Abstract
Endometriosis is a common benign gynecological disease, characterized by growth and proliferation of endometrial glands and stroma outside the uterus. With studies showing metabolic changes in various biofluids of endometriosis women, we have set upon to investigate whether endometrial tissue show differences in their metabolic profiles. 1H NMR analysis was performed on eutopic endometrial tissue of women with endometriosis and controls. Analysis was performed on spectral data and on relative concentrations of metabolites obtained from spectra using multivariate and univariate data analysis. Analysis shows that various energy, ketogenic and glucogenic metabolites have significant altered concentrations in various stages of endometriosis. In addition, altered tissue metabolites in minimal and mild stages of endometriosis were explored in serum of these patients to assess their role in disease diagnosis. For Stage I diagnosis alanine was found to have 90% sensitivity (true positives) and 58% specificity (true negatives). For Stage II diagnosis alanine, leucine, lysine, proline and phenylalanine showed significant altered levels in serum. While sensitivity of these serum metabolites varied between 69.2–100% the specificity values ranged between 58.3–91.7%. Further, a regression model generated with this panel of serum markers showed an improved sensitivity and specificity of 100% and 83%, respectively for Stage II diagnosis.
Subject terms: Diagnostic markers, Diagnostic markers
Introduction
Endometriosis is an estrogen dependent benign gynecological disorder and is defined by the presence of endometrial tissue consisting of glands and stroma outside the uterine cavity; commonly on the pelvic peritoneum, ovaries, and rectovaginal septum. Rarely, extra pelvic sites such as thorax1, cutaneous tissues2, bone3, and even the brain4 are also found to have infiltrating endometrial tissue growth. It is one of the most common causes of female infertility of unknown etiology which affects menstruating women in their reproductive age and may result from imbalance of the cellular equilibrium5. It affects 6–10% of women in their reproductive age, 50–60% adult and teenage women with complaints of pelvic pain and upto 50% of women reporting for infertility6. The symptoms of the disease include dysmenorrhoea, dyspareunia, dysuria and chronic abdominal pain which often appear late and overlap with other gynecological conditions such as pelvic adhesions, pelvic inflammatory disease, adenomyosis, ovarian cysts, and non-gynecological conditions such as inflammatory bowel syndrome, intestinal cystitis, irritable bowel syndrome etc6; often making diagnosis challenging. Currently, several diagnostic methods are available including ultrasonography and magnetic resonance imaging, nevertheless the gold standard for conclusive diagnosis remains surgical assessment of the pelvis by laparoscopy followed by biopsy7. Though CA-125 is the most popular biomarker for non-invasive diagnosis of endometriosis, several repeated studies have shown that CA-125 may be of more benefit in diagnosing Stages III–IV (advanced), than Stages I–II (mild)8.
Metabolomics is now increasingly being recognized as a powerful tool for elucidating pathogenesis and disease biomarker identification. This new ‘omics’ approach is used to perform global analysis of low molecular weight metabolites in various biological samples. An untargeted metabolomics approach provides an unbiased view of global metabolite changes in biological samples. The approach is hypotheses generating and exploratory in nature thereby helping in generating novel hypothesis or support older findings. Mass spectrometry (MS) and nuclear magnetic resonance (NMR) based platforms are the two most widely used analytical tools used for metabolomics studies. Unlike NMR, MS-based metabolomics provides better sensitivity for metabolomics research. With a combination of different ionization techniques and mass analyzers MS provides wide detection coverage of metabolites. NMR spectroscopy, on the other hand, is reproducible, quantitative and does not require extra steps for sample preparation, such as separation or derivatization9.
Despite several studies on metabolomics in various diseases, use of this approach is still sparse in endometriosis10–12. NMR and MS based metabolomics studies on endometrial fluid13, follicular fluid14, urine10, serum15 and plasma16 of endometriosis women have shown remarkable changes in their metabolite profile. Since endometrial tissue communicates with the blood directly or through extracellular fluids, several metabolites may be secreted into the blood stream. A circulatory diagnostic marker originating from the affected tissue is expected to be more reliable. The present study investigates 1H NMR based metabolic profiling of endometrial tissue to obtain a comprehensive idea of the metabolic perturbations associated with disease development and progression. The levels of altered tissue metabolites were further investigated in serum of paired endometriosis patients for their role in mild stage of the disease diagnosis.
Material and Methods
Subject selection
Necessary approvals were obtained from the Institutional Research Ethics Board of the Institute of Reproductive Medicine, Kolkata (IRM/IEC/BNC-IHP dated 30.11.2013); Institute of Post Graduate Medical Education & Research, Kolkata (Inst/IEC/551 dated 15.01.2014) and Indian Institute of Technology, Kharagpur, India (IIT/SRIC/AR/2013 dated 20.02.2013) prior to commencement of the study. All methods and experiments were performed in compliance and accordance to relevant guidelines and regulations. Endometriosis subjects were recruited from women presenting with endometriosis-associated symptoms and subsequently confirmed with laparoscopy followed by biopsy. Patients with age ≥40 years, ovarian tumors, polycystic ovarian syndrome, tubal block, anatomical abnormalities of the uterus, hydrosalphinx, pelvic inflammatory disease, other pelvic pathological conditions, leiomyoma, etc., were excluded from the study. Also, women who had received medical/hormonal supplementation during the last 3 months were excluded. 95 patients met the inclusion and exclusion criteria and were confirmed to have endometriosis. Staging was performed according to the revised American Society for Reproductive Medicine (initially named as AFS)17 criteria with Stage I classified as minimal (n = 20), Stage II as mild (n = 13), Stage III as moderate (n = 17) and Stage IV as severe endometriosis (n = 45).
