Abstract
Background
A number of genetic loci are associated with risk for Parkinson disease (PD) based on genome-wide association studies; however, the relationship between genetic variants and nigrostriatal degeneration, which is the structural correlate of Parkinsonism, has not been reported.
Objectives
We quantified nigrostriatal dopaminergic integrity with image analysis of putaminal tyrosine hydroxylase immunoreactivity in 492 brains with Lewy body disease and used this pathologic endophenotype to explore possible association with PD genetic variants.
Methods
The study cases had Lewy-related pathology and variable degrees of nigrostriatal degeneration. They were assigned to clinical subgroups according to their predominant clinical syndrome – Parkinsonism-predominant, Parkinsonism+dementia, and dementia-predominant. In addition to putaminal tyrosine hydroxylase immunoreactivity, semiquantitative scoring was used to assess substantia nigra neuronal loss. Twenty-nine PD genetic risk variants were genotyped on each case.
Results
Compared to controls, tyrosine hydroxylase immunoreactivity was reduced in Lewy body cases in dorsolateral (79%) and ventromedial (57%) putamen. The dorsolateral region was better preserved in dementia-predominant cases than in cases with Parkinsonism. Dorsolateral putaminal tyrosine hydroxylase immunoreactivity correlated with neuronal loss in the ventrolateral substantia nigra. Genetic analyses showed no significant association of PD risk variants with putaminal tyrosine hydroxylase immunoreactivity.
Conclusions
The results confirm regional differences in putaminal dopaminergic degeneration, and vulnerability of nigrostriatal pathway in Lewy body disorders with Parkinsonism. The lack of association with PD genetic risk variants suggests that they may not be associated with quantitative endophenotypes of nigrostriatal degeneration, but more likely related to risk of disease per se.
Keywords: Parkinson genetic risk variants, image analysis, Lewy body disease, putamen, tyrosine hydroxylase
Introduction
Lewy bodies are manifestations of a neurodegenerative disease process associated with a range of clinical presentations, including Parkinsonism and dementia. The most common clinical disorders with Lewy body disease (LBD) are Parkinson's disease (PD), PD with dementia (PDD) and dementia with Lewy bodies (DLB).1 A high proportion of Alzheimer disease (AD) patients also have LBs, but their contribution to the clinical syndrome is unclear.2 The neuropathologic features of Lewy body disorders include not only LBs, but also Lewy neurites, axonal spheroids and sparse glial inclusions,3 all of which contain aggregates of α-synuclein.4, 5 Striatal dopamine depletion is a striking neurochemical feature of PD that correlates with motoric deficits.6 Striatal dopamine loss is secondary to neurodegeneration of the nigrostriatal system, in particular loss of neurons in ventrolateral cell groups of the substantia nigra,4 with variable involvement of the medial region of the substantia nigra.7 A number of methods have been used to detect dopaminergic degeneration, including immunohistochemistry for tyrosine hydroxylase (TH-ir), vesicular monoamine transporter, and dopamine transporter.8, 9 Reduction in TH-ir is sensitive and can be detected in preclinical PD,10 sometimes referred to as incidental LBD.11, 12
Kordower and colleagues recently reported regional differences in nigrostriatal dopaminergic integrity in the putamen,8 with loss of TH-ir in the dorsolateral putamen within four years of symptomatic onset of PD; however, they limited their study to PD and did not include subjects with PDD or DLB. In the present study, we performed a similar regional analysis of nigrostriatal dopaminergic integrity using TH immunohistochemistry in pathologically-confirmed LBD patients with a range of clinical phenotypes and correlate these findings with genetic risk variants associated with PD. We also correlated putaminal TH-ir with estimates of neuronal loss in the substantia nigra. The first aim of our study was to clarify regional differences of putaminal dopaminergic degeneration with respect to clinical and pathologic features in LBD. The second aim was to explore possible genetic associations between dopaminergic neurodegeneration and PD genetic risk variants identified from genome-wide association studies. We tested the hypothesis that PD genetic variants, in addition to their previously demonstrated ability to alter risk of PD,13, 14 would also correlate with pathologic endophenotypes that are highly correlated with Parkinsonism. Our rationalization for including LBD cases not only with Parkinsonism, but also those with dementia as a contributing or primary clinical syndrome was the notion that PD genetic risk loci would track with nigrostriatal degeneration regardless of clinical features, given that the risk loci were identified in patients with Parkinsonism. To this end, we explored relationships between risk loci and quantitative measures of nigrostriatal dopaminergic degeneration, which is thought to be basis for the extrapyramidal signs in PD.
Materials and methods
Study samples
All cases had Lewy body pathology and were from the brain bank for neurodegenerative disorders at Mayo Clinic in Jacksonville, Florida. Cases with mutations in SNCA or LRRK2, and the cases with infarcts or hemorrhages in midbrain or putamen were excluded. For comparison, we also studied 17 age-, sex-, brain weight- and postmortem interval-matched neuropathologic controls with minimal or no Alzheimer and no Lewy-related pathology using the same methods. Brains were acquired from autopsies after consent of the legal next- of-kin or an individual with power-of-attorney. The Mayo Clinic Institutional Review Board has determined that studies with postmortem tissue are exempt from Human Subjects regulations.
Clinical classification of cases
We classified all LBD cases into three clinical subgroups according to their predominant antemortem syndrome ascertained from medical record review: Parkinsonism-predominant, Parkinsonism+dementia, and dementia-predominant. Parkinsonism-predominant cases included PD, as well as individuals misdiagnosed with a parkinsonian disorder without significant cognitive impairment, including multiple system atrophy (MSA) and progressive supranuclear palsy (PSP). None of the cases had neuropathologic features of MSA or PSP. Individuals with clinical diagnoses associated with both cognitive and motor impairment, including corticobasal syndrome (CBS), PDD and DLB, were included in the “Parkinsonism+dementia” group. Individuals with clinical diagnoses of Alzheimer type dementia or frontotemporal dementia (FTD) were included in the “dementia-predominant” group. Cases with incidental LBD presenting only with REM sleep behavior disorder or other non-motor features without dementia or Parkinsonism were not included.
