Abstract
Fibrates are hypolipidemic drugs that act as activators of peroxisome proliferator-activated receptor α (PPARα). In both humans and rodents, females were reported to be less responsive to fibrates than males. Previous studies on fibrates and PPARα usually involved male mice, but little has been done in females. The present study aimed to provide the first comprehensive analysis of the effects of clofibrate (CLOF) and PPARα on bile acid (BA) homeostasis in female mice. Study in WT male mice showed that a 4-day CLOF treatment increased liver weight, bile flow, and biliary BA excretion, but decreased total BAs in both serum and liver. In contrast, WT female mice were less susceptible to these CLOF-mediated responses observed in males. In WT female mice, CLOF decreased total BAs in the liver, but had little effect on the mRNAs of hepatic BA-related genes. Next, a comparative analysis between WT and PPARα-null female mice showed that lack of PPARα in female mice decreased total BAs in serum, but had little effect on total BAs in liver or bile. However, lack of PPARα in female mice increased mRNAs of BA synthetic enzymes (Cyp7a1, Cyp8b1, Cyp27a1, and Cyp7b1) and transporters (Ntcp, Oatp1a1, Oatp1b2, and Mrp3). Furthermore, the increase of Cyp7a1 in PPARα-null female mice was associated with an increase in liver Fxr-Shp-Lrh-1 signaling. In conclusion, female mice are resistant to CLOF-mediated effects on BA metabolism observed in males, which could be attributed to PPARα-mediated suppression in females on genes involved in BA synthesis and transport.
Keywords: biliary excretion, PPARα, bile acids, gender difference
Introduction
Bile acids (BAs) are metabolites of cholesterol and are well known for their detergent-like role in maintaining cholesterol soluble in bile and facilitating intestinal absorption of lipids and lipid-soluble vitamins (Chiang, 2009; Russell and Setchell, 1992). However, the detergent properties also render BA cytotoxicity at high concentrations (Pauli-Magnus and Meier, 2005). During the last decade, the discovery of two BA receptors, namely farnesoid X receptor (Fxr) and Takeda-G-protein-coupled receptor 5 (Tgr5), confers BAs a signaling role in regulating lipid and glucose metabolism, as well as mitochondrial activity and energy expenditure (Parks et al., 1999; Watanabe et al., 2006). Both the detergent (cytotoxic) properties and the signaling function of BAs require tight regulation of BA synthesis, transport and metabolism. Not surprisingly, alterations in BAs have been noted in various liver and intestinal diseases, such as cholestasis, steatosis, dyslipidemia, diabetes, inflammatory bowel diseases, and cardiovascular diseases (Hofmann, 2009a; Hofmann, 2009b).
Peroxisome proliferator-activated receptors (PPARs) are a family of nuclear hormone receptors that regulate genes involved in various metabolic processes (Gross et al., 2016). The PPAR family is comprised of three isoforms (α, β/δ and γ), among which PPARα is the predominant isoform in the liver. PPARα is known to have a broad female-specific repressive effect on hepatic expression of genes involved in steroid metabolism and immunity (Leuenberger et al., 2009). Cyp7b1, a BA-synthetic enzyme, is 4.1-fold higher in PPARα-null female livers than WT females (Leuenberger et al., 2009). However, whether lack of PPARα in female mice alters BA metabolism as well as the expression of other BA-related genes remains largely unknown.
Fibrates are a class of drugs that have been used for decades in the treatment of hypertriglyceridemia and combined hyperlipidemia (Mandard et al., 2004). Long-term use of fibrates has been associated with reduced BA synthesis, likely due to the decreased CYP7A1, the rate-limiting enzyme of BA biosynthesis (Bertolotti et al., 1995; Stahlberg et al., 1995). Fibrates reduced total BAs in serum of men, but not in women, suggesting a gender-related response (Trottier et al., 2011). Gender differences have also been reported in the responsiveness of rat liver to fibrates, with females less susceptible to peroxisome proliferation than male rats (Sundseth and Waxman, 1992). However, to our knowledge, it remains unknown whether PPARα mediates the gender-divergent effect of fibrates on BA homeostasis.
Although a variety of studies have investigated the effects of fibrates and PPARα activation on BA synthesis and transport, most studies were conducted in male mice. It remains largely unknown about the effects of PPARα activation on BA homeostasis in female mice. For this reason, the present study aimed to systematically decipher the female-related effects of PPARα activation or deficiency on BA homeostasis by using both wild-type (WT) and PPARα-null female mice treated with CLOF for four days. BA concentrations in liver, bile and serum, as well as the major pathways of BA regulation, synthesis, and transport were investigated.
Materials and Methods
Chemicals and Reagents
Taurocholic acid (TCA), tauro-β-muricholic acid (TMCA), tauromurideoxycholic acid (TMDCA), taurochenodeoxycholic acid (TCDCA), taurodeoxycholic acid (TDCA), taurolithocholic acid (TLCA), cholic acid (CA), α-muricholic acid (αMCA), β-muricholic acid (βMCA), ω-muricholic acid (ωMCA), murideoxycholic acid (MDCA), ursodeoxycholic acid (UDCA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), and lithocholic acid (LCA) were purchased from either Steraloids, Inc. (Newport, RI) or Sigma-Aldrich (St Louis, MO). Clofibric acid (CLOF) was purchased from Sigma-Aldrich Co. (St. Louis, MO). Chloroform, agarose, and ethidium bromide were purchased from AMRESCO Inc. (Solon, OH). RNA-Bee was purchased from TelTest Inc. (Friendswood, TX). All other chemicals, unless indicated, were purchased from Sigma-Aldrich Co. (St. Louis, MO).
Animals and Treatment
Adult female C57BL/6 mice were purchased from Charles River Laboratories Inc. (Wilmington, MA). PPARα-null mice were originally engineered by the laboratory of Dr. Frank J. Gonzalez (Lee et al., 1995) and backcrossed into the C57BL/6 background. All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Kansas Medical Center. All mice were housed in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited facility with a 12:12 hr light:dark cycle, and provided chow (Teklad Rodent Diet #8604, Harlan Teklad, Madison, WI) and water ad libitum. Wild-type (WT) and PPARα-null female mice (n=6–8 per group) were administered CLOF (300 mg/kg in corn oil, 5 ml/kg, i.p.) daily for 4 consecutive days. The vehicle control mice were treated with corn oil (5 ml/kg). The CLOF regimen used in the present study has been shown to activate PPARα and induce PPARα target genes in mouse liver (Petrick and Klaassen, 2007). All other groups investigated in the present study were given the corresponding corn oil vehicle (n>5 per group).
Sample Collection
At 24 hours after the fourth dose of CLOF, mice were anesthetized using ketamine (100 mg/kg, i.p.)/midazolam (5 mg/kg, i.p.) and the common bile duct of each mouse was cannulated with a 30-gauge needle attached to PE-10 tubing and collected for 40 min in pre-weighed tubes on ice. The volumes of bile were determined gravimetrically, using 1.0 for specific gravity.
Another set of mice (n=6–8/group) was anesthetized with 50 mg/kg pentobarbital at 24 hr after the last dose, blood was collected from the retro-orbital sinus of the mice, and serum was obtained by centrifuging blood at 6,000 g for 15 min. The livers with gallbladders removed, as well as ileum were harvested from the same animals, washed, snap-frozen in liquid nitrogen, and stored at −80°C.
