Abstract
Objectives
Inflammation and inflammatory conditions have been associated with pancreatic cancer risk and progression in a number of clinical, epidemiological, and animal model studies. The goal of the present study is to identify plasma markers of inflammation associated with survival of pancreatic cancer patients, and assess their joint contribution to patient outcome.
Methods
We measured circulating levels of four established markers of inflammation (C-reactive protein (CRP), interleukin-6 (IL-6), soluble tumor necrosis factor receptor type II (sTNF-RII), and macrophage inhibitory cytokine-1 (MIC-1)) in 446 patients enrolled in an ongoing prospective clinic-based study. Hazard ratios (HRs) and 95% confidence intervals (CI) for death were estimated using multivariate Cox proportional hazards models.
Results
Overall mortality was significantly increased in patients in the top quartile of CRP (HR = 2.52, 95% CI: 1.82–3.49), IL-6 (HR = 2.78, 95% CI: 2.03–3.81), sTNF-RII (HR = 2.00, 95% CI: 1.46–2.72), and MIC-1 (HR = 2.53, 95% CI: 1.83–3.50), compared to those in the bottom quartile (P-trend <0.0001 for all four comparisons). Furthermore, patients with higher circulating concentrations of all four cytokines had a median survival of 3.7 months; whereas, those with lower levels had a median survival of 19.2 months (HR = 4.55, 95% CI: 2.87–7.20, P-trend <0.0001).
Conclusion
Individual elevated plasma inflammatory cytokines are associated with significant and dramatic reductions in pancreatic cancer patient survival. Furthermore, we observed an independent combined effect of those cytokines on patient survival, suggesting that multiple inflammatory pathways are likely involved in PDAC progression. Future research efforts to target the inflammatory state using combination strategies in pancreatic cancer patients are warranted.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer death in the US, with a 5-year survival rate of only 8%1. Several pathologic characteristics obtained at surgery, such as tumor size, resection margin, and number of involved lymph nodes have been associated with reduced survival2. However, only 15–20% of PDAC patients are able to undergo resection3. Consequently, identification of additional biomarkers that can be easily assayed in all patients is urgently needed for elucidation of underlying mechanisms of disease progression and development of new therapeutic strategies.
Numerous studies have suggested a role of inflammation in pancreatic cancer development and progression4. Cytokines synthesized by the host, tumor cells, and stromal cells play a role in cellular proliferation, angiogenesis, and metastasis5,6. Furthermore, epidemiological studies reveal an association between several inflammation-associated conditions, such as diabetes7 and obesity8, with PDAC survival, further implicating inflammation in PDAC pathogenesis.
Elevated levels of individual inflammatory markers, including C-reactive protein (CRP), interleukine-6 (IL-6), macrophage inhibitory cytokine-1 (MIC-1), and tumor necrosis factor-α (TNF-α), as well as combined scores, such as the Glasgow Prognostic Score (GPS), or CRP to albumin ratio, have been associated with decreased survival in PDAC patients9–24. However, these studies were small and often did not account for potential confounding variables. Moreover, it is not known to what extent these inflammatory pathways, which have distinct roles in tumor development, such as cell proliferation, invasion, angiogenesis, and immune evasion5,6 might be involved in disease progression and patient survival. We therefore evaluated the individual and combined association of these four markers of inflammation with patient survival in a large prospective study of well-characterized PDAC patients.
Materials and methods
Study population
Patients were drawn from an ongoing clinic-based study at the Dana-Farber Cancer Institute (DFCI; Boston, MA). All new patients with a diagnosis of PDAC who were seen in the DFCI Gastrointestinal Cancer Center outpatient clinic were prospectively identified and enrolled to this cohort study between December 22, 2004 and June 16, 2014. Patients were eligible for the study if they had pathologically confirmed PDAC and were age 21 or older. The study was approved by the Dana-Farber/Harvard Cancer Center Institutional Review Board. All participants provided informed consent for their biological specimens and clinical data to be used for research.
Pancreatic cancer cases
A total of 1,038 DFCI patients were approached for consent between December 22, 2004 and June 18, 2014, and 743 (72%) agreed to participate (Supplementary Figure 1). Of patients that provided consent, 485 completed a questionnaire on medical history, medication use, lifestyle, and family history. There were no differences between patients who did or did not complete the questionnaire in regards to gender, age at diagnosis, race/ethnicity, or stage at diagnosis. Of the 485 patients who completed the questionnaire, 450 patients provided blood samples at an average of 1.4 months after diagnosis (Table 1). We excluded patients with missing information about height (n = 1), gender (n = 1), unknown stage at the time of blood draw (n = 1), and missing inflammatory cytokine data (n = 1).
Table 1.
Baseline characteristics of pancreatic cancer patients included in the study
| Characteristics | Pancreatic cancer cases(N = 446) |
|---|---|
| Mean (SD) | |
| Age at blood draw, years | 64.1 (10.4) |
| BMI, kg/m2 | 29.6 (74.2) |
| Physical activity, MET-hr/wk | 14.3 (25.1) |
| Median (SD) | |
| Time between diagnosis and blood draw, months | 1.4 (7.6) |
| Time between blood draw and survey, months | 0 (2.6) |
| Time between surgery and blood draw, monthsa | 1.6 (5.3) |
| Number of metastatic sitesb | 1.0 (0.7) |
| CA19-9 at blood draw, U/ml | 644 (274,892) |
| Gender, No. (%) | |
| Female | 211 (47) |
| Male | 235 (53) |
| Diabetes, No. (%) | |
| No | 279 (63) |
| Yes | 141 (32) |
| Unknown | 26 (6) |
| Cancer stage, No. (%) | |
| Localized | 12 (3) |
| Locally advanced | 100 (22) |
| Metastatic | 271 (61) |
| No. of evidence of disease | 63 (14) |
| Grade, No. (%) | |
| Well/moderately differentiated | 84 (19) |
| Poorly differentiated/undifferentiated | 96 (22) |
| Unknown | 266 (60) |
| Smoking status at blood draw, No. (%) | |
| Never | 194 (44) |
| Former | 216 (48) |
| Current | 32 (7) |
| Unknown | 4 (1) |
| Regular aspirin use at blood draw c , No. (%) | |
| No | 145 (33) |
| Yes | 142 (32) |
| Unknown | 159 (36) |
| Treatment d status at time of blood draw, No. (%) | |
| Treatment naive | 234 (53) |
| On treatment | 164 (37) |
| Post treatment | 48 (11) |
SD standard deviation, BMI body mass index, MET-hr metabolic equivalent of task-hour
aAmong patients who underwent surgical resection
bAmong patients with metastatic disease
cRegular use is defined as intake frequency of≥3 days/week
dIncludes chemotherapy and radiation
Exposure assessment
CRP, MIC-1, and sTNF-RII were assayed in the laboratory of Dr. Nader Rifai (Boston Children’s Hospital, Boston, MA). The sTNF-RII is an established surrogate measurement for TNF-α due to its role in TNF-α signaling, lower diurnal variation, and increased stability in frozen plasma25,26. Furthermore, unlike TNF-α levels of which tend to fluctuate, levels of sTNF-RII are stable over long periods of time27. CRP was measured using an immunoturbidimetric assay (Roche Diagnostics, Indianapolis, IN), with a limit of detection of 0.03 mg/L. MIC-1 and sTNF-RII were measured by an ELISA assay (R&D Systems, Minneapolis, MN), with a sensitivity of 4.36 pg/mL for MIC-1 and 0.6 pg/mL for sTNF-RII. IL-6 was measured as part of the 16-plex pro- and anti-inflammatory cytokine panel (Human Cytokine A Premixed Magnetic Luminex Performance Assay, R&D Systems, Minneapolis, MN). The sensitivity of the assay is 1.11 pg/mL. Coefficients of variation for each assay were calculated using 10% blinded duplicate samples, and ranged from 2.5% for CRP to 6.1% for sTNF-RII. Laboratory personnel was blinded to patient status.