Neuropathologic assessment
The diagnostic evaluation included quantitative assessment of neurofibrillary tangles and senile plaques with thioflavin S fluorescent microscopy, with assignment of Braak neurofibrillary tangle stage and Thal amyloid phase according to previously reported methods.15-17 Sections were cut from paraffin embedded tissue at 5 μm thickness and processed for α-synuclein immunohistochemistry (NACP, rabbit polyclonal, 1:3000; Mayo Clinic antibody) with a method that gives comparable results to other α-synuclein immunohistochemistry methods.18 Lewy body subtypes (brainstem, transitional, and diffuse (Table 1)) were assigned using published guidelines.1
Table 1. Demographic and pathologic characteristics in cases by major clinical syndromes.
| Dementia-predominant (N = 217) | Parkinsonism+ dementia (N = 195) | Parkinsonism-predominant (N = 80) | P-value for overall difference | Pair-wise comparison p-value | |||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Total | D vs P+D | D vs P | P+D vs P | ||||
| Demographic data | |||||||
| Age at death | 82 (77, 87) | 76 (72, 82) | 78 (73, 83) | <0.001 | <0.001 | <0.001 | 0.22 |
| Male (%) | 105 (48%) | 129 (66%) | 56 (70%) | <0.001 | <0.001 | 0.001 | 0.63 |
| Disease duration (N = 259) | 9 (6, 12) | 10 (6, 12) | 8 (5, 13) | 0.63 | N/A | N/A | N/A |
| Pathological data | |||||||
| Brain weight (g) | 1080 (942, 1200) | 1200 (1100, 1300) | 1180 (1080, 1300) | <0.001 | <0.001 | <0.001 | 0.032 |
| Postmortem interval (h) (N = 252) | 7 (4, 14) | 6 (4, 12) | 11 (6, 20) | 0.064 | 0.21 | 0.12 | 0.058 |
| Concurrent pathological AD (%) | 163/217(75%) | 42/195 (22%) | 7/80 (9%) | <0.001 | <0.001 | <0.001 | 0.019 |
| Braak NFT stage | V (IV, VI) | IV (II, IV) | III (II, III) | <0.001 | <0.001 | <0.001 | <0.001 |
| Thal amyloid phase | 5 (4, 5) | 3 (1, 5) | 1 (0, 3) | <0.001 | <0.001 | <0.001 | <0.001 |
| BLBD/TLBD/DLBD (% DLBD) | 25/91/101 (47%) | 12/58/125 (64%) | 18/31/31 (39%) | <0.001 | 0.001 | 0.055 | <0.001 |
| Substantia nigra neuronal loss | |||||||
| Ventrolateral | 2 (1.5, 2.5) | 3 (2.5, 3) | 3 (3, 3) | < 0.001 | < 0.001 | < 0.001 | 0.079 |
| Medial | 1.5 (1, 2.5) | 2.5 (1.5, 3) | 2.5 (1, 3) | < 0.001 | < 0.001 | < 0.001 | 0.42 |
| Putaminal TH-ir (%) | |||||||
| Dorsolateral | 6.2 (3.3, 12) | 2.9 (1.7, 5.6) | 2.8 (1.7, 4.6) | < 0.001 | < 0.001 | < 0.001 | 0.62 |
| Ventromedial | 10 (5.7, 14) | 10 (5.9, 14) | 9.3 (5.7, 13) | 0.86 | N/A | N/A | N/A |
Values are given as n (%) or median (interquartile range). Postmortem delay (h = hours); LBD = Lewy body disease; LBD types – brainstem (BLBD), transitional (TLBD), diffuse (DLBD); D = dementia-predominant; P+D = Parkinsonism+dementia; P = Parkinsonism-predominant; TH-ir = tyrosine hydroxylase immunoreactivity; AD = Alzheimer's disease; NFT = neurofibrillary tangle.
The putamen was evaluated at the level of the anterior commissure from a section made from the hemi-brain in a standardized dissection planedefined by three points infundibulum, uncus and posterior margin of the anterior commissure in the third ventricle. The level chosen for evaluation of the putamen was similar to that used in the previous report on regional differences in dopaminergic degeneration in PD.8 Digital images of the putamen were parcellated into dorsolateral and ventromedial areas,19 and dopaminergic degeneration was assessed quantitatively.
Quantification of striatal dopaminergic integrity
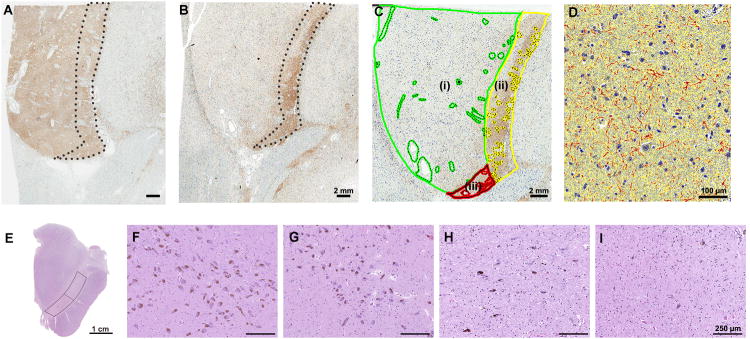
The basal ganglia section was processed for immunohistochemistry using a commercially available antibody to TH (rabbit polyclonal, 1:600; Affinity Bioreagents, Golden, CO, USA). The immunostained sections were captured by ScanScope XT (Aperio Technologies, Vista, CA, USA), and images were annotated with ImageScope (version 12.1). Regions of interest were manually edited to exclude artifacts, large blood vessels and their perivascular spaces, and large fiber bundles (Figure 1C). The putamen was subdivided into dorsolateral (Figure 1C (i)) and ventromedial regions similar to the regions studied by Kordower and colleagues.8 In order to standardize the two regions of interest, we developed operational rules. The ventromedial region consisted of two regions as follows: a medial portion was defined by a curvilinear line that started at the dorsal border of the putamen set at one-seventh of the length of the dorsal border and drawn parallel to the striatopallidal margin (Figure 1C (ii)) and a ventral wedge defined by a line between the lower one-tenth of the medial putamen and the outer margin of dorsolateral putamen (Figure 1C (iii)). Quantification of TH-ir used an algorithm that detected positive pixels (red) based upon optical density. It was expressed as a percentage, calculated as the positive pixels divided by the sum of inverse pixels (blue) and background pixels (yellow) (Figure 1D).
Figure 1.
Image analysis of TH-ir in subregions of putamen using ImageScope and semi-quantitative assessment of substantia nigra pigmented neurons. (A) and (B) show tyrosine hydroxylase immunohistochemistry of the putamen at the level of the anterior commissure. In a representative advanced LBD case (B) marked reduction of TH-ir is observed in dorsolateral putamen compared with a neurologically normal control (A). Note that TH-ir in the ventromedial putamen (dashed circle area) is preserved even in advanced LBD. (C): Green-circled area represents dorsolateral putamen (i). Ventrolateral putamen is the combined area of yellow-circled area (medial, (ii)) and red-circled area (ventral, (iii)). For image analysis, artifacts, large fiber tracts and blood vessels were manually edited (dashed circle) on each section. (D) A color deconvolution-based algorithm was used to measure TH-ir. A positive pixel count algorithm was customized to quantify brown immunoreactive pixels (red markup), subtracting inverse (blue) and background pixels (yellow). Transverse section of the unilateral midbrain (E). Warped rectangles show the lateral and medial substantia nigra. Semi-quantitative assessment of neuronal loss in the substantia nigra. Abundant pigmented neurons are seen in a neurologically normal control (Score 0) (F), compared with variable neuronal loss in LBD - mild (Score 1) (G), moderate (Score 2) (H) and severe (Score 3) (I). Scale bars: A - C = 2 mm; D = 100 μm; E = 1 cm; F – I = 250 μm.