Twenty four healthy fertile subjects were recruited from patients undergoing interval tubectomy (sterilization) and classified as controls. Women who had their last delivery during the 2–3 preceding years and having normal tubal pathology were included in the study. Exclusion criteria such as women ≥40 years of age and women receiving medical/hormonal supplementation during the last 3 months were considered while selecting control subjects. Blood and tissue samples were collected from both the study and control groups during mid secretory phase. Blood was drawn before anesthesia. Clinical information associated with each sample group is summarized in Table 1. Prior written informed consent was taken from all participants.
Table 1.
Clinical characteristics of samples included in the study.
| Characteristic (Tissue) | Healthy women (n = 24) | Endometriosis patients (n = 95) |
|---|---|---|
| Origin | Eastern region of India and Bangladesh | Eastern region of India and Bangladesh |
| Age (y), mean ± SD | 28.42 ± 3.2 | 29.37 ± 5.8 |
| BMI (kg/m2), mean ± SD | 26.17 ± 1.9 | 25.99 ± 1.5 |
| Stage of the disease | ||
| Stage I (Minimal) | — | 20 |
| (Stage II) (Mild) | — | 13 |
| (Stage II)I (Moderate) | — | 17 |
| Stage IV (Severe) | — | 45 |
Sample collection
Endometrial tissue was snap frozen and stored at −80 °C till further use. Blood samples were collected from women with endometriosis and controls, as mentioned previously15.
Sample preparation and metabolite extraction
Tissue
Metabolite extraction from tissue was performed according to Beckonert and coworkers18. Briefly, 100 mg of frozen tissue was weighed and grinded in a liquid nitrogen cooled mortar with 6% ice-cold perchloric acid. The sample was then vortexed and placed on ice for 10 min followed by centrifugation for 10 min at 12,000 g at 4 °C. The supernatant was then neutralized to pH 7.4 with 2 M potassium carbonate and left on ice for 30 min to precipitate the potassium perchlorate salts. The sample was then finally centrifuged, supernatant freeze–dried and stored at −80 °C. Before NMR acquisition, the tissue extracts were resuspended in 650 μl NMR buffer (100 mM sodium phosphate buffer, pH 7.4, in deuterated water (D2O), containing 1 mM sodium salt of 3-(trimethylsilyl) propionic-2,2,3,3,d4 acid (TSP). The samples were vortexed, centrifuged at 12,000 g for 5 min and finally 600 μl of the supernatant was transferred into an NMR tube.
Serum
Sample preparation for NMR analysis was performed as mentioned in our earlier study15. Briefly, serum samples were thawed and homogenized using a vortex mixer prior to NMR analysis. 200 µl of serum was mixed with 400 μl D2O containing 1 mM TSP. Following centrifugation (8000 rpm, 5 min), 600 µl of each sample was transferred into NMR tubes
1NMR data acquisition
NMR experiments were performed as explained in our earlier study15. Briefly, proton NMR spectra were recorded at 300 K using a 700 MHz Bruker Avance AV III spectrometer. CPMG spin-echo spectra were recorded with spectral width of 14 000 Hz and an acquisition time of 0.58 s. The pulse sequence used included a solvent pre-saturation routine for the suppression of the water signal. The resulting spectra were phased and baseline corrected, and chemical shifts referenced to TSP (δ = 0) by Bruker TOPSPIN 2.1 software. Normalization of spectrum to its total area was performed using MestReNova version 7.1.0 to limit the effect of concentration differences between samples. Minor chemical shifts due to pH alterations in tissue extracts and serum were adjusted and aligned using icoshift algorithm tool for MATLAB19,20. Individual metabolites were manually identified based on expected chemical-shift and coupling-constant values obtained from various sources, including earlier published articles, literatures and cross checked from the Human Metabolome Database (HMDB). Further, peak assignment was validated with COSY (Correlation spectroscopy) and TOCSY (Total correlation spectroscopy) spectra.
Multivariate analysis
Multivariate data analysis was performed on the full tissue spectra from 0.5–10 ppm in SIMCA 13.0.2 (Umetrics, Sweden). Water region from 4.5–5.15 ppm was excluded from the analysis. Unit variance scaling was applied to the data matrix to give equal weightage to all the variables. Following normalization and scaling, both the tissue and serum dataset was subjected to unsupervised and supervised multivariate analysis separately. Principal component analysis (PCA) was performed to detect inherent clustering and outliers in an unbiased manner. Partial least square discriminate analysis (PLS-DA) and a better classification model, orthogonal PLS-DA (OPLS-DA) was used to maximize class separation. Variables not relevant to class separation get removed in the OPLS-DA model. Therefore, only one predictive component is used for the discrimination between two classes21,22. To avoid over-fitting of the supervised models, the default 7-round cross-validation procedure in SIMCA software package was used. The reliability of the models was evaluated by the parameters R2 and Q2. R2 represents goodness of fit indicating explained variation in the dataset, whereas the cross validation parameters Q2 represent predictability of the model. Further, CV-ANOVA (analysis of variance testing of cross-validated predictive residuals) was carried out to ascertain the statistical significance of the OPLS-DA models. Significant variables were identified using S-line correlation plot.