Covariate assessment
Data on patient and disease characteristics, such as age at time of blood draw, albumin levels, gender, body mass index (BMI) at time of blood draw, date of diagnosis, stage, treatment history, and date of death were extracted from the medical record. Information on race, smoking status, physical activity, and aspirin and non-steroidal anti-inflammatory drug (NSAID) use at time of blood draw were extracted from the self-administered questionnaire. GPS was calculated as previously described9,24.
Statistical analysis
All inflammatory cytokines were log-transformed to improve normality. Correlation between cytokines was analyzed using Spearman correlation. We used the Wilcoxon rank-sum or Kruskal–Wallis test to evaluate differences in cytokine levels between two or more groups of interest.
We used the Cox proportional hazards model to evaluate the hazards ratios (HRs) and 95% confidence intervals (CIs) for mortality. Person-time was calculated as time between blood collection and death or last follow-up (November 16, 2016). To test the proportionality of hazards assumption, we evaluated the cross product of time and inflammatory cytokines. This test revealed a violation of the proportionality of hazards assumption which was addressed by including an interaction term between time and cytokine levels in the models, allowing calculation of HRs for different time points. Inflammatory cytokines were modeled as quartiles. To evaluate the trend of the association between inflammatory cytokines and survival across quartiles, we used the median of each quartile as a continuous variable in the model. In multivariate models, we adjusted a priori for age at blood collection, gender, grade (well differentiated, moderately differentiated, poorly differentiated, undifferentiated, unknown), cancer stage (localized/no evidence of disease, locally advanced, metastatic), treatment status (treatment naive, on treatment, post treatment), number of metastatic sites, BMI (continuous), and physical activity (continuous) at time of blood collection. We additionally examined potential confounding by smoking, aspirin use, NSAID use, and diabetes status at time of blood collection.
To investigate whether the combination of cytokine concentrations is more strongly related to mortality than each individual cytokine alone, we also evaluated the association between a combined inflammatory cytokine score and mortality. We calculated this combined score by summing the number of cytokines with levels above the population median. The score therefore ranged from 0 (no cytokines above the median) to 4 (all four cytokine levels above the median).
To compare discrimination between different survival models, we calculated the overall C-index. This metric is an extension of the receiver operating characteristic for the Cox proportional hazard model28.
We performed subgroup analyses to examine potential effect modification by age (<median of 64.5 years, ≥64.5 years), gender, BMI (<25 kg/m2, ≥25 kg/m2), diabetes (yes vs. no), smoking status (never vs. ever smoker), regular aspirin use (yes vs. no), grade of differentiation (well/moderately vs. poorly differentiated/undifferentiated), and metastatic status (non-metastatic vs. metastatic). We also performed a stratified analysis by treatment status (treatment naive vs. on/post treatment) for metastatic patients only. Statistical interaction was evaluated by including a cross-product term containing the stratification variable and inflammatory cytokine level (as quartiles) into the model and performing the likelihood ratio test.
Kaplan–Maier method and log-rank tests were used to illustrate and analyze survival curves. Statistical analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC). All P-values were two-sided.
Results
Characteristics of the study population are shown in Table 1. The study included 211 female (47%) and 235 (53%) male participants. The mean age at diagnosis was 64.1 years. At the time of blood draw, 3% of patients had localized disease, 22% had locally advanced disease, 61% had metastatic disease, and 14% of patients had no evidence of disease following surgical resection. Most patients (53%) were treatment naive at the time of blood draw, 37% were on active treatment with chemotherapy and/or radiation, and 11% were post treatment.
Median survival time in the entire patient cohort was 9.7 months (30.1 months for patients with no evidence of disease following surgical resection, 19.3 months with patients with localized disease, 12.4 months for those with locally advanced tumors, and 6.5 months for those with metastatic disease). Median follow-up was 9.3 months; at last follow-up, 413 patients (92.6%) had died.
We observed significant, but weak to moderate, correlations between cytokines, with correlation coefficients ranging from 0.12 (IL-6 and sTNF-RII) to 0.41 (MIC-1 and sTNF-RII) (Supplementary table 1). CRP levels differed significantly by time between diagnosis and blood draw, BMI, stage, and grade (Table 2). IL-6 levels were higher in regular aspirin users and patients with higher BMI. sTNF-RII levels were higher among older patients and patients with poorly differentiated and undifferentiated tumors. MIC-1 levels differed significantly by age, gender, time between diagnosis and blood draw, cancer stage, presence of diabetes, aspirin use, and treatment status.
Table 2.
