Abstract
Nitroxides are widely used in biology as antioxidants, spin labels, functional spin probes for pH, oxygen and thiol levels, and tissue redox status imaging using electron paramagnetic resonance (EPR); however, biological applications of nitroxides is hindered by fast bioreduction to EPR-silent hydroxylamines and rapid clearance. In this work, we have studied pyrrolidine nitroxides with acetoxymethoxycarbonyl groups which can undergo hydrolysis by cellular esterases to hydrophilic carboxylate derivatives resistant to bioreduction. Nitroxides containing acetoxymethoxycarbonyl groups were rapidly absorbed by cells from the media, 3,4-bis-(acetoxymethoxycarbonyl)-proxyl (DCP-AM2) and 3-(2-(bis(2-(acetoxymethoxy)-2-oxoethyl)amino)acetamido)-proxyl (DCAP-AM2) showing the strongest EPR signal of the cellular fraction. Remarkably, the EPR parameters of 3,4-dicarboxy-proxyl (DCP) and its mono- and di-acetoxymethyl esters are different, and consequent intracellular hydrolysis of acetoxymethoxycarbonyl groups in DCP-AM2 can be followed by EPR. To elucidate intracellular location of the resultant DCP, the mitochondrial fraction has been isolated. EPR measurements showed that mitochondria were the main place where DCP was finally accumulated. TEMPO derivatives showed expectedly much faster decay of EPR signal in the cellular fraction, compared to pyrrolidine nitroxides. It was found that supplementation of endothelial cells with 50 nM of DCP-AM2 completely normalised the mitochondrial superoxide level. Moreover, administration of DCP-AM2 to mice (1.4 mg/kg/day) resulted in substantial nitroxide accumulation in the tissues and significantly reduced hypertension. We found that hydroxylamine derivatives of dicarboxyproxyl nitroxide DCP-AM-H can be used for the detection of superoxide in vivo in angiotensin II model of hypertension. Infusion of DCP-AM-H in mice leads to accumulation of persistent EPR signal of nitroxide in the blood and vascular tissue in angiotensin II-infused wild-type but not in SOD2 overexpressing mice. Our data demonstrate that acetoxymethoxycarbonyl group containing nitroxides accumulate in mitochondria and demonstrate site-specific antioxidant activity.
Keywords: Nitroxide, electron paramagnetic resonance, superoxide, antioxidant, mitochondria, hypertension
Introduction
Nitroxides are widely used in biology as spin labels, functional spin probes for pH, oxygen and thiol levels, viscosity, molecular motion and tissue redox status imaging [1]. In addition, potential therapeutic applications of nitroxides attract much attention due to their unique antioxidant activity [2]. The main challenges in biological applications of nitroxides are related to their fast bioreduction to electron paramagnetic resonance (EPR)-silent hydroxylamines (>N-OH) and rapid excretion via kidney or via hepatobiliary system [3]. Recently, much attention has been drawn to sterically shielded nitroxides which demonstrate very high stability in model systems and in living cells [4]. Broad application of these nitroxides is, however, complicated due to complex multistep synthesis [5–7]. In addition, the introduction of bulky alkyl substituents make the compounds highly lipophilic or even insoluble in water, and in many cases, this is accompanied by a strong broadening of lines in the EPR spectra [5,8,9]. Specific methods of cellular targeting represent another approach to increase the efficacy of nitroxides application in biomedical studies. Intracellular accumulation retards clearance and can strongly increase the biological activity of nitroxides. Recently, we have described the antihypertensive activity of mitochondria-targeted piperidine and pyrrolidine nitroxides with triphenylphosphonium cationic groups [10] which are actively accumulated in cells and in mitochondria due to transmembrane electrostatic potential. Another approach implies the introduction of acetoxymethoxycarbonyl groups [11,12] which provide high cellular retention [13], very good tissue permeability and sustain EPR signal in vivo [14]. It should be noted that despite certain rate of bioreduction 2,2,5,5-tetramethyl-substituted pyrrolidine nitroxides represent the most stable class of nitroxides. There are numerous examples of successful application of 2,2,5,5-tetramethyl-substituted pyrrolidine nitroxides for in vivo EPR imaging and Overhauser-enhanced magnetic resonance imaging investigations in animals and ex vivo EPR measurements in animal tissues, see [15].
In contrast to triphenylphosphonium derivatives, nitroxides with acetoxymethoxycarbonyl groups passively diffuse to intracellular space where they undergo hydrolysis with intracellular esterases into highly hydrophilic carboxylate anions [16]. It has been shown that nitroxides with two carboxylate function are resistant to extrusion by cells via an organic anion transport mechanism [13] and they demonstrate protection from oxidative stress [17,18]. They can hardly diffuse back through the cellular membranes and therefore accumulate inside cells. However, the exact subcellular site where the acetoxymethoxycarbonyl group hydrolysis products are accumulated is yet unknown and antioxidant effects of acetoxymethyl esters of nitroxides in intact cells and in vivo have not been investigated. We have hypothesised that cellular accumulation of these nitroxides can protect cells and tissues from oxidative stress.
In this work, we provide evidence for their mitochondrial accumulation of carboxylate group containing pyrrolidine nitroxides upon treatment of intact cells with their acetoxymethyl esters, presumably due to the high activity of esterases/deacetylases in mitochondria (Figure 1). Preincubation of endothelial cells with acetoxymethoxycarbonyl groups containing pyrrolidine nitroxide prevents a H2O2-induced increase in cellular superoxide production. We have found that the new nitroxides with acetoxymethoxycarbonyl groups was shown to produce an antihypertensive effect comparable to that of mitochondria-targeted nitroxide mitoTEMPO [19]. Here, we also describe the application of acetoxymethoxycarbonyl group containing hydroxylamine for superoxide detection in cultured cells and in mice.
Figure 1.
Esterase reactions of 3,4-di(acetoxymethoxycarbonyl)-2,2,5,5-tetramethyl-1-pyrrolidinyloxyl (DCP-AM2) and mitochondrial accumulation of 3,4-dicarboxy-2,2,5,5-tetramethyl-1-pyrrolidinyloxyl (DCP).
