Abstract
The possible involvement of polyamines (PAs) in the chilling tolerance of cucumber (Cucumis sativus L. cv Jinchun No. 3 and cv Suyo) was investigated. Plants with the first expanded leaves were exposed to 3°C or 15°C in the dark for 24 h (chilling), and then transferred to 28°C/22°C under a 12-h photoperiod for another 24 h (rewarming). Chilling-tolerant cv Jinchun No. 3 showed a marked increase of free spermidine (Spd) in leaves, once during chilling and again during rewarming. Putrescine increased significantly during rewarming, but the increase of spermine was slight. Any of these PAs did not increase in chilling-sensitive cv Suyo during either period. PA-biosynthetic enzyme activities appear to mediate these differences between cultivars. Pretreatment of Spd to cv Suyo prevented chill-induced increases in the contents of hydrogen peroxide in leaves and activities of NADPH oxidases and NADPH-dependent superoxide generation in microsomes and alleviated chilling injury. Pretreatment of methylglyoxal-bis-(guanylhydrazone), a PA biosynthesis inhibitor, to chilled cv Jinchun No. 3 prevented Spd increase and enhanced microsomal NADPH oxidase activity and chilling injury. The results suggest that Spd plays important roles in chilling tolerance of cucumber, probably through prevention of chill-induced activation of NADPH oxidases in microsomes.
Mechanisms of chilling tolerance in plants have long been a subject of intensive studies, with a focus on membrane structure and function (Raison and Chapman, 1976; Lyons et al., 1979; Nishida and Murata, 1996). Enhanced chilling tolerance was reported in transgenic tobacco into which a gene for glycerol-3-phosphate acyl transferases or chloroplastic fatty acid desaturases from Arabidopsis was introduced (Murata et al., 1992; Kodama et al., 1994). Another mechanism involves cellular defense against membrane lipid peroxidation caused by a chill-induced increase in the generation of reactive oxygen species (ROS) such as superoxide, hydrogen peroxide, and hydroxyl radicals (Omran, 1980; Hodgson and Raison, 1991; Prasad et al., 1994). Chilling-tolerant plants are known to have more efficient antioxidant systems than chilling-sensitive ones (Walker and McKersie, 1993; Dipierro and Leonardis, 1997). Until now however, the mechanism of chill-induced ROS generation has remained to be clarified.
There is a growing interest in the possible involvement of polyamines (PAs) in the defense reaction of plants to various environmental stresses (Flores, 1990; Kumer et al., 1997; Bouchereau et al., 1999). PAs such as spermidine (Spd) and spermine (Spm) occur ubiquitously in plants, together with their diamine precursor putrescine (Put; Smith, 1985). Put is synthesized directly by decarboxylation of l-Orn in a reaction catalyzed by Orn decarboxylase (ODC). It is also synthesized by the decarboxylation of l-Arg by Arg decarboxylase (ADC), via agmatine and N-carba-moylputrescine intermediates. Addition of an aminopropyl moiety to one or both amino groups of Put by Spd and Spm synthases leads to the formation of Spd and Spm, respectively. The aminopropyl donor is decarboxylated S-adenosyl-Met derived from S-adenosyl-Met via the action of S-adenosyl-Met decarboxylase (SAMDC). Because of the polycationic nature at a physiological pH, PAs can bind strongly to the negative charges in cellular components such as nucleic acids, proteins, and phospholipids (Smith, 1985). Interactions of PAs with membrane phospholipids may stabilize the membranes under conditions of stress (Roberts et al., 1986).
It has been found that chilling-tolerant plants increase endogenous PA levels in response to chilling to a much greater extent than chilling-sensitive ones (Guye et al., 1986; Nadeau et al., 1987; Kramer and Wang, 1989, 1990; Lee, 1997). These findings indicate, but do not prove, the involvement of PAs in chilling tolerance of plants (Bouchereau et al., 1999). However, the mode of PA functions in enhancing the chilling tolerance of plants is not known.
We recently found that higher chilling tolerance of cucumber (Cucumis sativus) cultivars was closely related to a lower rate of ROS generation in leaves during chilling (3°C in darkness for 24 h) and subsequent rewarming (28°C/22°C under a 12-h photoperiod for 24 h; Shen et al., 1999a). Enzymatic and nonenzymatic antioxidant activities were not responsible for these differences between the chilling-tolerant and sensitive cultivars (Shen et al., 1999b). However, the rate of NADPH oxidation in 5,000 g of supernatant of leaf homogenate increased dramatically upon chilling in a chilling-sensitive cultivar, whereas no such increase was observed in a chilling-tolerant cultivar (Shen et al., 1999a). The NADPH-dependent superoxide generation rate in chilled leaves changed with time in close parallel to the NADPH oxidation rate. It is known that PAs are capable of protecting membranes against ROS-induced lipid peroxidation (Kitada et al., 1979; Tadolini et al., 1984; Tadolini, 1988). Ogata et al. (1996) recently found that PAs inhibited the activity of the superoxide-generating NADPH oxidases in human neutrophils. Their results provoked us to investigate the possible involvement of PAs in the chilling tolerance of cucumber cultivars. We describe herein the effect of chilling with or without pretreatment of exogenous PAs or a PA biosynthesis inhibitor on leaf PA levels, chilling injury, NADPH oxidation, and ROS generation rates of whole leaves and leaf microsomes in chilling-tolerant and sensitive cucumber cultivars.
