Abstract
Helicobacter pylori neutrophil-activating protein A subunit (NapA) has been identified as a virulence factor, a protective antigen and a potent immunomodulator. NapA shows unique application potentials for anti-H. pylori vaccines and treatment strategies of certain allergic diseases and carcinomas. However, appropriate production and utilization modes of NapA still remain uncertain to date. This work has established a novel efficient production and utilization mode of NapA by using L. lactis as an expression host and delivery vector, and demonstrated immune protective efficacy and immune modulatory activity of the engineered L. lactis by oral vaccination of mice. It was observed for the first time that H. pylori NapA promotes both polarized Th17 and Th1 responses, which may greatly affect the clinical application of NapA. This report offers a promising anti-H. pylori oral vaccine candidate and a potent mucosal immune modulatory agent. Meanwhile, it uncovers a way to produce and deliver the oral vaccine and immunomodulator by fermentation of food like milk, which might have striking effects on control of H. pylori infection, gastrointestinal cancers, and Th2 bias allergic diseases, including many food allergies.
Introduction
Helicobacter pylori infects over half of the global population, causing a variety of serious diseases including gastric carcinomas1. The infection rates were more than 50.8% in developing countries while over 34.7% in developed countries2. Current therapies, such as triple therapies, quintuple therapies and high-dose dual therapies, are based on using bismuth, proton pump inhibitors and antibiotics. These therapies are facing challenge of increasing resistance to the first-line antibiotics like clarithromycin, metronidazole and levofloxacin3–5. Vaccination has been proposed as the most promising strategy for control of this infection, but so far no commercial vaccine is available6.
In two days post challenge with H. pylori, as an innate immune response, infiltration of macrophages and neutrophils occurs in gastric glandular tissues7. By three weeks after infection, an adaptive immune response has arisen, and a large amount of T lymphocytes, besides macrophages and neutrophils, infiltrate into gastric submucosa and mucosa7. In an acute H. pylori infection, the immune response in mice is characterized by Th1 and Th17 activity, while in a chronic infection, it is marked by mixed Th1/Th2 activity7,8. Most of the infected individuals have no or little manifestation but carry this bacterium all their lives. Accumulated evidences support that anti-H. pylori protective immunity induced by vaccination is predominantly attributed to enhanced Th1 and especially Th17 responses9,10.
H. pylori neutrophil-activating protein A subunit (NapA) was originally identified as a virulence factor for its ability to mediate binding of H. pylori to gastric mucus, attract and activate neutrophils, and promote gastric inflammation11. Recently, the immune modulatory activity and potential applications of NapA have been investigated. NapA, as a Toll-like receptor-2 (TLR2) agonist, can activate dendritic cells (DCs), eliciting high IL-12 and low IL-10 secretion12,13. Stimulation of human neutrophils and monocytes with NapA can induce expression of IL-12 and IL-23, and thereby shift antigen-specific T cell responses from a Th2 to a Th1 phenotype, which is characterized by high levels of interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α) production13. NapA can also depress the Th2 response by activation of Treg cells14. These findings suggest that NapA might be a new tool for future preventive and therapeutic strategies aimed at redirecting Th2 to Th1 responses, for instance, in vaccinology, allergy and cancer immunotherapy13.
NapA plays dual roles in dealing with oxidative stress15. While NapA mediates damage to DNA by stimulating neutrophil to produce reactive oxide species, it protects DNA by combating oxidative stress with its ferroxidase center15,16. NapA neither has toxic effect on monocytes and neutrophils nor reduces their viability or lifespan, although it can enhance production of nitric oxide17,18. These data warrant approaches on application of NapA as a vaccine candidate or immunotherapy agent17.
In vaccine formulation, mucosal vaccination followed by systemic immunization with NapA significantly enhanced specific local and systemic immune responses19. NapA, used in combination with mucosal adjuvant or delivered by attenuated pathogens, can induce remarkable protection against H. pylori challenges by oral vaccination11,20. Although the immune efficacy is compromised when using only NapA in immunization, this protein is still considered as a major antigen candidate for anti-H. pylori vaccines11,20.
As reported, NapA has considerable efficacy on alleviating Th2-based allergic diseases like asthma21,22. NapA can drive Th1 inflammation and inhibit Th2 responses in allergic bronchial asthma. Both systemic and mucosal administrations of NapA are capable of reducing eosinophils, immunoglobulin E (IgE) and Th2 cytokines in bronchi22,23. Current evidences support NapA to be a novel treatment strategy for allergic diseases22,23.
Additionally, NapA has been used in treatment for many malignant tumors, such as bladder cancer, breast cancer, hepatoma and neuroendocrine tumor in animal models24–27. As observed, local administration of NapA induces bladder tumor necrosis by activating cytotoxic Th1 response26. Engineering oncolytic viruses like measles virus and adenovirus to express NapA can significantly enhance their antitumor activity27. The therapeutic effects of NapA have been attributed to its role in maturation of DCs, attraction of immune cells, polarized activation of antigen specific T cells, and induction of pro-inflammatory cytokine release13,24,28.
Currently, NapA is produced and applied mainly by purification from the products of recombinant E. coli strains, or by expression and delivery in attenuated pathogens like Salmonella typhimurium and measles virus29–31. The present problems include the dependence on unsafe mucosal adjuvant for the purified NapA to take protective effect, the purification productivity, the effect of purification on the bioactivity, and safety of the attenuated pathogens as delivery vectors for human use29–31. For further utilization of NapA, it is critical to exploit novel ways for production and delivery of this crucial protein.
In contrast to the attenuated pathogens, L. lactis NZ3900 is a food-grade probiotic bacterium. Moreover, L. lactis dose not colonize the digestive tract, and thus less probably leads to tolerance towards the delivered immunogens, compared with human commensal bacteria32. To date, studies have used L. lactis to produce H. pylori antigens like UreB, Cag7 and NapA, resulting in immune efficacy from no protection to reduced bacterial colonization in mice33–35. Besides, certain L. lactis strains isolated from the environment were proved capable of promoting Th1 bias immune response, and show allergy-protective in mice36,37. So far, the efficacy of production and delivery of NapA in L. lactis remains uncertain.
Here, an engineered L. lactis strain expressing NapA was genetically constructed and used for oral vaccination of mice, and thereby the protection against H. pylori challenges and immune modulation of mice were evaluated. This paper offers a novel efficient production and utilization mode of NapA, a promising oral vaccine candidate and a potent mucosal immunomodulator.
Results
Engineered L. lactis comprising napA
Gene sequencing showed that the sequence of the amplified napA gene was 432 bp in length (Supplementary Fig. 1) and identical to the published sequence (Genbank No. AY366361). It was confirmed by restrictive enzyme digestion and gene sequencing that the napA gene was exactly cloned in L. lactis, downstream of the nisin controlled promoter (Pnis) within the expression vector pNZ8110-lysM, generating pNZ8110-napA-lysM (Genbank No. KY385374). The schematic map of pNZ8110-napA-lysM is shown in Fig. 1.
Figure 1.
