Abstract
Simultaneous changes in several physiological factors may contribute to the large pharmacokinetic (PK) variability of vancomycin. This study was designed to systematically characterize the effects of multiple physiological factors to the altered PK of vancomycin observed in special populations. A vancomycin physiologically based pharmacokinetic (PBPK) model was developed as a PK simulation platform to quantitatively assess the effects of changes in physiologies to the PK profiles. The developed model predicted the concentration‐time profiles in healthy adults and diseased patients. The implementation of developmental changes in both renal and non‐renal elimination pathways to the pediatric model improved the predictability of vancomycin clearance. Simulated PK profiles with a 50% decrease in cardiac output (peak plasma concentration (Cmax), 59.9 ng/mL) were similar to those observed in patients before bypass surgery (Cmax, 55.1 ng/mL). The PBPK modeling of vancomycin demonstrated its potential to provide mechanistic insights into the altered disposition observed in patients who have changes in multiple physiological factors.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ The contribution of multiple physiological factors, which contributes to the large PK variability of vancomycin, has not been systematically characterized.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ A PBPK model could be used as a PK simulation platform to quantitatively assess the effects of changes in physiological parameters in special populations for vancomycin disposition.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ Simultaneous changes in covariates within a particular clinical situation may be further investigated using PBPK modeling. For example, the simulated vancomycin PK profiles in adults with a 50% decrease in cardiac output could explain clinical observations in adult patients before bypass surgery. Pediatric PBPK models implement developmental changes in renal and nonrenal elimination pathways that could predict vancomycin disposition observed in pediatric clinical studies.
HOW MIGHT THIS CHANGE DRUG DISCOVERY, DEVELOPMENT, AND/OR THERAPEUTICS?
☑ The PBPK models can provide a justification of changes in the PK profile of patients with multiple alterations in physiological factors (e.g., renal function, hemodynamic functions, and maturation). The complementary utility of PBPK modeling with clinical confirmation in special patient populations can be used to propose suitable dosing regimens to achieve safe and effective vancomycin concentrations that will improve clinical outcome.
Vancomycin is the cornerstone drug for serious infections caused by gram‐positive bacteria resistant to β‐lactam antibiotics. Vancomycin exposure–response is commonly predicted by the ratio of the area under the curve (AUC) to the minimum inhibitory concentration (MIC).1, 2 Accordingly, management of this pharmacokinetic/pharmacodynamic (PK/PD) relationship has been associated with clinical success. Vancomycin is eliminated primarily by glomerular filtration; therefore, alterations in renal function have an impact on the PKs. For example, the PK profiles of patients with impaired or immature renal functions differ from those in healthy adults.2, 3 Creatinine clearance (CCr) is often used as an informative predictor to describe the degree of renal impairment.2 It was reported that ∼85% of the variability in vancomycin clearance (CL) was explained by creatinine clearance in adult patients with various degrees of impaired renal function,4 which may vary in patients with different diagnoses and disease states. A patient's body weight and/or age is also used to indicate age‐dependent developmental changes in renal function.3
These factors are often implemented as covariates into vancomycin population PK models. However, these models do not fully explain the variability among patients. For example, interindividual variabilities were observed in PK parameters (e.g., central volume of distribution, clearance, etc.) for adult patients, which ranged from ∼20–60% of the relative standard error; however, the variability may rely on the patient populations, model structure, and the covariates.5, 6 The remaining unexplained variability may be influenced by other patient‐specific physiological parameters. Vancomycin is administered to critically ill patients with multiple underlying diseases, such as heart failure, chronic renal disease, and cirrhosis of the liver. It is also administered postoperatively for wound infection treatment.7, 8 Thus, multiple pathology and resultant changes to normal physiology may be the reason for the unexplained PK variability among patients.
Physiologically based pharmacokinetic (PBPK) modeling and simulation has been used as one of the tools that can quantitatively address the effect of multiple physiological changes in drug disposition.9 In this study, a PBPK model of vancomycin was developed as a PK simulation platform, in which renal clearance was implemented as the primary elimination pathway (∼85% of the total clearance), in order to provide mechanistic insight into factors contributing to the variability in vancomycin PK among patients with different diagnoses and disease states. The developed model was evaluated by comparing the simulated PK profiles with clinically observed data. Vancomycin is used for the treatment of bacterial infections, which may include inflammation in several populations, such as heart failure and pediatric patients. It has been proposed that such conditions may alter vancomycin PK due to changes in cardiac output, tissue penetration, and serum protein concentrations; however, quantitative approaches for these patients/conditions are limited. Therefore, in this study, sensitivity analyses were conducted to assess the impact of these changes quantitatively.
METHODS
The PBPK model was initially evaluated by comparing simulation results with clinical PK observations in healthy adults (Figure 1). The evaluated PBPK model was then applied to other populations for which vancomycin PK data were available in order to identify the changes in drug disposition and elimination pathways. Consequently, sensitivity analyses were conducted to determine the impact of cardiac output, tissue penetration, and free fraction levels on the PK profiles of vancomycin.
Figure 1.
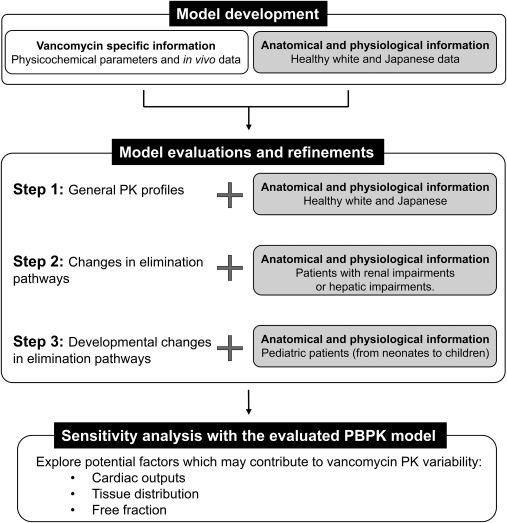
Schematic overview on the workflow of physiologically based pharmacokinetic (PBPK) model development and evaluation.
PBPK model development in adults
Simcyp simulator software (Simcyp, Sheffield, UK; version 16 for pediatric simulations and version 14 for the others) was used to develop a vancomycin PBPK model. Vancomycin‐specific parameters, such as molecular weight, pKa, in vitro protein binding, and in vivo renal clearance, were combined with anatomic and physiological system data. According to the report by Johnson and Rostami‐Hodijegan,10 the simulator allows for specific clinical trial designs to be replicated based on age, sex, dose, number of doses, and route of administration. Details of the clinical trials simulated in this study are summarized in Supplementary Tables S1–S4. Virtual subjects were generated based on reported demographic information through a correlated Monte‐Carlo approach, which ensures that realistic virtual subjects are generated. For example, in the model, height is generated based on age, and weight is calculated based on height. This strategy is designed to prevent implausible subjects with unrealistic weights in relation to heights from being created. Similarly, the organ volumes within the PBPK model are correlated based on body size. The integration of drug, system, and trial data is described in the reports by Jamei et al.11, 12 In this study, the relevant vancomycin‐specific parameters were collected from the literature. The collected data are summarized in Table 1.13, 14, 15, 17 The vancomycin compound model was evaluated in the healthy volunteers and the general Japanese population models, before being used in renally impaired, hepatic disease (liver cirrhosis), and pediatric population models (Figure 1). These population models, which are implemented in the Simcyp simulator, take into account population‐dependent anatomic and physiological changes. In the current study, age distributions were often based on the Weibull distribution for adult population models (e.g., healthy volunteer, Japanese, renal impairment, and liver cirrhosis population models) and uniform distributions for the pediatric population model. Physiological covariates cascade down from age to organ size, including blood flow level with an interconnection between each parameter. Additional variability is added to covariates to replicate the clinical physiological data, which may be represented as a coefficient of variation value using a log normal distribution. Regarding the prediction of vancomycin distribution, simulations were conducted based on a full PBPK model. A volume of distribution at steady state was predicted according to the method published by Poulin and Theil16 with correction by Berezhkovskiy.18
Table 1.