RESULTS
Effect of Chilling on Endogenous Free PA Contents in Leaves
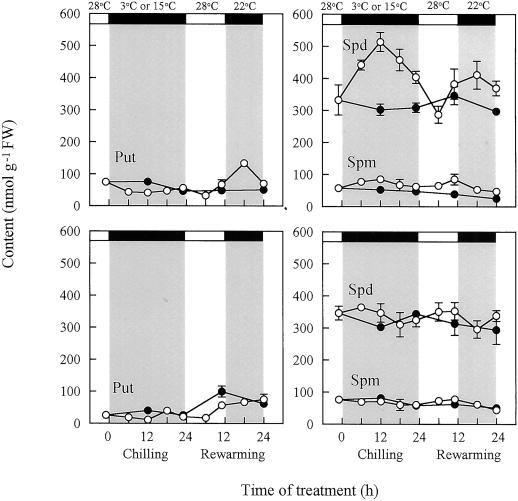
Put, Spd, and Spm were the major free PA species in cucumber leaves, Spd being the most abundant. A diamine cadaverine was undetectable irrespective of treatment temperatures. Effects of chilling treatment on PA contents in leaves differed greatly between the two cultivars (Fig. 1). In chilling-tolerant cv Jinchun No. 3, Spd content increased markedly upon chilling and there was little if any effect on either of other PAs, Put and Spm. During rewarming, Spd content increased again together with Put, with a peak at 18 h. The increase of Spm content was slight. On the other hand, chilling-sensitive cv Suyo did not show such typical changes in free PA contents as observed in cv Jinchun No. 3.
Figure 1.
Changes with time of free PA contents in leaves of cv Jinchun No. 3 (upper) and cv Suyo (lower) during chilling and rewarming. Temperature and light conditions are indicated on top of the panels. Error bars indicate se (n = 3). ○, 3°C; ●, 15°C during chilling treatment.
Effect of Chilling on PA-Biosynthetic Enzyme Activities in Leaves
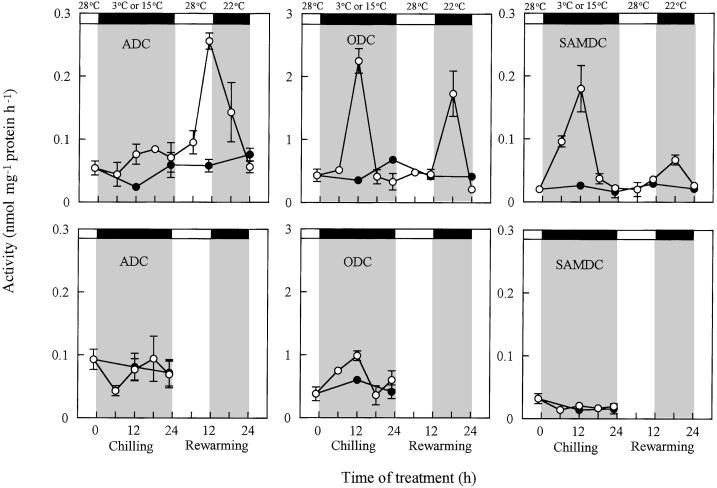
cv Jinchun No. 3 showed a transient, but marked, increase in leaf ODC and SAMDC activities during chilling (Fig. 2). Both of them increased 12 h after chilling. ADC activity increased slightly during chilling, but greatly during rewarming with a peak at the end of the light period. ODC and SAMDC activities also increased at the middle of the dark period after ADC activity began to decline. In contrast to cv Jinchun No. 3, cv Suyo did not show any significant increase in the activity of these PA-biosynthetic enzymes during chilling, except that ODC activity increased slightly during 6 to 12 h of the chilling period (Fig. 2). The enzyme activities in cv Suyo leaves during rewarming were not determined because the leaves did not show any increase in free PA contents.
Figure 2.
Changes with time of PA-biosynthetic enzyme activities in leaves of cv Jinchun No. 3 (top) and cv Suyo (bottom) during chilling and rewarming. Enzyme activities in cv Suyo leaves during rewarming were not determined. Temperature and light conditions are indicated on top of the panels. Error bars indicate se (n = 3). ○, 3°C; ●, 15°C during chilling treatment.
Effect of PA Pretreatment on Chilling Injury of Leaves
Leaves were not wilted at the end of chilling treatment in either cultivars, but cv Suyo leaves exhibited a slightly water-soaked appearance. Chilling injury symptoms of leaves, marginal and inner necrosis, developed after they were transferred to rewarming conditions. With PA-untreated plants, these chilling injury symptoms were considerably more severe in cv Suyo than cv Jinchun No. 3 (Table I). Chilled cv Suyo also had a higher malondialdehyde (MDA) content in leaves than chilled cv Jinchun No. 3, showing that chill-induced peroxidation of leaf membrane lipids was more severe in the former. Leaf wilting that occurred upon rewarming was also more severe in cv Suyo. Wilting was recovered within 1.5 h of rewarming in cv Jinchun No. 3 and 4 h in cv Suyo.
Table I.
Effect of exogenous PA pretreatment to leaves on the chilling injury of cucumber leaves
| Cultivar | Treatment | Necrotic Leaf Area | Leaf MDA Content |
|---|---|---|---|
| % | nmol g−1 fresh wt | ||
| Suyo | Water | 16.1 a | 18.5 a |
| 1 mm Put | 9.8 b | 9.5 b | |
| 1 mm Spd | 6.3 c | 8.5 b | |
| 1 mm Spm | 9.5 b | 8.7 b | |
| Jinchun No. 3 | Water | 6.8 a | 9.6 a |
| 1 mm Put | 6.0 a | 7.9 ab | |
| 1 mm Spd | 5.0 a | 6.3 b | |
| 1 mm Spm | 5.6 a | 8.7 ab |
Plants were treated with PAs or water and exposed to light at 28°C for 12 h before chilling at 3°C in the dark for 24 h. Chilling injury was assessed after 24 h of rewarming. Means with different letters within a column are significantly different at P < 0.05 based on Duncan’s multiple range test.
