Abstract
The monoclonal anti-immunoglobulin E (IgE) antibody, omalizumab, was the first drug approved for use in patients with chronic idiopathic/spontaneous urticaria (CIU/CSU) who remain symptomatic despite H1-antihistamine treatment. Omalizumab binds to free IgE, which lowers free IgE levels and causes FcεRI receptors on basophils and mast cells to be downregulated. It has been shown to improve symptoms of CIU/CSU, but its mechanism of action is not currently understood. Potential mechanisms in CIU/CSU include reducing mast cell releasability, reversing basopenia and improving basophil IgE receptor function, reducing activity of IgG autoantibodies against FcεRI and IgE, reducing activity of IgE autoantibodies against an antigen or autoantigen that has yet to be definitively identified, reducing the activity of intrinsically ‘abnormal’ IgE, and decreasing in vitro coagulation abnormalities associated with disease activity. However, none of these theories alone or in combination fully account for the pattern of symptom improvement seen with omalizumab therapy, and therefore, no one mechanism is likely to be the definitive mechanism of action. Additional research is needed to further clarify the involvement of omalizumab in relieving symptoms associated with the complex, multifactorial pathogenesis of CIU/CSU.
Keywords: chronic idiopathic urticaria, chronic spontaneous urticaria, omalizumab
Chronic idiopathic/spontaneous urticaria (CIU/CSU) is characterized by itching, burning, or painful evanescent wheals (hives) and/or angioedema, symptoms that present suddenly and are present most days of the week for at least 6 weeks (1, 2). Inducible urticarias can also be chronic, but can be provoked by physical stimuli (e.g., cold, friction, or pressure) or other factors (e.g., in cholinergic, aquagenic, or contact urticaria) (1, 2). With the exception of delayed pressure urticaria, the individual urticarial lesions in inducible urticarias appear shortly after provocation and usually fade within 4 h (typically 1–2 h) (1), whereas the typical lesions in CIU/CSU may persist for longer periods of time (3). The extended duration of lesions in CIU/CSU and delayed pressure urticaria may be attributed to a late-phase response similar to allergic reactions in which a lesion appears 4–10 h after a preceding immediate hive has disappeared (1, 4–8).
The prevalence of CIU/CSU is difficult to estimate as few studies have focused on it, and inconsistent classification of urticaria makes it difficult to compare individual studies (9). Up to 1.0% of the global population may suffer from CIU/CSU at any given time (9). In a retrospective study of insurance claims from a US commercial health plan, the prevalence of CIU/CSU was found to be 0.11% (10).
The wheals in patients with CIU/CSU are superficial swellings of the dermis that usually begin as skin lesions with red edges and a pale center, becoming pink as they mature (11). The pathology of active wheals shows degranulated mast cells and a dermal infiltrate with a variable number of lymphocytes, eosinophils, neutrophils, basophils, or macrophages (7, 12–14). Mediators such as histamine, platelet-activating factor, prostaglandin D2, and cytokines released from mast cells and endothelial cells contribute to the vasodilation, nerve activation, and cell recruitment seen in the wheals (15–20). Upregulation of endothelial cell adhesion molecules is typically present in skin affected by wheals, and preliminary findings suggest that patients with CIU/CSU show upregulation of genes involved in the recovery of the epidermal barrier, active inflammation, coagulation, and dermal repair (21–23).
Mast cells, the principal cells involved in wheal pathogenesis, express multiple receptors that are susceptible to activation, inducing their degranulation (e.g., chemokine, prostaglandin, Toll-like, or immunoglobulin receptors) (17, 24, 25). A key receptor in CIU/CSU is the immunoglobulin E (IgE) receptor FcεRI (26–28), although there is some evidence that it is not always involved in mast cell activation and histamine release (29), and multiple pathways leading to mast cell activation have been identified (30).
Here we review clinical data showing the pattern of symptoms and changes in biomarkers as well as data from in vitro studies that provide new insights into the potential mechanisms of action contributing to the efficacy of omalizumab, an anti-IgE antibody, in CIU/CSU. Previous reviews have focused on the utility of omalizumab as treatment for CIU/CSU and potential mechanisms of action, with an emphasis on mast cell priming via monomeric IgE and autoreactivity (31, 32). The current review expands on these and presents potential mechanisms related to the modulation of mast cell and basophil IgE receptor function, and possible mechanisms involving autoantigens or ‘abnormal’ IgE. We also present newly available data showing that omalizumab reduces FcεRI and IgE and normalizes gene expression in patients with CIU/CSU.
Omalizumab overview
Omalizumab for treatment of patients with CIU/CSU
Omalizumab is a recombinant DNA-derived humanized immunoglobulin G1K monoclonal antibody that selectively binds to human IgE (33). It was originally approved in the USA in 2003 for the treatment of moderate-to-severe persistent allergic asthma and then in the European Union (EU) in 2005 for treatment-resistant allergic asthma (33, 34).
Omalizumab was approved for use in patients with CIU/CSU in 2014 in both the USA and EU (33, 35, 36), and three phase 3 studies have confirmed it is efficacious in this population (37–39). It is the first drug approved for patients with CIU/CSU who remain symptomatic despite H1-antihistamine treatment (1, 2, 31, 35).
The reduction in inflammatory mediator release by omalizumab in patients with allergic disease requires a 95% reduction in serum IgE levels to modify allergen responses via receptor downregulation (40, 41). Thus, dosing for allergic asthma is determined by serum total IgE level and body weight to achieve this reduction in IgE (33). By contrast, CIU/CSU is not a classic allergen-driven disease and a fixed dose of omalizumab is approved for use in patients with CIU/CSU (37–39, 42). The mechanism of action that results in improvement of CIU/CSU symptoms is not entirely understood (32).
Known mechanism for omalizumab in allergic diseases
Omalizumab was developed to bind with high affinity to free IgE, thereby preventing allergen-specific IgE from attaching to FcεRI (40, 43). Omalizumab does not bind to cell surface IgE, so it does not directly activate mast cells or basophils (44, 45). The reduction in free IgE levels results in reduction in the number of FcεRI receptors on mast cells, basophils, and antigen-presenting cells (40, 41, 43, 46–48). Several studies have shown that a residual response to allergen triggering of mast cells and basophils remains, albeit with reduced mediator generation (40, 49).
The rate of change to suppress IgE, IgE receptors, and allergen responses varies by cell type (Fig. 1A, based on asthma dosing strategy studies) (40, 43, 50–54). Significant reduction in basophil FcεRI was noted as early as 3 days following the first dose of omalizumab in patients with allergic disease (40, 51). In patients with allergic rhinitis, FcεRI on basophils decreased by 88% at Day 7 but did not decrease on mast cells until Day 70, at which point acute allergen wheal size decreased (43). Clinical response to omalizumab in patients with peanut allergy was observed before Week 8, a time frame during which basophils, but not skin mast cells, were found to be suppressed in their allergen responses (52). Likewise, reductions in cat allergen nasal reactivity with omalizumab therapy occurred in an early time frame, before notable reductions in the size of the early-phase skin reaction or nasal mast cell mediator production (51, 53, 54).
Figure 1.
Time course of known cellular and clinical effects of omalizumab identified in studies focused on (A) patients with allergic response, mast cell diseases and (B) chronic urticaria. *MacGlashan et al. (2013) observed a 2.5- to 125-fold increase in basophil sensitivity after omalizumab treatment with sensitivity shifts noted at the midpoint and after 12 weeks of treatment.
In these allergen challenge models and often patients with CIU/CSU, omalizumab treatment led to symptom relief well before a reduction was seen in the size of the early-phase reaction of patients with allergic disease (37–39, 42, 55–58). Conversely, the late-phase reaction of allergen-induced responses resembles that seen in CIU/CSU (7). Also, acute administration of corticosteroids affects only the late-phase response of CIU/CSU (59) and has no effect upon allergen-induced mast cell degranulation. For example, one can administer skin tests to patients who are on steroid therapy. During antigen challenge in patients with allergic disease, omalizumab has a faster and greater effect on the skin latephase than the early-phase response and may act on infiltrating cells in addition to resident mast cells (53, 54). Thus, it is important to note that the insights gained from the effects of omalizumab on allergic reactions, particularly the responsiveness of mature skin mast cells, may not directly apply to CIU/CSU.
Rationale for evaluating omalizumab for CIU/CSU
It was theorized that omalizumab could prevent activation of mast cells and basophils in the approximately 40–45% of patients with CIU/CSU who may have an autoimmune component, by decreasing FcεRI density and preventing immunoglobulin G (IgG) antibody-mediated cross-linking of adjacent α-subunits or IgE itself (60). Further, there is evidence that patients with CIU/CSU have an abnormal basophil FcεRI pathway and that basophils are recruited to CIU/CSU lesions (26, 55, 61–63). Reversal of basopenia and basophil IgE receptor abnormalities, seen in natural remission of CIU/CSU, points toward basophils as an important contributor to disease (62, 64, 65). Omalizumab might target the uniquely abnormal basophil FcεRI phenotype and recruitment to the skin, as seen in patients with CIU/CSU.
