Abstract
Objectives
After subtotal colectomy, 40% of patients report chronic gastrointestinal symptoms and poor quality of life. Its etiology is unknown. We determined whether small intestinal bacterial overgrowth (SIBO) or small intestinal fungal overgrowth (SIFO) cause gastrointestinal symptoms after colectomy.
Methods
Consecutive patients with unexplained abdominal pain, gas, bloating and diarrhea (>1 year), and without colectomy (controls), and with colectomy were evaluated with symptom questionnaires, glucose breath test (GBT) and/or duodenal aspiration/culture. Baseline symptoms, prevalence of SIBO/SIFO, and response to treatment were compared between groups.
Results
Fifty patients with colectomy and 50 controls were evaluated. A significantly higher (p = 0.005) proportion of patients with colectomy, 31/50 (62%) had SIBO compared to controls 16/50 (32%). Patients with colectomy had significantly higher (p = 0.017) prevalence of mixed SIBO/SIFO 12/50 (24%) compared to controls 4/50 (8%). SIFO prevalence was higher in colectomy but not significant (p = 0.08). There was higher prevalence of aerobic organisms together with decreased anaerobic and mixed organisms in the colectomy group compared to controls (p = 0.008). Patients with colectomy reported significantly greater severity of diarrhea (p = 0.029), vomiting (p < 0.001), and abdominal pain (p = 0.05) compared to controls, at baseline. After antibiotics, 74% of patients with SIBO/SIFO in the colectomy and 69% in the control group improved (p = 0.69).
Conclusion
Patients with colectomy demonstrate significantly higher prevalence of SIBO/SIFO and greater severity of gastrointestinal symptoms. Colectomy is a risk factor for SIBO/SIFO.
Introduction
Colectomy is a common surgical procedure with an annual estimate of 235,000 procedures in the U.S.A1. It is performed for a variety of indications and can be lifesaving. However, up to 40% of patients undergoing subtotal colectomy report persistent gastrointestinal symptoms including gas, bloating, distension, pain and diarrhea2. A majority of these patients also report an impaired quality of life. The pathoetiology of these symptoms is unknown.
In a recent study, Singh et al., reported that 7/15 patients (47%) with severe slow-transit constipation and underlying neuropathy, and who underwent colectomy with ileorectal anastomosis, developed new onset of significant bloating, distension and flatulence3. Further testing revealed that all of these patients had SIBO and they responded to antibiotics. This observation suggested that colectomy may predispose patients to SIBO, but this concept has not been systematically assessed.
Small intestinal bacterial overgrowth (SIBO) or small intestinal fungal overgrowth (SIFO) is characterized by abdominal pain, distension, bloating and diarrhea, and the presence of an excessive amount of bacteria or fungus in the small bowel4–6. SIBO can be identified with either breath hydrogen tests or culture of duodenal fluid6–8, but quantitative culture of small bowel aspirate is the only method of identifying SIFO7.
Under normal physiological conditions several factors play a role in protecting the small intestine from bacterial colonization. These include a normal intestinal motility, especially the recurring cyclical migrating motor complex, gastric acid, mucous secretion, bile salts, and luminal immunoglobulins8–11. The ileocecal valve also serves as an important anatomical barrier between the ileum and cecum, and not only regulates the flow of chyme but also prevents the reflux of colonic contents into the small bowel12. In contrast, conditions that alter the normal gastric and small bowel function, such as opioids that inhibit motility, atrophic gastritis, Parkinson’s disease, diabetes, pseudo-obstruction, scleroderma, blind loop syndrome and use of PPI’s and others may each predispose to SIBO8,13,14.
The aim of this study was to assess the prevalence of SIBO and SIFO in a cohort of patients with chronic unexplained gastrointestinal symptoms following colectomy, and to compare this with a control group of patients with similar chronic symptoms but without previous colectomy. In addition, we assessed the prevalence of gastrointestinal symptoms in these patients at baseline and after treatment.
Methods
Patients
Consecutive adult patients who were referred to a specialist motility center over three years with unexplained chronic (>1 year) gastrointestinal symptoms such as gas, bloating, belching, diarrhea, and abdominal discomfort, and with a history of colectomy were evaluated. The control group consisted of patients with similar long-standing complaints of gas, bloating, pain and diarrhea but without colectomy, and any other gastrointestinal problems. Because of the overlapping nature of both upper and lower gastrointestinal symptoms and the presence of colectomy in one of our groups, they did not meet any of the Rome criteria for functional GI disorders. Patients were included if they had normal upper endoscopy, colonoscopy (except post-colectomy changes and intact anastomosis), computerized abdominal tomography scan, and normal hematology, biochemical profiles, tissue transglutaminase antibody, thyroid stimulating hormone, and normal right upper quadrant ultrasound scan. Patients with upper gut or small bowel surgery and those who were hospitalized or with serious cardiac, pulmonary, or neurologic comorbidities or with known intestinal obstruction or motility disorders such as scleroderma or pseudo-obstruction were excluded. All patients underwent either glucose breath test and/or duodenal aspirate with culture. In addition, they filled out a validated symptom questionnaire15.
