Abstract
Background
Interstitial lung abnormality (ILA) is found in 5–10% of the general population and is associated with increased mortality risk. Risk factors for ILA, including advanced age and smoking history also increase the risk for aortic stenosis (AS). Transcatheter aortic valve replacement (TAVR) has become an increasingly utilized intervention for patients with severe AS, and requires a high-resolution computed tomography (HRCT) of the chest to assess aortic valve dimensions.
Objectives
To determine the prevalence and clinical significance of ILA on HRCT performed in patients referred for TAVR.
Methods
Consecutive pre-TAVR HRCTs performed over a 5-year period were reviewed. ILA was defined as bilateral, nondependent reticular opacities. All-cause mortality among TAVR recipients was then compared between ILA cases and non-ILA controls matched 2:1 by age and gender using Cox proportional hazards regression and the Kaplan Meier estimator.
Results
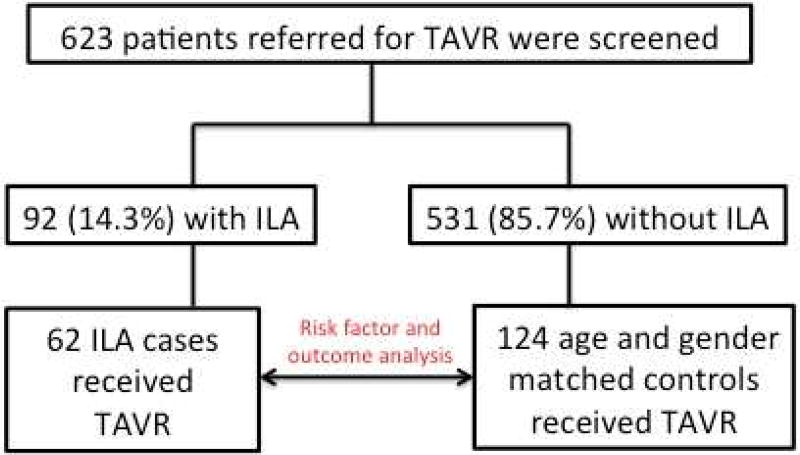
Of 623 HRCTs screened, ILA was detected in 92 (14.7%), including 62 patients that underwent TAVR. Among ILA cases, 17 (27.4%) had a typical or probable usual interstitial pneumonia pattern, suggesting a diagnosis of idiopathic pulmonary fibrosis. Survival was worse in ILA cases compared to non-ILA controls (p=0.008) and ILA was an independent predictor of mortality after multivariable adjustment (HR 3.29, 95% CI 1.34–8.08; p=0.009).
Conclusions
ILA is a common finding among patients with severe AS and is associated with increased mortality in those undergoing TAVR. Further research is needed to elucidate the biology underpinning this observation and determine whether ILA evaluation and risk stratification modulates this mortality risk.
MeSH terms: Interstitial lung disease, aortic stenosis, idiopathic interstitial pneumonia, transcatheter aortic valve replacement, interstitial lung abnormality
Introduction
Interstitial lung abnormality (ILA), defined as subclinical interstitial densities on computed tomography (CT) of the chest, may represent early interstitial lung disease (ILD) and has recently gained recognition as a clinically significant finding. ILA has been associated with decrements in pulmonary function, a high rate of progression and increased all-cause mortality.(1–4) Affecting 6–7% of the general population,(2, 4) ILA occurs more commonly with increasing age and in those with a smoking history. These risk factors also increase the likelihood of developing cardiovascular disease, including aortic stenosis.(5, 6)
Aortic stenosis (AS) affects 12% of the elderly population and severe disease results in high morbidity and mortality.(7–9) The treatment of severe AS includes medical therapy and surgical replacement of the aortic valve. Transcatheter aortic valve replacement (TAVR) has become an increasingly utilized alternative to surgical valve replacement, especially among those deemed to have high surgical risk.(10–12) Pre-TAVR high-resolution computed tomography (HRCT) of the chest is routinely performed to assess aortic root dimensions and valve size. Because such imaging includes contiguous lung views, incidental parenchymal findings such as nodules and emphysema are commonly reported.(13) These findings have been associated with a longer time to the TAVR procedure, a lower likelihood of undergoing TAVR, and worse overall outcome.(14)
In this investigation, we aimed to determine the prevalence and clinical significance of ILA among TAVR recipients. We hypothesized that co-morbid ILA would be associated with worse outcome in this patient population.
Methods
This retrospective cohort study was conducted at the University of California at Davis and was approved by our Institutional Review Board (protocol #928979), which provided a waiver of consent. A radiology database housing all CTs performed at UC-Davis was used to identify consecutive patients undergoing chest CT for pre-TAVR planning from January 1, 2012 to December 31, 2016. A junior radiologist (AK) screened all chest CTs performed during this period to identify ILA cases, defined as the presence of bilateral, nondependent reticular opacities. All positive studies, along with a random sample of negative studies (n=50), were reviewed by a thoracic radiologist (MK) with 7 years experience and blinded to clinical data to determine final ILA status and assess individual ILA features.