An initial case report in 2007 showed that three patients receiving omalizumab for the treatment of asthma also had symptomatic chronic urticaria (CU) that responded to treatment with omalizumab (66). A proof-of-concept study of omalizumab in patients with autoimmune-related CU, demonstrated by basophil studies or autologous skin testing, suggested that omalizumab may be an effective therapy in patients who are not adequately treated with antihistamines (60) and a second proof-of-concept study evaluated patients regardless of autoimmune status with similar success (55). These led to a phase 2 dose-ranging study that found omalizumab was well tolerated and efficacious (42), and phase 3 studies of more than 900 patients led to approval of omalizumab’s CIU/CSU indication (37–39).
Potential mechanisms of action in CIU/CSU
Lowering IgE levels and downregulating IgE receptors
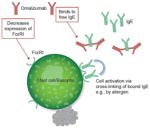
The ability of omalizumab to lower free IgE levels and downregulate FcεRI on mast cells and basophils is well established (40, 43, 49, 67). The reduction in FcεRI levels is the result of unbound FcεRI being degraded when they are not stabilized by binding to IgE (40, 41, 47, 48, 68).
A recent randomized controlled study found that baseline levels of FcεRI- and IgE-positive cells were higher in patients with CIU/CSU than in healthy volunteers, but after 12 weeks of omalizumab treatment levels in lesional and nonlesional skin were reduced to levels seen in healthy volunteers (57). Although the effect of omalizumab is clear, the baseline level of FcεRI- and IgE-positive cells in CIU/CSU may reflect the observation that IgE levels in the CIU/CSU group were 50% higher at baseline compared with the unmatched controls (69). Omalizumab treatment also altered the expression of genes associated with mast cell/leukocyte infiltration (FECER1G, C3AR1, CD93, S100A8, S100A9), increased oxidative stress (SOD2), vascularization (CYR61), and skin repair events (KRT6, KRT16A) in lesional skin to the levels seen in nonlesional skin and skin in healthy volunteers, as measured by microarray (70). Pooled data from all phase 3 studies showed that symptom reduction in CIU measured by 7-day sum of daily Urticaria Activity Scores (UAS7) was correlated with a reduction in free IgE levels relative to baseline using data collected at 12 and 24 weeks of omalizumab treatment (71). In patients with allergic disease, this combination of lowered IgE and FcεRI levels reduces allergen-stimulated responses of mast cells and basophils (43), despite an increase in the intrinsic sensitivity to allergen stimulation for basophils (49). How this relates to cell secretion in CIU/CSU, in which the agonist is not as well defined, is not yet clear.
It is of note that omalizumab therapy is successful in patients with or without a positive test for IgG autoantibodies against FcεRI or IgE, or IgE autoantibodies against thyroperoxidase (TPO) (37–39, 42, 55, 60, 72). In one phase 3 study, omalizumab therapy had a similar response in patients regardless of CU index status (39). Although a 24-h time point was not evaluated in phase 3 studies, a retrospective study reported that within 24 h >50% of patients experienced symptom control, defined as a reduction of ≥90% of UAS7 (58), suggesting that in some patients, symptom control may be achieved too quickly to be explained by IgE receptor downregulation (Fig. 1B) (40, 43, 73). Numerous studies have noted this rapidly responding subpopulation but report a much smaller percentage within the first week (37–39, 74, 75). Regardless, several additional theories need to be explored.
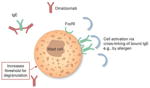
Reducing mast cell releasability
Cutaneous mast cells appear to have unique characteristics compared with mast cells in other locations (76, 77, 78). For example, human cutaneous mast cells degranulate in response to opiates and substance P and have complement receptors, unlike pulmonary mast cells, which do not (78, 79). Mast cell releasability, as assessed by compound 48/80-induced histamine responses via skin chambers, has been demonstrated to be increased in patients with CIU/CSU as compared with healthy controls (80–82); this enhanced releasability has been shown to reverse with CIU/CSU remission (80). Furthermore, levels of Mas-related gene X2 (MrgX2), a receptor expressed on human mast cells for basic proteins including compound 48/80 (83), are known to increase in the skin of patients with CIU/CSU (84). Separately, it has been noted that CD34+-derived mast cells of subjects with CIU spontaneously release histamine upon sensitization by IgE (27).
In skin biopsies from patients with allergic rhinitis, omalizumab reduced the number of available FcεRI receptors much more slowly on mast cells than on basophils (70 vs 7 days) (43). This difference in effect on mast cells vs basophils may be attributable to the relatively brief transit time of basophils in the circulation compared with the life span of tissue mast cells (85). The rate of receptor downregulation in skin mast cells of patients with CIU/CSU is less clear but appears to support the earlier findings in subjects with allergic disease for rate of receptor reductions (43, 54). Recent skin biopsy data in patients with CIU/CSU treated with fixed dose omalizumab did not show significant IgE-/FcεRI-positive cell reductions until Day 85, yet symptoms were reduced by Day 8 (57). Many patients experienced a major clinical effect of omalizumab in the phase 3 studies within 2 weeks (37–39), thus some other mechanism must contribute to omalizumab’s earlier effects. In the phase 3 studies, 70–77% of responders (UAS7 ≤ 6) appear to do so at 4 weeks. This clinical effect was seen in 36–51% of patients at Week 4 and 52–66% at Week 12 (74), the latter within the time frame of receptor downregulation. Studies of mast cell activation or releasability as mechanisms of omalizumab action are needed.
Evidence that further supports the theory that omalizumab can act to change mast cell releasability includes reports that omalizumab improves symptoms in patients with disorders of mast cells (86, 87). The response of two patients was slow, consistent with what is known about the time required for omalizumab to reduce receptors on mast cells compared with basophils (Fig. 1A) (87). Furthermore, a retrospective analysis of omalizumab therapy found several patients with cold urticaria and dermatographism had a complete response (58), yet these conditions do not include a late-phase response or a known role for basophils.
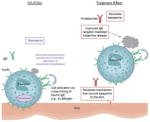
Reversing basopenia and improving basophil IgE receptor function
Evidence that shows basophils play a role in CIU/CSU pathogenesis includes findings that patients with CIU/CSU have basopenia, based on manual and flow cytometry basophil counts and histamine content in blood, which are not routine clinical procedures (61, 88–91). In clinical practice, basopenia can be evaluated by complete blood count (92).
Two observational studies of adult patients with active CIU/CSU found an inverse relationship between the number of basophils and disease severity (65, 88). In two observational studies of patients with CIU/CSU who had recurrent hives for more than 3 months, staining with BB1, a novel monoclonal antibody that recognizes basogranulin, identified that basophils are present in biopsies of nonlesional and lesional skin (7, 93). A recent study confirmed the role of basophil infiltration in CSU and demonstrates that there are slightly higher numbers of basophils in lesional compared with nonlesional skin (94). These findings suggest basopenia may reflect the recruitment of basophils to skin tissue; however, the recruitment pathways for this migration have not been identified (65, 95, 96). An evaluation of basophil infiltration in 24 skin diseases found that basophil accumulation in the skin and basophil activation in the bloodstream are relatively unique to CIU/CSU (12).
Treatment with omalizumab has been shown to increase basophil IgE receptor sensitivity to allergen-driven secretion in patients with allergic airways disease and peanut allergy (49, 97, 98). However, this finding does not directly relate to the mechanism of omalizumab in CIU/CSU because the baseline state of basophils is abnormal in CIU/CSU (26).
Several studies have found that basophils in patients with CIU/CSU released less histamine than healthy control subjects when they were tested with anti-IgE or anti-FcεRI antibodies, but not when exposed to agents that act independent of the FcεRI pathway (62, 63, 90, 95, 99, 100). This suggests that a selective defect in the FcεRI signaling pathway of basophils is likely in patients with CIU/CSU (62, 63, 90, 99, 100). An observational study seeking to identify the mechanism associated with this altered histamine release characterized two functional basophil phenotypes that have been identified among patients with CIU/CSU in approximately equal numbers: those who have normal histamine release (responders) and those who release significantly less histamine (nonresponders) (99). The abnormal responsiveness appears to be due to elevated expression of intracellular phosphatases, which dephosphorylate molecules essential for histamine release (99). In an observational study of 64 patients with CIU/CSU separated by functional basophil phenotypes based on their degree of histamine release, levels of IgG autoantibodies against FcεRI and IgE and functional basophil phenotype remained stable in those with active disease. IgG anti-FcεRI and anti-IgE autoantibody titers did not correspond to basophil functional phenotype, and when basophil IgE receptor function improved in patients who went into remission, no changes in the autoantibody titers were noted (64). A recent study showed that basophils of healthy donors cultured in serum from patients with active CIU/CSU displayed suppression of IgE receptor activation. The suppressive capacity of active CIU/CSU patient serum persisted after IgG or IgE depletion, but was not observed in cultures using serum from patients with CIU/CSU in remission (101). This suggests that active CIU/CSU skin disease may impart a suppressive factor in serum that suppresses basophil IgE function.
Additionally, phase 3 study participants at US sites had whole blood samples monitored for histamine content, an indirect measure of number of basophils. Overall increases in whole blood histamine content were noted in participants treated with omalizumab compared with placebo [(102), manuscript in preparation]. Parallel clinical improvement assessed using the itch severity score supports the concept that improvement of basopenia in active CIU/CSU is associated with reduced clinical symptoms, suggesting again that basophil recruitment to the skin is related to CIU/CSU symptom expression and a pathway that is altered by omalizumab (102).
