Diffuse large B-cell lymphoma (DLBCL) is an aggressive hematologic malignancy with more than two-thirds of the patients cured with first-line rituximab-based treatments. However, relapsing or refractory patients have a very poor survival despite salvage therapies.1 The PD-1/PD-L1 pathway plays a major role in peripheral tolerance and homeostasis by inhibiting T-cells activation. By expressing PD-L1 on their surface, the tumor cells as well as some non-malignant cells from the tumor microenvironment co-opt the immune checkpoint pathways to evade immune destruction.2 Immunotherapies targeting the PD-1/PD-L1 pathway have recently shown remarkable clinical benefit in subsets of solid cancer patients3 and in DLBCL patients undergoing autologous stem-cell transplant.4 Novel prognostic biomarkers are necessary to better identify the patients who would benefit the most from immunotherapy. We recently reported that the soluble PD-L1 protein expression (sPD-L1) was significantly increased in the blood of DLBCL patients versus healthy controls, and that elevated sPD-L1 was prognostic for overall survival (OS).5 Here, we confirmed these initial findings in an independent cohort of newly diagnosed DLBCL patients, evaluated tumor/microenvironment expression, and investigated the prognostic relevance of sPD-L1 on risk-stratified DLBCL patients when compared against established clinical, biological, and molecular prognostic markers.
The previously published discovery cohort consisted of 283 newly diagnosed aggressive DLBCL patients, age 18 to 60 years, from the French GOELAMS 075 multicenter randomized clinical trial (Supplemental Tables S1 and S2). The patients were treated with R-CHOP (51%) or R-CEEP/auto (49%). The five-year overall survival (OS) was estimated at 81.7% (95%CI 77.3–86.4). There was no OS difference between the two arms of treatment. The replication cohort consisted of 225 de novo DLBCL patients, age 18 to 92 years, all stages, from a prospective, observational cohort from the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (SPORE).6 The patients were treated by R-CHOP (84%) or R-CHOP-like (16%) treatments. The five-year OS estimate was 77.6% (95%CI 72.1–83.5). The matched control groups consisted of 60 French subjects and 98 US subjects, respectively. Plasma samples were collected pre-treatment using P100® tubes for the French study and EDTA tubes for the US study, and stored at −80°. The sPD-L1 production was measured in Rennes, France, for both cohorts using the enzyme-linked immunosorbent assay (PDCD1LG1 ELISA kit, USCN Life Science Inc, Wuhan, China), according to the manufacturer instructions (Supplementary Materials and Methods).
Soluble PD-L1 was significantly higher in pre-treatment plasma samples from DLBCL patients compared to matched controls in the US study (P<0.0001), replicating the original French study (Supplementary Figure S1). The cutpoint for sPD-L1 of DLBCL patients was defined as equal to twice the median value of the sPD-L1 levels in the respective matched control group (1357 pg/mL for the French cohort, 1652 pg/mL for the US cohort). Compared to their respective control groups, French DLBCL patients were 10.7 times more likely to have elevated sPD-L1 levels (95%CI 3.3–35.1) and US DLBCL patients, 8.8 (95%CI 4.4–19.6).
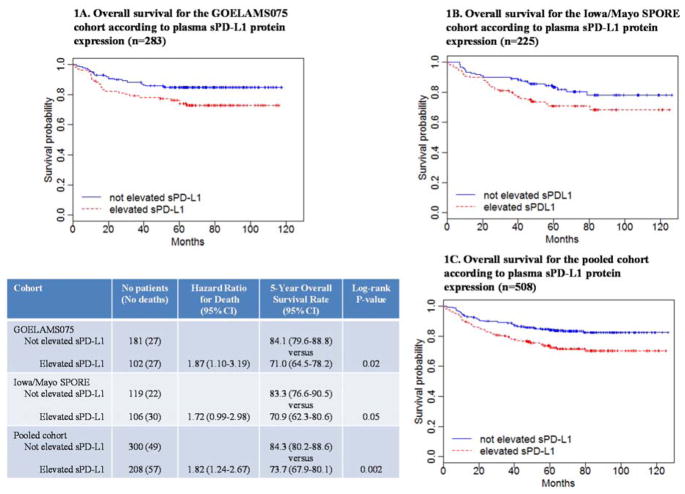
In the French cohort, elevated sPD-L1 levels were associated with inferior OS (HR=1.87; 95%CI 1.10–3.19), and this was replicated in the US cohort (HR=1.72; 95%CI 0.99–2.98). The five-year OS in patients with elevated sPD-L1 was (70.9%, 95%CI 62.3–80.6) in US and (71.0%, 95%CI 64.5–78.2) in French patients versus (83.3%, 95%CI 76.6–90.5) and (84.1%, 95%CI 79.6–88.8) in US and French patients with not elevated sPD-L1, respectively (Figure 1).