The Augusta University Medical Center Investigation Review Board approved the study, No. 659642-3 and the study was registered on clinical trials.gov—NCT03216239.
Glucose breath test
All patients were advised to consume a low carbohydrate diet for one day, avoid laxatives for one week and antibiotics for 6 weeks prior to the test. After an overnight fast, patients were asked to brush their teeth and rinse their mouth with an antiseptic mouthwash, at least 2 h before the test, to avoid false positive high basal levels from fermentation of substrate by oral bacteria. After obtaining a baseline breath sample, 75 grams of glucose dissolved in 250 ml of water was administered orally6,16. Subsequently, breath samples were collected at 15-minute intervals for the next 2 h. The samples were collected in a bag (QuinTron Instrument Company, Inc., WI) and alveolar gas was analyzed for both H2 and CH4 levels by chromatography (QuinTron Micro Analyzer, QuinTron Instrument Company, Inc., WI). The patients were also asked to score the presence and severity of nine gastrointestinal symptoms on a visual analog scale(0–3), throughout the breath test.
Duodenal aspiration and quantitative culture
All patients were required to be free of antibiotic use for 6 weeks prior to testing. Aspiration of distal duodenal fluid was performed during an upper endoscopy. The procedure was performed under aseptic precautions to minimize contamination7,16. A sterile 2 mm Liguory catheter (COOK Medical, Bloomington, IN, USA) was passed through the biopsy channel of the upper endoscope into the 3rd or 4th portions of the duodenum, followed by aspiration of 3–5 ml of duodenal fluid. Specimens were sent immediately to microbiology lab for standard aerobic, anaerobic and fungal cultures.
The duodenal aspirate specimens were plated for aerobic, anaerobic and fungal cultures. After vortexing the sample, the following agar plates were inoculated using a 0.001 calibrated loop: Blood, Chocolate, Maconkey, Columbia Naladixic Acid Agar (CAN) with blood, Anaerobe Blood, Phenyl Ethyl Alcohol (PEA), Anaerobic Remel which contains Paromycin and Vancomycin, Inhibitory Mold and Mycobiotic. They were then struck for colony count. The Blood and Chocolate agars were held at 37’ in CO2 for 5 days. Maconkey and CNA plates were held in O2 for 48 h before being discarded. Anaerobe media was incubated under anaerobic conditions for 5 days. Fungal plates were held for 4 weeks. Gram stains were reported.
Any bacterial growth ≥1,000 CFU was identified and reported out using colony count numeration. All organisms were listed in the physician report, except in very rare cases multiple (typically >3) organisms were isolated and reported as multiple growth of aerobes or anaerobes.
Identification of organisms including yeast were generally by mass spectrophotometry (Maldi-Time of Flight). Some organisms (i.e., Neisseria sp, Gram-positive bacilli resembling Diptheroids/Coryneforms, Lactobacillus species, Streptococcus viridans group, Staphylococcus coagulase negative, Rothia sp.) were identified based on gram stain, colonial morphology, or spot tests. Wherever appropriate antibiotic susceptibility panels were performed and reported. Culture plates were held for 10 days.
Symptom questionnaire
All patients completed a validated Likert-like bowel symptom questionnaire at their initial clinic visit that assessed nine symptoms: abdominal pain, belching, bloating, fullness, indigestion, nausea, diarrhea, vomiting and gas15,17. Patients were asked to rate the frequency, intensity and duration of each symptom on a 0–3 scale. Intensity: 0 = no symptoms, 1 = mild, 2 = moderate, 3 = severe symptoms. Frequency: 0 = None; 1 = Less than 1 episode/week, 2 = 1 episode/week, 3 = More than 1 episode/week. Duration: 0 = None, 1 = Less than 10 min, 2 = 10–30 min, 3 = Greater than 30 min. The total score for each symptom ranged from 0 to 9.
After establishing a diagnosis of SIBO with either GBT and/or duodenal cultures, patients were treated with appropriate antibiotics based on culture and antibiotic sensitivity, patient’s allergy profile and previous antibiotic use. Patients with SIFO were treated with antifungals and those with mixed SIBO/SIFO were treated with antibiotics and antifungals. Three months later, they either attended a follow up clinic visit or were contacted over the phone and asked to complete the aforementioned symptom questionnaire, and rate their overall gastrointestinal symptoms on a VAS scale (0 = very dissatisfied, 100 = completely satisfied.)