Univariate analysis
Univariate analysis was performed both for tissue and serum spectra. The statistically significant variables extracted were subjected to spectral integration using MestReNova version 7.1.0 s. Baseline corrections were carried out wherever necessary. Non-parametric Kruskal–Wallis test was used with the Dunn multiple comparisons test to identify the statistically significant metabolites. Spearman rank correlation analysis was performed between tissue metabolite levels and rAFS score of women with endometriosis to ascertain the role of statistically significant metabolites in disease progression. Spearman rank correlation analysis between serum and tissue was performed for the statistically significant serum metabolites in minimal/mild endometriosis cases. GraphPad Prism version 5.0 (GraphPad Software, San Diego California, USA) was used for these univariate analyses. Receiving operating characteristic (ROC) curve analysis for diagnostic potential study was performed using MedCalc software (version 12.2.1.0).
Results
Several metabolites from diverse chemical classes could be identified from the 1H NMR spectra of endometrial tissue samples. These metabolites included amino acids (valine, glycine, alanine, leucine, proline, isoleucine, glutamate, glutamine, aspartate, asparagine, threonine, tyrosine, phenylalanine, ornithine, creatine), organic acids (citrate, 3-hydroxybutyric acid, lactate, acetate, succinate, formate, taurine, nicotinurate), nucleosides/nucleotides (inosine, adenine, uracil, uridine) and sugars (glucose, myo-inositol, glycogen). The list of metabolites along with their chemical moieties and corresponding chemical shifts are provided in Supplementary Table 1. A representative 1H-NMR spectrum of endometrial tissue from an endometriosis patient and the assignment of the representative metabolites is provided in Supplementary Figure 1.
Multivariate analysis
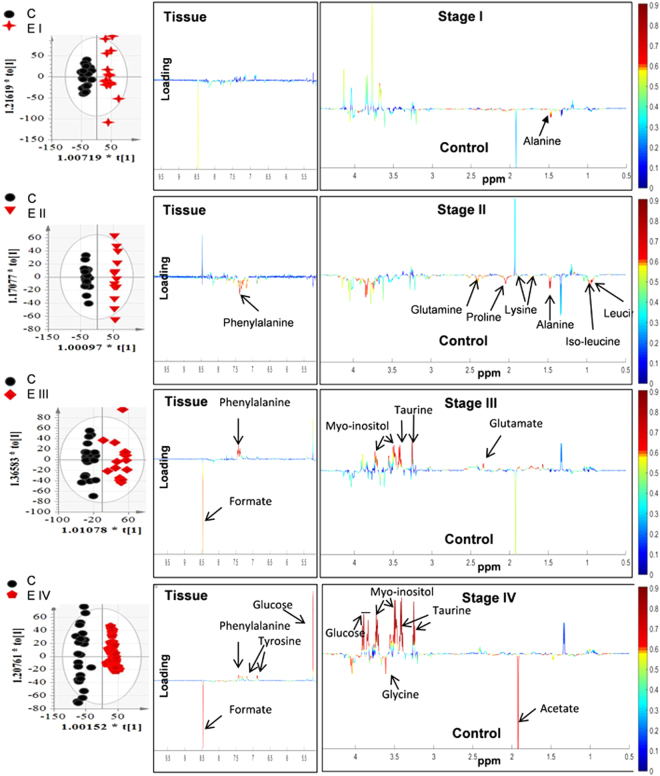
PCA analysis of tissue NMR spectra revealed clustering among the various stages of endometriosis and controls (Supplementary Figure 2). Supervised models such as PLS-DA (Supplementary Figure 2) and OPLS-DA (Fig. 1) showed improved discrimination with good model prediction parameters (Supplementary Table 2).
Figure 1.
OPLS-DA scores (left) and loadings plots for multivariate analysis with tissue samples collected from controls (n = 24) and stage I (n = 20), II (n = 13), III (n = 17) and IV (n = 45) of endometriosis patients. Results were from the 7-fold cross-validated models and colored scales were for the correlation coefficients (|r|) of variables.