Summary of physicochemical parameters, in vitro, and in vivo data of vancomycin from the literature
| Parameter | Value |
|---|---|
| Physicochemical parameter | |
| Molecular weight, g/mol | 1449.254a |
| logP | −3.1a |
| pKa | 2.18, 7.75, 8.89, 9.59, 10.4, 12b |
| Distribution | |
| Fraction unbound in serum | 0.4513 |
| Blood‐to‐plasma ratio | 0.55c |
| Plasma binding protein | Human serum albumin |
| Full PBPK model | |
| Vss (Poulin and Theil13) L/kg | 0.37 |
| Apparent Vss after i.v. administration, L/kg | 0.30 to 0.4313 |
| Elimination | |
| Total clearance, L/hr/70 kg | 6.78 ± 0.95d, e |
| Urine excretion ratio at 24 hours, % | 84.6 ± 7.3d |
| Renal clearance estimate, L/hr/70 kg | 5.73 ± 0.94d, f |
| Nonrenal clearance estimate, L/hr/70 kg | 1.05 ± 0.52d, g |
PBPK, physiologically based pharmacokinetic; Vss, volume of distribution at steady state.
Vancomycin was treated as an ampholyte and the lowest acidic pKa = 2.18 and the highest basic pKa = 8.9 were entered.
A system default value was used as described in Zhou et al.15
Data after intravenous single dose of vancomycin in Japanese healthy male volunteers, mean ± SD, n = 6 × 2 doses (0.5 and 1.0 g).13
Allometrically scaled clearance with standard body weight of 70 kg, which was calculated according to Anderson and Holford.14
Renal clearance was estimated using total clearance and urine excretion ratio at 24 hours.
Nonrenal clearance was estimated as a difference between total clearance and renal clearance.
Estimation of the nonrenal clearance of vancomycin
Eighty‐five percent (85%) of vancomycin was excreted into urine within 24 hours after administration in adult healthy volunteers.13 Therefore, it was assumed that vancomycin is excreted via renal and nonrenal elimination pathways. The nonrenal clearance was estimated by subtracting the renal clearance from the total clearance (CLnon‐renal, 1.05 L/hr/70 kg, as shown in Table 1 13–16). The estimated nonrenal clearance, which was assumed to be the hepatic clearance, was used to calculate the free hepatic intrinsic clearance (CLuint,H) with the following equations19:
where QH,B is hepatic blood flow (25.5% of cardiac output20). Cardiac output in adults is provided in the population library as a system parameter. In this study, 355.64 L/hr was used as a representative value of healthy volunteers. The free fraction of vancomycin in the blood and the nonrenal blood clearance (CLnonrenal,B) were calculated by dividing the free fraction (fu) of vancomycin in plasma/serum and CLnonrenal, respectively, with the blood to plasma ratio.
The unit of CLuint,H was changed to μL/min/million cells using the following scaling factors:
where liver weight and hepatocellularity are the population system parameters provided from the Simcyp population library. In this calculation, 1,736.96 g of liver weight21 and 126.27 million cells/g liver22 were used as representative values of healthy volunteers.
PBPK model evaluation using clinical pharmacokinetic data in adults
All simulations were carried out by using the respective reported clinical study designs with information regarding dose, administration route, study sample size, and demographics, such as age and sex. The parameters included in the simulation designs were specified to mimic the clinical studies. Parameters used for each simulation are summarized in Supplementary Table S1 (healthy white and Japanese subjects) and Supplementary Table S2 (healthy adults and renally impaired patients).
The evaluation of the developed PBPK models was performed by visual predictive check and by comparison of the PK parameters. In the visual predictive check, the observed systemic drug concentration–time profiles were overlaid with the simulated profiles. Clinically observed PK data were used to evaluate the developed PBPK model of vancomycin. When numerical data were not available, they were extracted from graphs in the original publications with the GetData Graph Digitizer version 2.26 (http://getdata-graph-digitizer.com). The predictability was assessed through a comparison of the observed and predicted maximum concentration (Cmax) and AUC values. The model evaluation using the observed vancomycin concentrations in plasma and epithelial lining fluid (ELF) reported by Lodise et al.23 was conducted based on the comparison of the predicted plasma and lung concentration profiles, respectively. The lung:plasma partition coefficient predicted by the Simcyp simulator was also evaluated by comparing the observed AUCELF,0–∞/AUCplasma,0–∞ ratio of vancomycin. In addition, the model evaluation using the observations reported by Brown et al.24 was conducted based on the simulation with Sim‐Cirrhosis CP‐A population file. The settings of age range (20–65 years) and dosing schedule (500 mg was infused intravenously for 30 minutes every 6 hours up to 7 days) in the simulation were designed according to the reported clinical study. The proportion of females was set as the default setting in the Simcyp simulator.
Evaluation of age‐dependent developmental changes in elimination pathways of vancomycin using clinical PK data in pediatric patients
Renal and nonrenal elimination were implemented as vancomycin clearance pathways. The renal clearance is commonly explained by a filtration process due to a lack of data showing the contribution of transporters on renal excretion in humans. In addition to the developmental changes in kidney growth, the maturation of the glomerular filtration rate (GFR) was described with the following equation in the Simcyp simulator, according to the method by Rhodin et al.25:
where age is chronological age (year) for a virtual subject. Unlike GFR, the ontogeny profile of the nonrenal elimination pathway is not available due to the lack of mechanistic information. The predicted age‐dependent clearances of vancomycin were compared with the clinically observed clearances in order to describe the ontogeny profile of nonrenal elimination. The developmental changes in the nonrenal elimination pathway were simulated for three different maturational patterns (slow, medium, and fast) that were predefined in Simcyp version 16. The fast ontogeny profile was selected for the nonrenal elimination pathway and was described by the following equation:
where Adultmax, Age50, n, and Fbirth were 1, 0.02, 1, and 0, respectively. The coefficient of variation for the GFR estimates was set at 30% as a default in the Simcyp simulator version 16. The Simcyp Pediatric simulator generates additional age‐dependent anatomic and physiological parameters for virtual pediatric subjects based on the equations reported by Johnson et al.20
All simulations with the pediatric platform, including evaluation of the ontogeny profile of the nonrenal elimination pathway, were conducted based on the trial design used in the respective clinical studies. The trial design used for each simulation is summarized in Supplementary Table S3 (US pediatric patients) and Supplementary Table S4 (Japanese pediatric patients).
Sensitivity analysis of cardiac output, tissue penetration, and free fraction of vancomycin in virtual subjects
Sensitivity analyses were conducted for cardiac output to explore the potential impact on the vancomycin PK profile. The cardiac output values were changed to 25% and 50% of the default value (set at 100%) for the Northern European white population. These reductions were based on patients who experienced a shocked condition (25%)26 or with heart failure (50%).27, 28 In the simulation, vancomycin (15 mg/kg) was administrated by intravenous infusion for 1 hour. The trial design included 300 virtual subjects (n = 15 virtual subjects × n = 20 trials) between 41 and 73 years of age with a female proportion of 0.27 (percentage of female to total subjects was 27%). The impact of tissue penetration (e.g., peripheral volume of distribution) was considered as part of the pathophysiology of acute bacterial infections. For example, the peripheral volume of distribution for an antimicrobial drug, daptomycin, was approximately twofold larger in subjects with acute bacterial infections compared with the uninfected subjects.29 Therefore, the impact of tissue penetration may vary due to an increase in vascular permeability and/or the collection of extracellular fluid at the site of infection. The sensitivity analysis was used to examine the tissue penetration of vancomycin and was conducted by changing the default Kp scalar, which is a compound‐specific parameter used to predict the tissue: plasma partition coefficient. The Kp scaler described in Table 1 was increased by 1.3‐fold and 1.6‐fold. The dosing regimens of vancomycin for two age groups (i.e., 2.6 days old and 3 years 11 months old) are summarized in Supplementary Table S3. The trial design included 300 virtual subjects (n = 5 virtual subjects × n = 60 trials) between 2.55 and 2.64 days of age (target postnatal age of virtual subjects was 2.6 days) and between 3.833 and 3.912 years of age (target postnatal age of virtual subjects was 3 years 11 months). The proportion of females was set as the default setting in the Simcyp simulator.