Data analyses
Patients were adjudicated as having SIBO if the culture showed bacterial concentration of ≥103 CFU/mL for aerobic or anaerobic organisms6,8,16,18. Glucose breath test was considered positive for SIBO if the following criteria were met: ≥20 PPM increase above baseline for H2, or ≥15 PPM increase above baseline for CH4, or ≥15 PPM increase above baseline for combined H2 and CH4 values7,16. The diagnosis of SIFO was made if the duodenal culture yielded a growth of fungal organisms at a concentration of ≥103 CFU/ml7,19.
Statistical analysis
Data are presented as mean ± SD unless otherwise stated. Continuous variables were compared using Student’s t-test for parametric data or Mann-Whitney U-test for non-parametric data. Categorical variables including prevalence in subgroups were compared using chi-square with Yate’s correction factor or Fisher’s exact test, as appropriate. Statistics were performed using Sigmaplot v12.2 (San Jose, CA) that checks for normal distribution. A p < 0.05 was considered statistically significant.
Results
Demographics
The colectomy group comprised of 50 patients (F/M = 41/9), mean age 52.3 years (range: 20–85), and with a mean duration of symptoms of 79.9 months. The indication for colectomy was colon inertia/slow-transit constipation (40%), colorectal carcinoma/polyps (14%), diverticular disease (14%), bowel obstruction (14%), Crohn’s disease (6%), and others (12%). Regarding the type of surgery, 28/50 (56%) had partial colectomy, 11/50 (22%) had subtotal colectomy and 11/50 (22%) had total colectomy. Three additional patients with colectomy were excluded because of recurrent hospitalization, small bowel surgeries and pseudo-obstruction syndromes. The control group comprised of 50 patients (F/M = 38/12) with a mean age of 49.9 years (range 18-88), and with a mean duration of symptoms of 77.6 months, and no history of bowel surgery. Two additional patients in the control group were excluded because of scleroderma, and bariatric surgery with blind loop syndrome. There were no differences in the demographic features between the two groups.
Symptom patterns
Patients with colectomy reported significantly higher severity of diarrhea (4.63 vs 2.98; p = 0.029), vomiting (2.54 vs 0.30; p < 0.001) and abdominal pain (7.25 vs 6.18; p = 0.05) compared to those without colectomy at baseline. Other gastrointestinal symptoms were not significantly different between the two groups, although their severity was generally higher in the colectomy group (Table 1).
Table 1.
Baseline gastrointestinal symptom scores in patients with and without colectomy
| Gastrointestinal symptom | Colectomy (N = 50) | Controls (N = 50) | p-value |
|---|---|---|---|
| Abdominal pain | 7.25 (2.1) | 6.18 (3.2) | 0.05 |
| Belching | 4.08 (3.1) | 3.42 (3.4) | 0.322 |
| Bloating | 6.21 (3.3) | 6.64 (3.3) | 0.515 |
| Fullness | 6.58 (3.3) | 6.02 (3.4) | 0.408 |
| Indigestion | 4.83 (3.2) | 4.20 (3.9) | 0.379 |
| Nausea | 5.64 (3.5) | 4.94 (3.7) | 0.334 |
| Diarrhea | 4.63 (3.8) | 2.98 (3.5) | 0.029 |
| Vomiting | 2.54 (3.6) | 0.30 (1.3) | <0.001 |
| Gas | 5.52 (3.6) | 5.50 (3.4) | 0.976 |
Data shown as mean (SD).
Glucose breath test
In the colectomy group, 48/50 patients underwent glucose breath testing and the remaining 2 subjects had duodenal aspiration alone. Of these, 21/48 (43.8%) tested positive for SIBO. Likewise, in the control group, 48/50 patients underwent glucose breath testing, and 2 had duodenal aspiration alone. Of these, 5/48 (10.4%) patients without colectomy tested positive for SIBO.
Duodenal aspirate/culture
In the colectomy group, 35 patients had duodenal aspirates performed, of whom 20/35 (57.1%) had SIBO. In the control group, 32 patients had duodenal aspirates of whom 12/32 (37.5%) had SIBO. In the colectomy group, 14/20 (70%) patients had >103 CFU/mL and 6/20 (30%) patients had >105 CFU/mL. In the control group, 10/12 (83%) patients had >103 CFU/mL and 2/12 (17%) patients had >105 CFU/mL. There was no difference (p = 0.8) between groups. Duodenal cultures grew a variety of organisms that are summarized in Table 2. There was significantly greater (p = 0.008) prevalence of aerobic organisms (45% vs 17%) including primarily Streptococcus species, Escherichia coli, Klebsiella pneumoniae in patients with colectomy compared to controls. With regards to the anaerobic organisms, the prevalence was 40% vs 58%, and with regards to mixed aerobic and anaerobic organisms, the prevalence was lower in the colectomy group at 15% vs 25%, when compared to the control group, (p = 0.008; Fig. 1).
Table 2.
