Abstract
Purpose
Osteoclast activity is an important factor in the pathogenesis of skeletal metastases and is a potential therapeutic target. This study aimed to determine if selective uptake of 99mTc-maraciclatide, a radiopharmaceutical targeting αvβ3 integrin, occurs in prostate cancer (PCa) bone metastases and to observe the changes following systemic therapy.
Methods
The study group comprised 17 men with bone-predominant metastatic PCa who underwent whole-body planar and single-photon emission computed tomography/computed tomography (SPECT/CT) imaging with 99mTc-maraciclatide before (n = 17) and 12 weeks after (n = 11) starting treatment with abiraterone. Tumour to normal bone (T:N) ratios, tumour to muscle (T:M) ratios and CT Hounsfield units (HU) were measured in up to five target metastases in each subject. An oncologist blinded to study scans assessed clinical responses up to 24 weeks using conventional criteria.
Results
Before treatment, metastases showed specific 99mTc-maraciclatide accumulation (mean planar T:N and T:M ratios 1.43 and 3.06; SPECT T:N and T:M ratios 3.1 and 5.19, respectively). Baseline sclerotic lesions (389–740 HU) showed lower T:M ratios (4.22 vs. 7.04, p = 0.02) than less sclerotic/lytic lesions (46–381 HU). Patients with progressive disease (PD; n = 5) showed increased planar T:N and T:M ratios (0.29 and 12.1%, respectively) and SPECT T:N and T:M ratios (11.9 and 20.2%, respectively). Patients without progression showed decreased planar T:N and T:M ratios (0.27 and −8.0%, p = 1.0 and 0.044, respectively) and SPECT T:N and T:M ratios (−21.9, and −27.2%, p = 0.3 and 0.036, respectively). The percentage change in CT HU was inversely correlated with the percentage change in SPECT T:M ratios (r = −0.59, p = 0.006).
Conclusions
99mTc-maraciclatide accumulates in PCa bone metastases in keeping with increased αvβ3 integrin expression. Greater activity in metastases with lower CT density suggests that uptake is related to osteoclast activity. Changes in planar and SPECT T:M ratios after 12 weeks of treatment differed between patients with and without PD and 99mTc-maraciclatide imaging may be a potential method for assessing early response.
Keywords: Prostate cancer, Bone metastases, 99mTc-Maraciclatide, Osteoclast
Introduction
Skeletal metastases are common in patients with prostate cancer (PCa) and are associated with significant morbidity and skeletal-related events [1]. However, effective systemic treatment is available leading to improvements in overall survival of several months to years [2]. This is longer than in most other types of cancer that have metastasized to the skeleton, and the management of PCa bone metastases therefore has a significant impact on healthcare resources [3]. It is recognized that conventional imaging, including 99mTc-diphosphonate bone scintigraphy or computed tomography (CT), is poor at determining treatment response or nonresponse at an early time point when patients can undergo therapeutic transition to second-line therapy that may be more effective, and can be saved from side effects of ineffective treatments [4, 5].
Whilst bone metastases from PCa are predominantly osteoblastic in nature, leading to sclerotic radiological morphology, it is known that they also demonstrate increased osteoclast activity that may be a major contributor to morbidity (e.g. pathological fracture) and which may be a therapeutic target for drugs including RANK ligand inhibitors (e.g. denosumab) and osteoclast inhibitors (e.g. bisphosphonates), that can decrease skeletal-related events [2]. Osteoclast activity is therefore a relevant process in the pathophysiology of bone metastases and may provide a useful target for detection and response assessment by imaging probes. Osteoclasts express higher levels of the αvβ3 integrin than any other cell types in the body [6] and this integrin heterodimer is involved in the adhesion of osteoclasts to the bone matrix [7]. It strongly binds the Arg-Gly-Asp (RGD) motif, which when radiolabelled, has been used to successfully image αvβ3 integrin expression related to osteoclast activity in animal models [8, 9]. 99mTc-Maraciclatide (99mTc-NC100692) is a cyclic peptide with a RGD binding site with high affinity for the αvβ3 integrin in vitro and in vivo, and has been investigated for detection of αvβ3 integrin overexpression in angiogenesis [10, 11].