Semi-quantitative assessment of substantia nigra pigmented neuronal loss
The midbrain was a transverse section at the level of the third nerve similar to that recommended for diagnostic evaluation of PD.4 Semi-quantitative assessment of substantia nigra cell groups was ascertained on hematoxylin and eosin stained sections at 100× magnification. We limited assessment to pigmented neurons of substantia nigra pars compacta, and divided it into two sectors – medial and ventrolateral (Figure 1E) – similar to regions used in previous clinicopathologic studies.7, 20 We used a human atlas of substantia nigra cell groups to identify ventrolateral and medial regions of the substantia nigra.21 The density of non-pigmented neurons was not taken into consideration for the semiquantitative scores based on a 4-point scale: 0 - none; 1 - mild; 2 - moderate; and 3 - severe.4
Genetic analyses
Genomic DNA was extracted from frozen cerebellar tissue by standard procedures. Genotyping was performed with a combination of Agena Bioscience Sequenom MassARRAY system (Agena Bioscience, San Diego, CA) and TaqMan SNP genotyping assays (Applied Bio-systems, Carlsbad, CA, USA) to determine genotype calls for 29 PD risk variants (Supplemental Table 1). PD risk variants were 28 single nucleotide polymorphisms (SNPs) previously identified as independent genetic risk factors for PD in a genome-wide association meta-analysis.13 An additional variant (TCEANC2 rs10788972) that was identified as a risk for PD in a genome-wide association analysis with pathologically-confirmed was also assessed. All call rates were > 95%, and there was no evidence of departure from Hardy-Weinberg equilibrium for any of the variants (all p > 0.01), except for HLA‐DQB rs13201101 (p < 0.001), which was driven by an excess of rare homozygotes.
Statistical analyses of genotyped SNPs with pathologic traits
Considering race and ethnicity as a potential confounding factors and the lack of variation in race and ethnicity in LBD cases, only non-Hispanic Caucasians were included in genetic association analyses. Examination of genetic associations with pathological endophenotypes was limited to two pathologic traits with continuous variables – dorsolateral and ventromedial putaminal TH-ir. The association of each SNP and dorsolateral and ventromedial putamen TH-ir was examined by linear regression models adjusted for age at death, sex, Braak neurofibrillary tangle stage, and Thal amyloid phase. These associations were also examined in a clinical subgroups of “any Parkinsonism” (Parkinsonism-predominant and Parkinsonism+dementia), as well as Parkinsonism+dementia and Parkinsonism-predominant, alone. Dorsolateral putaminal TH-ir was considered on logarithm scale and ventromedial putaminal TH-ir was considered on the square root scale in linear regression analysis owing to their skewed distributions. Using a Bonferroni correction to adjust for multiple testing for each different outcome, p-values of 0.0017 or lower were considered statistically significant. We also tested polygenic association with the aforementioned pathologic traits using a genetic risk score, which was calculated as described by Nalls and colleagues.13 Statistical analyses for genetic associations were performed using SAS (version 9.2; SAS Institute, Inc., Cary, NC).
Other statistical analyses
To compare demographic and neuropathologic variables between Parkinsonism-predominant, Parkinsonism+dementia, and dementia-predominant subgroups, a Chi-square test was used for categorical variables, and a Kruskal-Wallis rank sum test was used for ordered categorical or continuous variables. Given a significant overall difference between the three subgroups, pair-wise comparisons were made using a Chi-square test (categorical variables) or a Mann-Whitney test (ordered categorical or continuous variables). Spearman's test of correlation was used to examine the associations between striatal endophenotypes (putaminal TH-ir) and midbrain neuronal loss, overall and separately according to the three clinical subgroups.
Paired Wilcoxon signed rank tests were used to compare dorsolateral and ventromedial putaminal TH-ir. We used a Bonferroni correction to adjust for multiple testing separately for each group of similar statistical tests. P-values ≤ 0.0038 (comparisons of demographic and pathologic variables between the three subgroups) and ≤ 0.0031 (correlations between putaminal TH-ir and midbrain neuronal loss) were considered as significant. For comparisons of demographic and pathologic variables between the dementia-predominant, Parkinsonism+dementia, and Parkinsonism-predominant subgroups, if a significant (P ≤ 0.0028) overall difference was identified, pair-wise tests were then performed using a second Bonferroni correction, where P ≤ 0.0167 was considered as significant. All statistics other than the genetic analyses were performed with SigmaPlot (ver 12.0, Systat Software Inc., Chicago, IL).
Results
Demographic and clinical characteristics of the autopsy cohort
A total of 492 cases of pathologically-confirmed LBD were included in the study. Most had common antemortem clinical syndromes (PD (N = 29), PDD (N = 72), DLB (N = 111) and AD (N = 201)), while 65 cases (13%) had uncommon clinical presentations, including FTD (N = 9), CBS (N = 7), MSA (N = 19), PSP (N = 30) and others (N = 14). All cases were categorized into three clinical subgroups: Parkinsonism-predominant (N = 80), Parkinsonism+dementia (N = 195), and dementia-predominant (N = 217). The clinical and pathologic characteristics of the three subgroups are summarized in Table 1. The dementia-predominant subgroup had female predominance (52%), as well as higher median age at death (81 years old) compared to the other subgroups. In contrast, Parkinsonism-predominant, and Parkinsonism+dementia subgroups were younger (median ages of 78 and 76 years old, respectively) and had a male predominance (70% and 66%, respectively).
Pathologic characteristics of the clinical subgroups
Brain weights differed significantly between the three clinical subgroups (p < 0.001) and were least in the dementia-predominant subgroup (median: 1080 g). Postmortem intervals were not significantly different across the subgroups (p = 0.064). In all clinical subgroups, the most frequent Lewy body type was diffuse cortical type; the distribution of Lewy body types differed between the clinical subgroups (p < 0.001). Diffuse Lewy body pathology was most frequent in the Parkinsonism+dementia subgroup (64%), and lowest in the Parkinsonism-predominant subgroup (39%) (Table 1).
Of the 492 LBD cases included in this study, 212 cases (43%) had intermediate-to-high AD neuropathologic change.22 As might be expected, the frequency of co-morbid AD pathology was greatest in the dementia-predominant subgroup (75%), and lower in Parkinsonism+dementia (22%) and Parkinsonism-predominant (9%) subgroups. Alzheimer neuropathologic change summarized by Braak stage and Thal phase showed gradation in decreasing order from dementia-predominant to Parkinsonism+dementia to Parkinsonism-predominant, with significant differences between each subgroup. Following this order, the frequency of putaminal amyloid (Thal phases 3 or greater) varied; 90%, 70% and 45%, respectively (data not shown). The Parkinsonism-predominant group had the lowest Braak stage (median: III, interquartile range: II-III) and Thal phase (median: 1, interquartile: 0-3).