Next, the sensitivity analyses were conducted for the free fraction level of vancomycin to assess its effects on the AUC of total and free vancomycin. The binding of vancomycin to plasma/serum protein has been reported to range from 10–50%.30 The PBPK simulations with the fixed free fraction of vancomycin were initially conducted to evaluate the predictability of a correlation between ratio of total and free AUC to MIC (AUC/MIC) vs. total trough concentration in pediatric patients. The predicted correlation was compared to the clinically observed correlation reported by De Cock et al.31 The free fraction of vancomycin was fixed at 0.715, as reported.31 In this simulation, vancomycin (15 mg/kg) was administered intravenously over 1 hour four times a day for 7 days to virtual pediatric subjects. The total and free AUC/MIC were calculated according to the method described by De Cock et al.31 The trial design included 1,000 virtual pediatric subjects (n = 10 virtual subjects × n = 100 trials) between 0.033 and 15 years of age. The proportion of females was set at the default setting in the Simcyp simulator. The sensitivity analysis was conducted by changing the free fraction level from 0.45 to 0.75 using 100 virtual pediatric subjects (n = 10 virtual subjects × n = 10 trials) based on the simulation conditions previously described above (i.e., dosing regimen, age range, and female ratio).
RESULTS
Development of vancomycin PBPK model
An initial PBPK model was developed using vancomycin‐specific physicochemical parameters, as summarized in Table 1. The volume of distribution at steady state (Vss) for vancomycin was estimated to be 0.37 L/kg, which was comparable to the clinical observations (0.30–0.43 L/kg) in adults.13 Because a high urinary excretion ratio indicates that vancomycin is predominantly eliminated unchanged in the urine, a nonrenal elimination pathway was incorporated into the vancomycin model to explain the remaining minor clearance route. This residual clearance was described as the biliary clearance in the model based on the large molecular weight of vancomycin and the findings reported by Currie and Lemos‐Filho.32 The CLuint,H was estimated to be 0.18 μL/min/million cells through the back‐calculation from the in vivo nonrenal clearance.
The developed vancomycin compound file was evaluated using clinical PK data reported in six different clinical studies in healthy adult white and Japanese subjects. The simulation results of the vancomycin concentration‐time profiles are shown in Figure 2 a–g 13, 23, 33, 34 with the observed data (individual observations in Figure 2 a,b,c,g; mean observational data in Figure 2 d–f). The observed data were predominantly within the 5th to 95th percentile range of the simulations. The ratios of predicted to observed Cmax and AUC ranged from 0.79–1.23 (Supplementary Table S5). The mean ratio values for Cmax and AUC were 0.96 and 1.08, respectively. The concentrations of vancomycin in the lungs were also simulated (inserted figure within Figure 2 g). The partition coefficient of vancomycin between the lung and plasma was estimated to be 0.62. The predicted values were similar to the clinical observations indicating that the AUCELF,0–∞/AUCplasma,0–∞ ratio of vancomycin was 0.41 ± 0.17 (mean ± SD, n = 9 excluding one outlier23), in which the AUC ratio was used as the partition coefficient of vancomycin in the lungs.
Figure 2.
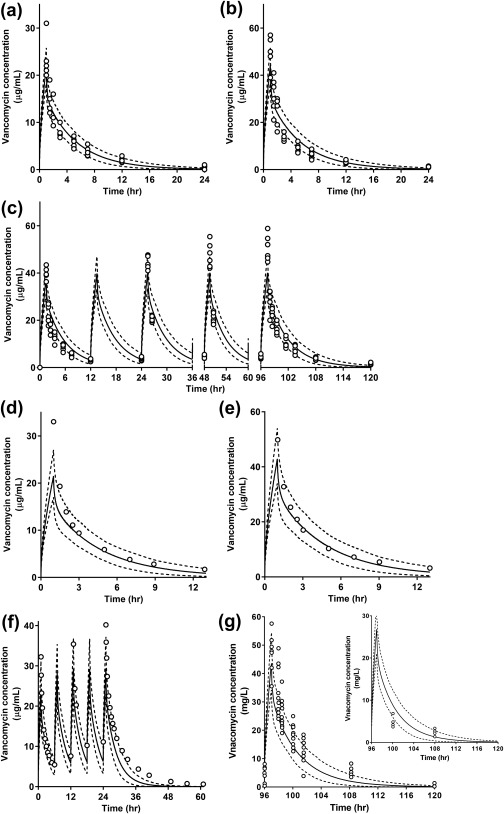
Observed and simulated system concentration‐time profiles of vancomycin in healthy volunteers through physiologically based pharmacokintic (PBPK) modeling. Open circles represent the observed data from reported clinical studies: (a–c) Nakashima et al.13 (1992); (d and e) Boeckh et al.33 (1988); (f) Healy et al.34 (1987); and (g) Lodise et al.23 (2011). Solid and dashed lines represent the mean and 5th/95th percentiles of the simulation the simulation results, respectively. In g, the small figure located on the right side shows lung concentration‐time profiles of vancomycin. Parameter settings used for each simulation are summarized in Supplementary Table S1.
Model evaluation with renal impairment and liver cirrhosis system models
Evaluation of a base PBPK model was conducted using PK observations in healthy Japanese volunteers and patients to assess an applicability of the input data for renal elimination. The PK profile of clinical data obtained from healthy Japanese volunteers were compared to the PK profiles simulated by using healthy white system data (Figure 3 a 35) and general Japanese system data (Figure 3 b 35), in which system data indicates population‐specific anatomic and physiological parameters. The two levels of creatinine clearance (CCr) for evaluation of renal impairment were mild to moderate with CCr ≥30 to <70 mL/min (Figure 3 c 35) and severe with CCr ≥15 to <30 mL/min (Figure 3 d 35). Each classification was determined in accordance to US Food and Drug Administration guidelines.36 The ratios of predicted to observed Cmax and AUC∞ ranged from 0.62–0.94 in healthy adults, and 0.73–1.36 in patients with renal impairment (Supplementary Table S6). The mean ratio values for Cmax and AUC were 1.05 each in the patients with renal impairment. The PK profiles of vancomycin were also simulated using the liver cirrhosis population model (Child‐Pugh scores for mild level of cirrhosis, score level A; CP‐A), which is implemented in the Simcyp software. The clinical observations in patients with abnormal liver functions were predominantly within the 5th to 95th percentiles of these simulations (Figure 3 e 24).
Figure 3.