Identification of statistically significant metabolites
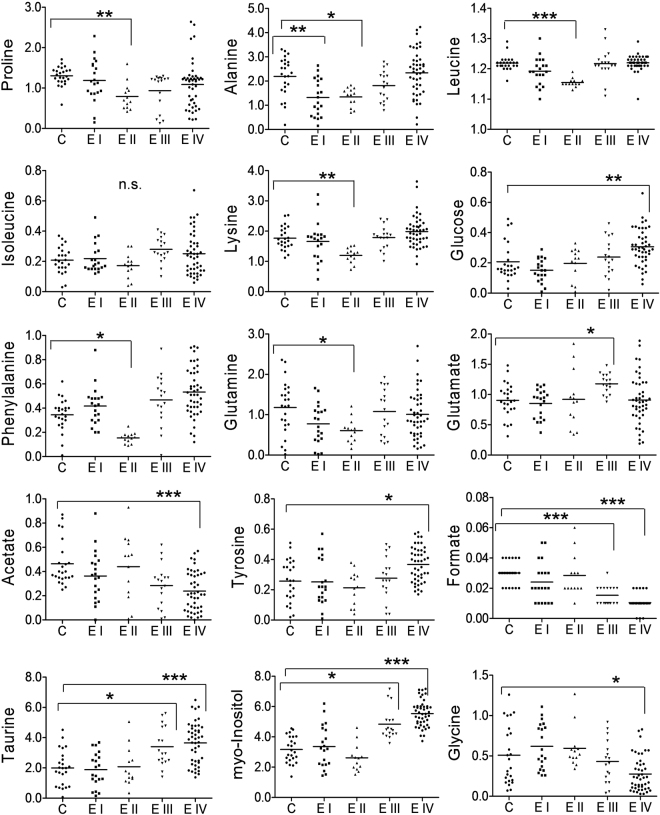
A two variable extraction procedure was used to extract the most relevant tissue metabolites responsible for disease classification. First, S-line correlation plot was used to identify the most significant variables responsible for class separation in tissue (Fig. 1). The variables with higher values of correlation coefficient (denoted by red color) were the major metabolites contributing maximum towards class separation. These metabolites were then subjected to relative quantitative analysis using their individual integral values. Metabolite such as isoleucine which was found to be significant in the first step is now ruled out from the list of relevant metabolites, owing to their insignificant p value during univariate analysis (Fig. 2). Further, the statistically relevant tissue metabolites in early (minimal/mild) endometriosis were identified in serum NMR spectra and subjected to relative quantitative analysis using their individual integral values. The relevant tissue and serum metabolites were subjected to correlation analysis. While alanine, lysine, phenylalanine and leucine showed negative correlation, proline showed positive correlation (Fig. 3). The diagnostic potential of these markers were assessed in serum of minimal and mild endometriosis patients (Table 2 and Fig. 4). A partial least squares (PLS) regression model with a combination of the panel of serum markers followed by ROC analysis showed improved sensitivity and specificity of 100% and 83% for Stage II diagnosis.
Figure 2.
Results of metabolite integral values for selected tissue metabolites. Horizontal lines represent group means. Kruskal-Wallis test was used with the Dunn multiple comparisons test. Levels of statistical significance are as follows: *p < 0.05; **p < 0.01; ***p < 0.001.
Figure 3.
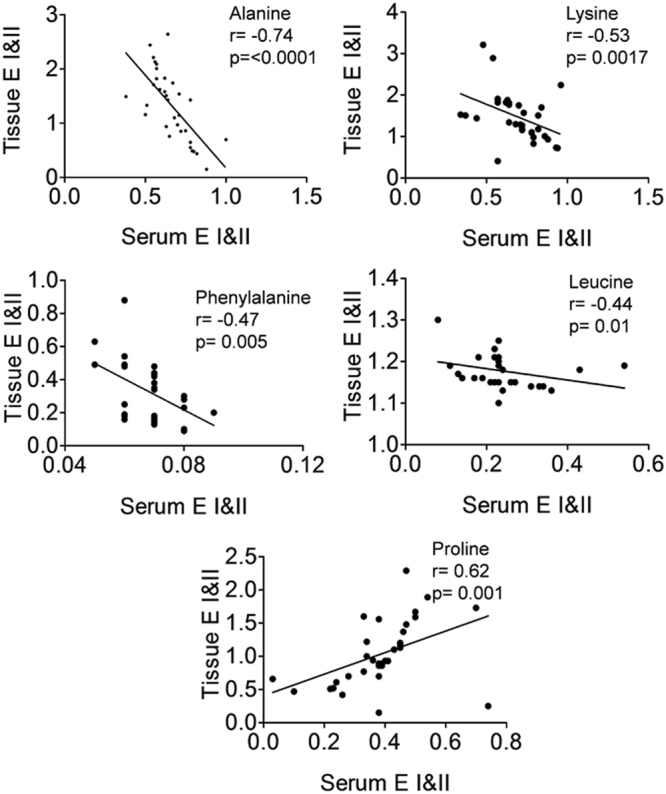
Results of correlation analysis between tissue and serum metabolite expression levels. Non-parametric Spearman correlation test was used.
Table 2.
Diagnostic potential of the identified serum markers.
| Metabolite | Sensitivity (%) | Specificity (%) |
|---|---|---|
| Alanine (Stage I) | 90 | 58.3 |
| Alanine (Stage II) | 84.6 | 58.3 |
| Leucine (Stage II) | 69.2 | 91.7 |
| Lysine (Stage II) | 69.2 | 83.3 |
| Proline (Stage II) | 92.3 | 87.5 |
| Phenylalanine (Stage II) | 100 | 75 |
| Multi panel metabolite (Stage II) | 100 | 83 |
Figure 4.