Neuronal loss in the ventrolateral substantia nigra was more marked than in the medial substantia nigra. Both the ventrolateral and the medial substantia nigra had significantly greater neuronal loss in Parkinsonism-predominant and Parkinsonism+dementia subgroups than in the dementia-predominant subgroup.
Striatal dopaminergic integrity
To put the LBD cases in context, neurologically normal controls processed identically showed robust TH-ir in both dorsolateral and ventromedial putamen (dorsolateral: median 21, interquartile range 14-31; ventromedial: 23, interquartile range 17-25), and no regional difference between the two regions (p = 0.84). In contrast, there was significant decrease in TH-ir in the putamen in brains with LBD (dorsolateral: median 4, interquartile range: 2-8, range: 0.3-42, ventromedial: median 10, interquartile range: 6-14, range: 0.3-29) with a notable degree of variation in these measure. TH-ir in ventromedial putamen was relatively preserved compared to the dorsolateral putamen (representative case is shown in Figure 1B), with reduction in dorsolateral of 79% compared to 57% for ventromedial putamen. Among the clinical subgroups, TH-ir in the dorsolateral putamen was better preserved in the dementia-predominant subgroup (median 6, interquartile range 3-12) compared to Parkinsonism+dementia (median 3, interquartile range 2-6) and Parkinsonism-predominant subgroups (median 3, interquartile range 2-5) (Table 1). There was no difference in TH-ir in the ventromedial putamen across clinical subgroups (p = 0.86) (Table 1).
Correlation between putaminal TH-ir with midbrain neuronal loss
When considered as a whole, TH-ir in the dorsolateral putamen had an inverse correlation with neuronal loss in both ventrolateral substantia nigra (r = -0.46, p = 2.0×10-7) and medial substantia nigra (r = -0.32, p = 5.7×10-12) (Table 2). This finding also held true when considering the three clinical subgroups. In contrast, TH-ir in the ventromedial putamen correlated with neuronal loss in ventrolateral substantia nigra only for overall group (r = -0.15, p = 0.0007), but did not correlate with it for any of the three clinical subgroups. TH-ir in the ventromedial putamen correlated with neuronal loss in medial substantia nigra for overall (r = -0.21, p = 5.8×10-6), Parkinsonism+dementia (r = -0.22, p = 0.003) and Parkinsonism-predominant subgroups (r = -0.31, p = 0.003).
Table 2. Correlation between putamen TH immunoreactivity and midbrain neuronal loss.
| Substantia nigra neuronal loss | Putaminal TH immunoreactivity | ||
|---|---|---|---|
| Dorsolateral | Ventromedial | ||
| Ventrolateral region | All patients | r = -0.46 | r = -0.15 |
| p = 2.0 × 10-7 | p = 0.0007 | ||
|
| |||
| Dementia-only | r = -0.30 | -0.15 | |
| p = 6.6 × 10-7 | p = 0.031 | ||
|
| |||
| Parkinsonism+dementia | r = -0.35 | r = -0.12 | |
| p = 5.8 × 10-7 | p = 0.10 | ||
|
| |||
| Parkinsonism-only | r = -0.43 | r = -0.26 | |
| p = 9.9 × 10-5 | p = 0.02 | ||
|
| |||
| Medial region | All patients | r = -0.32 | r = -0.21 |
| 5.7 × 10-12 | 5.8 × 10-6 | ||
|
| |||
| Dementia | r = -0.21 | r = -0.15 | |
| p = 0.002 | p = 0.038 | ||
|
| |||
| Parkinsonism+dementia | r = -0.25 | r = -0.22 | |
| p = 0.0007 | p = 0.003 | ||
|
| |||
| Parkinsonism | r = -0.26 | r = -0.31 | |
| p = 0.024 | p = 0.003 | ||
TH = tyrosine hydroxylase. P-values are from Spearman's test of correlation. (P-values ≤ 0.0031 are considered statistically significant after Bonferroni correction.)
Associations of PD genetic risk variants with TH-ir
Genetic analyses were performed on 473 of the 492 cases (96%). A summary of allele and genotype counts and frequencies are shown in Supplemental Table 1; observed allele frequencies were very similar to those previously reported.13, 14 Regarding the association between PD genetic risk variants and two continuous variable pathologic endophenotypes (dorsolateral and ventromedial putaminal TH-ir), no significant associations were detected after Bonferroni correction in the overall LBD series (Table 3). In subanalyses for groups showing “any Parkinsonism” (Parkinsonism-predominant or Parkinsonism+dementia) or “Parkinsonism-predominant,” one variant of SNCA (rs3910105) showed nominally significant association with the ventromedial putamen TH-ir (p = 0.009) in the Parkinsonism-predominant subgroup; however, none of the variants showed significant association with any of the pathologic traits after correction (Table 4, Supplemental Table 2). The median PD genetic risk score was 3.1 (range: 2.0-4.9), and there was no significant association between it and any of the pathologic traits (all P ≥ 0.13). The lack of association with PD genetic risk score was similar when examining “any Parkinsonism” (Parkinsonism+dementia and Parkinsonism-predominant), Parkinsonism+dementia, and Parkinsonism-predominant subgroups, separately (data not shown).
Table 3. Association between each variant and dorsolateral and ventral medial putamen TH-ir in the overall LBD series.