In this study our hypotheses were that 99mTc-maraciclatide specifically accumulates in PCa bone metastases and that changes in uptake correlate with treatment response to systemic therapy. Our aims were to measure uptake of 99mTc-maraciclatide in patients with de-novo or progressive bone metastases from PCa, to correlate uptake and changes in uptake with CT density (Hounsfield units, HU), as a surrogate for the underlying osteoblastic and osteoclastic activity, and to determine if changes in uptake at 12 weeks could act as a marker of treatment response.
Materials and methods
The study group comprised 17 consecutive men (mean age 73.4 years) with de-novo or progressive bone-predominant metastatic PCa scheduled for treatment with abiraterone. Informed consent was obtained from all individual participants included in the study. Ethical and radiation committee (ARSAC) approvals were obtained for the study. Six patients did not undergo 12-week imaging, in two due to patient choice and in four due to lack of tracer availability. In 11 patients who had follow-up 99mTc-maraciclatide imaging at 12 weeks, a clinical oncologist blinded to the 99mTc-maraciclatide imaging defined the clinical response to abiraterone using conventional clinical (including pain score), biochemical (prostate-specific antigen, alkaline phosphatase) and imaging results (CT and bone scan) up to 24 weeks after the start of therapy. Clinical response was recorded as progressive disease (PD) or non-PD, i.e. stable disease, partial response or complete response.
Patients underwent whole-body planar scintigraphy 45 min after injection of 740 MBq 99mTc-maraciclatide using a dual-headed gamma camera (Philips, Cleveland, OH) with low-energy high-resolution collimators and a 20% energy window centred on 140 keV, before and 12 weeks after starting abiraterone. An additional single-photon emission CT/CT (SPECT/CT) scan of a target lesion was then acquired. The acquisition parameters for the SPECT component were 20 s/frame and 128 projections over 360° and the images were reconstructed with an ordered subsets expectation maximization algorithm with five iterations over eight subsets. The acquisition parameters for the CT component were 100 mAs/slice, 120 kV, pitch 1.188 and rotation time 0.75 s.
Regions of interest (ROI) were drawn manually around up to five target lesions per patient on anterior and posterior planar scans to calculate the geometric mean activity. If a lesion could only be visualized on either the anterior or the posterior view, then only this ROI was used for subsequent analysis. A contralateral ROI of the same area was placed over a normal area of bone and if the lesion was in the midline, e.g. the spine, then the nearest normal midline vertebra was used to define an area of normal skeletal uptake. A tumour to normal bone (T:N) ratio was calculated for each metastasis. ROIs were also placed over muscle in the right lateral thigh to calculate a geometric mean muscle activity and hence a tumour to muscle (T:M) ratio. For SPECT imaging T:N and T:M ratios were calculated for each of the five lesions within the field of view with counts averaged for all the slices in which tumour activity was visible. For muscle activity the erector spinae muscle was used when the lateral thigh was not in the field of view. The same lesions were analysed in the 11 patients who had 12-week scans and the mean percentage change in each 99mTc-maraciclatide uptake ratio was also recorded. Mean CT HU were recorded for each lesion on the corresponding CT component of the SPECT/CT acquisition on pretreatment and 12-week scans. No attempt was made to measure an index of global skeletal involvement as previously reported for bone scintigraphy [12], as planar T:N ratios and normal skeletal uptake are lower than those from standard bone scintigraphy, precluding this approach.
Changes in uptake ratios were compared between patients with clinical PD and those with non-PD and the significance of differences in uptake ratios and in percentage change in uptake ratios between those below and above the median baseline CT HU value and between those below and above the median percentage change in CT HU, respectively, were evaluated using Student’s t test or the Mann-Whitney U test depending on whether or not the data were normally distributed as tested by the Shapiro-Wilk test. Spearman’s test was used to determine correlations between percentage change in CT HU and percentage change in 99mTc-maraciclatide uptake. Statistical tests were performed with IBM SPSS Statistics, version 24.