A recent study comparing omalizumab treatment with placebo in patients with CIU/CSU found that mean peripheral blood basophil counts increased as early as the first 8 days and continued to Day 85 of treatment in patients taking omalizumab (69). Similarly, mean IgE bound to basophils and FcεRI expression on basophils were noticeably reduced by Day 8, remaining suppressed throughout the omalizumab treatment period (57, 69). Interestingly, the timing for the changes in blood basophils aligned with the onset of symptom relief seen on Day 8 of omalizumab treatment, which is earlier than when major changes to skin IgE receptors have been seen in skin biopsies (Fig. 1B) (57, 69). In a separate study with omalizumab in patients with CIU/CSU, Gober et al. found that this therapeutic antibody improved basophil IgE receptor-mediated histamine release (55). While evidence supports a role for basophils in CIU/CSU, the exact origin of the basophil abnormalities in this disease is under active study. Whether these basophil abnormalities are pathogenic in the subpopulation in whom they are evident or are a consequence of having CIU/CSU is unclear. Regardless, omalizumab may reverse a mechanism that recruits basophils to the skin and suppresses their IgE receptor pathway.
Reducing activity of IgG autoantibodies against FcεRI and IgE
The approved fixed dose of omalizumab for use in patients with CIU/CSU reflects that CIU/CSU is not a classic allergen-driven disease (37–39, 42). However, it is a long-held theory that IgG autoantibodies against FcεRI and/or IgE could play a role in approximately 40–45% of patients with CU (16, 28, 103–107). In the most common situation (35– 40%), an IgG autoantibody directed to the α-subunit of FcεRI causes α-subunits to cross-link and leads to in vitro histamine release from basophils and mast cell degranulation in some patients (28, 60, 105). A smaller fraction of patients with CU (5–10%) has functional IgG anti-IgE autoantibodies that release histamine from basophils (106).
The presence of autoantibodies against thyroid antigens (discussed below), FcεRI, and IgE in patients with CIU/CSU, suggests that CIU/CSU is associated with autoimmunity or that there is an autoimmune subset of CIU/CSU in which autoantibodies might contribute to pathogenesis (108, 109). Omalizumab could theoretically remove the effect of the autoimmune antigen by lowering the level of available surface IgE or IgE receptors. In support of this theory, levels of FcεRI- and IgE-positive skin cells in patients with CIU/CSU decreased in lesional and nonlesional skin following treatment with omalizumab (69). However, the timing of this decrease was not seen within the timeframe of the onset of symptom relief at Day 8 of treatment. Clinical efficacy was statistically significant at Week 2, but notable cellular effects were not statistically significant until Day 85 (Fig. 1B) (69). Additionally, grouped data from three phase 3 studies showed that 1 week after starting omalizumab a partial response was experienced by approximately 10% of patients, and after 2 weeks roughly 30% and 15% of patients had a partial and complete response, respectively (74), which is earlier than changes occur in receptor/functional responsiveness of cutaneous mast cells. As noted earlier, omalizumab therapy is successful in patients with or without a positive CU index test for autoantibodies against IgE and FcεRI (37–39, 42, 55, 72). Also, titers of IgG autoantibodies to IgE and FcεRI remain stable as patients enter natural remission (64).
Autoimmunity cannot explain why the drug works in patients with CIU/CSU who lack IgE-anti-TPO autoantibodies and IgG autoantibodies to IgE and FcεRI (72) or why anti-TPO, anti-IgE, and anti-FcεRI autoantibodies can be present in patients with other conditions in the absence of urticaria or in healthy controls (110–112). Yet we know that with age, abnormal, disease-associated autoantibodies (antinuclear antibodies, rheumatoid factor) increase in healthy individuals with no corresponding clinical manifestations (113, 114). Further, autoimmune phenomena in rheumatoid arthritis (anticyclic citrulline antibodies) or systemic lupus erythematosus (SLE; anti-dsDNA) are rarely affected by therapies that control symptoms (115). Related issues are disparate reports of the incidence of anti-FcεRI in nonurticarial patients and disagreement regarding methods utilized (immunoblot, binding assays, ELISA, autologous skin test, or basophil histamine release). For example, one study reported positives by ELISA in 0/41 healthy controls, 3/15 patients with SLE, 16/45 with dermatomyositis, and 106/281 with CU (116). A histamine release assay was performed on a fraction of those in each category, but the only positives were in those with CU, suggesting that the histamine release assay is more specific for CU (116). In keeping with these findings, positive histamine release assays were reported in 54/104 patients with CU and 0/35 patients with nonurticarial allergies seen consecutively in a clinical practice (117). In contrast, a report that demonstrated significant positive basophil histamine release assays in non-CIU/CSU controls (5/22) suggested that a positive histamine release cannot reliably point to CIU/CSU (112), while a separate study found positives by histamine release assay in 3/20 normal controls, 6/26 patients with SLE, and 9/27 with CU (110). Thus, specificity and pathogenicity of these antibodies are not clear even though they are strongly associated with CIU/CSU and are functional.
Reducing activity of IgE autoantibodies against an unknown autoantigen
Elevated levels of IgE autoantibodies against TPO are present in 54% of patients with CIU/CSU (109). While it is unclear whether the twofold difference observed between patients with CIU/CSU and controls is clinically relevant, there is a clear association between CIU/CSU and autoimmune thyroid dysfunction (118–120).
Although there is no direct evidence of the tissue presence of TPO antigens in CIU/CSU, omalizumab could lead to a reduction in the levels of these IgE autoantibodies and/or a decrease in IgE receptor density on mast cells, thus inhibiting mast cell activation (42, 56). As for the more traditionally measured IgG anti-TPO, omalizumab therapy is equally successful in patients who are positive for IgG anti-TPO as compared to those who are anti-TPO negative (38, 42, 56).
Studies are needed to evaluate TPO antigen presence in skin, IgE-anti-TPO as an inducer of mast cell degranulation, and the use of omalizumab in patients with CIU/CSU and no IgE-anti-TPO autoantibodies (56). The incidence of IgE anti-TPO in patients with Hashimoto’s thyroiditis and no urticaria is not known, although they must have IgG-anti-TPO or IgG antithyroglobulin (109).
Reducing activity of intrinsically ‘abnormal’ IgE
‘Abnormal’ IgE may contribute to symptoms in a subset of patients with cold-induced urticaria (107). Passive transfer experiments found that symptoms of urticaria could be induced in healthy patients by injecting them with serum from patients with cold-induced urticaria (121, 122). Further studies found that in some cases, the serum factors that initiated symptoms were associated with IgE (123–125).
The involvement of an IgE cryoglobulin has been disproven, and the association of a cold-inducible antigen in skin has not yet been demonstrated; therefore, transfer of an intrinsically ‘abnormal’ IgE has been suggested as the potential mechanism (126). Additionally, experiments using reverse passive transfer, in which the serum was injected after healthy skin was exposed to the stimulus that induces urticaria symptoms, failed to produce symptoms, suggesting a cold-induced conformational change in bound IgE is needed (121). The potential of such an intrinsically ‘abnormal’ IgE as a component of the pathogenesis of CIU/CSU is then supported by findings that omalizumab was effective in treating a patient with cold-induced urticaria (127). Complete responses have also since been reported in three of six patients with cold urticaria and six of seven with dermatographism (58). These disorders have in common rapid mast cell secretion of histamine, no cellular infiltrate, and reports of successful passive transfer due to IgE.
Similar to the theory that omalizumab reduces the activity of IgE autoantibodies, omalizumab may help to reduce the opportunity for ‘abnormal’ IgE to stimulate mast cells and basophils. Recent ex vivo observations that a high concentration of omalizumab catalyzes rapid dissociation of bound IgE from human basophils isolated from patients with allergic disease could explain rapid responses to omalizumab in patients with CIU/CSU if the IgE were an autoantibody to any endogenous antigen or if the antibody was in fact causing or catalyzing cutaneous mast cell secretion. However, whether these findings are physiologically relevant in the clinical setting, where concentrations of omalizumab used are lower, is unknown (128). It has also been proposed that a combination of signals—not just IgE—is required to activate mast cells in patients with urticaria (121).
Decreasing the role of coagulation involvement
Because of the tight interplay between coagulation and inflammation, coagulation may play a role in the pathology of CU (129–136) and thus may be relevant to CIU/CSU. A series of studies have found that increased levels of prothrombin cleavage fragments resulting from an activated extrinsic coagulation pathway may play a role in the pathogenesis of CU. Plasma markers of thrombin generation were elevated in patients with active CU, and these markers decrease during remission (130–133). Accelerated thrombin generation might activate mast cells and increase the permeability of skin (137, 138).
In patients with CU, eosinophils are the main cells expressing tissue factor (139), which activates the coagulation cascade that leads to thrombin formation (132). In skin lesions of patients with CU, tissue factor was strongly expressed by upper dermal inflammatory cells, with increased immunoreactivity compared with normal controls (132). Furthermore, association of disease severity with the activation of the coagulation pathway was observed (132). Eosinophils can be activated by IgG autoantibodies against FcεRII/CD23 that are found in some patients with CIU/CSU (140). However, the activation might be secondary to the activation of mast cells by FcεRI and IgE autoantibodies or unknown factors (133). It is proposed that eosinophils may play a role in CIU/CSU pathology only in patients who have IgG antibodies against FcεRII (whose incidence is unknown) but not against FcεRI and IgE (141).