Duodenal culture results in colectomy patients versus controls
| Colectomy | Controls | |
|---|---|---|
| Culture results (≥103 CFU/mL) | N = 35 (%) | N = 19 (%) |
| Streptococcus species | 8 (23) | 5 (26) |
| Escherichia coli | 4 (11) | 1 (5) |
| Klebsiella pneumoniae | 4 (11) | 2 (11) |
| Staphylococcus aureus | 2 (6) | 0 (0) |
| Lactobacilli | 3 (9) | 1 (5) |
| Citrobacter | 1 (3) | 0 (0) |
| Neisseria | 1 (3) | 1 (5) |
| Rothia species | 1 (3) | 0 (0) |
| Coryneform | 1 (3) | 0 (0) |
| Bacteroides | 2 (6) | 0 (0) |
| Peptostreptococcus | 1 (3) | 0 (0) |
| Anaerobic Gram-positive cocci | 2 (6) | 0 (0) |
| Anaerobic Gram-negative bacilli | 1 (3) | 0 (0) |
| Veillonella | 1 (3) | 3 (16) |
| Diphtheroids | 0 (0) | 1 (5) |
| Microaerophilic streptococci | 0 (0) | 1 (5) |
| Stenotrophomonas maltophilia | 0 (0) | 1 (5) |
| Haemophilus influenzae | 0 (0) | 1 (5) |
| Coagulase-negative staphylococci | 0 (0) | 1 (5) |
| Serratia marcescens | 0 (0) | 1 (5) |
| Othersa | 3 (9) | 0 (0) |
a Reported as multiple anaerobic gram-positive and gram-negative organisms
Fig. 1.
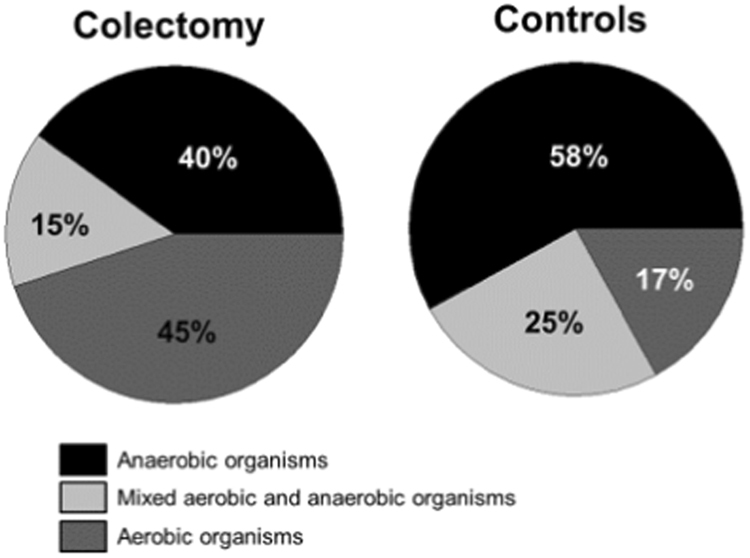
The prevalence of aerobic and/or anaerobic organisms in patients with colectomy compared to controls
Prevalence of SIBO/SIFO
The overall prevalence of SIBO (positive GBT or positive duodenal aspirate) was significantly higher (p = 0.005) in the colectomy group when compared to controls (62% vs 32%; Fig. 2). Table 3 summarizes our data on the prevalence of SIBO based on GBT, duodenal cultures or both. In addition, in the colectomy group 12/50 (24%) had SIBO and SIFO compared to 4/50 (8%) in those without colectomy (p = 0.017). The overall prevalence of SIFO was also higher in the colectomy group compared to controls but this finding was not significant (28% vs 12%; p = 0.08; Fig. 2). Moreover, two patients in each group had SIFO alone. Most (61%) grew candida albicans and a smaller percentage (39%) grew Candida glabrata. Furthermore, we found that the prevalence of SIBO/SIFO was not influenced by those with or without a history of slow-transit constipation (p = 0.94), and the presence/absence of IC valve (p = 0.26).
Fig. 2.
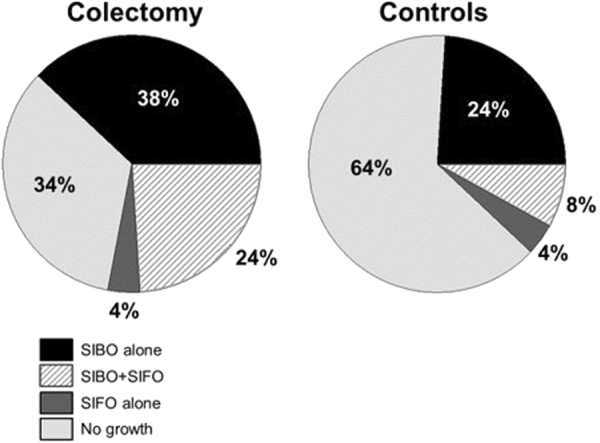
The prevalence of SIBO and/or SIFO in patients with colectomy and controls without colectomy
Table 3.
