Abstract
The oculomotor system is the motor system of choice for many neuroscientists studying motor control and learning because of its simplicity, easy control of inputs (e.g., visual stimulation), and precise control and measurement of motor outputs (eye position). This is especially true in primates, which are easily trained to perform oculomotor tasks. Here we provide the first detailed characterization of the oculomotor performance of trained squirrel monkeys, primates used extensively in oculomotor physiology, during saccade and smooth pursuit tasks, and compare it to that of the rhesus macaque. We found that both primates have similar oculomotor behavior but the rhesus shows a larger oculomotor range, better performance for horizontal saccades above 10 degrees, and better horizontal smooth pursuit gain to target velocities above 15 deg/s. These results are important for interspecies comparisons and necessary when selecting the best stimuli to study motor control and motor learning in the oculomotor systems of these primates.
Keywords: Non-human primate, Eye movement, Comparative, Smooth pursuit, Saccades
Introduction
For more than half a century, neuroscientists have used saccade and pursuit eye movements as models to study a wide range of brain functions such as motor control, attention, and learning (Straube et al. 1997; Shinoda et al. 2008). Saccades are fast eye movements driven by a positional retinal error and are conceptually similar to other ballistic movements like reaching. Smooth pursuit is a slow eye movement driven by retinal motion error and is conceptually similar to other slow movements with sensory feedback like drawing. Saccade and pursuit physiology research have predominantly chosen squirrel monkeys and macaques (e.g., rhesus monkeys) as primate animal models. Each animal model presents its own advantages and disadvantages. For example, macaques are easy to train and are better to use when the experiment demands full control of the motor output, but these animals are costly, making it unrealistic to use them for histology studies that require a large sample of animals. Squirrel monkeys are harder to train than macaques and have traditionally been used when precise control of eye movements is not necessary. An advantage of squirrel monkeys is that they have been the primate of choice for anatomical studies, and as a result, there is an abundance of squirrel monkey anatomical data available (e.g., Moratalla et al. 1992; Moschovakis et al. 1996).
Unlike rhesus macaques, the behavior of squirrel monkeys trained in oculomotor tasks has not been characterized in detail. This lack of information could present a problem when data obtained from trained squirrel monkeys are generalized to other primate species. In this study, we fill the gap by examining the oculomotor behavior of trained squirrel monkeys during the performance of saccade and pursuit tasks and by comparing the oculomotor performance to that of the rhesus macaque. Our findings suggest that, while the squirrel monkey is capable of performing the same basic set of tasks as the rhesus, the squirrel monkey performs these tasks less consistently and within a narrower range of eye positions.
Methods
Animal preparation and surgical methods
We include data from three 4–8-year-old squirrel monkeys (Saimiri sciureus; 083, 087, and 099) and one 5-year-old rhesus macaque (Macaca mulatta; Monkey A). All animals underwent at least one surgical operation where we implanted an eye coil to measure eye movements and a head post for head fixation. Briefly, animals were anesthetized using gas anesthesia (isofluorane 1–2%), and a stainless steel (squirrel monkeys) or titanium (rhesus macaque) head post was implanted using standard techniques (Stoet and Snyder 2004; Blazquez et al. 2007). In the same surgical procedure, we implanted a 3–4 turns coil (insulated stainless steel wire) in the left or right eye. After a minimum recovery period of 3–4 weeks, animals were used for behavioral studies. All surgical methods and experimental protocols were approved by the Washington University Committee on Animal Care and performed in accordance with the National Institutes of Health guidelines.
Experimental setup
Animals were head fixed and comfortably seated in a primate chair during the experiments, and their eye movements were monitored using a search coil system (Primelec, Regensdorf, Switzerland; CNC Engineering, Seattle, WA). Our visual stimulus consisted of a green (squirrel monkeys) or red (rhesus monkey) laser projected on a white screen placed in front of the animal (45 cm and front projected in squirrel monkeys; 50 cm and back projected in the rhesus monkey) which subtended approximately 0.3 degrees of visual angle in both species. Visual stimuli were presented to the animals while in a room under dim lighting conditions. For squirrel monkeys, however, the room was more illuminated, although still dim, because our squirrel monkeys tended to fall asleep in the dark or under very dim ambient light. The tasks were controlled by a PC computer and a Power 1401 through a custom-made program written in Spike2 (Cambridge Electronic Design, UK).