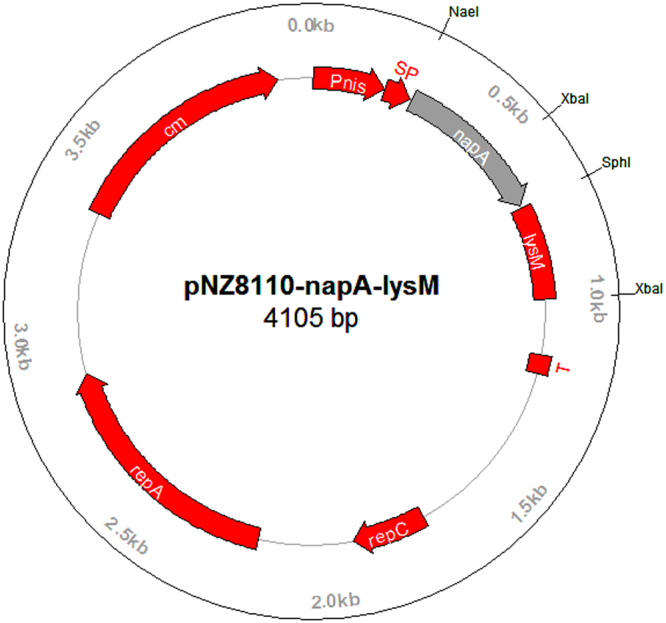
The schematic maps of the recombinant plasmid pNZ8110-napA-lysM. Pnis, nisA promoter; SP, signal sequence of usp45 gene; napA, napA gene coding region; T, terminator; repA and repC, replication gene A and C; cm, chloramphenicol resistance gene; lysM, the anchor motif of L. lactis acmA gene.
Expression and immunoreactivity of NapA
The expression of NapA in engineered L. lactis was induced with nisin. SDS-PAGE assays of the cell lysates resulted in that a predominant band was present at approximately 14 kD position in the cell lysate and the cell wall protein samples of the engineered strain, while absent at the corresponding positions in the controls (Fig. 2a). No remarkable difference was detected in culture supernatant samples between the engineered strain and the control (Fig. 2b). The expressed NapA constitutes 15% of the cell lysate proteins of the engineered L. lactis. Western blotting assays showed that the 14 kD protein expressed in the engineered L. lactis yielded positive immunoreaction with the mouse anti-H. pylori sera (Fig. 2c). Figure 2a–c were cropped from Supplementary Fig. 2a–c. These findings demonstrated efficient expression of NapA in the engineered L. lactis and the antigenicity of the recombinant NapA.
Figure 2.
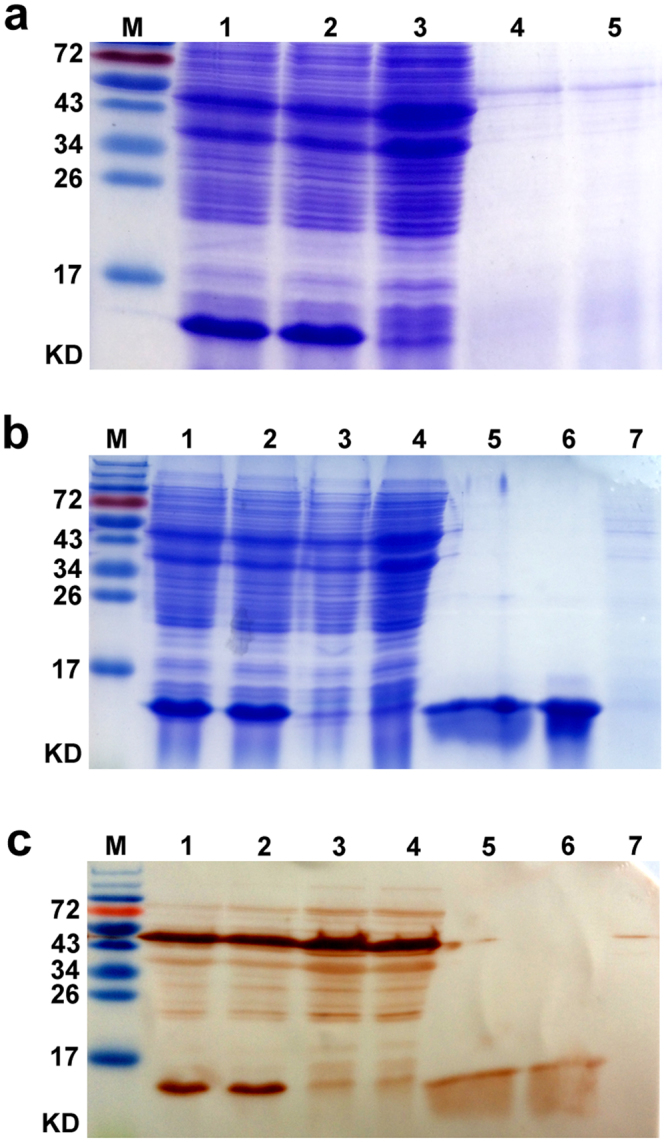
SDS-PAGE (a,b) and western blotting analysis (c) of L. lactis cell lysate, cell wall and cutrure supernatant proteins. The L. lactis strains were induced to express NapA using nisin. M, Protein markers. (a) Lane 1, 2, Cell lysates of L. lactis NZ3900 (pNZ8110-napA-lysM); Lane 3, Cell lysates of NZ3900 (pNZ8110-lysM); Lane 4, 5, Culture supernatant of NZ3900 (pNZ8110-napA-lysM) and NZ3900 (pNZ8110-lysM), respectively. (b) Lane 1, 2, Cell lysates of NZ3900 (pNZ8110-napA-lysM); Lane 3, 4, Cell lysates of NZ3900 (pNZ8110-lysM); Lane 5, 6, Cell wall proteins of NZ3900 (pNZ8110-napA-lysM); Lane 7, Cell wall proteins of NZ3900 (pNZ8110-lysM). (c) Lane 1, 2, Cell lysates of NZ3900 (pNZ8110-napA-lysM); Lane 3, 4, Cell lysates of NZ3900 (pNZ8110-lysM); Lane 5, 6, Cell wall proteins of NZ3900 (pNZ8110- napA-lysM); Lane 7, Cell wall proteins of NZ3900 (pNZ8110-lysM). (a–c) were cropped from Supplementary Fig. 2a–c. SDS-PAGE and westernblot assays showed that the recombinant NapA protein was detectable both in cell lysate and cell wall protein samples, and possessed immunoreactivity with mouse anti-H. pylori sera.
Immunization of mice and antibody assays
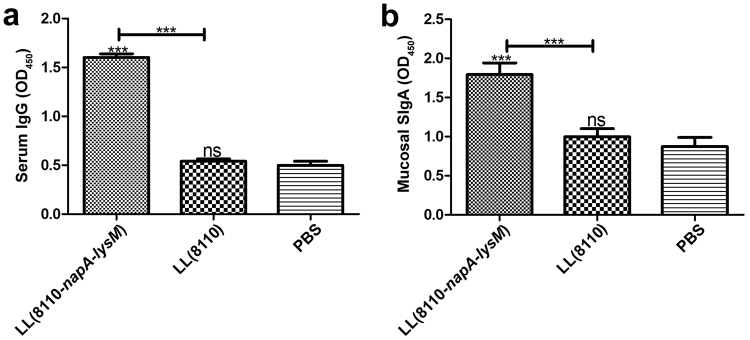
The NapA was purified from E. coli TB1 (pMAL-c2x-linker-napA), resulting in product with a purity of estimated 90%. The purified NapA (4 μg/mL) was used to coat ELISA microplates. The sera (1:50 diluted) and fecal samples were tested by ELISA for NapA-specific antibodies. As results, both fecal SIgA and serum IgG levels were higher in the LL(8110-napA-lysM) group than in the LL(8110) group and the PBS group (P < 0.05) (Fig. 3). These data indicated that oral immunization with the engineered L. lactis expressing NapA evoked significantly elevated local and systemic humoral immune responses in mice.