Plasma inflammatory biomarkers according to selected patient and tumor characteristics
| Characteristics | CRP (mg/L) (n = 437) median (IQ range) | P-valueb | IL-6 (pg/mL) (n = 427) median (IQ range) | P-valueb | sTNF-RII (pg/mL) (n = 423) median (IQ range) | P-valueb | MIC-1 (pg/mL) (n = 434) median (IQ range) | P- Valueb |
|---|---|---|---|---|---|---|---|---|
| Age a | ||||||||
| <64.5 years | 8.1 (24.9) | 2.6 (2.4) | 3443.9 (2060.2) | 1698.2 (1819.5) | ||||
| ≥64.5 years | 7.3 (20.6) | 0.47 | 3.0 (2.2) | 0.19 | 3726.6 (2390.5) | 0.01 | 2064.8 (1931.4) | 0.005 |
| Time between diagnosis and blood draw a | ||||||||
| <1.4 months | 10.8 (24.2) | 2.8 (2.4) | 3508.0 (2309.6) | 1719.5 (1689.2) | ||||
| ≥1.4 months | 5.4 (17.7) | 0.002 | 2.8 (2.2) | 0.29 | 3730.7 (1890.7) | 0.07 | 1995.0 (1938.5) | 0.01 |
| BMI a | ||||||||
| <25.3 kg/m2 | 5.4 (18.7) | 2.6 (1.8) | 3531.7 (2619.3) | 1837.2 (1824.2) | ||||
| ≥25.3 kg/m2 | 12.1 (24.0) | <0.0001 | 3.0 (2.4) | 0.001 | 3641.2 (2619.3) | 0.30 | 1938.2 (2002.4) | 0.41 |
| Physical activity a | ||||||||
| <3.9 MET-hr/wk | 7.4 (24.4) | 2.9 (2.4) | 3512.2 (2213.7) | 2055.1 (2039.1) | ||||
| ≥3.9 MET-hr/wk | 6.7 (25.5) | 0.87 | 2.5 (1.7) | 0.71 | 3606.8 (1827.2) | 0.29 | 1937.0 (1413.4) | 0.40 |
| Gender | ||||||||
| Male | 9.5 (24.5) | 2.8 (2.6) | 3660.3 (2015.4) | 2022.5 (2462.1) | ||||
| Female | 7.2 (18.7) | 0.13 | 2.8 (1.8) | 0.87 | 3493.4 (2395.8) | 0.59 | 1773.4 (1656.8) | 0.03 |
| Diabetes | ||||||||
| No | 7.4 (20.6) | 2.8 (2.2) | 3547.0 (2174.2) | 1819.2 (1744.2) | ||||
| Yes | 9.0 (26.9) | 0.44 | 2.8 (2.3) | 0.92 | 3644.6 (2129.6) | 0.41 | 2221.1 (2811.6) | 0.01 |
| Cancer stage | ||||||||
| Localized | 4.5 (10.3) | 1.8 (2.1) | 3490.5 (2103.0) | 1443.1 (1397.0) | ||||
| Locally advanced | 4.9 (16.0) | 2.8 (1.9) | 3290.3 (1827.9) | 1727.1 (1924.3) | ||||
| Metastatic | 12.6 (30.5) | 2.8 (2.5) | 3803.6 (2182.2) | 2165.3 (1991.8) | ||||
| NED | 2.9 (8.5) | <0.0001 | 2.7 (2.3) | 0.14 | 3497.0 (1973.2) | 0.05 | 1390.7 (901.5) | <0.0001 |
| Grade | ||||||||
| Well/moderately differentiated | 4.1 (15.3) | 2.7 (2.4) | 3112.0 (1454.8) | 1575.3 (1529.7) | ||||
| Poorly differentiated/undifferentiated | 11.1 (31.3) | 0.01 | 3.0 (2.7) | 0.16 | 3944.5 (2586.0) | 0.005 | 1975.2 (1846.0) | 0.09 |
| Smoking | ||||||||
| Never | 8.0 (21.8) | 2.8 (2.2) | 3570.1 (2040.3) | 1877.2 (1943.7) | ||||
| Past | 7.8 (23.0) | 2.8 (2.5) | 3714.3 (2070.0) | 1949.8 (2034.3) | ||||
| Current | 11.7 (22.4) | 0.55 | 2.8 (2.9) | 0.39 | 3569.5 (1722.8) | 0.30 | 1734.6 (2031.2) | 0.85 |
| Regular aspirin use c | ||||||||
| Non-users | 5.5 (24.0) | 2.5 (1.6) | 3509.2 (1826.6) | 1869.3 (1451.4) | ||||
| Users | 10.6 (22.9) | 0.09 | 3.0 (2.5) | 0.01 | 3768.1 (2708.8) | 0.03 | 2180.8 (2586.0) | 0.04 |
| Regular NSAID use c | ||||||||
| Non-users | 6.6 (25.0) | 2.8 (2.2) | 3661.9 (2090.5) | 2064.8 (1746.5) | ||||
| Users | 8.9 (34.2) | 0.21 | 2.8 (1.5) | 0.96 | 3522.4 (1950.8) | 0.59 | 1769.7 (1344.3) | 0.44 |
| Treatment status d | ||||||||
| Treatment naive | ||||||||
| On/post treatment | 8.8 (21.1) | 2.8 (2.1) | 3529.3 (2182.0) | 1672.5 (1611.1) | ||||
| 6.9 (23.7) | 0.74 | 2.8 (2.2) | 0.11 | 3700.3 (2082.3) | 0.53 | 2145.8 (2580.3) | <0.0001 | |
CRP, C-reactive protein; IL-6, interleukin-6; MIC-1, macrophage inhibitory cytokine-1; sTNF-RII, tumor necrosis factor receptor 2; IQ, interquartile range; BMI, body mass index; MET-hr, metabolic equivalent of task-hour; NED, no evidence of disease, NSAID, nonsteroidal anti-inflammatory drug
aCut point determined by the median value
bCalculated using Wilcoxon rank-sum, or Kruskal–Wallis test
cRegular use is defined as intake frequency of ≥3 days/week
dIncludes chemotherapy and radiation
Bold value denote significance P-value ≤ 0.05
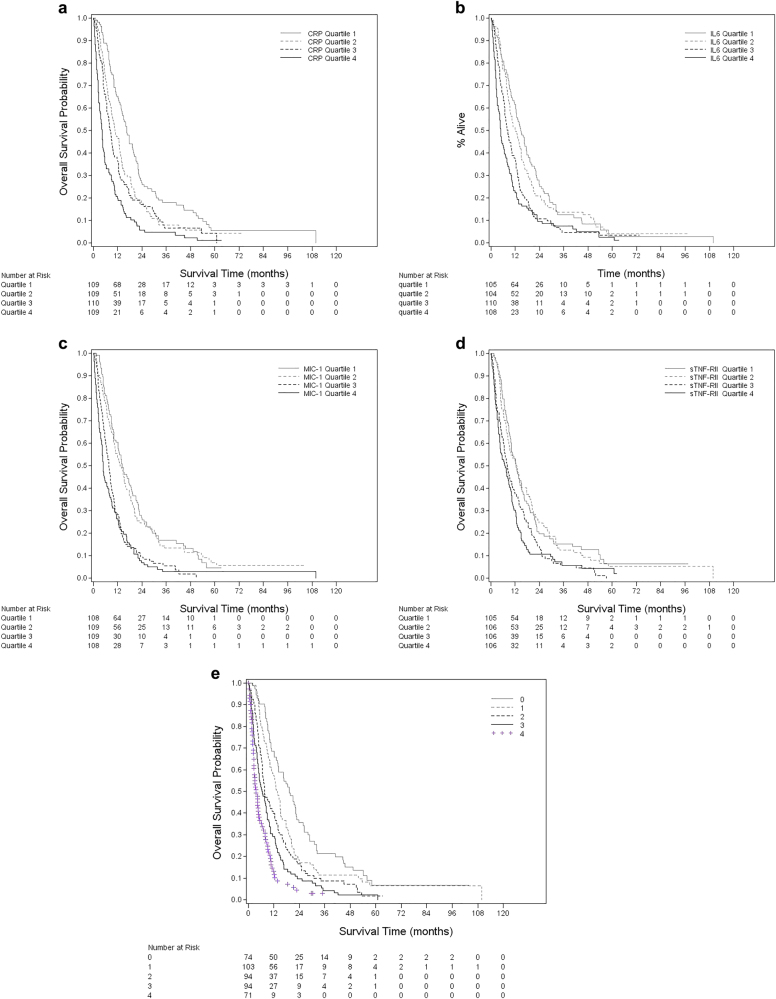
For each measured cytokine, patients with levels in the highest quartile had significantly worse survival (log-rank P-value <0.0001) (Fig. 1a–d). In the multivariate model, compared to patients in the lowest quartile, the mortality hazard of patients in the highest quartiles at the median survival time of 9.5 months was 2.52 (95% CI: 1.82–3.49) for CRP, 2.78 (95% CI: 2.03–3.81) for IL-6, 2.00 (95% CI: 1.46–2.72) for sTNF-RII, and 2.53 (95% CI: 1.83–3.50) for MIC-1 (Table 3). Moreover, there was a significant linear trend across quartiles (P-trend <0.0001 for all four cytokines). Further adjustment for diabetes, smoking, aspirin use, and non-aspirin NSAID use did not alter the associations (data not shown). We observed similar HRs for death across cytokine quartiles at 6 and 12 months (Supplementary Tables 2 and 3).