Pretreatment of cv Suyo with PAs at 1 mm alleviated chilling injury, as judged by a low level of necrotic area and MDA content in the first leaves (Table I). Leaf wilting was also alleviated by PA pretreatment. Spd showed greater effects than Spm and Put, the latter two PAs having similar effects. Furthermore, Spd promoted the growth of chilled cv Suyo plants in a greenhouse; Spd-pretreated plants had only 14.3% lower dry mass than unchilled plants after 7 d, whereas dry mass was 42.5% lower in Spd-untreated plants. On the other hand, PAs were almost ineffective in alleviating chilling injury of cv Jinchun No. 3, although Spd-pretreated leaves had significantly lower MDA than control.
Effect of Methylglyoxal-Bis-(Guanylhydrazone) (MGBG) Pretreatment with or without PAs on PA Contents and Chilling Injury in cv Jinchun No. 3 Leaves
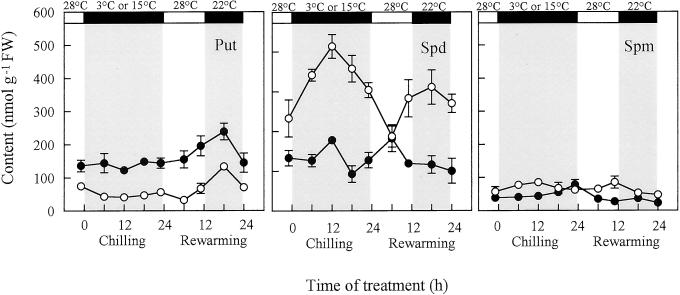
The above results are indicative of possible involvement of Spd in the high chilling tolerance of cv Jinchun No. 3 as compared with cv Suyo. If this is the case, treatment of a Spd biosynthesis inhibitor to cv Jinchun No. 3 before chilling may reduce its chilling tolerance. To confirm this hypothesis, 5 mm MGBG, a SAMDC inhibitor, was sprayed onto cv Jinchun No. 3 plants 2 h before chilling. PA analysis on MGBG-pretreated leaves revealed that MGBG canceled the chill-induced increase in free Spd content during chilling and rewarming (Fig. 3). MGBG-pretreated leaves had a much higher Put content than untreated leaves before and during chilling. The increase of Put content during rewarming was not canceled by MGBG pretreatment.
Figure 3.
Effect of 5 mm MGBG pretreatment to cv Jinchun No. 3, 2 h before chilling, on changes with time of free PA contents in chilled leaves during chilling and rewarming. Temperature and light conditions are indicated on top of the panels. Error bars indicate se (n = 3). ●, Pretreated with MGBG; ○, control (redrawn from Fig. 1).
Chilling injury of cv Jinchun No. 3 leaves was enhanced significantly by MGBG pretreatment to a similar extent to cv Suyo leaves, as judged by a similar level of leaf necrosis and MDA content (Table II). Leaf wilting was also promoted by MGBG pretreatment. These effects of MGBG were reversed by the concomitant treatment with 3 mm Spd. However, Put failed to reverse the detrimental effects of MGBG.
Table II.
Effect of MGBG pretreatment to leaves with or without PAs on the chilling injury of cv Jinchun No. 3 leaves
| Treatment | Necrotic Leaf Area | Leaf MDA Content |
|---|---|---|
| % | nmol g−1 fresh wt | |
| Water | 6.4 c | 8.3 c |
| 5 mm MGBG | 15.6 a | 17.2 a |
| 5 mm MGBG + 2 mm Put | 14.0 a | 15.9 a |
| 5 mm MGBG + 2 mm Spd | 8.7 b | 10.6 b |
Plants were treated with MGBG with or without PAs or with water and exposed to light at 28°C for 2 h before chilling at 3°C in the dark for 24 h. Chilling injury was assessed after 24 h of rewarming. Means with different letters within a column are significantly different at P < 0.05 based on Duncan's multiple range test.
Effect of Spd and MGBG Pretreatment on NADPH Oxidation, Hydrogen Peroxide Content, and NADPH-Dependent Superoxide Generation in Chilled Leaves and Microsomes
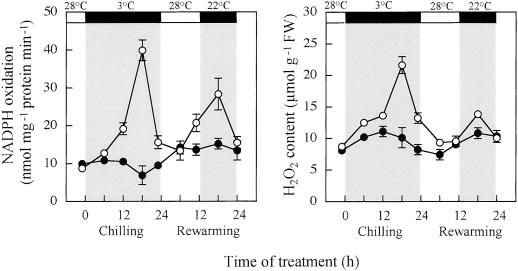
Effects of 1 mm Spd pretreatment to cv Suyo, 12 h before chilling, on the rate of NADPH oxidation in the 600 g of supernatant of leaf homogenate and leaf hydrogen peroxide content were investigated. In Spd-untreated leaves the NADPH oxidation rate increased markedly in the late chilling period, reaching a peak after 18 h and then decreased rapidly (Fig. 4A). During rewarming it increased again with a peak after 18 h of rewarming. In contrast, Spd-pretreated leaves showed much lower rates during chilling and rewarming. Hydrogen peroxide content in both of Spd-untreated and Spd-pretreated leaves changed with time in a similar manner to the NADPH oxidation rate, during the chilling and rewarming periods (Fig. 4B).
Figure 4.
Effect of 1 mm Spd pretreatment to cv Suyo, 12 h before chilling, on changes with time of NAPDH oxidation rate in 600 g of supernatants of leaf homogenate (A) and leaf hydrogen peroxide content (B) during chilling and rewarming. Temperature and light conditions are indicated on top of the panels. Error bars indicate se (n = 3). ●, Pretreated with Spd; ○, control.