Figure 3.
ELISA tests for NapA-specific serum IgG (a) and fecal SIgA (b) levels. The blood and feces of mice was sampled one week after the last oral immunization. The sera (1:50 diluted) and fecal samples were tested for NapA-specific antibodies. ns, no significance; *P < 0.05; **P < 0.01; ***P < 0.001, compared with the PBS or designated group. This figure showed that oral immunization with the engineered L. lactis expressing NapA evoked significantly elevated fecal SIgA and serum IgG responses in mice.
Cultivation of splenocytes and evaluation of cytokines
ELISA assays of cytokines in the splenocyte culture supernatant resulted in that the group vaccinated with the engineered L. lactis had significantly elevated IL-2, IL-12, IL-17, IL-23 and INF-γ levels and reduced production of IL-4, compared with PBS group or LL(8110) group (Fig. 4). The IL-8 production level in the vaccinated group was significantly higher than that in the PBS group (Fig. 4d). These results demonstrated the effect of the engineered strain on redirection of Th2 to Th1 response. Notably, the markedly enhanced IL-23 and IL-17 expression in the vaccinated mice demonstrated a polarized Th17 response driven by the L. lactis-delivered NapA. The effect of H. pylori NapA on Th17 response was observed for the first time, which may greatly affect the clinical application of NapA.
Figure 4.

Assessment of cytokine expression levels of splenocytes. One week post immunization, splenocytes were separated from the mice and cultivated for 3 d upon irritation with H. pylori cell lysate antigens. The culture medium supernatant was tested for the cytokines by ELISA. ns, no significance; *P < 0.05; **P < 0.01; ***P < 0.001, compared with the PBS or designated group. This figure showed that by oral vaccination the engineered L. lactis producing NapA induced significantly elevated production of IL-2, IL12 and INF-γ, and reduced expression of IL-4, demonstrating the effect of the engineered strain on redirection of Th2 to Th1 response. Notably, the markedly enhanced IL-23 and IL-17 expression in the vaccinated mice demonstrated a polarized Th17 response driven by the L. lactis-delivered NapA.
H. pylori challenge and gastric examination
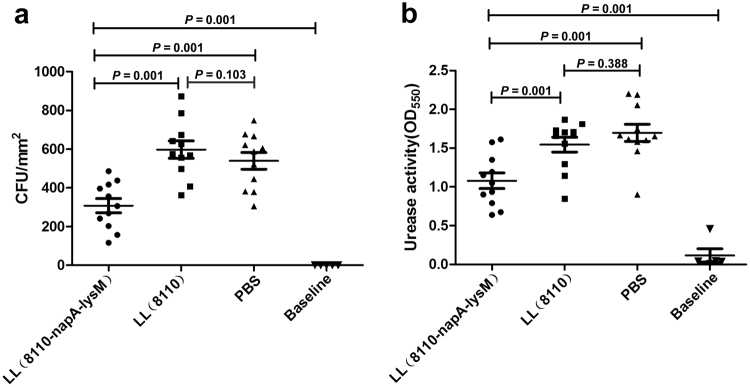
The gastric examination by bacterial cultivation and urease activity assays showed that the gastric H. pylori burden and urease activity in LL(8110-napA-lysM) group were significantly lower than those in LL(8110) group and PBS group, but higher than those in Baseline group (n = 10) (Fig. 5). The results showed that the oral vaccination with the L. lacitis-delivered NapA was capable of significantly reducing the bacterial load in the stomachs of the mice challenged with H. pylori, but unable to protect the mice from infection.
Figure 5.
Evaluation of H. pylori colonization in mice by quantitative cultivation (a) and urease activity assays (b). The gastric walls of mice were sampled one week after the last challenge with H. pylori. The bacterial colonization was assessed by rapid urease tests and quantitative bacterial culture. *P < 0.05; **P < 0.01; ***P < 0.001, compared with the PBS or designated group. Baseline, the values of the BALB/c mice (n = 10) untreated with either L. lactis or H. pylori. The results showed that the NapA delivered by L. lacitis significantly reduced the bacterial load in the stomachs of the vaccinated mice.
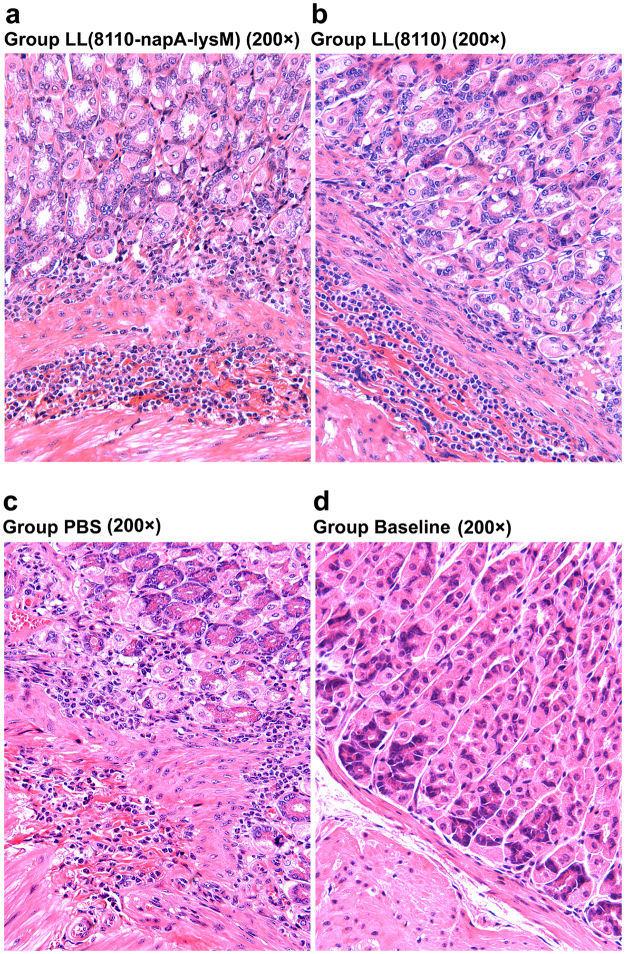
Pathological analysis was carried out to evaluate gastric inflammatory responses. Infiltration of lymphocytes and polymorph nuclear cells in gastric mucosa and submucosa were observed following the challenges in both the immunized mice and the controls administered with L. lactis (pNZ8110) or PBS (Fig. 6). However, no significant difference in the extent of inflammation was detected between the immunized group and the unimmunized H. pylori-challenged groups (Fig. 7). The Baseline group has significantly lower level of inflammatory changes than all the H. pylori-gavaged groups (P < 0.01). These findings indicated the oral immunization with the engineered L. lactis did not cause enhanced inflammation responses to H. pylori challenges.
Figure 6.
