Abstract
Rifaximin is a non-systemic, broad-spectrum antibiotic that acts against gram-positive, gram-negative, and anaerobic bacteria. Clinical studies indicate that rifaximin is beneficial in treating irritable bowel syndrome (IBS). The mechanism responsible for the beneficial effects of rifaximin is not clear. In a recent study, we reported that rifaximin alters the bacterial population in the ileum of rats, leading to a relative abundance of Lactobacillus species. These changes prevent gut inflammation and visceral hyperalgesia caused by chronic stress. To more closely mirror human clinical studies in which rifaximin is used to treat IBS symptoms, we performed additional studies and showed that rifaximin reversed mucosal inflammation and barrier dysfunction evoked by chronic stress. These beneficial effects were accompanied by a striking increase in the abundance of Lactobacillaceae and a marked reduction in the number of segmented filamentous bacteria after rifaximin treatment. These microbial changes may contribute to the antiinflammatory effects of rifaximin on the intestinal mucosa.
Keywords: Lactobacillus, segmented filamentous bacteria, tight junction protein, inflammatory cytokines, gut microbiota, irritable bowel syndrome, psychological stress
Irritable bowel syndrome (IBS) is a common gastrointestinal disorder affecting 10–15% of the population in the Western world. The pathophysiology of IBS is not well understood.1–3 Many IBS patients have motility abnormalities and/ or visceral hypersensitivity. Recent studies indicate that IBS may be associated with subclinical mucosal inflammation in the ileum and colon, which is characterized by cellular infiltration of CD3 and CD25 lymphocytes and mast cells.4–8 These inflammatory changes are accompanied by increased cytokines in the mucosa or peripheral circulation.9,10 There is accumulating evidence that patients with IBS also have increased intestinal permeability.11,12 These patients with impaired barrier function have an increased functional bowel disorder severity index (FBDSI) score and are hypersensitive to painful stimuli.12 It is conceivable that sustained enhancement of paracellular permeability may facilitate the passage of luminal bacteria or their metabolic products through the barrier, leading to mucosal inflammation and visceral hypersensitivity.
Alteration of the gut microbiota may play an important role in the pathogenesis of IBS symptoms. Up to 20–25% of patients develop IBS symptoms following an episode of enteric infection.7,13 Epidemiological studies suggest that frequent antibiotic use may be a risk factor for IBS.14 Furthermore, IBS patients frequently have intestinal bacterial overgrowth.15 Considered together, these observations suggest that gut dysbiosis may play an important role in IBS. In fact, deep molecular sequencing studies of fecal samples show significant alteration of the microbial community in IBS.16–19 These changes may contribute to both the pathogenesis and the generation of symptoms in IBS.20,21 Administration of probiotics has proven to be effective in improving IBS symptoms22–24 and preventing stress-induced intestinal abnormalities and visceral hypersensitivity.25,26 Hence, treatments aimed at altering or modifying the gut microbiota are an attractive strategy in the treatment of IBS.
Rifaximin is a semisynthetic derivative of rifamycin with an additional benzimidazole ring that prevents its absorption from the gastrointestinal tract.27,28 The drug has in vitro activity against gram-positive, gram-negative, and anaerobic bacteria. Besides being approved by the Food and Drug Administration for traveler’s diarrhea and mild hepatic encephalopathy, rifaximin has been reported to be beneficial in treating IBS.29–31 Meta-analysis has shown rifaximin to be more efficacious than placebo for IBS symptom improvement, with a therapeutic gain of 9.9% and a number needed to treat (NNT) of 10.2.31 The mechanism responsible for its beneficial effects is not clear.