Results
In the 11 patients who underwent baseline and 12-week imaging, the clinical reference standard indicated PD in five patients and non-PD in six patients (stable disease in five, treatment response in one). There was no significant difference in percentage change in serum calcium or alkaline phosphatase levels between those with PD (2.4% and 23.6%, respectively) and those with non-PD (−0.7% and 10.4%, respectively). Metastatic bone lesions showed accumulation of 99mTc-maraciclatide with mean planar T:N and T:M ratios of 1.43 (± 0.27) and 3.06 (± 0.83), respectively, and SPECT T:N and T:M ratios of 3.1 (± 1.7) and 5.19 (± 2.77), respectively, before treatment in the 17 patients (Fig. 1). In these scans, the most sclerotic lesions (389–740 HU, mean 583 HU) showed lower 99mTc-maraciclatide T:M ratios (4.22 vs. 7.04, p = 0.02) than the less sclerotic or lytic lesions (46–381 HU, mean 223 HU; Fig. 2).
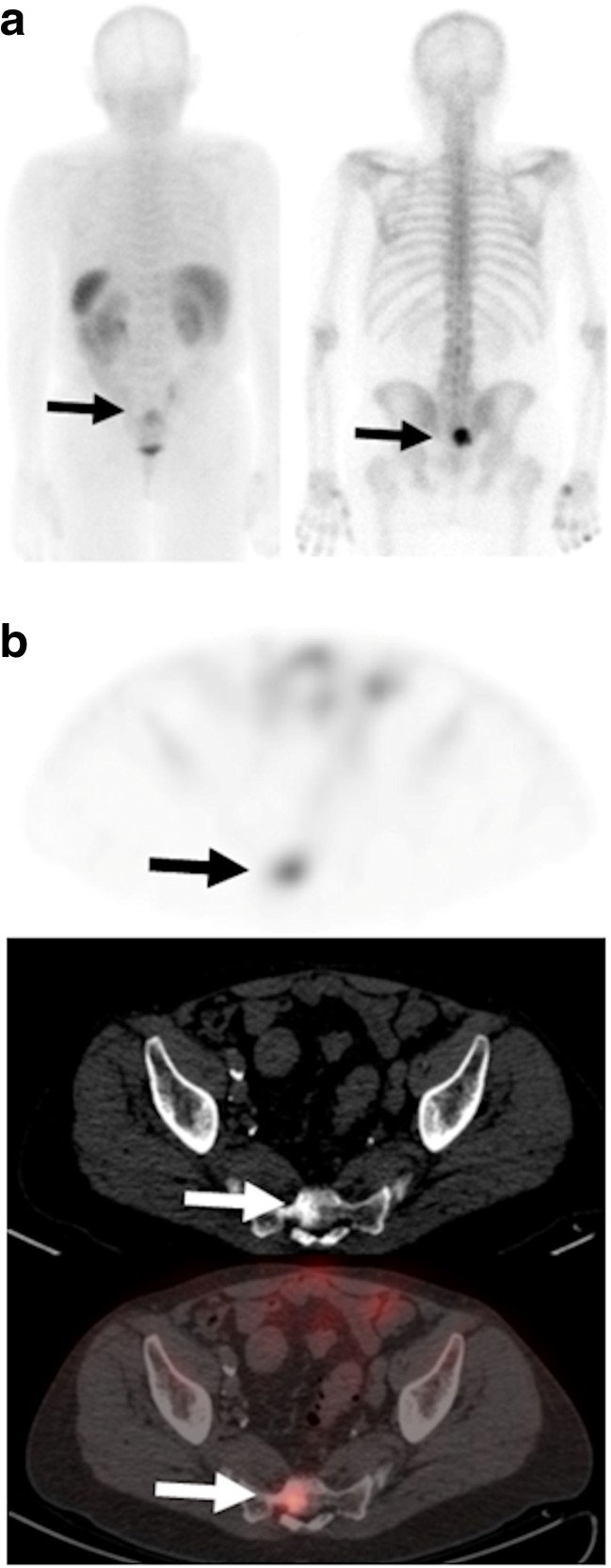
Fig. 1.
