Abstract
The objective of the study was to analyze the relationship between patient characteristics and the health-related quality of life (HRQoL) among patients with hepatitis C at the start of treatment, 2–12 weeks of treatment and ≥3 months post treatment using Short-Form 36 (SF-36). The eight domains and two composite scores of SF-36 were analyzed using 236 individuals. Compared to US general population norms, on average, the physical health scores were significantly lower for the studied hepatitis C population, while the differences related to mental health were between zero and small. For a physical health composite score, the treatment effect was between medium and large (0.70, 0.66, and 0.64 at the baseline and follow-ups), and for a mental health composite score it was close to zero. After controlling for demographic factors, the mixed-effects models demonstrated that HRQoL significantly improved only for general health during the treatment and vitality during post treatment. The strongest predictor of HRQoL at the two follow-up periods was HRQoL at baseline of the same domain. The ordinal logistic regressions showed that at the baseline, the strongest negative predictors of HRQoL in most of the domains were hypertension, diabetes, high BMI, high number of comorbidities including pulmonary comorbidities, low hemoglobin, and public health insurance. Considering that the improvement in HRQoL sustained after treatment only for a mental (vitality) domain, the main determinants of quality of life of the patients with hepatitis C were comorbidities.
Introduction
There have been many studies that had reported increased incidence of comorbidities for HCV patients. It has been shown that they were more likely to have hepatic steatosis and comorbidities related to metabolic syndrome including diabetes and atherosclerosis1. A cross-sectional study of surveys from a national database, National Health and Nutrition Examination Survey (NHANES III), demonstrated that patients with hepatitis C and older than 40 are three times more likely to have type 2 diabetes than those without HCV infection2. There are many other studies supporting an increased incidence of diabetes type 2 for HCV patients3,4, and it has been suggested that the sustained virological response may reduce the risk for several comorbidities including type 2 diabetes5, renal and cardiovascular complications6,7.
There have been many previous reports on quality of life of patients with chronic hepatitis C infection8–11. Most of these studies had compared patient-reported outcomes (health-related quality of life (HRQoL) on quality of life at the baseline and follow-ups12 or quality of life of HCV patients with general population13 or quality of life between subpopulations or treatment regimens14. There are only very few studies evaluating the strength of the association of HRQoLs with the patient characteristics and baseline comorbidities14.
Previous studies had demonstrated that most of the sum scores of SF-36 have non-normal distribution (e.g., beta-binomial or unknown distribution)15–17. The HRQoLs questionnaires consist of multiple items used to derive sum scores. However, sum scores are discrete and have only very few levels of outcome. For this reason, application of parametric techniques such as t-tests or linear regressions even for studies with a large sample size might be problematic15,17. Perhaps this could be an explanation for the paucity of such studies. We believe that the results of quality of life studies might be difficult to interpret if they were based on parametric statistical techniques, which may not be recommended to evaluate the strength of the association of HRQoLs and patient characteristics15. The analysis becomes even more complex when the association of patient characteristics differs between different components of physical and mental health domains. Also, there are conflicting results related to differences in HRQoL between the hepatitis C population and the average population norms. While some studies showed that the hepatitis C population has a higher difference from the average population in physical health, the others showed a higher difference in mental health13,14.
The reported literature on this topic is highly variable in their statistical analysis and this complicates comparisons of the results across the studies. Most of the studies did not use a standardized measure of the difference in HRQoL from a baseline or from an average population. Several studies reported a difference in absolute score or a normalized score using different normalization techniques or in % from a normalized score. These reporting differences limit the interpretation of the size of the effect in terms of clinical significance (i.e., statistical significance vs. clinically meaningful difference). Although it might be easy to get a low p-value for minor differences with a large sample size study, these differences may not be clinically meaningful. It is important to note that many previous studies on the quality of life using large datasets have not provided interpretation in terms of clinical significance. In this study, we described our results in terms of a relative difference (i.e., a so-called “effect size”) by categorizing it as small, medium or large, and hopefully this will help better clinical interpretation of our results.
Our study was motivated by these current issues and discrepancies in analysis and reporting the results in HRQoL. The objective of our study was to determine whether HRQoL of hepatitis C population is significantly different from the US population norms in both physical and mental components using SF-36. We also wanted to examine the relationship of comorbidities and HRQoL, and wanted to assess whether HRQoL improved, compared to baseline, and after hepatitis C cure. We hypothesized that hepatitis C population has significantly lower scores in both physical and mental health dimensions, and that hepatitis C cure improve both dimensions of the HRQoL.