In studies using rodent mast cells, thrombin has been shown to induce mast cell degranulation (138, 142). However, there are no human studies showing that thrombin induces mast cell degranulation (141) and ‘active’ thrombosis has never been demonstrated to be present in CIU/CSU (129). A confounding observation is that the same abnormality in markers of thrombin formation is present in hereditary angioedema types I and II, for which the pathogenesis is clear (143, 144), and there is no clinical thrombosis. Here, coagulation abnormalities could reflect endothelial cell activation related to bradykinin formation, suggesting abnormalities that reflect increased vascular permeability regardless of cause.
Of the potential mechanisms discussed, in the absence of supporting data, this is the most speculative in terms of a direct/indirect role for omalizumab. Nonetheless, it is plausible that the coagulation system is implicated in CIU/CSU, so its inclusion is important.
Summary
Although it is clear that omalizumab is an effective treatment for many patients with CIU/CSU, the mechanism of action remains elusive. Much has been learned to date, yet no current theories, alone or in combination, can explain all aspects of the mechanisms that underlie the efficacy of omalizumab for patients with CIU/CSU (Table 1). Known mechanisms of mast cell activation or releasability, or downregulation of IgE receptors do not explain all the effects of omalizumab. The reversal of basopenia and improvement of basophil IgE receptor function observed with use of omalizumab do not explain the effect on mast cells and other cells involved in CIU/CSU lesions. Furthermore, the incidence of IgG autoantibodies against FcεRI and IgE, and IgE autoantibodies against an antigen or autoantigen that has yet to be definitively identified does not exceed 50%, and the extent of overlap with those who have basophil abnormalities is not known. The rate of symptom relief does not align with the rate of omalizumab’s effect on mast cell receptors affected by IgG autoantibodies. Potential reduction in intrinsically ‘abnormal’ IgE has been considered, but symptoms of CIU/CSU may require more than one initiating component. Finally, much is yet to be learned about the potential role of coagulation involvement in CIU/CSU.
Table 1.
Summary of potential mechanisms
| Theory | Supporting evidence | Conflicting evidence | Illustration |
|---|---|---|---|
| Lowers IgE levels and downregulates IgE receptors |
|
|
|
| Reduces mast cell releasability |
|
|
|
| Reverses basopenia and improves basophil IgE receptor function |
|
|
|
| Reduces activity of IgG autoantibodies against FcεRI and IgE |
|
|
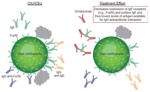
|
| Reduces activity of IgE autoantibodies against an autoantigen |
|
|
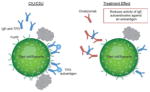
|
| Reduces activity of intrinsically ‘abnormal’ IgE |
|
|
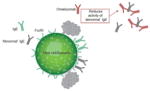
|
| Decreases the role of coagulation involvement |
|
|
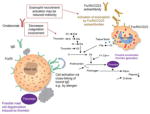
|
CIU/CSU, chronic idiopathic/spontaneous urticaria; CU, chronic urticaria; HAE, hereditary angioedema; IgE, immunoglobulin E; TPO, thyroper-oxidase.
Given that subsets of patients with CIU/CSU may have different disease mechanisms that are not yet fully understood, the challenge of finding evidence for more than one mechanism is great. Additional research is needed to further explore each of these potential explanations for the mechanism of omalizumab (e.g., the role of mast cells vs basophils, cell reactivity, autoantibodies, and potential unknown serum factors) and the likely interplay among the theories.
Additional areas for future study may include the ability of omalizumab to inhibit the release of inflammatory cytokines, chemokines, and common mediators involved in wheal pathogenesis from cutaneous mast cells, basophils, and the vasculature. When antihistamine- and leukotriene-resistant patients are studied, approximately 40% have a complete response to omalizumab (i.e., no urticaria) with a total response rate of approximately 50–70% after 12 weeks. A small fraction respond extraordinarily quickly (i.e., within a few days), while the remainder improve more gradually, within 2–10 weeks (37–39). Thus, unique mechanisms may be relevant for subpopulations of patients with CIU/CSU, and studies providing definitive evidence about omalizumab’s mechanism of action should help define the underlying pathogenesis of this vexing disorder.
Acknowledgments
Funding
Medical writing and editorial assistance in the development of this manuscript were provided by Sarah Thornburg at JK Associates, Inc., Conshohocken, PA, USA, a part of the Fishawack Group of Companies, and this service was supported by Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA, and Genentech, Inc., South San Francisco, CA, USA.
Footnotes
Author contributions
All authors prepared the report, contributed to data interpretation, and critically revised and approved the submitted manuscript.
Conflict of interests
Allen P. Kaplan is a consultant for Novartis/Genentech, and he also adjudicates allergic reactions due to antihypertensive agents and drugs for diabetes for Novartis. Ana M. Giménez-Arnau is a medical advisor for Uriach Pharma, Genentech, and Novartis. She has received research grants from Intendis—Bayer, Uriach Pharma, and Novartis and has participated in educational activities sponsored by Uriach Pharma, Novartis, Genentech, Menarini, GSK, MSD, Almirall, and Leo Pharma. Sarbjit S. Saini has received grant support from the National Institutes of Health, Novartis, and Astra-Zeneca; he is a consultant to Genentech, Medimmune, Novartis, Ono Pharma, and Regeneron.
References
- 1.Bernstein JA, Lang DM, Khan DA, Craig T, Dreyfus D, Hsieh F, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270–1277. doi: 10.1016/j.jaci.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 2.Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014;69:868–887. doi: 10.1111/all.12313. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan AP. Chronic urticaria: pathogenesis and treatment. J Allergy Clin Immunol. 2004;114:465–474. doi: 10.1016/j.jaci.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 4.Davis KC, Mekori YA, Kohler PF, Schocket AL. Late cutaneous reactions in patients with delayed pressure urticaria. J Allergy Clin Immunol. 1984;73:810–812. doi: 10.1016/0091-6749(84)90451-2. [DOI] [PubMed] [Google Scholar]
- 5.Dolovich J, Ruhno J, Sauder DN, Ahlstedt S, Hargreave FE. Isolated late cutaneous skin test response to ampicillin: a distinct entity. J Allergy Clin Immunol. 1988;82:676–679. doi: 10.1016/0091-6749(88)90982-7. [DOI] [PubMed] [Google Scholar]
- 6.Mekori YA, Dobozin BS, Schocket AL, Kohler PF, Clark RA. Delayed pressure urticaria histologically resembles cutaneous late-phase reactions. Arch Dermatol. 1988;124:230–235. [PubMed] [Google Scholar]
- 7.Ying S, Kikuchi Y, Meng Q, Kay AB, Kaplan AP. TH1/TH2 cytokines and inflammatory cells in skin biopsy specimens from patients with chronic idiopathic urticaria: comparison with the allergen-induced late-phase cutaneous reaction. J Allergy Clin Immunol. 2002;109:694–700. doi: 10.1067/mai.2002.123236. [DOI] [PubMed] [Google Scholar]
- 8.Solley GO, Gleich GJ, Jordon RE, Schroeter AL. The late phase of the immediate wheal and flare skin reaction. Its dependence upon IgE antibodies. J Clin Invest. 1976;58:408–420. doi: 10.1172/JCI108485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maurer M, Weller K, Bindslev-Jensen C, Gimenez-Arnau A, Bousquet PJ, Bousquet J, et al. Unmet clinical needs in chronic spontaneous urticaria. A GA(2) LEN task force report. Allergy. 2011;66:317–330. doi: 10.1111/j.1398-9995.2010.02496.x. [DOI] [PubMed] [Google Scholar]
- 10.Broder MS, Raimundo K, Antonova E, Chang E. Resource use and costs in an insured population of patients with chronic idiopathic/spontaneous urticaria. Am J Clin Dermatol. 2015;16:313–321. doi: 10.1007/s40257-015-0134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saini SS. Chronic spontaneous urticaria: etiology and pathogenesis. Immunol Allergy Clin North Am. 2014;34:33–52. doi: 10.1016/j.iac.2024.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito Y, Satoh T, Takayama K, Miyagishi C, Walls AF, Yokozeki H. Basophil recruitment and activation in inflammatory skin diseases. Allergy. 2011;66:1107–1113. doi: 10.1111/j.1398-9995.2011.02570.x. [DOI] [PubMed] [Google Scholar]