Fig. 1. Patient survival by quartiles of inflammatory cytokines.
A combined inflammatory score was created by adding number of inflammatory markers with the value above the population median
Table 3.
Hazard ratiosa for death by inflammatory biomarker levels
| HR (95% CI) | |||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P-trendc | |
| CRP (N = 437) | |||||
| Range (mg/L) | 0.05–2.34 | 2.35–7.72 | 7.81–24.26 | 24.42–183.74 | |
| Person-months | 2356 | 1616 | 1376 | 865 | |
| Cases/deaths | 109/97 | 109/101 | 110/100 | 109/106 | |
| Median OS, months | 16.6 | 10.7 | 8.4 | 4.3 | |
| Age-adjusted | 1.00 (Ref) | 1.66 (1.22–2.27) | 1.98 (1.45–2.71) | 3.28 (2.40–4.49) | <.0001 |
| Multivariate-adjustedb | 1.00 (Ref) | 1.48 (1.08–2.04) | 2.08 (1.52–2.86) | 2.52 (1.82–3.49) | <.0001 |
| IL-6 (N = 427) | |||||
| Range (pg/ml) | 0.17–1.86 | 1.89–2.76 | 2.78–4.15 | 4.17–165.73 | |
| Person-months | 1905 | 1791 | 1299 | 1030 | |
| Cases/deaths | 105/95 | 104/95 | 110/102 | 108/103 | |
| Median OS, months | 14.8 | 12.1 | 8.4 | 4.9 | |
| Age-adjusted | 1.00 (Ref) | 1.18 (0.86–1.62) | 1.83 (1.35–2.48) | 2.41 (1.78–3.26) | <.0001 |
| Multivariate-adjustedb | 1.00 (Ref) | 1.11 (0.80–1.54) | 2.22 (1.62–3.03) | 2.78 (2.03–3.81) | <.0001 |
| sTNF-RII (N = 423) | |||||
| Range (pg/ml) | 989.36–2655.95 | 2657.81–3591.33 | 3606.79–4771.55 | 4825.31–9918.17 | |
| Person-months | 1811 | 1938 | 1300 | 1107 | |
| Cases/deaths | 105/90 | 106/97 | 106/104 | 106/100 | |
| Median OS, months | 12.5 | 12.8 | 8.1 | 7.0 | |
| Age-adjusted | 1.00 (Ref) | 1.05 (0.77–1.43) | 1.59 (1.18–2.14) | 1.90 (1.41–2.56) | <.0001 |
| Multivariate-adjustedb | 1.00 (Ref) | 1.05 (0.76–1.45) | 1.44 (1.06–1.94) | 2.00 (1.46–2.72) | <.0001 |
| MIC-1 (N = 434) | |||||
| Range (pg/ml) | 344.40–1215.34 | 1216.57–1896.73 | 1897.19–3172.78 | 3183.01–31829.82 | |
| Person-months | 2021 | 2054 | 1119 | 999 | |
| Cases/deaths | 108/95 | 109/98 | 109/104 | 108/104 | |
| Median OS, months | 14.1 | 13.2 | 7.6 | 4.9 | |
| Age-adjusted | 1.00 (Ref) | 1.20 (0.87–1.64) | 2.30 (1.69–3.12) | 2.61 (1.91–3.56) | <.0001 |
| Multivariate-adjustedb | 1.00 (Ref) | 1.34 (0.97–1.86) | 2.06 (1.50–2.84) | 2.53 (1.83–3.50) | <.0001 |
CRP, C-reactive protein; IL-6, interleukin-6; MIC-1, macrophage inhibitory cytokine-1; sTNF-RII, tumor necrosis factor receptor 2. OS, overall survival. HR, hazard ratio. CI, confidence interval
aHazard ratios at the median survival time of our cohort (9.5 months)
bAdjusted for age at blood draw, gender, grade (well differentiated, moderately differentiated, poorly differentiated, undifferentiated, and unknown), stage (localized/no evidence of disease, locally advanced, and metastatic), treatment status (naive, on, and post), number of metastatic sites, and BMI (continuous) at blood draw and physical activity (continuous)
cP-trend values calculated by entering quartile medians as continuous variables in Cox proportional hazards model
We also examined the association between CRP levels and mortality using the conventional CRP cutoff of 10 mg/L. Compared to patients with CRP ≤ 10 mg/L, those with CRP >10 mg/L had a twofold increase in the risk of death (multivariate HR: 2.00, 95% CI: 1.62–2.47). When simultaneously adjusting for all four cytokines in the multivariate model, we observed a continued significant mortality hazard for patients in the highest quartile of CRP (HR: 1.59, 95% CI: 1.07–2.37), IL-6 (HR: 1.80, 95% CI: 1.23–2.65), and MIC-1 (HR: 1.80, 95% CI: 1.22–2.67), while the association between sTNF-RII and survival was no longer significant (HR: 1.10, 95% CI: 0.75–1.59). Compared to patients with an inflammatory score of 0 and median survival 19.2 months, those with a score of 4 had a median survival of only 3.7 months and adjusted HR of 4.55 (95% CI: 2.87–7.20; P-trend <0.0001) (Table 4, Fig. 1e).