| Association with lateral putamen TH-ir | Association with medial putamen TH-ir | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variant | Nearest gene | MAF | Regression coefficient (95% CI) | P-value | Regression coefficient (95% CI) | P-value |
| rs10788972 | TCEANC2 | 47% | -0.05 (-0.22, 0.12) | 0.60 | 0.06 (-0.15, 0.26) | 0.58 |
| rs35749011 | GBA/SYT11 | 3% | -0.10 (-0.43, 0.24) | 0.57 | -0.07 (-0.47, 0.33) | 0.73 |
| rs114138760 | GBA/SYT11 | 2% | -0.03 (-0.44, 0.37) | 0.88 | 0.08 (-0.41, 0.57) | 0.74 |
| rs823118 | RAB7L1/NUCKS1 | 45% | -0.02 (-0.19, 0.14) | 0.80 | 0.03 (-0.17, 0.23) | 0.76 |
| rs10797576 | SIPA1L2 | 14% | 0.10 (-0.08, 0.27) | 0.29 | 0.08 (-0.14, 0.29) | 0.48 |
| rs6430538 | ACMSD/TMEM163 | 42% | -0.03 (-0.19, 0.14) | 0.76 | -0.05 (-0.25, 0.14) | 0.60 |
| rs1955337 | STK39 | 12% | -0.08 (-0.26, 0.11) | 0.41 | 0.05 (-0.17, 0.27) | 0.66 |
| rs12637471 | MCCC1 | 20% | -0.01 (-0.17, 0.16) | 0.95 | -0.10 (-0.29, 0.10) | 0.32 |
| rs34311866 | TMEM175 | 24% | 0.07 (-0.09, 0.23) | 0.39 | 0.10 (-0.09, 0.30) | 0.29 |
| rs34884217 | TMEM175 | 8% | 0.09 (-0.12, 0.31) | 0.39 | 0.12 (-0.14, 0.38) | 0.37 |
| rs11724635 | BST1 | 44% | -0.02 (-0.19, 0.15) | 0.81 | 0.00 (-0.20, 0.21) | 0.98 |
| rs6812193 | FAM47E/SCARB2 | 39% | 0.04 (-0.12, 0.21) | 0.60 | 0.02 (-0.18, 0.21) | 0.88 |
| rs356182 | SNCA | 34% | 0.00 (-0.16, 0.16) | 0.98 | 0.01 (-0.18, 0.20) | 0.94 |
| rs3910105 | SNCA | 50% | 0.09 (-0.09, 0.27) | 0.33 | 0.02 (-0.20, 0.24) | 0.86 |
| rs9275326 | HLA-DQB1 | 8% | -0.08 (-0.29, 0.14) | 0.48 | -0.04 (-0.30, 0.22) | 0.76 |
| rs13201101 | HLA-DQB1 | 5% | -0.23 (-0.51, 0.04) | 0.10 | -0.08 (-0.42, 0.25) | 0.62 |
| rs199347 | GPNMB | 39% | 0.04 (-0.12, 0.20) | 0.65 | 0.05 (-0.15, 0.24) | 0.63 |
| rs591323 | FGF20 | 26% | -0.13 (-0.28, 0.03) | 0.12 | -0.14 (-0.33, 0.05) | 0.15 |
| rs117896735 | INPP5F | 1% | -0.05 (-0.52, 0.42) | 0.84 | -0.19 (-0.76, 0.38) | 0.52 |
| rs329648 | MIR4697 | 36% | -0.04 (-0.20, 0.12) | 0.65 | -0.15 (-0.34, 0.04) | 0.13 |
| rs76904798 | LRRK2 | 13% | -0.01 (-0.19, 0.17) | 0.91 | -0.06 (-0.28, 0.16) | 0.61 |
| rs11060180 | CCDC62 | 48% | 0.09 (-0.09, 0.26) | 0.34 | 0.08 (-0.13, 0.30) | 0.44 |
| rs11158026 | GCH1 | 32% | -0.08 (-0.23, 0.08) | 0.34 | -0.15 (-0.34, 0.04) | 0.12 |
| rs2414739 | VPS13C | 28% | -0.05 (-0.21, 0.10) | 0.50 | -0.17 (-0.35, 0.02) | 0.077 |
| rs14235 | BCKDK/STX1B | 42% | -0.06 (-0.22, 0.10) | 0.49 | 0.09 (-0.11, 0.28) | 0.37 |
| rs11868035 | SREBF/RAI1 | 31% | -0.01 (-0.17, 0.15) | 0.90 | 0.00 (-0.19, 0.19) | 0.98 |
| rs17649553 | MAPT | 23% | 0.05 (-0.11, 0.21) | 0.51 | 0.09 (-0.11, 0.28) | 0.38 |
| rs12456492 | RIT2 | 32% | -0.06 (-0.21, 0.10) | 0.47 | -0.15 (-0.33, 0.04) | 0.13 |
| rs55785911 | DDRGK1 | 37% | -0.04 (-0.20, 0.12) | 0.64 | 0.02 (-0.18, 0.21) | 0.86 |
Regression coefficients; 95% CIs; and p-values result from linear regression models adjusted for age at death, sex, Braak stage and Thal phase where dorsolateral putamen TH-ir. A regression coefficient above zero indicates that dorsolateral putamen or ventromedial TH-ir was higher for patients with a copy of the minor allele of the given variant. (After applying a Bonferroni correction for multiple testing, p-values of 0.0017 or lower are considered as statistically significant.) TH-ir = tyrosine hydroxylase immunoreactivity; MAF = minor allele frequency.
Table 4. Association between each variant and dorsolateral and ventromedial putamen TH-ir in the subset of patients with any Parkinsonism (Parkinsonism predominant or dementia and Parkinsonism).
| Association with lateral putamen TH-ir | Association with medial putamen TH-ir | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variant | Nearest gene | MAF | Regression coefficient (95% CI) | P-value | Regression coefficient (95% CI) | P-value |
| rs10788972 | TCEANC2 | 47% | -0.03 (-0.27, 0.21) | 0.81 | 0.09 (-0.21, 0.39) | 0.56 |
| rs35749011 | GBA/SYT11 | 3% | -0.07 (-0.48, 0.34) | 0.75 | 0.00 (-0.51, 0.52) | 0.99 |
| rs114138760 | GBA/SYT11 | 2% | 0.27 (-0.25, 0.78) | 0.31 | 0.35 (-0.30, 1.00) | 0.30 |
| rs823118 | RAB7L1/NUCKS1 | 45% | -0.11 (-0.34, 0.11) | 0.33 | 0.01 (-0.27, 0.29) | 0.95 |
| rs10797576 | SIPA1L2 | 14% | 0.18 (-0.06, 0.41) | 0.13 | 0.15 (-0.15, 0.45) | 0.32 |
| rs6430538 | ACMSD/TMEM163 | 42% | 0.06 (-0.15, 0.28) | 0.56 | 0.11 (-0.16, 0.38) | 0.41 |
| rs1955337 | STK39 | 12% | -0.05 (-0.28, 0.18) | 0.68 | 0.03 (-0.27, 0.32) | 0.85 |
| rs12637471 | MCCC1 | 20% | 0.04 (-0.18, 0.26) | 0.74 | -0.02 (-0.30, 0.25) | 0.87 |
| rs34311866 | TMEM175 | 24% | 0.09 (-0.12, 0.30) | 0.39 | 0.13 (-0.13, 0.40) | 0.33 |
| rs34884217 | TMEM175 | 8% | 0.09 (-0.21, 0.39) | 0.57 | 0.12 (-0.26, 0.50) | 0.53 |
| rs11724635 | BST1 | 44% | -0.05 (-0.28, 0.19) | 0.70 | -0.07 (-0.36, 0.22) | 0.62 |
| rs6812193 | FAM47E/SCARB2 | 39% | 0.02 (-0.20, 0.24) | 0.84 | -0.14 (-0.41, 0.13) | 0.32 |
| rs356182 | SNCA | 34% | -0.08 (-0.30, 0.13) | 0.43 | -0.03 (-0.30, 0.24) | 0.82 |
| rs3910105 | SNCA | 50% | 0.06 (-0.18, 0.30) | 0.63 | 0.02 (-0.28, 0.33) | 0.88 |
| rs9275326 | HLA-DQB1 | 8% | -0.09 (-0.37, 0.20) | 0.56 | 0.00 (-0.37, 0.36) | 0.99 |
| rs13201101 | HLA-DQB1 | 5% | 0.05 (-0.30, 0.40) | 0.77 | 0.23 (-0.21, 0.67) | 0.30 |
| rs199347 | GPNMB | 39% | 0.09 (-0.12, 0.31) | 0.40 | 0.05 (-0.22, 0.32) | 0.73 |
| rs591323 | FGF20 | 26% | -0.15 (-0.36, 0.06) | 0.16 | -0.14 (-0.40, 0.13) | 0.31 |
| rs117896735 | INPP5F | 1% | 0.09 (-0.56, 0.74) | 0.78 | -0.43 (-1.25, 0.39) | 0.31 |
| rs329648 | MIR4697 | 36% | -0.12 (-0.33, 0.09) | 0.27 | -0.21 (-0.47, 0.05) | 0.12 |
| rs76904798 | LRRK2 | 13% | -0.04 (-0.29, 0.21) | 0.74 | -0.01 (-0.33, 0.30) | 0.94 |
| rs11060180 | CCDC62 | 48% | 0.05 (-0.19, 0.29) | 0.69 | 0.08 (-0.22, 0.38) | 0.58 |
| rs11158026 | GCH1 | 32% | -0.05 (-0.26, 0.16) | 0.65 | -0.17 (-0.43, 0.10) | 0.22 |
| rs2414739 | VPS13C | 28% | -0.02 (-0.23, 0.19) | 0.85 | -0.06 (-0.32, 0.20) | 0.65 |
| rs14235 | BCKDK/STX1B | 42% | -0.02 (-0.24, 0.20) | 0.83 | 0.15 (-0.12, 0.43) | 0.28 |
| rs11868035 | SREBF/RAI1 | 31% | 0.03 (-0.18, 0.24) | 0.78 | 0.03 (-0.24, 0.29) | 0.85 |
| rs17649553 | MAPT | 23% | 0.00 (-0.21, 0.22) | 0.97 | 0.02 (-0.25, 0.29) | 0.91 |
| rs12456492 | RIT2 | 32% | -0.11 (-0.32, 0.09) | 0.29 | -0.20 (-0.46, 0.06) | 0.14 |
| rs55785911 | DDRGK1 | 37% | -0.13 (-0.34, 0.09) | 0.24 | -0.06 (-0.32, 0.21) | 0.68 |