- 13.Sabroe RA, Poon E, Orchard GE, Lane D, Francis DM, Barr RM, et al. Cutaneous inflammatory cell infiltrate in chronic idiopathic urticaria: comparison of patients with and without anti-FcεRI or anti-IgE autoantibodies. J Allergy Clin Immunol. 1999;103:484–493. doi: 10.1016/s0091-6749(99)70475-6. [DOI] [PubMed] [Google Scholar]
- 14.Natbony SF, Phillips ME, Elias JM, Godfrey HP, Kaplan AP. Histologic studies of chronic idiopathic urticaria. J Allergy Clin Immunol. 1983;71:177–183. doi: 10.1016/0091-6749(83)90096-9. [DOI] [PubMed] [Google Scholar]
- 15.Heavey DJ, Kobza-Black A, Barrow SE, Chappell CG, Greaves MW, Dollery CT. Prostaglandin D2 and histamine release in cold urticaria. J Allergy Clin Immunol. 1986;78:458–461. doi: 10.1016/0091-6749(86)90033-3. [DOI] [PubMed] [Google Scholar]
- 16.Ferrer M, Kinet JP, Kaplan AP. Comparative studies of functional and binding assays for IgG anti-FcεRIα (alpha-subunit) in chronic urticaria. J Allergy Clin Immunol. 1998;101:672–676. doi: 10.1016/s0091-6749(98)70176-9. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan AP, Greaves M. Pathogenesis of chronic urticaria. Clin Exp Allergy. 2009;39:777–787. doi: 10.1111/j.1365-2222.2009.03256.x. [DOI] [PubMed] [Google Scholar]
- 18.Maurice PD, Barr RM, Koro O, Greaves MW. The effect of prostaglandin D2 on the response of human skin to histamine. J Invest Dermatol. 1987;89:245–248. doi: 10.1111/1523-1747.ep12471120. [DOI] [PubMed] [Google Scholar]
- 19.Ormerod AD, Kobza Black A, Dawes J, Murdoch RD, Koro O, Barr RM, et al. Prostaglandin D2 and histamine release in cold urticaria unaccompanied by evidence of platelet activation. J Allergy Clin Immunol. 1988;82:586–589. doi: 10.1016/0091-6749(88)90968-2. [DOI] [PubMed] [Google Scholar]
- 20.Saarinen JV, Harvima RJ, Horsmanheimo M, Harvima IT. Modulation of the immediate allergic wheal reaction in the skin by drugs inhibiting the effects of leukotriene C4 and prostaglandin D2. Eur J Clin Pharmacol. 2001;57:1–4. doi: 10.1007/s002280000249. [DOI] [PubMed] [Google Scholar]
- 21.Curto-Barredo LNL, Puigdecanet E, Jansen N, Ruberg S, Pujol RM, Santamaria-Babi L, et al. Gene expression profiling in chronic spontaneous urticaria. J Invest Dermatol. 2014;134:s73. [Google Scholar]
- 22.Patel OP, Giorno RC, Dibbern DA, Andrews KY, Durairaj S, Dreskin SC. Gene expression profiles in chronic idiopathic (spontaneous) urticaria. Allergy Rhinol. 2015;6:101–110. doi: 10.2500/ar.2015.6.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye YM, Kim BE, Shin YS, Park HS, Leung DY. Increased epidermal filaggrin in chronic idiopathic urticaria is associated with severity of urticaria. Ann Allergy Asthma Immunol. 2014;112:533– 538. doi: 10.1016/j.anai.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morimoto K, Shirata N, Taketomi Y, Tsuchiya S, Segi-Nishida E, Inazumi T, et al. Prostaglandin E2-EP3 signaling induces inflammatory swelling by mast cell activation. J Immunol. 2014;192:1130–1137. doi: 10.4049/jimmunol.1300290. [DOI] [PubMed] [Google Scholar]
- 25.Suurmond J, Dorjee AL, Knol EF, Huizinga TW, Toes RE. Differential TLRinduced cytokine production by human mast cells is amplified by FcvarεRI triggering. Clin Exp Allergy. 2015;45:788–796. doi: 10.1111/cea.12509. [DOI] [PubMed] [Google Scholar]
- 26.Vonakis BM, Saini SS. New concepts in chronic urticaria. Curr Opin Immunol. 2008;20:709–716. doi: 10.1016/j.coi.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saini SS, Paterniti M, Vasagar K, Gibbons SP, Jr, Sterba PM, Vonakis BM. Cultured peripheral blood mast cells from chronic idiopathic urticaria patients spontaneously degranulate upon IgE sensitization: Relationship to expression of Syk and SHIP-2. Clin Immunol. 2009;132:342–348. doi: 10.1016/j.clim.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hide M, Francis DM, Grattan CE, Hakimi J, Kochan JP, Greaves MW. Autoantibodies against the high-affinity IgE receptor as a cause of histamine release in chronic urticaria. N Engl J Med. 1993;328:1599–1604. doi: 10.1056/NEJM199306033282204. [DOI] [PubMed] [Google Scholar]
- 29.Bossi F, Frossi B, Radillo O, Cugno M, Tedeschi A, Riboldi P, et al. Mast cells are critically involved in serum-mediated vascular leakage in chronic urticaria beyond high-affinity IgE receptor stimulation. Allergy. 2011;66:1538–1545. doi: 10.1111/j.1398-9995.2011.02704.x. [DOI] [PubMed] [Google Scholar]
- 30.Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan AP, Popov TA. Biologic agents and the therapy of chronic spontaneous urticaria. Curr Opin Allergy Clin Immunol. 2014;14:347–353. doi: 10.1097/ACI.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 32.Chang TW, Chen C, Lin CJ, Metz M, Church MK, Maurer M. The potential pharmacologic mechanisms of omalizumab in patients with chronic spontaneous urticaria. J Allergy Clin Immunol. 2015;135:337–342. doi: 10.1016/j.jaci.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 33.Xolair® (omalizumab) US prescribing information 2016. 2014 Available from: https://www.gene.com/download/pdf/xolair_prescribing.pdf.
- 34.European Medicines Agency. Xolair: European public assessment report - Product Information Summary of product characteristics. 2015 [cited January 19, 2015]; Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000606/WC500057298.pdf.
- 35.Goldenberg MM. Pharmaceutical approval update. P T. 2014;39:415–423. [PMC free article] [PubMed] [Google Scholar]
- 36.European Medicines Agency. Xolair-H-C-606-II-48: EPAR - Assessment Report - Variation. 2014 [cited January 19, 2015]; Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000606/WC500164453.pdf.
- 37.Kaplan A, Ledford D, Ashby M, Canvin J, Zazzali JL, Conner E, et al. Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J Allergy Clin Immunol. 2013;132:101–109. doi: 10.1016/j.jaci.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 38.Maurer M, Rosen K, Hsieh HJ, Saini S, Grattan C, Gimenez-Arnau A, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368:924–935. doi: 10.1056/NEJMoa1215372. [DOI] [PubMed] [Google Scholar]
- 39.Saini SS, Bindslev-Jensen C, Maurer M, Grob JJ, Bulbul Baskan E, Bradley MS, et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on h1 antihistamines: a randomized, placebo controlled study. J Invest Dermatol. 2015;135:67–75. doi: 10.1038/jid.2014.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacGlashan DW, Jr, Bochner BS, Adelman DC, Jardieu PM, Togias A, McKenzie-White J, et al. Down-regulation of FcεRI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol. 1997;158:1438–1445. [PubMed] [Google Scholar]
- 41.Saini SS, MacGlashan DW, Jr, Sterbinsky SA, Togias A, Adelman DC, Lichtenstein LM, et al. Down-regulation of human basophil IgE and FCεRIα surface densities and mediator release by anti-IgE-infusions is reversible in vitro and in vivo. J Immunol. 1999;162:5624–5630. [PubMed] [Google Scholar]
- 42.Saini S, Rosen KE, Hsieh HJ, Wong DA, Conner E, Kaplan A, et al. A randomized, placebo-controlled, dose-ranging study of single-dose omalizumab in patients with H1-antihistamine-refractory chronic idiopathic urticaria. J Allergy Clin Immunol. 2011;128:567–573. doi: 10.1016/j.jaci.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Beck LA, Marcotte GV, MacGlashan D, Togias A, Saini S. Omalizumab-induced reductions in mast cell Fce psilon RI expression and function. J Allergy Clin Immunol. 2004;114:527–530. doi: 10.1016/j.jaci.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 44.Presta LG, Lahr SJ, Shields RL, Porter JP, Gorman CM, Fendly BM, et al. Humanization of an antibody directed against IgE. J Immunol. 1993;151:2623–2632. [PubMed] [Google Scholar]
- 45.Shields RL, Whether WR, Zioncheck K, O’Connell L, Fendly B, Presta LG, et al. Inhibition of allergic reactions with antibodies to IgE. Int Arch Allergy Immunol. 1995;107:308–312. doi: 10.1159/000237010. [DOI] [PubMed] [Google Scholar]
- 46.Schroeder JT, Bieneman AP, Chichester KL, Hamilton RG, Xiao H, Saini SS, et al. Decreases in human dendritic cell-dependent T(H)2-like responses after acute in vivo IgE neutralization. J Allergy Clin Immunol. 2010;125:896–901. doi: 10.1016/j.jaci.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 47.MacGlashan D, Jr, Xia HZ, Schwartz LB, Gong J. IgE-regulated loss, not IgE-regulated synthesis, controls expression of FcεRI in human basophils. J Leukoc Biol. 2001;70:207–218. [PubMed] [Google Scholar]