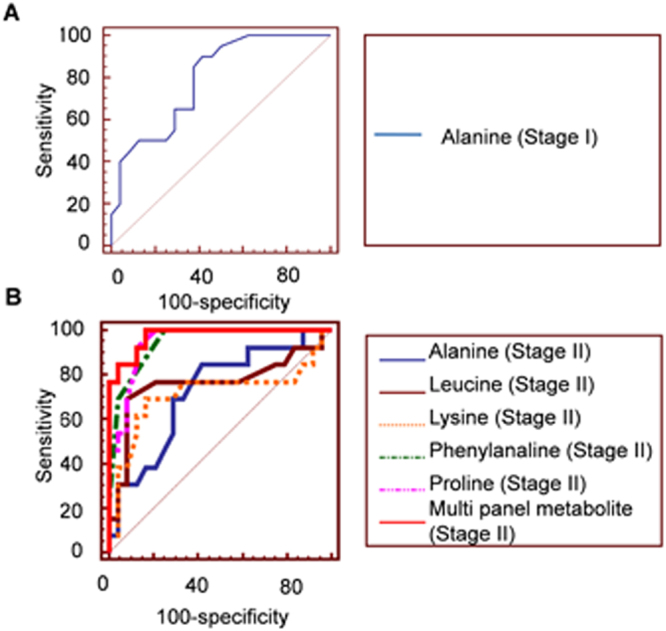
ROC curve analysis of the identified serum metabolite markers for (A) Stage I and (B) Stage II diagnosis.
Discussion
Though it is now widely accepted that metabolomics promises immense potential understanding pathogenesis, diagnosis and therapy monitoring of many diseases; use of this approach in endometriosis is still at its nascent stage. This approach, nevertheless, has gained momentum, as evident by three studies investigating endometriosis-associated metabolic changes in serum15,16 and urine samples10. We here study the metabolite changes in endometrial tissue of women with endometriosis (Stage I–IV) using 1H NMR based metabolomics. Since CA-125 is routinely used and is quite efficient in diagnosis of stage III & IV of endometriosis, our subsequent objective was to identify a panel of metabolites which can be used for non-invasive diagnosis of Stage I & II of the disease. We, therefore, investigated the levels of tissue metabolites in serum which were found to be relevant to Stage I & II disease pathology. To the best of our knowledge this is the first report on endometrial tissue metabolite profile of women with endometriosis.
Although the precise mechanism of disease development remains unknown, according to Sampson’s hypothesis, endometriosis is established when retrograde endometrial cells implant within the pelvic cavity. It is now widely accepted that there are fundamental abnormal changes within the eutopic endometrium of women with endometriosis compared to normal endometrium of healthy women. Several studies have shown that eutopic endometrium has enhanced ability of proliferation, angiogenesis, and implantation and, thus, greater probability of escaping the unfavorable conditions of the ectopic environment. Therefore, the character of eutopic endometrium determines the fate of the backward-flowing endometrial tissue in the pelvic cavity23. The present study, therefore, attempts to study the metabolic changes in eutopic endometrium of women with endometriosis. It is well accepted that endometriosis exhibits features similar to that of malignancy24,25. Though a benign condition, endometriosis appears to act like cancer characterized by cell invasion, uncontrolled growth, neo-vascularization and a decrease in the number of cells due to apoptosis26. Increasing evidences suggest that markers for these tumorigenic properties are also expressed in eutopic tissue27–30 indicating that endometrium in these patients may already have been affected before they migrate to the peritoneum resulting in endometriosis31; thus supporting the retrograde menstruation hypothesis. Taurine is a β-amino acid which is found abundantly in various mammalian tissues, and its concentration has been found to be increased in several tumors32–34. Elevated taurine levels in tumors are associated with increased proliferative activity and cell density35. Another metabolite, myo-Inositol, is correlated with tumor cell density36. While its level is reported to be downregulated in cancer with a negative correlation with taurine37,38, several other studies suggest increased levels of both myo-inositol and taurine in tumors of the prostate and squamous cell carcinoma39,40. Following the analogy of endometriosis with the neoplastic process, increased levels of these two metabolites in the tissue of advanced stages of endometriosis (Figs 1 and 2) is associated with the higher proliferative activity seen in endometriotic lesions. Further, both taurine and myo-inositol showed strong positive correlation with rAFS scores (Supplementary figure 3) suggesting their role in disease progression. Also, our observation of higher taurine levels in endometriosis women is in agreement with an earlier metabolomics study performed on urine samples of endometriosis patients10. Endometriosis is associated with angiogenesis41 which is further associated with elevated de novo nucleotide synthesis42. Formate is an integral metabolite of purine biosynthesis. Owing to increased angiogenesis and tissue growth in women with advanced stages of endometriosis, it is likely that the formate molecules are incorporated into the purine nucleotide synthesis leading to its depletion. This may be the possible explanation for decreased formate levels observed in endometrial tissue of women with severe endometriosis.
Several amino acids were found to be dysregulated in tissue of various stages of endometriosis women compared to healthy controls. Alteration in glutamine and glutamate level was observed in Stage II and Stage III of endometriosis patients. Both glutamine and glutamate are glucogenic amino acids and are considered to be vital for maintenance of cell function43. They can be used for a diverse array of processes44 according to the demands of the body during severe illness. A recent study has infact associated altered glutamine and glutamate levels to endometriosis-associated pelvic pain45. A number of amino acids including alanine, lysine, phenylalanine and leucine showed significantly lower levels in endometrial tissue of women with endometriosis relative to healthy controls. This may be due to the fact that endometriosis results from physiological mechanism of tissue injury and repair46. The catabolic state induced in response to injury in endometriosis leads to increased breakdown of endogenous protein and release of free amino acids in circulation. This is further supported by our results where we observed an inverse relationship between tissue and serum levels for alanine, lysine, phenylalanine and leucine. Another amino acid, proline was also found to have significant lower levels in tissue, but showed positive correlation with serum level of Stage II endometriosis. In advanced stages of the disease, though not statistically significant, a general downward trend was observed in tissue samples (Fig. 1). A recent report proposes that the degradation of proline occurs only through proline dehydrogenase and is associated with reactive oxygen species (ROS) generation47. With increasing evidence suggesting that ROS may be involved in endometriosis progression48, it may be worthwhile to further explore the role of proline in the pathogenesis of the disease.