Figure 1.
Kaplan-Meier overall survival estimates with respect to plasma sPD-L1 at diagnosis, for the French and the US cohorts considering a sPD-L1 cut-off of 1357 pg/mL and 1652 pg/mL respectively (Panel A and B, respectively), and for the pooled cohort (Panel C).
Since the French results were replicated in the US cohort, we next pooled both cohorts to increase power for multivariate and subset analyses (Supplemental Table S1). In the pooled cohort, 54% were intermediate-risk and 9% high-risk IPI patients. The median overall follow-up was 65 months (range, 1–127). The five-year OS estimate was 80.0% (95%CI 76.5–83.6) and the median OS had not yet been reached. At the univariate level, elevated sPD-L1, high-intermediate and high-risk IPI, bone marrow involvement, abnormal AMLPI7 (Absolute Monocyte-to-Lymphocyte Prognostic Index), and abnormal NLR8 (Neutrophil-to-Lymphocyte Ratio) were associated with inferior OS (Supplementary Table S3). There was no OS difference between GCB and non-GCB (P=0.77). Using each cohort-specific cutpoints, patients in the pooled analysis with elevated sPD-L1 had a statistically inferior OS compared to not elevated sPD-L1 (five-year OS, 73.7% (95%CI 67.9–80.1) vs. 84.3% (95%CI 80.2–88.6); P=0.002; Figure 1). The hazard ratio for elevated sPD-L1 was 1.82 (95%CI 1.24–2.67; unadjusted model).
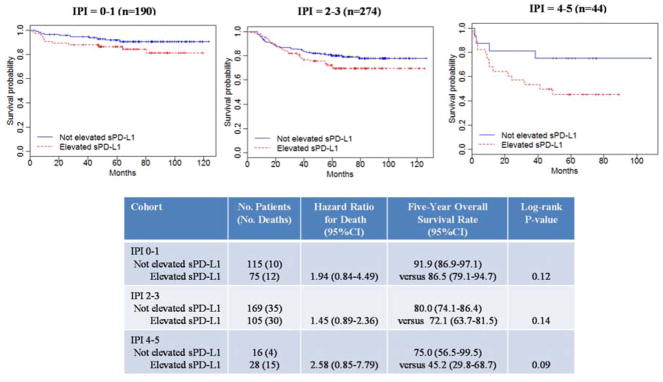
Patients with elevated sPD-L1 tended toward inferior OS compared to not elevated sPD-L1 regardless of IPI (Figure 2). The adverse effect of elevated sPD-L1 was strongest among high-risk IPI (HR=2.58, 95%CI 0.85–7.79) and did identify a subgroup with 5-year OS <50%. However, the interpretation should be tempered by the small numbers of patients and events in this group.
Figure 2.
Kaplan-Meier overall survival estimates of plasma sPD-L1 levels for the pooled cohort, according to IPI.
For either the French or US cohort, elevated sPD-L1 plus abnormal AMLPI or NLR tended toward an unfavorable outcome. This was not true for normal AMLPI or NLR, except for normal NLR French patients for who elevated sPD-L1 tended to be of inferior prognosis (Supplemental Figure S2). In the pooled cohort, patients with elevated versus not elevated sPD-L1 had a significantly worse OS among patients with abnormal AMLPI (HR=1.94, 95%CI 1.27–2.95; P=0.002) or abnormal NLR (HR=1.97, 95%CI 1.20–3.21; P=0.007) (Supplementary Figure S3). Only GCB DLBCLs with elevated sPD-L1 had a significantly inferior survival compared to not elevated sPD-L1 (Supplemental Figure S4).
AMLPI rather than NLR was included in the multivariate model as 92% of abnormal NLR cases were abnormal AMLPI patients (vs. 65% inversely). COO was excluded due to over 40% of patients with missing data. In the multivariate model, elevated sPD-L1 (HR=1.65; 95%CI 1.10–2.45; P=0.016), age (HR=1.42 per 10-year increment; 95%CI 1.20–1.66; P<0.0001), high-risk IPI (HR=2.95; 95%CI 1.51–5.74; P=0.002), abnormal AMLPI (HR=2.28; 95%CI 1.26–4.15; P=0.007), and bone marrow involvement (HR=1.68; 95%CI 1.07–2.66; P=0.025) all remained statistically significant.