- 48.Prussin C, Griffith DT, Boesel KM, Lin H, Foster B, Casale TB. Omalizumab treatment downregulates dendritic cell FcεRI expression. J Allergy Clin Immunol. 2003;112:1147–1154. doi: 10.1016/j.jaci.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Macglashan DW, Jr, Saini SS. Omalizumab increases the intrinsic sensitivity of human basophils to IgE-mediated stimulation. J Allergy Clin Immunol. 2013;132:906–911. doi: 10.1016/j.jaci.2013.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arm JP, Bottoli I, Skerjanec A, Floch D, Groenewegen A, Maahs S, et al. Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clin Exp Allergy. 2014;44:1371–1385. doi: 10.1111/cea.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eckman JA, Sterba PM, Kelly D, Alexander V, Liu MC, Bochner BS, et al. Effects of omalizumab on basophil and mast cell responses using an intranasal cat allergen challenge. J Allergy Clin Immunol. 2010;125:889–895. doi: 10.1016/j.jaci.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Savage JH, Courneya JP, Sterba PM, Macglashan DW, Saini SS, Wood RA. Kinetics of mast cell, basophil, and oral food challenge responses in omalizumab-treated adults with peanut allergy. J Allergy Clin Immunol. 2012;130:1123–1129. doi: 10.1016/j.jaci.2012.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paterniti MO, Breslin LM, Courneya JP, Sterba PM, Hamilton RG, MacGlashan DW, Jr, et al. Differences in effects of omalizumab on late-phase responses to allergen challenge in the skin and nose at the time of basophil hyporesponsiveness. J Invest Dermatol. 2014;134:1743–1744. doi: 10.1038/jid.2013.541. [DOI] [PubMed] [Google Scholar]
- 54.Ong YE, Menzies-Gow A, Barkans J, Benyahia F, Ou TT, Ying S, et al. Anti-IgE (omalizumab) inhibits late-phase reactions and inflammatory cells after repeat skin allergen challenge. J Allergy Clin Immunol. 2005;116:558–564. doi: 10.1016/j.jaci.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 55.Gober LMSP, Eckman JA, Saini SS. Effect of anti-IgE (Omalizumab) in Chronic Idiopathic Urticaria (CIU) patients. J Allergy Clin Immunol. 2008;121:S147. [Google Scholar]
- 56.Maurer M, Altrichter S, Bieber T, Biedermann T, Brautigam M, Seyfried S, et al. Efficacy and safety of omalizumab in patients with chronic urticaria who exhibit IgE against thyroperoxidase. J Allergy Clin Immunol. 2011;128:202–209. doi: 10.1016/j.jaci.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 57.Metz M, Staubach P, Bauer A, Brehler R, Gericke J, Ashton-Chess J, et al. Omalizumab normalizes levels of high affinity immunoglobulin E receptor-positive skin cells in patients with chronic spontaneous urticaria: a randomized, double-blind, placebo-controlled study. J Invest Dermatol. 2014;134:S30–S38. [Google Scholar]
- 58.Metz M, Ohanyan T, Church MK, Maurer M. Omalizumab is an effective and rapidly acting therapy in difficult-to-treat chronic urticaria: a retrospective clinical analysis. J Dermatol Sci. 2014;73:57–62. doi: 10.1016/j.jdermsci.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 59.Charlesworth EN, Kagey-Sobotka A, Schleimer RP, Norman PS, Lichtenstein LM. Prednisone inhibits the appearance of inflammatory mediators and the influx of eosinophils and basophils associated with the cutaneous late-phase response to allergen. J Immunol. 1991;146:671– 676. [PubMed] [Google Scholar]
- 60.Kaplan AP, Joseph K, Maykut RJ, Geba GP, Zeldin RK. Treatment of chronic autoimmune urticaria with omalizumab. J Allergy Clin Immunol. 2008;122:569–573. doi: 10.1016/j.jaci.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 61.Rorsman H. Basophilic leucopenia in different forms of urticaria. Acta Allergol. 1962;17:168–184. doi: 10.1111/j.1398-9995.1962.tb02937.x. [DOI] [PubMed] [Google Scholar]
- 62.Kern F, Lichtenstein LM. Defective histamine release in chronic urticaria. J Clin Invest. 1976;57:1369–1377. doi: 10.1172/JCI108405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Greaves MW, Plummer VM, McLaughlan P, Stanworth DR. Serum and cell bound IgE in chronic urticaria. Clin Allergy. 1974;4:265–271. doi: 10.1111/j.1365-2222.1974.tb01384.x. [DOI] [PubMed] [Google Scholar]
- 64.Eckman JA, Hamilton RG, Gober LM, Sterba PM, Saini SS. Basophil phenotypes in chronic idiopathic urticaria in relation to disease activity and autoantibodies. J Invest Dermatol. 2008;128:1956–1963. doi: 10.1038/jid.2008.55. [DOI] [PubMed] [Google Scholar]
- 65.Oliver ET, Sterba PM, Saini SS. Interval shifts in basophil measures correlate with disease activity in chronic spontaneous urticaria. Allergy. 2015;70:601–603. doi: 10.1111/all.12578. [DOI] [PubMed] [Google Scholar]
- 66.Spector SL, Tan RA. Effect of omalizumab on patients with chronic urticaria. Ann Allergy Asthma Immunol. 2007;99:190–193. doi: 10.1016/S1081-1206(10)60644-8. [DOI] [PubMed] [Google Scholar]
- 67.Oliver JM, Tarleton CA, Gilmartin L, Archibeque T, Qualls CR, Diehl L, et al. Reduced FcεRI-mediated release of asthma-promoting cytokines and chemokines from human basophils during omalizumab therapy. Int Arch Allergy Immunol. 2010;151:275–284. doi: 10.1159/000250436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Borkowski TA, Jouvin MH, Lin SY, Kinet JP. Minimal requirements for IgE-mediated regulation of surface FcεRI. J Immunol. 2001;167:1290–1296. doi: 10.4049/jimmunol.167.3.1290. [DOI] [PubMed] [Google Scholar]
- 69.Metz M, Staubach P, Bauer A, Brehler R, Gericke J, Kangas M, et al. Omalizumab normalizes levels of high affinity IgE receptor-positive skin cells in patients with chronic spontaneous urticaria: a randomized, double-blind, placebo-controlled study. 44th Annual ESDR Meeting; Copenhagen, Denmark. 2014. [Google Scholar]
- 70.Metz MTR, Letzkus M, Hartmann N, Staubach P, Bauer A, Brehler R, et al. Omalizumab normalizes gene expression in lesional skin of patients with chronic spontaneous urticaria: results from a randomized, double-blind, placebo-controlled study. European Academy of Dermatology and Venereology 2015; 2015; June 2015; Copenhagen, Denmark. 2015. [Google Scholar]
- 71.Novartis. Data on File. [Google Scholar]
- 72.Ferrer M, Gamboa P, Sanz ML, Goikoetxea MJ, Cabrera-Freitag P, Javaloyes G, et al. Omalizumab is effective in nonautoimmune urticaria. J Allergy Clin Immunol. 2011;127:1300–1302. doi: 10.1016/j.jaci.2010.12.1085. [DOI] [PubMed] [Google Scholar]
- 73.Gomez G, Jogie-Brahim S, Shima M, Schwartz LB. Omalizumab reverses the phenotypic and functional effects of IgE-enhanced FcεRI on human skin mast cells. J Immunol. 2007;179:1353–1361. doi: 10.4049/jimmunol.179.2.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaplan A, Ferrer M, Bernstein JA, Antonova E, Trzaskoma B, Raimundo K, et al. Timing and duration of omalizumab response in patients with chronic idiopathic/spontaneous urticaria. J Allergy Clin Immunol. 2016;137:474–481. doi: 10.1016/j.jaci.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 75.Kaplan A, Antonova E, Trzaskoma B, Raimundo K, Rosen K, Omachi TA, et al. Response patterns in chronic idiopathic/spontaneous urticaria (CIU/CSU) patients treated with omalizumab for 24 weeks in two randomized, double-blind, placebocontrolled clinical trials (ASTERIA I and GLACIAL) J Allergy Clin Immunol. 2015;135:AB127. [Google Scholar]
- 76.Chang TW, Shiung YY. Anti-IgE as a mast cell-stabilizing therapeutic agent. J Allergy Clin Immunol. 2006;117:1203–1212. doi: 10.1016/j.jaci.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 77.Fukuzumi T, Waki N, Kanakura Y, Nagoshi J, Hirota S, Yoshikawa K, et al. Differences in irradiation susceptibility and turnover between mucosal and connective tissue-type mast cells of mice. Exp Hematol. 1990;18:843–847. [PubMed] [Google Scholar]
- 78.Fureder W, Agis H, Willheim M, Bankl HC, Maier U, Kishi K, et al. Differential expression of complement receptors on human basophils and mast cells. Evidence for mast cell heterogeneity and CD88/C5aR expression on skin mast cells. J Immunol. 1995;155:3152–3160. [PubMed] [Google Scholar]
- 79.Church MK, Benyon RC, Lowman MA, Hutson PA, Holgate ST. Allergy or inflammation? From neuropeptide stimulation of human skin mast cells to studies on the mechanism of the late asthmatic response. Agents Actions. 1989;26:22–30. doi: 10.1007/BF02126554. [DOI] [PubMed] [Google Scholar]