BA Quantification
Sample extraction and quantification of individual BAs were performed according to an UPLC-MS/MS method described previously (Alnouti et al., 2008; Zhang and Klaassen, 2010).
Multiplex Suspension Array
Total RNA was isolated using RNA-Bee reagent (Tel-Test Inc., Friendswood, TX) and concentrations were quantified spectrophotometrically at 260 nm. Integrity and quality of RNA samples were evaluated by formaldehyde-agarose gel electrophoresis. Mutiplex suspension arrays (Panomics/Affymetrix, Fremont, CA) were performed to quantify the mRNA expression. Individual gene accession numbers can be accessed at www.panomics.com (sets #21330 and #21383). Samples were analyzed using a Bio-Plex 200 System Array reader with Luminex 100 xMAP technology, and the data were acquired using Bio-Plex Data Manager version 5.0 (Bio-Rad, Hercules, CA). Assays were performed according to the manufacturer’s protocol. The mRNA data were normalized to Rpl13a mRNA and presented as fold change relative to the WT control group.
Statistical Analysis
Data were analyzed with a two-way analysis of variance, followed by Duncan’s post-hoc test. Statistical significance was set at p<0.05. Bars represent mean ± S.E. (n=6–8 per group).
Results
Effects of PPARα activation and deficiency on liver weight and bile flow in female mice
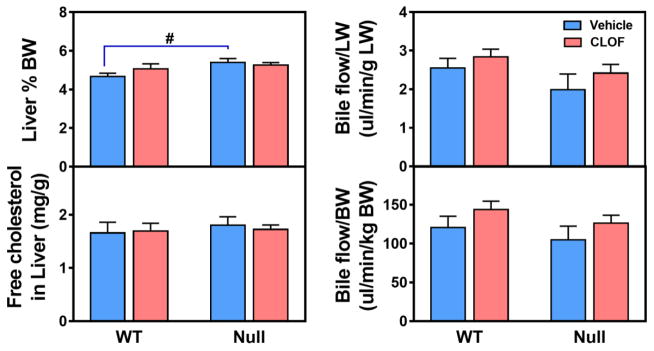
PPARα has been proposed to play a role in regulating liver weight and bile flow (Kutz et al., 1979). Our previous study showed that CLOF could activate PPARα in both male and female WT mice, but not in PPARα-null mice (Aleksunes and Klaassen, 2012). Cyp4a14, a well-established PPARα target, is female predominant, due to a combined effect of male-pattern growth hormone and androgens (Zhang and Klaassen, 2013). Cyp4a14 in female PPARα-null mice was decreased to the similar level of male WT mice, suggesting a critical role of PPARα in maintaining Cyp4a14 in female mice. Due to the different basal levels, Cyp4a14 was induced about 200-fold in male WT mice and 3-fold in female WT mice. Nevertheless, male and female WT mice had a similar level of Cyp4a14 mRNA in livers after CLOF treatment (Aleksunes and Klaassen, 2012). In our study in WT male mice, CLOF was shown to increase both liver weight (10%↑) and bile flow (about 20%↑) (Zhang et al., 2017). In contrast, the current data showed that CLOF had no effect on liver weight or bile flow in WT female mice (Figure 1). Compared to WT female mice, PPARα-null female mice had enlarged liver weight (17%↑). CLOF and PPARα have been shown to regulate cholesterol homeostasis (Grundy et al., 1972). The current data revealed that free cholesterol was not altered by either CLOF or PPARα deficiency in female mice (Figure 1 and Supplemental Table 1). Therefore, in contrast to male mice, CLOF did not influence the liver weight or bile flow in female mice, whereas PPARα deficiency in female mice resulted in an increase in liver weight.
Figure 1. Effects of PPARα activation and deficiency on liver weight, hepatic free cholesterol, and bile flow.
PPARα activation was achieved by a four-day treatment of CLOF (300 mg/kg, i.p.). The other groups of mice were administered with vehicle control (corn oil, i.p.). Bile was collected for 40 minutes from age-matched WT and PPARα-null female mice (n>5 per treatment group) for 40 minutes. Liver weight (LW) is expressed as a percent of bodyweight (BW). Hepatic free cholesterol was assayed by using a WAKO free cholesterol kit. Bile flow rates were normalized to liver weight (LW) and body weight (BW), respectively. Data represent means ± S.E.M. *p<0.05 versus vehicle treatment group; #p<0.05 versus WT group.
Effects of PPARα activation and deficiency on liver BA concentrations in female mice
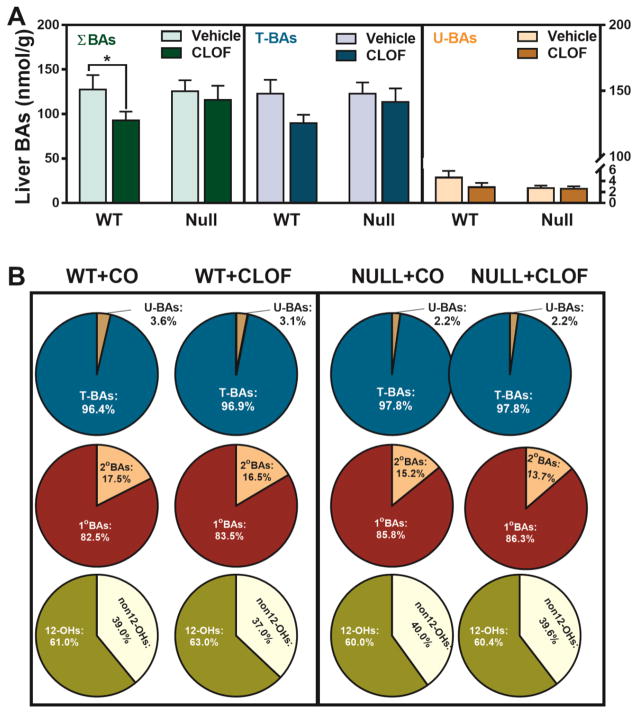
CLOF significantly decreased CDCA (63%↓), Tα+βMCA (33%↓) and TUDCA (46%↓) in WT female mice (Figure 2). As a result, CLOF decreased the concentration of total BAs (27%↓) in livers of WT female mice (Figure 3A). The proportions of conjugated BAs, primary BAs, and 12-OH BAs are shown in Figure 3B. CLOF given to WT female mice had little effect on the proportions of major BA categories.
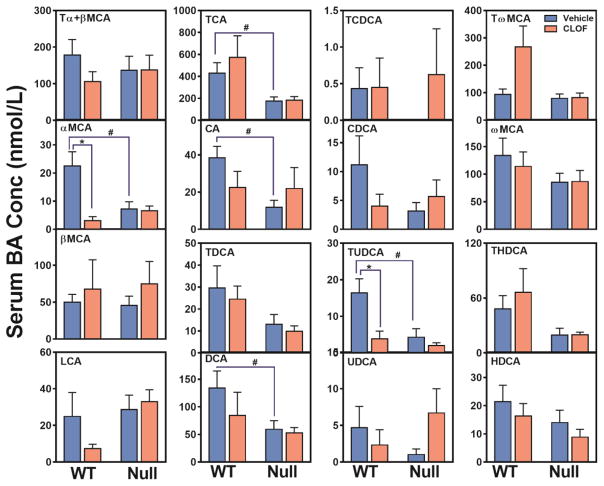
Figure 2. Effects of PPARα activation and deficiency on liver BA concentrations.