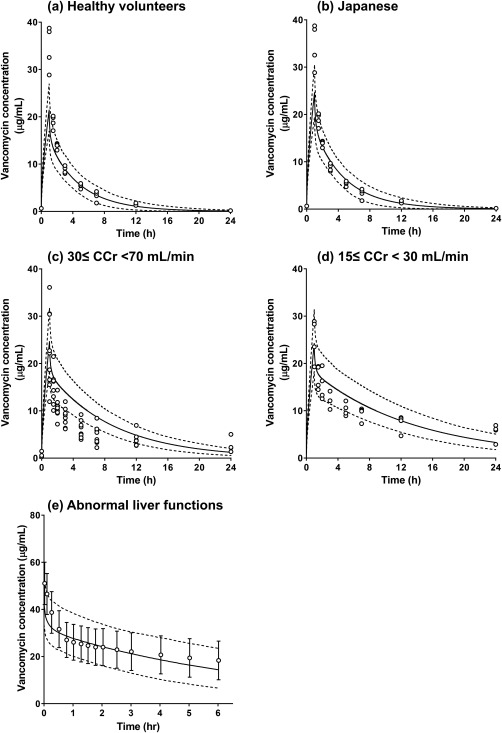
Observed and simulated system concentration‐time profiles of vancomycin in healthy volunteers, patients with renal impairment, and patients with abnormal liver functions through physiologically based pharmacokintic (PBPK) modeling. Open circles represent the observed data from reported clinical studies: (a–d) Takenaka et al.35 (1993); (e) Brown et al.24 (1983). Solid and dashed lines represent the mean and 5th/95th percentiles of the simulation results, respectively. Simulations were conducted with: (a) Sim‐Healthy Volunteer; (b) Sim‐Japanese; (c) Sim‐RenalGFR_30‐60; (d) Sim‐RenalGFR_less_30; (e) Sim‐CirrhosisCP‐A population files implemented in the system. Parameter settings used for each simulation are summarized in the Methods Section and Supplementary Table S2. CCr, creatinine clearance.
Pediatric PBPK model including developmental changes in the nonrenal elimination pathway
In this study, renal and nonrenal elimination pathways were incorporated into the vancomycin model. Maturational change in GFR, which was reported by Rhodin et al.25 was used; however, the nonrenal elimination pathway was unknown. Consequently, the age‐dependent developmental changes in the nonrenal elimination pathway of vancomycin were examined by comparing the reported relationship between postconceptional age and total CL. The fast maturational pattern, which was predefined in the Simcyp software, reasonably captured the observed data of vancomycin CL in the pediatric population when compared to the slow and medium settings. The developed base model combined with the pediatric system platform was evaluated using data reported from two different clinical PK studies conducted in US and Japanese pediatric patients. The simulation results of the vancomycin concentration‐time profiles after a single dose are shown in Figure 4 a–f 37, 38 with the mean observed data for pediatric patients who ranged from 2.6 days to 7.6 years (7 years 7 months) of age.37 The simulations reasonably traced vancomycin concentration‐time profiles observed in US pediatric patients. The ratio of predicted to observed PK data ranged from 0.85–1.69 for Cmax and 0.54–1.52 for AUC (Supplementary Table S7). The mean ratio values for Cmax and AUC were 1.16 and 1.09, respectively.
Figure 4.
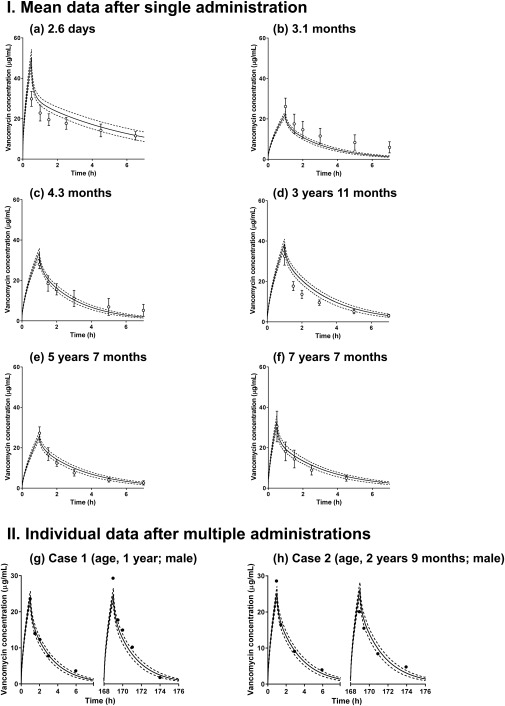
Observed and simulated system concentration‐time profiles of vancomycin in US and Japanese pediatric patients through physiologically based pharmacokintic (PBPK) modeling. Solid and dashed lines represent the mean and 5th/95th percentiles of the simulation results, respectively. I (a–f) Open circles represent the observed mean data from reported clinical studies.37 Parameter settings used for each simulation in this study are summarized in Supplementary Table S3. II (g and h) Closed circles represent the observed individual data from reported clinical studies.38 Parameter settings used for each simulation are summarized in Supplementary Table S4.
The simulation results of the vancomycin concentration‐time profiles after multiple dosing are shown in Figure 4 g,h for two individual Japanese pediatric patients aged 1 year and 2.8 years (2 years 9 months), respectively.38 The predicted Cmax and AUC were within a twofold range of the observed data for each individual patient (Supplementary Table S8). The mean ratio values for Cmax and AUC were 1.10 and 1.01, respectively. The age‐dependent predictability of Cmax and AUC was determined by combining data from the US and Japanese pediatric patients (Figure 5, 37, 38). A clear relationship between age and predictability was not observed.
Figure 5.
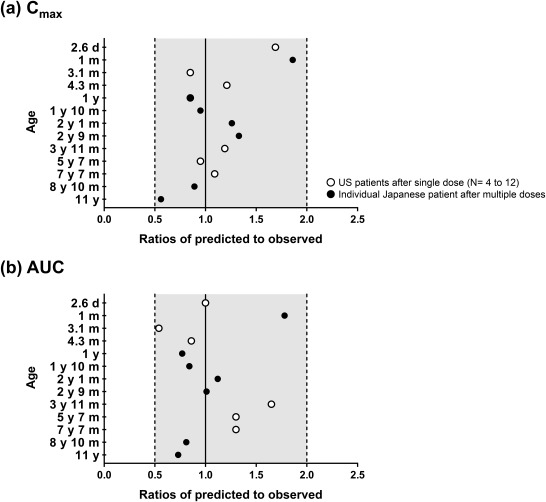
Comparison between vancomycin predicted and observed values for the ratio of the maximum concentration (Cmax) (a) and the area under the plasma concentration time curve (AUC) (b) in each age group. Regarding observed pharmacokinetic (PK) parameters, PK data after single administration in US patients (N = 4–12, open circles)37 and multiple administration in individual Japanese patients (closed circles)38 were used to calculate the ratio.
The impact of cardiac output and tissue‐plasma partition coefficient on vancomycin pharmacokinetics in virtual subjects
The impact of cardiac output on vancomycin PK was also assessed in adults. The cardiac output was changed to 25% and 50% of the default value (set at 100%). Vancomycin concentration‐time profiles were changed depending on a decrease in cardiac output, although corresponding changes in AUC were negligible (Figure 6 a 8). Simulation of vancomycin PK profiles, with 50% decrease in cardiac output, was comparable to clinical observations in patients before bypass surgery. A 50% decrease in cardiac output resulted in a 19% increase in Cmax (just after the end of infusion), but changes in concentrations at 2 hours and 12 hours after starting the infusion were <10%.
Figure 6.