Table 4.
Hazard ratiosa for death by combined inflammatory marker score
| Inflammatory scoreb | ||||||
|---|---|---|---|---|---|---|
| HR (95% CI) | ||||||
| 0 | 1 | 2 | 3 | 4 | P-trendc | |
| Person-months | 1765 | 1790 | 1240 | 959 | 460 | |
| Cases/deaths | 74/65 | 103/90 | 94/88 | 94/92 | 71/68 | |
| Median OS, months | 19.2 | 13.8 | 7.6 | 6.5 | 3.7 | |
| Age-adjusted | 1.00 (Ref) | 1.73 (1.16–2.57) | 2.39 (1.60–3.56) | 3.36 (2.25–5.03) | 4.53 (2.90–7.09) | <.0001 |
| Multivariate-adjustedd | 1.00 (Ref) | 1.82 (1.21–2.73) | 2.60 (1.72–3.95) | 3.52 (2.32–5.35) | 4.55 (2.87–7.20) | <.0001 |
HR, hazard ratio; CI, confidence interval; OS, overall survival
aHazard ratios at 9.5 months (median survival time)
bCreated by adding number of inflammatory markers with a value above the study population median
cP-trend calculated by entering inflammatory score as continuous variables in Cox proportional hazards model
dAdjusted for age at blood draw, gender, grade (well differentiated, moderately differentiated, poorly differentiated, undifferentiated, and unknown), stage (localized/no evidence of disease, locally advanced, and metastatic), treatment status (naive, on, and post), number of metastatic sites, BMI (continuous) at blood draw and physical activity (continuous)
The model including CRP had a discriminatory index of 0.80 (95% CI: 0.71–0.88), comparable to those of GPS (C-index = 0.76, 95% CI: 0.66–0.85) and CRP to albumin ratio (C-index = 0.74, 95% CI: 0.64–0.83) (Supplementary Tables 4 and 5).
The significant association between increasing cytokine levels and worse mortality was consistent across most subgroups of known prognostic characteristics (Table 5). We observed a significant interaction between CRP and BMI (P-interaction = 0.03), and between CRP and aspirin use (P-interaction = 0.001), with association with worse mortality being stronger among patients with BMI ≤25 kg/m2 and among aspirin users.
Table 5.
Hazard ratiosa for death among selected patient subgroups
| CRP | IL-6 | sTNF-RII | MIC-1 | |
|---|---|---|---|---|
| >10 mg/L vs. ≤ 10 mg/L | Q4 vs. Q1 | Q4 vs. Q1 | Q4 vs. Q1 | |
| HR (95% CI)b | HR (95% CI)b | HR (95% CI)b | HR (95% CI)b | |
| Age c | ||||
| ≤64.5 years | 2.09 (1.53–2.86) | 3.96 (2.48–6.33) | 2.21 (1.41–3.45) | 3.10 (1.96–4.90) |
| >64.5 years | 2.00 (1.46–2.72) | 1.78 (1.12–2.82) | 2.18 (1.36–3.49) | 2.41 (1.44–4.04) |
| P-interactiond | 0.96 | 0.05 | 0.93 | 0.78 |
| BMI c | ||||
| ≤25 kg/m2 | 2.83 (1.98–4.05) | 4.70 (2.75–8.03) | 2.52 (1.52–4.19) | 2.56 (1.57–4.17) |
| >25 kg/m2 | 1.73 (1.23–2.31) | 2.53 (1.60–4.00) | 1.65 (1.10–2.49) | 2.52 (1.60–3.96) |
| P-interactiond | 0.03 | 0.12 | 0.12 | 0.49 |
| Gender | ||||
| Male | 1.89 (1.40–2.55) | 2.66 (1.74–4.07) | 2.93 (1.90–4.51) | 3.31 (2.10–5.24) |
| Female | 2.44 (1.77–3.37) | 3.22 (1.90–5.43) | 1.41 (0.88–2.23) | 2.40 (1.45–3.99) |
| P-interactiond | 0.10 | 0.84 | 0.17 | 0.49 |
| History of diabetes | ||||
| No | 1.91 (1.44–2.54) | 2.93 (1.90–4.52) | 1.67 (1.12–2.48) | 2.06 (1.33–3.19) |
| Yes | 2.65 (1.74–4.02) | 4.25 (2.31–7.82) | 3.27 (1.70–6.29) | 5.20 (2.67–10.13) |
| P-interactiond | 0.64 | 0.90 | 0.83 | 0.37 |
| Metastatic disease | ||||
| No | 1.47 (0.95–2.27) | 2.29 (1.18–4.47) | 1.33 (0.74–2.39) | 1.65 (0.93–2.93) |
| Yes | 2.10 (1.59–2.76) | 2.40 (1.58–3.66) | 1.72 (1.13–2.62) | 2.92 (1.88–4.53) |
| P-interactiond | 0.15 | 0.29 | 0.96 | 0.11 |
| Number of metastatic sites e | ||||
| 1 | 1.99 (1.42–2.79) | 2.32 (1.37–3.92) | 1.59 (0.93–2.72) | 2.91 (1.75–4.84) |
| >1 | 2.05 (1.16–3.63) | 2.44 (1.05–5.72) | 1.70 (0.72–4.01) | 6.41 (1.96–21.10) |
| P-interactiond | 0.60 | 0.43 | 0.25 | 0.30 |
| Grade | ||||
| Well/moderately differentiated | 2.42 (1.23–4.76) | 2.41 (1.07–5.42) | 3.77 (1.55–9.14) | 2.82 (1.11–7.21) |
| Poorly differentiated/undifferentiated | 2.56 (1.49–4.41) | 5.20 (2.16–12.52) | 2.30 (1.05–5.05) | 3.23 (1.45–7.18) |
| P-interactiond | 0.81 | 0.05 | 0.29 | 0.63 |
| Smoking status | ||||
| Never smokers | 2.15 (1.52–3.03) | 2.40 (1.47–3.93) | 2.40 (1.51–3.82) | 2.54 (1.52–4.23) |
| Ever smokers | 1.99 (1.48–2.66) | 3.43 (2.16–5.47) | 1.73 (1.10–2.71) | 2.93 (1.86–4.63) |
| P-interactiond | 0.70 | 0.31 | 0.19 | 0.33 |
| Regular aspirin use f | ||||
| No | 1.59 (1.05–2.41) | 4.97 (2.55–9.70) | 2.03 (1.04–3.96) | 4.25 (2.03–8.91) |
| Yes | 3.01 (1.91–4.75) | 2.42 (1.32–4.45) | 3.16 (1.68–5.94) | 3.23 (1.62–6.43) |
| P-interactiond | 0.001 | 0.34 | 0.35 | 0.62 |
| Treatment status g | ||||
| Treatment naive | 2.08 (1.52–2.84) | 2.88 (1.84–4.51) | 1.70 (1.09–2.64) | 2.02 (1.28–3.18) |
| On/post treatment | 2.14 (1.54–2.95) | 2.97 (1.82–4.83) | 2.29 (1.44–3.64) | 2.81 (1.63–4.82) |
| P-interactiond | 0.43 | 0.72 | 0.60 | 0.54 |
CRP, C-reactive protein; IL-6, interleukin-6; MIC-1, macrophage inhibitory cytokine-1; sTNF-RII, tumor necrosis factor receptor 2; HR, hazard ratio; CI, confidence interval; BMI, body mass index
aHazard ratios at 9.5 months (median survival time)