Levels of chronic life stress predict clinical outcome in IBS.32 A clinical study demonstrated that psychological factors most clearly predict the development of IBS symptoms after gastroenteritis.33 These patients often show mucosal inflammation and impaired barrier function.9,11,12,34 Abdominal pain and discomfort, which are common IBS symptoms, are believed to reflect visceral hypersensitivity, which can be induced by chronic psychological stress. Similar to IBS patients, in rodent models of visceral hypersensitivity, psychological stress has also been related to alteration of the gut microbiota, mucosa inflammation and gut barrier function.35–37
Recognizing there is no perfect animal model for IBS, we believe it is reasonable to use an animal model of visceral hyperalgesia induced by chronic water avoidance stress (WAS) to determine if rifaximin alters gut microbiota, prevents intestinal inflammation, and improves gut barrier function. Our studies provide evidence that rifaximin reduces mucosal inflammation and improves gut barrier function in rats subjected to water avoidance or repeat restraint stress. These effects are accompanied by normalization of visceral hypersensitivity.38 A summary of our original study38 is as follows: We used 2 rat models of chronic stress to examine the effects of rifaximin on gut microbiota and mucosal inflammation. Repeated exposure to water avoidance stress for 10 d or repeated restraint stress for 7 d each induced mucosal inflammation characterized by an increase in IL-17, IL-6, and TNFα gene expression in the distal ileal tissue and a marked increase in gut permeability. This was accompanied by a reduction in gene and protein expression of the tight junction protein occludin, indicating impairment of tight junction integrity. Behavioral pain studies showed marked enhancement of vasomotor response induced by colorectal distention in rats subjected to both forms of stress. These changes were accompanied by a significant reduction of several less abundant groups of bacteria including Erysipelotrichaceae and Clostridiaceae, contributing to a lower within community diversity (α-diversity). Concurrent oral administration of rifaximin altered the composition of bacterial communities in the ileum, leading to a relative abundance of Lactobacillus. These changes were associated with amelioration of mucosal inflammation and normalization of visceral hypersensitivity. The beneficial effects of rifaximin to ameliorate mucosal inflammation evoked by chronic stress are unlikely to be nonspecific effects due to the reduction of ileal bacteria load. As a control, we examined the effects of neomycin, another nonsystemic, broad-spectrum antibiotic. Although both oral rifaximin and neomycin in WAS rats reduced the ileal bacteria content and altered the microbial community, neomycin caused an increase in the relative abundance of Proteobacteria attributed to Enterobacteriaceae and Enterococcacceae, whereas rifaximin evoked a marked increase in Lactobacillus spp. In contrast to rifaximin, repeated oral gavage of neomycin was ineffective in preventing mucosal inflammation, barrier dysfunction, and the development of visceral hypersensitivity induced by WAS.
Our observations38 extend previous findings that psychological stressors can alter bacterial community structure leading to reduced species richness and diversity.35,39,40 Other studies indicate that commensal bacteria can influence the expression of host genes whose products regulate the mucosal barrier function, nutrient absorption, xenobiotic metabolism, and angiogenesis.41 Hence it appears that disruption of the delicate and mutually beneficial relationship between the intestinal microbiota and the host may contribute to mucosal inflammation and barrier dysfunction.41
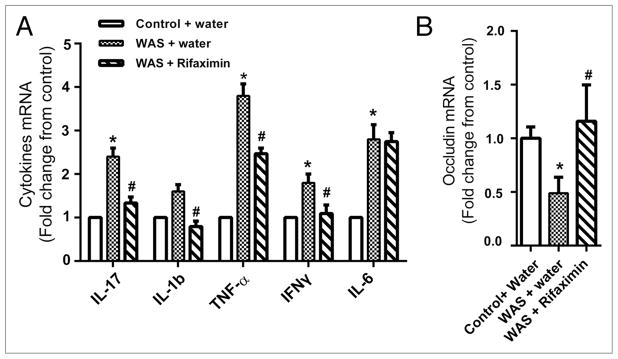
In our original study,38 we reported that oral administration of rifaximin prevented mucosal inflammation, barrier impairment, and visceral hyperalgesia in chronically stress rats. To more closely mirror human clinical studies in which rifaximin was used to treat IBS symptoms, we performed additional unpublished studies to determine if rifaximin could reverse mucosal inflammation and barrier dysfunction evoked by chronic stress. Experiments were performed on adult Wistar rats (200–225 g). The rats were housed in plastic cages, 3 per cage, and maintained on a 12 h light:12 h dark cycle. All experimental procedures were performed in accordance with the National Institutes of Health guidelines and were approved by the University Committee on Use and Care of Animals at the University of Michigan. Repeated exposure of rats to WAS was conducted as described previously.38 The rats were placed on a block in the middle of a Plexiglass tank filled with sterile water (25 °C) to 1 cm below the platform height. The rats were maintained on the block for 1 h daily for 10 consecutive days. Sham WAS rats which served as controls were placed similarly in a tank but without water for 1 h daily for 10 d. On day 11, the WAS rats were treated with an oral gavage of either rifaximin (150 mg/kg) or water twice daily, 6 h apart, for 10 consecutive days. We examined the gene expression levels of five inflammatory cytokines involved with mucosal inflammation in distal ileal tissues: interleukin (IL)-17, IL-6, tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and IL-1β. Quantitative reverse transcription PCR showed a significant increase in IL-17, IL-6, IL-1β, TNFα, and INFγ in the ileal tissue of WAS-treated rats compared with sham WAS–treated rats on day 20 (10 d after WAS) (Fig. 1A) (n = 6, P < 0.05). Rifaximin treatment given 10 d after stress treatment reversed and normalized IL-17, IL-1β, TNFα, and INFγ (Fig. 1A) (n = 6, P < 0.05). This was accompanied by normalization of the gene expression of occludin, which is a tight junction protein frequently used as a marker of mucosal integrity42 (Fig. 1B, 1-way ANOVA/ Bonferroni posttest, P < 0.05).