a 99mTc-maraciclatide posterior planar image (left) shows uptake in a sacral metastasis (arrows; T:N ratio 1.81, T:M ratio 4.79) that is also demonstrated on the 99mTc-methylene diphosphonate bone scan (right). b 99mTc-maraciclatide uptake is visible on the axial SPECT image (top) and the fused SPECT/CT image (bottom; T:N ratio 8.0, T:M ratio 10.0), and is visible on the CT bone windows (centre; HU = 381)
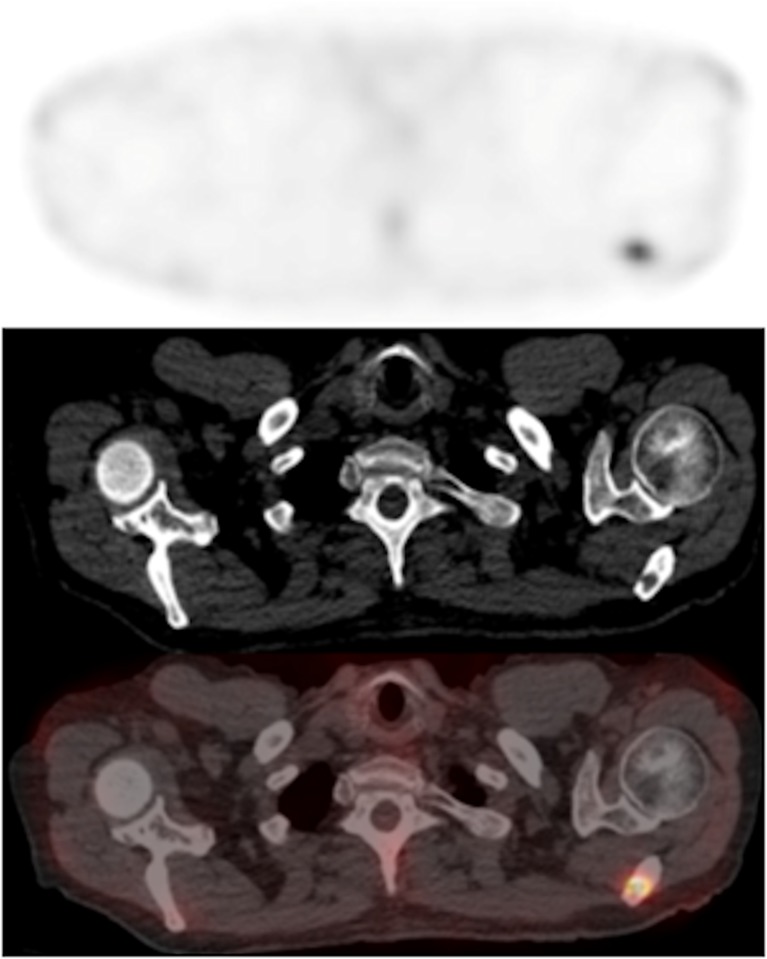
Fig. 2.
Axial SPECT image (top) and axial fused SPECT/CT image (bottom) show 99mTc-maraciclatide uptake in a left scapula metastasis (T:N ratio 8.25, T:M ratio 5.91) that appears lytic on the CT image (centre; HU = 46)
Five patients with PD showed a mean percentage increase in planar T:N and T:M ratios and SPECT T:N and T:M ratios (0.29, 12.1, 11.9 and 20.2%, respectively) whereas six patients with non-PD showed no change or a reduction in T:M ratios (0.27, −8.0, −21.9, −27.2%, respectively; Fig. 3). Changes in planar and SPECT T:M ratios were significantly different between patients with PD and those with non-PD (p = 0.044 and 0.036, respectively) whereas changes in T:N ratios were not significantly different (p = 1.0 and 0.3, respectively). Percentage change in CT HU in individual lesions between baseline and 12 weeks showed an inverse correlation with percentage change in SPECT T:M ratios (r = −0.59, p = 0.006). Percentage change in SPECT T:M ratios in lesions with an increase in CT HU (5.8% to 290.9%) were significantly different from those with a decrease in CT HU (−7.9 to −67.1%) with median percentage changes of −30.9% and 3.9%, respectively (p = 0.016).