Methods and statistical analysis
Patients
We assessed the mental and physical health status of patients undergoing hepatitis C treatment using the SF-36 questionnaires at baseline, during 2–12 weeks of treatment and ≥3 months post treatment. The data were collected from patients attending the HCV Clinic at the Mercy Medical Center in Baltimore over the period of 2014–2017 after getting the Institutional Review Board approval. During this period, 236 patients participated in the survey. Majority of the patients received sofosbuvir/ledipasvir (157, 67%) or sofosbuvir/simeprevir (41, 17%) combination. The demographic and other baseline characteristics of the patients are shown in Table 1. The SF-36 questionnaires that included 36 questions evaluating mental and health status were administered at baseline, 2–12 weeks of treatment and ≥3 months post treatment (i.e., ≥3 months after the treatment has stopped). The questionnaire was completed by 236 patients at baseline, 219 during the treatment phase and 149 at ≥3 months post treatment. For the period of 2–12 weeks of treatment, the median time since the start of the treatment was 44 days (with 25th and 75th percentiles being at 35 and 54 days). For the period of ≥3 months post treatment, the median time since the end of the treatment was 185 days (25th and 75th percentiles being at 108 and 236 days). The questionnaire was completed by 236 patients at baseline, 219 during the treatment phase and 149 at ≥3 months post treatment. Only three patients did not achieve a sustained viral response at the end of treatment.
Table 1.
The characteristics of the study population with hepatitis C
| Baseline visit | 2–12 weeks treatment | ≥3 Months post treatment | |
|---|---|---|---|
| N | 236 | 219 | 149 |
| Age, mean (SD) | 59.6 (7.7) | 59.4 (7.8) | 60.2 (7.9) |
| Race, n (%) | |||
| African-American | 106 (45%) | 95 (44%) | 71 (48%) |
| Caucasian | 121 (52%) | 116 (54%) | 74 (50%) |
| Other | 9 (3%) | 8 (2%) | 4 (2%) |
| Female, n (%) | 86 (36%) | 80 (37%) | 52(35%) |
| Diabetes, n (%) | 57 (24%) | 54 (25%) | 36(24%) |
| Hypertension, n (%) | 106 (45%) | 100 (46%) | 73(49%) |
| Treatment status: naive, n (%) | 130 (55%) | 121 (55%) | 79(53%) |
| Number of comorbidities, mean (SD) | 3.2 (1.7) | 3.2 (1.7) | 3.3 (1.8) |
| Hemoglobin, mean (SD) | 13.8 (2) | 13.8 (2) | 13.8 (1.6) |
| BMI, mean (SD) | 30.1 (6.4) | 30.2 (6.5) | 29.8 (6.4) |
| Insurance, n (%) | |||
| Private | 112 (47%) | 105 (48%) | 70 (47%) |
| Medicare | 84 (36%) | 75 (34%) | 55 (37%) |
| Medicaid | 31 (13%) | 30 (14%) | 17 (11%) |
| Missing | 9 (4%) | 9 (4%) | 7 (5%) |
| Medication, n (%) | |||
| Sofosbuvir/ledipasvir | 157(67%) | 143 (66%) | 103 (70%) |
| Sofosbuvir/simeprevir | 41 (17%) | 40 (18%) | 24 (16%) |
| Other | 38 (16%) | 36 (16%) | 22 (15%) |
| Hepatitis C genotype, n (%) | |||
| 1 | 226 (96%) | 210 (96%) | 144 (97%) |
| Others | 10 (4%) | 9 (4%) | 5 (3%) |
| Fibrosis stage, n (%) | |||
| 0 | 7 (3%) | 7 (3%) | 6 (4%) |
| 1 | 32 (14%) | 27 (12%) | 18 (12%) |
| 2 | 88 (37%) | 81 (37%) | 53 (36%) |
| 3 | 36 (15%) | 33 (15%) | 25 (17%) |
| 4 | 73 (31%) | 71 (32%) | 47 (32%) |
The survey questions were used to construct eight domains of SF-36 and two summary scores reflecting mental and physical health using a standard procedure18. The missing values were imputed if <50% of missing values were within a domain. After that the responses were transformed to a 0 to 100 scale. The Physical Health Component Score (PCS) and the Mental Health Component Score (MCS) were derived from the eight domains by standardizing using the mean and a standard deviation of the US population norm. Then, PCS and MCS were calculated as the weighted sum of standardized scores. The mixed-effects models were used to test if there were differences in HRQoL before, during and after the treatment for eight domains and composite scores. This is a robust tool to model the data with missing values. A compound symmetry variance-covariance structure was the best fit to our data as the correlation was similar between the three-time periods within a subject. Multiple comparisons were done by estimating the least-squared means and using Tukey–Kramer adjustment for confidence intervals.
The ordinal logistic regressions were used to study the associations between the outcome at baseline and patients’ characteristics. This method was chosen because the outcome was measured on an ordinal scale, and the scores have bounded or skewed distributions. The tests which assumed normality and constant variances (e.g., t-test, linear regression) are not recommended for these sum score measures based on our judgment15–17. Most of the models were proportional odds models as the proportional odds assumption was not violated for most of the regressions; if this assumption was not met, a nonproportional odds model was used. The following covariates were tested for significance: age, gender, ethnicity, insurance, treatment status (naive vs. non-responder), hepatitis C genotype, hypertension, diabetes, number of comorbidities including pulmonary, renal and cardiac, body mass index (BMI), and hemoglobin.