Materials and methods
Materials
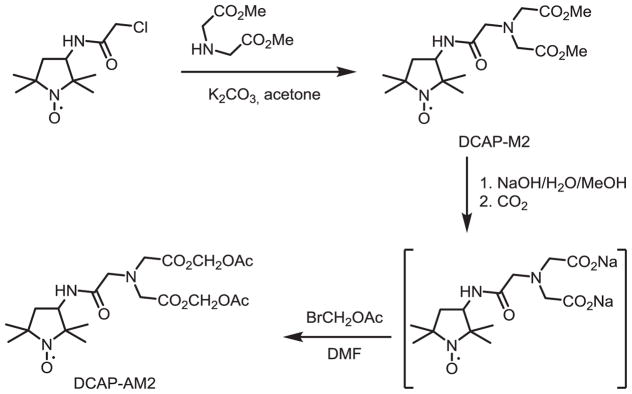
Nitroxide radicals TEMPOL and mitoTEMPO were purchased from Enzo Life Sciences (San Diego, CA), bromomethyl acetate was purchased from Sigma-Aldrich (St. Louis, MO). The 3,3-dicarboxy-2,2,5,5-tetra-methylpyrrolidine-1-oxyl (DCP) was prepared in analogy to original method [14,20] with some modifications described in [21]. The synthesis of nitroxides DCP-AM, DCP-AM2 [14] and DCAP-AM2 and of corresponding hydroxylamine DCP-AM-H is shown in Schemes 1 and 2.
Scheme 1.
Synthesis of DCP-AM2 and DCP-AM.
Scheme 2.
Synthesis of 3-(2-(Bis(2-methoxy-2-oxoethyl)amino)acetamido)-2,2,5,5-tetramethyl pyrrolidine-1-oxyl (DCAP-AM2).
trans-3,4-Di(acetoxymethoxycarbonyl)-2,2,5,5-tetra-methylpyrrolidin-1-oxyl (DCP-AM2) and 3-acetoxy-methoxycarbonyl-4-carboxy-2,2,5,5-tetramethylpyr-rolidin-1-oxyl (DCP-AM)
Anhydrous Na2CO3 (1.0 g, 9.4 mmol) was added portion-wise to a stirred solution of trans-3,4-dicarboxy-2,2,5,5-tetramethylpyrrolidin-1-oxyl (DCP) (600 mg, 2.6 mmol) in dry DMSO (2 ml). After CO2 release ceased (ca. 15 min), bromoethyl acetate (0.6 ml, 6.1 mmol) was added drop-wise (1.5 h) and the suspension was allowed to stand overnight at 40 °C. The reaction mixture was poured into water (25 ml) and extracted with Et2O, the extract dried with Na2SO4 and ether was distilled off in vacuum to give DCP-AM2, 407 mg, 41%, m.p. 81–83 (Et2O), IR (KBr) 1769, 1742 [14]. The structure of this nitroxide is confirmed with spectral data of bis(acetoxymethyl) 1-acetoxy-2,2,5,5-tetramethylpyrrolidine-3,4-dicarboxylate (DCP-AM2-Ac).
The remaining water solution was carefully acidified to pH 3–4 with NaHSO4 and extracted with ethyl acetate – propanol-2 mixture 9:1, the extract was dried with Na2SO4, the solvent was distilled off in vacuum and the residue was crystallised from hexane-ethyl acetate mixture to give DCP-AM, 250 mg, 31%, m.p. 133–135 (hexane-ethyl acetate 2:1), IR (KBr) 1766, 1733 (C=O), calculated for C13H2ONO7:C 51.65, H 6.67, N 4.63; found: C 51.38, H 6.70, N 4.57.
((Acetoxymethoxy)carbonyl)-1-hydroxy-2,2,5,5-tetra-methylpyrrolidine-3-carboxylic acid (DCP-AM-H)
The compound was prepared from nitroxide DCP-AM using previously described procedure [22], in brief, the catalyst (Pd/C, 1%, 20 mg per mmol of nitroxide) was added to a 0.2 M solution of DCP-AM in methanol and the mixture vigorously stirred in hydrogen atmosphere. After the calculated volume of hydrogen was absorbed the catalyst was filtered off and the methanol was distilled off in a vacuum. The residue was purified via precipitation from methanol with Et2O, yield 70%, m.p. 190–195, IR (KBr) 1756, 1620 br (CO); NMR 1H δ (CD3)2SO: 0.84, 0.89, 1.16 and 1.19 each s 3H (2,2,5,5-Me4), 2.49 s 3H (OAc), 2.88 and 2.94 each 1H AB J 8 Hz (3,4-CH), 5.61 and 5.72 each 1H br s (CH2), 7.6 br s 1H (OH); NMR 13C δ (CD3)2SO: 20.84, 21.52, 21.96, 27.33 and 27.56 (CH3), 51.40 and 51.57 (3,4-CH), 62.90 and 62.97 (C2 and C5), 79.56 (OCH2O), 169.72 (COO−), 170.92 and 172.98 (C=O). Calculated for C13H21NO7:C 51.48, H 6.98, N 4.62; found: C 51.26, H 7.02, N 4.91.