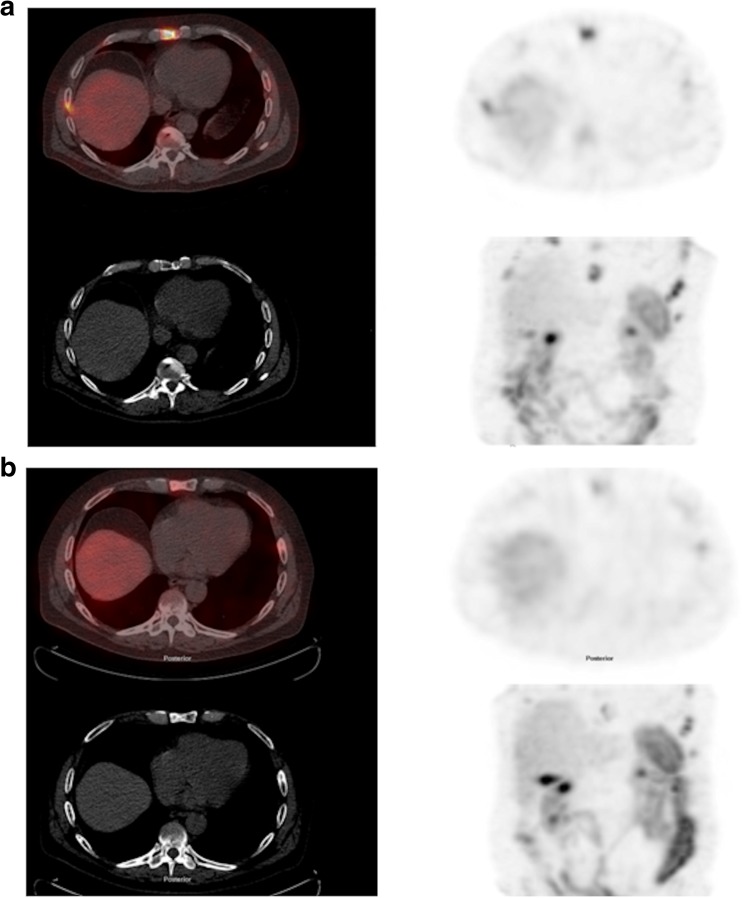
Fig. 3.
Fused axial SPECT/CT image (top left), CT image (bottom left), axial SPECT image (top right) and coronal maximum intensity image (bottom left) show a sternal lesion in a patient with a treatment response: a before treatment (T:N ratio 8.66, T:M ratio 11.97, HU = 155), b 12 weeks after starting treatment (T:N ratio 4.42, T:M ratio 5.18, HU = 413)
Discussion
This study showed that 99mTc-maraciclatide, a tracer that is known to target the αvβ3 integrin [10], specifically accumulates in metastatic bone lesions in patients with PCa. The uptake of 99mTc-maraciclatide (SPECT T:M ratio) was higher in the untreated lowest density bone metastases than in those with higher density, as measured by CT HU, suggesting that uptake is related to the degree of osteoclast activity. Previous in vitro experiments with an alternative positron emission tomography (PET) αvβ3 integrin targeting tracer, 64Cu-CB-TE2A-c(RGDyK), have shown specific uptake in osteoclasts and a correlation with osteoclast numbers in a mouse model of parathyroid hormone-induced osteolysis in the calvaria [8]. Similarly, the same PET tracer has shown specific uptake in a mouse model of osteolytic bone metastases [9], and a different PET tracer, 68Ga-DOTA-E-[c(RGDfK)]2, has shown specific accumulation in a rat model of breast cancer bone metastasis [13]. In humans, a different 99mTc-RGD tracer (99mTc-3PRGD2) has been shown to detect bone metastases from lung cancer with high sensitivity and with better specificity than 99mTc-methylene diphosphonate scintigraphy [14]. A PET tracer, 18F-galacto-RGD, has been found to accumulate in metastatic PCa with a T:M ratio of 2.8 ± 1.3 leading to the detection of 58 out of 74 metastases in 12 patients [15].
In our study, patients who had clinical PD by 24 weeks showed significantly different percentage changes in planar and SPECT T:M ratios at 12 weeks (12.1% and 20.2% increases, respectively) compared with those with non-PD (−8.0% and −27.2% decreases, p = 0.044 and 0.036, respectively) suggesting that 99mTc-maraciclatide may be useful for determining early treatment response. A difference in percentage change in 99mTc-maraciclatide uptake (SPECT T:M ratio) was also seen between lesions that showed an increase in CT HU and those that showed a decrease (p = 0.016), and the inverse correlation also suggests that 99mTc-maraciclatide activity may be related to underlying changes in the bone microenvironment following treatment, including changes in osteoclast activity. These data are supported by the previously mentioned preclinical study of osteolytic metastases in a transgenic mouse model that showed reductions in uptake of 64Cu-CB-TE2A-c(RGDyK) following treatment with an osteoclast inhibitor, the bisphosphonate zolendronate [9]. Human skeletal diseases that are known to exhibit greater osteoclast activity than PCa, such as multiple myeloma [16] or periprosthetic osteolysis [17], would provide relevant clinical models to further test this hypothesis.