Diagnostic yield of Glucose Breath Test (GBT) and Duodenal Aspirate/Culture (DA) in patients with colectomy and controls
| GBT only | DA only | GBT+/DA+ | GBT+/DA− | GBT−/DA+ | GBT−/DA− | |
|---|---|---|---|---|---|---|
|
Colectomy,
N = 50 | ||||||
| SIBO+, N = 31 | 8 | 2 | 10 | 3 | 8 | 0 |
| SIBO−, N = 19 | 12 | 0 | 0 | 0 | 0 | 7 |
|
Controls,
N = 50 | ||||||
| SIBO+, N = 16 | 2 | 1 | 1 | 2 | 10 | 0 |
| SIBO−, N = 34 | 16 | 1 | 0 | 0 | 0 | 17 |
DA duodenal aspirate and culture, GBT glucose breath test, SIBO small intestinal bacterial overgrowth, + positive test, − negative test
Effects of antibiotic/antifungal treatment on gastrointestinal symptoms
Patients who received antibiotics reported significant improvement in the prevalence and severity of gastrointestinal symptoms when compared to their baseline symptoms, both in the colectomy group (Table 4a), and in the control group (Table 4b). The following antibiotics were prescribed for SIBO based on the microbial sensitivity and the patients’ allergy profile: Rifaximin, Amoxicillin, Amoxicillin-Clavulanate, Cotrimoxazole, Cephalosporin, Metronidazole, Tinidazole, Ciprofloxacin, Levofloxacin and Tetracycline. Oral Fluconazole and very rarely Itraconazole was given for treatment of SIFO. In the colectomy group, of the 2 patients with SIFO only, one reported 70% improvement and the other no improvement. Likewise, in the control group, of the 2 patients with SIFO only, one reported 33% improvement and the other 50% improvement. During follow up, overall 74% and 69% of patients with SIBO/SIFO in the colectomy and control groups respectively (p = 0.69), reported improvement in symptoms, and on a VAS scale, overall gastrointestinal symptom satisfaction after treatment averaged a rating of 61% for the colectomy group and 42% for controls (0 = very dissatisfied, 100 = completely satisfied).
Table 4a.
Symptom prevalence and severity score before and after treatment in the colectomy group
| SIBO (N = 19) | SIBO/SIFO (N = 12) | |||||
|---|---|---|---|---|---|---|
| Symptoms | Prevalence | Severity | Improvement | Prevalence | Severity | Improvement |
| Abdominal pain | 100% | 7.7 | 53% | 92% | 6.8 | 40% |
| Belching | 61% | 3.6 | 33% | 83% | 4.2 | 48% |
| Bloating | 94% | 6.4 | 44% | 100% | 7.5 | 47% |
| Fullness | 83% | 6.1 | 41% | 92% | 7.5 | 48% |
| Indigestion | 67% | 4.0 | 45% | 92% | 6.7 | 49% |
| Nausea | 78% | 5.6 | 41% | 92% | 7.8 | 53% |
| Diarrhea | 78% | 5.8 | 26% | 83% | 5.7 | 34% |
| Vomiting | 28% | 1.6 | 56% | 42% | 3.0 | 67% |
| Gas | 83% | 6.0 | 45% | 100% | 6.3 | 41% |
Table 4b.
Symptom prevalence and severity score before and after treatment in the control group
| SIBO (N = 12) | SIBO/SIFO (N = 4) | |||||
|---|---|---|---|---|---|---|
| Symptoms | Prevalence | Severity | Improvement | Prevalence | Severity | Improvement |
| Abdominal pain | 100% | 7.1 | 31% | 100% | 8.3 | 44% |
| Belching | 64% | 4.5 | 46% | 75% | 3.7 | 45% |
| Bloating | 91% | 7.1 | 31% | 100% | 8.7 | 54% |
| Fullness | 100% | 6.5 | 21% | 100% | 8.7 | 58% |
| Indigestion | 73% | 4.9 | 30% | 75% | 5.7 | 47% |
| Nausea | 91% | 5.4 | 26% | 100% | 7.7 | 48% |
| Diarrhea | 45% | 3.2 | 34% | 100% | 7.3 | 59% |
| Vomiting | 0% | 0.0 | NA | 0% | 0.0 | NA |
| Gas | 40% | 6.4 | 30% | 75% | 5.3 | 43% |
Discussion
Over a 3-year period, we investigated a consecutive series of patients referred to our tertiary care center with refractory gastrointestinal symptoms including abdominal pain, gas, bloating, distension and diarrhea, following colectomy. In this colectomy group, we found a significant and two-fold higher prevalence of SIBO (62%) when compared to a control group of patients with similar chronic gastrointestinal complaints but without colectomy (32%). We also found significant differences in the type of bacterial flora, with a predominance of aerobic bacterial organisms and fewer anaerobic organisms in post-colectomy SIBO patients when compared to the controls. The duodenal cultures grew a variety of organisms including primarily Streptococcus species, Escherichia coli, Klebsiella pneumoniae and Lactobacilli.