Histological observations of mouse gastric tissues following H. pylori challenge. The gastric slide specimens were prepared by paraffin section and hematoxylin-eosin (HE) staining. There was infiltration of lymphocytes and polymorph nuclear cells in gastric mucosa and submucosa in both the vaccinated mice (a) and the controls treated with L. lactis (pNZ8110) (b) or PBS (d–f). No obvious inflammation response was observed in most mice (7/8) of the Baseline group given neither immunization nor challenge (c).
Figure 7.
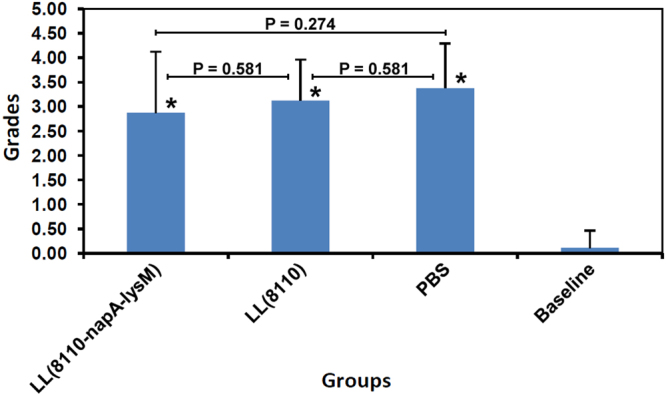
Histological grading of mouse gastric inflammatory changes. The extent of gastric inflammation was graded using the method previously reported57. No significant difference in the inflammatory response was detected between the immunized group (2.88 ± 1.25, n = 8) and the unimmunized groups, LL(8110) group (3.13 ± 0.83, n = 8) and PBS group (3.38 ± 0.92, n = 8) (P > 0.05). The Baseline group had significantly lower level of inflammatory changes (0.13 ± 0.35, n = 8) than all the H. pylori-gavaged groups (P = 0.00).
Discussion
Epidemiology studies show inverse correlation between H. pylori infection and frequency of asthma, which brings up a disputed issue on whether eradicating this infection is to benefit asthma patients or not38. In fact, it is hardly acceptable to leave H. pylori, as a class I carcinogen, in human body without deadline, at least for the cases with clinical manifestations. One possible solution might be using certain H. pylori components, like NapA, to replace the Helicobacter infection for depression of Th2 responses. Similarly, for other Th2 predominant allergic diseases, immunotherapies might also be established by using H. pylori NapA.
Among the possible administration routines, mucosal delivery of NapA should be first considered, due to the high safety, easy manipulation and rationality, especially for mucosal allergies and local cancers of digestive, respiratory and urinary tracts39. However, till now, no efficient production and safe utilization modes of NapA have been defined for its practical application. This study successfully produced and delivered NapA via using the safe L. lactis expression system, constituting a considerable step toward human use of this crucial protein.
The L. lactis NZ3900/pNZ8110 is a nisin-controlled expression system (NICE). The food-grade safe NZ3900 strain has been engineered carrying the genes encoding the regulators of bacterial responses to nisin concentration in the culture medium, while the plasmid vector pNZ8110 contains the nisin-inducible promoter32. The plasmid pNZ8110-lysM was constructed in our previous study, by introduction of the anchor motif lysM of L. lacits acmA gene into pNZ8110 (GenBank No. KY385375). pNZ8110-lysM can express heterogeneous proteins as intracellular, extracellular or surface displayed molecules, depending on which site the exogenous genes are inserted.
In this work, the napA gene was cloned into the pNZ8110-lysM at the site NaeI downstream of the signal peptide (SP) encoding region to make the NapA expressed in fusion with SP as a secreted protein. The termination codon of napA was deleted for the capablity of fusion expression with other genes. As a result of the deletion of the termination codon, a peptide of 12 amino acids encoded by the recombinant plasmid was added to the C end of the expressed NapA. Although the gene sequencing indicated that the recombinant plasmid pNZ8110-napA-lysM had been accurately constructed, SDS-PAGE assays showed that NapA was detectable in both the whole cell lysate and the cell wall exfoliation proteins, but not in the culture supernatant proteins. Similarly, previous studies observed that although NapA and UreB in nature are cytoplasmic proteins, they are also detectable in the outer membrane proteins of H. pylori40,41. Hence, these proteins were thought to be capable of adhering to bacterial membrane40,41. Another interesting finding is that the NapA expression product has a lower molecular weight than the expected (14 kD vs 16 kD), the mechanism underlying which needs further investigation.
Previous studies reported that production of heteroproteins in L. lactis might result in rather low expression levels, therefore some reports showed the expression product only by western blot assays, but not by SDS-PAGE33,42,43. The expressed NapA in the present study constituted 15% of the L. lactis cell lysate proteins, demonstrating the high expression efficiency of this engineered strain34,42. The westernblotting analysis confirmed that the recombinant NapA expressed in L. lactis had the antigenicity of its natural form. These findings suggested that the engineered strain could be a novel source of NapA.
Up till now, it remains unclear how many CFU and times of treatments are most suitable for oral vaccination with the L. lactis-delivered NapA. To confirm whether or not the L. lactis expressing NapA is capable of inducing desired immune efficacy, it is reasonable for this study to use a relatively higher dose and frequency of treatments, and the results indicated that the immunization regimens used here were effective.
In the present study, vaccination with the engineered L. lactis elicited markedly elevated systemic and mucosal immune responses, and thus significantly reduced bacterial colonization in the stomachs of the H. pylori-challenged mice. These results indicates that delivery of NapA by L. lactis is capable of evoking significant immune protection in mice, evidencing that L. lactis can be an efficient oral vaccine vehicle with adjuvant activity to induce protective immunity against H. pylori33.
To explore the mechanism underlying the immune protection and modulation, we assayed cytokine expression by splenocytes of the immunized mice. As shown, vaccination with the engineered L. lacis induced significantly elevated levels of IL-2, IL-12 and INF-γ, and reduced expression of IL-4, demonstrating the competence of the engineered L. lactis in redirection of Th2 to Th1 response. The enhanced IL-8 level in the vaccinated mice supports that the engineered strain has acquired the ability of NapA to attract neutrophils and promote inflammation. The increased IL-10 production might be due to the activation of Treg by NapA14,44.
Although certain previous studies indicate that H. pylori normally elicits an innate immune response, followed by a Th1 response, there are also evidences supporting that this bacterium can increase a Th2 or induce a mixed Th1/Th2 adaptive immune response in mice45. In the present study, a Th2 response was noticed in the unimmunized mice challenged with H. pylori, owing to the high IL-4 level in the PBS group. Our findings support existence of a Th2 or a mixed Th1/Th2 immune response to H. pylori challenges.
The elevated IL-23 and IL-17 expression levels demonstrated polarized Th17 responses. Since it has been evidenced that anti-H. pylori protective immunity is predominantly attributed to Th1 and especially Th17 responses, the immune profiles of the immunized mice herein may explain the immune protection caused by the engineered L. lactis9,46. Notably, the present study is the first report on that NapA of H. pylori evokes a Th17-biased response, although a previous observation demonstrated the ability of Borrelia burgdorferi NapA to drive a Th17 response47.