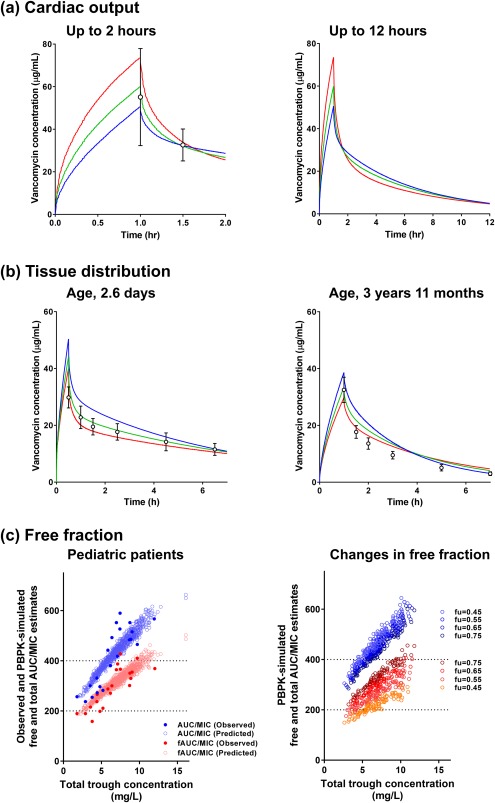
Impact of changes in (a) cardiac output; (b) tissue penetration; (c) free fraction of vancomycin on predicted system concentration‐time profiles in virtual subjects. a Cardiac output was changed from 100% (blue line) to 50% (green line) to 25% (red line) of the default value (set at 100%) for the Northern European white population and the simulation was conducted with modified values, as described in the Methods section. Open circles with a bar represents the mean ± SD of vancomycin concentrations up to 2 hours (left) and 24 hours (right) after starting infusion observed in patients before artery bypass surgery.8 b Tissue penetration of vancomycin was modified by changing a Kp scalar, which is a scalar applied to all predicted tissue: plasma partition coefficient. The Kp scalar was increased to 1.3‐fold (green line) and 1.6‐fold (red line) compared to the original setting estimated in this study (blue line, Table 1), and the simulation was conducted with modified values, as described in the Methods section. Open circles with bars represent the mean ± SD of vancomycin concentrations observed in pediatric patients aged 2.6 days old and 4.3 months old.37 c Ratio of total and free area under the curve (AUC) to minimum inhibitory concentration (MIC) was predicted using the physiologically based pharmacokinetic (PBPK) model of vancomycin. Total and free AUC/MIC are represented by blue and red symbol, respectively. Left: The free fraction of vancomycin was fixed as reported by De Cock et al.31 The PBPK model‐predicted values (open circles) were overlaid with the clinical data reported by De Cock et al.31 (closed circles). Right: The free fraction of vancomycin was changed from 0.45 to 0.75.
In addition, the effect of tissue penetration of vancomycin was examined by modifying the Kp scalar (1.3 to 1.6‐fold increases in Kp scalar; Figure 6 b 37). An increase in the Kp scalar resulted in a decrease in vancomycin concentration in the distribution phase. The increase in the Kp scalar improved the fitting of the distribution phase observed in US pediatric patients at postnatal days 2.6 of age and 3 years 11 months of age.37
A correlation between total and free AUC/MIC levels vs. total trough concentrations predicted by the PBPK model of vancomycin captured the clinical observations, in which the free fraction of vancomycin was fixed at the value reported in the clinical study31 (Figure 6 c, left). The predicted free AUC/MIC level was more sensitive to changes in free fraction levels when compared to the total AUC/MIC (Figure 6 c, right).
DISCUSSION
In this study, a vancomycin PBPK model was developed to systematically assess the impact of changes in multiple physiological parameters on vancomycin PK profiles. The model was developed and evaluated using clinical PK observations from healthy volunteers, patients with organ dysfunction, and pediatric patients. The renal clearance, which assumed a filtration process that was calculated by GFR and free fraction, was implemented as the primary elimination pathway in the current vancomycin PBPK model. The contribution of GFR in virtual Japanese subjects was assessed by using predicted vancomycin CL after a single intravenous dose of vancomycin (0.5 g) during the model development process. The PBPK simulation demonstrated that 91% of the variability in vancomycin CL was explained by GFR. Sequentially, sensitivity analyses were conducted to explore potential contributing factors to altered vancomycin PBPK disposition within a particular clinical situation. Based on the PK predictions generated by the model, GFR affects the clearance of vancomycin in patients with renal impairment and in pediatric patients with maturational change in elimination pathways. Sensitivity analyses indicated that changes in the cardiac output and tissue penetration of vancomycin may affect the distribution of vancomycin. In general, the predictability of the PK profile in special patient populations relies on system knowledge (i.e., population characteristics in terms of anatomic and physiological parameters). Therefore, PBPK models that used patient‐specific changes in physiologies could elucidate case‐based clinical PK observations.
After intravenous administration, vancomycin is distributed to several tissues (i.e., lungs, brain, heart, bone, fat, etc.) via passive diffusion.23, 30, 39 In the current study, the lung:plasma partition coefficient of vancomycin was predicted to be 0.62 at steady state. The total AUCELF,0–∞/AUCplasma,0–∞ ratio was reported to be 0.41 ± 0.17 (mean ± SD, n = 9 excluding one outlier) after multiple dosing in healthy volunteers.23 Cruciani et al.40 reported that the mean penetration of total vancomycin into the lungs ranged from 0.24–0.41 at several time points in elderly patients with lung carcinoma, relapsing pneumothorax, or tubercular abscess. Although large variability was observed, the penetration level in elderly patients indicates a lower trend compared with healthy volunteers. As observed in plasma/serum, the age and disease state of the patients may affect the free fraction of vancomycin in the lungs. The next challenge would be to assess the differences in lung penetration of vancomycin due to age and/or disease with consideration for free fraction of vancomycin in the lungs.
In the brain, the plasma partition coefficient of vancomycin was predicted to be 0.61. The concentration in cerebrospinal fluid (CSF) is considered to be the same as the free fraction in the brain; therefore, CSF penetration was predicted to be 0.14. The value was predicted using in vitro fu,brain = 0.23 from fu,serum = 0.45 according to the relationship between in vitro log fu,brain and log fu,plasma,41 in which free fraction in serum is assumed to be the same as in plasma. Although the observed data were highly variable,30 the predicted value was comparable to the observed data (0–0.18) for the patients with uninflamed meninges. A study by Djukic et al.42 suggests that there was a significant positive relationship between the lipid‐water partition coefficient at pH = 7.0 (i.e., log D) and the ratio of AUCCSF to AUCserum for 27 drugs, which included vancomycin in patients with inflamed meninges.42 According to that study,42 the predicted ratio of AUCCSF to AUCserum for vancomycin ranged from 0.03–0.1. These findings are similar to the current PBPK simulated data; therefore, the current prediction, which is based on the physicochemical property of the drug, could be used as an alternative method to predict the penetration of vancomycin into the CSF. In addition to the lungs and brain, further evaluation is needed to assess the penetration of vancomycin in each tissue compartment in order to gain a better understanding of the target concentrations through PBPK simulation.
Vancomycin is predominantly eliminated through the renal filtration process. Similar to the clinical observations in renally impaired patients, the predicted AUC of vancomycin increased with a decrease in CCr.35 This confirms that renal function has an important impact on vancomycin exposure. Hepatic elimination seems to have a limited contribution to the PK of vancomycin due to a lack of metabolic data. Nevertheless, Brown et al.24 (1983) reported that vancomycin PK in patients with abnormal liver functions was significantly different from that in patients with normal liver function. In their report, geometric means of CCr and albumin concentrations in patients with abnormal liver functions (n = 9) showed 30% and 10% decreases, respectively, when compared to patients with normal liver function (n = 6). Similar decreases in CCr and albumin concentrations were found in the liver cirrhosis system model (Child‐Pugh scores for mild level of cirrhosis, score level A; CP‐A). The CP‐A model is designed to show a 30% decrease in GFR and 10% decrease in albumin concentration compared with normal subjects, due to hepato‐renal syndrome observed in cirrhosis.43 Interestingly, these physiological changes theoretically have an opposite effect on drug clearance. A decrease in GFR results in a decrease in the clearance. On the other hand, a decrease in albumin concentration results in an increase in clearance through an increase in free fraction of the drug. In this study, simulation with the CP‐A system model was well‐predicted for the PK profile of vancomycin in patients with abnormal liver function who showed an increase in vancomycin trough concentration.24 Therefore, multiple physiological changes that are due to liver dysfunction showed a potential to influence the distribution and elimination of vancomycin. The results also showed the trend that the central tendency of elimination rate in the virtual CP‐A population is slightly higher than that in the observations. This is probably due to a difference in data distribution between the virtual generated population and the clinical population consisting of nine patients, which hampers the capturing of the normal distribution. From these observations, understanding such patient‐oriented physiological data improves the PBPK model and helps to elucidate the underlying mechanisms influencing the PK profile. In addition to the patient physiological data, disease progression should be considered when assessing the PK of vancomycin. For example, the progression of liver cirrhosis (i.e., CP‐A, CP‐B, and CP‐C) may influence the degree of changes in physiological factors, such as GFR, serum albumin concentration, and cardiac output.43 The PBPK modeling and simulations would be beneficial for such patients having simultaneous physiological changes.