All CTs reviewed included complete lung series with contiguous 1mm cuts in the inspiratory supine position. Chest CTs were scored for usual interstitial pneumonia (UIP) subtype (typical UIP, probable UIP, indeterminate for UIP or inconsistent with UIP),(15) ILA features (reticulation, honeycombing, traction bronchiectasis/bronchiolectasis and ground-glass opacity), and predominant distribution of ILA in the zonal (upper, mid-lung, lower or diffuse) and transverse planes (peripheral, bronchovascular or diffuse). Concurrent emphysema, pleural effusion and pulmonary edema were also assessed. A patient was considered to have pulmonary fibrosis when traction bronchiectasis/bronchiolectasis or honeycombing was present.
The electronic medical record was retrospectively reviewed to extract pertinent clinical data, including past medical history (ILD, chronic obstructive pulmonary disease (COPD), demographics (age, gender, race), smoking history, spirometry (forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and FEV1/FVC ratio), whether TAVR was performed and what type of anesthesia was employed. Vital status was determined by review of medical records and the social security death index. A control cohort was assembled using a random number generator to randomly select TAVR recipients without ILA and match them 2:1 to ILA cases by age and gender.
Statistical Analysis
Continuous variables are reported as means with standard deviation (SD) or medians with interquartile range depending on variable distribution and are compared using a two-tailed student’s t-test or rank sum test, as appropriate. Categorical variables are reported as counts and percentages and compared using the Chi-square test or Fisher’s exact test, as appropriate. ILA risk factors were assessed using univariable and multivariable conditional logistic regression. Survival analysis was performed using univariate and multivariable Cox regression along with an unadjusted log rank test and plotted using the Kaplan-Meier survival estimator. Multivariable Cox models were adjusted for age, gender, race and smoking history. Two multivariable models were chosen, one with and one without spirometry data, which was not performed in a large minority of patients. Two-year survival was assessed with survival time defined as time from TAVR to death, loss-to-follow-up or end of study period (Jan 31, 2017). Patients lost to follow-up were censored at the time of last contact. Statistical significance was defined as p<0.05. All statistical analyses were performed using Stata (StataCorp. 2013. Release 13. College Station, TX).
Results
Of 623 chest CTs screened, 92 (14.7%) had features of ILA. Of the 92 patients with ILA, 62 underwent TAVR and were included in the final analysis (Figure 1). Groups were similar with regard to age, gender and race, but ILA cases had significantly more individuals with a smoking history (69.4% vs. 50.8%; p=0.02) and supplemental oxygen use (24.2% vs. 12.1%; p=0.03) compared to non-ILA controls (Table 1). Only 15 (24.2%) ILA cases had a documented history of ILD and there was significantly more emphysema on HRCT among ILA cases compared to non-ILA controls (45.2% vs 13.7%, p<0.001) despite a similar number of patients with documented COPD between groups. Groups were similar with regard FVC (% predicted) and FEV1 (% predicted). ILA cases had a higher mean FEV1/FVC ratio compared to non-ILA controls (0.71 vs. 0.66; p=0.02), but a large minority of patients did not undergo spirometry in each group. The median time from HRCT to TAVR was similar between groups, as was the percent of patients undergoing general anesthesia.
Figure 1.
Consort diagram
Table 1.
Clinical Characteristic Between ILA cases and controls
| Variable | ILA Cases (n=62)* |
Controls (n=124)** |
p-value |
|---|---|---|---|
|
| |||
| Age in years, mean ±SD | 83.3 (6.7) | 82.7 (6.2) | 0.56 |
| Male, n (%) | 38 (61.3) | 76 (61.3) | 1 |
| White race, n (%) | 50 (80.7) | 108 (87.1) | 0.25 |
| Ever Smoker, n (%) | 43 (69.4) | 63 (50.8) | 0.02 |
| Pack-years, median [IQR] | 21 (13–40) | 20 (4–40) | 0.18 |
| Supplemental oxygen use | 15 (24.2) | 15 (12.1) | 0.03 |
| Documented ILD | 14 (22.6) | 0 (0) | <0.001 |
| Emphysema on HRCT | 28 (45.2) | 17 (13.7) | <0.001 |
| Documented COPD | 32 (51.6) | 64 (59.8) | 0.3 |
| Spirometry | |||
| FEV1 (% predicted), mean ±SD | 88.7 (28.3) | 81.5 (26.3) | 0.14 |
| FVC (% predicted), mean ±SD | 89 (23.5) | 88.2 (23.4) | 0.85 |
| FEV1/FVC ratio, mean ±SD | 0.71 (0.12) | 0.66 (0.12) | 0.02 |
| Days to TAVR, median [IQR] | 34 (13–56) | 34 (19–82) | 0.88 |
| Received general anesthesia, n (%) | 50 (80.7) | 98 (79) | 0.79 |
Exception for n: spirometry (n=44)
Exception for n: spirometry (n=107)
Unadjusted ILA risk factors among TAVR recipients (Table 2) included smoking history (OR 2.64, 95% CI 1.25–5.62; p=0.01), supplemental oxygen use (OR 2.43, 95%CI 1.06–5.55; p=0.04), emphysema on HRCT (OR 6.77, 95% CI 2.75–16.64; p<0.001) and FEV1/FVC ratio >0.7 (OR 2.45, 95% CI 1.08–5.54; p=0.03). Race and other spirometry variables were not associated with differential ILA risk. After multi-variable adjustment, emphysema and FEV1/FVC ratio >0.7 remained independent predictors of ILA, but smoking history and supplemental oxygen use did not.