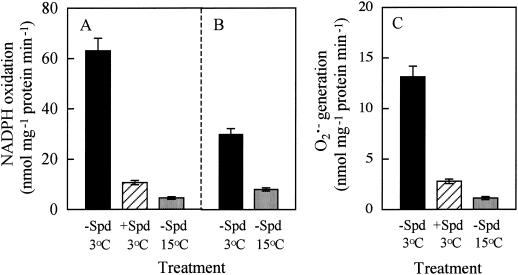
Since microsomes are rich in NADPH-preferring oxidases, we investigated the effect of chilling on the NADPH oxidation and NADPH-dependent superoxide generation activities of leaf microsomes. It was found that microsomes in chilled cv Suyo leaves exhibited a marked increase in the NADPH oxidation activity 16 h after chilling (Fig. 5A) and also 18 h after rewarming following chilling for 24 h (Fig. 5B). The NADPH-dependent superoxide generation activity of microsomes also increased appreciably 16 h after chilling (Fig. 5C). This chill-induced increase of microsomal NADPH oxidation and superoxide generation activities did not occur in Spd-pretreated leaves (Fig. 5, A and C). On the contrary, microsomes in chilled cv Jinchun No. 3 leaves had a slightly higher NADPH oxidation activity than those in control 16 h after chilling (Fig. 6). However, MGBG pretreatment caused a substantial increase in the NADPH oxidation activity of chilled leaf microsomes. The NADPH oxidation by microsomes prepared from cv Suyo leaves chilled for 16 h was completely inhibited by diphenylene iodonium (40 μm), and significantly stimulated by flavin adenine dinucleotide and flavin mononucleotide (25 μm each), added to the reaction mixture (data not shown).
Figure 5.
NADPH oxidation (A and B) and NADPH-dependent superoxide generation (C) activities of cv Suyo leaf microsomes as affected by chilling with or without 1 mm Spd pretreatment. Microsomes were prepared 16 h after chilling (A and C) or 18 h after rewarming following chilling for 24 h (B). Error bars indicate se (n = 4).
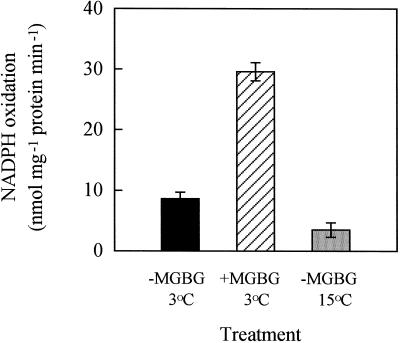
Figure 6.
NADPH oxidation activity of cv Jinchun No. 3 leaf microsomes as affected by chilling with or without 5 mm MGBG pretreatment. Microsomes were prepared 16 h after chilling. Error bars indicate se (n = 4).
DISCUSSION
Free Spd content in leaves increased markedly during chilling and rewarming in chilling-tolerant cv Jinchun No. 3, but not in chilling-sensitive Suyo (Fig. 1). In addition, pretreatment of cv Suyo with PAs alleviated chilling injury, with Spd being most effective (Table I). Pretreatment of cv Jinchun No. 3 with a PA biosynthesis inhibitor MGBG inhibited Spd accumulation in chilled leaves (Fig. 3) and enhanced chilling injury, which was reversed by the concomitant treatment with Spd (Table II). These results support the hypothesis that Spd is involved in the chilling tolerance of cucumber cultivars. Lee et al. (1995) have shown that the increase of abscisic acid content is responsible for the chill-induced increase of Put content in rice plants. It is known that abscisic acid and possibly ethylene are involved in the chilling tolerance of plants (Rikin and Richmond, 1976; Ciardi et al., 1997; Morgan and Drew, 1997). However, Spd pretreatment did not affect the contents of abscisic acid and 1-aminocyclopropane-1-carboxylic acid, a precursor of ethylene, in chilled leaves of cv Suyo, and abscisic acid content did not increase in leaves of cv Jinchun No. 3 during chilling (data not shown). These results exclude the possibility that these hormones mediate the Spd effect.
As compared with Spd, contribution of Spm to the chilling tolerance of cv Jinchun No. 3 seems small because the increase of Spm contents in chilled leaves was much smaller (Fig. 1). However, pretreatment of Spm at the same concentration as Spm was effective in alleviating chilling injury of cv Suyo (Table I). Kramer and Wang (1989) observed a great increase of Spm contents in cold-hardened zucchini squash fruits during cold storage and direct treatment of fruits with Spm prior to cold storage resulting in reduced chilling injury. Thus Spm may have the potential for counteracting a mechanism involved in chilling injury of plants. It is probable that functioning of Spm may depend on the level of its increase upon chilling.
Guye et al. (1986), Nadeau et al. (1987), and Lee et al. (1997) have shown that Put is primarily responsible for the chilling tolerance of bean, wheat, and rice, respectively. In cucumber however, Put does not seem to play a role by itself because MGBG promoted chilling injury of cv Jinchun No. 3 while causing a substantial increase in Put (Table II; Fig. 3) and exogenous Put failed to reverse the MGBG effect (Table II). Amelioration of chilling injury of cv Suyo by exogenous Put (Table I) could be attributable to its conversion to Spd before chilling treatment.
There is little doubt that PA-biosynthetic enzyme activities have mediated the difference of PA contents in chilled leaves between cv Jinchun No. 3 and cv Suyo. In cv Jinchun No. 3, ADC activity did not increase during chilling, whereas ADC and ODC activities increased during rewarming (Fig. 2). Generally, ADC is considered to relate more closely to stress reactions of plants than is ODC (Galston, 1983; Lee et al., 1997). However, Kramer and Wang (1990) showed that elevated activity of ODC, and not ADC, was responsible for chill-induced Put accumulation in zucchini squash fruits. In our study the nature of stress during rewarming can be different from that during chilling. Hence, stress responsive Put-biosynthetic enzymes could be different with plant species and kinds of stresses.