Behavioral training and tasks
We used a standard water restriction protocol to provide the animals with motivation to perform the behavioral tasks. Animals were trained to perform saccade and pursuit tasks using a window size of 2–3 degrees radius. During the saccade task, the initial fixation point was in the center of the screen and the target jumped to a new location in one of the four cardinal directions (up, down, left, and right). Several variations of the pursuit task were employed. In order to reduce contamination of saccades in the pursuit data, the first two variations used the step-ramp protocol (Rashbass 1961), in which the target was initially displaced in the opposite direction of the subsequent pursuit movement to a position such that at the time of the initiation of the eye movement, the target was near the fixation point. In the first variation, the target started at the center of the screen and moved in one of the four cardinal directions. In the second variation, the target started at a position 5–8 degrees eccentric from center in one of the four cardinal directions and the target moved to the opposite side of the screen, passing through the center. The data from these first two variations were collapsed together for subsequent analysis. In the third variation, the target was sinusoidally oscillated at different frequencies in the range of 0.1–2.0 Hz with a displacement of ±8 degrees and the monkey was rewarded for following the target for at least one second, but sometimes up to 2 s, depending on task demands. With the exception of the sinusoidal pursuit task, which was run continuously for at least 30 cycles for frequencies of 0.4 Hz or greater and 10 cycles for frequencies below 0.4 Hz, saccade and pursuit trials were randomly presented in the four cardinal directions and consisted of a random fixation time (500–800 ms) followed by displacement of the target and a final fixation (400–500 ms). During saccade and pursuit tasks, we allowed a grace period of 400 and 200 ms, respectively, for the eye to reach the target at the beginning of each trial. The squirrel monkey setup had a maximum range for target eccentricities of 10 degrees horizontal and 12 degrees vertical, while in the macaque setup, the range of target movement extended beyond 20 degrees both horizontally and vertically. In addition to eye movements recorded during task performance, we also recorded the oculomotor behavior during spontaneous eye movements in the absence of a defined visual target, called here the “free viewing condition.”
Analysis methods
Data were acquired using a Power 1401 and Spike2 software and imported into Matlab (Mathworks, Natick, MA, USA) for offline analysis. Eye position and laser position were acquired as analog signals at 500 Hz, and task events (e.g., reward, laser ON, laser OFF, etc.) were acquired as digital events. Eye position was filtered using a Savitzky-Golay filter (four-point window and second-degree polynomial). First and second derivatives were calculated to obtain eye velocity and acceleration traces. We calibrated the eye using 8 degree saccades, assuming that perfect fixation occurs 400 ms after the first saccade to the target; if the initial saccade under- or overshot the target, this gave sufficient time to generate a corrective saccade to the target. Saccade detection was automated and then manually inspected. Eye velocities rising above and falling below 50 deg/s were detected as saccade onset and offset, respectively. Pursuit latencies were calculated by detecting the first point at which the eye velocity increased half a standard deviation above the mean of the noise and remained above this threshold value for at least 100 ms. In addition, all trials were manually inspected prior to inclu sion and, if necessary, corrected by eye using the velocity trace, which needed to be done for approximately 15% of the trials. Saccade gain was expressed as the ratio between absolute target displacement and absolute change in eye position. Pursuit velocities were calculated as either (1) the mean velocity in a 20 ms window chosen at different times from pursuit onset (step-ramp pursuit; see “Results”) or (2) as the amplitude of a sine function fit to the cycle averaged eye velocity by least squares (sinusoidal pursuit). All sta tistical analyses and curve fittings were performed over at least 50 data points, often corresponding to several experimental days.
Results
The majority of the data presented here were obtained in the squirrel monkey. Data obtained in the macaque are shown only for comparison unless stated otherwise; macaque oculomotor behavior has been extensively described in other studies (e.g., Lisberger and Westbrook 1985; Stone and Lisberger 1990).
Overall performance
Although squirrel monkeys frequently generate 100% correct trials in a block as macaques do, they tire quickly and tend to nap for several minutes several times during experimental sessions lasting about 2 h. We quantified the perseverance of the animals in performing oculomotor tasks as the percentage of correct trials during the entire session and the time interval between two consecutive correct trials. The rationale behind measuring perseverance in this way is based on our experience training these animals: fully trained animals committed to perform the tasks show good performance over the entire session and are eager to fixate as soon as the fixation point appears. Thus, not only do they have a higher percentage of good trials overall, but the interval between two consecutive good trials is shorter and less variable. Squirrel monkeys performed at 70.9 ± 11.9% correct during an entire session with an average of 606 ± 137 correct trials per session, and a mean good trial to good trial interval of 14.5 ± 24.0 s. On the other hand, the median interval was 1.5 s. This indicates that the squirrel monkeys often jumped relatively quickly from one trial to the next but that there were a large number of longer intervals, captured in the higher mean, and were a large amount of variability. By comparison, our rhesus macaque performed at 90.3 ± 8.1%, correct during an entire session with an average of 1,392 ± 410 correct trials per session, and a mean good trial to good trial interval of 3.7 ± 2.4 s (median 450 ms). Clearly, macaques show not only more good trials per session but also shorter and less variable intervals between good trials than squirrel monkeys when performing these tasks. It is also worth noting that our rhesus was eager to perform more trials if given extra time at the end of a session, but our squirrel monkeys generally were not, indicating that the disparity in perseverance between species is probably even greater than our data suggest.