The contribution of other pathways was assumed to describe ∼10% of the total clearance. Due to the paucity of data related to the mechanism of nonrenal elimination, the developmental changes were explored for the pediatric population. The fast ontogeny profile was chosen to describe a developmental change in nonrenal elimination of vancomycin. An age‐dependent change in renal elimination function, indicated by GFR, has been well‐established in neonates and infants.25 Predicted PK parameters (Cmax and AUC) were within a twofold range of the observed data in the US pediatric patients, but the current simulations slightly over‐predicted the distribution phase in the two patients who were evaluated. A study by Pennington et al.44 has shown that an abnormal increase in body temperature due to inflammation might affect the PK of antibiotics. The data showed that etiocholanolone‐stimulated fever reduces gentamicin serum concentration without changes in half‐life and renal clearance, which indicates an enhancement of tissue penetration for antibiotics. The clinical PK observations in the US patients, which were used in this study, indicated that some patients may have had a fever during treatment.37 The current study showed 1.3‐fold to 1.6‐fold increases in tissue‐plasma partition coefficient resulting in a reasonable match with the Cmax of vancomycin observed in the 2.6 day old and 3 years 11 month old patients. The estimated increase of 1.3‐fold to 1.6‐fold in tissue penetration of vancomycin is similar to the 1.6‐fold increase in vancomycin ELF: plasma ratio between patients with high and low albumin concentration in ELF, where inflammation conditions may be linked to these modifications.45 Inflammation may also contribute to the distribution and clearance of vancomycin. For example, the renal clearance of vancomycin was enhanced in rats with carcinogen‐induced osteosarcoma, in which the plasma concentrations of interleukin‐1β and interleukin‐6 were elevated46; however, human data are unavailable. From these results, the unstable nature of common occurrences, such as infection‐induced fever, may influence the PK profile of vancomycin, which may correlate with the efficacy of vancomycin.1
The advantage of PBPK modeling is that “what‐if” scenarios can be used to explore the most likely cause of altered disposition in a specific patient population. In this study, the sensitivity analysis was conducted focusing on changes in cardiac output in order to consider the vancomycin PK profile in patients with heart failure. The simulations of the vancomycin PK profiles with 50% decrease in cardiac output compared to a normal subject showed similar results compared with clinical observations in patients before bypass surgery.8 This reduction in cardiac output seems to be reasonable because the quantified fractional reduction in renal blood flow was reported at 78%, 55%, and 63% of normal blood flow in mild, moderate, and severe chronic heart failure patients, respectively.27, 28 In the Simcyp software, the renal blood flow is set at 17–19% of cardiac output. The PBPK simulations for vancomycin concentration‐time profiles varied between subjects with and without heart failure. A decrease in cardiac output resulted in an increase in Cmax of vancomycin, but changes in the AUC0–∞ were negligible in virtual adults with normal renal function. In addition, the changes in concentrations at 2 and 12 hours after starting infusion were <10% in virtual adults. These concentrations are often used as peak (at 1 hour after the end of infusion) and trough concentrations (predose) in clinical settings. From the predicted changes in these concentrations simulated by the PBPK model, a Bayesian estimator, using only these two measurements, may seem difficult to reproduce PK profiles of vancomycin in patients with cardiac failure. Another consideration is that patients with heart failure often have renal dysfunction. The simulation using the developed model demonstrated that peak and trough concentrations of vancomycin increased due to reductions in both cardiac output and renal function (Supplementary Figure S1). These predictions should be examined in patients with advanced cardiac disease due to the impact of changes in renal function and cardiac output. Further evaluation using clinical observations in patients with cardiac failure is needed to understand the disease‐dependent and condition‐dependent effects on PK profiles.
The unbound concentration of vancomycin is pharmacologically active and influenced by the free fraction level of vancomycin, which shows large variability among patients.31, 47 A potential cause of the variability is changes in binding protein levels due to the disease and age of patients. The concentration of albumin, to which vancomycin predominantly binds, was influenced by inflammation and renal disease.48, 49, 50 In addition, the age‐dependent increase in albumin level was observed in the pediatric population.20 Therefore, the free fraction of vancomycin in pediatric patients with inflammation may vary depending on the relative contribution of albumin to the total proteins bound to vancomycin. In this study, the clearance of vancomycin was assumed to be the sum of renal clearance based on glomerular filtration (as a renal elimination pathway) and hepatic biliary excretion clearance (as a nonrenal elimination pathway), which were calculated based on free vancomycin. Therefore, the predicted free AUC/MIC values were sensitive to changes in the free fraction of vancomycin in the virtual pediatric population. The alteration in free AUC/MIC due to disease and age may affect the PDs of vancomycin.
A limitation of the current study is the lack of information in humans regarding the role of transporters in vancomycin renal clearance. The overall renal CL of vancomycin is ∼5.73 L/hr/70 kg. The calculated fu*GFR estimates a CL of 3.38 L/hr/70 kg due to filtration; therefore, there is a potential of active tubular secretion for vancomycin. Shimada et al.46 reported that the potential contribution of tubular secretion/re‐absorption to vancomycin renal transport was influenced by cytokine levels due to osteosarcoma in rats. The article also indicated that the renal clearance of vancomycin was inhibited by cimetidine, which is a substrate for the organic cation transporters in control rats.46 However, as previously mentioned, there are a lack of data showing the contribution of transporters on renal excretion in humans when compared with animals. Therefore, further investigations regarding the contribution of renal transporters on vancomycin CL would improve the current PBPK model and increase our understanding about vancomycin renal elimination pathways.
In summary, a PBPK model simulated the altered PK profiles of vancomycin caused by multiple physiological factors, which included age and organ dysfunction in the renal, hepatic, and cardiovascular systems. The simulations from the developed platform demonstrated: (1) the potential impact of changes in cardiac output on vancomycin distribution (sensitive to peak concentration of vancomycin); (2) potential variability in the volume of distribution due to inflammation; and (3) impact of changes in free fraction level of vancomycin on free AUC/MIC. The next step in this process is to assess the current findings from the PBPK models and simulations using a wide variety of vancomycin PK data, which will be obtained from several hospitals, with a special consideration given to disease progression. The clinical confirmation will enhance the findings from the current PBPK simulations. These findings from patient‐oriented predictions using PBPK modeling will facilitate an understanding of the underlying mechanisms in inter‐patient variability. It will also support the optimization of vancomycin dosing regimens in a wide range of pediatric and adult patient populations with various disease states such as patients with heart failure having renal dysfunction and pediatric populations undergoing renal maturation.
SOURCE OF FUNDING
Brooks T. McPhail is supported by National Institutes of Health (NIH) T32 grant (3T32HD069054‐07S2).