Bis(acetoxymethyl) 1-acetoxy-2,2,5,5-tetramethylpyrrolidine-3,4-dicarboxylate (DCP-AM2-Ac)
The nitroxide DCP-AM2 (200 mg, 0.53 mmol) was subjected to hydrogenation according to the above procedure. After the calculated volume of hydrogen was absorbed the catalyst was filtered off and the methanol was distilled off in a vacuum. The residue was dissolved in dry chloroform (10 ml), then acetic anhydride (0.5 ml, 5.3 mmol) and anhydrous sodium acetate (0.5 g, 6.1 mmol) were immediately added and the mixture was heated to reflux for 0.5 h. The precipitate was filtered off, the solvent was distilled off in vacuum and the residue was dissolved in hexane and cooled to −5 °C, the colourless crystals of DCP-AM2-Ac were filtered off to yield 115 mg (52%), m.p. 55–58, IR (KBr) 1767 (C=O); NMR 1H δ CDCl3: 1.06 br s and 1.31 s each 6H (2,2,5,5-Me4), 2.07 s 9H (OAc3), 3.32 br m 2H (3,4-CH), 5.67 and 5.80 each 1H AB J 4 Hz; NMR 1H δ (CD3)2CO: 1.03 s and 1.28 br s each 6H (2,2,5,5-Me4), 2.05 s 6H (OAc2), 2.06 s 3H (OAc), 3.23 br m 2H (3,4-CH), 5.68 and 5.81 each 1H AB J 5.8 Hz; NMR 13C δ (CD3)2CO: 16.6 br, 25.37 br, 25.85 br and 26.92 br (2,2,5,5-CH3), 17.80 and 19.44 (CH3, Ac), 49.95 br and 52.04 br (3,4-CH), 63.34 br and 64.83 br (C2 and C5), 79.10 (OCH2O), 168.85, 169.26 and 169.32 (C=O) (some signals are broadened due to slow inversion of N-OAcgroup, [23]). Calculated for C18H27NO10:C 52.06, H 6.50, N 3.12; found: C 51.79, H 6.52, N 3.36.
3-(2-(Bis(2-methoxy-2-oxoethyl)amino)acetamido)-2,2,5,5-tetramethylpyrrolidin-1-oxyl
To a stirred solution of 3-chloro acetamido-Proxyl [10] (0.483 g, 3 mmol) in acetone (10 ml), potassium carbonate (0.828 g, 6 mmol) and iminodiacetic acid dimethyl ester (0.700 g, 3 mmol) were added. After 6 h of stirring at room temperature, the reaction mixture was diluted with water (20 ml) and extracted with CHCl3 (2 × 15 ml). Organic layers were dried by Na2SO4 and evaporated to give DCAP-M2 as an orange oil, yield 1.055 g (98%). The crude product was used for the synthesis of the nitroxide DCAP-AM2 without further purification (see below). IR (neat): 1682 (O=C-NH), 1739 (O=C-OMe). Calculated for C16H28N3O6 C 53.62, H 7.87, N 11.72; found: C 53.81, H 7.81, N 11.66.
3-(2-(Bis(2-(acetoxymethoxy)-2-oxoethyl)amino) acetamido)-2,2,5,5-tetramethylpyrrolidin-1-oxyl (DCAP-AM2)
A solution of NaOH (0.098 g, 2.428 mmol) in water (2 ml) was added to a stirred solution of DCAP-M2 (0.435 g, 1.214 mmol) in methanol (5 ml). The reaction mixture was allowed to stand at ambient temperature for 3 h. Then gaseous CO2 was bubbled through the solution until pH reached 7.5. The solvents were distilled off in a vacuum and the residue was suspended in DMF (5 ml) and the solution of bromoethylacetate (0.391 g, 2.426 mmol) in DMF (3 ml) added. The mixture was stirred at ambient temperature for 24 h, then brine was added (20 ml) and the resulting mixture was extracted with CHCl3 (3 × 20 ml). Chloroform was distilled off in a vacuum and the residue was separated by column chromatography (silica gel, EtOAc) to give DCAP-AM2 as an orange oil (0.450 g, 76%), IR (neat): 1672 (O = C-NH), 1764 (O = C-OMe). Calculated for C20H32N3O10:C 50.63, H 6.80, N 8.86; found: C 50.65, H 6.75, N 8.76.
Stock solutions of cyclic hydroxylamine DCP-AM-H (5 mM) was prepared in argon-purged DMSO/Saline (10:90) and containing 1 mM DTPA. All other reagents were obtained from Sigma-Aldrich.
Cell culture
Human aortic endothelial cells (HAEC) were purchased from Lonza (Chicago, IL) and cultured in EGM-2 medium supplemented with 2% FBS but without antibiotics. On the day before the study, the FBS concentration was reduced to 1%.
Superoxide measurements using HPLC
Cells were cultured up to 80% confluence. Stock solution of MitoSOX (4 mM) was prepared in DMSO and was diluted in KHB buffer to a final concentration of 2 μM MitoSOX. Cells were loaded with MitoSOX for 20 min. Next, buffer was aspirated and scraped cells were mixed with methanol (300 μl) and homogenised with a glass pestle. The cell homogenate was passed through a 0.22 μm syringe filter and methanol filtrates were analysed by HPLC according to previously published protocols [24]. MitoSOX oxidation products, mito-2-hydroxyethidium and mitoethidium, were separated using a C-18 reverse-phase column (Nucleosil 250–4.5 mm) and a mobile phase containing 0.1% tri-fluoroacetic acid and an acetonitrile gradient (from 37 to 47%) at a flow rate of 0.5 ml/min. Ethidium and 2-hydroxyethidium were detected with a fluorescence detector using an emission wavelength of 580 nm and an excitation of 480 nm. Production of mitochondrial superoxide was measured using MitoSOX and HPLC following accumulation of superoxide specific product, mito-2-hydroxyethidium, as described previously [19].
Animal experiments
Transgenic mice expressing mitochondria-targeted catalase (mCAT), SOD2 overexpressing mice (TgSOD2) and their wild-type littermates (Jackson Labs.) are on C57BL/ 6J background. Hypertension was induced by a low-suppressor dose of Ang II as described previously [25]. In order to test the therapeutic potential of nitroxides, mice were infused angiotensin II osmotic minipump (0.7 mg/kg/day) and received a second minipump for infusion of vehicle, TEMPOL, DCP-AM2 or DCAP-AM2 (1.5 mg/kg/day). Blood pressure was monitored by the tail cuff method as previously described [26,27]. In additional experiments, C57BL/6J mice were injected with 100 μl of 2.5 mM mitoTEMPO or DCAP-AM2. Following 60-min mice were sacrificed, blood and kidney were collected for analysis of nitroxide stability and tissue accumulation. Vanderbilt Institutional Animal Care and Use Committee approved the procedures.