Spontaneous saccade eye movements
We measured the natural range of eye movements for both species as the range in degrees that contain the middle 90% of all fixations during the free viewing condition (two tail distribution) for a minimum of 30 min of spontaneous eye movements, during which the monkeys were kept alert. These data were accumulated from several recording sessions for each monkey. Figure 1a shows representative traces of horizontal spontaneous eye movement from a squirrel monkey (083; solid line) and a macaque (Monkey A; dashed line). We measured the eye movement range in two conditions, while the animals faced the experimental room and while they faced the projecting screen. In the first situation, animals had a diversity of objects of interest in the visual field including the experimenter, PC monitor, and instruments, located at a distance between 1.5 and 2.0 m. The horizontal and vertical eye movement range of squirrel monkeys while facing the experimental room was 26 degrees (-15.8 to 10.4) and 26.3 degrees (-12.2 to 14.14) respectively (Fig. 1b). In the same conditions, our macaque had a range of eye movements of 62.0 degrees (-28.5 to 33.4) horizontally and 53.8 degrees (-30.1 to 23.7) vertically. The eye movement range is substantially smaller for squirrel monkeys when the animals faced the white projecting screen; 15.5 degrees horizontally and 25.7 degrees vertically. Themacaque, however, showed a similar range of eye movements in both conditions, covering 53.8 degrees horizontally and 52.2 degrees vertically during spontaneous eye movements facing the projecting screen. Thus, the eye movement range of squirrel monkeys is two to three times smaller than that of the macaque. This is consistent with reports from head unrestrained squirrel monkeys in which a combination of head and eye saccades are engaged beyond horizontal saccade amplitudes of approximately 10 degree eccentricity (McCrea and Gdowski 2003).
Fig. 1.
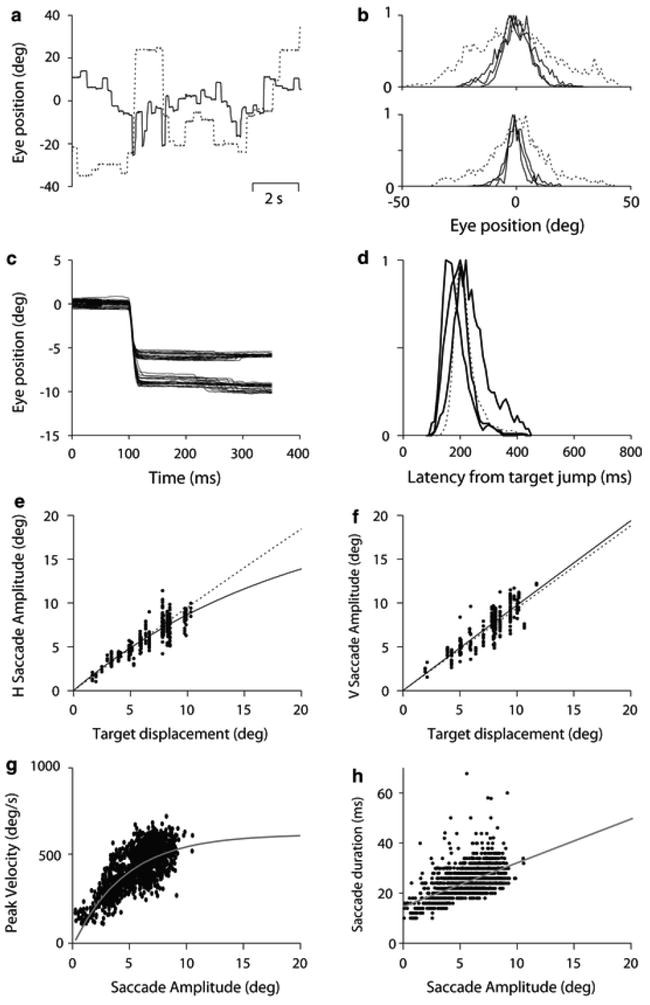
Eye movements in the free viewing condition (a, b) and during visually guided saccade task (c–h). a Exemplar raw data during the free viewing condition from squirrel monkey 083 (solid lines) and rhesus macaque (dashed lines). b Histograms showing the frequency of eye positions used for fixation in the free viewing condition normalized with respect to the most frequent eye position. On the top, we show data obtained while the animals faced the recording room, and on the bottom while the animals faced the projecting screen. Solid lines show the data obtained in each of our squirrel monkeys, and the dashed lines the data obtained in the rhesus macaque. c Raw data of horizontal saccade eye movements of squirrel monkey 099 for target displacements of 6 and 10 degrees aligned with the initiation of saccades. Notice that while in response to 6 degrees target displacement, the eye could either overshoot or undershoot, target displacements of 10 degrees always resulted in hypometric saccades. d Histogram showing the distributions of saccade latencies for the three squirrel monkeys used in this study (solid curves) and the rhesus macaque (dashed curve). e, f Show the relationship between the target displacement and corresponding saccade amplitude for horizontal and vertical saccades, respectively. Each dot represents a single saccade, the solid curve the fitting of the data, and the dashed line the fitting corresponding to similar data obtained from the rhesus macaque. g, h Show data obtained in squirrel monkeys and plot the relationship between saccade amplitude and peak velocity (g), or saccade duration (h) representative of the saccade main sequence. Gray lines show the fittings of the data.