Next we studied PD-L1 expression on the tumor cells and the non-malignant cells of the tumor microenvironment. We focused on the US cohort for which a monoclonal PD-L1 antibody was used. Seventy-two cases were successfully stained (GCB, n=32; non-GCB, n=21; no COO available otherwise). PD-L1 was expressed on tumor cells for 43 cases (60%) and on non-malignant cells of the tumor microenvironment for 44 cases (61%). PD-L1 levels expressed on tumor cells and in the microenvironment were positively correlated (Pearson’s coefficient r=0.85, P<0.0001). Patients expressing ≥5% tumor PD-L1 were somewhat more likely to have elevated sPD-L1 levels (65% versus 55%); expression versus no expression of PD-L1 in the tumor microenvironment showed similar sPD-L1 levels (59% versus 64%) (Supplemental Table S4). Tumor PD-L1 was not associated with OS (HR=0.90, 95%CI 0.33–2.45; P=0.84), while PD-L1 expressed in the tumor microenvironment had a better prognosis (HR=0.50, 95%CI 0.18–1.37; P=0.18).
In this paper, we validated prior findings5 that DLBCL patients have a higher level of sPD-L1 at diagnosis compared to matched healthy controls, and that elevated levels of sPD-L1 is an adverse prognostic factor for OS in newly diagnosed DLBCL patients treated with rituximab plus anthracycline-based therapy. These findings extend the prior results, as the US cohort is more representative of the entire DLBCL population, covering all ages and clinical presentation.
The addition of rituximab to conventional chemotherapy has significantly improved the survival of DLBCL patients and the standard IPI score is no longer capable of discriminating patients with less than 50% overall survival.9 In each IPI risk group of the pooled cohort, elevated sPD-L1 was associated with a poor OS. Interestingly, elevated sPD-L1 in high-risk IPI patients was able to identify patients with a much poorer outcome. In the multivariate model, sPD-L1 was independent of IPI, age, bone marrow involvement, and AMLPI or NLR. Focusing on the 18 DLBCLs with high-risk IPI, age >60 and abnormal AMLPI, elevated sPD-L1 patients were more likely to die (91% deaths) compared to not elevated sPD-L1 patients (29%). This may suggest that very high-risk DLBCLs might be identified using clinical features and minimally-invasive measurements of blood components, even though this association would require confirmation on a larger cohort.
Soluble PD-L1 was not correlated with PD-L1 expression on tumor cells nor on non-malignant cells in the tumor microenvironment. The production of sPD-L1 likely involves a complex mechanism not directly related to PD-L1 expression at the tumor site. A hypothesis may be that sPD-L1 is released by circulating myeloïd-derived suppressor cells.10
We found no association between the PD-L1 expression on tumor cells and OS. This is consistent with a recent report on R-CHOP treated Korean DLBCL patients that used the same PD-L1 antibody,11 but is not universal.12 We reported that DLBCL patients expressing PD-L1 on non-malignant cells tended to have a better outcome than those with no expression. Macrophages express PD-L1 and, tumor-infiltrating macrophages were associated with a favorable outcome in DLBCLs only when rituximab was given.13 These findings were also controversial.14 Overall, this indicates that the role of the tumor microennvironment needs to be further assessed.15
In summary, pre-treatment soluble PD-L1 was confirmed to be an adverse prognostic factor, independent of IPI, in de novo R-CHOP treated DLBCs. In the light of the remarkable success of immunotherapies targeting the PD-1/PD-L1 immune checkpoint in relapsed/refractory lymphomas, sPD-L1 should be further evaluated for its predictive capacity regarding response in patients treated with anti-PD-1 or anti-PD-L1.
Supplementary Material
Acknowledgments
This study is supported by the French Health Minister (BMS_LyTrans project; NCT01287923), the National Institute of Cancer (INCa Translational Research 2010), the French Lymphoma Study Association (LYSA), the French National Agency for Research (ANR Carnot/CALYM), the National Cancer Institute (P50 CA97274, R01 CA92153), and the Henry J Predolin Foundation. Concerning the French cohort, we are indebted to the LYSA pathology platform (Hôpital Henri Mondor, Créteil) for immunohistochemical experiments; the Centre de Ressources Biologiques CRB-Santé (BB-0033-00056) of the Rennes hospital for providing high quality samples. This work was partly supported by a French National Cancer Institute post-doctoral fellowship (grant number PDOC-RC-GO/07-003) to the first author (D.R.).