Table 2.
Variables Predicting ILA in Patients Undergoing TAVR
| Characteristic | Unadjusted | Adjusted Model 1 (n=120) | Adjusted Model 2* (n=186) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| n | OR | p-value | 95% CI |
OR | p-value | 95% CI |
OR | p-value | 95% CI |
|
| White Race | 186 | 0.62 | 0.25 | 0.27–1.41 | 0.57 | 0.51 | 0.11–3.02 | 0.52 | 0.18 | 0.20–1.34 |
| Ever Smoker | 186 | 2.64 | 0.01 | 1.25–5.62 | 1.79 | 0.43 | 0.43–7.29 | 1.41 | 0.44 | 0.59–3.38 |
| Supplemental oxygen use | 186 | 2.43 | 0.04 | 1.06–5.55 | 3.75 | 0.07 | 0.88–16.0 | 2.06 | 0.12 | 0.82–5.17 |
| Emphysema on HRCT | 186 | 6.77 | <0.001 | 2.75–16.64 | 7.58 | 0.002 | 2.09–27.5 | 5.86 | <0.001 | 2.25–15.2 |
| Spirometry | ||||||||||
| FEV1 < 80% predicted | 120 | 0.55 | 0.14 | 0.25–1.22 | 0.37 | 0.26 | 0.06–2.1 | |||
| FVC < 80% predicted | 120 | 0.75 | 0.49 | 0.33–1.71 | 0.84 | 0.84 | 0.16–4.56 | |||
| FEV1/FVC ratio >0.7 | 120 | 2.45 | 0.03 | 1.08–5.54 | 3.84 | 0.03 | 1.16–12.8 | |||
Model 1 without spirometry
variable adjustment
When assessing ILA features on chest CT (Table 3), 13 patients (21.0%) had definite UIP, 4 (6.4%) had probable UIP, 36 (58.1%) had features indeterminate for UIP and 9 (14.5%) had features inconsistent with UIP. Features of pulmonary fibrosis were observed in 27 (43.5%) patients. All patients had reticulation, 18 (29%) had honeycombing, 15 (24.2%) had traction bronchiectasis/bronchiolectasis, 17 (27.4%) had ground glass opacity and 28 (45.2%) had concurrent emphysema. Most patients had either lower lung zone predominant (46.8%) or diffuse involvement (50%) in the zonal plane, and peripheral involvement (93.6%) in the transverse plane. Pleural effusion was observed in 14 (22.6%) patients and pulmonary edema in 8 (12.9%) patients.
Table 3.
HRCT ILA Characteristics
| Variable | TAVR ILA Cohort (n=62) |
|---|---|
|
| |
| Pattern, n (%) | |
| Typical UIP | 13 (21.0) |
| Probable UIP | 4 (6.4) |
| Indeterminate for UIP | 36 (58.1) |
| Inconsistent with UIP | 9 (14.5) |
| Pulmonary Fibrosis, n (%) | 27 (43.5) |
| Reticulation, n (%) | 62 (100) |
| Honeycombing, n (%) | 18 (29) |
| Traction bronchiectasis, n (%) | 15 (24.2) |
| Ground glass opacity, n (%) | 17 (27.4) |
| Emphysema, n (%) | 28 (45.2) |
| Zonal Distribution, n (%) | |
| Upper/mid lung zone | 2 (3.2) |
| Lower lung zone | 29 (46.8) |
| Diffuse | 31 (50) |