bAdjusted for age at blood draw, gender, grade, (well differentiated, moderately differentiated, poorly differentiated, and unknown), stage (localized/no evidence of disease, locally advanced, and metastatic), treatment status (naive, on treatment, and post treatment), number of metastatic sites, BMI (continuous) at blood draw, physical activity (continuous), and excluding the stratifying variable
cMedian population values
dP-interaction was calculated by entering a cross-product term of stratifying variable and inflammatory cytokine (quartiles) into Cox proportional hazards model
eAmong patients with metastatic disease
fRegular use is defined as intake frequency of ≥3 days/week
gRestricted to metastatic patients
To evaluate whether higher levels of inflammatory cytokines simply reflect a greater burden of disease, we adjusted for disease stage, number of metastatic sites and CA19-9 levels in the multivariate model and observed no significant change of the estimate. In addition, associations were similar by metastatic disease and number of metastatic sites (P-interaction >0.25) (Table 5).
Discussion
In this large prospective clinic-based study of 446 PDAC patients, subjects with higher levels of each of CRP, IL-6, and MIC-1 experienced a significant increase in overall mortality. Moreover, patients with elevations of all four markers combined had the largest comparative increase in mortality, suggesting involvement of multiple activated inflammatory pathways in PDAC progression. Compared to patients with all cytokines below the population median, those with all four cytokines above the population median had an almost fivefold increased hazard of death, with median survival of 4 vs. 19 months. These associations were independent of known prognostic factors, such as tumor stage, grade, number of metastatic sites, and CA19-9 levels. Furthermore, except for sTNF-RII, the effects of these cytokines on survival are mutually independent.
The inverse associations of CRP and IL-6 with patient survival in our study are consistent with previously reported findings10,12,13,19,20,22,23. We further showed that the associations of these two cytokines are independent from each other, as well as from MIC-1 and sTNF-RII. While we observed a significant association between survival and sTNF-RII, a surrogate for TNF-α, the association was attenuated after adjusting for CRP, IL-6 and MIC-1. This therefore argues against an independent effect of TNF-α on survival, as has been previously suggested11. Our finding of decreased survival among patients in the top quartile of MIC-1 compared to those in the lowest quartile is also consistent with previous studies29,30; however, distinct from those prior studies, we were able to comprehensively adjust for potential confounders. We also found that the influence of high-MIC-1 levels was independent of CRP, IL-6, and sTNF-RII. To our knowledge, no previous studies have assessed the combined contribution of those four inflammatory cytokines on PDAC patient survival.
Several explanations have been proposed to address the relationship between inflammatory cytokines and survival. High levels of circulating cytokines may result from the systemic response of host to tumor, reflecting the tumor burden. CRP is produced by the liver as part of the acute phase response31, and its levels correlate with cancer progression13,20,22,23. However, adjusting for clinical markers of aggressive disease did not change our observation. Moreover, in stratified analyses the associations between inflammatory cytokines and patient mortality were similar across subgroups of tumor burden.
Extensive experimental evidence supports the importance of inflammatory cytokines in tumor growth and progression, both by acting directly on tumor cells and by modifying the tumor microenvironment5. IL-6 exerts its protumorigenic effects by activating several signaling pathways involved in PDAC, such as JAK-STAT3, Ras-MAPK, and PI3K-Akt, leading to increased cellular proliferation, angiogenesis, and metastatic potential32. IL-6 is one of the key factors in the development of muscle wasting, or cachexia33, which is responsible for about one third of PDAC-associated deaths and decreased response to treatment34. Furthermore, it was shown that IL-6 leads to formation of desmoplastic stroma35, a dense extracellular matrix which acts as a physical barrier for effective drug delivery36. MIC-1, a member of the human transforming growth factor (TGF)-β superfamily, has both anti- and protumorigenic roles in colon, breast, prostate, and melanoma cancers37. While differences in MIC-1 levels between PDAC patients and healthy controls have previously been reported16,38,39, little is known about the molecular pathways underlying this association. Furthermore, it was shown that both IL-640,41 and MIC-142 attenuate T-cell-mediated anti-tumor immune response. In PDAC43, as well as other cancers, MIC-1 overexpression is associated with increased resistance to chemotherapy drugs37. However, it is unlikely that resistance to treatment explains the effect of MIC-1 in our study, since there was no difference in survival in treatment naive or on/post treatment group by MIC-1 levels (Table 5).