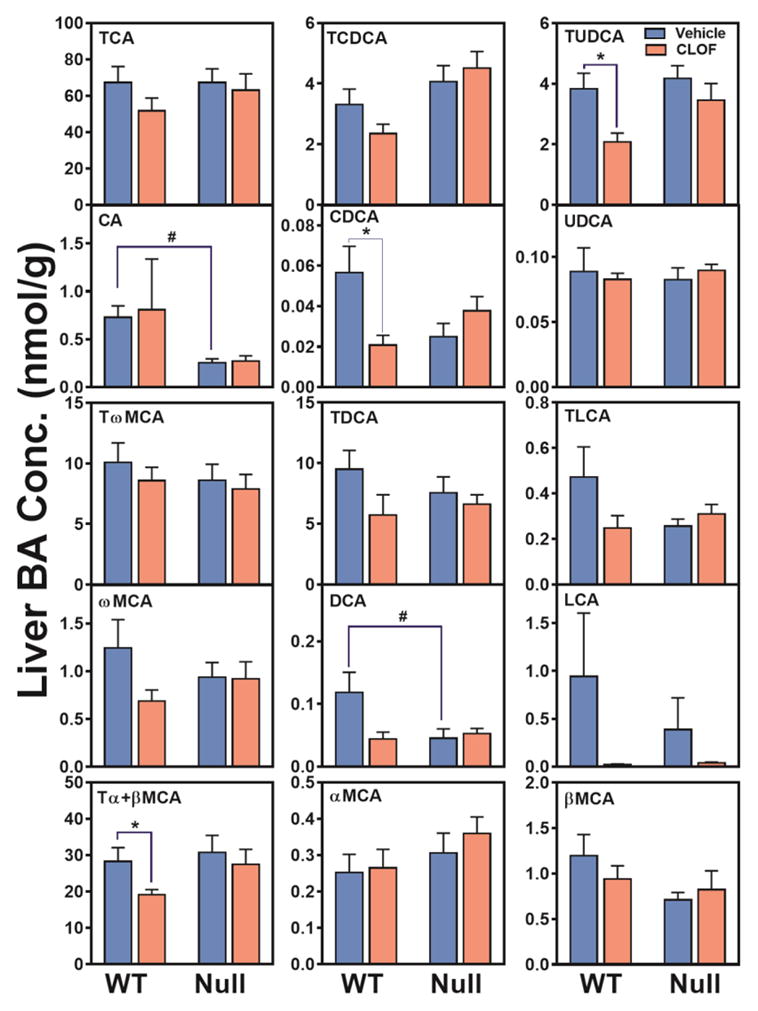
Concentrations of individual BAs in liver were quantified by UPLC-MS/MS. Data represent means ± S.E.M. (n>5 per group). *p<0.05 versus vehicle treatment group; #p<0.05 versus WT group.
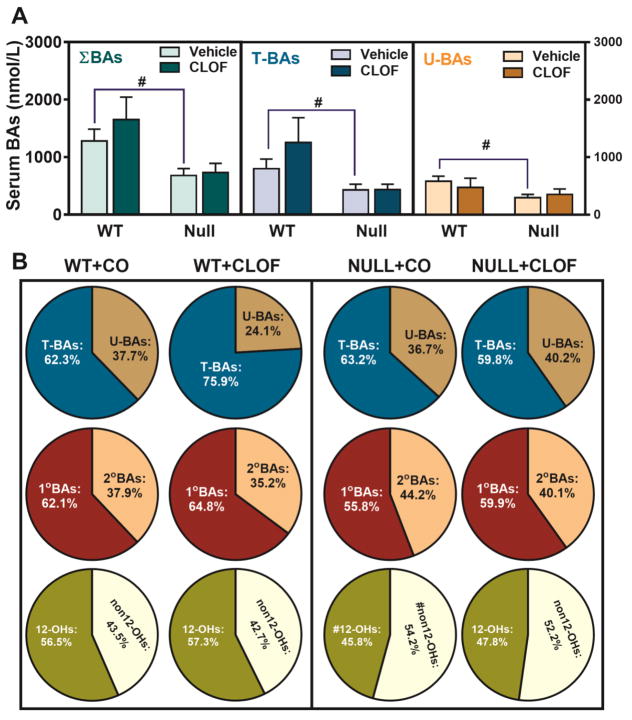
Figure 3. Effects of PPARα activation and deficiency on liver total BAs and BA composition.
(A) Liver total BAs, total conjugated BAs, and total unconjugated BAs were calculated by summation of corresponding BAs. ΣBAs represent the summation of total BAs. T-BAs are total taurine-conjugated BAs. U-BAs are total unconjugated BAs. (B) The composition of major BA categories in the liver was calculated as the percentage of total BAs. Data represent means ± S.E.M. (n>5 per group). *p<0.05 versus vehicle treatment group; #p<0.05 versus WT group.
Compared to WT female mice, the livers of PPARα-null female mice had similar levels of individual BAs, except that CA and DCA was significantly decreased (about 60%↓) (Figure 2). Although the total BAs were similar between WT and PPARα-null female mice, the proportion of primary BAs tended to be higher in PPARα-null female mice. In contrast to WT female mice, CLOF had little effect on BA concentrations or the proportions of major BA categories in the liver of PPARα-null female mice. In conclusion, CLOF given to WT female mice decreased total BAs in the liver without altering the proportions of BA categories, whereas the overall effect of PPARα deficiency in female mice was minimal on the concentrations of BAs in the liver.