- 80.Jacques P, Lavoie A, Bedard PM, Brunet C, Hebert J. Chronic idiopathic urticaria: profiles of skin mast cell histamine release during active disease and remission. J Allergy Clin Immunol. 1992;89:1139–1143. doi: 10.1016/0091-6749(92)90297-f. [DOI] [PubMed] [Google Scholar]
- 81.Bedard PM, Brunet C, Pelletier G, Hebert J. Increased compound 48/80 induced local histamine release from nonlesional skin of patients with chronic urticaria. J Allergy Clin Immunol. 1986;78:1121–1125. doi: 10.1016/0091-6749(86)90260-5. [DOI] [PubMed] [Google Scholar]
- 82.Brunet C, Bedard PM, Hebert J. Effects of H1-antihistamine drug regimen on histamine release by nonlesional skin mast cells of patients with chronic urticaria. J Allergy Clin Immunol. 1990;86:787–793. doi: 10.1016/s0091-6749(05)80184-8. [DOI] [PubMed] [Google Scholar]
- 83.Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, et al. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun. 2006;349:1322–1328. doi: 10.1016/j.bbrc.2006.08.177. [DOI] [PubMed] [Google Scholar]
- 84.Fujisawa D, Kashiwakura J, Kita H, Kikukawa Y, Fujitani Y, Sasaki-Sakamoto T, et al. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J Allergy Clin Immunol. 2014;134:622–633. doi: 10.1016/j.jaci.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 85.MacGlashan D. Loss of receptors and IgE in vivo during treatment with anti-IgE antibody. J Allergy Clin Immunol. 2004;114:1472–1474. doi: 10.1016/j.jaci.2004.07.064. [DOI] [PubMed] [Google Scholar]
- 86.Carter MC, Robyn JA, Bressler PB, Walker JC, Shapiro GG, Metcalfe DD. Omalizumab for the treatment of unprovoked anaphylaxis in patients with systemic mastocytosis. J Allergy Clin Immunol. 2007;119:1550–1551. doi: 10.1016/j.jaci.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 87.Molderings GJ, Raithel M, Kratz F, Azemar M, Haenisch B, Harzer S, et al. Omalizumab treatment of systemic mast cell activation disease: experiences from four cases. Intern Med. 2011;50:611–615. doi: 10.2169/internalmedicine.50.4640. [DOI] [PubMed] [Google Scholar]
- 88.Grattan CE, Dawn G, Gibbs S, Francis DM. Blood basophil numbers in chronic ordinary urticaria and healthy controls: diurnal variation, influence of loratadine and prednisolone and relationship to disease activity. Clin Exp Allergy. 2003;33:337– 341. doi: 10.1046/j.1365-2222.2003.01589.x. [DOI] [PubMed] [Google Scholar]
- 89.Grattan CE, Walpole D, Francis DM, Niimi N, Dootson G, Edler S, et al. Flow cytometric analysis of basophil numbers in chronic urticaria: basopenia is related to serum histamine releasing activity. Clin Exp Allergy. 1997;27:1417–1424. doi: 10.1046/j.1365-2222.1997.1630972.x. [DOI] [PubMed] [Google Scholar]
- 90.Sabroe RA, Francis DM, Barr RM, Black AK, Greaves MW. Anti-Fc(episilon) RI auto antibodies and basophil histamine releasability in chronic idiopathic urticaria. J Allergy Clin Immunol. 1998;102:651–658. doi: 10.1016/s0091-6749(98)70283-0. [DOI] [PubMed] [Google Scholar]
- 91.Saini SSVK, Haung F, Gibbons SP, Vonakis BM. Signaling defects in basophils in chronic urticaria. J Allergy Clin Immunol. 2003;111:S178–S179. [Google Scholar]
- 92.Magen E, Mishal J, Zeldin Y, Schlesinger M. Clinical and laboratory features of antihistamine-resistant chronic idiopathic urticaria. Allergy Asthma Proc. 2011;32:460–466. doi: 10.2500/aap.2011.32.3483. [DOI] [PubMed] [Google Scholar]
- 93.Caproni M, Giomi B, Volpi W, Melani L, Schincaglia E, Macchia D, et al. Chronic idiopathic urticaria: infiltrating cells and related cytokines in autologous seruminduced wheals. Clin Immunol. 2005;114:284–292. doi: 10.1016/j.clim.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 94.Kay AB, Ying S, Ardelean E, Mlynek A, Kita H, Clark P, et al. Elevations in vascular markers and eosinophils in chronic spontaneous urticarial weals with low-level persistence in uninvolved skin. Br J Dermatol. 2014;171:505–511. doi: 10.1111/bjd.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saini SS. Basophil responsiveness in chronic urticaria. Curr Allergy Asthma Rep. 2009;9:286–290. doi: 10.1007/s11882-009-0040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oliver ET, Sterba PM, Devine K, Vonakis BM, Saini SS. Altered expression of chemoattractant receptor-homologous molecule expressed on T(H)2 cells on blood basophils and eosinophils in patients with chronic spontaneous urticaria. J Allergy Clin Immunol. 2016;137:304–306. doi: 10.1016/j.jaci.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 97.MacGlashan DW, Jr, Savage JH, Wood RA, Saini SS. Suppression of the basophil response to allergen during treatment with omalizumab is dependent on 2 competing factors. J Allergy Clin Immunol. 2012;130:1130–1135. doi: 10.1016/j.jaci.2012.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zaidi AK, Saini SS, MacGlashan DW., Jr Regulation of Syk kinase and FcRbeta expression in human basophils during treatment with omalizumab. J Allergy Clin Immunol. 2010;125:902–908. doi: 10.1016/j.jaci.2009.12.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vonakis BM, Vasagar K, Gibbons SP, Jr, Gober L, Sterba PM, Chang H, et al. Basophil FcεRI histamine release parallels expression of Src-homology 2-containing inositol phosphatases in chronic idiopathic urticaria. J Allergy Clin Immunol. 2007;119:441–448. doi: 10.1016/j.jaci.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 100.Luquin E, Kaplan AP, Ferrer M. Increased responsiveness of basophils of patients with chronic urticaria to sera but hypo-responsiveness to other stimuli. Clin Exp Allergy. 2005;35:456–460. doi: 10.1111/j.1365-2222.2005.02212.x. [DOI] [PubMed] [Google Scholar]
- 101.Sterba PM, Hamilton RG, Saini SS. Suppression of basophil FcvarεRI activation by serum from active chronic idiopathic/spontaneous urticaria (CIU/CSU) subjects. J Invest Dermatol. 2015;135:1454–1456. doi: 10.1038/jid.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saini SSRK, Hsieh HJ, Sterba PM, Courneya JP, Hulter H, Chen H. Whole blood histamine concentration response to omalizumab in patients with chronic idiopathic/spontaneous urticaria: post hoc analysis of ASTERIA I, ASTERIA II and GLACIAL studies. J Allergy Clin Immunol. 2014;133:AB117. [Google Scholar]
- 103.Niimi N, Francis DM, Kermani F, O’Donnell BF, Hide M, Kobza-Black A, et al. Dermal mast cell activation by autoantibodies against the high affinity IgE receptor in chronic urticaria. J Invest Dermatol. 1996;106:1001–1006. doi: 10.1111/1523-1747.ep12338544. [DOI] [PubMed] [Google Scholar]
- 104.Kikuchi Y, Kaplan AP. Mechanisms of autoimmune activation of basophils in chronic urticaria. J Allergy Clin Immunol. 2001;107:1056–1062. doi: 10.1067/mai.2001.115484. [DOI] [PubMed] [Google Scholar]
- 105.Fiebiger E, Maurer D, Holub H, Reininger B, Hartmann G, Woisetschlager M, et al. Serum IgG autoantibodies directed against the alpha chain of FcεRI: a selective marker and pathogenetic factor for a distinct subset of chronic urticaria patients? J Clin Invest. 1995;96:2606–2612. doi: 10.1172/JCI118325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tong LJ, Balakrishnan G, Kochan JP, Kinet JP, Kaplan AP. Assessment of autoimmunity in patients with chronic urticaria. J Allergy Clin Immunol. 1997;99:461– 465. doi: 10.1016/s0091-6749(97)70071-x. [DOI] [PubMed] [Google Scholar]
- 107.Gruber BL, Baeza ML, Marchese MJ, Agnello V, Kaplan AP. Prevalence and functional role of anti-IgE autoantibodies in urticarial syndromes. J Invest Dermatol. 1988;90:213–217. doi: 10.1111/1523-1747.ep12462239. [DOI] [PubMed] [Google Scholar]
- 108.Konstantinou GN, Asero R, Ferrer M, Knol EF, Maurer M, Raap U, et al. EAACI taskforce position paper: evidence for autoimmune urticaria and proposal for defining diagnostic criteria. Allergy. 2013;68:27–36. doi: 10.1111/all.12056. [DOI] [PubMed] [Google Scholar]