Figure 1.
Treatment with rifaximin (150 mg/kg, twice daily, oral gavage) reversed increased expression of inflammatory cytokines and abnormal tight junction protein in the ileal tissues evoked by chronic WAS. mRNA levels of cytokines and occludin were measured with real-time quantitative RT-PCR. Data are presented as fold change in each target mRNA level relative to expression in control samples after normalization of GAPDH. Compared with chronic treatment with vehicle, ten days of oral gavage of rifaximin decreased the IL-17, IL-1β, Interferon-γ and TNF-α mRNA levels and increased the occludin mRNA level in rats previously subjected to chronic WAS. (n = 6 in each group, *P < 0.05 WAS compared with control; #P < 0.05 rifaximin compared with vehicle treated rats subjected to WAS)
Quantitative polymerase chain reaction (PCR) and 454 pyrosequencing were used to analyze bacterial 16S rRNA in ileal contents from the rats. The sequencing methods were detailed in the supplementary methods of the original article.38 The open-source, platform-independent, community supported software program, Mothur (http://www.mothur.org) was used to bin 16S rRNA gene sequences into operational taxonomic units (OTUs) and phylotypes following the Schloss standard operating procedure (http://www.mothur.org/wiki/Schloss_sop). Phylotypes were assigned at the level of the phylum and family. Within community diversity (α-diversity) was calculated using the Shannon diversity index applied to the normalized phylotype data. Statistical analyses (Conover-Inman post hoc test for multiple comparisons) were performed using Systat 13. In control (Sham-treated) rats, the three major phyla in the ileal bacterial community are Bacteroidetes, Firmicutes and Proteobacteria with Firmicutes being the most abundant. At the family level, Peptostreptococcaceae, Lactobacillaceae, and unclassified Clostridiales are the most abundant. WAS did not alter the bacterial community composition at the phylum level; however, there was an obvious reduction of several less abundant family groups including Erysipelotrichaceae and Clostridiaceae.
Rifaximin treatment decreased the total bacterial number of 16S copies, resulting in an 84% reduction in the total bacterial load. Although there were no changes in the phylum level, changes were obvious at the family level. There was a relative reduction of the abundance of Clostridiaceae, Erysipelotrichaceae, and Peptostreptococcaceae. This was accompanied by a striking increase in abundance of Lactobacillaceae (identified as Lactobacillus spp.) and a marked reduction in the number of segmented filamentous bacteria after rifaximin treatment of WAS rats (Fig. 2). These changes in microbial communities may contribute to the anti-inflammatory effects of rifaximin on the intestinal mucosa. Lactobacillus helveticus has been shown to prevent bacterial translocation and improve gut barrier function in rats following chronic psychological stress.43 Other studies show that Lactobacillus paracasei and its products generated during fermentation restore normal mucosa permeability and reduce stress-induced visceral pain in rodents.25 Furthermore, Lactobacillus spp. also have been reported to downregulate proinflammatory cytokines IL-6 and TNFα in Crohn’s disease.44,45 Recent studies report that Lactobacillus reuteri is able to convert dietary L-histidine into an immunoregulatory signal, histamine, which suppresses proinflammatory TNF production via modulation of PKA and ERK signaling. This provides a mechanism to explain the antiinflammatory property of Lactobacillus spp.46
Figure 2.