Effects of PPARα activation and deficiency on biliary excretion of BAs in female mice
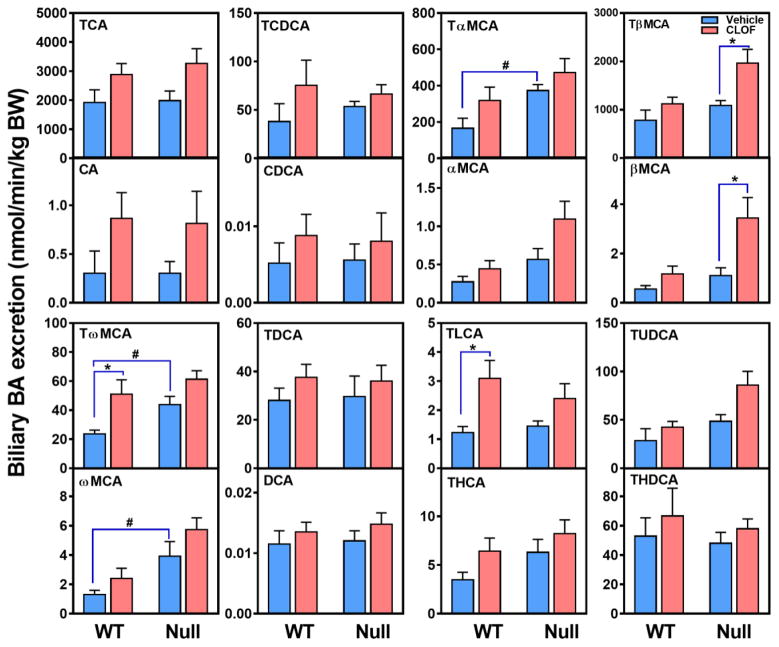
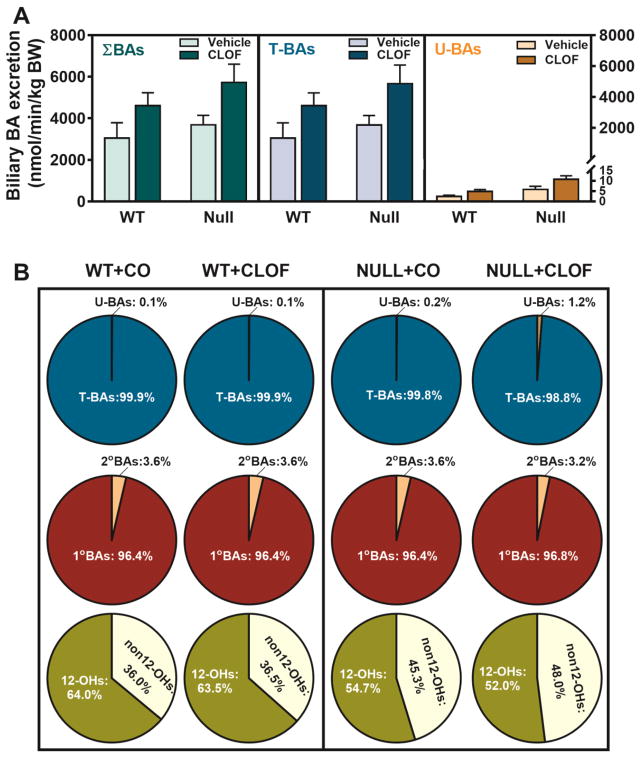
BAs are a major constituent of bile, and also a key factor that determines bile flow. Although CLOF tended to increase the biliary excretion of most BAs, only TLCA (151%↑) and TωMCA (116%↑) in WT female mice were significantly increased (Figure 4). Consequently, CLOF tended to increase, but not significantly, the biliary excretion of total BAs and total conjugated BAs in WT female mice (Figure 5A). Furthermore, CLOF had little effect on the proportions of major BA categories in the bile of WT female mice (Figure 5B).
Figure 4. Effects of PPARα activation and deficiency on the biliary excretion of BAs.
Individual BAs in the bile were quantified by UPLC-MS/MS, and the biliary excretion rate was normalized to body weight. Data represent means ± S.E.M. *p<0.05 versus vehicle treatment group; #p<0.05 versus WT group.
Figure 5. Effects of PPARα activation and deficiency on the biliary excretion of total BAs and BA composition.
(A) Biliary excretion of total BAs, total conjugated BAs, and total unconjugated BAs were calculated by summation of corresponding BAs. ΣBAs represent the summation of total BAs. T-BAs are total taurine-conjugated BAs. U-BAs are total unconjugated BAs. (B) The composition of major BA categories in the liver was calculated as the percentage of total BAs. Data represent means ± S.E.M. (n>5 per group). *p<0.05 versus vehicle treatment group; #p<0.05 versus WT group.
Compared with WT female mice, PPARα-null female mice had higher biliary excretion of TαMCA (126%↑), TωMCA (86%↑), and ωMCA (200%↑) (Figure 4). PPARα deficiency in female mice had little effect on the biliary excretion of total BAs, but markedly decreased the ratio of CA-derived BAs (12-OHs) to CDCA-derived BAs (non12-OHs) (Figure 5B). Finally, the current data showed that CLOF given to PPARα-null female mice significantly increased the biliary excretion of TβMCA (81%↑) and βMCA (215%↑) (Figure 4). As a result, CLOF tended to increase, but not significantly, the biliary excretion of total BAs and total conjugated BAs in PPARα-null female mice. To summarize, CLOF tended to increase the biliary excretion of total BAs without altering the BA hydrophobic profile in both WT and PPARα-null female mice, whereas lack of PPARα in female mice resulted in a BA composition with less 12-OH BAs in the bile.
Effects of PPARα activation and deficiency on serum BA concentrations in female mice
In WT female mice, CLOF resulted in a marked decrease in αMCA (86%↓) and TUDCA (77%↓) (Figure 6). Nevertheless, due to a non-significant increase of other BAs such as TCA and TωMCA, CLOF did not alter total BAs, total conjugated BAs, or total unconjugated BAs in serum of WT female mice (Figure 7A). The relative proportion of the major BA categories in serum is shown in Figure 7B. In serum of WT female mice, CLOF tended to increase the percentages of conjugated BAs from 62.3% to 75.9%, but had little effect on primary BAs and 12-OH BAs, which were about 62.1% and 56.5% of the total BAs, respectively.
Figure 6. Effects of PPARα activation and deficiency on serum BA concentrations.
Concentrations of individual BAs in serum were quantified by UPLC-MS/MS. Data represent means ± S.E.M. (n>5 per group). *p<0.05 versus vehicle treatment group; #p<0.05 versus WT group.
Figure 7. Effects of PPARα activation and deficiency on serum total BAs and BA composition.
(A) Serum total BAs, total conjugated BAs, and total unconjugated BAs were calculated by summation of corresponding BAs. ΣBAs represent the summation of total BAs. T-BAs are total taurine-conjugated BAs. U-BAs are total unconjugated BAs. (B) The composition of major BA categories was calculated as the percentage of total BAs. Data represent means ± S.E.M. (n>5 per group). *p<0.05 versus vehicle treatment group; #p<0.05 versus WT group.
Compared to WT female mice, PPARα-null female mice had lower TCA (59%↓), CA (87%↓), DCA (56%↓), αMCA (68%↓), and TUDCA (74%↓) in serum, resulting in a marked decrease (47%↓) in serum total BAs, total conjugated BAs (46%), and total unconjugated BAs (48%) (Figure 6 and 7A). Furthermore, lack of PPARα in female mice was shown to decrease the percentages of both primary BAs (62.1%→55.8%) and 12-OH BAs (56.5%→45.8%) (Figure 7B). Unlike in WT female mice, CLOF did not produce statistically significant alterations in serum BAs in PPARα-null female mice. Interestingly, CLOF tended to decrease the percentage of conjugated BAs (63.2%→59.8%) in serum of PPARα-null female mice, while increasing the percentage of primary BAs (55.8%→59.9%). To summarize, the overall effect of CLOF on BAs in serum of WT female mice was relatively small, whereas PPARα deficiency in female mice decreased total BAs in serum, characterized by decreased primary and 12-OH BAs.
Effects of PPARα activation and deficiency on mRNAs of BA-synthetic enzymes and transporters in liver
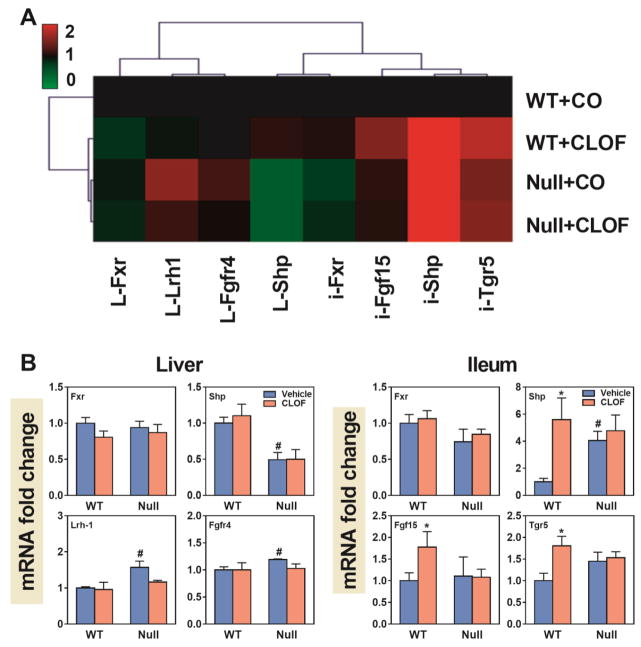
The relative changes in mRNA expression of genes involved in liver BA biosynthesis and transport are shown in a heatmap (Figure 8A). WT female mice showed a gene expression pattern different than that of PPARα-null female mice. CLOF produced little alterations in gene expression in either WT or PPARα-null female mice.