Regression coefficients; 95% CIs; and p-values result from linear regression models adjusted for age at death, sex, Braak stage and Thal phase. A regression coefficient above zero indicates that dorsolateral or ventromedial putamen TH-ir was higher for patients with a copy of the minor allele of the given variant. (After applying a Bonferroni correction for multiple testing, p-values of 0.0017 or lower are considered as statistically significant.) TH-ir = tyrosine hydroxylase immunoreactivity; MAF = minor allele frequency.
Discussion
In the present study, the integrity of nigrostriatal dopaminergic innervation was measured by image analysis of putaminal TH-ir in a large number of brains with pathologically-confirmed Lewy body pathology and a range of clinical presentations. We found significant regional differences in vulnerability of the putamen to dopaminergic neurodegeneration, with dorsolateral more affected than ventromedial, as noted by others.8 This fits with evidence that the dorsolateral putamen has projections from the most vulnerable neuronal cell group in the substantia nigra, the ventrolateral cell group. While degeneration was less severe in the ventromedial putamen, it was significantly different from neurologically normal controls. Moreover, there was inverse correlation between ventromedial putamen dopaminergic innervation and neuronal loss in the medial region of substantia nigra, while the correlation was weak or absent for ventrolateral substantia nigra neuronal loss. These observations fit with evidence that this part of the putamen receives dopaminergic innervation from less vulnerable neurons in the medial region of substantia nigra that are affected only in later stages of PD.8
Dopaminergic nerve terminal loss in the dorsolateral putamen was greater in parkinsonian subgroups (Parkinsonism-predominant and Parkinsonism+dementia) than in the dementia-predominant subgroup. In contrast, no differences were detected across clinical subgroups for ventromedial putamen TH-ir. These findings might suggest that estimates of substantia nigra neuronal loss and dorsolateral putaminal dopaminergic integrity measured objectively with digital microscopy would be excellent endophenotypes for Parkinsonism.
The failure to find association with PD genetic risk variants identified from GWAS studies suggests that genetic variants may be related to risk of PD, but not pathologic endophenotypes (i.e. nigrostriatal degeneration). Among clinical PD cohorts, a number of previous studies have linked specific genetic variants with clinical phenotypes 23-28 and it has been reported that using the polygenic risk score can correlate with motor progression in PD and potentially age-at-onset.29-31 In addition, relatively rare GBA mutations28 have been linked with progression of both cognition and motor deficits in PD while APOE has been linked to cognition28 and common SNCA variants with motor deficits. 23 This discordance, could be in part due to clinical heterogeneity or the nature of assessing end-stage pathology. It is also worth noting that GBA and alternate SNCA alleles have been implicated in cognitive measures versus susceptibility to PD29, thus again demonstrating the need for large unbiased genome-wide approaches using nigrostriatal degeneration as the endophenotype.
Interestingly, our findings are quite different from the effects of APOE genotype on both risk for AD32 and on severity of Alzheimer-related pathologic endophenotypes.33 Bennett and colleagues found a robust association between APOE4 and pathologic traits (p = 9 × 10-24), which was many fold greater than the association with risk of clinical diagnosis of AD in the same cohort (p = 3 × 10-7). Apolipoprotein E may contribute to AD risk and pathogenesis through multiple pathways.34 It remains unclear how many of the PD susceptibility gene variants contribute to risk of PD. The results of the present study seem to suggest that they do not associate with severity of nigrostriatal dopaminergic degeneration. PD is a multifactorial disease process affected by genetic, epigenetic and environmental factors.35-37
Additionally, we considered several factors that might have weakened the correlation. First, the selected common variants were identified from GWAS studies based on a case-control approach. This method selects for variants enriched in the disease group compared to healthy controls and might fail to detect genetic variants that contribute to disease severity or heterogeneity of PD, such as nigrostriatal neurodegeneration assessed in the present study. This study has shown the need for unbiased genome-wide approaches (e.g. GWAS or whole-genome sequencing) to resolve genetic variation that can drive this pathology. Second, the possibly weak effects of individual genetic variants; to overcome this, we also created a weighted genetic risk score similar to that used by Nalls and colleagues13 based upon the effect size of the genetic variant and the frequency of the variant. Unfortunately, the genetic risk score also failed to show association with the quantitative endophenotype, dorsolateral putaminal TH-ir, which is highly correlated with Parkinsonism.8 Third, interactions of other genetic and non-genetic factors may have masked genetic association with pathologic traits. It has been emphasized that interplay of these factors may play a key role in function or dysfunction of susceptibility genes.35, 36, 38 Soldoner and colleagues recently identified a specific risk variant (rs356168) of the α-synuclein gene (SNCA) that has cis-regulatory effects on a non-coding distal enhancer element, that could contribute the PD pathogenesis.38 Attempts to disentangle the contribution of these modifier factors on the effects of genetic variants identified from case-control studies may be needed. These studies may also include other candidate genetic determinants of neurodegenerative pathologies (e.g. hits from AD GWAS) as a large number of patients with Alzheimer's disease have Lewy body pathology at end-stage. It is likely a large unbiased strategy using GWAS or whole-genome sequencing approaches will help dissect the genetic architecture of nigrostriatal degeneration. Finally, power to detect associations between genetic variants and TH-ir was limited in the relatively small Parkinsonism predominant subgroup (N = 80), and therefore the possibility of a type II error is important to consider.