Relative abundance of selected phylotypes identified from bacterial communities in the terminal ileum. Error bars represent SEM; WAS = water avoidance stress, N.D. = not detected. * is significantly different than sham WAS; # is significantly different than WAS (Conover–Inman post hoc test for multiple comparisons, P < 0.05).
The reduction in the number of segmented filamentous bacteria (SFB) by rifaximin may also contribute to the antiinflammatory action of rifaximin. Colonization of the small intestine of mice with SFB has been shown to be sufficient to reduce the appearance of CD4+ T helper cells that produce IL-17 and IL-22 (Th 17 cells) in the lamina propria.47 This was correlated with increased expression of genes associated with inflammation. Other studies show that SFB are able to stimulate germinal center reactions leading to a specific IgA antibody response.48 These mechanisms may act in concert to promote intestinal inflammation. Suppression of SFB by rifaximin may contribute to the reduced inflammation observed in our studies.
Rectal sensitivity to colorectal distension (CRD) was used to evaluate visceral hyperalgesia. The protocol for measuring visceromotor response (VMR) to CRD is detailed in our original article.38 The effects of stress and/or rifaximin treatment on the VMR to CRD were analyzed by comparing post-stress or post-treatment measurements with baseline values at each distension pressure using 2-way repeated measures analysis of variance (ANOVA), followed by Bonferroni post-test comparisons. As shown in Figure 3, both control and WAS rats showed pressure-dependent increases in VMR to CRD on day 0 and day 21, chronic WAS induced greater increase in VMR to CRD compared with sham WAS rats. Repeated oral gavage of rifaixmin significantly attenuated the increased VMR to CRD induced by WAS at 40 and 60 mmHg (P < 0.05, n = 6–8). Therefore our studies show that rifaximin not only alters bacterial population in the ileum and reverses intestinal inflammation, but it also normalizes visceral sensory abnormalities. This is not surprising as a cause-and-effect relationship between mucosal barrier alterations and visceral hypersensitivity has been described in a rodent model and in which chemical blockade of enhanced stress-induced paracellular permeability was accompanied by reduced sensitivity to colonic distension.49 The nature of the mediators released from the gut mucosa remains to be identified.
Figure 3.
Effects of chronic stress and antibiotics on VMR to CRD. EMG amplitude expressed as mean change from baseline (sham WAS) after WAS. Rifaximin (150 mg/kg, twice daily, oral gavage) markedly attenuated the increased VMR to CRD induced by WAS at 40 and 60 mmHg (n = 6 in each group, *P < 0.05 compared with the sham WAS, #P < 0.05 compared with WAS).
In summary, we demonstrated for the first time that rifaximin reduces ileal bacteria load, and alters microbial communities, resulting in a dominance of Lactobacillus spp. and suppression of SFB in WAS rats. These bacterial changes are accompanied by amelioration of mucosal inflammation and normalization of visceral hypersensitivity. Our data suggest that rifaximin may be beneficial in treating the symptomatic patient with IBS, but also imply that rifaximin prophylaxis may be beneficial in preventing a stress-induced flare. These findings may explain the benefits of rifaximin in the treatment of IBS.
Acknowledgments
Grant support: These studies were supported by the National Institutes of Health grants R01 DK058913 (CO) and P30 DK34933 (CO).