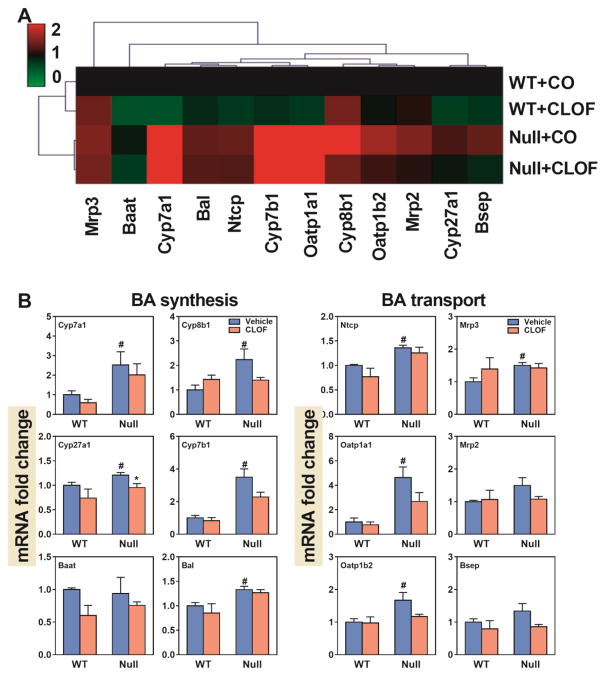
Figure 8. Effects of PPARα activation and deficiency on mRNA expression of BA-synthetic enzymes and transporters in the liver.
(A) Heat map of genes involved in BA synthesis and transport in the liver. The colors on the heatmap corresponded to the relative changes in average gene expression. Red represents up-regulation while green represents down-regulation. (2) QuantiGene Plex 2.0 Assay was performed to measure the gene expression of BA-synthetic enzymes (Cyp7a1, Cyp8b1, Cyp27a1, Cyp7b1, Bal, and Baat) and BA transporters (Ntcp, Oatp1a1, Oatp1b2, Mrp3, Mrp2 and Bsep). Relative mRNA levels were calculated with vehicle controls set as 100%. Data represent means ± S.E.M. *p<0.05 versus vehicle treatment group; #p<0.05 versus WT group.
As shown in Figure 8B, CLOF had little effect on BA synthetic enzymes in WT female mice, except for a tendency of decrease in Cyp7a1 (40%↓), the rate-limiting enzyme of BA biosynthesis in the liver. BAs require transporters for their enterohepatic circulation. As shown in Figure 8B, CLOF had little effect on either hepatic uptake (Ntcp and Oatp1b2) or efflux (Mrp2, Mrp3 and Bsep) BA transporters in WT female mice.
Compared to WT female mice, PPARα-null female mice had higher expression of BA synthetic enzymes, including Cyp7a1 (3-fold↑), Cyp7b1 (2.5 fold↑), Cyp27a1 (20%↑), Cyp8b1 (120%↑) and Bal (33%↑). Furthermore, PPARα-null female mice had higher expression of BA transporters, such as Ntcp (35%↑), Oatp1a1 (360%↑), Oatp1b2 (67%↑), and Mrp3 (50%↑). In PPARα-null female mice, CLOF significantly decreased Cyp27a1 (21%↓), and had little effect on liver BA transporters. In summary, the overall effect of CLOF on the hepatic expression of genes involved in BA synthesis and transport was minimal in WT female mice, whereas PPARα deficiency in female mice increased both BA-synthetic enzymes and transporters.
Effects of PPARα activation and deficiency on mRNAs of genes important for BA and cholesterol transport in ileum
Though PPARα deficiency produced both increased and decreased effects on intestine transporters, the gene expression pattern in WT female mice was similar to that of PPARα-null mice (Figure 9A). CLOF produced a distinct gene expression pattern in the intestine of both WT and PPARα-null female mice.
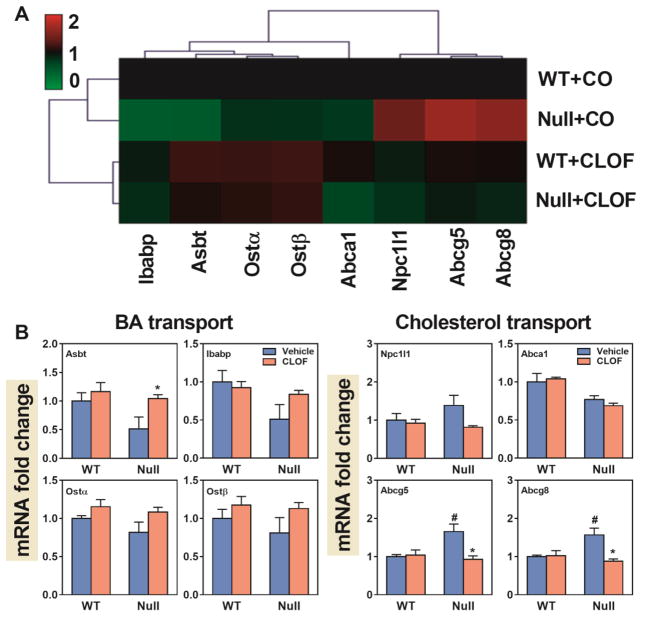
Figure 9. Effects of PPARα activation and deficiency on mRNA expression of BA and cholesterol transporters in the ileum.
(A) Heat map of genes involved in BA and cholesterol transport in the ileum. The colors on the heatmap corresponded to the relative changes in average gene expression. Red represents up-regulation while green represents down-regulation. (2) QuantiGene Plex 2.0 Assay was performed to measure the gene expression of BA transporters (Asbt, Ibabp, Ostα, and Ostβ) and cholesterol transporters (Npc1l1, Abca1, Abcg5, and Abcg8). Relative mRNA levels were calculated with vehicle controls set as 100%. Data represent means ± S.E.M. *p<0.05 versus vehicle treatment group; #p<0.05 versus WT group.
In WT female mice, CLOF had little effect on BA or cholesterol transporters in the ileum (Figure 9B). Compared to WT female mice, PPARα-null female mice had higher cholesterol transporters, namely Abcg5 (65%↑) and Abcg8 (57%↑). Interestingly, CLOF decreased Abcg5 and Abcg8 in PPARα-null female mice back to a similar level as in WT females. To summarize, CLOF had little effect on BA and cholesterol transporters in the ileum of WT female mice, whereas PPARα-null mice markedly increased cholesterol efflux transporters (Abcg5 and Abcg8).