In addition, we found a higher prevalence of small intestinal fungal overgrowth (SIFO) in patients with colectomy when compared to the control patients. The most common fungus that was cultured was candida species. This finding reaffirms recent studies that SIFO is another important component of the small intestinal overgrowth syndromes19. A recent article showed that fungus may co-exist and interact with bacteria in the gut and form fungal–bacterial biofilms in the GI tract20. Further studies are required to explore the role of fungus in overgrowth syndromes, and in particular whether they are co-pathogens and cause greater morbidity as opposed to either SIBO or SIFO alone.
The glucose breath test is a simple, widely available and non-invasive method of diagnosis of SIBO, and it was positive in 44% of patients with colectomy and 11% of patients without colectomy. Although specific, GBT has low sensitivity for diagnosis of SIBO6,8,16. Consequently, if the GBT is negative, and there is a high index of clinical suspicion for SIBO, such as in the post-colectomy population, further testing with duodenal aspiration and quantitative culture should be considered. This study further confirms previous observations that duodenal culture has a higher yield for diagnosis of SIBO/SIFO than GBT8,16. All patients diagnosed with SIBO and/or SIFO should be treated with antibiotics and/or antifungals and are likely to benefit symptomatically as observed in our study. Also, the improvement in symptoms of SIBO observed here is similar to those reported in other studies of SIBO treatment with antibiotics such as norfloxacin and rifaximin21–23.
Colectomy combined with ileocolonic anastomosis has become the procedure of choice in the surgical management of colon cancer, refractory constipation, ulcerative colitis, familial polyposis and others24–28. Morphological studies have shown that the adaptation of the terminal ileum to its neorectum function is accompanied by a progressive transformation to a colonic type mucosa29. Typically, the bacterial fermentation of both the endogenous mucus and the undigested carbohydrates normally occurs in the large intestine resulting in short chain fatty acids (SCFAs), carbon dioxide, hydrogen, and methane30. However, in patients with SIBO, this process occurs prematurely in the small bowel causing gas, bloating, distention and diarrhea, symptoms that are often mislabeled as IBS31.
The ileocecal valve serves as an important barrier and gatekeeper that prevents reflux of colonic contents into the small bowel. In contrast, conditions that favor low ileocecal valve pressure or loss of the ileocecal barrier, such as following colectomy and construction of an ileocolonic anastomosis, may allow transmigration of bacteria from the colon and predispose patients to the development of SIBO32,33.
Can symptoms alone help with a diagnosis of SIBO? Our detailed analyses revealed that no single symptom or clusters of symptoms at baseline could identify patients who have SIBO. Interestingly, patients with SIFO also share a similar set of symptoms as those with SIBO. Furthermore, symptoms alone could not differentiate between patients with or without colectomy at baseline, or between those with positive and negative SIBO/SIFO. Thus, the two groups were well matched for symptom presentation, but symptoms alone were poor predictors for the presence of bacterial and/or fungal overgrowth, irrespective of the underlying predisposing mechanism.
The limitations of our study include the secondary analysis of prospectively collected data and potential referral bias, since all of these patients were evaluated at a tertiary care gastrointestinal motility center. Consequently, our observations may not reflect the prevalence of this condition in the general population. Also, there is no gold standard for the diagnosis of SIBO/SIFO, and our method of aspiration and quantitative culture cannot definitively exclude the risk of contamination of aspirates. However, we have tried to minimize this by using standard sterile techniques, and the procedure was performed by a single experienced operator. Also, we did not repeat the breath test or aspirate after treatment as the study was not designed for this purpose. The treatment with appropriate antibiotics and/or antifungals was based on breath test results, culture positivity, patients’ drug allergy profile, and insurance coverage and previous antibiotic use, and not with a single drug, as this was a non-randomized treatment study. The significant improvement in symptoms however, after antibiotics and antifungals, support the likely causal association with SIBO/SIFO.
In conclusion, our study demonstrates a significantly higher prevalence of SIBO/SIFO in a cohort of patients with colectomy and chronic unexplained gastrointestinal symptoms. This finding implies that colectomy is a significant risk factor for the development of SIBO/SIFO. Although patients with colectomy had greater baseline severity of symptoms, by themselves they were poor predictors of SIBO/SIFO. Therefore, the use of breath tests and/or duodenal aspirate/culture is essential for confirming a diagnosis of SIBO/SIFO. Treatment with antibiotics and/or antifungals led to significant improvement of symptoms in these patients.
Study Highlights
What Is Current Knowledge
Colectomy is a common surgical procedure. About 40% of patients undergoing subtotal colectomy report persistent gastrointestinal symptoms and an impaired quality of life.