In addition, we measured the intersaccadic intervals of both species during the free viewing condition to see if we could detect a difference in saccade frequency related to the differences in body size, and thus likely the metabolic rates, of the two species. Such a result would be in line with our observation that squirrel monkeys are generally highly active in their cages, whereas rhesus macaques tend to be more sedentary. Indeed, the median intersaccadic intervals for our squirrel monkeys were 196, 148, and 238 ms, corresponding to median saccade frequencies of 5.1, 6.8, and 4.2 saccades per second (083, 087, 099), while our rhesus macaque had a median intersaccadic interval of 262 ms (3.8 saccades per second). A second macaque we tested for confirmation (monkey B) had similar values to monkey A (316 ms, 3.2 saccades per second). This suggests a scaling of saccade frequency with body size within our dataset.
However, comparison with available data in humans (a primate species approximately one order of magnitude larger than macaques), reveals a more complicated picture. For instance, Roos et al. (2008) reported median intersaccadic intervals in humans of approximately 212 ms (4.7 saccades per second) during spontaneous eye movements, which is between the values we found for squirrel and rhesus monkeys. This discrepancy could be due to differences in experimental conditions and analysis methods or it could reveal that saccade frequency does not scale with body size within the larger primate order.
Saccades
Latency
To prevent contamination of express saccades or other forms of prediction, we removed from our data those trials with saccade latencies below 100 ms and those preceded by trials with an identical target displacement direction. Figure 1c shows representative raw data from a squirrel monkey (099), and Fig. 1d normalized histograms of saccade latencies for all monkeys. The average saccade latency for squirrel monkeys was between 180 and 243 ms (193 ± 41 for monkey 083, 181 ± 45 for monkey 087 and 243 ± 64 for monkey 099) with modes of 200, 143, and 205 for monkey 083, 087, and 099, respectively. For comparison, our macaque showed an average latency of 213 ± 36 ms and a mode at 198 ms. The differences between modes and means are likely due to the skew of the latency distributions.
Saccade accuracy
We measured saccade accuracy as the difference between the eye position at the end of the initial saccade to a target and the target position, and we measured saccade gain as the change in eye position divided by the change in laser position. Figure 1e and f show saccade amplitude for horizontal and vertical target displacements. Left and right saccade gains were grouped together because they showed no statistical difference (P[0.17; for 8–10 deg saccades). Up and down saccades also showed no statistical difference and were grouped as well (P[0.43 for 8–10 deg saccades). Squirrel monkeys showed average horizontal saccade gains above 0.9 for target displacements less than 8 degrees (Fig. 1e, f), with gains dropping 15.1 ± 7% for 9–10 degrees saccades. Vertical saccade gains, however, were near unity for all target displacements used in the squirrel monkey experiments (gain of 0.96 ± 0.15 for saccades between 10 and 12 degrees, Fig. 1f). Macaques are known to have saccade gains near 1 for saccades up to 20 degrees (Robinson et al. 1993). In support, our macaque showed average saccade gains of 1.03 ± 0.07, 0.99 ± 0.05, and 0.97 ± 0.06 for target displacement of 10, 15, and 20 degrees (dotted lines in e and f).