In spite of the transient increase in ODC activity during chilling, there was no significant accumulation of free Put in cv Jinchun No. 3 leaves (Figs. 1 and 2). The increase of the sum of Put, Spd, and Spm during chilling may well be ascribed to enhanced ODC activity. Thus it is most likely that Put has been metabolized quickly to Spd and/or Spm during chilling. However, we cannot rule out the possibility that free Put was converted to conjugated and/or bound forms. The protective effect of PAs against the damage of superoxides has been considered to depend on their prior conversion to perchloric acid-soluble conjugated PAs (Bouchareau et al., 1999). Also, direct binding of free PAs to membrane lipids may contribute to membrane stabilization under stressful conditions (Roberts et al., 1986). Further study is needed concerning the distribution of various forms of PAs in leaf cells as affected by chilling and exogenous PA application.
Synthesis of Spd, catalyzed by Spd synthase (Slocum, 1991), is regulated mainly at the level of SAMDC (Greenburg and Cohen, 1985; Noh and Minocha, 1994). Spd content in chilled cv Jinchun No. 3 leaves changed with time in close parallel to SAMDC activity (Fig. 3). Thus it seems that SAMDC plays an important role in regulating the chill-induced Spd accumulation in chilling-tolerant cucumber cultivars. Rorat et al. (1997) recently found that cold storage of chilling-tolerant potato tubers induced the expression of mRNAs for two different SAMDC isozymes. To clarify whether the increased activity of SAMDC in chilled cv Jinchun No. 3 leaves involves chill-induced gene expression deserves further investigation.
Chilled cv Suyo showed enhanced NADPH oxidation in 600 g of supernatant of leaf homogenate, once during chilling and again during rewarming (Fig. 4). This pattern was quite similar to that obtained in the previous study with 5,000 g of supernatant (Shen et al., 1999a). It is known that microsomes are rich in NADPH-preferring oxidases (Goeptar et al., 1995), so we determined the NADPH oxidation activity of leaf microsomes. The results showed that it increased markedly in cv Suyo during chilling and also rewarming as compared with control, whereas in cv Jinchun No. 3 the increase was slight (Figs. 5 and 6). The NADPH oxidation by microsomes was completely inhibited by a micromolar concentration of diphenylene iodonium and stimulated by flavin nucleotides, indicating the NADPH oxidation was mediated by NADPH oxidases (Goeptar et al., 1995). Thus we conclude that chilling induces the activation of NADPH oxidases of leaf microsomes in chilling-sensitive cucumber cultivars.
Activation of NADPH oxidases is known to elicit a massive generation of superoxide anions in various biological membranes (Vianello and Marci, 1991; Ogawa et al., 1997), which causes peroxidation of membrane lipids (Asada et al., 1977). Increases of ROS generation and resultant peroxidation of membrane lipids in chilled leaves are common to chilling-sensitive species and cultivars (Omran, 1980; Prasad et al., 1994; Saruyama and Tanida, 1995). Accumulation of hydrogen peroxide in cv Suyo leaves during chilling (Fig. 4) is demonstrative of the actual increase of ROS generation in chilled cv Suyo leaves. Hydrogen peroxide content decreased significantly during the late chilling to the early rewarming periods when the necrotic lesions began to develop. In the previous study this decrease of hydrogen peroxide was accompanied by a marked increase of highly reactive hydroxyl radical generation (Shen et al., 1999a), which is considered primarily responsible for lipid peroxidation (Halliwell and Gutteridge, 1990). Thus it seems most likely that activation of superoxide-generating NADPH oxidases takes an important part in the chill-induced ROS generation and thus the chilling injury of plants.
Spd pretreatment to cv Suyo suppressed the chill-induced increase in not only NADPH oxidase activity, but also NADPH-dependent superoxide generation activity of leaf microsomes (Fig. 5). Accumulation of hydrogen peroxide and MDA in intact leaves was also prevented (Fig. 4; Table I). On the other hand, MGBG pretreatment to chilled cv Jinchun No. 3 caused a substantial increase in NADPH oxidase activity of leaf microsomes (Fig. 6) and MDA content in intact leaves (Table II). These results strongly suggest that Spd acts as a cellular membrane protectant against chill-induced lipid peroxidation through prevention of superoxide-generating NADPH oxidase activation. Kitada et al. (1979) and Ogata et al. (1996) have observed that Spd and Spm inhibit NADPH oxidase activity in rat liver microsomes and human neutrophils, respectively. Kramer and Wang (1989) indicated that PAs could protect membrane lipids from chill-induced peroxidation. To our knowledge the present report is the first to demonstrate that Spd can counteract the chill-induced activation of NADPH oxidases in plant microsomes. Although the cause of increased NADPH oxidase activity and hydrogen peroxide generation in chilled cv Suyo leaves during the later rewarming period (Figs. 4 and 5) is not known, our results indicate that Spd can also counteract a kind of oxidative stresses imposed on chilled leaves during rewarming. The mode of Spd action on the NADPH oxidase activity, together with the mechanism of chill-induced activation of the enzyme, needs further investigation.
In summary, the present study provides evidence that PAs, Spd in particular, are involved in the chilling tolerance of cucumber cultivars. The primary function of Spd is probably the inhibition of chill-induced activation of the microsomal NADPH oxidases and consequential ROS generation. The superoxide-generating NADPH oxidases and also PAs are localized in various cellular organelles (Babior et al., 1997; Kumer et al., 1997). Thus investigations into the subcellular distribution of the increased oxidase activity and Spd in chilled cucumber leaves may help better the understanding of the mechanism of chilling injury and the role of Spd in the chilling tolerance of plants.