| Transverse Distribution, n (%) | |
| Peripheral | 58 (93.6) |
| Bronchovascular | 3 (4.8) |
| Diffuse | 1 (1.6) |
| Pleural effusion, n (%) | 14 (22.6) |
| Pulmonary edema, n (%) | 8 (12.9) |
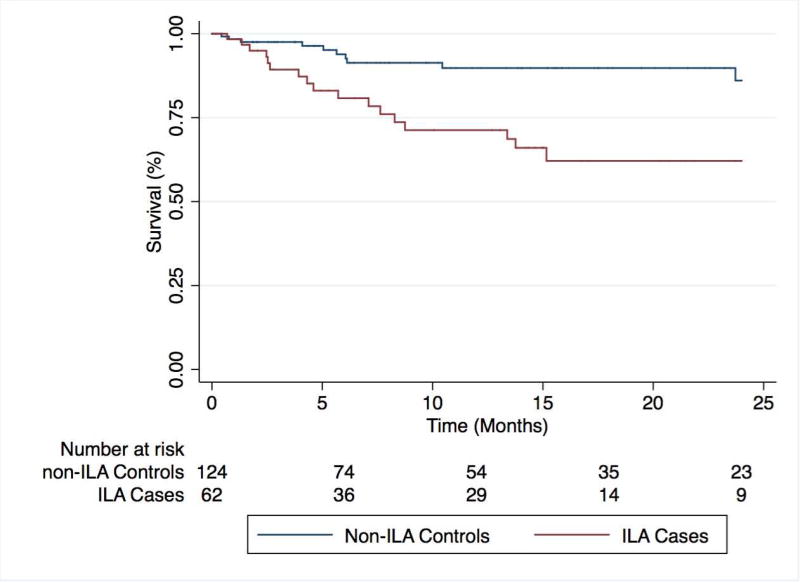
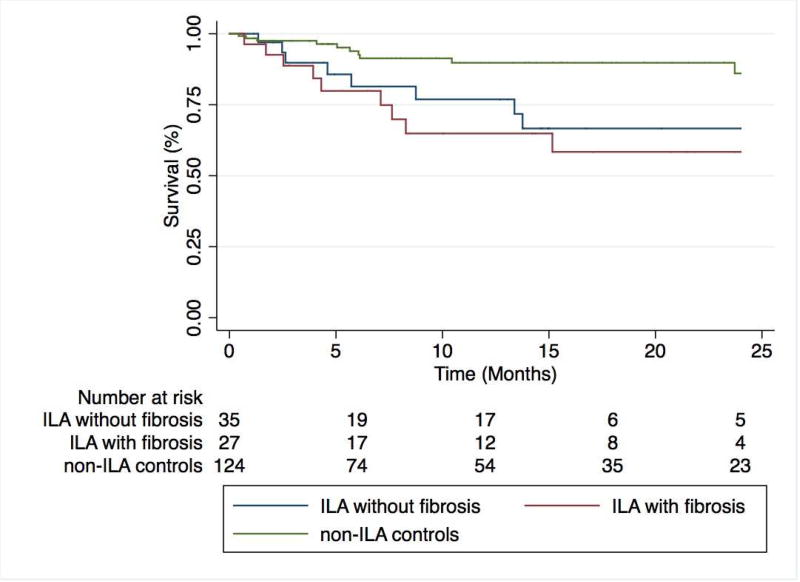
ILA cases displayed significantly worse survival than matched controls (p<0.001)(Figure 2). In unadjusted Cox regression analysis (Table 4), predictors of mortality included the presence of ILA on HRCT (HR 3.49; 95% CI 1.60–7.63; p=0.002), emphysema on HRCT (HR 2.63, 95% CI 1.23–5.61; p=0.01) and supplemental oxygen use (HR 2.27, 95% CI 1.02–5.06; p=0.049). ILA and emphysema on HRCT remained independent predictors of mortality after multivariable adjustment that did not include spirometry data, while only ILA (HR 3.29, 95% CI 1.34–8.08; p=0.009) remained an independent predictor of mortality after multivariable adjustment that included spirometry data. Among ILA cases, survival did not differ between UIP subtypes (p=0.11) or when stratifying by the presence of pulmonary fibrosis (p=0.58) (Figure 3).
Figure 2.
Two-year survival among ILA cases and non-ILA control subjects undergoing TAVR. ILA cases display significantly worse survival than matched non-ILA controls (p<0.001).
Table 4.
Variables Predicting Survival in Patients Undergoing TAVR
| Unadjusted | Adjusted Model 1 (n=151)* | Adjusted Model 2 (n=186)** | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Characteristic | n | HR | p-value | 95% CI |
HR | p- value |
95% CI |
HR | p-value | 95% CI |
| ILA on HRCT | 186 | 3.49 | 0.002 | 1.60–7.63 | 4.02 | 0.01 | 1.34–12.1 | 3.29 | 0.009 | 1.34–8.08 |
| Emphysema on HRCT | 186 | 2.63 | 0.01 | 1.23–5.61 | 2.15 | 0.2 | 0.66–6.96 | 2.73 | 0.04 | 1.06–7.05 |
| Supplemental oxygen use | 186 | 2.27 | 0.049 | 1.02–5.06 | 2.11 | 0.19 | 0.70–6.41 | 2.47 | 0.051 | 0.99–6.16 |
| General Anesthesia | 186 | 1.18 | 0.76 | 0.41–3.44 | 0.91 | 0.89 | 0.24–3.44 | 1.16 | 0.8 | 0.38–4.56 |
| Spirometry | ||||||||||
| FEV1 < 80% predicted | 151 | 1.38 | 0.45 | 0.60–3.21 | 2.48 | 0.26 | 0.52–11.9 | |||
| FVC < 80% predicted | 151 | 1.13 | 0.78 | 0.47–2.69 | 0.74 | 0.67 | 0.19–2.92 | |||
| FEV1/FVC ratio >0.7 | 151 | 1.8 | 0.18 | 0.77–4.21 | 1.73 | 0.35 | 0.55–5.41 | |||
Adjusted for age, gender, race, smoking history and unadjusted variables above
Model 1 without spirometry variable adjustment
Figure 3.