Saccade Main Sequence
The characteristic relationship between saccade parameters that defines the main sequence in humans and macaques was also true for squirrel monkeys. We used data from visually guided saccades to construct the main sequence shown in Fig. 1g, h. All saccades shared the same main sequence independent of the saccade direction (the population data containing the residuals were not significantly different when fitting separately each single direction or all directions; ANOVA P>0.45). In general, our squirrel monkeys generated faster and shorter saccades than our macaque. Thus, 4–5 degrees and 8–9 degrees saccades had peak velocities of 378 ± 77 and 515 ± 70 deg/s, respectively, and durations of 23.3 ± 5 and 28.4 ± 4 ms in squirrel monkeys, which are significantly faster than that of our macaque (ANOVA single factor P ≪ 0.01 and P ≪ 0.01 for 4–5 and 8–9 degrees saccades, respectively): 257 ± 49 and 411 ± 83 deg/s peak velocity with 26.2 ± 5.7 and 33.3 ± 7.64 ms duration for 4–5 and 8–9 degrees saccade, respectively. This discrepancy is likely animal specific not species specific. The literature shows a highly variable saccade main sequence in macaques, with peak velocities resembling both that of our squirrel monkeys (Robinson et al. 1993) and that of our rhesus macaque (Straube et al. 1997).
Smooth Pursuit task
We compared the pursuit behavior of the two species using two different forms of pursuit, step-ramp, and sinusoidal, to give us a more complete picture of the commonalities and differences between the two species. This is important because both types of pursuit are commonly used in oculomotor studies. Step-ramp pursuit gives us important information about the initiation of pursuit, such as latency and open loop gain, whereas sinusoidal pursuit gives us important information about the frequency response and steady-state gain of the pursuit system. Figure 2a shows representative eye position traces for horizontal eye movements from one of our squirrel monkeys (087) during three different velocities of step-ramp pursuit.
Fig. 2.
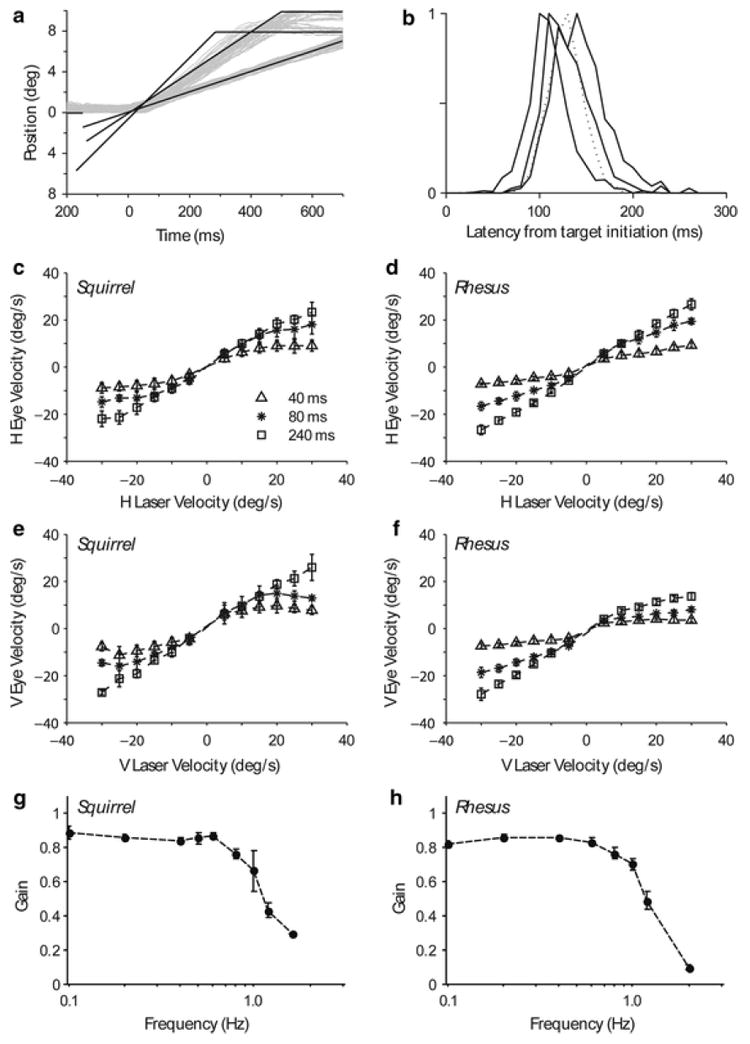
Pursuit eye movements. a Horizontal pursuit eye position traces of squirrel monkey 087 for target velocities of 10, 20, and 30 deg/s. b Histogram showing the distributions of pursuit latencies for the three squirrel monkeys used in this study (solid curves) and the rhesus macaque (dashed curves). c–f Curves showing maximum eye velocities achieved at different target velocities during three phases of pursuit averaged across all squirrel monkeys (c, e) and the macaque (d, f) for horizontal (c, d) and vertical (e, f) pursuit: early open loop (40 ms), late open loop (80 ms), and closed loop (240 ms). g–h Bode plots showing pursuit gain at different frequencies for ±8 deg sinusoidal target motion averaged across all squirrel monkeys (g) and the macaque (h). All error bars are SD.