MATERIALS AND METHODS
Plant Material
Cucumber (Cucumis sativus) cv Jinchun No. 3 (a Chinese cultivar) and cv Suyo (a Japanese cultivar) were used in this study. cv Jinchun No. 3 is more chilling-tolerant than cv Suyo (Shen et al., 1999a). The seedlings, which were raised in a greenhouse, were transplanted at the cotyledonary stage to clay pots containing commercial nursery soil. They were then grown in a growth chamber kept at 28°C/22°C (day/night) under a 12-h photoperiod. Light was provided by metal halide lamps with 250 μmol m−2 s−1 photosynthetic photon flux density on plant canopy. Aerial humidity fluctuated between 60% and 75% relative humidity (RH). Plants with the first expanded leaves were used as experimental materials. At least three different plants were used for each of the following determinations.
Chilling Treatment and Chilling Injury Assessment
For chilling treatment, one group of plants was moved at the end of the day from the growth chamber to a dark incubator kept at 3°C ± 0.2°C and near 100% RH for 24 h. Another group of plants, placed for 24 h in a dark incubator kept at 15°C ± 1°C and between 60% and 75% RH, served as the control. Following treatments, the pots were dipped in a water bath (approximately 20°C) for 10 min to raise the soil temperature and the plants were transferred to the initial growth chamber for another 24 h (rewarming).
The first leaves were sampled periodically during chilling and rewarming. They were then frozen in liquid nitrogen and stored at −80°C in tightly sealed plastic vials until analysis except NADPH oxidation and NADPH-dependent superoxide generation rate determinations that were conducted immediately after sampling. Chilling injury was assessed in terms of the necrotic area percentage of the first leaves and their MDA content at the end of the rewarming period. MDA content has been used as an indication of lipid peroxidation due to increased ROS generation (Seel et al., 1991). MDA was determined by a color reaction with thiobarbituric acid (Heath and Packer, 1968).
PAs and PA Biosynthesis Inhibitor Pretreatment
For PA pretreatment, leaves were sprayed with 1 mm Put, Spd, or Spm (hydrochloride salts) 12 h before chilling. In experiments with a PA biosynthesis inhibitor leaves were sprayed with 5 mm MGBG, the SAMDC inhibitor, with or without 2 mm Put or Spd, 2 h before chilling. The solutions were all supplemented with 0.01% (v/v) Tween 20 as a detergent. Control plants for these treatments were sprayed with 0.01% (v/v) Tween 20 solution.
PA Analysis
Free PAs were quantified by the method of Flores and Galston (1982). Leaves were homogenized in 0.5 m perchloric acid (4 mL g−1 fresh weight). The homogenate was centrifuged at 40,000g for 20 min, and the supernatant was passed through a cation exchange column (50W-X4, H+ form, Bio-Rad, Hercules, CA) to remove amino acids and neutral substances (Corbin et al., 1989). After washing the column successively with 0.7 m NaCl in 100 mm phosphate buffer (pH 8.0), water, and 1 m HCl, PAs were eluted with 6 m HCl. They were analyzed as benzoylated derivatives via HPLC equipped with a UV detector. Inertsil ODS-2 (4.6 × 250 mm, GL Science, Tokyo) was used as a column and 58% (v/v) methanol in 1% (v/v) acetic acid was used as an isocratic eluting solvent.
Assay of ADC, ODC, and SAMDC
Activities of ADC, ODC, and SAMDC were determined by the method of Lee (1997), with minor modifications. Leaves were homogenized in a chilled mortar with 25 mm potassium phosphate (pH 8.0), containing 0.1 mm EDTA, 0.1 mm phenylmethylsulfonyl fluoride, and 25 mm ascorbic acid. After centrifuging at 30,000g for 15 min, the homogenate was added to a reaction vial containing l-[U-14C]Arg in 200 mm Tris [tris(hydroxymethyl)aminomethane]-HCl (pH 8.5), l-[U-14C]Orn in 200 mm Tris-HCl (pH 8.0), or S-adenosyl-l-(carboxyl-14C) Met in 200 mm potassium phosphate (pH 7.5), for ADC, ODC, and SAMDC assays, respectively. After incubation for 30 min at 37°C, the reaction was stopped by adding 10% (w/v) trichloroacetic acid. 14CO2 was recovered in 2 m NaOH in a center well of the reaction vial and the radioactivity was counted by a liquid scintillation counter. Nonenzymatic decarboxylation of radioactive substrates was subtracted.
Assay of NADPH Oxidation and NADPH-Dependent Superoxide Generation Rates and Hydrogen Peroxide Content
Leaves were homogenized with 50 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]-KOH‘ (pH 7.8) containing 250 mm Suc and 0.1 mm EDTA. The homogenate was filtered through Miracloth (Calbiochem, San Diego) and the filtrate was centrifuged at 600g for 15 min. To obtain microsomes, the 600 g of supernatant was centrifuged at 42,000g for 20 min and the resultant supernatant at 140,000g for 1 h. The final pellet was suspended in the above buffer to obtain microsomes at about 1 mg protein mL−1. To determine NADPH oxidation rate, an aliquot of the 600 g of supernatant or microsomal preparations was added to a reaction mixture consisting of 50 mm HEPES-KOH (pH 7.8), 100 μm EDTA, and 1 μm KCN in a final volume of 1 mL (Pinton et al., 1994). KCN was added to block peroxidase activity. Reactions were initiated by the addition of 100 μm NADPH. The NADPH oxidation rate was based on a decrease of A340 after incubation at 30°C for 5 min. The NADPH-dependent superoxide-generation rate in microsomes was determined by measuring the rate of superoxide dismutase-inhibitory ferricytochrome c reduction in the presence of NADPH (Cakmak and Marschner, 1988). The reaction mixture was 50 mm HEPES-KOH (pH 7.8), 100 μm EDTA, 1 μm KCN, and 0.75 mm ferricytochrome c in a final volume of 1 mL. Hydrogen peroxide in leaves was extracted in 5% (w/v) trichloroacetic acid and quantified by the method of Brennan and Frenkel (1977). Protein was determined by the method of Bradford (1976).