Two-year survival among ILA cases stratified by the presence of pulmonary fibrosis. ILA cases with and without features of pulmonary fibrosis on high-resolution computed tomography display similar survival patterns (p=0.58), but significantly worse survival than non-ILA control subjects (p=0.003).
Discussion
In this investigation, we showed that 15% of patients with severe AS referred for TAVR had features of ILA on HRCT. Additionally, we showed that among TAVR recipients, ILA was an independent predictor of mortality. To our knowledge, this study is among the first to specifically assess ILA epidemiology and clinical significance in patients with AS. Our findings suggest that ILA is common among those with AS and that this high-risk population may benefit from additional pulmonary evaluation prior to TAVR intervention. Our findings are also consistent with other studies demonstrating increased mortality risk among individuals with ILA.(2, 4)
The prevalence of ILA among TAVR referrals was higher than that reported in the general population, which is estimated at 6–7%.(2, 4) It was also higher than that observed in COPD (2, 3) and lung cancer-screening cohorts,(16–18) all of which reported a prevalence of <10%. The advanced age of those referred for TAVR likely explains a portion of these findings, given the anticipated morphologic changes in the aging lung on CT,(19) and the age-related increased prevalence of common ILDs such as idiopathic pulmonary fibrosis and unclassifiable idiopathic interstitial pneumonia.(20, 21) This effect may also be compounded by the high percentage of smokers in our cohort, which has also been shown to increase the risk of ILA and is a well-established risk factor for IPF.(1, 3, 16, 22) Interestingly, despite >50% of patients endorsing a smoking history in this study, emphysema was only observed in 13.7% of control subjects compared to 45.2% of ILA cases, suggesting that many ILA cases had combined pulmonary fibrosis and emphysema (CPFE). The opposing forces of emphysema-mediated obstruction and ILD-mediated restriction that occurs in CPFE may explain why mean spirometry measures were normal in both groups.(23–25) Full PFTs, including body plethysmography and diffusion capacity testing, may better identify those with CPFE, as a disproportionate reduction in diffusion capacity often results.(23–25)
Though ILA may represent early ILD, a specific ILD etiology could not be ascertained for most patients given the retrospective nature of this investigation. The UIP subtypes observed on HRCT suggest that at least 1 in 4 patients with ILA may have had IPF, as UIP and probable UIP on high-resolution CT are strongly predictive of IPF in patients without an alternate ILD etiology.(26–29) No patients in our cohort reported the use of IPF therapy at the time of TAVR, which have been shown to reduce IPF progression(30, 31) and possibly prevent acute exacerbations.(32, 33) This raises the question of whether the use of anti-fibrotic therapy in those meeting consensus criteria for IPF(27) could modulate the increased mortality risk we observed. We also observed similar survival patterns amongst ILA cases irrespective of whether pulmonary fibrosis was present on HRCT, suggesting that pulmonary fibrosis alone could not be used to sufficiently risk stratify these patients.
The biology underpinning worse outcomes among TAVR recipients with ILA remains unclear, but like other centers,(34) the majority of patients in our cohort underwent TAVR with general anesthesia. Previous studies have shown that ventilation strategy may influence outcomes in patients with ILD,(35, 36) so it is possible that intraoperative hyperoxia or ventilation-induced lung injury may have played a role. The use of general anesthesia was not associated with differential mortality risk; however there were relatively few patients undergoing conscious sedation with whom to compare outcomes. Additionally, most TAVRs performed under conscious sedation in our study occurred after 2015, leaving less follow-up time for outcomes modeling.
Our study has several limitations. First, this was a single center study, which may limit generalizability. Next was the retrospective nature of the investigation, which detects only association, and not causation. Another limitation was the large minority of patients with missing spirometry data, which limited our ability to conduct multivariable adjusted modeling that included these variables. We addressed this by presenting two final multivariable adjusted models. A final limitation was the inability to determine ILD etiology in those with ILA. This stemmed from the nature of the patients referrals in our study, which were overwhelmingly from outside institutions. However, even among those with documented ILD, it is recognized that diagnostic agreement for ILD etiology between community physicians and academic pulmonologists with ILD expertise is poor.(37) Additionally, our findings suggested that ILD etiology may be less important than the general presence of ILA in these patients.