Effects of PPARα activation and deficiency on mRNAs of genes involved in BA signaling
Fxr is a BA receptor that plays a critical role in maintaining BA homeostasis. In the liver, activation of Fxr induces the expression of Shp, which inhibits Lrh-1-mediated transcription of Cyp7a1 (Goodwin et al., 2000). In the intestine, Fxr activation induces Fgf15, which binds to its receptor (Fgfr4) in the liver to suppress Cyp7a1 (Inagaki et al., 2005). Tgr5 is also a BA receptor and its activation in the ileum has a critical role in regulating energy homeostasis (Duboc et al., 2014). The relative changes in the mRNA expression of genes involved in BA signaling are shown in Figure 10A. WT female mice showed a gene expression pattern distinct from that of the other three groups, suggesting that BA signaling in WT female mice was altered by both fibrate and PPARα deficiency. In contrast, CLOF-treated PPARα-null female mice had a similar gene expression pattern as that of untreated PPARα-null females.
Figure 10. Effects of PPARα activation and deficiency on mRNA expression of genes involved in BA signaling.
(A) Heat map of genes involved in BA signaling pathways in liver and ileum. The colors on the heatmap corresponded to the relative changes in average gene expression. Red represents up-regulation while green represents down-regulation. (2) QuantiGene Plex 2.0 Assay was performed to measure the gene expression of Fxr, Shp, Lrh-1 and Fgfr4 in the liver and Fxr, Shp, Fgf15, and Tgr5 in the ileum. Relative mRNA levels were calculated with vehicle controls set as 100%. Data represent means ± S.E.M. *p<0.05 versus vehicle treatment group; #p<0.05 versus WT group.
As shown in Figure 10B, CLOF markedly increased Shp (460%↑), Fgf15 (77%↑) and Tgr5 (81%) in the ileum of WT female mice. PPARα deficiency produced a decrease in Shp (51%↓) as well as an increase in Lrh-1 (57%↑) and Fgfr4 (19%↑) in the liver. Additionally, PPARα deficiency markedly increased Shp (300%↑) in the ileum of female mice. Unlike in WT female mice, CLOF had little effect on BA signaling in PPARα-null female mice. To summarize, CLOF appears to stimulate BA signaling in the ileum of WT female mice, whereas PPARα deficiency in female mice tends to activate Fxr-Shp-Lrh-1 signaling in the liver.
Discussion
Previous studies have shown that activation of PPARα alters BA homeostasis by regulating genes involved in BA synthesis, conjugation, and transport (Li and Chiang, 2009). However, a comparative analysis between WT and PPARα-null mice has demonstrated that PPARα has a female-specific repressive effect on the expression of a broad range of genes in the liver (Leuenberger et al., 2009). In particular, Cyp7b1, an enzyme involved in BA synthesis, was 4.1-fold higher in livers of PPARα-null female mice than in WT females. This prompted us to hypothesize that PPARα activation might have gender-divergent effects on BA homeostasis in mice.
Despite its significant cholesterol lowering effect in hyperlipidemia patients, the clinical use of CLOF has been dampened by the increased risk of gallstone formation (Bateson et al., 1978). Nevertheless, CLOF has been widely used in rodents to activate PPARα (Moffit et al., 2006). Liver enlargement and peroxisome proliferation are two prominent markers in rodents treated with CLOF and other fibrates (Hess et al., 1965). Our previous study showed that CLOF significantly increased liver weight in male mice (Zhang et al., 2017). In contrast, the current data demonstrate that CLOF did not change liver weights in female mice. Thus, female mice were less responsive to CLOF-induced liver enlargement than males. This was consistent with a previous report that female rats are less susceptible to CLOF-induced peroxisome proliferation than males (Sundseth and Waxman, 1992). The gender-divergent effect of CLOF on liver enlargement is likely due to the intrinsic female-specific suppressive effect of PPARα on hepatic genes, in particular cytochrome P450s (Leuenberger et al., 2009). This is supported by the observation that liver weights of PPARα-null female mice were increased about 10% compared to age-matched WT females.
The inductive effect of CLOF on gallstone formation is primarily a result of increased cholesterol saturation in bile (Bateson et al., 1978). The relationship of bile flow and biliary BAs to cholesterol saturation in bile has been studied extensively. Individual BAs have distinct hydrophobicity and critical micelle concentrations, which make both the concentration and composition of BAs important determinants for cholesterol saturation (Hofmann and Roda, 1984). In our previous study, CLOF significantly increased bile flow and biliary BA excretion in WT male mice (Zhang et al., 2017). In contrast, the current study showed that CLOF did not alter bile flow in WT female mice. Although CLOF significantly increased the biliary excretion of two CDCA-derived BAs (TLCA and TωMCA) in WT female mice, the ratio of CA-derived BAs to CDCA-derived BAs, which determines the overall BA-mediated cholesterol solubility in bile, remained unchanged in CLOF-treated females. Interestingly, compared to WT females, PPARα-null female mice exhibited a decrease in the ratio of CA-derived BAs to CDCA-derived BAs, largely due to the increased TαMCA, TωMCA, and ωMCA. CA has been reported to enhance cholesterol absorption and increase the risk of gallstone diseases (Murphy et al., 2005; Wang et al., 1999; Woollett et al., 2004). Due to the decreased composition of CA-derived BAs, the potential risk of gallstone formation is expected to be lower in PPARα-null female mice.
The regulatory effect of fibrates on BA homeostasis has been extensively reviewed, providing data to support the claim of PPARα as a pharmacological target for the treatment of cholestatic disorders (Ghonem et al., 2015; Li and Chiang, 2009). A gender-different response of fenofibrate on BA concentrations in the serum of humans was observed, with the total serum BAs markedly decreased in men while remained unchanged in women. This suggests that BA homeostasis in females are less susceptible to fibrate treatment than in males (Trottier et al., 2011). In our previous study, CLOF given to WT male mice resulted in a decrease in serum BAs. In contrast, the current study showed that CLOF had little effect on serum total BAs or the composition of major BA categories in WT female mice. Compared to WT female mice, PPARα-null female mice had a decrease in serum concentrations of most BAs, resulting in a marked decrease in total BAs in the serum. As a result, a marked decrease in the ratio of CA-derived BAs to CDCA-derived BAs was observed in PPARα-null female mice. Therefore, the current study in mice recapitulates the gender-divergent effect of fibrates on BAs in the serum of humans, and strongly suggests that a PPARα-mediated mechanism is likely involved in the poor responses of females to fibrate treatment.