Despite its limitations, we believe that there are strengths of our study: 1) it is based on a large autopsy-proven cohort of LBD cases; 2) the endophenotype for nigrostriatal integrity, TH-ir in the putamen, was objectively measured with digital pathology and an unbiased color deconvolution algorithm; and 3) all the currently known PD genetic variants identified by clinical and autopsy genome-wide association studies were included in the statistical analysis of endophenotypes.
In summary, our anatomical findings regarding the regional anatomy of putamen dopaminergic innervation confirm and extend previous observations on selective vulnerability of striatonigral and mesolimbic dopaminergic systems in LBD. Failure to find genetic correlates with quantitative endophenotypes that tract closely with extrapyramidal signs in PD may suggest that PD genetic risk factors influence risk of PD, but not the severity of pathologic processes linked to Parkinsonism, which is unlike findings in Alzheimer's disease. Further studies are needed to understand the pathogenetic mechanisms of PD genetic risk loci, including expanding studies of pathologic endophenotypes such as those used in this study to larger multicenter cohorts of pathologically confirmed PD.
Supplementary Material
Suppl. Table 1. Allele and genotype counts and frequencies. Genotype 1; 2 and 3 represents homozygous major allele; heterozygous allele and homozygous minor allele, respectively.
Suppl. Table 2. Association between each variant and dorsolateral and ventromedial putamen TH-ir in the subset of Parkinsonism-predominant patients. Regression coefficients; 95% CIs; and p-values result from linear regression models adjusted for age at death, sex, Braak stage and Thal phase where dorsolateral putamen TH-ir. A regression coefficient above zero indicates that dorsolateral putamen TH-ir was higher for patients with a copy of the minor allele of the given variant. After applying a Bonferroni correction for multiple testing, p-values of 0.0017 or lower are considered as statistically significant. TH-ir = tyrosine hydroxylase immunoreactivity; MAF = minor allele frequency.
Acknowledgments
We are grateful to family members for granting permission for autopsy studies on their loved ones. Without their generous donation, these studies would have been impossible. We would also like to thank the expert technical assistance of Linda Rousseau and Virginia Phillips for histology and Monica Castanedes-Casey for immunohistochemistry. This study was supported by the Michael J. Fox Foundation, Udall Center of Excellence in Parkinson's Disease Research (P50 NS072187), The Little Family Foundation, Mayo Clinic AD and related dementias genetics program, and the Mangurian Foundation Lewy Body Dementia Program at Mayo Clinic.
Dr. Ross reports no disclosures. He receives research support from the NIH (P50-NS072187; R01-NS078086)
Dr. Dickson reports no disclosures. He receives research support from the NIH (P50‐AG016574; P50-NS072187; P01-AG003949) and the Robert E. Jacoby Professorship.
Dr. Dickson is an editorial board member of Acta Neuropathologica, Annals of Neurology, Brain, Brain Pathology, and Neuropathology, and he is editor in chief of American Journal of Neurodegenerative Disease, and International Journal of Clinical and Experimental Pathology.
Footnotes
Financial disclosure of all authors: Dr. Kasanuki reports no disclosures.
Mr. Heckman reports no disclosures.
Ms. Diehl reports no disclosures.
Dr. Murray reports no disclosures.
Dr. Koga reports no disclosures.
Dr. Soto reports no disclosures.
Author Contributions: KK: Acquisition, analysis and interpretation of data; drafting of manuscript; execution of the statistical analysis; writing of the first draft
MGH: Execution of the statistical analysis
Review and critique; interpretation of data
NND: Analysis and interpretation of data; review and critique
MEM: Acquisition
analysis and interpretation of data; review and critique
SK: Review and critique
AS: Analysis and interpretation of data
OAR: Study concept and design; acquisition
analysis and interpretation of data; review and critique
DWD: Study concept and design; interpretation of data; review and critique
References
- 1.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 2.Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. Journal of neuropathology and experimental neurology. 2012;71(4):266–273. doi: 10.1097/NEN.0b013e31824b211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wakabayashi K, Hayashi S, Yoshimoto M, Kudo H, Takahashi H. NACP/alpha-synuclein-positive filamentous inclusions in astrocytes and oligodendrocytes of Parkinson's disease brains. Acta neuropathologica. 2000;99(1):14–20. doi: 10.1007/pl00007400. [DOI] [PubMed] [Google Scholar]
- 4.Dickson DW, Braak H, Duda JE, et al. Neuropathological assessment of Parkinson's disease: refining the diagnostic criteria. The Lancet Neurology. 2009;8(12):1150–1157. doi: 10.1016/S1474-4422(09)70238-8. [DOI] [PubMed] [Google Scholar]
- 5.Ince PG. Dementia with Lewy Bodies and Parkinson's Disease Dementia. In: Dickson DW, Weller RO, editors. Neurodegeneration: The Molecular Pathology of Dementia and Movement Disorders. Second. Oxford, UK: Wiley-Blackwell; 2011. [Google Scholar]
- 6.Cheng HC, Ulane CM, Burke RE. Clinical progression in Parkinson disease and the neurobiology of axons. Annals of neurology. 2010;67(6):715–725. doi: 10.1002/ana.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rinne JO, Rummukainen J, Paljarvi L, Rinne UK. Dementia in Parkinson's disease is related to neuronal loss in the medial substantia nigra. Annals of neurology. 1989;26(1):47–50. doi: 10.1002/ana.410260107. [DOI] [PubMed] [Google Scholar]
- 8.Kordower JH, Olanow CW, Dodiya HB, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain : a journal of neurology. 2013;136(Pt 8):2419–2431. doi: 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hedreen JC. Tyrosine hydroxylase-immunoreactive elements in the human globus pallidus and subthalamic nucleus. The Journal of comparative neurology. 1999;409(3):400–410. doi: 10.1002/(sici)1096-9861(19990705)409:3<400::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 10.DelleDonne A, Klos KJ, Fujishiro H, et al. Incidental Lewy body disease and preclinical Parkinson disease. Archives of neurology. 2008;65(8):1074–1080. doi: 10.1001/archneur.65.8.1074. [DOI] [PubMed] [Google Scholar]