Our results carry multiple potential clinical and translational implications. First, CRP, IL-6, and MIC-1 assays are inexpensive and non-invasive. Therefore, their prognostic potential should be further investigated in future studies. Furthermore, they could be used to identify patients who may benefit most from anti-inflammatory strategies. Indeed, interest in the use of circulating inflammatory cytokines as predictors of treatment efficacy was first suggested by the RECAP trial, a randomized phase II study of capecitabine with or without ruxolitinib (JAK/STAT inhibitor) in patients with refractory PDAC that showed a survival benefit with ruxolitinib in patients with high CRP or modified GPS15. Unfortunately, subsequent phase III trials did not confirm this finding, but have led to research efforts in other novel inflammation-mediated pathways, including inhibitors of TBK1, which regulates a KRAS-driven autocrine cytokine circuit44, immunomodulatory agents such as CCR2 antagonists (ClinicalTrials.gov identifier NCT02732938), and vitamin D receptor VDR analogs, which have been shown to reprogram the tumor microenvironment45. It is conceivable that levels of inflammatory cytokines may play a future role in helping to select the patients who are most likely to benefit from these novel agents.
Advantages of our study include the prospective study design, large number of patients, as well as detailed information on clinical, pathological, treatment, and lifestyle factors. We were also able to evaluate a variety of inflammatory cytokines singly and in combination. However, several limitations exist. We were not able to evaluate pancreatic cancer-specific mortality, but 95% of pancreatic cancer patients present with incurable disease at diagnosis, therefore it is highly unlikely that our patients died of other causes. Furthermore, our study consisted of predominantly white participants treated at a tertiary academic center, and therefore our results may not be generalizable to the overall population of pancreatic cancer patients. Reassuringly, though, the median survival of our study population is reflective of PDAC patients overall. Finally, while circulating inflammatory cytokines have the advantage of being easily measurable and accessible in patients, plasma levels may not adequately reflect inflammatory activity within the tumor or its microenvironment. Fortunately, recent technological advances that allow tumor RNA sequencing in bulk or on single-cell populations will pave the way toward elucidating the exact origin and mechanism of the high-inflammatory state of PDAC patients.
In conclusion, increasing levels of circulating inflammatory cytokines were associated with significantly decreased survival of patients with pancreatic cancer in this large prospective clinic-based study. The potential prognostic value of these markers, as well as their utility for patient selection for novel anti-inflammatory and immunomodulatory agents, should be evaluated in future studies. Moreover, efforts to understand the pathogenesis of the high-inflammatory state and development of novel agents to decrease inflammation in PDAC patients are warranted.
Study Highlights
What is current knowledge
Inflammation and inflammatory conditions have been associated with increased risk of pancreatic cancer
Individual inflammatory cytokines have been associated with survival of pancreatic cancer patients
What is new here
The effect of inflammatory cytokines on pancreatic cancer patient survival is mutually independent and additive, suggesting that multiple inflammatory pathways are involved in PDAC progression
Electronic supplementary material
Conflict of interest
Guarantor of the article: Kimmie Ng.
Specific author contributions: Study conception and design: A.B., N.S., D.A.B., K.W., C.S.F., K.N. Data collection and/or assembly: A.B., N.S., N.P.N., M.M.Z., N.R., M.W.W., L.K.B., D.A.R., V.M.-O., B.M.W., M.H.K., C.S.F., K.N. Data analysis and/or interpretation: A.B., N.S., D.A.R., V.M.-O., Y.C., S.Z., E.M.P., B.M.W., M.K., D.A.B., K.W., C.S.F., K.N. Manuscript writing: A.B., N.P.N., M.M.Z., N.R., Y.C., S.Z., E.M.P., K.N. Approval of the final manuscript version: all authors.
Financial support:This work was supported by the National Institutes of Health research grants DF/HCC SPORE in Gastrointestinal Cancer-P50CA127003 (A.B., C.S.F.), Department of Defense CA130288, Celgene, Lustgarten Foundation for Pancreatic Cancer Research (C.S.F., B.M.W.), Perry S. Levy Fund for Gastrointestinal Cancer Research (B.M.W., C.S.F.), Broman Fund for Pancreatic Cancer Research (K.N.), Pappas Family Research Fund for Pancreatic Cancer (C.S.F.), the Noble Effort Fund, the Peter R. Leavitt Family Fund, and Promises for Purple (B.M.W.); and Robert T. and Judith B. Hale Fund for Pancreatic Cancer Research (A.B., B.M.W., C.S.F.).
Potential competing interests: The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article 10.1038/s41424-018-0008-5 contains supplementary material, which is available to authorized users.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Hartwig W, et al. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann. Surg. 2011;254:311–319. doi: 10.1097/SLA.0b013e31821fd334. [DOI] [PubMed] [Google Scholar]
- 3.Li D, et al. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 4.Greer JB, Whitcomb DC. Inflammation and pancreatic cancer: an evidence-based review. Curr. Opin. Pharmacol. 2009;9:411–418. doi: 10.1016/j.coph.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutat. Res. 2008;659:15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Roshani R, McCarthy F, Hagemann T. Inflammatory cytokines in human pancreatic cancer. Cancer Lett. 2014;345:157–163. doi: 10.1016/j.canlet.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Yuan C, et al. Survival among patients with pancreatic cancer and long-standing or recent-onset diabetes mellitus. J. Clin. Oncol. 2015;33:29–35. doi: 10.1200/JCO.2014.57.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan C, et al. Prediagnostic body mass index and pancreatic cancer survival. J. Clin. Oncol. 2013;31:4229–4234. doi: 10.1200/JCO.2013.51.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afaneh C, et al. Pancreatic cancer surgery and nutrition management: a review of the current literature. Hepatobiliary Surg. Nutr. 2015;4:59–71. doi: 10.3978/j.issn.2304-3881.2014.08.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellone G, et al. Cytokine expression profile in human pancreatic carcinoma cells and in surgical specimens: implications for survival. Cancer Immunol. Immunother. 2006;55:684–698. doi: 10.1007/s00262-005-0047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dima SO, et al. An exploratory study of inflammatory cytokines as prognostic biomarkers in patients with ductal pancreatic adenocarcinoma. Pancreas. 2012;41:1001–1007. doi: 10.1097/MPA.0b013e3182546e13. [DOI] [PubMed] [Google Scholar]
- 12.Ebrahimi B, et al. Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis. Cancer. 2004;101:2727–2736. doi: 10.1002/cncr.20672. [DOI] [PubMed] [Google Scholar]