Although CLOF decreased total BAs in livers of WT female mice, it caused no statistically significant alterations in the expression of genes involved in BA synthesis and transport, except that Cyp7a1 tended to decrease about 40%. Nevertheless, this may provide a possible explanation for the reduced liver BAs in CLOF-treated WT female mice. Cyp7a1 is known to be regulated by both liver Fxr-Shp and intestine Fxr-fgf15 signaling pathways. In WT female mice, CLOF had little effect on liver Fxr-Shp signaling genes, whereas in the ileum CLOF increased Shp (4.6-fold↑), Fgf15 (77%↑) and Tgr5 (81%↑). Therefore, the BA lowering effect of CLOF in livers of WT female mice is likely attributed to the CLOF-mediated activation of intestinal Fxr-Fgf15 signaling, which is associated with a tendency of decrease in Cyp7a1 expression in the liver. It should be noted that CLOF decreased the genes involved in the intestinal Fxr-Fgf15 signaling in WT male mice, which was associated with a significant increase in Cyp7a1 mRNA (Zhang et al., 2017). Thus, the present study suggests that CLOF also has a gender-specific effect on intestinal gene expression. Compared to WT female mice, a globally increased hepatic expression of BA synthetic enzymes and transporters was observed in PPARα-null female mice. However, there were no alterations in total BAs in the liver of PPARα-null female mice. Cyp7a1 was increased about 3-fold in PPARα-null female mice, which was associated with a marked increase in Lrh-1, a transcription factor that has been shown to be critical for induction of Cyp7a1 transcription (Out et al., 2011). Although there were no alterations in the concentration of total BAs in the liver, PPARα-null female mice should have an increased amount of BAs in the liver when compared to WT female mice, because the ratio of liver to body weight was increased about 17% in PPARα-null female mice compared to WT female mice (Figure 1). This is associated with the increased Cyp7a1 in PPARα-null female mice. Given our findings on the gender-different role of PPARα in regulating Cyp7a1 and other BA synthetic enzymes, both the BA pool and fecal BA excretion should be determined in a future study to further evaluate the gender-specific role of PPARα in maintaining the homeostasis of BAs.
In addition to cytochrome P450s, the current study also showed that PPARα in female mice had a suppressive effect on major liver BA transporters, in particular BA uptake transporters (Ntcp and Oatp1b2). PPARα deficiency in female mice produced a significant increase in Ntcp and Oatp1b2, which might explain why PPARα-null female mice had lower serum BAs than WT females. Interestingly, the most prominent alterations in PPARα-null female mice were Cyp7b1 and Oatp1a1, both of which show a male-predominant expression in the liver (Leuenberger et al., 2009; Zhang et al., 2012). As shown in Supplemental Figure 1, PPARα did not suppress Cyp7b1 mRNA in livers of male mice. Leuenberger et al. (2009) showed that sumoylation of the ligand-binding domain of PPARα contributes to the PPARα-mediated female specific suppression on Cyp7b1. However, the removal of PPARα suppression in female mice could not bring Cyp7b1 back to a similar level as in male mice, suggesting other mechanisms, such as STAT5 signaling and hormones, are also involved in regulating the male-predominant expression of Cyp7b1 (Davey et al., 1999). It should be noted that PPARα also suppresses Oatp1a1 mRNA in livers of male mice (Supplemental Figure 1). Furthermore, Oatp1a1 mRNA in female PPARα-null mice was increased to the same level in male wild type mice. This suggests that the regulatory mechanism for PPARα-mediated suppression of Oatp1a1 is different from that for Cyp7b1. In the ilea of female mice, PPARα deficiency had little effect on intestinal BA transporters, but markedly increased two cholesterol efflux transporters (Abcg5 and Abcg8). This might indicate that PPARα deficiency influences cholesterol concentrations in female mice, which is consistent with the previous finding that PPARα-null mice had lower plasma steroid levels than WT females (Leuenberger et al., 2009). Interestingly, the concentrations of free cholesterol in liver were not altered by either CLOF or PPARα deficiency in female mice. This is not surprising because PPARα also plays a critical role in BA synthesis and biliary excretion of cholesterol (Grundy et al., 1972). It is worth investigating the potential gender difference in PPARα-mediated regulation of cholesterol homeostasis in a future study.
In summary, WT female mice are less susceptible to CLOF-induced peroxisome proliferation and biliary BA excretion in males, as evidenced by their unchanged liver weights and biliary BA composition. CLOF had little effect on serum total BAs in WT female mice, recapitulating the effect of fibrates on serum BAs in women. Despite minimal effects on hepatic gene expression in WT female mice, CLOF decreased total BAs in their livers, likely due to the reduced Cyp7a1 caused by activation of the intestinal Fxr-Fgf15 signaling pathway. The present study also confirmed the suppressive effect of PPARα on gene expression in livers of female mice. Collectively, the present findings strongly support a female-specific role of PPARα in regulating BA homeostasis, and provide a new perspective for the use of fibrates in pharmacology and toxicology.
Supplementary Material
Highlights.
Female mice were resistant to clofibrate-induced effects on bile acid metabolism observed in males.
PPARα deficiency in female mice induced mRNAs of genes involved in bile acid synthesis and transport in the liver.
The increase of Cyp7a1 in PPARα-null female mice was associated with an increase in liver Fxr-Shp-Lrh-1 signaling.
Clofibrate had little effect on bile acid homeostasis and mRNAs of bile acid-related genes in PPARα-null mice.
Acknowledgments
Funding Information
This work was supported by the National Institutes of Health grants (R01 ES025708, R01 ES009649, and F32 DK092069) and the Children’s Mercy Startup fund for ILC.
The authors would like to thank all members of the Klaassen laboratory for technical assistance with blood and tissue collection.
Abbreviations
- 1°BAs
Primary bile acids
- 2°BAs
secondary bile acids
- 12-OH
12-hydroxylated bile acid
- Asbt
apical sodium-dependent bile acid transporter
- BA
bile acid
- Baat
bile acid CoA:amino acid N-acyltransferase
- Bal
bile acid CoA ligase
- Bsep
bile salt export pump
- CA
cholic acid
- CLOF
clofibrate
- Fxr
farnesoid X receptor
- Fgf
fibroblast growth factor
- MCA
muricholic acid
- Ntcp
Na (+)-taurocholate cotransporting polypeptide
- Npc1l1
Nieman-Picl c1-like 1
- Oatp
organic anion transporting polypeptide
- Oat
organic anion transporter
- Ost
organic solute transporter
- PPARα
peroxisome proliferator-activated receptor α
- ΣBAs
total bile acids
- Shp
small heterodimer partner
- T-BAs
taurine-conjugated bile acdis
- U-BAs
unconjugated bile acids
- UPLC-MS/MS
ultraperformance liquid chromatography-tandem mass spectrometry
Footnotes
Conflict of Interest
All authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleksunes LM, Klaassen CD. Coordinated regulation of hepatic phase I and II drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPARalpha-, and Nrf2-null mice. Drug metabolism and disposition: the biological fate of chemicals. 2012;40:1366–1379. doi: 10.1124/dmd.112.045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnouti Y, Csanaky IL, Klaassen CD. Quantitative -profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC -MS/MS. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2008;873:209–217. doi: 10.1016/j.jchromb.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson MC, Maclean D, Ross PE, Bouchier IA. Clofibrate therapy and gallstone induction. The American journal of digestive diseases. 1978;23:623–628. doi: 10.1007/BF01072597. [DOI] [PubMed] [Google Scholar]