The exact role of Th17-biased response in tumor immunity remains unclear48. Some studies have documented that Th17 response is associated with reduced tumor progression, improved survival in patients with epithelial cancers, and potent antitumor efficacy in mice49. Others suggested that Th17 cells and related cytokines might have both antitumor and pro-tumor functions, and the links between Th17 cells and carcinogenesis are highly dependent on the context50. The findings of NapA’s function to up-regulate Th17 response are of significance for exploring its application.
In the past decades, incidence rate of allergic diseases, especially food allergies, have been continually increasing51. As reported, certain L. lactis isolates can prevent allergic inflammatory reactions, through endosomal recognition of L. lactis and its RNA by dendritic cells36. Engineered L. lactis strains producing peanut allergens were observed to modulate peanut-induced allergic immune responses in mice by redirection of Th2 to Th1 response37. As shown in our work, treatment with L. lactis NZ3900 containing the empty plasmid vector can also cause significantly reduced IL-4 production, compared with gavages with PBS. This result is in accord with the previous reports36,37. The IL-4 level in LL(8110-napA-lysM) group was significantly lower than that in the LL(8110) group, indicating the function of NapA to modulate the Th1/Th2 responses. Additionally, activation of Treg by L. lactis might contribute to the remarkably lowered IL-2 level in LL(8110) group, compared with the PBS group. These data demonstrate L. lactis as an efficient mucosal delivery vector for treating Th2-based allergic diseases36,37.
Presently, frequent occurrences of cancers have also become critical public health problems52. Biotherapies, such as bacillus Calmette-Guerin (BCG) therapy, have been used for treatment of certain cancers, and brought attractive efficacy, however, new alternative drugs are urgently needed53. In future, the engineered L. lactis expressing NapA might be applied as an immune modulatory agent, for instance, in treatment of certain immune dysregulation and carcinomas. Given that it is used in fermentation of food like milk, the lactic acid bacterium engineered here and its derivatives might have striking effect on control of these disease. The mucosal administration routine by which it takes effect is also attracted.
In the histological analysis, no significant difference was detected between the immunized group and the controls by biopsy of the gastric tissues, although the enhanced IL-8 levels in the vaccinated mice suggested the potential presence of post-immunization inflammation. On one hand, the vaccination with the engineered L. lactis might decrease inflammation by reducing the H. pylori colonization. On the other hand, NapA might induce post-immunization inflammation through polarizing Th1 immune response and elevating IL-8 expression level. The multiple functions of NapA may contribute to the histological changes observed here.
Additionally, even though L. lactis has been generally regarded as safe for human use, its effect on the gastro-intestinal microbiota, suspected by the administration of high doses of L. lactis, still needs further investigation. In future, the long-term effect of use of the engineered L. lactis as a potential oral vaccine candidate should also be defined. Moreover, if the immunity induced by the vaccination is incapable of lasting for enough time, the vaccination will have to be performed regularly to maintain the effect.
In summary, this work has established a novel efficient production and utilization mode of H. pylori NapA, demonstrated for the first time the ability of NapA to promote both Th17 and Th1 polarization, and offered an engineered L. lactis strain, which can be a promising anti-H. pylori oral vaccine candidate, a potent mucosal immune modulatory agent, and a potential antitumor strategy.
Methods
Bacteria and cultivation
L. lactis NZ3900, H. pylori MEL-Hp27, H. pylori 11637, E. coli TB1(pMAL-c2x-linker –napA) and plasmid pNZ8110-lysM were used in this work (Supplementary Table 1). L. lactis, H. pylori and E. coli were cultivated using GM17 medium, sheep blood containing Brucella agar plate and Luria Broth medium, respectively, as previously described54,55.
Polymerase chain reaction of napA
The primers used for PCR amplification of napA were 5′-GCATGCCGGC ATGAAAACATTTGAAAT-3′ (Sense) and 5′-CGTGCATGCAGCTAAATGGGCTTG CAGCA-3′ (Antisense) with the endonuclease sites NaeI and SphI underlined. Genomic DNA was isolated from H. pylori MEL-Hp27 using alkaline lysis method, and used as the PCR template. PCR profile included 30 cycles of 94 °C for 1 min, 50 °C for 30 s and 72 °C for 2 min.
Construction of L. lactis expressing napA
By restriction endonuclease digestion with NaeI and SphI (TaKaRa, China) and ligation reaction, the napA gene was ligated with pNZ8110-lysM and used for transformation of NZ3900 by electrophoration54. The recombinants were obtained by chloramphenicol resistance selection and PCR detection of napA, and identified by restriction digestion and gene sequencing54. The L. lactis strain carrying napA gene was referred to as L. lactis NZ3900 (pNZ8110-napA-lysM).
Production of NapA in L. lactis
The engineered L. lactis was grown and induced for expression of NapA by adding 40 μg/L nisin to the culture at OD600 ≈ 0.35 and incubation for 5 h. The cellular lysate samples were prepared by centrifugation and supersonication54.
Samples of the culture supernatant were prepared from 50 mL of culture. The supernatant was obtained by 1 × 104 rpm at 4 °C for 20 min, and filtered through 0.22 µm filter. The proteins in the filtrate were precipitated by adding trichloroacetic acid (100 mL/L), incubating at 4 °C for 16 h and centrifugation at 1 × 104 rpm at 4 °C for 30 min. The pellet was resuspended in 8 mL acetone, centrifugated at 1 × 104 rpm at 4 °C for 20 min, and kept at room temperature in fume hood until dry. The protein sample was added 360 μL PBS, kept at 4 °C for 3 h, and centrifugated at 1 × 104 rpm 4 °C for 10 min. The supernatant was separated and used as samples of the culture supernatant.
Samples of bacterial cell wall proteins were prepared from 10 mL of culture. The cultivated bacteria were pelleted by 4.3 × 103 g at 4 °C for 5 min, and washed with TES solution (50 mM Tris-HCl, 1 mM EDTA, 20% sucrose) for twice, and then suspended in 200 μL TES-LMR (30 g/L lysozyme, 0.1 g/L RNase), kept at 37 °C for 2 h with mixing at intervals for several times. The supernatant was separated by centrifugation at 2.5 × 104 g 4 °C for 10 min, added ice pre-cooling trichloroacetic acid at 160 g/L, kept in ice for 20 min. The precipitate was obtained by centrifugation at 1.15 × 104 g, 4 °C for 10 min, suspended in 100 μL of 50 mM NaOH solution, kept at 4 °C for 12 h, and the supernatant was separated as the cell wall protein samples.
The expression products were identified by electrophoresis and western blotting assays using mouse anti-H. pylori antisera as the detector antibody as reported previously35.
Oral vaccination of mice and challenge with H. pylori
Specific pathogen free BALB/c mice, aged six weeks, were purchased from Henan Experimental Animal Center (Zhengzhou, China). The mice were assigned at random to 4 groups (Table 1). Equal amounts of male and female mice were included in every group, raised separately and housed 3~4 each cage. For LL(8110-napA-lysM) group (n = 22) and LL(8110) group (n = 22), the mice were treated by gavage with cell suspensions at a dose of 250 μL of L. lactis NZ3900 (pNZ8110-napA-lysM) (5 × 1010 CFU/mL) and NZ3900 (pNZ8110) (5 × 1010 CFU/mL), respectively, on day 0, 7, 14, 21, 28 and 35. For PBS group (n = 22), the mice were administered with equal volumes of PBS instead of the cell suspensions. The mice of Baseline group (n = 10) were untreated with L. lactis, PBS or H. pylori. The mice underwent fasting for 12 h and water deprivation for 4 h prior to the gavages, and were given food and drinking water 4 h after irrigation. The animal experiments have acquired approval of Institutional Review Board at Zhengzhou University, and performed under the ARRIVE guidelines.