Latency
The response latency is an important variable when studying smooth pursuit behavior because it indicates the time period required for visual motion information to be processed and influence the behavior. This time period is called the open loop period of the pursuit system (Lisberger and Westbrook 1985). Figure 2b shows normalized latency histograms for all monkeys. Squirrel monkeys have mean pursuit latencies and modes between 100 and 150 ms (110 ± 23, 142 ± 29, and 129 ± 25 ms for monkeys 083, 087, and 099, with modes of 104, 137, and 112 ms). Macaques have been reported to have latencies as short as 100 ms, but most frequently around 120–150 ms. In line with this, our macaque had a mean and mode pursuit latency of 127 ± 19 ms and 126 ms, respectively, which is within the range of latencies we encountered in the squirrel monkey. Pursuit latency in squirrel monkeys and the macaque depended on the direction and speed of the target (2 factor ANOVA P ≪ 0.01, and P ≪ 0.01) but the direction effect was not systematic when comparing individual monkeys. There was, however, a significant difference within two of the three squirrel monkeys and the macaque when comparing latencies for the two slowest and two fastest speeds (083: P = 0.5; 087: P = 0.03; 099: P = 0.03; A: P ≪ 0.001; Mann–Whitney U Test). The trend was for shorter latencies at lower velocities.
Pursuit velocity
Figure 2c–f shows horizontal and vertical pursuit velocities measured during the step-ramp pursuit task at different intervals from the initiation of pursuit, using target velocities up to 30 deg/s. We segmented the pursuit behavior into three time periods, corresponding to the early (40 ms) and late (80 ms) periods of the open loop and the closed loop period (240 ms) (Lisberger and Westbrook 1985), and averaged together all data falling within these epochs for the three squirrel monkeys and, separately, for the macaque. For the squirrel monkeys, the maximum eye velocity achieved during the first 40 ms of horizontal pursuit was 9.1 ± 2.3 deg/s, even with target velocities of 30 deg/s. Higher velocities were reached in the late period of the open loop, such that the pursuit system could compensate well for target velocities up to about 15 deg/s, reaching maximum velocities of about 16.5 ± 3.1 deg/s for 30 deg/s target motion. The closed loop horizontal pursuit period provided an increase in performance, but saturated with maximum eye velocities of 22.7 ± 3.8 deg/s for 30 deg/s target motion. Eye velocities were higher during the closed loop period for vertical compared to horizontal pursuit, with no clear saturation at the highest velocities used in this study (30 deg/s). Our rhesus had lower initial eye accelerations than the squirrel monkeys so that during the early phase of the open loop period, it was unable to compensate for the target velocities we employed. However, our rhesus was better able to match higher target velocities during the closed loop period, generating pursuit velocities of 26.7 ± 2.2 deg/s at 30 deg/s target velocities, which was significantly different than the closed loop performance of the squirrel monkeys at the two highest velocities (P = 0.003, Mann–Whitney U Test). Our macaque had an obvious deficit in upward pursuit (see Fig. 2f) that is consistent with naturally occurring pursuit deficits reported by others (Grasse and Lisberger 1992), so we were only able to do a species comparison of vertical pursuit performance for downward target motion. During downward pursuit, both species were better able to match target velocities than they were during horizontal and there was no significant species difference in closed loop performance at the two highest velocities (P = 0.37, Mann–Whitney U Test). These results are consistent with the average steady-state gains measured during horizontal sinusoidal pursuit (Fig. 2g–h). In the squirrel monkey, steady-state gains were approximately 0.85 at lower frequencies of target motion (0.1 Hz: 0.89 ± 0.07, 0.2 Hz: 0.86 ± 0.09, 0.4 Hz: 0.84 ± 0.1, 0.6 Hz: 0.86 ± 0.03), but began to drop around 0.8 Hz (0.75 ± 0.09), corresponding to peak velocities of 40 deg/s at the ±8 deg target displacements (Fig. 2g). In the rhesus, steady-state gains were almost identical to those of the squirrel monkeys, mirroring the drop-off around 0.8 Hz, and showing a similar roll off (Fig. 2h). These sinusoidal data suggest that the smooth pursuit performance of the rhesus macaque during ramp pursuit would be degraded at higher velocities than what we tested, which is consistent with our squirrel monkey data (but see Cullen et al. 1991) and with earlier reports in rhesus (Lisberger et al. 1981).