Conclusion
ILA is a commonly encountered HRCT finding in patients referred for TAVR and is associated with an increased risk of mortality in patients undergoing TAVR. While the precise etiology of individual ILAs remains unclear, the CT patterns observed suggest a high percentage of patients with undiagnosed IPF and other forms of pulmonary fibrosis. Given the poor outcomes associated with delayed ILD center referral,(35) along with recent advances in IPF therapeutics,(36–39) cardiologists should consider a referral to an ILD center for those patients with ILA prior to TAVR intervention, as this has the potential to modulate outcomes in this patient population.
Highlights.
Interstitial lung abnormalities are commonly observed in patients undergoing TAVR and are more common in this patient population than the general population
The presence of interstitial lung abnormalities are associated with worse outcome in those undergoing TAVR
Pulmonology evaluation should be considered for patients referred for TAVR who are found to have interstitial lung abnormalities, as pulmonary risk stratification and may help improve outcomes
Acknowledgments
Funding
This study was funded by a grant from the National Heart Lung and Blood Institute (K23HL138190) and by the UC-Davis Gordon Wong Endowment.
MK has received speaking fees from Boehringer Ingelheim. TS has received consultancy fees from Abbott and Millipede. GW has received speaking fees from Edwards Lifesciences. DWB has received consultancy fees from Millipede. JMO has received speaking fees and served on advisory boards for Genentech and Boehringer Ingelheim.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contribution
MK and AK reviewed chest CTs for this study and contributed to conceptualization, interpretation of results and manuscript preparation. SA and EL extracted clinical data for this study and contributed to interpretation of results. EF and MJ assisted with IRB submission, interpretation of results and manuscript preparation. CM extracted clinical data for this study and contributed to interpretation of results. TS, GW, WB and JS performed TAVR, collected clinical data and assisted with interpretation of results. JMO contributed to the conceptualization, data analysis, interpretation of results and manuscript preparation.
Conflict of Interest Statement
AK, SA, EL, EF, MJ, CM, GW and JS have no declarations.
References
- 1.Lederer DJ, Enright PL, Kawut SM, Hoffman EA, Hunninghake G, van Beek EJ, Austin JH, Jiang R, Lovasi GS, Barr RG. Cigarette smoking is associated with subclinical parenchymal lung disease: The multi-ethnic study of atherosclerosis (mesa)-lung study. American journal of respiratory and critical care medicine. 2009;180:407–414. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Putman RK, Hatabu H, Araki T, Gudmundsson G, Gao W, Nishino M, Okajima Y, Dupuis J, Latourelle JC, Cho MH, El-Chemaly S, Coxson HO, Celli BR, Fernandez IE, Zazueta OE, Ross JC, Harmouche R, Estepar RS, Diaz AA, Sigurdsson S, Gudmundsson EF, Eiriksdottir G, Aspelund T, Budoff MJ, Kinney GL, Hokanson JE, Williams MC, Murchison JT, MacNee W, Hoffmann U, O'Donnell CJ, Launer LJ, Harrris TB, Gudnason V, Silverman EK, O'Connor GT, Washko GR, Rosas IO, Hunninghake GM Evaluation of CLtIPSEI, Investigators CO. Association between interstitial lung abnormalities and all-cause mortality. JAMA : the journal of the American Medical Association. 2016;315:672–681. doi: 10.1001/jama.2016.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, Estepar RS, Lynch DA, Brehm JM, Andriole KP, Diaz AA, Khorasani R, D'Aco K, Sciurba FC, Silverman EK, Hatabu H, Rosas IO Investigators CO. Lung volumes and emphysema in smokers with interstitial lung abnormalities. The New England journal of medicine. 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araki T, Putman RK, Hatabu H, Gao W, Dupuis J, Latourelle JC, Nishino M, Zazueta OE, Kurugol S, Ross JC, San Jose Estepar R, Schwartz DA, Rosas IO, Washko GR, O'Connor GT, Hunninghake GM. Development and progression of interstitial lung abnormalities in the framingham heart study. American journal of respiratory and critical care medicine. 2016;194:1514–1522. doi: 10.1164/rccm.201512-2523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindroos M, Kupari M, Heikkila J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: An echocardiographic study of a random population sample. J Am Coll Cardiol. 1993;21:1220–1225. doi: 10.1016/0735-1097(93)90249-z. [DOI] [PubMed] [Google Scholar]