Table 1.
Grouping of the BALB/c mice and treatments.
| Group | n | Treatments | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0~35d | 42d | 42d | 42d | 42d | 42d | 42~51d | 58d | 58d | ||
| Gavage with L. lactis or PBS 6 times | Divide into 2 Sub- groups n | EUT | Sample blood | Sample spleen cells | Sample feces | H. pylori challenges 4 times | EUT | Sample gastric tissues | ||
| LL(8110- napA-lysM) | 22 | √ | 11 | √ | √ | √ | √ | × | × | × |
| 11 | × | × | × | × | √ | √ | √ | |||
| LL(8110) | 22 | √ | 11 | √ | √ | √ | √ | × | × | × |
| 11 | × | × | × | × | √ | √ | √ | |||
| PBS | 22 | √ | 11 | √ | √ | √ | √ | × | × | × |
| 11 | × | × | × | × | √ | √ | √ | |||
| Baseline | 10 | × | × | × | × | × | × | × | √ | √ |
Note: √ received the treatment. × Not given the treatment. EUT euthanasia.
Seven days after the last vaccination, blood and intestinal feces samples were collected for half of the mice from each group as described below, while the remained mice were used in the H. pylori challenge experiment (Table 1). H. pylori 11637 grown on culture plate for 3 d were harvested and resuspended in culture medium to yield bacterial suspension of 1 × 1010 CFU/mL. The mice were fasted overnight, and received intragastric gavage with 250 μL sodium hydrogen carbonate (0.3 g/L) estimated 30 min before receiving challenges. The mice of all the 3 groups were treated by gavage with 200 μL H. pylori suspension each on day 7, 10, 13 and 16 post-immunization. Fasting and water deprivation were carried out in all the mice as mentioned above. The PBS group was used as a positive control group.
Blood and intestinal feces sampling
Blood samples were fetched from orbital sinus and kept at 4 °C for 16 ~ 20 h, and then sera were separated and stored in aliquots at −20 °C. Intestinal feces was sampled following separation of the spleens (see details below), 100 mg of feces was fetched from the intestine of each mouse, and then 1 mL of PBS containing proteinase inhabitor (Phenylmethanesulfonyl fluoride, 0.1 mM) was injected by the duodenum to wash the intestinal wall. The PBS eluate was recovered and mixed with the feces. The mixture was kept at 4 °C for overnight (14~16 h). The supernatant of the mixture was separated via centrifugation at 1.2 × 104 rpm for 10 min, and kept at −20 °C.
ELISA detection of antibodies
NapA was purified by amylose affinity chromatography from IPTG-induced E. coli TB1 (pMAL-c2x-linker-napA)56. The specific serum IgG and fecal SIgA antibodies were quantified by ELISA using the purified NapA as the detector antigen52. Briefly, 96-well microplates (Beijing Solarbio, China) were coated with NapA, and the ELISA signals were developed using biotinylated goat anti-mouse IgG (Abcam, USA), goat anti-mouse SIgA (Abcam, USA) and PNPP substrate (Beijing Solarbio, China). The absorbance of each well at 450 nm (OD450) was read by a Microplate Reader (Tecan Sunrise, CH), and designed as indicators of the specific SIgA and IgG levels.
Splenocytes cultivation and antigenic irritation
After blood sampling, the mice were sacrificed by spinal dislocation and soaked in 75% ethanol solution for 3 min, and then the spleens were harvested. The spleens were homogenized and passed through a 20 mesh strainer. The filtered splenocyte suspension was collected and treated with erythrocyte lysing buffer. The splenocytes were washed with D-hanks solution, and suspended to 5 × 106 cells/mL in 10% calf serum containing RPMI-1640 medium. The suspension of splenocytes were added into 24-well culture plates, 400 μL each well. H. pylori cell lysate antigens were added at 10 μg/mL to the wells as irritation agent. The cell cultivation was performed at 37 °C, 5% CO2 for 72 h.
Assessment of cytokines
The supernatant of splenocyte culture medium was separated at 72 h post cultivation by centrifugation at 3000 rpm for 20 min, and tested for quantification of cytokines interleukin (IL)-2, 4, 8, 10, 12, 17, 23 and interferon (IFN)-γ using ELISA kits (Mlbio, Shanghai, China), as instructed by the manufacturer.
Evaluation of H. pylori infection
On day 7 post the challenge, the mice were fasted overnight and sacrificed by spinal dislocation. Two round pieces of the gastric wall, 4 mm in diameter, were dissected from the gastric pyloric antrum using a puncher for each mouse, homogenized on a 200 mesh strainer, and washed with 1 mL preservation fluid (containing 100 g/L sucrose and 500 mL/L fetal bovine serum). The filtrate were recovered, and used as gastric samples for urease activity assays and bacterial cultivation.
For urease activity assays, 100 μL of each gastric sample was placed in 500 μL of urea broth medium (urea 20 g/L, soy peptone 1.0 g/L, NaCl 5.0 g/L, KH2PO4 2.0 g/L, glucose 1.0 g/L, phenol red 7.5 × 10−2 g/L), and kept at 37 °C for 5 h. The final values were read at 550 nm wavelength.
For quantitative bacterial culture, 100 μL of each gastric sample was plated onto Columbia agar plates with sheep blood and antibiotics, and cultured for 4 d as previously described53. Bacterial counts were expressed as colony forming unit (CFU) per square millimetre of gastric wall.
Additionally, ten BALB/c mice, free of the immunization and bacterial challenges, were employed for determining the base line of urease activity and H. pylori colonization levels of the mice.
Gastric histological examination
Histological examination was performed for eight mice per group to evaluate gastric inflammation responses. In brief, after being sampled as mentioned above, the stomach tissue remained was fixed using 10% neutral formaldehyde solution, and checked by paraffin section and hematoxylin-eosin (HE) staining. The gastritis of the mice was graded using the method previously reported57.
Statistical analysis
The data were analyzed using the software package SPSS 21.0. The measurement data were expressed as means ± standard deviation , and compared by one-way variance analysis. The pairwise comparisons of mean values were carried out using the least significant deviation methods (LSD). The difference was considered as significant at P < 0.05.
Electronic supplementary material
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (81773495), China Postdoctoral Science Foundation (200801273) and Henan Innovation Center of Molecular Diagnosis and Laboratory Medicine (XTCX-2015-ZD2).