For this analysis of ordinal logistic regressions, the data were split into ordered categories (using percentiles) based upon their underlying distribution. Most of the domain and summary scores were split into five 20% groups. For two domains of social function and role physical the data were split by 25%, and for role emotional by 33%. Thus, the ordinal logistic regression modeled a cumulative probability over the groups ordered from high scores to low. The t-tests were used to compare the differences between the US population norms and the mean of the studied hepatitis C population.
For interpretation of the results in this study, we used both absolute differences and also standardized (relative) differences in outcome. We interchangeably refer to the standardized (relative) and also absolute differences further in the text as to an effect size. According to Page19, “The effect size is one of the most important indicators of clinical significance. It reflects the magnitude of the difference in outcomes between groups; a greater effect size indicates a larger difference between experimental and control groups.” He also suggested that in clinical studies, a format for reporting should include not only absolute differences but also a relative difference (expressed in %) and also an effect size. We agree with him, particularly because many SF-36 studies reported normalized differences that do not give insights into clinical significance, and thus are incomparable across the studies.
There are different cutoff levels to quantify the differences in outcome (or an effect size) in the literature. First, for an absolute difference, using SF-36 surveys, a small effect size (or small clinical difference) is considered to be about 4–10 points, medium effect size is the difference between 10 and 17 and a large effect size is the difference of 17 and above20,21. Secondly, the relative (standardized differences) and the cutoff levels were introduced by Cohen22 and Sawilowsky23. According to Cohen22, the standardized difference, d is defined as the difference between two means (or change in outcome) divided by a standard deviation. The cutoff levels are the following: a very small difference is d = 0.01, small is d = 0.2, medium is d = 0.5, and large is d = 0.8 as suggested by Cohen22 and Sawilowsky23. Thus, we further associate a small effect size with a small clinical significance, and a large effect size with a large clinical significance.
Results
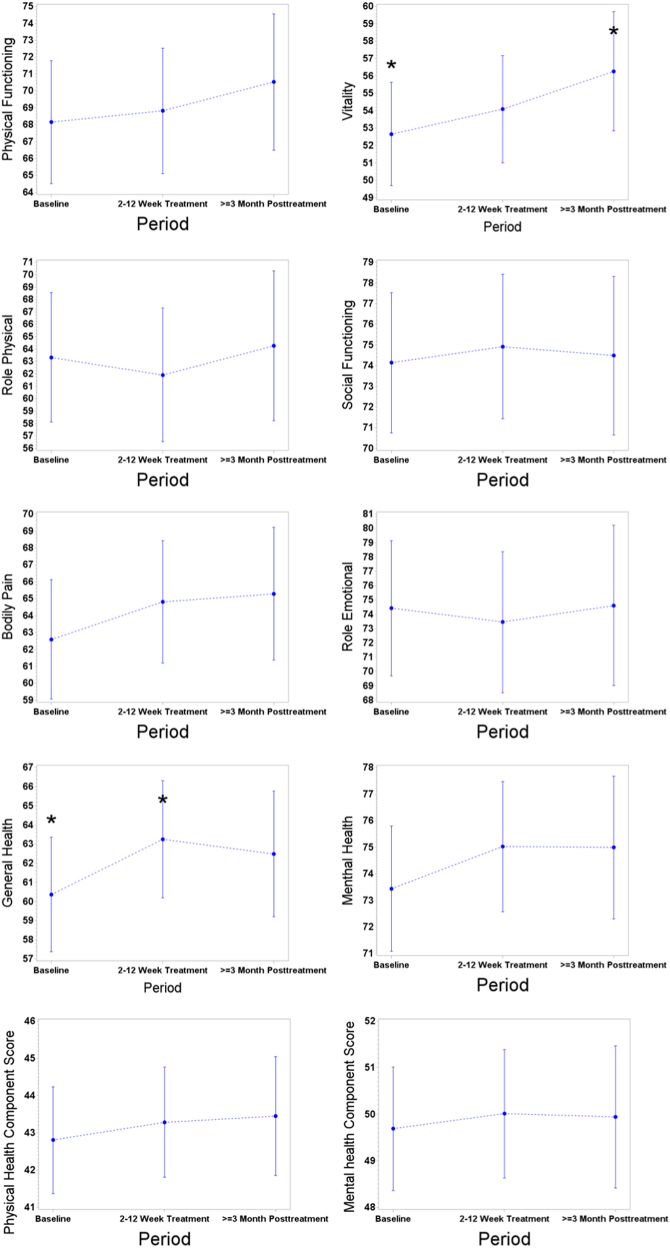
The mixed-effects models were used to compare the difference from the baseline in eight domains and two composite scores. At 2–12 weeks of treatment, all domains related to physical health and mental health improved except for the role physical and role emotional domains but most of the dimensions were not significantly different from the baseline (Fig. 1). The highest significant difference from the baseline was observed for a general health domain (2.9, p = 0.01). At ≥3 months post treatment, the highest difference from the baseline was for a vitality domain (3.6, p = 0.03). Our highest achieved differences are of a small size as they are below a 4-point-difference20. These results were not affected by non-responders to a therapy as there were only three non-responders. Also removing these patients from the analysis did not change our findings.