- Bertolotti M, Concari M, Loria P, Abate N, Pinetti A, Guicciardi ME, Carulli N. Effects of different phenotypes of hyperlipoproteinemia and of treatment with fibric acid derivatives on the rates of cholesterol 7 alpha-hydroxylation in humans. Arteriosclerosis, thrombosis, and vascular biology. 1995;15:1064–1069. doi: 10.1161/01.atv.15.8.1064. [DOI] [PubMed] [Google Scholar]
- Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey HW, Wilkins RJ, Waxman DJ. STAT5 signaling in sexually dimorphic gene expression and growth patterns. American journal of human genetics. 1999;65:959–965. doi: 10.1086/302599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duboc H, Tache Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2014;46:302–312. doi: 10.1016/j.dld.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghonem NS, Assis DN, Boyer JL. Fibrates and cholestasis. Hepatology. 2015;62:635–643. doi: 10.1002/hep.27744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Molecular cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- Gross B, Pawlak M, Lefebvre P, Staels B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nature reviews Endocrinology. 2016 doi: 10.1038/nrendo.2016.135. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Ahrens EH, Jr, Salen G, Schreibman PH, Nestel PJ. Mechanisms of action of clofibrate on cholesterol metabolism in patients with hyperlipidemia. Journal of lipid research. 1972;13:531–551. [PubMed] [Google Scholar]
- Hess R, Staubli W, Riess W. Nature of the hepatomegalic effect produced by ethyl-chlorophenoxy-isobutyrate in the rat. Nature. 1965;208:856–858. doi: 10.1038/208856a0. [DOI] [PubMed] [Google Scholar]
- Hofmann AF. Bile acids: trying to understand their chemistry and biology with the hope of helping patients. Hepatology. 2009a;49:1403–1418. doi: 10.1002/hep.22789. [DOI] [PubMed] [Google Scholar]
- Hofmann AF. The enterohepatic circulation of bile acids in mammals: form and functions. Frontiers in bioscience. 2009b;14:2584–2598. doi: 10.2741/3399. [DOI] [PubMed] [Google Scholar]
- Hofmann AF, Roda A. Physicochemical properties of bile acids and their relationship to biological properties: an overview of the problem. Journal of lipid research. 1984;25:1477–1489. [PubMed] [Google Scholar]
- Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell metabolism. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Kutz K, Schulte A, Just C, Lindstaedt H, Reiter B. Bile formation and biliary lipid composition under the influence of clofibrate and phenobarbital pretreatment in the rat. Naunyn-Schmiedeberg’s archives of pharmacology. 1979;308:171–177. doi: 10.1007/BF00499061. [DOI] [PubMed] [Google Scholar]
- Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, Fernandez-Salguero PM, Westphal H, Gonzalez FJ. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Molecular and cellular biology. 1995;15:3012–3022. doi: 10.1128/mcb.15.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuenberger N, Pradervand S, Wahli W. Sumoylated PPARalpha mediates sex-specific gene repression and protects the liver from estrogen-induced toxicity in mice. The Journal of clinical investigation. 2009;119:3138–3148. doi: 10.1172/JCI39019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Chiang JY. Regulation of bile acid and cholesterol metabolism by PPARs. PPAR research. 2009;2009:501739. doi: 10.1155/2009/501739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandard S, Muller M, Kersten S. Peroxisome proliferator-activated receptor alpha target genes. Cellular and molecular life sciences: CMLS. 2004;61:393–416. doi: 10.1007/s00018-003-3216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffit JS, Aleksunes LM, Maher JM, Scheffer GL, Klaassen CD, Manautou JE. Induction of hepatic transporters multidrug resistance-associated proteins (Mrp) 3 and 4 by clofibrate is regulated by peroxisome proliferator-activated receptor alpha. The Journal of pharmacology and experimental therapeutics. 2006;317:537–545. doi: 10.1124/jpet.105.093765. [DOI] [PubMed] [Google Scholar]
- Murphy C, Parini P, Wang J, Bjorkhem I, Eggertsen G, Gafvels M. Cholic acid as key regulator of cholesterol synthesis, intestinal absorption and hepatic storage in mice. Bba-Mol Cell Biol L. 2005;1735:167–175. doi: 10.1016/j.bbalip.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Out C, Hageman J, Bloks VW, Gerrits H, Sollewijn Gelpke MD, Bos T, Havinga R, Smit MJ, Kuipers F, Groen AK. Liver receptor homolog-1 is critical for adequate up-regulation of Cyp7a1 gene transcription and bile salt synthesis during bile salt sequestration. Hepatology. 2011;53:2075–2085. doi: 10.1002/hep.24286. [DOI] [PubMed] [Google Scholar]
- Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- Pauli-Magnus C, Meier PJ. Hepatocellular transporters and cholestasis. Journal of clinical gastroenterology. 2005;39:S103–110. doi: 10.1097/01.mcg.0000155550.29643.7b. [DOI] [PubMed] [Google Scholar]
- Petrick JS, Klaassen CD. Importance of hepatic induction of constitutive androstane receptor and other transcription factors that regulate xenobiotic metabolism and transport. Drug metabolism and disposition: the biological fate of chemicals. 2007;35:1806–1815. doi: 10.1124/dmd.107.015974. [DOI] [PubMed] [Google Scholar]
- Russell DW, Setchell KD. Bile acid biosynthesis. Biochemistry. 1992;31:4737–4749. doi: 10.1021/bi00135a001. [DOI] [PubMed] [Google Scholar]
- Stahlberg D, Reihner E, Rudling M, Berglund L, Einarsson K, Angelin B. Influence of bezafibrate on hepatic cholesterol metabolism in gallstone patients: reduced activity of cholesterol 7 alpha-hydroxylase. Hepatology. 1995;21:1025–1030. doi: 10.1002/hep.1840210421. [DOI] [PubMed] [Google Scholar]
- Sundseth SS, Waxman DJ. Sex-dependent expression and clofibrate inducibility of cytochrome P450 4A fatty acid omega-hydroxylases. Male specificity of liver and kidney CYP4A2 mRNA and tissue -specific regulation by growth hormone and testosterone. The Journal of biological chemistry. 1992;267:3915–3921. [PubMed] [Google Scholar]
- Trottier J, Caron P, Straka RJ, Barbier O. Profile of serum bile acids in noncholestatic volunteers: gender-related differences in response to fenofibrate. Clinical pharmacology and therapeutics. 2011;90:279–286. doi: 10.1038/clpt.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DQ, Lammert F, Cohen DE, Paigen B, Carey MC. Cholic acid aids absorption, biliary secretion, and phase transitions of cholesterol in murine cholelithogenesis. The American journal of physiology. 1999;276:G751–760. doi: 10.1152/ajpgi.1999.276.3.G751. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- Woollett LA, Buckley DD, Yao L, Jones PJ, Granholm NA, Tolley EA, Tso P, Heubi JE. Cholic acid supplementation enhances cholesterol absorption in humans. Gastroenterology. 2004;126:724–731. doi: 10.1053/j.gastro.2003.11.058. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Klaassen CD. Effects of feeding bile acids and a bile acid sequestrant on hepatic bile acid composition in mice. Journal of lipid research. 2010;51:3230–3242. doi: 10.1194/jlr.M007641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Klaassen CD. Hormonal regulation of Cyp4a isoforms in mouse liver and kidney. Xenobiotica; the fate of foreign compounds in biological systems. 2013;43:1055–1063. doi: 10.3109/00498254.2013.797622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lickteig AJ, Csanaky IL, Klaassen CD. Clofibrate decreases bile acids in livers of male mice by increasing biliary bile acid excretion in a PPARalpha-dependent manner. Toxicological sciences: an official journal of the Society of Toxicology. 2017 doi: 10.1093/toxsci/kfx191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Limaye PB, Lehman-McKeeman LD, Klaassen CD. Dysfunction of organic anion transporting polypeptide 1a1 alters intestinal bacteria and bile acid metabolism in mice. PloS one. 2012;7:e34522. doi: 10.1371/journal.pone.0034522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.