The main limitation of this study is that we had no direct measurement of the proportion of uptake of 99mTc-maraciclatide that was specific to osteoclast activity or what degree of uptake may have been tumour-specific or endothelial/angiogenesis-specific. Indeed it is known that PCa cell lines derived from bone metastases express the αvβ3 integrin [18] and we cannot exclude an effect on baseline measurements from previous therapy in the subset of our patients recruited with PD following previous treatment. Nevertheless, the inverse correlations we observed with CT density measured in HUs that is known to be related to osteoclastic and osteoblastic phenotypes before treatment and, in particular, the changes with treatment, support the hypothesis that 99mTc-maraciclatide uptake is related to osteoclast activity through targeting the αvβ3 integrin. Planar T:N and T:M ratios are prone to greater measurement error than SPECT ratios but the planar ratios are nevertheless of interest in this first study of 99mTc-maraciclatide imaging of skeletal metastases in PCa in providing a quantitative description of uptake and a comparator for future studies. Secondly, abiraterone is not known to have a direct anti-osteoclastic effect and it is likely that the changes in 99mTc-maraciclatide uptake we observed in bone metastases were in part the result of a secondary downstream reduction in osteoclast activity following the antitumour action of abiraterone caused by inhibition of androgen synthesis [19].
Conclusions
99mTc-maraciclatide accumulates in bone metastases from PCa in keeping with increased expression of αvβ3 integrin, and shows an inverse correlation with CT density, suggesting a relationship with osteoclast activity. In addition, changes in 99mTc-maraciclatide uptake after 12 weeks of systemic therapy with abiraterone differ between patients with subsequent PD and non-PD and this may therefore be a potential method for predicting clinical response that deserves further study.
Acknowledgements
The authors acknowledge financial support from the King’s College London/University College London Comprehensive Cancer Imaging Centres funded by Cancer Research UK and the Engineering and Physical Sciences Research Council in association with the Medical Research Council and the Department of Health, the Wellcome Trust EPSRC Centre for Medical Engineering at King’s College London and Prostate Cancer UK, and the support of the National Institute of Health Research Clinical Research Network. The tracer was supplied by GE Healthcare Ltd. (UK).
Funding
This study was financially supported by the King’s College London/University College London Comprehensive Cancer Imaging Centres funded by Cancer Research UK and the Engineering and Physical Sciences Research Council in association with the Medical Research Council and the Department of Health (C1519/A16463) and Prostate Cancer UK (PA12–04) and was supported by the National Institute of Health Research Clinical Research Network (NIHR CRN).
Compliance with ethical standards
Conflicts of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration ♠cof Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Halabi S, Kelly WK, Ma H, Zhou H, Solomon NC, et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol. 2016;34:1652–1659. doi: 10.1200/JCO.2015.65.7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coleman RE. Bone cancer in 2011: prevention and treatment of bone metastases. Nat Rev Clin Oncol. 2011;9:76–78. doi: 10.1038/nrclinonc.2011.198. [DOI] [PubMed] [Google Scholar]
- 3.Hoefeler H, Duran I, Hechmati G, Garzon Rodriguez C, Lüftner D, et al. Health resource utilization associated with skeletal-related events in patients with bone metastases: results from a multinational retrospective – prospective observational study – a cohort from 4 European countries. J Bone Oncol. 2014;3:40–48. doi: 10.1016/j.jbo.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clamp A, Danson S, Nguyen H, Cole D, Clemons M. Assessment of therapeutic response in patients with metastatic bone disease. Lancet Oncol. 2004;5:607–616. doi: 10.1016/S1470-2045(04)01596-7. [DOI] [PubMed] [Google Scholar]