Analysis of cellular accumulation of nitroxides
Cellular accumulation of nitroxides was determined by EPR analysis of cellular fractions. For this purpose, confluent HAECs were incubated in KHB for 60 min with corresponding nitroxide (5 μM) at 37 °C. Then cells were collected and nitroxide concentration was measured by EPR in the incubation buffer, cellular media after incubation and cellular fractions.
EPR measurements
EPR measurements were performed in 50 μl glass capillary tubes at room temperature using the Bruker EMX spectrometer. Spectrometer settings were as follows: field sweep, 60 G; microwave frequency, 9.82 GHz; microwave power, 20 mW; modulation amplitude, 1 G; conversion time, 164 ms; time constant 328 ms; sweep time, 168 s; receiver gain, 1 × 105; the number of scans, 4. The rate of superoxide formation was measured by monitoring the amplitude of the low-field component of the EPR spectrum as previously described [28]. The concentration of nitroxide was determined from a calibration curve for intensity of the EPR signal of 3-carbox-yproxyl at various known concentrations. The rate of superoxide production was calculated from the accumulation of nitroxide, obtained from the EPR time scan. For this purpose, the EPR kinetics was analysed using linear regression and WinEPR software (BrukerBiospin Corp, Billerica, MA).
Statistics
Experiments were analysed using the Student–Newman–Keuls post-hoc test and analysis of variance (ANOVA). p levels <.05 were considered significant.
Results
Cellular accumulation of nitroxides
Incubation of cultured endothelial cells with DCP-AM2 (5 μM) lead to the robust disappearance of nitroxide EPR signal in the cell media within 60 min. Conversely, analysis of cellular pellet showed significant growth of nitroxide EPR signal. Analysis of EPR spectra showed that hyperfine coupling constants in cells and cell media are different from those in the buffer (Figure 2). We reasoned that esterase-mediated cleavage of DCP-AM2 can lead to accumulation of mono-ester DCP-AM and dicarboxylic acid DCP which may have distinct hyperfine coupling constants (Figure 1). Indeed, EPR analysis of purified nitroxides showed remarkable differences in nitrogen hyperfine coupling constant between DCP-AM2, DCP-AM and DCP as 15.6, 15.9 and 16.1 G, correspondingly. Comparison of nitrogen hyperfine coupling constants of purified nitroxides and experimental EPR spectra indicates accumulation of DCP in the cellular pellet while DCP-AM was detected in the cell media following incubation of endothelial cells with DCP-AM2. In contrast, incubation of endothelial cells with DCP did not lead to significant consumption of DCP nitroxide from the buffer and only minor accumulation of DCP in cells was observed (Figure 2). Interestingly, incubation of cells with mitoTEMPO lead to significant absorption of nitroxide from the buffer; however, no significant EPR signal of mitoTEMPO in cells was found due to rapid cellular bioreduction [10].
Figure 2.
Incubation of cells with DCP-AM2 is accompanied by cellular accumulation of DCP. The figure shows typical EPR spectra observed in at least three independent experiments.
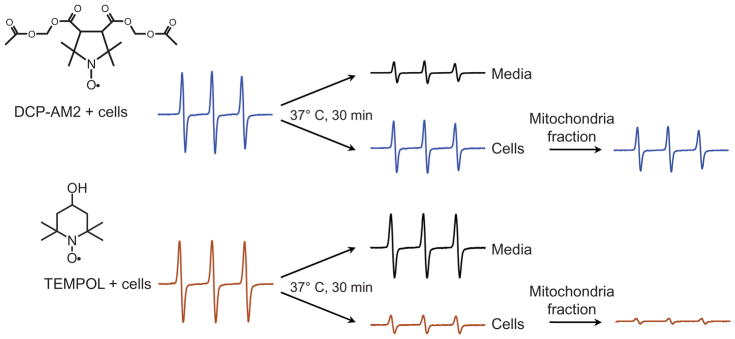
Mitochondrial accumulation of nitroxides
To determine the exact subcellular site of DCP accumulation endothelial cells were incubated with DCP-AM2 (10 μM) for 30 min and then EPR signal was measured in cell pellet and in mitochondria fraction isolated from these cells. EPR signal of mitochondrial fraction was similar to one from intact cells indicating that major part of the DCP-AM2 derived nitroxide was accumulated in mitochondria. A similar analysis of cellular pellet of TEMPOL treated cells showed minor accumulation of nitroxide and negligible EPR signal in mitochondria due to passive diffusion of TEMPOL (Figure 3).
Figure 3.
EPR analysis of cellular nitroxide accumulation in HAECs treated with DCP-AM2 and TEMPOL. The figure shows typical EPR spectra observed in at least three independent experiments.
Cellular bioreduction of nitroxides
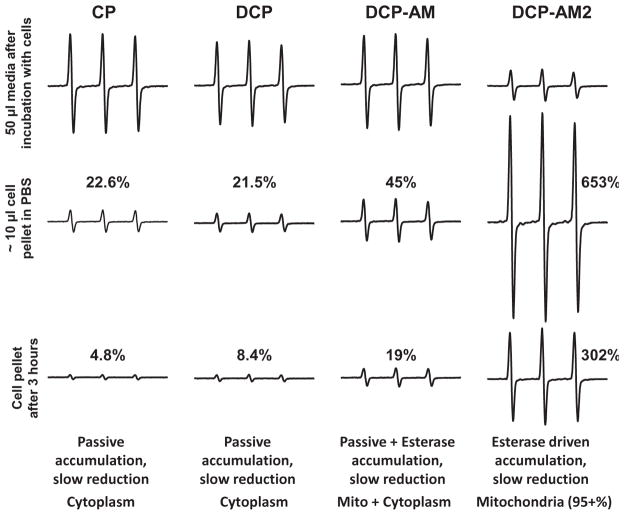
Our data indicate that mitochondrial accumulation of lipophilic cation mitoTEMPO leads to rapid reduction of nitroxide into EPR silent hydroxylamine (Figure 2). Expectedly, pyrrolidine nitroxides show much higher resistance to reduction. In next experiment, we have incubated endothelial cells with 3-carboxyproxyl (CP), DCP, DCP-AM or DCP-AM2 for 60 min at 37 °C. It was found that incubation of cells with CP and DCP resulted in a small accumulation of nitroxides in cells (22.6 and 21.5% compared to buffer signal) which was substantially reduced 3 h after cells isolation (4.8 and 8.4% correspondingly). Incubation of cells with DCP-AM lead to accumulation of twice as much nitroxide compared to DCP (45%), however, the intensity of the signal was substantially reduced (19%) 3 h after cells isolation. As expected, DCP-AM2 showed the most robust cellular accumulation (653%) which was reduced only two-fold 3 h later (Figure 4). These data are in line with the passive diffusion of the nitroxides CP, DCP, DCP-AM and DCP-AM2 into cells: the rate of their permeation through the membrane depends on nitroxide lipophilicity. However, permeation of DCP-AM and DCP-AM2 is almost irreversible due to their intracellular conversion into DCP. Higher intracellular stability of DCP as compared to CP is contradictory to previously published data [29,30] and may be due to a significant contribution of hydrophobic cellular compartments (e.g. mitochondrial membrane) to bioreduction of nitroxides in living cells.