Discussion
Our results show that the oculomotor behavior of squirrel monkeys and rhesus macaques is very similar during spontaneous eye movements and during the execution of saccade and pursuit tasks; however, there are several important differences: (1) Rhesus macaques show more perseverance in performance during experimental sessions. In short, they work harder and for longer periods of time. (2) Squirrel monkeys have a narrower oculomotor range than macaques. (3) Squirrel monkey saccadic eye movements become inaccurate for horizontal target displacements above 8 degrees, while rhesus macaques maintain accurate saccadic eye movements up to at least 20 degree eccentricities. (4) Similarly, horizontal pursuit gains in the squirrel monkey during the closed loop period fall below unity for target velocities above 15 deg/s, while vertical pursuit gain in the closed loop period is near unity for the range of target velocities used in this study (up to 30 deg/s). Rhesus macaques, however, have no problem matching target velocities of 30 deg/s during the closed loop period for both horizontal and vertical laser movements. Our results justify the comparison of behavioral and neuronal data obtained from both animal models, but caution that these comparisons have to be done within the limitations of each species.
The finding that macaques have a larger oculomotor range than squirrel monkeys suggests that head movements need to be engaged more frequently for image stabilization in squirrel monkeys than in rhesus macaques (Freedman and Sparks 1997; McCrea and Gdowski 2003). This might be the result of adaptive differences to optimize the oculomotor systems of the two species for their different physical properties; squirrel monkeys are small animals (between 0.6 and 1 kg) and head movements have to overcome smaller inertial forces than macaques (usually above 6 kg). It is likely that the larger range of near unity saccade and pursuit gains of the rhesus macaque is the direct consequence of a more prominent role of eye movements for gaze control.
The behavioral differences noted above are perhaps not surprising because there are differences in the oculomotor systems of squirrel and rhesus monkeys at the neuronal level, which could reflect different strategies for gaze stabilization between the two species and ultimately result in the different behavioral responses we observed during oculomotor tasks. For instance, neck proprioceptor and vestibular information are combined in squirrel monkeys such that many neurons in the vestibular nuclei code both 503 vestibular and neck proprioceptor signals (Gdowski et al. 2000). In the rhesus macaque, however, vestibular nuclei neurons that carry vestibular signals do not code neck proprioception (Roy and Cullen 2001). This difference in wiring in the gaze stabilization pathways could be related to the behavioral observation noted above that squirrel monkeys rely to a greater extent than rhesus macaques on head movements during gaze shifts (McCrea and Gdowski 2003). Furthermore, we suggest that the smaller compensatory range of eye velocities during horizontal pursuit in the squirrel monkey is not due to a limitation of the motor or premotor system, because squirrel monkeys can generate eye movements above 100 deg/s during optokinetic stimulation (Blazquez et al. 2007), but a limitation in the pathways responsible for generating smooth pursuit responses. For example, a low working range in the neuronal population decoding of middle temporal area (MT) neurons could make visual motion predictions unreliable for high target velocities.
Our results reveal similarities and differences in the oculomotor behavior of squirrel monkeys and rhesus macaques that may be critical when comparing neuronal and behavioral data from both species. Both animal species can perform oculomotor tasks, and both share similar oculomotor behavior; however, comparison should be done carefully within the linear limits of the oculomotor behavior of each species. For example, our data argue that neuronal responses to horizontal saccades above 8 degrees eccentricity or fixations above 14 degrees eccentricities might undergo saturation effects in squirrel monkeys but not in rhesus macaques.