The underlying cause for these symptoms is unclear.
What Is New Here
The prevalence of SIBO/SIFO is significantly higher in patients with unexplained gastrointestinal symptoms following colectomy when compared to controls.
Colectomy with ileocolonic anastomosis is a significant risk factor for the development of SIBO/SIFO.
Symptoms alone are poor predictors of SIBO/SIFO, and breath tests and/or duodenal aspirate/culture is key for establishing this diagnosis.
Treatment with antibiotics and/or antifungals results in significant improvement of symptoms.
Acknowledgements
We sincerely thank H. Smith for superb secretarial assistance and Collier Badger and Arie Mack for assistance with breath tests and Nicole Martinez De Andino with patient assessment.
Conflict of interest
Guarantor of the article: Satish S.C. Rao, MD., Ph.D., FRCP (LON).
Specific author contributions: Satish SC Rao—Study concept and design, performing duodenal aspiration, breath test interpretations, data acquisition, data collection, study recruitment, data analysis and interpretation, manuscript preparation, critical revision, and important intellectual content and final approval. G. Tan, MD: Data collection and analysis of controls, manuscript preparation. H. Abdulla, MD: Data collection, data analysis and interpretation, manuscript preparation. S. Yu, MD: Study recruitment, Interpretation breath tests, IRB, Data analysis and interpretation, manuscript preparation. P. Leelasinjaroen, MD: Data collection and analysis, manuscript preparation. S. Larion, MD: Data analysis and statistics, manuscript preparation. All authors are affiliated and located at Augusta University and all authors have approved the final draft submitted.
Financial support: none.
Potential competing interest: none.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Agency for Healthcare Research and Quality (AHRQ): HCUPnet: healthcare cost and utilization project. Rockville, MD: AHRQ, http://hcupnet.ahrq.gov/. Accessed 2 August 2017.
- 2.Knowles CH, Scott M, Lunniss PJ. Outcome of colectomy for slow transit constipation. Ann. Surg. 1999;230:627–638. doi: 10.1097/00000658-199911000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh S, Heady S, Coss-Adame E, Rao SSC. Clinical utility of colonic manometry in slow transit constipation. Neurogastro Motil. 2013;25:487–495. doi: 10.1111/nmo.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grace E, Shaw C, Whelan K, Andreyev HJN. Review article: small intestinal bacterial overgrowth – prevalence, clinical features, current and developing diagnostic tests and treatment. Aliment. Pharmacol. Ther. 2013;38:674–688. doi: 10.1111/apt.12456. [DOI] [PubMed] [Google Scholar]
- 5.Khoshini R, Dai SD, Lezcano S, Pimental M. A systematic review of diagnostic tests for small intestinal bacterial overgrowth. Dig. Dis. Sci. 2008;53:1443–1454. doi: 10.1007/s10620-007-0065-1. [DOI] [PubMed] [Google Scholar]
- 6.Rezai A, et al. Hydrogen and methane-based breath testing in gastrointestinal disorders: the north american consensus. Am. J. Gastroenterol. 2017;112:775–784. doi: 10.1038/ajg.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erdogan A, Lee Y, Sifuentes H, Rao SS. Small intestinal fungal overgrowth (SIFO): a cause of gastrointestinal symptoms. Gastroenterology. 2014;146:S358. doi: 10.1016/S0016-5085(14)61294-4. [DOI] [Google Scholar]
- 8.Jacobs C, Coss Adame E, Attaluri A, Rao SSC. Dysmotility and proton pump inhibitor use are independent risk factors for small intestinal bacterial and/or fungal overgrowth. Alim Pharmacol. Ther. 2013;37:1103–1111. doi: 10.1111/apt.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Justus PG, et al. Altered myoelectric activity in the experimental blind loop syndrome. J. Clin. Invest. 1983;72:1064–1071. doi: 10.1172/JCI111031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawson AM, Isselbacher KJ. Studies on lipid metabolism in the small intestine with observations on the role of bile salts. J. Clin. Invest. 1960;39:730–740. doi: 10.1172/JCI104090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown WR. Relationships between immunoglobulins and the intestinal epithelium. Gastroenterology. 1978;75:129–138. [PubMed] [Google Scholar]