LITERATURE CITED
- Asada K, Takahashi M, Tanaka K, Nakano N. Formation of active oxygen and its fate in chloroplasts. In: Hayashi O, Asada K, editors. Biological and Medical Aspects of Active Oxygen. Tokyo: Japan Science Societies Press; 1977. pp. 45–63. [Google Scholar]
- Babior BM, Benna JE, Chanock SJ, Smith RM. The NADPH oxidase of leukocytes: the respiratory burst. In: Scandalios JG, editor. Oxidative Stress and the Molecular Biology of Antioxidant Defenses. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 737–783. [Google Scholar]
- Bouchereau A, Aziz A, Larher F, Martin-Tanguy J. Polyamines and environmental challenges: recent development. Plant Sci. 1999;140:103–125. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brennan T, Frenkel C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 1977;59:411–416. doi: 10.1104/pp.59.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakmak I, Marschner H. Zinc-dependent changes in ESR signals, NADPH oxidase and plasma membrane permeability in cotton roots. Physiol Plant. 1988;73:182–186. [Google Scholar]
- Ciardi JA, Deikman J, Orzolek MD. Increased ethylene synthesis enhances chilling tolerance in tomato. Physiol Plant. 1997;101:333–340. [Google Scholar]
- Corbin JL, Marsh BH, Peters GA. An improved method for analysis of polyamines in plant tissue by precolumn delivatization with ophthalaldehide and separation by high performance liquid chromatography. Plant Physiol. 1989;90:434–439. doi: 10.1104/pp.90.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipierro S, Leonardis SD. The ascorbate system and lipid peroxidation in stored potato (Solanum tuberosum L.) tubers. J Exp Bot. 1997;48:779–783. [Google Scholar]
- Flores HE. Polyamines and plant stress. In: Alscher RG, Cumming JR, editors. Stress Responses in Plants: Adaptation and Acclimation Mechanisms. New York: Wiley-Liss; 1990. pp. 217–239. [Google Scholar]
- Flores HE, Galston AW. Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol. 1982;69:701–706. doi: 10.1104/pp.69.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galston AW. Polyamines as modulators of plant development. BioScience. 1983;33:382–388. [Google Scholar]
- Goeptar AR, Scheerens H, Vermeulen NPE. Oxygen and xenobiotic reductase activities of cytochrome P450. Crit Rev Toxicol. 1995;25:25–65. doi: 10.3109/10408449509089886. [DOI] [PubMed] [Google Scholar]
- Greenburg ML, Cohen SS. Dicycloheximine-induced shift of biosynthesis from spermidine to spermine in plant protoplasts. Plant Physiol. 1985;78:568–575. doi: 10.1104/pp.78.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guye MG, Vigh L, Wilson JM. Polyamine titre in relation to chilling-sensitivity in Phaseolus sp. J Exp Bot. 1986;37:1036–1043. [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Ed 2. Oxford: Clarendon Press; 1990. [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplast: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hodgson RA, Raison JK. Superoxide production by thylakoids during chilling and its implication in the susceptibility of plants to chilling-induced photoinhibition. Planta. 1991;183:222–228. doi: 10.1007/BF00197792. [DOI] [PubMed] [Google Scholar]
- Kitada M, Igarashi K, Hirose S, Kitagawa H. Inhibition by polyamines of lipid peroxide formation in rat liver microsomes. Biochem Biophys Res Commun. 1979;87:388–394. doi: 10.1016/0006-291x(79)91808-4. [DOI] [PubMed] [Google Scholar]
- Kodama H, Hamada T, Horiguchi G, Nishimura M, Iba K. The enhancement of cold tolerance by expression of a gene for chloroplast ω-desaturase in transgenic tobacco. Plant Physiol. 1994;105:601–605. doi: 10.1104/pp.105.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer GF, Wang CY. Correlation of reduced chilling injury with increased spermine and spermidine levels in zucchini squash. Physiol Plant. 1989;76:479–484. [Google Scholar]
- Kramer GF, Wang CY. Effects of chilling and temperature preconditioning on the activity of polyamine biosynthetic enzymes in zucchini. J Plant Physiol. 1990;136:115–119. [Google Scholar]
- Kumer A, Altabella T, Taylor MA, Tiburcio AF. Recent advances in polyamine research. Trend Plant Sci. 1997;2:124–130. [Google Scholar]
- Lee TM. Polyamine regulation of growth and chilling tolerance of rice (Oryza sativa L.) roots cultured in vitro. Plant Sci. 1997;122:111–117. [Google Scholar]
- Lee TM, Lur HS, Chu C. Abscisic acid and putrecine accumulation in chilling-tolerant rice cultivars. Crop Sci. 1995;35:502–508. [Google Scholar]
- Lee TM, Lur HS, Chu C. Role of abscisic acid in chilling tolerance of rice (Oryza sativa L.) seedlings: II. Modulation of free polyamine levels. Plant Sci. 1997;126:1–10. [Google Scholar]
- Lyons JM, Raison JK, Steponkus PL. The plant membrane in response to low temperature: an overview. In: Lyons JM, Graham D, Raison JK, editors. Low Temperature Stress in Crop Plants: The Role of the Membrane. London: Academic Press; 1979. pp. 1–24. [Google Scholar]
- Morgan PW, Drew MC. Ethylene and plant response to stress. Physiol Plant. 1997;100:620–630. [Google Scholar]