- 11.Beach TG, Adler CH, Sue LI, et al. Reduced striatal tyrosine hydroxylase in incidental Lewy body disease. Acta neuropathologica. 2008;115(4):445–451. doi: 10.1007/s00401-007-0313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickson DW, Fujishiro H, DelleDonne A, et al. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson's disease. Acta neuropathologica. 2008;115(4):437–444. doi: 10.1007/s00401-008-0345-7. [DOI] [PubMed] [Google Scholar]
- 13.Nalls MA, Pankratz N, Lill CM, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson's disease. Nature genetics. 2014;46(9):989–993. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beecham GW, Dickson DW, Scott WK, et al. PARK10 is a major locus for sporadic neuropathologically confirmed Parkinson disease. Neurology. 2015;84(10):972–980. doi: 10.1212/WNL.0000000000001332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta neuropathologica. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 16.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 17.Murray ME, Lowe VJ, Graff-Radford NR, et al. Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer's disease spectrum. Brain : a journal of neurology. 2015;138(Pt 5):1370–1381. doi: 10.1093/brain/awv050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beach TG, White CL, Hamilton RL, et al. Evaluation of alpha-synuclein immunohistochemical methods used by invited experts. Acta neuropathologica. 2008;116(3):277–288. doi: 10.1007/s00401-008-0409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2002;18(8):386–404. doi: 10.1007/s00381-002-0604-1. [DOI] [PubMed] [Google Scholar]
- 20.Gibb WR, Lees AJ. Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1991;54(5):388–396. doi: 10.1136/jnnp.54.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nieuwenhuys R, Voogd J, Huijzen Cv. The human central nervous system. 4th. New York: Springer; 2008. [Google Scholar]
- 22.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta neuropathologica. 2012;123(1):1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper CA, Jain N, Gallagher MD, et al. Common variant rs356182 near SNCA defines a Parkinson's disease endophenotype. Annals of clinical and translational neurology. 2017;4(1):15–25. doi: 10.1002/acn3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang G, Huang Y, Chen W, et al. Variants in the SNCA gene associate with motor progression while variants in the MAPT gene associate with the severity of Parkinson's disease. Parkinsonism & related disorders. 2016;24:89–94. doi: 10.1016/j.parkreldis.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 25.Davis AA, Andruska KM, Benitez BA, Racette BA, Perlmutter JS, Cruchaga C. Variants in GBA, SNCA, and MAPT influence Parkinson disease risk, age at onset, and progression. Neurobiology of aging. 2016;37:209.e201–207. doi: 10.1016/j.neurobiolaging.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oczkowska A, Kozubski W, Lianeri M, Dorszewska J. Mutations in PRKN and SNCA Genes Important for the Progress of Parkinson's Disease. Current genomics. 2013;14(8):502–517. doi: 10.2174/1389202914666131210205839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ritz B, Rhodes SL, Bordelon Y, Bronstein J. alpha-Synuclein genetic variants predict faster motor symptom progression in idiopathic Parkinson disease. PloS one. 2012;7(5):e36199. doi: 10.1371/journal.pone.0036199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huertas I, Jesus S, Garcia-Gomez FJ, et al. Genetic factors influencing frontostriatal dysfunction and the development of dementia in Parkinson's disease. PloS one. 2017;12(4):e0175560. doi: 10.1371/journal.pone.0175560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davis MY, Johnson CO, Leverenz JB, et al. Association of GBA Mutations and the E326K Polymorphism With Motor and Cognitive Progression in Parkinson Disease. JAMA neurology. 2016;73(10):1217–1224. doi: 10.1001/jamaneurol.2016.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Escott-Price V, Nalls MA, Morris HR, et al. Polygenic risk of Parkinson disease is correlated with disease age at onset. Annals of neurology. 2015;77(4):582–591. doi: 10.1002/ana.24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pihlstrom L, Toft M. Cumulative genetic risk and age at onset in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2015;30(12):1712–1713. doi: 10.1002/mds.26366. [DOI] [PubMed] [Google Scholar]
- 32.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 33.Bennett DA, De Jager PL, Leurgans SE, Schneider JA. Neuropathologic intermediate phenotypes enhance association to Alzheimer susceptibility alleles. Neurology. 2009;72(17):1495–1503. doi: 10.1212/WNL.0b013e3181a2e87d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanekiyo T, Xu H, Bu G. ApoE and Abeta in Alzheimer's disease: accidental encounters or partners? Neuron. 2014;81(4):740–754. doi: 10.1016/j.neuron.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scholz SW, Mhyre T, Ressom H, Shah S, Federoff HJ. Genomics and bioinformatics of Parkinson's disease. Cold Spring Harbor perspectives in medicine. 2012;2(7):a009449. doi: 10.1101/cshperspect.a009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Labbe C, Lorenzo-Betancor O, Ross OA. Epigenetic regulation in Parkinson's disease. Acta neuropathologica. 2016;132(4):515–530. doi: 10.1007/s00401-016-1590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ascherio A, Schwarzschild MA. The epidemiology of Parkinson's disease: risk factors and prevention. The Lancet Neurology. 2016;15(12):1257–1272. doi: 10.1016/S1474-4422(16)30230-7. [DOI] [PubMed] [Google Scholar]
- 38.Soldner F, Stelzer Y, Shivalila CS, et al. Parkinson-associated risk variant in distal enhancer of alpha-synuclein modulates target gene expression. Nature. 2016;533(7601):95–99. doi: 10.1038/nature17939. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Table 1. Allele and genotype counts and frequencies. Genotype 1; 2 and 3 represents homozygous major allele; heterozygous allele and homozygous minor allele, respectively.
Suppl. Table 2. Association between each variant and dorsolateral and ventromedial putamen TH-ir in the subset of Parkinsonism-predominant patients. Regression coefficients; 95% CIs; and p-values result from linear regression models adjusted for age at death, sex, Braak stage and Thal phase where dorsolateral putamen TH-ir. A regression coefficient above zero indicates that dorsolateral putamen TH-ir was higher for patients with a copy of the minor allele of the given variant. After applying a Bonferroni correction for multiple testing, p-values of 0.0017 or lower are considered as statistically significant. TH-ir = tyrosine hydroxylase immunoreactivity; MAF = minor allele frequency.