Figure 4.
Comparison of cellular accumulation and cellular bioreduction of pyrrolidine nitroxides with carboxyl and acetoxymethoxycarbonyl groups. The figure shows typical EPR spectra observed in at least three independent experiments.
In additional experiments, we have compared cellular accumulation and bioreduction of DCP-AM2 [14] and newly synthesised nitroxide DCAP2-AM2. It was found that both nitroxides showed robust accumulation in cells, however, DCP-AM2 derived nitroxide showed higher resistance to bioreduction compared to DCAP-AM2 hydrolysis products (Figure 5).
Figure 5.
Cellular bioreduction of DCP-AM2 and DCAP-AM2 derivatives. The figure shows typical EPR spectra observed in at least three independent experiments.
Inhibition of mitochondrial superoxide by mitochondria-targeted nitroxides
We have previously shown that mitochondria-targeted superoxide dismutase mimetic mitoTEMPO reduces mitochondrial superoxide in endothelial cells treated with hormone angiotensin II or H2O2 while untargeted analogue TEMPOL was not effective [10,19]. In this work, we have compared the antioxidant effect of mitoTEMPO and DCP-AM2 on mitochondrial superoxide level. For this aim, cultured human endothelial cells were supplemented with a low dose of mitoTEMPO or DCP-AM2 (50 nM) for 60 min and then treated with 100 μM H2O2 to induce production of mitochondrial superoxide as it was previously described [10]. Following 60 min treatment with H2O2 cells were washed twice with Krebs-HEPES buffer and incubated with mitochondria-targeted superoxide probe MitoSOX for 20 min. Mitochondrial superoxide was measured by HPLC analysis of superoxide specific product mito-2-hydroxyethidium [19]. As expected H2O2 significantly increased mitochondrial superoxide production in nitroxide-free cells, however, supplementation with either mitoTEMPO or DCP-AM2 reduced superoxide production to control level (Figure 6).
Figure 6.
Effect of mitoTEMPO and DCP-AM2 on mitochondrial superoxide in H2O2-treated cells. Cultured human aortic endothelial cells were loaded with mitoTEMPO or DCP-AM2 (50 nM) for 60 min and then treated with 100 μM H2O2 for 60 min at 37 °C in Krebs-Hepes buffer. Then cells were washed twice with Krebs-Hepes buffer and supplemented with 1 μM MitoSOX and incubated for 20 min. Accumulation of superoxide specific product mito-2-hydroxyethidium was measured by HPLC as described previously [19]. *p <.01 vs. Control, **p <.05 vs. Ang II (n = 6).
In vivo experiments with acetoxymethoxycarbonyl pyrrolidine nitroxides
Similarly to DCP-AM and other acetoxymethoxycarbonyl group containing nitroxides [12–14,31], DCAP-AM2 should be an efficient agent for loading of nitroxide into tissues of living animals. To test this hypothesis, DCAP-AM2 or mitoTEMPO were injected in mice (100 μl 2.5 mM, i.p.) and 60-min later mice were sacrificed to collect blood and kidney for EPR analysis. Analysis of frozen tissue showed robust accumulation of nitroxide in DCAP-AM2 treated mice which was several times higher compared to the accumulation of mitoTEMPO (Figure 7(A)). Thus, in agreement to our in vitro data DCAP-AM2 provides efficient tissue delivery, the nitroxide shows high stability and retention.
Figure 7.
In vivo nitroxide accumulation, antihypertensive effect and superoxide detection by pyrrolidine nitroxides with acetoxymethoxycarbonyl groups. (A) C57Bl/6J mice were injected with 100 μl of 2.5 mM mitoTEMPO or DCAP-AM2. Following 60-min mice were sacrificed, blood and kidney were frozen for EPR analysis. (B,C) Mice were infused with DCAP-AM2, DCP-AM2 or TEMPOL using osmotic minipump (1.5 mg/kg/day). Blood pressure was measured by tail-cuff method. (D) In vivo detection of superoxide using DCP-AM-H (100 μl 2.5 mM, i.p. 60 min). *p <.01 vs. Control, **p <.01 vs. Ang II (n =6–8).
We have previously shown that mitochondria targeted nitroxides are very potent antihypertensive agents [10]. In this work, we have tested if a low dose of DCAP-AM2 (1.5 mg/kg/day) attenuates hypertension in the angiotensin II animal model. C57BL/6J mice were implanted with two osmotic minipump: one containing angiotensin II to induce hypertension, second pump with mitochondria-targeted superoxide mimetic DCAP-AM2 for 14 d (Figure 7(B)). As expected, angiotensin II infusion increased blood pressure to 157 ± 7 mm Hg and treatment with DCAP-AM2 significantly reduced blood pressure to 136 ± 5 mm Hg similarly to previously reported antihypertensive effect of mitoTEMPO [19]. Of note, treatment of healthy control mice with mitochondria-targeted nitroxides such as DCAP-AM2 did not affect basal blood pressure because superoxide does not play a role in the regulation of blood pressure in normotensive subjects [19].