Contributor Information
Shane A. Heiney, Division Department of Psychology, University of Pennsylvania, Solomon Labs, 3720 Walnut St, Philadelphia, PA, 19104, USA
Pablo M. Blazquez, Division Department of Otolaryngology, School of Medicine, Washington University, 4566 Scott Avenue, Box 8115, St. Louis, MO, 63110, USA
References
- Blazquez PM, Davis-Lopez de Carrizosa MA, Heiney SA, Highstein SM. Neuronal substrates of motor learning in the velocity storage generated during optokinetic stimulation in the squirrel 538 monkey. J Neurophysiol. 2007;97:1114–1126. doi: 10.1152/jn.00983.2006. [DOI] [PubMed] [Google Scholar]
- Crawford TJ, Bennett D, Lekwuwa G, Shaunak S, Deakin JF. Cognition and the inhibitory control of saccades in schizophrenia and Parkinson’s disease. Prog Brain Res. 2002;140:449–466. doi: 10.1016/S0079-6123(02)40068-4. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Belton T, McCrea RA. A non-visual mechanism for voluntary cancellation of the vestibulo-ocular reflex. Exp Brain Res. 1991;83:237–252. doi: 10.1007/BF00231150. [DOI] [PubMed] [Google Scholar]
- Freedman EG, Sparks DL. Eye-head coordination during head-unrestrained gaze shifts in rhesus monkeys. J Neurophysiol. 1997;77:2328–2348. doi: 10.1152/jn.1997.77.5.2328. [DOI] [PubMed] [Google Scholar]
- Gdowski GT, Boyle R, McCrea RA. Sensory processing in the vestibular nuclei during active head movements. Arch Ital Biol. 2000;138:15–28. [PubMed] [Google Scholar]
- Grasse KL, Lisberger SG. Analysis of a naturally occurring asymmetry in vertical smooth pursuit eye movements in a monkey. J Neurophysiol. 1992;67:164–179. doi: 10.1152/jn.1992.67.1.164. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Westbrook LE. Properties of visual inputs that initiate horizontal smooth pursuit eye movements in monkeys. J Neurosci. 1985;5:1662–1673. doi: 10.1523/JNEUROSCI.05-06-01662.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberger SG, Evinger C, Johanson GW, Fuchs AF. Relation-ship between eye acceleration and retinal image velocity during foveal smooth pursuit in man and monkey. J Neurophysiol. 1981;46:229–249. doi: 10.1152/jn.1981.46.2.229. [DOI] [PubMed] [Google Scholar]
- Moratalla R, Quinn B, DeLanney LE, Irwin I, Langston JW, Graybiel AM. Differential vulnerability of primate caudate-putamen and striosome-matrix dopamine systems to the neurotoxic effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci. 1992;89:3859–3863. doi: 10.1073/pnas.89.9.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschovakis AK, Scudder CA, Highstein SM. The microscopic anatomy and physiology of the mammalian saccadic system. Prog Neurobiol. 1996;50:133–254. doi: 10.1016/s0301-0082(96)00034-2. [DOI] [PubMed] [Google Scholar]
- Rashbass C. The relationship between saccadic and smooth tracking eye movements. J Physiol. 1961;159:326–338. doi: 10.1113/jphysiol.1961.sp006811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson FR, Straube A, Fuchs AF. Role of the caudal fastigial nucleus in saccade generation. II. Effects of muscimol inactivation. J Neurophysiol. 1993;70:1741–1758. doi: 10.1152/jn.1993.70.5.1741. [DOI] [PubMed] [Google Scholar]
- Roos JCP, Calandrini DM, Carpenter RHS. A single mechanism for the timing of spontaneous and evoked saccades. Exp Brain Res. 2008;187:283–293. doi: 10.1007/s00221-008-1304-1. [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Passive activation of neck proprioceptive inputs does not influence the discharge patterns of vestibular nuclei neurons. Ann NY Acad Sci. 2001;942:486–489. doi: 10.1111/j.1749-6632.2001.tb03776.x. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Sugiuchi Y, Izawa Y, Takahashi M. Neural circuits for triggering saccades in the brainstem. Prog Brain Res. 2008;171:79–85. doi: 10.1016/S0079-6123(08)00611-0. [DOI] [PubMed] [Google Scholar]
- Stoet G, Snyder LH. Single neurons in posterior parietal cortex of monkeys encode cognitive set. Neuron. 2004;42:1003–1012. doi: 10.1016/j.neuron.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Stone LS, Lisberger SG. Visual responses of Purkinje cells in the cerebellar flocculus during smooth-pursuit eye movements in monkeys. I. Simple spikes. J Neurophysiol. 1990;63:1241–1261. doi: 10.1152/jn.1990.63.5.1241. [DOI] [PubMed] [Google Scholar]
- Straube A, Fuchs AF, Usher S, Robinson FR. Characteristics of saccadic gain adaptation in rhesus macaques. J Neurophysiol. 1997;77:874–895. doi: 10.1152/jn.1997.77.2.874. [DOI] [PubMed] [Google Scholar]