Fig. 1. The means for SF-36 domains and composite scores after controlling for clinical and demographic variables at baseline, 2–12 weeks of treatment and ≥3-month post treatment (* = significant differences).
.
The ordinal logistic regressions demonstrated that the score at the baseline was the strongest predictor of the outcome at the two follow-up periods. Thus, including the baseline as a covariate in an ordinal logistic regression with a score at a follow-up period as an outcome resulted in a loss of significance of the other covariates for most of the regressions while the baseline covariate stayed significant. For this reason, and because most of the scores were not significantly different from the baseline, we provided results of the relationship between an outcome at baseline and the clinical predictors as covariates (Table 2). The strongest negative predictors of HRQoL in most of the domains were presence of hypertension, high BMI, high number of comorbidities and pulmonary comorbidity, low hemoglobin, and public health insurance. Only one domain, a general health was significantly related to a type 2 diabetes.
Table 2.
The odds ratios for the associations of the baseline comorbidities and the other baseline characteristics with the SF-36 scores before the start of the treatment
| Independent variable | OR | Lower 95% CI | Upper 95% CI | p-value (a) |
|---|---|---|---|---|
| Physical functioning | ||||
| Hypertension | 0.42 | 0.26 | 0.67 | 0.0002 |
| Medicaid vs. Medicare ins | 1.74 | 0.84 | 3.61 | <0.0001 |
| Private vs. Medicare ins. | 4.08 | 2.42 | 6.89 | <0.0001 |
| Pulmonary comorbidity | 0.41 | 0.22 | 0.78 | 0.0062 |
| BMI | 0.95 | 0.92 | 0.99 | 0.0106 |
| Number of comorbidities | 0.79 | 0.69 | 0.91 | 0.0013 |
| Role physical | ||||
| BMI | 0.95 | 0.92 | 0.99 | 0.0106 |
| Number of comorbidities | 0.79 | 0.69 | 0.91 | 0.0013 |
| Medicaid vs. Medicare ins | 1.1 | 0.51 | 2.35 | <0.0001 |
| Private vs. Medicare ins | 3.52 | 2.01 | 6.16 | <0.0001 |
| Pulmonary comorbidity | 0.43 | 0.22 | 0.84 | 0.0131 |
| Bodily pain | ||||
| Hypertension | 0.6 | 0.38 | 0.94 | 0.0274 |
| Medicaid vs. Medicare ins | 0.67 | 0.32 | 1.41 | <0.0001 |
| Private vs. Medicare ins. | 2.67 | 1.59 | 4.47 | <0.0001 |
| BMI | 0.96 | 0.93 | 1 | 0.0283 |
| Number of comorbidities | 0.8 | 0.7 | 0.92 | 0.0021 |
| General health | ||||
| Diabetes | 0.51 | 0.3 | 0.88 | 0.0151 |
| Hypertension | 0.53 | 0.33 | 0.84 | 0.0075 |
| Cardiac comorbidity | 0.33 | 0.13 | 0.84 | 0.0195 |
| Medicaid vs. Medicare ins | 1.35 | 0.64 | 2.83 | 0.0001 |
| Private vs. Medicare ins. | 3.02 | 1.78 | 5.1 | 0.0001 |
| Renal comorbidity | 0.42 | 0.18 | 0.95 | 0.0385 |
| Pulmonary comorbidity | 0.27 | 0.14 | 0.52 | <0.0001 |
| BMI | 0.96 | 0.93 | 1 | 0.0305 |
| Hemoglobin | 1.26 | 1.1 | 1.44 | 0.001 |
| Number of comorbidities | 0.67 | 0.58 | 0.78 | <0.0001 |
| Vitality | ||||