In additional experiments, we have analysed the anti-hypertensive effect of DCP-AM2 and compared with untargeted nitroxide TEMPOL. Tempol at high dose is a potent antihypertensive agent, however, low dose of TEMPOL is not effective [19]. We hypothesised that low dose of mitochondria-targeted DCP-AM2 will attenuate hypertension while a similar dose of untargeted nitroxide TEMPOL will not reduce blood pressure in angiotensin II-infused mice. In order to test this hypothesis, C57BL/6J mice were implanted with osmotic mini-pumps containing angiotensin II, DCAP-AM2 (1.5 mg/ kg/day) or a similar dose of TEMPOL. It was found that angiotensin II infusion increased blood pressure to 162 ± 8 mm Hg, treatment with DCP-AM2 reduced blood pressure to 134 ± 8 mm Hg but TEMPOL did not reduce hypertension in angiotensin II infused mice (Figure 7(C)). The above studies showing that DCAP-AM2 and DCP-AM2 can reduce hypertension directly demonstrate novel antihypertensive effect of acetoxymethoxycarbonyl pyrrolidine nitroxides.
In vivo superoxide detection using acetoxymethoxycarbonyl derivatives of 1-hydroxy-pyrrolidine
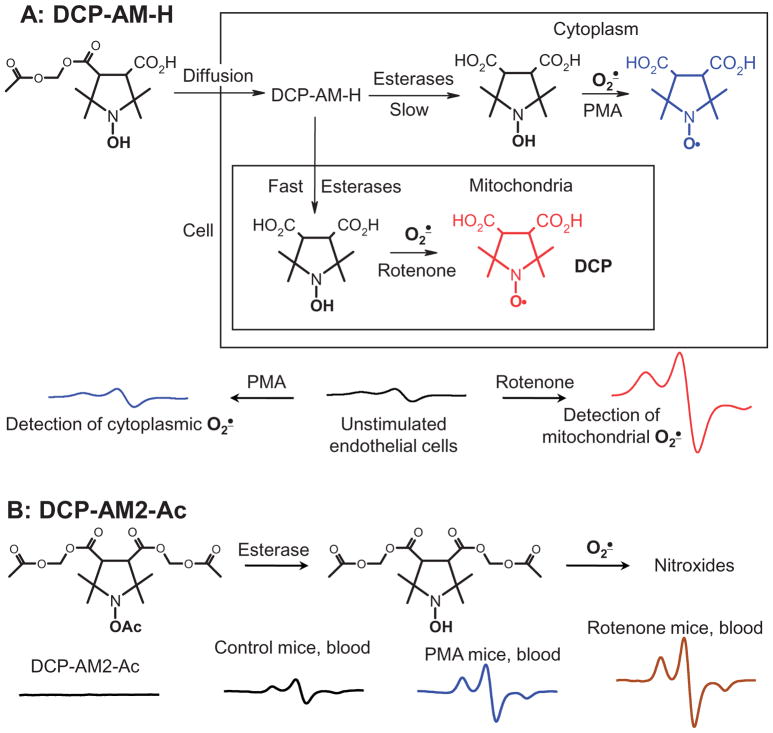
We have previously described that hypertension is associated with increased production of mitochondrial superoxide which can be detected using mitochondria-targeted hydroxyamine mitoTEMPO-H [19,28]. Reaction of mitoTEMPO-H with superoxide leads to accumulation of nitroxide mitoTEMPO which can be measured using EPR. However, mitoTEMPO is rapidly reduced back to hydroxyamine mitoTEMPO-H (Figure 2) [10] and this reverse conversion hinders detection of superoxide. In this work, we have tested if new hydroxylamine spin probe DCP-AM-H can be used for mitochondrial super-oxide detection in vivo. Our in vitro experiments in endothelial cells confirmed detection of mitochondrial superoxide in rotenone-treated cells (Figure 8(A)) [28]. For this aim, we have used C57BL/6J wild-type mice (WT), transgenic mice with overexpression of mitochondrial superoxide dismutase (SOD2) and mice expressing mitochondria-targeted catalase (mCAT). We have previously shown that mitochondrial superoxide is increased in vessels of angiotensin II-induced hypertension in wild type mice but superoxide production is not increased in SOD2 or mCAT mice and they are protected from hypertension [19,32,33]. Following 14 d of angiotensin II infusion mice were injected with DCP-AM-H (100 μl 2.5 mM, i.p.) and 60-min later mice were sacrificed to collect blood and aorta for EPR analysis. Analysis of frozen tissues showed robust accumulation of nitroxide in angiotensin II-infused hypertensive mice in the blood and aortas of wild-type C57BL/6J mice; however, angiotensin II infusion in SOD2 and mCAT mice did not show increased nitroxide production compared to normotensive wild-type control mice (Figure 7(D)) since we have previously shown that angiotensin II does not increase mitochondrial superoxide in SOD2 and mCAT mice [32]. These data support mitochondrial detection of superoxide in vivo using a DCP-AM-H probe.
Figure 8.
Detection of cytoplasmic and mitochondrial superoxide by DCP-AM-H and DCP-AM-Ac. (A) Cultured human aortic endothelial cells were incubated with rotenone (10 μM) or PMA (10 μM) for 30 min prior to supplementation with DCP-AM-H (100 μM) for 30 min. (B) In vivo detection of superoxide using DCP-AM2-Ac. Mice were injected with vehicle (100 μl DMSO/2-propanediol 1:9), Rotenone (100 μl, 0.4 mM, i.p. 60 min) or PMA (100 μl 2 mM, i.p., 60 min) prior to injection of DCP-AM2-Ac (100 μl 10 mM, i.p., 120 min). The figure shows typical EPR spectra observed in at least three independent experiments.