Tissue metabolite levels may or may not be reflected in circulation. For study of diagnostic potential of the early stage (Stage I/II) dysregulated tissue metabolites, their levels were studied in serum of paired samples. It was encouraging to find that five of the dysregulated tissue amino acids were also found to have altered levels in serum of these patients. Interestingly, while tissue showed lower levels for alanine, lysine, phenylalanine and leucine, serum samples showed an inverse relationship for these metabolites. It was, therefore, not surprising to find a significant negative correlation between tissue and serum levels of alanine, lysine, phenylalanine and leucine (Fig. 3). A positive correlation was observed for proline. Further, for minimally invasive diagnosis, the diagnostic potential of these five metabolites was assessed in serum. For Stage I diagnosis alanine was found to have 90% sensitivity (true positives) and 58% specificity (true negatives). For Stage II diagnosis maximum sensitivity was observed for phenylalanine, however its specificity was found to be poor. Leucine showed maximum specificity of 91.7%, however its sensitivity was again found to be poor (69.2%). It is now being widely recognized that a panels of markers would have better diagnostic potential compared with individual markers49. This is in accordance with our findings for Stage II diagnosis where we find sensitivity and specificity of 100% and 83%, respectively for the PLS model developed using the combined panel of markers.
Conclusion
In summary, we have for the first time identified characteristic metabolites in eutopic endometrial tissue of various stages of endometriosis. The metabolic perturbations mediated by the metabolites which can significantly discriminate between endometriosis and controls are discussed. Further, the relevant tissue metabolites are correlated with the corresponding serum metabolites in early stage of the disease for investigating their role in early diagnosis. Five serum amino acids, proline, alanine, lysine, phenylalanine and leucine were found to be suitable for endometriosis diagnosis. Future studies will focus on the validation of these results in larger cohorts of patients. Also, a follow-up study to track the levels of the metabolites from the onset of endometriosis could help improve its clinical management. In addition, inclusion of additional patient groups with other conditions associated with pelvic inflammation will help in deciphering the cross-talk between endometriosis and associated pelvic inflammation. It is also important to mention that for robust assessment of these markers, their expression levels in endometriosis patients receiving hormone medications should also be investigated. Furthermore, there is a need to compare the present findings, which are restricted to the secretory phase, to the candidate metabolites’ expression in the proliferative phase for developing a comprehensive diagnostic test for endometriosis.
Electronic supplementary material
Acknowledgements
Council of Scientific and Industrial Research, India (grant no. 27(0303)/14/EMR-II Dated: 25/04/2014) is acknowledged for funding support. The facilities provided by the National Facility for High Field NMR located at TIFR and supported by the Department of Science and Technology (DST) are also gratefully acknowledged. The authors would like to acknowledge Mr. Khushman Taunk (SMST, IIT Kharagpur) for providing valuable inputs during manuscript preparation. The authors are grateful to all the volunteers who participated in this study.
Author Contributions
Conceived the study: K.C., B.N.C., S.D.G., M.D.; designed the study: M.D., K.C.; involved in clinical sample preparation: M.D., E.S., S.K.J.; performed the experiments and analyzed the data: M.D., M.J., D.D., U.S.; drafted the manuscript: M.D., B.S., M.M., K.C.; provided clinical samples: B.N.C., C.D.R.; provided chemicals and reagents: K.C., S.D.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
2/12/2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
Contributor Information
Mainak Dutta, Email: mainak@dubai.bits-pilani.ac.in.
Koel Chaudhury, Email: koel@smst.iitkgp.ernet.in.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-23954-7.