- 13.Falconer JS, et al. Acute-phase protein response and survival duration of patients with pancreatic cancer. Cancer. 1995;75:2077–2082. doi: 10.1002/1097-0142(19950415)75:8<2077::AID-CNCR2820750808>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 14.Haruki K, et al. The C-reactive protein to albumin ratio predicts long-term outcomes in patients with pancreatic cancer after pancreatic resection. World J. Surg. 2016;40:2254–2260. doi: 10.1007/s00268-016-3491-4. [DOI] [PubMed] [Google Scholar]
- 15.Hurwitz HI, et al. Randomized, double-blind, phase II Study of Ruxolitinib or Placebo in combination with capecitabine in patients with metastatic pancreatic cancer for whom therapy with gemcitabine has failed. J. Clin. Oncol. 2015;33:4039–4047. doi: 10.1200/JCO.2015.61.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaur S, et al. Potentials of plasma NGAL and MIC-1 as biomarker(s) in the diagnosis of lethal pancreatic cancer. PLoS ONE. 2013;8:e55171. doi: 10.1371/journal.pone.0055171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Z, et al. Prognostic value of the CRP/Alb ratio, a novel inflammation-based score in pancreatic cancer. Ann. Surg. Oncol. 2017;24:561–568. doi: 10.1245/s10434-016-5579-3. [DOI] [PubMed] [Google Scholar]
- 18.McMillan DC, Canna K, McArdle CS. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br. J. Surg. 2003;90:215–219. doi: 10.1002/bjs.4038. [DOI] [PubMed] [Google Scholar]
- 19.Mroczko B, et al. Diagnostic usefulness of serum interleukin 6 (IL-6) and C-reactive protein (CRP) in the differentiation between pancreatic cancer and chronic pancreatitis. J. Clin. Lab. Anal. 2010;24:256–261. doi: 10.1002/jcla.20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papadoniou N, et al. Prognostic factors in patients with locally advanced (unresectable) or metastatic pancreatic adenocarcinoma: a retrospective analysis. Anticancer Res. 2008;28:543–549. [PubMed] [Google Scholar]
- 21.Poch B, et al. Systemic immune dysfunction in pancreatic cancer patients. Lange. Arch. Surg. 2007;392:353–358. doi: 10.1007/s00423-006-0140-7. [DOI] [PubMed] [Google Scholar]
- 22.Szkandera J, et al. Validation of C-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patients. Br. J. Cancer. 2014;110:183–188. doi: 10.1038/bjc.2013.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tingstedt B, et al. Predictive factors in pancreatic ductal adenocarcinoma: role of the inflammatory response. Scand. J. Gastroenterol. 2007;42:754–759. doi: 10.1080/00365520601058452. [DOI] [PubMed] [Google Scholar]
- 24.Yamada S, et al. Clinical implication of inflammation-based prognostic score in pancreatic cancer: glasgow prognostic score is the most reliable parameter. Medicine. 2016;95:e3582. doi: 10.1097/MD.0000000000003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diez-Ruiz A, et al. Soluble receptors for tumour necrosis factor in clinical laboratory diagnosis. Eur. J. Haematol. 1995;54:1–8. doi: 10.1111/j.1600-0609.1995.tb01618.x. [DOI] [PubMed] [Google Scholar]
- 26.Liu S, et al. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch. Intern. Med. 2007;167:1676–1685. doi: 10.1001/archinte.167.15.1676. [DOI] [PubMed] [Google Scholar]
- 27.Epstein MM, et al. Temporal stability of serum concentrations of cytokines and soluble receptors measured across two years in low-risk HIV-seronegative men. Cancer Epidemiol. Biomark. Prev. 2013;22:2009–2015. doi: 10.1158/1055-9965.EPI-13-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat. Med. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 29.Lerner L, et al. Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients. J. Cachex-. Sarcopenia Muscle. 2015;6:317–324. doi: 10.1002/jcsm.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou YF, et al. Combined detection of serum UL16-binding protein 2 and macrophage inhibitory cytokine-1 improves early diagnosis and prognostic prediction of pancreatic cancer. Oncol. Lett. 2014;8:2096–2102. doi: 10.3892/ol.2014.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ceciliani F, Giordano A, Spagnolo V. The systemic reaction during inflammation: the acute-phase proteins. Protein Pept. Lett. 2002;9:211–223. doi: 10.2174/0929866023408779. [DOI] [PubMed] [Google Scholar]
- 32.Ara T, Declerck YA. Interleukin-6 in bone metastasis and cancer progression. Eur. J. Cancer. 2010;46:1223–1231. doi: 10.1016/j.ejca.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan CR, et al. Pancreatic cancer cachexia: a review of mechanisms and therapeutics. Front. Physiol. 2014;5:88. doi: 10.3389/fphys.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachmann J, et al. Cachexia worsens prognosis in patients with resectable pancreatic cancer. J. Gastrointest. Surg. 2008;12:1193–1201. doi: 10.1007/s11605-008-0505-z. [DOI] [PubMed] [Google Scholar]
- 35.Mews P, et al. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut. 2002;50:535–541. doi: 10.1136/gut.50.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ireland L, et al. Chemoresistance in pancreatic cancer is driven by stroma-derived insulin-like growth factors. Cancer Res. 2016;76:6851–6863. doi: 10.1158/0008-5472.CAN-16-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mimeault M, Batra SK. Divergent molecular mechanisms underlying the pleiotropic functions of macrophage inhibitory cytokine-1 in cancer. J. Cell Physiol. 2010;224:626–635. doi: 10.1002/jcp.22196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koopmann J, et al. Serum macrophage inhibitory cytokine 1 as a marker of pancreatic and other periampullary cancers. Clin. Cancer Res. 2004;10:2386–2392. doi: 10.1158/1078-0432.CCR-03-0165. [DOI] [PubMed] [Google Scholar]
- 39.Mohamed AA, et al. Evaluation of circulating ADH and MIC-1 as diagnostic markers in Egyptian patients with pancreatic cancer. Pancreatology. 2015;15:34–39. doi: 10.1016/j.pan.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 40.Kitamura H, et al. IL-6-STAT3 controls intracellular MHC class II alphabeta dimer level through cathepsin S activity in dendritic cells. Immunity. 2005;23:491–502. doi: 10.1016/j.immuni.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Park SJ, et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J. Immunol. 2004;173:3844–3854. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- 42.Roth P, et al. GDF-15 contributes to proliferation and immune escape of malignant gliomas. Clin. Cancer Res. 2010;16:3851–3859. doi: 10.1158/1078-0432.CCR-10-0705. [DOI] [PubMed] [Google Scholar]
- 43.Ji H, et al. Twist promotes invasion and cisplatin resistance in pancreatic cancer cells through growth differentiation factor 15. Mol. Med. Rep. 2015;12:3841–3848. doi: 10.3892/mmr.2015.3867. [DOI] [PubMed] [Google Scholar]
- 44.Zhu Z, et al. Inhibition of KRAS-driven tumorigenicity by interruption of an autocrine cytokine circuit. Cancer Discov. 2014;4:452–465. doi: 10.1158/2159-8290.CD-13-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sherman MH, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.