CONFLICT OF INTEREST
Trevor N. Johnson is an employee of Simcyp (a Certara company). The other authors declared no conflict of interests.
AUTHOR CONTRIBUTIONS
C.E., T.N.J., B.T.M., A.A.V., and T.F. wrote the manuscript. C.E., T.N.J., and T.F. designed the research. C.E., B.T.M., and T.F. performed the research. C.E., T.N.J., and T.F. analyzed the data. C.E. contributed new reagents/analytical tools.
Supporting information
Figure S1. Impact of changes in cardiac output and renal function on vancomycin PK profiles in virtual adult subjects.
The cardiac output values were changed to 50% of the default value (set at 100%) in the Sim‐North European Caucasian and the Sim_Renal GFR 30‐60 population models (SimCYP simulator, version 16). In the simulation, vancomycin (15 mg/kg) was administrated by intravenous infusion for 1 hour. Doses were given twice a day (q12h) for virtual Caucasian (blue, 100% cardiac output; green, 50% cardiac output) and once a day (q24h) for virtual subject with renal impairment (red). The trial design included 300 virtual subjects (n=15 virtual subjects × n=20 trials) between 41‐73 years of age with a female proportion of 0.27 (percentage of female to total subjects was 27%). The solid and dashed lines indicate the mean and 5th‐95th percentiles of the simulations by PBPK modeling, respectively. The open circles with bars represent the mean ± S.D. of observed vancomycin concentrations (up to 2.5 hours after starting infusion, left) in patients before artery bypass surgery1.
Reference:
1. Kitzes‐Cohen R, Farin D, Piva G, Ivry S, Sharony R, Amar R, et al. Pharmacokinetics of vancomycin administered as prophylaxis before cardiac surgery. Therapeutic drug monitoring 2000, 22(6): 661–667.
Supplemental Tables
Supplemental Data
References
- 1. Álvarez, R. , López Cortés, L.E. , Molina, J. , Cisneros, J.M. & Pachón, J. Optimizing the clinical use of vancomycin. Antimicrob. Agents Chemother. 60, 2601–2609 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stockmann, C. et al Vancomycin pharmacokinetic models: informing the clinical management of drug‐resistant bacterial infections. Expert Rev. Anti Infect. Ther. 12, 1371–1388 (2014). [DOI] [PubMed] [Google Scholar]
- 3. de Hoog, M. , Mouton, J.W. & van den Anker, J.N. Vancomycin: pharmacokinetics and administration regimens in neonates. Clin. Pharmacokinet. 43, 417–440 (2004). [DOI] [PubMed] [Google Scholar]
- 4. Moellering, R.C. Jr, Krogstad, D.J. & Greenblatt, D.J. Vancomycin therapy in patients with impaired renal function: a nomogram for dosage. Ann. Intern. Med. 94, 343–346 (1981). [DOI] [PubMed] [Google Scholar]
- 5. Lim, H.S. , Chong, Y.P. , Noh, Y.H. , Jung, J.A. & Kim, Y.S. Exploration of optimal dosing regimens of vancomycin in patients infected with methicillin‐resistant Staphylococcus aureus by modeling and simulation. J. Clin. Pharm. Ther. 39, 196–203 (2014). [DOI] [PubMed] [Google Scholar]
- 6. Mangin, O. , Urien, S. , Mainardi, J.L. , Fagon, J.Y. & Faisy, C. Vancomycin pharmacokinetic and pharmacodynamic models for critically ill patients with post‐sternotomy mediastinitis. Clin. Pharmacokinet. 53, 849–861 (2014). [DOI] [PubMed] [Google Scholar]
- 7. Cristallini, S. et al New regimen for continuous infusion of vancomycin in critically ill patients. Antimicrob. Agents Chemother. 60, 4750–4756 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kitzes‐Cohen, R. et al Pharmacokinetics of vancomycin administered as prophylaxis before cardiac surgery. Ther. Drug Monit. 22, 661–667 (2000). [DOI] [PubMed] [Google Scholar]
- 9. Huang, S.M. & Rowland, M. The role of physiologically based pharmacokinetic modeling in regulatory review. Clin. Pharmacol. Ther. 91, 542–549 (2012). [DOI] [PubMed] [Google Scholar]
- 10. Johnson, T.N. & Rostami‐Hodjegan, A. Resurgence in the use of physiologically based pharmacokinetic models in pediatric clinical pharmacology: parallel shift in incorporating the knowledge of biological elements and increased applicability to drug development and clinical practice. Paediatr. Anaesth. 21, 291–301 (2011). [DOI] [PubMed] [Google Scholar]
- 11. Jamei, M. , Marciniak, S. , Feng, K. , Barnett, A. , Tucker, G. & Rostami‐Hodjegan, A. The Simcyp population‐based ADME simulator. Expert Opin. Drug Metab. Toxicol. 5, 211–223 (2009). [DOI] [PubMed] [Google Scholar]
- 12. Jamei, M. et al The simcyp population based simulator: architecture, implementation, and quality assurance. In Silico Pharmacol. 1, 9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Food and Drug Administration. F71966566, Baxter. Warnings and precautions, thromboembolic disorders. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/050671s018lbl.pdf.
- 14. Zhou, W. et al Predictive performance of physiologically based pharmacokinetic and population pharmacokinetic modeling of renally cleared drugs in children. CPT Pharmacometrics Syst. Pharmacol. 5, 475–483 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakashima, M. , Katagiri, K. & Oguma, T. Phase I studies on vancomycin hydrochloride for injection. Chemotherapy 40, 210–224 (1992). [Google Scholar]
- 16. Anderson, B.J. & Holford, N.H. Mechanism‐based concepts of size and maturity in pharmacokinetics. Annu. Rev. Pharmacol. Toxicol. 48, 303–332 (2008). [DOI] [PubMed] [Google Scholar]
- 17. Poulin, P. & Theil, F.P. Prediction of pharmacokinetics prior to in vivo studies. 1. Mechanism‐based prediction of volume of distribution. J. Pharm. Sci. 91, 129–156 (2002). [DOI] [PubMed] [Google Scholar]
- 18. Berezhkovskiy, L.M. Volume of distribution at steady state for a linear pharmacokinetic system with peripheral elimination. J. Pharm. Sci. 93, 1628–1640 (2004). [DOI] [PubMed] [Google Scholar]
- 19. Salem, F. , Johnson, T.N. , Abduljalil, K. , Tucker, G.T. & Rostami‐Hodjegan, A. A re‐evaluation and validation of ontogeny functions for cytochrome P450 1A2 and 3A4 based on in vivo data. Clin. Pharmacokinet. 53, 625–636 (2014). [DOI] [PubMed] [Google Scholar]
- 20. Johnson, T.N. , Rostami‐Hodjegan, A. & Tucker, G.T. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin. Pharmacokinet. 45, 931–956 (2006). [DOI] [PubMed] [Google Scholar]
- 21. Johnson, T.N. , Tucker, G.T. , Tanner, M.S. & Rostami‐Hodjegan, A. Changes in liver volume from birth to adulthood: a meta‐analysis. Liver Transpl. 11, 1481–1493 (2005). [DOI] [PubMed] [Google Scholar]
- 22. Barter, Z.E. et al Scaling factors for the extrapolation of in vivo metabolic drug clearance from in vitro data: reaching a consensus on values of human microsomal protein and hepatocellularity per gram of liver. Curr. Drug Metab. 8, 33–45 (2007). [DOI] [PubMed] [Google Scholar]
- 23. Lodise, T.P. , Drusano, G.L. , Butterfield, J.M. , Scoville, J. , Gotfried, M. & Rodvold, K.A. Penetration of vancomycin into epithelial lining fluid in healthy volunteers. Antimicrob. Agents Chemother. 55, 5507–5511 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brown, N. et al Effects of hepatic function on vancomycin clinical pharmacology. Antimicrob. Agents Chemother. 23, 603–609 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rhodin, M.M. et al Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr. Nephrol. 24, 67–76 (2009). [DOI] [PubMed] [Google Scholar]