References
- 1.Talley NJ, Zinsmeister AR, Van Dyke C, Melton LJ., 3rd Epidemiology of colonic symptoms and the irritable bowel syndrome. Gastroenterology. 1991;101:927–34. doi: 10.1016/0016-5085(91)90717-y. [DOI] [PubMed] [Google Scholar]
- 2.Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, Whitehead WE, Janssens J, Funch-Jensen P, Corazziari E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–80. doi: 10.1007/BF01303162. http://dx.doi.org/10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 3.Saito YA, Locke GR, Talley NJ, Zinsmeister AR, Fett SL, Melton LJ., 3rd A comparison of the Rome and Manning criteria for case identification in epidemiological investigations of irritable bowel syndrome. Am J Gastroenterol. 2000;95:2816–24. doi: 10.1111/j.1572-0241.2000.03192.x. http://dx.doi.org/10.1111/j.1572-0241.2000.03192.x. [DOI] [PubMed] [Google Scholar]
- 4.Barbara G, De Giorgio R, Stanghellini V, Cremon C, Corinaldesi R. A role for inflammation in irritable bowel syndrome? Gut. 2002;51(Suppl 1):i41–4. doi: 10.1136/gut.51.suppl_1.i41. http://dx.doi.org/10.1136/gut.51.suppl_1.i41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–5. doi: 10.1126/science.1106442. http://dx.doi.org/10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 6.Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–83. doi: 10.1053/gast.2002.33579. http://dx.doi.org/10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]
- 7.Spiller RC. Postinfectious irritable bowel syndrome. Gastroenterology. 2003;124:1662–71. doi: 10.1016/s0016-5085(03)00324-x. http://dx.doi.org/10.1016/S0016-5085(03)00324-X. [DOI] [PubMed] [Google Scholar]
- 8.Barbara G, Stanghellini V, De Giorgio R, Corinaldesi R. Functional gastrointestinal disorders and mast cells: implications for therapy. Neurogastroenterol Motil. 2006;18:6–17. doi: 10.1111/j.1365-2982.2005.00685.x. http://dx.doi.org/10.1111/j.1365-2982.2005.00685.x. [DOI] [PubMed] [Google Scholar]
- 9.Gwee KA, Collins SM, Read NW, Rajnakova A, Deng Y, Graham JC, McKendrick MW, Moochhala SM. Increased rectal mucosal expression of interleukin 1beta in recently acquired post-infectious irritable bowel syndrome. Gut. 2003;52:523–6. doi: 10.1136/gut.52.4.523. http://dx.doi.org/10.1136/gut.52.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinan TG, Quigley EM, Ahmed SM, Scully P, O’Brien S, O’Mahony L, O’Mahony S, Shanahan F, Keeling PW. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304–11. doi: 10.1053/j.gastro.2005.11.033. http://dx.doi.org/10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 11.Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101:1288–94. doi: 10.1111/j.1572-0241.2006.00672.x. http://dx.doi.org/10.1111/j.1572-0241.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146:41–6. doi: 10.1016/j.pain.2009.06.017. http://dx.doi.org/10.1016/j.pain.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji S, Park H, Lee D, Song YK, Choi JP, Lee SI. Post-infectious irritable bowel syndrome in patients with Shigella infection. J Gastroenterol Hepatol. 2005;20:381–6. doi: 10.1111/j.1440-1746.2005.03574.x. http://dx.doi.org/10.1111/j.1440-1746.2005.03574.x. [DOI] [PubMed] [Google Scholar]
- 14.Quigley EM. Bacterial flora in irritable bowel syndrome: role in pathophysiology, implications for management. J Dig Dis. 2007;8:2–7. doi: 10.1111/j.1443-9573.2007.00277.x. http://dx.doi.org/10.1111/j.1443-9573.2007.00277.x. [DOI] [PubMed] [Google Scholar]
- 15.Posserud I, Stotzer PO, Björnsson ES, Abrahamsson H, Simrén M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut. 2007;56:802–8. doi: 10.1136/gut.2006.108712. http://dx.doi.org/10.1136/gut.2006.108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. http://dx.doi.org/10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:512–9. e114–5. doi: 10.1111/j.1365-2982.2009.01427.x. [DOI] [PubMed] [Google Scholar]