- 12.Phillips SF, Quigley EM, Kumar D, Kamath PS. Motility of the ileocolonic junction. Gut. 1988;29:390–406. doi: 10.1136/gut.29.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sachdev AH, Pimentel M. Gastrointestinal bacterial overgrowth: pathogenesis and clinical significance. Ther. Adv. Chronic Dis. 2013;4:223–231. doi: 10.1177/2040622313496126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan AH, et al. Small intestinal bacterial overgrowth in Parkinson’s disease. Parkinsonism Relat. Disord. 2014;20:535–540. doi: 10.1016/j.parkreldis.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Choi Y, Kraft N, Zimmerman B, Jackson M, Rao S. Fructose intolerance in IBS and utility of fructose-restricted diet. J. Clin. Gastroenterol. 2008;42:233–238. doi: 10.1097/MCG.0b013e318126c154. [DOI] [PubMed] [Google Scholar]
- 16.Erdogan A, et al. Small intestinal bacterial overgrowth: duodenal aspiration vs glucose breath test. Neurogastroenterol. Motil. 2015;27:481–489. doi: 10.1111/nmo.12516. [DOI] [PubMed] [Google Scholar]
- 17.Erdogan A, et al. What is the optimal threshold for an increase in hydrogen and methane levels with glucose breath test (GBT) for detection of small intestinal bacterial overgrowth (SIBO)? Gastroenterology. 2014;146:S532. doi: 10.1016/S0016-5085(14)61927-2. [DOI] [Google Scholar]
- 18.Rezai A, Pimental M, Rao S. How to test and treat small intestinal bacterial overgrowth: an evidence-based approach. Curr. Gastroenterol. Rep. 2016;18:8.1–11. doi: 10.1007/s11894-015-0482-9. [DOI] [PubMed] [Google Scholar]
- 19.Erdogan A, Rao SS. Small intestinal fungal overgrowth. Curr. Gastroenterol. Rep. 2015;17:16.1–7. doi: 10.1007/s11894-015-0436-2. [DOI] [PubMed] [Google Scholar]
- 20.Sam QH, Chang MW, Chai LYA. The fungal microbiome and its interaction with gut bacteria in the host. Int. J. Mol. Sci. 2017;18:330–341. doi: 10.3390/ijms18020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghoshal UC, Baba CS, Ghoshal U. Low-grade small intestinal bacterial overgrowth is common in patients with non-alcoholic steatohepatitis on quantitative jejunal aspirate culture. Indian J. Gastroenterol. 2017;36:390–399. doi: 10.1007/s12664-017-0797-6. [DOI] [PubMed] [Google Scholar]
- 22.Ghoshal UC, Mittal B, Singh R. Functional dyspepsia is associated with GNβ3 C825T and CCK-AR T/C polymorphism. Eur. J. Gastroenterol. Hepatol. 2016;28:226–232. doi: 10.1097/MEG.0000000000000557. [DOI] [PubMed] [Google Scholar]
- 23.Gatta L, Scarpignato C. Systematic review with meta-analysis: rifaximin is effective and safe for the treatment of small intestine bacterial overgrowth. Aliment. Pharmacol. Ther. 2017;45:604–616. doi: 10.1111/apt.13928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Z, et al. Ileocolonic anastomosis after right hemicolectomy for colon cancer: functional end-to-end or end-to-side? World J. Surg. Oncol. 2014;12:306. doi: 10.1186/1477-7819-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nyam DC, Pemberton JH, Ilstrup DM, Rath DM. Long-term results of surgery for chronic constipation. Dis. Colon. Rectum. 1997;40:273–279. doi: 10.1007/BF02050415. [DOI] [PubMed] [Google Scholar]
- 26.Wofford SA, Verne GN. Approach to patients with refractory constipation. Curr. Gastroenterol. Rep. 2000;2:389–394. doi: 10.1007/s11894-000-0038-4. [DOI] [PubMed] [Google Scholar]
- 27.da Luz Moreira A, Kiran RP, Lavery I. Clinical outcomes of ileorectal anastomosis for ulcerative colitis. Br. J. Surg. 2010;97:65–69. doi: 10.1002/bjs.6809. [DOI] [PubMed] [Google Scholar]
- 28.Nieuwenhuis MH, et al. Genotype-phenotype correlations as a guide in the management of familial adenomatous polyposis. Clin. Gastroenterol. Hepatol. 2007;5:374–378. doi: 10.1016/j.cgh.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Lerch MM. Postoperative adaptation of the small intestine after total colectomy and J-pouch-anal anastamosis. Dis. Colon. Rectum. 1989;32:600–608. doi: 10.1007/BF02554181. [DOI] [PubMed] [Google Scholar]
- 30.Cummings JH, et al. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ringel-Kulka T, et al. Altered colonic bacterial fermentation as a potential pathophysiological factor in irritable bowel syndrome. Am. J. Gastroenterol. 2015;110:1339–1346. doi: 10.1038/ajg.2015.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller LS, et al. Ileocecal valve dysfunction in small intestinal bacterial overgrowth: a pilot study. World J. Gasteroenterol. 2012;18:6801–6808. doi: 10.3748/wjg.v18.i46.6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roland BC, et al. Low ileocecal valve pressure is significantly associated with small intestinal bacterial overgrowth (SIBO) Dig. Dis. Sci. 2014;59:1269–1277. doi: 10.1007/s10620-014-3166-7. [DOI] [PubMed] [Google Scholar]