- 26. Macnab, M.S. , Macrae, D.J. , Guy, E. , Grant, I.S. & Feely, J. Profound reduction in morphine clearance and liver blood flow in shock. Intensive Care Med. 12, 366–369 (1986). [DOI] [PubMed] [Google Scholar]
- 27. Leithe, M.E. , Margorien, R.D. , Hermiller, J.B. , Unverferth, D.V. & Leier, C.V. Relationship between central hemodynamics and regional blood flow in normal subjects and in patients with congestive heart failure. Circulation 69, 57–64 (1984). [DOI] [PubMed] [Google Scholar]
- 28. Rasool, M.F. , Khalil, F. & Läer, S. Predicting stereoselective disposition of carvedilol in adult and pediatric chronic heart failure patients by incorporating pathophysiological changes in organ blood flows – a physiologically based pharmacokinetic approach. Drug Metab. Dispos. 44, 1103–1115 (2016). [DOI] [PubMed] [Google Scholar]
- 29. Dvorchik, B. , Arbeit, R.D. , Chung, J. , Liu, S. , Knebel, W. & Kastrissios, H. Population pharmacokinetics of daptomycin. Antimicrob. Agents Chemother. 48, 2799–2807 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rybak, M.J. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin. Infect. Dis. 2006, 42(suppl 1), S35–S39 (2006). [DOI] [PubMed] [Google Scholar]
- 31. De Cock, P.A . et al Impact of vancomycin protein binding on target attainment in critically ill children: back to the drawing board? J. Antimicrob. Chemother. 72, 801–804 (2017). [DOI] [PubMed] [Google Scholar]
- 32. Currie, B.P. & Lemos‐Filho, L. Evidence for biliary excretion of vancomycin into stool during intravenous therapy: potential implications for rectal colonization with vancomycin‐resistant enterococci. Antimicrob. Agents Chemother. 48, 4427–4429 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boeckh, M. , Lode, H. , Borner, K. , Höffken, G. , Wagner, J. & Koeppe, P. Pharmacokinetics and serum bactericidal activity of vancomycin alone and in combination with ceftazidime in healthy volunteers. Antimicrob. Agents Chemother. 32, 92–95 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Healy, D.P. , Polk, R.E. , Garson, M.L. , Rock, D.T. & Comstock, T.J. Comparison of steady‐state pharmacokinetics of two dosage regimens of vancomycin in normal volunteers. Antimicrob. Agents Chemother. 31, 393–397 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takenaka, T. et al Pharmacokinetics of vancomycin and dosage planning in patients with renal insufficiency. Chemotherapy 41, 1079–1089 (1993). [Google Scholar]
- 36.U.S. Food and Drug Administration. Guidance for industry providing clinical evidence of effectiveness for human drugs and biological products. <http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072127.pdf>.
- 37. Schaad, U.B. , McCracken, G.H. Jr & Nelson, J.D. Clinical pharmacology and efficacy of vancomycin in pediatric patients. J. Pediatr. 96, 119–126 (1980). [DOI] [PubMed] [Google Scholar]
- 38. Fujii, R. et al Prospective study to examine the clinical efficacy of vancomycin hydrochloride against pediatric infections caused by methicillin‐cephem resistant Staphylococcus aureus. Chemotherapy 42, 863–870 (1994). [Google Scholar]
- 39. Martin, C. et al Penetration of vancomycin into mediastinal and cardiac tissues in humans. Antimicrob. Agents Chemother. 38, 396–399 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cruciani, M. et al Penetration of vancomycin into human lung tissue. J. Antimicrob. Chemother. 38, 865–869 (1996). [DOI] [PubMed] [Google Scholar]
- 41. Liu, X. et al Use of a physiologically based pharmacokinetic model to study the time to reach brain equilibrium: an experimental analysis of the role of blood‐brain barrier permeability, plasma protein binding, and brain tissue binding. J. Pharmacol. Exp. Ther. 313, 1254–1262 (2005). [DOI] [PubMed] [Google Scholar]
- 42. Djukic, M. , Munz, M. , Sörgel, F. , Holzgrabe, U. , Eiffert, H. & Nau, R. Overton's rule helps to estimate the penetration of anti‐infectives into patients' cerebrospinal fluid. Antimicrob. Agents Chemother. 56, 979–988 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Johnson, T.N. , Boussery, K. , Rowland‐Yeo, K. , Tucker, G.T. & Rostami‐Hodjegan, A. A semi‐mechanistic model to predict the effects of liver cirrhosis on drug clearance. Clin. Pharmacokinet. 49, 189–206 (2010). [DOI] [PubMed] [Google Scholar]
- 44. Pennington, J.E. , Dale, D.C. , Reynolds, H.Y. & MacLowry, J.D. Gentamicin sulfate pharmacokinetics: lower levels of gentamicin in blood during fever. J. Infect. Dis. 132, 270–275 (1975). [DOI] [PubMed] [Google Scholar]
- 45. Lamer, C. et al Analysis of vancomycin entry into pulmonary lining fluid by bronchoalveolar lavage in critically ill patients. Antimicrob. Agents Chemother. 37, 281–286 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shimada, I. et al Enhanced renal clearance of vancomycin in rats with carcinogen‐induced osteosarcoma. Anticancer Res. 32, 823–829 (2012). [PubMed] [Google Scholar]
- 47. Oyaert, M. et al Factors impacting unbound vancomycin concentrations in different patient populations. Antimicrob. Agents Chemother. 59, 7073–7079 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grossman, S.H. , Davis, D. , Kitchell, B.B. , Shand, D.G. & Routledge, P.A. Diazepam and lidocaine plasma protein binding in renal disease. Clin. Pharmacol. Ther. 31, 350–357 (1982). [DOI] [PubMed] [Google Scholar]
- 49. Moshage, H.J. , Janssen, J.A. , Franssen, J.H. , Hafkenscheid, J.C. & Yap, S.H. Study of the molecular mechanism of decreased liver synthesis of albumin in inflammation. J. Clin. Invest. 79, 1635–1641 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Don, B.R. & Kaysen, G. Serum albumin: relationship to inflammation and nutrition. Semin. Dial. 17, 432–437 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Impact of changes in cardiac output and renal function on vancomycin PK profiles in virtual adult subjects.
The cardiac output values were changed to 50% of the default value (set at 100%) in the Sim‐North European Caucasian and the Sim_Renal GFR 30‐60 population models (SimCYP simulator, version 16). In the simulation, vancomycin (15 mg/kg) was administrated by intravenous infusion for 1 hour. Doses were given twice a day (q12h) for virtual Caucasian (blue, 100% cardiac output; green, 50% cardiac output) and once a day (q24h) for virtual subject with renal impairment (red). The trial design included 300 virtual subjects (n=15 virtual subjects × n=20 trials) between 41‐73 years of age with a female proportion of 0.27 (percentage of female to total subjects was 27%). The solid and dashed lines indicate the mean and 5th‐95th percentiles of the simulations by PBPK modeling, respectively. The open circles with bars represent the mean ± S.D. of observed vancomycin concentrations (up to 2.5 hours after starting infusion, left) in patients before artery bypass surgery1.
Reference:
1. Kitzes‐Cohen R, Farin D, Piva G, Ivry S, Sharony R, Amar R, et al. Pharmacokinetics of vancomycin administered as prophylaxis before cardiac surgery. Therapeutic drug monitoring 2000, 22(6): 661–667.
Supplemental Tables
Supplemental Data


