantly by high concentration (10 μM). The results may suggest that CMTCs are more sensitive to gemcitabine but more difficult to kill than HMTCs. We optimized the FASP method to increase the protein recovery and used it to prepare PANC-1 cells for mass spectrometry analysis. In total, 4102 proteins were identified by LC–MS/MS, where 35 proteins were up-regulated with 27 down-regulated in CMTCs relative to HMTCs. We verified the quantitative mass spectrometry results of three key proteins CD44, PPP2R1A, and TP53 in cell proliferation by Western blot. IPA suggests that the cancer microvesicles might participate in the pathways of REAJ and ILK in PANC-1 cells to promote cell migration and proliferation.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health under grant R01GM49500 (D.M.L.) and the National Cancer Institute under grants P5OCA130810 and R21CA189775 (D.M.L.). We acknowledge the assistance of the Wayne State University Proteomics Core that is supported through National Institutes of Health grants P30 ES020957, P30 CA 022453, and S10 OD010700.
Footnotes
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jproteo-me.8b00014.
Figure S-1: Morphology and size distribution comparison of microvesicles captured by antibody-conjugated gold beads from pancreatic cancer patients and healthy controls Figure S-2: A Venn diagram of five groups, where each group consists of two patients. Figure S-3: The canonical integrin linked kinase (ILK) pathway. Figure S-4: The remodeling of epithelial adherens junctions (REAJ) pathway. Table S-1: Proteins not identified in HMTCs but in PANC-1 cells without exposure. Table S-3: Differentially expressed proteins between cancer microvesicles and healthy microvesicles. (PDF)
Table S-2: Quantitative proteome analysis of HMTCs and CMTCs. (XLSX)
References
- 1.Cappello F, Logozzi M, Campanella C, Bavisotto CC, Marcilla A, Properzi F, Fais S. Exosome levels in human body fluids: A tumor marker by themselves? Eur J Pharm Sci. 2017;96:93–98. doi: 10.1016/j.ejps.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Kleeff J, Costello E, Jackson R, Halloran C, Greenhalf W, Ghaneh P, Lamb RF, Lerch MM, Mayerle J, Palmer D, Cox T, Rawcliffe CL, Strobel O, Buchler MW, Neoptolemos JP. The impact of diabetes mellitus on survival following resection and adjuvant chemotherapy for pancreatic cancer. Br J Cancer. 2016;115:887–894. doi: 10.1038/bjc.2016.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bach DH, Hong JY, Park HJ, Lee SK. The role of exosomes and miRNAs in drug-resistance of cancer cells. Int J Cancer. 2017;141:220–230. doi: 10.1002/ijc.30669. [DOI] [PubMed] [Google Scholar]
- 4.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 5.Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell. 2016;30:836–848. doi: 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muralidharan-Chari V, Clancy JW, Sedgwick A, D’SouzaSchorey C. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci. 2010;123:1603–1611. doi: 10.1242/jcs.064386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan J, Ding M, Xu K, Yang C, Mao LJ. Exosomes in diagnosis and therapy of prostate cancer. Oncotarget. 2017;8:97693–97700. doi: 10.18632/oncotarget.18532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whiteside TL. Tumor-Derived Exosomes and Their Role in Cancer Progression. Adv Clin Chem. 2016;74:103–141. doi: 10.1016/bs.acc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han L, Xu J, Xu Q, Zhang B, Lam EW, Sun Y. Extracellular vesicles in the tumor microenvironment: Therapeutic resistance, clinical biomarkers, and targeting strategies. Med Res Rev. 2017;37:1318–1349. doi: 10.1002/med.21453. [DOI] [PubMed] [Google Scholar]