- 6.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular health study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 7.Bach DS. Prevalence and characteristics of unoperated patients with severe aortic stenosis. The Journal of heart valve disease. 2011;20:284–291. [PubMed] [Google Scholar]
- 8.Perera S, Wijesinghe N, Ly E, Devlin G, Pasupati S. Outcomes of patients with untreated severe aortic stenosis in real-world practice. N Z Med J. 2011;124:40–48. [PubMed] [Google Scholar]
- 9.Osnabrugge RL, Mylotte D, Head SJ, Van Mieghem NM, Nkomo VT, LeReun CM, Bogers AJ, Piazza N, Kappetein AP. Aortic stenosis in the elderly: Disease prevalence and number of candidates for transcatheter aortic valve replacement: A meta-analysis and modeling study. J Am Coll Cardiol. 2013;62:1002–1012. doi: 10.1016/j.jacc.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S Investigators PT. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. The New England journal of medicine. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 11.Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG Investigators P. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. The New England journal of medicine. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 12.Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, Chetcuti S, Gleason T, Heiser J, Lange R, Merhi W, Oh JK, Olsen PS, Piazza N, Williams M, Windecker S, Yakubov SJ, Grube E, Makkar R, Lee JS, Conte J, Vang E, Nguyen H, Chang Y, Mugglin AS, Serruys PW, Kappetein AP Investigators S. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. The New England journal of medicine. 2017;376:1321–1331. doi: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- 13.Hussien AF, Jeudy J, Kligerman SJ, White CS. Thoracic incidental findings in preoperative computed tomography evaluation for transcatheter aortic valve implantation (tavi) Journal of thoracic imaging. 2016;31:183–188. doi: 10.1097/RTI.0000000000000208. [DOI] [PubMed] [Google Scholar]
- 14.Showkathali R, Sen A, Brickham B, Dworakowski R, Wendler O, MacCarthy P. "Incidental findings" during tavi work-up: More than just an inconvenience. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2015;11:465–469. doi: 10.4244/EIJY14M06_04. [DOI] [PubMed] [Google Scholar]
- 15.Lynch DA, Sverzellati N, Travis WD, Brown KK, Colby TV, Galvin JR, Goldin JG, Hansell DM, Inoue Y, Johkoh T, Nicholson AG, Knight SL, Raoof S, Richeldi L, Ryerson CJ, Ryu JH, Wells AU. Diagnostic criteria for idiopathic pulmonary fibrosis: A fleischner society white paper. The Lancet Respiratory medicine. 2017 doi: 10.1016/S2213-2600(17)30433-2. [DOI] [PubMed] [Google Scholar]
- 16.Jin GY, Lynch D, Chawla A, Garg K, Tammemagi MC, Sahin H, Misumi S, Kwon KS. Interstitial lung abnormalities in a ct lung cancer screening population: Prevalence and progression rate. Radiology. 2013;268:563–571. doi: 10.1148/radiol.13120816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salvatore M, Henschke CI, Yip R, Jacobi A, Eber C, Padilla M, Knoll A, Yankelevitz D. Journal club: Evidence of interstitial lung disease on low-dose chest ct images: Prevalence, patterns, and progression. AJR American journal of roentgenology. 2016;206:487–494. doi: 10.2214/AJR.15.15537. [DOI] [PubMed] [Google Scholar]
- 18.Sverzellati N, Guerci L, Randi G, Calabro E, La Vecchia C, Marchiano A, Pesci A, Zompatori M, Pastorino U. Interstitial lung diseases in a lung cancer screening trial. The European respiratory journal. 2011;38:392–400. doi: 10.1183/09031936.00201809. [DOI] [PubMed] [Google Scholar]
- 19.Copley SJ. Morphology of the aging lung on computed tomography. Journal of thoracic imaging. 2016;31:140–150. doi: 10.1097/RTI.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 20.Navaratnam V, Fleming KM, West J, Smith CJ, Jenkins RG, Fogarty A, Hubbard RB. The rising incidence of idiopathic pulmonary fibrosis in the u.K. Thorax. 2011;66:462–467. doi: 10.1136/thx.2010.148031. [DOI] [PubMed] [Google Scholar]
- 21.Patterson KC, Shah RJ, Porteous MK, Christie JD, D'Errico CA, Chadwick M, Triano MJ, Deshpande C, Rossman MD, Litzky LA, Kreider M, Miller WT., Jr Interstitial lung disease in the elderly. Chest. 2017;151:838–844. doi: 10.1016/j.chest.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: A risk factor for idiopathic pulmonary fibrosis. American journal of respiratory and critical care medicine. 1997;155:242–248. doi: 10.1164/ajrccm.155.1.9001319. [DOI] [PubMed] [Google Scholar]
- 23.Cottin V, Nunes H, Brillet PY, Delaval P, Devouassoux G, Tillie-Leblond I, Israel-Biet D, Court-Fortune I, Valeyre D, Cordier JF. Combined pulmonary fibrosis and emphysema: A distinct underrecognised entity. The European respiratory journal. 2005;26:586–593. doi: 10.1183/09031936.05.00021005. [DOI] [PubMed] [Google Scholar]