| Hypertension | 0.52 | 0.33 | 0.83 | 0.0062 |
| Pulmonary comorbidity | 0.3 | 0.15 | 0.57 | 0.0003 |
| BMI | 0.94 | 0.9 | 0.97 | 0.0004 |
| Number of comorbidities | 0.71 | 0.61 | 0.82 | <0.0001 |
| Social functioning | ||||
| Hypertension | 0.62 | 0.39 | 0.98 | 0.0428 |
| Medicaid vs. Medicare ins | 1.2 | 0.57 | 2.51 | 0.0004 |
| Private vs. Medicare ins | 2.79 | 1.64 | 4.73 | 0.0004 |
| Pulmonary comorbidity | 0.47 | 0.25 | 0.9 | 0.0227 |
| Number of comorbidities | 0.77 | 0.67 | 0.89 | 0.0004 |
| Role emotional | ||||
| Medicaid vs. Medicare ins | 0.82 | 0.36 | 1.87 | 0.0025 |
| Private vs. Medicare ins | 2.6 | 1.4 | 4.85 | 0.0025 |
| Pulmonary comorbidity | 0.42 | 0.2 | 0.87 | 0.0197 |
| Number of comorbidities | 0.81 | 0.69 | 0.96 | 0.0146 |
| Mental health | ||||
| Creatinine | 2.75 | 1.1 | 6.92 | 0.0313 |
| Number of comorbidities | 0.78 | 0.67 | 0.9 | 0.0005 |
| Mental Health Component Summary Score | ||||
| Number of comorbidities | 0.82 | 0.71 | 0.95 | 0.007 |
| Physical Health Component Summary Score | ||||
| Hypertension | 0.53 | 0.33 | 0.85 | 0.0084 |
| Medicaid vs. Medicare ins | 1.76 | 0.82 | 3.77 | <0.0001 |
| Private vs. Medicare ins | 4.77 | 2.75 | 8.27 | <0.0001 |
| Pulmonary comorbidity | 0.36 | 0.19 | 0.7 | 0.0024 |
| BMI | 0.95 | 0.91 | 0.98 | 0.0042 |
| Number of comorbidities | 0.78 | 0.67 | 0.9 | 0.0008 |
aOnly significant independent variables are shown. The intercepts were omitted
For interpretation of the results, we focused on the physical health composite score (PCS) as this is a summarized measure of the other physical health domains, and it is often used to compare across studies and populations. The presence of hypertension decreases the odds of increasing the PCS score by two deciles by a factor of 0.53. It means that for example for subjects with a PCS score in a group between 60th to 80th percentiles, hypertension decreases the odds of increasing the PCS score up to the next level (i.e., the level of 80–100 percentile, which is the highest level) by a factor of 0.53. An individual having a private insurance vs. Medicare has 4.77 times increase in the odds of increasing the PCS score by two deciles, while Medicaid has 1.76 times increase in the odds of increasing the PCS score by two deciles. We used two deciles for the interpretation because the data were split by 20% for this analysis (see Methods and Statistical Analysis section).
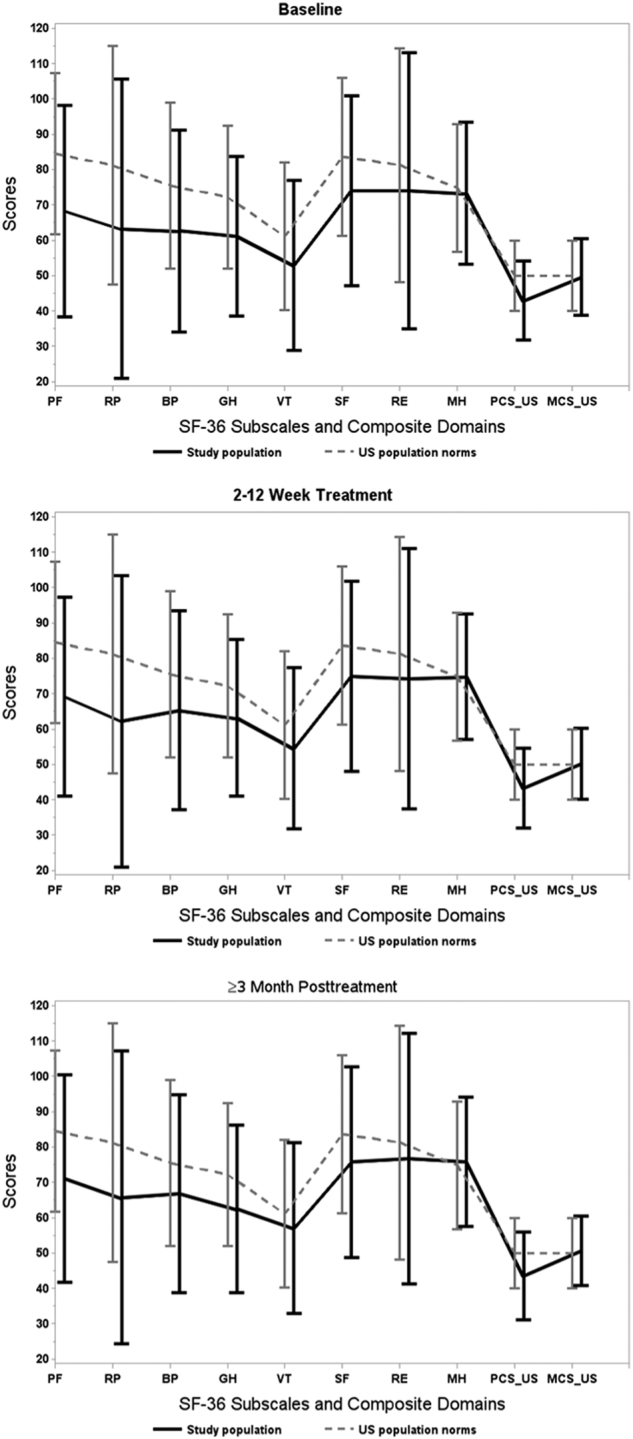
Comparing to a US general population, for all physical health domains and a composite score, the average scores were significantly lower for the studied hepatitis C population at p < 0.05 using t-tests. The size of the standardized mean differences was between the medium (i.e., with a relative difference of ≥0.5) and large (i.e., with a relative difference of 0.8 and above) according to Cohen22 and Sawilowsky23 (Table 3, see also Fig. 2).