- 10.Deng G, Qu J, Zhang Y, Che X, Cheng Y, Fan Y, Zhang S, Na D, Qu X, Liu Y. Gastric cancer-derived exosomes promote peritoneal metastasis by destroying the mesothelial barrier. FEBS Lett. 2017;591:2167–2179. doi: 10.1002/1873-3468.12722. [DOI] [PubMed] [Google Scholar]
- 11.Pan L, Liang W, Fu M, Huang ZH, Li X, Zhang W, Zhang P, Qian H, Jiang PC, Xu WR, Zhang X. Exosomes-mediated transfer of long noncoding RNA ZFAS1 promotes gastric cancer progression. J Cancer Res Clin Oncol. 2017;143:991–1004. doi: 10.1007/s00432-017-2361-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Deng T, Liu R, Bai M, Zhou L, Wang X, Li S, Wang X, Yang H, Li J, Ning T, Huang D, Li H, Zhang L, Ying G, Ba Y. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat Commun. 2017;8:15016. doi: 10.1038/ncomms15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnarumma E, Fiore D, Nappa M, Roscigno G, Adamo A, Iaboni M, Russo V, Affinito A, Puoti I, Quintavalle C, Rienzo A, Piscuoglio S, Thomas R, Condorelli G. Cancer-associated fibroblasts release exosomal microRNAs that dictate an aggressive phenotype in breast cancer. Oncotarget. 2017;8:19592–19608. doi: 10.18632/oncotarget.14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucchetti D, Calapa F, Palmieri V, Fanali C, Carbone F, Papa A, De Maria R, De Spirito M, Sgambato A. Differentiation Affects the Release of Exosomes from Colon Cancer Cells and Their Ability to Modulate the Behavior of Recipient Cells. Am J Pathol. 2017;187:1633–1647. doi: 10.1016/j.ajpath.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Lobb RJ, van Amerongen R, Wiegmans A, Ham S, Larsen JE, Möller A. Exosomes derived from mesenchymal non-small cell lung cancer cells promote chemoresistance. Int J Cancer. 2017;141:614–620. doi: 10.1002/ijc.30752. [DOI] [PubMed] [Google Scholar]
- 16.Nitsche U, Kong B, Balmert A, Friess H, Kleeff J. Should every patient with pancreatic cancer receive perioperative/neoadjuvant therapy? Indian J Med Paediatr Oncol. 2016;37:211–213. doi: 10.4103/0971-5851.195731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heid I, Steiger K, Trajkovic-Arsic M, Settles M, Esswein MR, Erkan M, Kleeff J, Jager C, Friess H, Haller B, Steingotter A, Schmid RM, Schwaiger M, Rummeny EJ, Esposito I, Siveke JT, Braren RF. Co-clinical Assessment of Tumor Cellularity in Pancreatic Cancer. Clin Cancer Res. 2017;23:1461–1470. doi: 10.1158/1078-0432.CCR-15-2432. [DOI] [PubMed] [Google Scholar]
- 18.Klein-Scory S, Tehrani MM, Eilert-Micus C, Adamczyk KA, Wojtalewicz N, Schnolzer M, Hahn SA, Schmiegel W, Schwarte-Waldhoff I. New insights in the composition of extracellular vesicles from pancreatic cancer cells: implications for biomarkers and functions. Proteome Sci. 2014;12:50. doi: 10.1186/s12953-014-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan S, Han B, Gao S, Wang X, Wang Z, Wang F, Zhang J, Xu D, Sun B. Exosome-encapsulated microRNAs as circulating biomarkers for colorectal cancer. Oncotarget. 2017;8:60149–60158. doi: 10.18632/oncotarget.18557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiam K, Wang T, Watson DI, Mayne GC, Irvine TS, Bright T, Smith L, White IA, Bowen JM, Keefe D, Thompson SK, Jones ME, Hussey DJ. Circulating Serum Exosomal miRNAs As Potential Biomarkers for Esophageal Adenocarcinoma. J Gastrointest Surg. 2015;19:1208–1215. doi: 10.1007/s11605-015-2829-9. [DOI] [PubMed] [Google Scholar]