In additional experiments, we have tested if novel acyl-protected hydroxylamine DCP-AM2-Ac can be used for detection of cellular superoxide. Cyclic hydroxylamine are relatively unstable and can be contaminated with nitroxide signal before reaction with superoxide which acyl protected hydroxylamine are activated intracellularly to produce hydroxylamine at the site of superoxide production and hydroxylamine accumulation can be measured by oxidation with NaIO4 [34]. Indeed, the stock solution of DCP-AM2-Ac did not show the presence of nitroxide radicals, however, EPR analysis of blood from mice injected with the DCP-AM2-Ac showed robust nitroxide accumulation. Furthermore, treatment with NADPH oxidase agonist PMA resulted in a two-fold increase of EPR signal in the blood while complex I inhibitor rotenone raised EPR signal by three-fold (Figure 8(B)). These data support detection of cellular superoxide using a DCP-AM2-Ac probe.
Discussion
This study shows the first evidence that acetoxymethoxycarbonyl groups provide efficient transport of nitroxides to mitochondria. Di-anionic pyrrolidine nitroxides formed due to intracellular hydrolysis of ester groups are retained in the mitochondria of endothelial cells and show remarkable stability. This leads to accumulation of the nitroxides in mitochondria, thereby providing a high concentration of antioxidant and SOD-mimetic at the place where a major part of cellular superoxide is produced. As a result, the strong therapeutic effect is produced. This new mechanism of mitochondria targeting is alternative to transmembrane electrostatic potential-driven accumulation of triphenyl-phosphonium cations. It has been shown that of trans-membrane potential-driven accumulation is theoretically limited to a 1000-fold increase in concentration [35]. In our experiments, the acetoxymethyl esters provided more than 600-fold higher concentration of nitroxide inside the cell compared to extracellular space. Remarkably, this mechanism is not dependent on mitochondria function. Presumably, the new structures can be designed combining the above two mechanisms, which would provide much more efficient mitochondrial accumulation.
Conclusion
The mitochondria-targeted nitroxides DCP-AM2 and DACP-AM2, therefore, represent new antihypertensive agents. Cyclic hydroxylamine probe DCP-AM-H turned out a useful spin probe for mitochondrial superoxide in the blood cells and aortic tissue in hypertension.
Acknowledgments
We thank Dr. Roman Uzhachenko for help with mice experiments.
Funding
This work was supported by funding from National Institute of Health [PO1 HL129941], by American Heart Association [16GRNT31230017] and Russian Foundation for Basic Research [17-03-01132-a].
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Bobko AA, Eubank TD, Voorhees JL, Efimova OV, Kirilyuk IA, Petryakov S, et al. In vivo monitoring of pH, redox status, and glutathione using L-band EPR for assessment of therapeutic effectiveness in solid tumors. Magn Reson Med. 2012;67(6):1827–1836. doi: 10.1002/mrm.23196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soule BP, Hyodo F, Matsumoto K, Simone NL, Cook JA, Krishna MC, Mitchell JB. Therapeutic and clinical applications of nitroxide compounds. Antioxid Redox Signal. 2007;9(10):1731–1743. doi: 10.1089/ars.2007.1722. [DOI] [PubMed] [Google Scholar]
- 3.Kinoshita Y, Yamada K, Yamasaki T, Mito F, Yamato M, Kosem N, et al. In vivo evaluation of novel nitroxyl radicals with reduction stability. Free Radic Biol Med. 2010;49(11):1703–1709. doi: 10.1016/j.freeradbiomed.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 4.Jagtap AP, Krstic I, Kunjir NC, Hänsel R, Prisner TF, Sigurdsson ST. Sterically shielded spin labels for in-cell EPR spectroscopy: analysis of stability in reducing environment. Free Radic Res. 2015;49(1):78–85. doi: 10.3109/10715762.2014.979409. [DOI] [PubMed] [Google Scholar]
- 5.Paletta JT, Pink M, Foley B, Rajca S, Rajca A. Synthesis and reduction kinetics of sterically shielded pyrrolidine nitroxides. Org Lett. 2012;14(20):5322–5325. doi: 10.1021/ol302506f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Paletta JT, Berg K, Reinhart E, Rajca S, Rajca A. Synthesis of unnatural amino acids functionalized with sterically shielded pyrroline nitroxides. Org Lett. 2014;16(20):5298–5300. doi: 10.1021/ol502449r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasaki K, Ito T, Fujii HG, Sato S. Synthesis and reduction kinetics of five ibuprofen-nitroxides for ascorbic acid and methyl radicals. Chem Pharm Bull (Tokyo) 2016;64(10):1509–1513. doi: 10.1248/cpb.c16-00347. [DOI] [PubMed] [Google Scholar]
- 8.Bobko AA, Kirilyuk IA, Gritsan NP, Polovyanenko DN, Grigor'ev IA, Khramtsov VV, Bagryanskaya EG. EPR and quantum chemical studies of the pH-sensitive imidazo-line and imidazolidine nitroxides with bulky substituents. Appl Magn Reson. 2010;39(4):437–451. doi: 10.1007/s00723-010-0179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirilyuk IA, Polienko YF, Krumkacheva OA, Strizhakov RK, Gatilov YV, Grigor'ev IA, Bagryanskaya EG. Synthesis of 2,5-bis(spirocyclohexane)-substituted nitroxides of pyrroline and pyrrolidine series, including thiol-specific spin label: an analogue of MTSSL with long relaxation time. J Org Chem. 2012;77(18):8016–8027. doi: 10.1021/jo301235j. [DOI] [PubMed] [Google Scholar]
- 10.Dikalova AE, Kirilyuk IA, Dikalov SI. Antihypertensive effect of mitochondria-targeted proxyl nitroxides. Redox Biol. 2015;4:355–362. doi: 10.1016/j.redox.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kao JP, Rosen GM. Esterase-assisted accumulation of 3-carboxy-2,2,5,5-tetramethyl-1-pyrrolidinyloxyl into lymphocytes. Org Biomol Chem. 2004;2(1):99–102. doi: 10.1039/b310467b. [DOI] [PubMed] [Google Scholar]