Table 3.
The standardized mean differences between the study population and the US general population norms
| Subscales and composite domains | Baseline | 2–12 weeks treatment | ≥3 months post treatment |
|---|---|---|---|
| Physical Functioning | −0.69 | −0.66 | −0.57 |
| Role Physical | −0.52 | −0.55 | −0.45 |
| Bodily Pain | −0.53 | −0.43 | −0.36 |
| General Health | −0.54 | −0.44 | −0.48 |
| Vitality | −0.39 | −0.31 | −0.19 |
| Social Functioning | −0.42 | −0.38 | −0.35 |
| Role Emotional | −0.21 | −0.21 | −0.14 |
| Mental Health | −0.08 | 0.00 | 0.06 |
| SF-36 composite domains | |||
| Physical Health Component Score | −0.70 | −0.66 | −0.64 |
| Mental Health Component Score | −0.04 | 0.01 | 0.06 |
The mental domains and composite scores are shown in bold. The negative differences indicate a lower score for the study population compared to the US general population
Fig. 2. The average values for SF-36 domains and composite scores for the study population and the US population norms.
.
The differences between the US general population norms and the studied population were smaller for mental health compared to physical health dimensions. The size of the standardized mean difference was between very low and medium (Table 3). Although the scores improved at ≥3 months post treatment for the vitality and social functioning domains, they were yet significantly lower compared to US general population. For the role emotional domain, the standardized mean differences from the US population were small and significant at baseline, but at ≥3 months post treatment, the differences were smaller and insignificant (0.14). The differences from the US general population norm were insignificant for the mental health domain and a mental health composite summary score at all three periods and were close to zero.
Discussion
In this study, we have demonstrated that HCV patients have considerably lower physical health and a comparable mental health with the US general population. These findings are more likely to be related to increased comorbidities seen in HCV population3–7. The differences between the studied hepatitis C population and the US population norm in physical health were between medium and large. The differences, however, were between zero and small for the mental health domain.
Our findings contribute to a pool of diverse findings about differences in hepatitis C population when compared to population averages. In contrast to our study, a small study13 had previously shown lower differences in physical functioning domains (except for general health) than mental health domain between HCV population and Australian population averages. A study that analyzed 3425 subjects from clinical trials reported that HCV population had similar physical and mental summary health scores (50–51) to the general US population norms15. Another study, similarly to our results, showed that most of the physical health domains were significantly lower for hepatitis C group relative to the matched controls for both before and after diagnosis10.
In our study, during the follow-up, the largest and significant improvements were observed for a general health and vitality domain with a size of 2.9 (at 2–12 weeks of treatment) and 3.6 points at 3-month post treatment, which corresponds to a small size of the standardized difference (0.19 and 0.20, respectively)22,23. Our results are consistent with a study of three randomized clinical trials reported by Bernstein et al12. who demonstrated that the highest change from the baseline was for a vitality domain among virologic responders. The same findings were reported for the other clinical trials that included subjects who achieved cure with hepatitis C treatment14.
For composite summary scores PCS and MCS, the changes between the baseline and a post treatment period were very small. After controlling for demographic factors, the absolute changes from the baseline at ≥3 months post treatment were 0.65 for a physical health summary score PCS and 0.25 for a mental health summary score MCS. These effects are very small (and equivalent to a 0.09 and 0.032 of a standardized difference, respectively, i.e., of a very small size). Alternatively, we can also provide a difference from the baseline expressed in % as suggested by Page19. It will give us an estimate of 1.5% change from a baseline for PCS (from 42.8 to 43.45) and 0.05% for MCS (from 49.68 to 49.94), demonstrating again that the treatment effect was very small.
However, comparisons across the studies, and interpretation of different studies in terms of clinical importance, might be problematic as different studies reported results using diverse scales (original or normalized in different ways). Also, a minimal clinically important difference remains unknown for many studies of SF-36, and only several studies reported it as a difference of a size of 5 to 10 on an original scale21. Thus, using a standardized difference might assist in interpretation of the findings and facilitate comparisons across the studies.
In comparison to the other studies, interferon-based clinical trials, Bernstein et al12. reported changes in summary scores at 72 weeks relative to the baseline for responders (2.2 for physical health and 2 for mental health). A recent study, analysis of 3425 subjects from clinical trials, also reported (normalized) percent changes between about 3 and 5 for these two summary scores depending upon treatment regimens14. However, as we suggested earlier, we cannot compare the estimated effects across the studies or interpret the size of the treatment effect considering the use of different scales.