- 21.Troyer RM, Ruby CE, Goodall CP, Yang L, Maier CS, Albarqi HA, Brady JV, Bathke K, Taratula O, Mourich D, Bracha S. Exosomes from Osteosarcoma and normal osteoblast differ in proteomic cargo and immunomodulatory effects on T cells. Exp Cell Res. 2017;358:369–376. doi: 10.1016/j.yexcr.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 22.An M, Lohse I, Tan Z, Zhu J, Wu J, Kurapati H, Morgan MA, Lawrence TS, Cuneo KC, Lubman DM. Quantitative Proteomic Analysis of Serum Exosomes from Patients with Locally Advanced Pancreatic Cancer Undergoing Chemoradiotherapy. J Proteome Res. 2017;16:1763–1772. doi: 10.1021/acs.jproteome.7b00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J, Tan Z, Lubman DM. Exosome enrichment of human serum using multiple cycles of centrifugation. Electrophoresis. 2015;36:2017–2026. doi: 10.1002/elps.201500131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coombes JD, Schevzov G, Kan CY, Petti C, Maritz MF, Whittaker S, Mackenzie KL, Gunning PW. Ras Transformation Overrides a Proliferation Defect Induced by Tpm3.1 Knockout. Cell Mol Biol Lett. 2015;20:626–646. doi: 10.1515/cmble-2015-0037. [DOI] [PubMed] [Google Scholar]
- 25.Han Y, Zhao J, Huang R, Xia M, Wang D. Omics-Based Platform for Studying Chemical Toxicity Using Stem Cells. J Proteome Res. 2018;17:579–589. doi: 10.1021/acs.jproteome.7b00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel GK, Khan MA, Bhardwaj A, Srivastava SK, Zubair H, Patton MC, Singh S, Khushman M, Singh AP. Exosomes confer chemoresistance to pancreatic cancer cells by promoting ROS detoxification and miR-155-mediated suppression of key gemcitabine-metabolising enzyme, DCK. Br J Cancer. 2017;116:609–619. doi: 10.1038/bjc.2017.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wisniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6:359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 28.An M, Zou X, Wang Q, Zhao X, Wu J, Xu LM, Shen HY, Xiao X, He D, Ji J. High-confidence de novo peptide sequencing using positive charge derivatization and tandem MS spectra merging. Anal Chem. 2013;85:4530–4537. doi: 10.1021/ac4001699. [DOI] [PubMed] [Google Scholar]
- 29.Zhao M, An M, Wang Q, Liu X, Lai W, Zhao X, Wei S, Ji J. Quantitative proteomic analysis of human osteoblast-like MG-63 cells in response to bioinert implant material titanium and polyetheretherketone. J Proteomics. 2012;75:3560–3573. doi: 10.1016/j.jprot.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 30.Yang R, Liu X, Thakolwiboon S, Zhu J. Protein Markers Associated with an ALDH Sub-Population in Colorectal Cancer. J Proteomics Bioinf. 2016;9:238–247. doi: 10.4172/jpb.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu J, Zhu J, Yin H, Liu X, An M, Pudlo NA, Martens EC, Chen GY, Lubman DM. Development of an Integrated Pipeline for Profiling Microbial Proteins from Mouse Fecal Samples by LC-MS/MS. J Proteome Res. 2016;15:3635–3642. doi: 10.1021/acs.jproteome.6b00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao X, Wang Q, Wang S, Zou X, An M, Zhang X, Ji J. Citric acid-assisted two-step enrichment with TiO2 enhances the separation of multi- and monophosphorylated peptides and increases phosphoprotein profiling. J Proteome Res. 2013;12:2467–2476. doi: 10.1021/pr301061q. [DOI] [PubMed] [Google Scholar]
- 33.Vykoukal J, Sun N, Aguilar-Bonavides C, Katayama H, Tanaka I, Fahrmann JF, Capello M, Fujimoto J, Aguilar M, Wistuba, Taguchi A, Ostrin EJ, Hanash SM. Plasma-derived extracellular vesicle proteins as a source of biomarkers for lung adenocarcinoma. Oncotarget. 2017;8:95466–95480. doi: 10.18632/oncotarget.20748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiba M, Watanabe N, Watanabe M, Sakamoto M, Sato A, Fujisaki M, Kubota S, Monzen S, Maruyama A, Nanashima N, Kashiwakura I, Nakamura T. Exosomes derived from SW480 colorectal cancer cells promote cell migration in HepG2 hepatocellular cancer cells via the mitogen-activated protein kinase pathway. Int J Oncol. 2016;48:305–312. doi: 10.3892/ijo.2015.3255. [DOI] [PubMed] [Google Scholar]