- Murata N, Ishizaki-Nishizawa O, Higashi S, Hayashi H, Tasaka Y, Nishida I. Genetically engineered alteration in the chilling sensitivity of plants. Nature. 1992;356:710–713. [Google Scholar]
- Nadeau P, Delaney S, Chouinard L. Effect of cold hardening on the regulation of polyamine levels in wheat (Triticum aestivum L.) and alfalfa (Medicago sativa L.) Plant Physiol. 1987;84:73–77. doi: 10.1104/pp.84.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida I, Murata N. Chilling sensitivity in plants and cyanobacteria: the crucial contribution of membrane lipid. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:541–568. doi: 10.1146/annurev.arplant.47.1.541. [DOI] [PubMed] [Google Scholar]
- Noh BW, Minocha SC. Expression of a human S-adenosylmethionine decarboxylase cDNA in transgenic tobacco and its effects on polyamine biosynthesis. Transgen Res. 1994;3:26–35. doi: 10.1007/BF01976024. [DOI] [PubMed] [Google Scholar]
- Ogata K, Nishimoto N, Uhlinger DJ, Igarashi K, Takeshita M. Spermine suppresses the activation of human neutrophil NADPH oxidase in cell-free and semi-recombinant systems. Biochem J. 1996;313:549–554. doi: 10.1042/bj3130549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K, Kanematsu S, Asada K. Generation of superoxide anion and localization of CuZn-superoxide dismutase in the vascular tissue of spinach hypocotyls: their association with lignification. Plant Cell Physiol. 1997;38:1118–1126. doi: 10.1093/oxfordjournals.pcp.a029096. [DOI] [PubMed] [Google Scholar]
- Omran RG. Peroxide levels and the activities of catalase, peroxidase, and indoleacetic acid oxidase during and after chilling cucumber seedlings. Plant Physiol. 1980;65:407–408. doi: 10.1104/pp.65.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton R, Cakmak I, Marschner H. Zinc deficiency enhanced NAD(P)H-dependent superoxide radical production in plasma membrane vesicles isolated from roots of bean plants. J Exp Bot. 1994;45:45–50. [Google Scholar]
- Prasad TK, Anderson MD, Martin BA, Stewart CR. Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell. 1994;6:65–74. doi: 10.1105/tpc.6.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison JK, Chapman EA. Membrane phase changes in chilling sensitive Vigna radiata and their significance to growth. Aust J Plant Physiol. 1976;3:291–299. [Google Scholar]
- Rikin A, Richmond AE. Amelioration of chilling injuries in cucumber seedlings by abscisic acid. Physiol Plant. 1976;38:95–97. [Google Scholar]
- Roberts DR, Dumdroff EB, Thompson JE. Exogenous polyamines alter membrane fluidity in bean leaves: a basis for potential misinterpretation of their true physiological role. Planta. 1986;167:395–401. doi: 10.1007/BF00391345. [DOI] [PubMed] [Google Scholar]
- Rorat T, Irzykowski W, Grygorowicz WJ. Identification and expression of novel cold induced genes in potato (Solanum sogorandinum) Plant Sci. 1997;124:69–78. [Google Scholar]
- Saruyama H, Tanida M. Effect of chilling on activated oxygen-scavenging enzymes in low temperature-sensitive and -tolerant cultivars of rice (Oryza sativa L.) Plant Sci. 1995;109:105–113. [Google Scholar]
- Seel W, Hendry G, Atherton N, Lee J. Radical formation and accumulation in vivo, in desiccation tolerant and intolerant mosses. Free Rad Res Commun. 1991;15:133–141. doi: 10.3109/10715769109049133. [DOI] [PubMed] [Google Scholar]
- Shen W, Nada K, Tachibana S. Oxygen radical generation in chilled leaves of cucumber (Cucumis sativus L.) cultivars with different tolerance to chilling temperature. J Japan Soc Hortic Sci. 1999a;68:780–787. [Google Scholar]
- Shen W, Nada K, Tachibana S. Effect of chilling treatment on enzymic and nonenzymic antioxidant activities in leaves of chilling tolerant and chilling sensitive cucumber (Cucumis sativus L.) cultivars. J Japan Soc Hortic Sci. 1999b;68:967–973. [Google Scholar]
- Slocum RD. Polyamine biosynthesis in plants. In: Slocum RD, Flores HE, editors. Biochemistry and Physiology of Polyamine in Plants. Boca Raton, FL: CRC Press; 1991. pp. 23–40. [Google Scholar]
- Smith TA. Polyamines. Annu Rev Plant Physiol. 1985;36:117–143. [Google Scholar]
- Tadolini B. Polyamine inhibition of lipoperoxidation: the influence of polyamines on iron oxidation in the presence of compounds mimicking phospholipid polar heads. Biochem J. 1988;249:33–36. doi: 10.1042/bj2490033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadolini B, Cabrini L, Landi L, Varani E, Pasquali P. Polyamine binding to phospholipid vesicles and inhibition of lipid peroxidation. Biochem Biophys Res Commun. 1984;122:550–555. doi: 10.1016/s0006-291x(84)80068-6. [DOI] [PubMed] [Google Scholar]
- Vianello A, Marci F. Generation of superoxide anion and hydrogen peroxide at the surface of plant cells. J Bioener Biomembr. 1991;23:409–423. doi: 10.1007/BF00771012. [DOI] [PubMed] [Google Scholar]
- Walker MA, McKersie BD. Role of ascorbate-glutathione antioxidant system in chilling resistance of tomato. J Plant Physiol. 1993;141:234–239. [Google Scholar]