Author Contributions
X.P. constructed the engineered L. lactis strain, performed the Western blot analysis and animal experimentation. R.Z., G.D. and L.Z. designed the project, interpreted the data and wrote the manuscript. R.Z., N.S., C.W. and S.C. were involved in the immunization of mice and evaluation of immune efficacy. Q.F. and Y.X. helped to carry out the genetic construction of the recombinant strain and animal experiments. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-24879-x.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nagy P, Johansson S, Molloy-Bland M. Systematic review of time trends in the prevalence of Helicobacter pylori infection in China and the USA. Gut Pathog. 2016;8:8. doi: 10.1186/s13099-016-0091-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zamani M, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2018;47:868–876. doi: 10.1111/apt.14561. [DOI] [PubMed] [Google Scholar]
- 3.Dargiene G, et al. Primary antibiotic resistance of Helicobacter pylori strains among adults and children in a tertiary referral centre in Lithuania. APMIS. 2018;126:21–28. doi: 10.1111/apm.12752. [DOI] [PubMed] [Google Scholar]
- 4.Abdoh Q, et al. Helicobacter pylori resistance to antibiotics at the An-Najah National University Hospital: a cross-sectional study. Lancet. 2018;391(Suppl 2):S32. doi: 10.1016/S0140-6736(18)30398-2. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y, Zhu Y, Lu NH. Novel and effective therapeutic regimens for helicobacter pylori in an era of increasing antibiotic resistance. Front. Cell. Infect. Microbiol. 2017;7:168. doi: 10.3389/fcimb.2017.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talebi Bezmin Abadi A. Vaccine against Helicobacter pylori: Inevitable approach. World J. Gastroenterol. 2016;22:3150–3157. doi: 10.3748/wjg.v22.i11.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Algood HM, Gallo-Romero J, Wilson KT, Peek RM, Jr., Cover TL. Host response to Helicobacter pylori infection before initiation of the adaptive immune response. FEMS Immunol. Med. Microbiol. 2007;51:577–586. doi: 10.1111/j.1574-695X.2007.00338.x. [DOI] [PubMed] [Google Scholar]
- 8.Akhiani AA, Schön K, Franzén LE, Pappo J, Lycke N. Helicobacter pylori-specific antibodies impair the development of gastritis, facilitate bacterial colonization, and counteract resistance against infection. J. Immunol. 2004;172:5024–5033. doi: 10.4049/jimmunol.172.8.5024. [DOI] [PubMed] [Google Scholar]
- 9.Ding H, et al. Th1-mediated immunity against Helicobacter pylori can compensate for lack of Th17 cells and can protect mice in the absence of immunization. PLoS One. 2013;8:e69384. doi: 10.1371/journal.pone.0069384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amedei A, et al. The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses. J. Clin. Invest. 2006;116:1092–1101. doi: 10.1172/JCI27177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu HW. Helicobacter pylori neutrophil-activating protein: from molecular pathogenesis to clinical applications. World J. Gastroenterol. 2014;20:5294–5301. doi: 10.3748/wjg.v20.i18.5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pachathundikandi SK, Lind J, Tegtmeyer N, El-Omar EM, Backert S. Interplay of the gastric pathogen Helicobacter pylori with toll-like receptors. Biomed. Res. Int. 2015;2015:192420. doi: 10.1155/2015/192420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramachandran M, Jin C, Yu D, Eriksson F, Essand M. Vector-encoded Helicobacter pylori neutrophil-activating protein promotes maturation of dendritic cells with Th1 polarization and improved migration. J. Immunol. 2014;193:2287–2296. doi: 10.4049/jimmunol.1400339. [DOI] [PubMed] [Google Scholar]
- 14.Sehrawat A, Sinha S, Saxena A. Helicobacter pylori neutrophil-activating protein: a potential Treg modulator suppressing allergic asthma? Front. Microbiol. 2015;6:493. doi: 10.3389/fmicb.2015.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang G, Hong Y, Olczak A, Maier SE, Maier RJ. Dual Roles of Helicobacter pylori NapA in inducing and combating oxidative stress. Infect. Immun. 2006;74:6839–6846. doi: 10.1128/IAI.00991-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokoyama H, Fujii S. Structures and metal-binding properties of Helicobacter pylori neutrophil-activating protein with a di-nuclear ferroxidase center. Biomolecules. 2014;4:600–615. doi: 10.3390/biom4030600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mobarez AM, Soleimani N, Tavakoli-Yaraki M, Farhangi B. Evaluation of nitric oxide production and proliferation activity of recombinant protein of Helicobacter pylori on macrophages. Microb. Pathog. 2016;100:149–153. doi: 10.1016/j.micpath.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Cappon A, et al. Helicobacter pylori-derived neutrophil-activating protein increases the lifespan of monocytes and neutrophils. Cell. Microbiol. 2010;12:754–764. doi: 10.1111/j.1462-5822.2010.01431.x. [DOI] [PubMed] [Google Scholar]
- 19.Vajdy M, et al. Enhanced mucosal and systemic immune responses to Helicobacter pylori antigens through mucosal priming followed by systemic boosting immunizations. Immunology. 2003;110:86–94. doi: 10.1046/j.1365-2567.2003.01711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kountouras J, et al. Potential implications of Helicobacter pylori-related neutrophil-activating protein. World J. Gastroenterol. 2012;18:489–490. doi: 10.3748/wjg.v18.i5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, et al. A Recombinant DNA plasmid encoding the sil-4r-nap fusion protein suppress airway inflammation in an ova-induced mouse model of asthma. Inflammation. 2016;39:1434–1440. doi: 10.1007/s10753-016-0375-6. [DOI] [PubMed] [Google Scholar]
- 22.Amedei A, Codolo G, Del Prete G, de Bernard M, D’Elios MM. The effect of Helicobacter pylori on asthma and allergy. J. Asthma Allergy. 2010;3:139–147. doi: 10.2147/JAA.S8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Elios MM, et al. Helicobacter pylori, asthma and allergy. FEMS Immunol. Med. Microbiol. 2009;56:1–8. doi: 10.1111/j.1574-695X.2009.00537.x. [DOI] [PubMed] [Google Scholar]
- 24.Iankov ID, et al. Expression of immunomodulatory neutrophil-activating protein of Helicobacter pylori enhances the antitumor activity of oncolytic measles virus. Mol. Ther. 2012;20:1139–1147. doi: 10.1038/mt.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang T, et al. Antitumor and immunomodulatory effects of recombinant fusion protein rMBP-NAP through TLR-2 dependent mechanism in tumor bearing mice. Int. Immunopharmacol. 2015;29:876–883. doi: 10.1016/j.intimp.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 26.Codolo, G., Munari, F., Fassan, M. & de Bernard, M. Evaluation of the efficacy of the H. pylori protein HP-NAP as a therapeutic tool for treatment of bladder cancer in an orthotopic murine model. J. Vis. Exp. (99), e52743 (2015). [DOI] [PMC free article] [PubMed]