- 18.Rajilić-Stojanović M, Biagi E, Heilig HG, Kajander K, Kekkonen RA, Tims S, de Vos WM. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141:1792–801. doi: 10.1053/j.gastro.2011.07.043. http://dx.doi.org/10.1053/j.gastro.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 19.Jeffery IB, O’Toole PW, Öhman L, Claesson MJ, Deane J, Quigley EM, Simrén M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997–1006. doi: 10.1136/gutjnl-2011-301501. http://dx.doi.org/10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- 20.Nobaek S, Johansson ML, Molin G, Ahrné S, Jeppsson B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1231–8. doi: 10.1111/j.1572-0241.2000.02015.x. http://dx.doi.org/10.1111/j.1572-0241.2000.02015.x. [DOI] [PubMed] [Google Scholar]
- 21.Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95:3503–6. doi: 10.1111/j.1572-0241.2000.03368.x. http://dx.doi.org/10.1111/j.1572-0241.2000.03368.x. [DOI] [PubMed] [Google Scholar]
- 22.O’Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O’Sullivan GC, Kiely B, Collins JK, Shanahan F, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–51. doi: 10.1053/j.gastro.2004.11.050. http://dx.doi.org/10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 23.Kajander K, Hatakka K, Poussa T, Färkkilä M, Korpela R. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: a controlled 6-month intervention. Aliment Pharmacol Ther. 2005;22:387–94. doi: 10.1111/j.1365-2036.2005.02579.x. http://dx.doi.org/10.1111/j.1365-2036.2005.02579.x. [DOI] [PubMed] [Google Scholar]
- 24.Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, Quigley EM. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325–32. doi: 10.1136/gut.2008.167270. http://dx.doi.org/10.1136/gut.2008.167270. [DOI] [PubMed] [Google Scholar]
- 25.Ait-Belgnaoui A, Han W, Lamine F, Eutamene H, Fioramonti J, Bueno L, Theodorou V. Lactobacillus farciminis treatment suppresses stress induced visceral hypersensitivity: a possible action through interaction with epithelial cell cytoskeleton contraction. Gut. 2006;55:1090–4. doi: 10.1136/gut.2005.084194. http://dx.doi.org/10.1136/gut.2005.084194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eutamene H, Lamine F, Chabo C, Theodorou V, Rochat F, Bergonzelli GE, Corthésy-Theulaz I, Fioramonti J, Bueno L. Synergy between Lactobacillus paracasei and its bacterial products to counteract stress-induced gut permeability and sensitivity increase in rats. J Nutr. 2007;137:1901–7. doi: 10.1093/jn/137.8.1901. [DOI] [PubMed] [Google Scholar]
- 27.Huang DB, DuPont HL. Rifaximin--a novel antimicrobial for enteric infections. J Infect. 2005;50:97–106. doi: 10.1016/j.jinf.2004.05.019. http://dx.doi.org/10.1016/j.jinf.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 28.Scarpignato C, Pelosini I. Experimental and clinical pharmacology of rifaximin, a gastrointestinal selective antibiotic. Digestion. 2006;73(Suppl 1):13–27. doi: 10.1159/000089776. http://dx.doi.org/10.1159/000089776. [DOI] [PubMed] [Google Scholar]
- 29.Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, Shaw AL, Bortey E, Forbes WP TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32. doi: 10.1056/NEJMoa1004409. http://dx.doi.org/10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- 30.Pimentel M, Park S, Mirocha J, Kane SV, Kong Y. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann Intern Med. 2006;145:557–63. doi: 10.7326/0003-4819-145-8-200610170-00004. http://dx.doi.org/10.7326/0003-4819-145-8-200610170-00004. [DOI] [PubMed] [Google Scholar]
- 31.Menees SB, Maneerattannaporn M, Kim HM, Chey WD. The efficacy and safety of rifaximin for the irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2012;107:28–35. doi: 10.1038/ajg.2011.355. quiz 36 http://dx.doi.org/10.1038/ajg.2011.355. [DOI] [PubMed] [Google Scholar]