- 35.Harris DA, Patel SH, Gucek M, Hendrix A, Westbroek W, Taraska JW. Exosomes released from breast cancer carcinomas stimulate cell movement. PLoS One. 2015;10:e0117495. doi: 10.1371/journal.pone.0117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye SB, Li ZL, Luo DH, Huang BJ, Chen YS, Zhang XS, Cui J, Zeng YX, Li J. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget. 2014;5:5439–5452. doi: 10.18632/oncotarget.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.An M, Shen H, Cao J, Pei X, Chang Y, Ma S, Bao J, Zhang X, Bai X, Ma Y. The alteration of H4-K16ac and H3-K27met influences the differentiation of neural stem cells. Anal Biochem. 2016;509:92–99. doi: 10.1016/j.ab.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Zhu J, He J, Liu Y, Simeone DM, Lubman DM. Identification of glycoprotein markers for pancreatic cancer CD24+CD44+ stem-like cells using nano-LC-MS/MS and tissue microarray. J Proteome Res. 2012;11:2272–2281. doi: 10.1021/pr201059g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei D, Parsels LA, Karnak D, Davis MA, Parsels JD, Marsh AC, Zhao L, Maybaum J, Lawrence TS, Sun Y, Morgan MA. Inhibition of protein phosphatase 2A radiosensitizes pancreatic cancers by modulating CDC25C/CDK1 and homologous recombination repair. Clin Cancer Res. 2013;19:4422–4432. doi: 10.1158/1078-0432.CCR-13-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takasugi M, Okada R, Takahashi A, Virya Chen D, Watanabe S, Hara E. Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nat Commun. 2017;8:15729. doi: 10.1038/ncomms15728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Persad S, Dedhar S. The role of integrin-linked kinase (ILK) in cancer progression. Cancer Metastasis Rev. 2003;22:375–384. doi: 10.1023/a:1023777013659. [DOI] [PubMed] [Google Scholar]
- 42.Xing Y, Cui L, Kang Q. Silencing of ILK attenuates the abnormal proliferation and migration of human Tenon’s capsule fibroblasts induced by TGF-beta2. Int J Mol Med. 2016;38:407–416. doi: 10.3892/ijmm.2016.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoganathan N, Yee A, Zhang Z, Leung D, Yan J, Fazli L, Kojic DL, Costello PC, Jabali M, Dedhar S, Sanghera J. Integrin-linked kinase, a promising cancer therapeutic target: biochemical and biological properties. Pharmacol Ther. 2002;93:233–242. doi: 10.1016/s0163-7258(02)00192-4. [DOI] [PubMed] [Google Scholar]
- 44.Carmichael J, Fink U, Russell RC, Spittle MF, Harris AL, Spiessi G, Blatter J. Phase II study of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer. 1996;73:101–105. doi: 10.1038/bjc.1996.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colosia A, Khan S, Hackshaw MD, Oglesby A, Kaye JA, Skolnik JM. A Systematic Literature Review of Adverse Events Associated with Systemic Treatments Used in Advanced Soft Tissue Sarcoma. Sarcoma. 2016;2016:1–13. doi: 10.1155/2016/3597609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pokutta S, Weis WI. Structure and mechanism of cadherins and catenins in cell-cell contacts. Annu Rev Cell Dev Biol. 2007;23:237–261. doi: 10.1146/annurev.cellbio.22.010305.104241. [DOI] [PubMed] [Google Scholar]
- 47.Yuan Z, Wong S, Borrelli A, Chung MA. Down-regulation of MUC1 in cancer cells inhibits cell migration by promoting E-cadherin/catenin complex formation. Biochem Biophys Res Commun. 2007;362:740–746. doi: 10.1016/j.bbrc.2007.08.074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.