- 27.Ramachandran M, Yu D, Wanders A, Essand M, Eriksson F. An infection-enhanced oncolytic adenovirus secreting H. pylori neutrophil-activating protein with therapeutic effects on neuroendocrine tumors. Mol. Ther. 2013;21:2008–2018. doi: 10.1038/mt.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iankov ID, Haralambieva IH, Galanis E. Immunogenicity of attenuated measles virus engineered to express Helicobacter pylori neutrophil-activating protein. Vaccine. 2011;29:1710–1720. doi: 10.1016/j.vaccine.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang YC, et al. High yield purification of Helicobacter pylori neutrophil- activating protein overexpressed in Escherichia coli. BMC Biotechnol. 2015;15:23. doi: 10.1186/s12896-015-0136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez-Duarte OG, et al. Protection of mice against gastric colonization by Helicobacter pylori by single oral dose immunization with attenuated Salmonella typhimurium producing urease subunits A and B. Vaccine. 1998;16:460–471. doi: 10.1016/S0264-410X(97)00247-8. [DOI] [PubMed] [Google Scholar]
- 31.Sun B, Li ZS, Tu ZX, Xu GM, Du YQ. Construction of an oral recombinant DNA vaccine from H. pylori neutrophil activating protein and its immunogenicity. World J. Gastroenterol. 2006;12:7042–7046. doi: 10.3748/wjg.v12.i43.7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mierau I, Kleerebezem M. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl. Microbiol. Biotechnol. 2005;68:1–13. doi: 10.1007/s00253-005-0107-6. [DOI] [PubMed] [Google Scholar]
- 33.Gu Q, Song D, Zhu M. Oral vaccination of mice against Helicobacter pylori with recombinant Lactococcus lactis expressing urease subunit B. FEMS Immunol. Med. Microbiol. 2009;56:197–203. doi: 10.1111/j.1574-695X.2009.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee MH, Roussel Y, Wilks M, Tabaqchali S. Expression of Helicobacter pylori urease subunit B gene in Lactococcus lactis MG1363 and its use as a vaccine delivery system against H. pylori infection in mice. Vaccine. 2001;19:3927–3935. doi: 10.1016/S0264-410X(01)00119-0. [DOI] [PubMed] [Google Scholar]
- 35.Zhang R, et al. An engineered Lactococcus lactis strain exerts significant immune responses through efficient expression and delivery of Helicobacter pylori Lpp20 antigen. Biotechnol. Lett. 2016;38:2169–2175. doi: 10.1007/s10529-016-2209-x. [DOI] [PubMed] [Google Scholar]
- 36.Stein K, et al. Endosomal recognition of Lactococcus lactis G121 and its RNA by dendritic cells is key to its allergy-protective effects. J. Allergy Clin. Immunol. 2016;139:667–678. doi: 10.1016/j.jaci.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 37.Ren C, et al. Modulation of peanut-induced allergic immune responses by oral lactic acid bacteria-based vaccines in mice. Appl. Microbiol. Biotechnol. 2014;98:6353–6364. doi: 10.1007/s00253-014-5678-7. [DOI] [PubMed] [Google Scholar]
- 38.D’Elios MM, de Bernard M. To treat or not to treat Helicobacter pylori to benefit asthma patients. Expert Rev. Respir. Med. 2010;4:147–150. doi: 10.1586/ers.10.9. [DOI] [PubMed] [Google Scholar]
- 39.Rosales-Mendoza S, Angulo C, Meza B. Food-Grade organisms as vaccine biofactories and oral delivery vehicles. Trends Biotechnol. 2016;34:124–136. doi: 10.1016/j.tibtech.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Namavar F, Sparrius M, Veerman EC, Appelmelk BJ, Vandenbroucke-Grauls CM. Neutrophil-activating protein mediates adhesion of Helicobacter pylori to sulfated carbohydrates on high-molecular-weight salivary mucin. Infect. Immun. 1998;66:444–447. doi: 10.1128/iai.66.2.444-447.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phadnis SH, et al. Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis. Infect. Immun. 1996;64:905–912. doi: 10.1128/iai.64.3.905-912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang XJ, Feng SY, Li ZT, Feng YM. Expression of Helicobacter pylori hspA gene in Lactococcus lactis NICE system and experimental study on its immunoreactivity. Gastroenterol. Res. Pract. 2015;2015:750932. doi: 10.1155/2015/750932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim SJ, et al. Oral administration of Lactococcus lactis expressing Helicobacter pylori Cag7-ct383 protein induces systemic anti-Cag7 immune response in mice. FEMS Immunol. Med. Microbiol. 2009;57:257–268. doi: 10.1111/j.1574-695X.2009.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braga WM, Atanackovic D, Colleoni GW. The role of regulatory T cells and TH17 cells in multiple myeloma. Clin. Dev. Immunol. 2012;2012:293479. doi: 10.1155/2012/293479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu YZ, Tan G, Wu F, Zhi FC. H. pylori attenuates TNBS-induced colitis via increasing mucosal Th2 cells in mice. Oncotarget. 2017;8:73810–73816. doi: 10.18632/oncotarget.17962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flach CF, Östberg AK, Nilsson AT, Malefyt Rde W, Raghavan S. Proinflammatory cytokine gene expression in the stomach correlates with vaccine-induced protection against Helicobacter pylori infection in mice: an important role for interleukin-17 during the effector phase. Infect. Immun. 2011;79:879–886. doi: 10.1128/IAI.00756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Codolo G, et al. Structure and immunomodulatory property relationship in NapA of Borrelia burgdorferi. Biochim. Biophys. Acta. 2010;1804:2191–2197. doi: 10.1016/j.bbapap.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Kaewkangsadan V, et al. Crucial contributions by T lymphocytes (effector, regulatory, and checkpoint inhibitor) and cytokines (TH1, TH2, and TH17) to a pathological complete response induced by neoadjuvant chemotherapy in women with breast cancer. J. Immunol. Res. 2016;2016:4757405. doi: 10.1155/2016/4757405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilke CM, et al. Th17 cells in cancer: help or hindrance? Carcinogenesis. 2011;32:643–649. doi: 10.1093/carcin/bgr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat. Rev. Immunol. 2010;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin Z, et al. Home Dampness Signs in Association with Asthma and Allergic Diseases in 4618 Preschool Children in Urumqi, China-The Influence of Ventilation/Cleaning Habits. PLoS One. 2015;10:e0134359. doi: 10.1371/journal.pone.0134359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsoi KK, Hirai HW, Chan FC, Griffiths S, Sung JJ. Cancer burden with ageing population in urban regions in China: projection on cancer registry data from World Health Organization. Br. Med. Bull. 2017;121:83–94. doi: 10.1093/bmb/ldw050. [DOI] [PubMed] [Google Scholar]
- 53.D’Agostino D, et al. Therapy for non-muscle invasive bladder cancer: HP-NAP. Urologia. 2012;79:142–148. doi: 10.5301/RU.2012.9189. [DOI] [PubMed] [Google Scholar]
- 54.Chen SY, Zhang RG, Duan GC, Shi JX. Food-grade expression of Helicobacter pylori ureB subunit in Lactococcus lactis and its immunoreactivity. Curr. Microbiol. 2011;62:1726–1731. doi: 10.1007/s00284-011-9920-6. [DOI] [PubMed] [Google Scholar]
- 55.Ma YJ, Duan GC, Zhang RG, Fan QT, Zhang WD. Mutation of iceA in Helicobacter pylori compromised IL-8 induction from human gastric epithelial cells. J. Basic Microbiol. 2010;50:S83–88. doi: 10.1002/jobm.200900410. [DOI] [PubMed] [Google Scholar]
- 56.Kang QZ, Duan GC, Fan QT, Xi YL. Fusion expression of Helicobacter pylori neutrophil-activating protein in E. coli. World J. Gastroenterol. 2005;11:454–456. doi: 10.3748/wjg.v11.i3.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guo L, et al. Oral immunization with a multivalent epitope-based vaccine, based on NAP, Urease, HSP60, and HpaA, provides therapeutic effect on H. pylori infection in mongolian gerbils. Front. Cell. Infect. Microbiol. 2017;7:349. doi: 10.3389/fcimb.2017.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.