- 12.Hu HP, Sosnovsky G, Li SW, Rao NU, Morse PD, 2nd, Swartz HM. Development of nitroxides for selective localization inside cells. Biochim Biophys Acta. 1989;1014(3):211–218. doi: 10.1016/0167-4889(89)90214-0. [DOI] [PubMed] [Google Scholar]
- 13.Rosen GM, Burks SR, Kohr MJ, Kao JP. Synthesis and biological testing of aminoxyls designed for long-term retention by living cells. Org Biomol Chem. 2005;3(4):645–648. doi: 10.1039/b415586f. [DOI] [PubMed] [Google Scholar]
- 14.Miyake M, Shen J, Liu S, et al. Acetoxymethoxycarbonyl nitroxides as electron paramagnetic resonance proimaging agents to measure O2 levels in mouse brain: a pharmacokinetic and pharmacodynamic study. J Pharmacol Exp Ther. 2006;318(3):1187–1193. doi: 10.1124/jpet.106.106245. [DOI] [PubMed] [Google Scholar]
- 15.Bačić G, Pavićević A, Peyrot F. In vivo evaluation of different alterations of redox status by studying pharmacokinetics of nitroxides using magnetic resonance techniques. Redox Biol. 2016;8:226–242. doi: 10.1016/j.redox.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sano H, Naruse M, Matsumoto K, Oi T, Utsumi H. A new nitroxylprobe with high retention in the brain and its application for brain imaging. Free Radic Biol Med. 2000;28(6):959–969. doi: 10.1016/s0891-5849(00)00184-2. [DOI] [PubMed] [Google Scholar]
- 17.Rayner CL, Bottle SE, Gole GA, Ward MS, Barnett NL. Real-time quantification of oxidative stress and the protective effect of nitroxide antioxidants. Neurochem Int. 2016;92:1–12. doi: 10.1016/j.neuint.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 18.de la Fuente-Núñez C, Reffuveille F, Fairfull-Smith KE, Hancock RE. Effect of nitroxides on swarming motility and biofilm formation, multicellular behaviors in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2013;57(10):4877–4881. doi: 10.1128/AAC.01381-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107(1):106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathew AE, Dodd JR. Synthesis of substituted, 2,2,5,5-tetramethylpyrrolidin-l-oxyl spin labels-PH sensitivity studies. J Heterocycl Chem. 1985;22(1):225–228. [Google Scholar]
- 21.Gorodetsky AA, Kirilyuk IA, Khramtsov VV, Komarov DA. Functional electron paramagnetic resonance imaging of ischemic rat heart: monitoring of tissue oxygenation and pH. Magn Reson Med. 2016;76(1):350–358. doi: 10.1002/mrm.25867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan’shole VV, Kirilyuk IA, Grigor’ev IA, Morozov SV, Tsentalovich YuP. Antioxidative properties of nitroxyl radicals and hydroxyamines in reactions with triplet and deaminated kynurenine. Russ Chem Bull. 2010;59(1):66–74. [Google Scholar]
- 23.Yordanov AT, Yamada K, Krishna MC, Russo A, Yoo J, English S, et al. Acyl-protected hydroxylamines as spin label generators for EPR brain imaging. J Med Chem. 2002;45(11):2283–2288. doi: 10.1021/jm0105169. [DOI] [PubMed] [Google Scholar]
- 24.Dikalov S, Griendling KK, Harrison DG. Measurement of reactive oxygen species in cardiovascular studies. Hypertension. 2007;49(4):717–727. doi: 10.1161/01.HYP.0000258594.87211.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dikalova A, Clempus R, Lassègue B, Cheng G, McCoy J, Dikalov S, et al. Nox1 overexpression potentiates angio-tensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112(17):2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 26.Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension. 1995;25(5):1111–1115. doi: 10.1161/01.hyp.25.5.1111. [DOI] [PubMed] [Google Scholar]
- 27.Widder JD, Guzik TJ, Mueller CF, Clempus RE, Schmidt HH, Dikalov SI, et al. Role of the multidrug resistance protein-1 in hypertension and vascular dysfunction caused by angiotensin II. Arterioscler Thromb Vasc Biol. 2007;27(4):762–768. doi: 10.1161/01.ATV.0000259298.11129.a2. [DOI] [PubMed] [Google Scholar]
- 28.Dikalov SI, Kirilyuk IA, Voinov M, Grigor'ev IA. EPR detection of cellular and mitochondrial superoxide using cyclic hydroxylamines. Free Radic Res. 2011;45(4):417–430. doi: 10.3109/10715762.2010.540242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keana JF, Pou S, Rosen GM. Nitroxides as potential contrast enhancing agents for MRI application: influence of structure on the rate of reduction by rat hepatocytes, whole liver homogenate, subcellular fractions, and ascorbate. Magn Reson Med. 1987;5(6):525–536. doi: 10.1002/mrm.1910050603. [DOI] [PubMed] [Google Scholar]
- 30.Keana JF, Van Nice FL. Influence of structure on the reduction of nitroxide MRI contrast-enhancing agents by ascorbate. Physiol Chem Phys Med NMR. 1984;16(6):477–480. [PubMed] [Google Scholar]
- 31.Redler G, Barth ED, Bauer KS, Kao J, Rosen GM, Halpern HJ. In vivo electron paramagnetic resonance imaging of differential tumor targeting using cis-3,4-di(acetoxymethoxycarbonyl)-2,2,5,5-tetramethyl-1-pyrro-lidinyloxyl. Magn Reson Med. 2014;71(4):1650–1656. doi: 10.1002/mrm.24813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Itani HA, Dikalova AE, McMaster WG, Nazarewicz RR, Bikineyeva AT, Harrison DG, Dikalov SI. Mitochondrial cyclophilin D in vascular oxidative stress and hypertension. Hypertension. 2016;67(6):1218–1227. doi: 10.1161/HYPERTENSIONAHA.115.07085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dikalova AE, Itani HA, Nazarewicz RR, McMaster WG, Flynn CR, Uzhachenko R, et al. Sirt3 impairment and SOD2 hyperacetylation in vascular oxidative stress and hypertension. Circ Res. 2017;121(5):564–574. doi: 10.1161/CIRCRESAHA.117.310933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itoh O, Aoyama M, Yokoyama H, Obara H, Ohya H, Kamada H. Sensitive ESR determination of intracellular oxidative stress by using acyl-protected hydroxylamines as new spin reagents. Chem Lett. 2000;29(4):304–305. [Google Scholar]
- 35.Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]