- 32.Bennett EJ, Tennant CC, Piesse C, Badcock CA, Kellow JE. Level of chronic life stress predicts clinical outcome in irritable bowel syndrome. Gut. 1998;43:256–61. doi: 10.1136/gut.43.2.256. http://dx.doi.org/10.1136/gut.43.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gwee KA, Leong YL, Graham C, McKendrick MW, Collins SM, Walters SJ, Underwood JE, Read NW. The role of psychological and biological factors in postinfective gut dysfunction. Gut. 1999;44:400–6. doi: 10.1136/gut.44.3.400. http://dx.doi.org/10.1136/gut.44.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guilarte M, Santos J, de Torres I, Alonso C, Vicario M, Ramos L, Martínez C, Casellas F, Saperas E, Malagelada JR. Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut. 2007;56:203–9. doi: 10.1136/gut.2006.100594. http://dx.doi.org/10.1136/gut.2006.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF, Dinan TG. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263–7. doi: 10.1016/j.biopsych.2008.06.026. http://dx.doi.org/10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 36.Bailey MT, Dowd SE, Parry NM, Galley JD, Schauer DB, Lyte M. Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infect Immun. 2010;78:1509–19. doi: 10.1128/IAI.00862-09. http://dx.doi.org/10.1128/IAI.00862-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, Pothoulakis C, McRoberts JA, Mayer EA. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42–53. doi: 10.1152/ajpgi.00500.2004. http://dx.doi.org/10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- 38.Xu D, Gao J, Gillilland M, 3rd, Wu X, Song I, Kao JY, Owyang C. Rifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology. 2014;146:484, e4. doi: 10.1053/j.gastro.2013.10.026. http://dx.doi.org/10.1053/j.gastro.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun Y, Zhang M, Chen CC, Gillilland M, 3rd, Sun X, El-Zaatari M, Huffnagle GB, Young VB, Zhang J, Hong SC, et al. Stress-induced corticotropin-releasing hormone-mediated NLRP6 inflammasome inhibition and transmissible enteritis in mice. Gastroenterology. 2013;144:1478–87. e1–8. doi: 10.1053/j.gastro.2013.02.038. http://dx.doi.org/10.1053/j.gastro.2013.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. http://dx.doi.org/10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136:2003–14. doi: 10.1053/j.gastro.2009.01.075. http://dx.doi.org/10.1053/j.gastro.2009.01.075. [DOI] [PubMed] [Google Scholar]
- 42.Brun P, Castagliuolo I, Di Leo V, Buda A, Pinzani M, Palù G, Martines D. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518–25. doi: 10.1152/ajpgi.00024.2006. http://dx.doi.org/10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 43.Zareie M, Johnson-Henry K, Jury J, Yang PC, Ngan BY, McKay DM, Soderholm JD, Perdue MH, Sherman PM. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut. 2006;55:1553–60. doi: 10.1136/gut.2005.080739. http://dx.doi.org/10.1136/gut.2005.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borruel N, Carol M, Casellas F, Antolín M, de Lara F, Espín E, Naval J, Guarner F, Malagelada JR. Increased mucosal tumour necrosis factor alpha production in Crohn’s disease can be downregulated ex vivo by probiotic bacteria. Gut. 2002;51:659–64. doi: 10.1136/gut.51.5.659. http://dx.doi.org/10.1136/gut.51.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Llopis M, Antolin M, Carol M, Borruel N, Casellas F, Martinez C, Espín-Basany E, Guarner F, Malagelada JR. Lactobacillus casei downregulates commensals’ inflammatory signals in Crohn’s disease mucosa. Inflamm Bowel Dis. 2009;15:275–83. doi: 10.1002/ibd.20736. http://dx.doi.org/10.1002/ibd.20736. [DOI] [PubMed] [Google Scholar]
- 46.Thomas CM, Hong T, van Pijkeren JP, Hemarajata P, Trinh DV, Hu W, Britton RA, Kalkum M, Versalovic J. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One. 2012;7:e31951. doi: 10.1371/journal.pone.0031951. http://dx.doi.org/10.1371/journal.pone.0031951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–98. doi: 10.1016/j.cell.2009.09.033. http://dx.doi.org/10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talham GL, Jiang HQ, Bos NA, Cebra JJ. Segmented filamentous bacteria are potent stimuli of a physiologically normal state of the murine gut mucosal immune system. Infect Immun. 1999;67:1992–2000. doi: 10.1128/iai.67.4.1992-2000.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ait-Belgnaoui A, Bradesi S, Fioramonti J, Theodorou V, Bueno L. Acute stress-induced hypersensitivity to colonic distension depends upon increase in paracellular permeability: role of myosin light chain kinase. Pain. 2005;113:141–7. doi: 10.1016/j.pain.2004.10.002. http://dx.doi.org/10.1016/j.pain.2004.10.002. [DOI] [PubMed] [Google Scholar]