References
- 1.Lee CH, Huang YC, Huang SF, Wu YK, Kuo KT. Thoracic endometriosis: rare presentation as a solitary pulmonary nodule with eccentric cavitations. Thorax. 2009;64:919–920. doi: 10.1136/thx.2008.111294. [DOI] [PubMed] [Google Scholar]
- 2.Attia L, et al. A case of cutaneous endometriosis developed on an abdominal scar. Tunis Med. 2009;88:841–843. [PubMed] [Google Scholar]
- 3.Oei SG, Peters AA, Welvaart K, Bode PJ, Fleuren GJ. Aggressive endometriosis in bone. Lancet. 1992;339:1477–1478. doi: 10.1016/0140-6736(92)92067-p. [DOI] [PubMed] [Google Scholar]
- 4.Sarma D, et al. Cerebellar endometriosis. AJR Am J Roentgenol. 2004;182:1543–1546. doi: 10.2214/ajr.182.6.1821543. [DOI] [PubMed] [Google Scholar]
- 5.Olive DL, Schwartz LB. Endometriosis. N Engl J Med. 1993;328:1759–1769. doi: 10.1056/NEJM199306173282407. [DOI] [PubMed] [Google Scholar]
- 6.Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 8.May KE, et al. Peripheral biomarkers of endometriosis: a systematic review. Human reproduction update. 2010;16:651–674. doi: 10.1093/humupd/dmq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emwas AH. The strengths and weaknesses of NMR spectroscopy and mass spectrometry with particular focus on metabolomics research. Methods Mol Biol. 2015;1277:161–193. doi: 10.1007/978-1-4939-2377-9_13. [DOI] [PubMed] [Google Scholar]
- 10.Vicente-Munoz S, et al. Nuclear magnetic resonance metabolomic profiling of urine provides a noninvasive alternative to the identification of biomarkers associated with endometriosis. Fertil Steril. 2015;104:1202–1209. doi: 10.1016/j.fertnstert.2015.07.1149. [DOI] [PubMed] [Google Scholar]
- 11.Vouk K, et al. Discovery of phosphatidylcholines and sphingomyelins as biomarkers for ovarian endometriosis. Hum Reprod. 2012;27:2955–2965. doi: 10.1093/humrep/des152. [DOI] [PubMed] [Google Scholar]
- 12.Lee YH, et al. Dysregulated sphingolipid metabolism in endometriosis. J Clin Endocrinol Metab. 2014;99:E1913–1921. doi: 10.1210/jc.2014-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dominguez F, et al. Lipidomic profiling of endometrial fluid in women with ovarian endometriosisdagger. Biol Reprod. 2017;96:772–779. doi: 10.1093/biolre/iox014. [DOI] [PubMed] [Google Scholar]
- 14.Cordeiro, F. B. et al. Lipidomics analysis of follicular fluid by ESI-MS reveals potential biomarkers for ovarian endometriosis. J Assist Reprod Genet (2015). [DOI] [PMC free article] [PubMed]
- 15.Dutta M, et al. A metabonomics approach as a means for identification of potential biomarkers for early diagnosis of endometriosis. Mol Biosyst. 2012;8:3281–3287. doi: 10.1039/c2mb25353d. [DOI] [PubMed] [Google Scholar]
- 16.Lee YH, et al. Limited value of pro-inflammatory oxylipins and cytokines as circulating biomarkers in endometriosis - a targeted ‘omics study. Sci Rep. 2016;6:26117. doi: 10.1038/srep26117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril67, 817–821 (1997). [DOI] [PubMed]
- 18.Beckonert O, et al. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat Protoc. 2007;2:2692–2703. doi: 10.1038/nprot.2007.376. [DOI] [PubMed] [Google Scholar]
- 19.Savorani F, Tomasi G, Engelsen SB. icoshift: A versatile tool for the rapid alignment of 1D NMR spectra. J Magn Reson. 2010;202:190–202. doi: 10.1016/j.jmr.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Tomasi G, Savorani F, Engelsen SB. icoshift: An effective tool for the alignment of chromatographic data. J Chromatogr A. 2011;1218:7832–7840. doi: 10.1016/j.chroma.2011.08.086. [DOI] [PubMed] [Google Scholar]
- 21.Trygg J, Wold S. Orthogonal projections to latent structures (O-PLS) Journal of Chemometrics. 2002;16:119–128. [Google Scholar]
- 22.Bylesjo M, et al. Orthogonal projections to latent structures as a strategy for microarray data normalization. BMC Bioinformatics. 2007;8:207. doi: 10.1186/1471-2105-8-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H, Lang JH. Is abnormal eutopic endometrium the cause of endometriosis? The role of eutopic endometrium in pathogenesis of endometriosis. Medical science monitor: international medical journal of experimental and clinical research. 2011;17:RA92–99. doi: 10.12659/MSM.881707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin J, Zhang X, Chen Y. Mutagen sensitivity as a susceptibility marker for endometriosis. Hum Reprod. 2003;18:2052–2057. doi: 10.1093/humrep/deg393. [DOI] [PubMed] [Google Scholar]
- 25.Karanjgaokar VC, Murphy DJ, Samra JS, Mann CH. Malignant transformation of residual endometriosis after hysterectomy: a case series. Fertil Steril. 2009;92(2037):e2019–2021. doi: 10.1016/j.fertnstert.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Capobianco, A. & Rovere-Querini, P. Endometriosis, a disease of the macrophage. Front Immunol4, 9 (2013). [DOI] [PMC free article] [PubMed]
- 27.Hayrabedyan S, Kyurkchiev S, Kehayov I. Endoglin (cd105) and S100A13 as markers of active angiogenesis in endometriosis. Reprod Biol. 2005;5:51–67. [PubMed] [Google Scholar]