- 5.Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402–1418. doi: 10.1200/JCO.2015.64.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teitelbaum SL. Osteoclasts and integrins. Ann N Y Acad Sci. 2006;1068:95–99. doi: 10.1196/annals.1346.017. [DOI] [PubMed] [Google Scholar]
- 7.Ross FP, Chappel J, Alvarez JI, Sander D, Butler WT, Farach-Carson MC, et al. Interactions between the bone matrix proteins osteopontin and bone sialoprotein and the osteoclast integrin alpha v beta 3 potentiate bone resorption. J Biol Chem. 1993;268:9901–9907. [PubMed] [Google Scholar]
- 8.Sprague JE, Kitaura H, Zou W, Ye Y, Achilefu S, Weilbaecher KN, et al. Noninvasive imaging of osteoclasts in parathyroid hormone-induced osteolysis using a 64Cu-labeled RGD peptide. J Nucl Med. 2007;48:311–318. [PMC free article] [PubMed] [Google Scholar]
- 9.Wadas TJ, Deng H, Sprague JE, Zheleznyak A, Weilbaecher KN, Anderson CJ. Targeting the alphavbeta3 integrin for small-animal PET/CT of osteolytic bone metastases. J Nucl Med. 2009;50:1873–1880. doi: 10.2967/jnumed.109.067140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bach-Gansmo T, Danielsson R, Saracco A, Wilczek B, Bogsrud TV, Fangberget A, et al. Integrin receptor imaging of breast cancer: a proof-of-concept study to evaluate 99mTc-NC100692. J Nucl Med. 2006;47:1434–1439. [PubMed] [Google Scholar]
- 11.Hua J, Dobrucki LW, Sadeghi MM, Zhang J, Bourke BN, Cavaliere P, et al. Noninvasive imaging of angiogenesis with a 99mTc-labeled peptide targeted at avb3 integrin after murine hindlimb ischemia. Circulation. 2005;111:3255–3260. doi: 10.1161/CIRCULATIONAHA.104.485029. [DOI] [PubMed] [Google Scholar]
- 12.Imbriaco M, Larson SM, Yeung HW, Mawlawi OR, Erdi Y, Venkatraman ES, et al. A new parameter for measuring metastatic bone involvement by prostate cancer: the bone scan index. Clin Cancer Res. 1998;4:1765–1772. [PubMed] [Google Scholar]
- 13.Mühlhausen U, Komljenovic D, Bretschi M, Leotta K, Eisenhut M, Semmler W, et al. A novel PET tracer for the imaging of αvβ3 and αvβ5 integrins in experimental breast cancer bone metastases. Contrast Media Mol Imaging. 2011;6:413–420. doi: 10.1002/cmmi.435. [DOI] [PubMed] [Google Scholar]
- 14.Miao W, Zheng S, Dai H, Wang F, Jin X, Zhu Z, et al. Comparison of 99mTc-3PRGD2 integrin receptor imaging with 99mTc-MDP bone scan in diagnosis of bone metastasis in patients with lung cancer: a multicenter study. PLoS One. 2014;9:e111221. doi: 10.1371/journal.pone.0111221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beer AJ, Schwarzenböck SM, Zantl N, Souvatzoglou M, Maurer T, Watzlowik P, et al. Non-invasive assessment of inter- and intrapatient variability of integrin expression in metastasized prostate cancer by PET. Oncotarget. 2016;7:28151–28159. doi: 10.18632/oncotarget.8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galson DL, Silbermann R, Roodman GD. Mechanisms of multiple myeloma bone disease. Bonekey Rep. 2012;1:135. doi: 10.1038/bonekey.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purdue PE, Koulouvaris P, Potter HG, Nestor BJ, Sculco TP. The cellular and molecular biology of periprosthetic osteolysis. Clin Orthop Relat Res. 2007;454:251–261. doi: 10.1097/01.blo.0000238813.95035.1b. [DOI] [PubMed] [Google Scholar]
- 18.Putz E, Witter K, Offner S, Stosiek P, Zippelius A, Johnson J, et al. Phenotypic characteristics of cell lines derived from disseminated cancer cells in bone marrow of patients with solid epithelial tumors: establishment of working models for human micrometastases. Cancer Res. 1999;59:241–248. [PubMed] [Google Scholar]
- 19.Rehman Y, Rosenberg JE. Abiraterone acetate: oral androgen biosynthesis inhibitor for treatment of castration-resistant prostate cancer. Drug Des Devel Ther. 2012;6:13–18. doi: 10.2147/DDDT.S15850. [DOI] [PMC free article] [PubMed] [Google Scholar]