The differences in HRQoL between the baseline and post treatment compared to the other studies might be partly explained by the differences in population characteristics. Our population had different demographics as compared to the other two large studies where most of the population was Caucasians. Our study population included 52% Caucasians and 45% African-Americans. Additionally, our population had much lower physical health scores at baseline relative to the average US population when compared to the registration trials where physical scores were almost similar to the average US population (14; see supplementary Table 1). It might be one of the strongest explanations of our results as most of our patients who came for treatment were not young and in a compromised health (with an average number of comorbidities of three, see Table 1). Thus, a cure improved (slightly) only a mental component (vitality) but not a physical component.
In our study, the strongest negative predictors of quality of life in most of the domains were presence of hypertension, high BMI, pulmonary comorbidities, and a high number of comorbidities, low hemoglobin, and public health insurance. Our findings suggest that multiple comorbidities seen in hepatitis C population may determine the quality of life, and hepatitis C cure may play only a smaller role in a patient-reported outcome than previously reported.
Unlike the combined data from clinical trials, our study has a smaller sample size. Our sample size, however, was adequate to detect clinically meaningful differences. For example, for the moderate effect size (i.e., a 10-point difference), which might be more clinically meaningful compared to a small effect size (e.g., a 4-point difference) it requires from 27 to 92 samples depending upon a domain, and it requires from 8 to 24 samples depending upon a domain to detect a large effect size (i.e., 15-point difference and above) when comparing two populations20. We have also verified if our results might have been influenced by missing data at a follow-up. The patients that did not fill out SF_36 at ≥3 months follow-up was comparable in terms of the physical and mental component at a baseline with the patients who filled out the survey, because the differences between the two groups were small and insignificant using t-tests. Thus, we did not expect that these data at follow-up were missing not at random. Additionally, we used a sensitivity analysis, and it did not reveal considerable differences between the reported results either.
The strength of our study is that we evaluated the relationship between the HRQoL and patient characteristics, which has been rarely addressed but is of a high importance particularly because our hepatitis C population was already in a relatively poor health at the baseline. We have also recommended a use of the standardized difference, which might help interpretation of the results to distinguish between a statistical significance and a meaningful clinical importance, particularly because there are no definitive conclusions in the literature regarding a minimal clinically important difference in SF-36 for hepatitis C patients.
We conclude that our study demonstrated that the studied hepatitis C population has a poorer physical health and an average mental health in all eight domains relative to the US population norm. Considering that the study showed a relatively low improvement in mental health domains and insignificant improvement in physical health domains as a response to a hepatitis C cure using patient-reported outcomes, we emphasize an importance of identifying and improving comorbidities at a baseline as a way of reducing health burden on patients with hepatitis C, especially because several of them including hypertension, BMI, and diabetes might be controlled using preventive and therapeutic measures.
We also would like to be cautious about extending our results to a wider HCV population because our study population was already aged with multiple comorbidities at the baseline. Since HCV may cause or worsen metabolic or cardiovascular complications, it is possible that early treatment may have a better impact on long-term quality of life1–7. Additional studies should investigate a patient-reported health outcome based on the duration of HCV infection as several studies had demonstrated that treatment reduced the incidence of cardiovascular events, hospital visits, and other health-related issues. Also, there are several studies that pooled a large number of HCV patients, and it would be useful if they could transform their treatment effects into effects that might be better related to a clinical significance and improve interpretability of the results (e.g., in terms of the effect size or odds ratios). That way we will be able to further verify, with a higher precision, the treatment effects on the patient-reported health outcome.
Study Highlights
WHAT IS KNOWN
Quality of life (HRQoL) is considered to be poor in those with HCV and it is thought to be related to HCV infection.
Multiple comorbidiites are common in those with HCV infection, but the strength of the association of HRQoLs with the patient's baseline comorbidities has not been adequately explored.
WHAT IS NEW HERE
Baseline comorbidities in HCV patients predict their pre-treatment HRQoL.
The strongest predictor of post-treatment HRQoL is their baseline HRQoL suggesting that the main determinants of HRQoL in patients with hepatitis C are baseline comorbidities.
Conflict of interest
Guarantor of the article: Paul J. Thuluvath: I am accepting full responsibility for the conduct of the study. I had access to the data and had control of the decision to publish.
Specific author contributions: Planning and conducting the study: P.J.T. and Y.S. Collecting and/or interpreting data: P.J.T. and Y.S. Statistical analysis: Y.S. Drafting the manuscript: P.J.T. P.J.T. and Y.S. have approved the final draft of the manuscript.
Financial support: None.