- 24.Kurashima K, Takayanagi N, Tsuchiya N, Kanauchi T, Ueda M, Hoshi T, Miyahara Y, Sugita Y. The effect of emphysema on lung function and survival in patients with idiopathic pulmonary fibrosis. Respirology. 2010;15:843–848. doi: 10.1111/j.1440-1843.2010.01778.x. [DOI] [PubMed] [Google Scholar]
- 25.Ryerson CJ, Hartman T, Elicker BM, Ley B, Lee JS, Abbritti M, Jones KD, King TE, Jr, Ryu J, Collard HR. Clinical features and outcomes in combined pulmonary fibrosis and emphysema in idiopathic pulmonary fibrosis. Chest. 2013;144:234–240. doi: 10.1378/chest.12-2403. [DOI] [PubMed] [Google Scholar]
- 26.Brownell R, Moua T, Henry TS, Elicker BM, White D, Vittinghoff E, Jones KD, Urisman A, Aravena C, Johannson KA, Golden JA, King TE, Jr, Wolters PJ, Collard HR, Ley B. The use of pretest probability increases the value of high-resolution ct in diagnosing usual interstitial pneumonia. Thorax. 2017 doi: 10.1136/thoraxjnl-2016-209671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE, Jr, Kondoh Y, Myers J, Muller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schunemann HJ. An official ats/ers/jrs/alat statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. American journal of respiratory and critical care medicine. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raghu G, Lynch D, Godwin JD, Webb R, Colby TV, Leslie KO, Behr J, Brown KK, Egan JJ, Flaherty KR, Martinez FJ, Wells AU, Shao L, Zhou H, Pedersen PS, Sood R, Montgomery AB, O'Riordan TG. Diagnosis of idiopathic pulmonary fibrosis with high-resolution ct in patients with little or no radiological evidence of honeycombing: Secondary analysis of a randomised, controlled trial. The Lancet Respiratory medicine. 2014;2:277–284. doi: 10.1016/S2213-2600(14)70011-6. [DOI] [PubMed] [Google Scholar]
- 29.Lynch DA, Sverzellati N, Travis WD, Brown KK, Colby TV, Galvin JR, Goldin JG, Hansell DM, Inoue Y, Johkoh T, Nicholson AG, Knight SL, Raoof S, Richeldi L, Ryerson CJ, Ryu JH, Wells AU. Diagnostic criteria for idiopathic pulmonary fibrosis: A fleischner society white paper. The Lancet Respiratory medicine. 2018;6:138–153. doi: 10.1016/S2213-2600(17)30433-2. [DOI] [PubMed] [Google Scholar]
- 30.King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, Lederer DJ, Nathan SD, Pereira CA, Sahn SA, Sussman R, Swigris JJ, Noble PW, Group AS. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. The New England journal of medicine. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 31.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, Noble PW, Selman M, Taniguchi H, Brun M, Le Maulf F, Girard M, Stowasser S, Schlenker-Herceg R, Disse B, Collard HR. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. The New England journal of medicine. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 32.Ley B, Swigris J, Day BM, Stauffer JL, Raimundo K, Chou W, Collard HR. Pirfenidone reduces respiratory-related hospitalizations in idiopathic pulmonary fibrosis. American journal of respiratory and critical care medicine. 2017;196:756–761. doi: 10.1164/rccm.201701-0091OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Churg A, Muller NL, Wright JL. Respiratory bronchiolitis/interstitial lung disease: Fibrosis, pulmonary function, and evolving concepts. Arch Pathol Lab Med. 2010;134:27–32. doi: 10.5858/134.1.27. [DOI] [PubMed] [Google Scholar]
- 34.Hagmeyer L, Randerath W. Smoking-related interstitial lung disease. Deutsches Arzteblatt international. 2015;112:43–50. doi: 10.3238/arztebl.2015.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez-Perez ER, Yilmaz M, Jenad H, Daniels CE, Ryu JH, Hubmayr RD, Gajic O. Ventilator settings and outcome of respiratory failure in chronic interstitial lung disease. Chest. 2008;133:1113–1119. doi: 10.1378/chest.07-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi SM, Lee J, Park YS, Cho YJ, Lee CH, Lee SM, Yoon HI, Yim JJ, Lee JH, Yoo CG, Lee CT, Kim YW, Park JS. Postoperative pulmonary complications after surgery in patients with interstitial lung disease. Respiration; international review of thoracic diseases. 2014;87:287–293. doi: 10.1159/000357046. [DOI] [PubMed] [Google Scholar]
- 37.Flaherty KR, Andrei AC, King TE, Jr, Raghu G, Colby TV, Wells A, Bassily N, Brown K, du Bois R, Flint A, Gay SE, Gross BH, Kazerooni EA, Knapp R, Louvar E, Lynch D, Nicholson AG, Quick J, Thannickal VJ, Travis WD, Vyskocil J, Wadenstorer FA, Wilt J, Toews GB, Murray S, Martinez FJ. Idiopathic interstitial pneumonia: Do community and academic physicians agree on diagnosis? American journal of respiratory and critical care medicine. 2007;175:1054–1060. doi: 10.1164/rccm.200606-833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]