- 109.Altrichter S, Peter HJ, Pisarevskaja D, Metz M, Martus P, Maurer M. IgE mediated autoallergy against thyroid peroxidase – a novel pathomechanism of chronic spontaneous urticaria? PLoS One. 2011;6:e14794. doi: 10.1371/journal.pone.0014794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cho CB, Stutes SA, Altrich ML, Ardoin SP, Phillips G, Ogbogu PU. Autoantibodies in chronic idiopathic urticaria and nonurticarial systemic autoimmune disorders. Ann Allergy Asthma Immunol. 2013;110:29–33. doi: 10.1016/j.anai.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chan YC, Ramadani F, Santos AF, Pillai P, Ohm-Laursen L, Harper CE, et al. “Auto-anti-IgE”: naturally occurring IgG anti-IgE antibodies may inhibit allergen-induced basophil activation. J Allergy Clin Immunol. 2014;134:1394–1401. doi: 10.1016/j.jaci.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eckman JA, Hamilton RG, Saini SS. Independent evaluation of a commercial test for “autoimmune” urticaria in normal and chronic urticaria subjects. J Invest Dermatol. 2009;129:1584–1586. doi: 10.1038/jid.2008.416. [DOI] [PubMed] [Google Scholar]
- 113.Nilsson BO, Skogh T, Ernerudh J, Johansson B, Lofgren S, Wikby A, et al. Antinuclear antibodies in the oldest-old women and men. J Autoimmun. 2006;27:281–288. doi: 10.1016/j.jaut.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 114.van Schaardenburg D, Lagaay AM, Otten HG, Breedveld FC. The relation between class-specific serum rheumatoid factors and age in the general population. Br J Rheumatol. 1993;32:546–549. doi: 10.1093/rheumatology/32.7.546. [DOI] [PubMed] [Google Scholar]
- 115.Reveille JD. Predictive value of autoantibodies for activity of systemic lupus erythematosus. Lupus. 2004;13:290–297. doi: 10.1191/0961203303lu1015oa. [DOI] [PubMed] [Google Scholar]
- 116.Fiebiger E, Hammerschmid F, Stingl G, Maurer D. Anti-FcεRIα autoantibodies in autoimmune-mediated disorders. Identification of a structure-function relationship. J Clin Invest. 1998;101:243–251. doi: 10.1172/JCI511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kaplan AP, Joseph K. Basophil secretion in chronic urticaria: autoantibody-dependent or not? J Allergy Clin Immunol. 2007;120:729–730. doi: 10.1016/j.jaci.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 118.Confino-Cohen R, Chodick G, Shalev V, Leshno M, Kimhi O, Goldberg A. Chronic urticaria and autoimmunity: associations found in a large population study. J Allergy Clin Immunol. 2012;129:1307–1313. doi: 10.1016/j.jaci.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 119.Sugiyama A, Nishie H, Takeuchi S, Yoshinari M, Furue M. Hashimoto’s disease is a frequent comorbidity and an exacerbating factor of chronic spontaneous urticaria. Allergol Immunopathol. 2015;43:249–253. doi: 10.1016/j.aller.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 120.Kikuchi Y, Fann T, Kaplan AP. Antithyroid antibodies in chronic urticaria and angioedema. J Allergy Clin Immunol. 2003;112:218. doi: 10.1067/mai.2003.1605. [DOI] [PubMed] [Google Scholar]
- 121.Sherman WB, Seebohm PM. Passive transfer of cold urticaria. J Allergy. 1950;21:414–424. doi: 10.1016/0021-8707(50)90017-7. [DOI] [PubMed] [Google Scholar]
- 122.Samsoe-Jensen T. Cold urticaria; report of a case: passive transfer and in vitro experiments with skin cells. Acta Derm Venereol. 1955;35:107–110. [PubMed] [Google Scholar]
- 123.Houser DD, Arbesman CE, Ito K, Wicher K. Cold urticaria. Immunologic studies. Am J Med. 1970;49:23–33. doi: 10.1016/s0002-9343(70)80110-3. [DOI] [PubMed] [Google Scholar]
- 124.Kaplan AP, Beaven MA. In vivo studies of the pathogenesis of cold urticaria, cholinergic urticaria, and vibration-induced swelling. J Invest Dermatol. 1976;67:327– 332. doi: 10.1111/1523-1747.ep12514352. [DOI] [PubMed] [Google Scholar]
- 125.Akiyama T, Ushijima N, Anan S, Takahashi I, Yoshida H. A case of cold urticaria due to a serum factor belonging to the IGE class. J Dermatol. 1981;8:139–143. doi: 10.1111/j.1346-8138.1981.tb02580.x. [DOI] [PubMed] [Google Scholar]
- 126.Kaplan AP, Garofalo J, Sigler R, Hauber T. Idiopathic cold urticaria: in vitro demonstration of histamine release upon challenge of skin biopsies. N Engl J Med. 1981;305:1074–1077. doi: 10.1056/NEJM198110293051808. [DOI] [PubMed] [Google Scholar]
- 127.Boyce JA. Successful treatment of cold-induced urticaria/anaphylaxis with anti-IgE. J Allergy Clin Immunol. 2006;117:1415– 1418. doi: 10.1016/j.jaci.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 128.Eggel A, Baravalle G, Hobi G, Kim B, Buschor P, Forrer P, et al. Accelerated dissociation of IgE-FcεRI complexes by disruptive inhibitors actively desensitizes allergic effector cells. J Allergy Clin Immunol. 2014;133:1709–1719. doi: 10.1016/j.jaci.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tedeschi A, Kolkhir P, Asero R, Pogorelov D, Olisova O, Kochergin N, et al. Chronic urticaria and coagulation: pathophysiological and clinical aspects. Allergy. 2014;69:683–691. doi: 10.1111/all.12389. [DOI] [PubMed] [Google Scholar]
- 130.Asero R, Tedeschi A, Riboldi P, Griffini S, Bonanni E, Cugno M. Severe chronic urticaria is associated with elevated plasma levels of D-dimer. Allergy. 2008;63:176–180. doi: 10.1111/j.1398-9995.2007.01514.x. [DOI] [PubMed] [Google Scholar]
- 131.Takahagi S, Mihara S, Iwamoto K, Morioke S, Okabe T, Kameyoshi Y, et al. Coagulation/fibrinolysis and inflammation markers are associated with disease activity in patients with chronic urticaria. Allergy. 2010;65:649–656. doi: 10.1111/j.1398-9995.2009.02222.x. [DOI] [PubMed] [Google Scholar]
- 132.Asero R, Tedeschi A, Coppola R, Griffini S, Paparella P, Riboldi P, et al. Activation of the tissue factor pathway of blood coagulation in patients with chronic urticaria. J Allergy Clin Immunol. 2007;119:705–710. doi: 10.1016/j.jaci.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 133.Asero R, Tedeschi A, Riboldi P, Cugno M. Plasma of patients with chronic urticaria shows signs of thrombin generation, and its intradermal injection causes wheal-and-flare reactions much more frequently than autologous serum. J Allergy Clin Immunol. 2006;117:1113–1117. doi: 10.1016/j.jaci.2005.12.1343. [DOI] [PubMed] [Google Scholar]
- 134.Fujii K, Usuki A, Kan-No Y, Ohgou N. Elevation of circulating thrombin-antithrombin III complex and fibrin degradation products in urticaria: a laboratory finding unrelated to intravascular coagulopathy. J Dermatol. 2008;35:308–310. doi: 10.1111/j.1346-8138.2008.00474.x. [DOI] [PubMed] [Google Scholar]
- 135.Takeda T, Sakurai Y, Takahagi S, Kato J, Yoshida K, Yoshioka A, et al. Increase of coagulation potential in chronic spontaneous urticaria. Allergy. 2011;66:428–433. doi: 10.1111/j.1398-9995.2010.02506.x. [DOI] [PubMed] [Google Scholar]
- 136.Wang F, Tang H, Xu JH, Kang KF. Activation of the blood coagulation cascade is involved in patients with chronic urticaria. J Allergy Clin Immunol. 2009;123:972–973. doi: 10.1016/j.jaci.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 137.Schaeffer RC, Jr, Gong F, Bitrick MS, Jr, Smith TL. Thrombin and bradykinin initiate discrete endothelial solute permeability mechanisms. Am J Physiol. 1993;264:H1798–H1809. doi: 10.1152/ajpheart.1993.264.6.H1798. [DOI] [PubMed] [Google Scholar]
- 138.Razin E, Marx G. Thrombin-induced degranulation of cultured bone marrow-derived mast cells. J Immunol. 1984;133:3282–3285. [PubMed] [Google Scholar]
- 139.Cugno M, Marzano AV, Tedeschi A, Fanoni D, Venegoni L, Asero R. Expression of tissue factor by eosinophils in patients with chronic urticaria. Int Arch Allergy Immunol. 2009;148:170–174. doi: 10.1159/000155748. [DOI] [PubMed] [Google Scholar]
- 140.Puccetti A, Bason C, Simeoni S, Millo E, Tinazzi E, Beri R, et al. In chronic idiopathic urticaria autoantibodies against FcεRII/CD23 induce histamine release via eosinophil activation. Clin Exp Allergy. 2005;35:1599–1607. doi: 10.1111/j.1365-2222.2005.02380.x. [DOI] [PubMed] [Google Scholar]
- 141.Asero R, Cugno M, Tedeschi A. Eosinophils in chronic urticaria: supporting or leading actors? World Allergy Organ J. 2009;2:213–217. doi: 10.1097/WOX.0b013e3181bb965f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dugina TN, Kiseleva EV, Glusa E, Strukova SM. Activation of mast cells induced by agonists of proteinase-activated receptors under normal conditions and during acute inflammation in rats. Eur J Pharmacol. 2003;471:141–147. doi: 10.1016/s0014-2999(03)01752-7. [DOI] [PubMed] [Google Scholar]
- 143.Cugno M, Cicardi M, Bottasso B, Coppola R, Paonessa R, Mannucci PM, et al. Activation of the coagulation cascade in C1-inhibitor deficiencies. Blood. 1997;89:3213–3218. [PubMed] [Google Scholar]
- 144.Kaplan AP. Enzymatic pathways in the pathogenesis of hereditary angioedema: the role of C1 inhibitor therapy. J Allergy Clin Immunol. 2010;126:918–925. doi: 10.1016/j.jaci.2010.08.012. [DOI] [PubMed] [Google Scholar]