- 28.Donnez J, Smoes P, Gillerot S, Casanas-Roux F, Nisolle M. Vascular endothelial growth factor (VEGF) in endometriosis. Hum Reprod. 1998;13:1686–1690. doi: 10.1093/humrep/13.6.1686. [DOI] [PubMed] [Google Scholar]
- 29.Bourlev V, et al. The relationship between microvessel density, proliferative activity and expression of vascular endothelial growth factor-A and its receptors in eutopic endometrium and endometriotic lesions. Reproduction. 2006;132:501–509. doi: 10.1530/rep.1.01110. [DOI] [PubMed] [Google Scholar]
- 30.Wang HB, et al. Expression of vascular endothelial growth factor receptors in the ectopic and eutopic endometrium of women with endometriosis. Zhonghua Yi Xue Za Zhi. 2005;85:1555–1559. [PubMed] [Google Scholar]
- 31.Carvalho L, Podgaec S, Bellodi-Privato M, Falcone T, Abrao MS. Role of eutopic endometrium in pelvic endometriosis. J Minim Invasive Gynecol. 2011;18:419–427. doi: 10.1016/j.jmig.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 32.Chan EC, et al. Metabolic profiling of human colorectal cancer using high-resolution magic angle spinning nuclear magnetic resonance (HR-MAS NMR) spectroscopy and gas chromatography mass spectrometry (GC/MS) J Proteome Res. 2009;8:352–361. doi: 10.1021/pr8006232. [DOI] [PubMed] [Google Scholar]
- 33.El-Sayed S, et al. An ex vivo study exploring the diagnostic potential of 1H magnetic resonance spectroscopy in squamous cell carcinoma of the head and neck region. Head & Neck. 2002;24:766–772. doi: 10.1002/hed.10125. [DOI] [PubMed] [Google Scholar]
- 34.Srivastava S, et al. Taurine - a possible fingerprint biomarker in non-muscle invasive bladder cancer: A pilot study by 1H NMR spectroscopy. Cancer Biomark. 2010;6:11–20. doi: 10.3233/CBM-2009-0115. [DOI] [PubMed] [Google Scholar]
- 35.Griffin JL, Shockcor JP. Metabolic profiles of cancer cells. Nat Rev Cancer. 2004;4:551–561. doi: 10.1038/nrc1390. [DOI] [PubMed] [Google Scholar]
- 36.Lehtimaki KK, et al. Metabolite changes in BT4C rat gliomas undergoing ganciclovir-thymidine kinase gene therapy-induced programmed cell death as studied by 1H NMR spectroscopy in vivo, ex vivo, and in vitro. J Biol Chem. 2003;278:45915–45923. doi: 10.1074/jbc.M306209200. [DOI] [PubMed] [Google Scholar]
- 37.Miccoli P, et al. Metabolomics approach to thyroid nodules: a high-resolution magic-angle spinning nuclear magnetic resonance-based study. Surgery. 2012;152:1118–1124. doi: 10.1016/j.surg.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 38.Torregrossa L, et al. Toward the reliable diagnosis of indeterminate thyroid lesions: a HRMAS NMR-based metabolomics case of study. J Proteome Res. 2012;11:3317–3325. doi: 10.1021/pr300105e. [DOI] [PubMed] [Google Scholar]
- 39.Swanson MG, et al. Proton HR-MAS spectroscopy and quantitative pathologic analysis of MRI/3D-MRSI-targeted postsurgical prostate tissues. Magnetic Resonance in Medicine. 2003;50:944–954. doi: 10.1002/mrm.10614. [DOI] [PubMed] [Google Scholar]
- 40.Tripathi P, et al. Delineating metabolic signatures of head and neck squamous cell carcinoma: phospholipase A2, a potential therapeutic target. Int J Biochem Cell Biol. 2012;44:1852–1861. doi: 10.1016/j.biocel.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rocha AL, Reis FM, Taylor RN. Angiogenesis and endometriosis. Obstet Gynecol Int. 2013;2013:859619. doi: 10.1155/2013/859619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vandekeere S, Dewerchin M, Carmeliet P. Angiogenesis Revisited: An Overlooked Role of Endothelial Cell Metabolism in Vessel Sprouting. Microcirculation. 2015;22:509–517. doi: 10.1111/micc.12229. [DOI] [PubMed] [Google Scholar]
- 43.Newsholme P, et al. Glutamine and glutamate as vital metabolites. Braz J Med Biol Res. 2003;36:153–163. doi: 10.1590/s0100-879x2003000200002. [DOI] [PubMed] [Google Scholar]
- 44.Newsholme P, Procopio J, Lima MM, Pithon-Curi TC, Curi R. Glutamine and glutamate–their central role in cell metabolism and function. Cell Biochem Funct. 2003;21:1–9. doi: 10.1002/cbf.1003. [DOI] [PubMed] [Google Scholar]
- 45.As-Sanie S, et al. Functional Connectivity Is Associated With Altered Brain Chemistry in Women With Endometriosis-Associated Chronic Pelvic Pain. J Pain. 2016;17:1–13. doi: 10.1016/j.jpain.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leyendecker G, Wildt L, Mall G. The pathophysiology of endometriosis and adenomyosis: tissue injury and repair. Arch Gynecol Obstet. 2009;280:529–538. doi: 10.1007/s00404-009-1191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phang JM, Liu W, Hancock CN, Fischer JW. Proline metabolism and cancer: emerging links to glutamine and collagen. Curr Opin Clin Nutr Metab Care. 2015;18:71–77. doi: 10.1097/MCO.0000000000000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ngo C, et al. Reactive oxygen species controls endometriosis progression. Am J Pathol. 2009;175:225–234. doi: 10.2353/ajpath.2009.080804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lanišnik Rižner Tea. Noninvasive biomarkers of endometriosis: myth or reality? Expert Review of Molecular Diagnostics. 2014;14(3):365–385. doi: 10.1586/14737159.2014.899905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.