Potential competing interests: None.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Negro F. Facts and fictions of HCV and comorbidities: Steatosis, diabetes mellitus, and cardiovascular diseases. Hepatology. 2014;61:S69–S78. doi: 10.1016/j.jhep.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Mehta SH, et al. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann. Intern Med. 2000;133:592–599. doi: 10.7326/0003-4819-133-8-200010170-00009. [DOI] [PubMed] [Google Scholar]
- 3.Lin YJ, et al. Chronic hepatitis C virus infection and the risk for diabetes: a community-based prospective study. Liver Int. 2017;37:179–186. doi: 10.1111/liv.13194. [DOI] [PubMed] [Google Scholar]
- 4.Moucari R, et al. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology. 2008;134:416–423. doi: 10.1053/j.gastro.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Arase Y, et al. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology. 2009;49:739–744. doi: 10.1002/hep.22703. [DOI] [PubMed] [Google Scholar]
- 6.Hsu YC, et al. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology. 2014;59:1293–1302. doi: 10.1002/hep.26892. [DOI] [PubMed] [Google Scholar]
- 7.Petta S, et al. Hepatitis C virus infection is associated with increased cardiovascular mortality: A meta-analysis of observational studies. Gastroenterology. 2016;150:145–155. doi: 10.1053/j.gastro.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Foster GR, Goldin RD, Thomas HC. Chronic hepatitis C infection causes a significant reduction in quality of life in the absence of cirrhosis. Hepatology. 1997;27:209–212. doi: 10.1002/hep.510270132. [DOI] [PubMed] [Google Scholar]
- 9.Morais-de-Jesus M, Daltro-Oliveira R, Miranda Pettersen K. Hepatitis C Virus Infection as a traumatic experience. PLoS ONE. 2014;9:e110529. doi: 10.1371/journal.pone.0110529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strauss E, et al. Altered quality of life in the early stages of chronic hepatitis C is due to the virus itself. Clin. Res. Hepatol. Gastroenterol. 2014;38:40–45. doi: 10.1016/j.clinre.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Younossi ZM, et al. Superiority of interferon-free regimens for chronic hepatitis C: The effect on health-related quality of life and work productivity. Med. (Baltim.) 2017;96:e5914. doi: 10.1097/MD.0000000000005914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernstein D, Kleinman L, Barker CM, Revicki DA, Green J. Relationship of health-related quality of life to treatment adherence and sustained response in chronic hepatitis C patients. Hepatology. 2002;35:704–708. doi: 10.1053/jhep.2002.31311. [DOI] [PubMed] [Google Scholar]
- 13.Rodger AJ, Jolley D, Thompson SC, Lanigan A, Crofts N. The impact of diagnosis of hepatitis C virus on quality of life. Hepatology. 1999;30:1299–1301. doi: 10.1002/hep.510300504. [DOI] [PubMed] [Google Scholar]
- 14.Younossi ZM, Stepanova M, Henry L, Nader F, Hunt S. An in-depth analysis of patient-reported outcomes in patients with chronic hepatitis C treated with different anti-viral regimens. Am. J. Gastroenterol. 2016;111:808–816. doi: 10.1038/ajg.2016.99. [DOI] [PubMed] [Google Scholar]
- 15.Arostegui I, Núñez-Antón V, Quintana JM. Statistical approaches to analyse patient-reported outcomes as response variables: an application to health-related quality of life. Stat. Methods Med. Res. 2012;21:189–214. doi: 10.1177/0962280210379079. [DOI] [PubMed] [Google Scholar]
- 16.Najera-Zuloaga J., Lee D., Arostegui I. Comparison of beta-binomial regression model approaches to analyze health-related quality of life data. SM IN MR 2017; 10.1177/0962280217690413. [DOI] [PubMed]
- 17.Nooraeea N. Statistical Methods for Marginal Inference from Multivariate Ordinal Data. Doctoral Thesis. 205p, University of Groningen (2015).
- 18.Ware J. E., Snow K. K., Kosinski M., Gandek B. SF36 Health Survey, Manual and Interpretation Guide. Boston. MA: New England Medical Center, the Health Institute, 1993.
- 19.Page P. Beyond statistical significance: Clinical interpretation of rehabilitation research literature. Int J. Sports Phys. Ther. 2014;9:726–736. [PMC free article] [PubMed] [Google Scholar]
- 20.Prieto L. Estimating sample sizes for studies using the SF-36 health survey. J. Epidemiol. Community Health. 1996;50:473–474. doi: 10.1136/jech.50.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward M, Guthrie L, Alba M. Clinically important changes in Short Form-36 scales for use in rheumatoid arthritis clinical trials: The impact of low responsiveness. Arthritis Care Res. 2014;66:1783–1789. doi: 10.1002/acr.22392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen J. A power primer. Psychol. Bull. 1992;112:155–159. doi: 10.1037/0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 23.Sawilowsky S. A different future for social and behavioral science research. J. Mod. Appl. Stat. Methods. 2013;2:128–132. doi: 10.22237/jmasm/1051747860. [DOI] [Google Scholar]