Footnotes
Disclaimers
The authors declare no conflict of interest.
Contributors
T.F. raised the fund. I.A. did the sPD-L1 measurements. D.R. did the data analysis. M.J.M. and J.R.C. contributed to the statistics. D.R., J.R.C. and T.F. did the data interpretation and wrote the manuscript. N.M. designed the parent French study. J.R.C. designed the US study. G.L., and M.P. did the French database update and immunohistochemistry. A.L.F. did the immunohistochemistry on the MER cohort. C.P. did the blood sample collection. I.A., A.L.F, M.J.M., C.P., T.M.H., S.M.A., B.K.L., K.T., T.E.W., T.L., S.L.S., M.R. and N.M. contributed to the writing and approving the manuscript.
References
- 1.Friedberg JW. Relapsed/Refractory Diffuse Large B-Cell Lymphoma. ASH Educ Program Book. 2011;2011:498–505. doi: 10.1182/asheducation-2011.1.498. [DOI] [PubMed] [Google Scholar]
- 2.Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A. Programmed death-1 pathway in cancer and autoimmunity. Clin Immunol. 2014;153:145–152. doi: 10.1016/j.clim.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Chen DS, Mellman I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Armand P, Nagler A, Weller EA, Devine SM, Avigan DE, Chen Y-B, et al. Disabling Immune Tolerance by Programmed Death-1 Blockade With Pidilizumab After Autologous Hematopoietic Stem-Cell Transplantation for Diffuse Large B-Cell Lymphoma: Results of an International Phase II Trial. J Clin Oncol. 2013;31:4199–4206. doi: 10.1200/JCO.2012.48.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossille D, Gressier M, Damotte D, Maucort-Boulch D, Pangault C, Semana G, et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia. 2014;28:2367–2375. doi: 10.1038/leu.2014.137. [DOI] [PubMed] [Google Scholar]
- 6.Drake MT, Maurer MJ, Link BK, Habermann TM, Ansell SM, Micallef IN, et al. Vitamin D Insufficiency and Prognosis in Non-Hodgkin’s Lymphoma. J Clin Oncol. 2010;28:4191–4198. doi: 10.1200/JCO.2010.28.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilcox RA, Ristow K, Habermann TM, Inwards DJ, Micallef INM, Johnston PB, et al. The absolute monocyte and lymphocyte prognostic score predicts survival and identifies high-risk patients in diffuse large-B-cell lymphoma. Leukemia. 2011;25:1502–1509. doi: 10.1038/leu.2011.112. [DOI] [PubMed] [Google Scholar]
- 8.Proctor MJ, McMillan DC, Morrison DS, Fletcher CD, Horgan PG, Clarke SJ. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer. 2012;107:695–699. doi: 10.1038/bjc.2012.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziepert M, Hasenclever D, Kuhnt E, Glass B, Schmitz N, Pfreundschuh M, et al. Standard International Prognostic Index Remains a Valid Predictor of Outcome for Patients With Aggressive CD20+ B-Cell Lymphoma in the Rituximab Era. J Clin Oncol. 2010;28:2373–2380. doi: 10.1200/JCO.2009.26.2493. [DOI] [PubMed] [Google Scholar]
- 10.Azzaoui I, Uhel F, Rossille D, Pangault C, Dulong J, Le Priol J, et al. T-cell defect in diffuse large B-cell lymphomas involves expansion of myeloid-derived suppressor cells. Blood. 2016;128:1081–1092. doi: 10.1182/blood-2015-08-662783. [DOI] [PubMed] [Google Scholar]
- 11.Kwon D, Kim S, Kim P-J, Go H, Nam SJ, Paik JH, et al. Clinicopathological analysis of programmed cell death 1 and programmed cell death ligand 1 expression in the tumour microenvironments of diffuse large B cell lymphomas. Histopathology. 2015 doi: 10.1111/his.12882. [DOI] [PubMed] [Google Scholar]
- 12.Kiyasu J, Miyoshi H, Hirata A, Arakawa F, Ichikawa A, Niino D, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. 2015;126:2193–2201. doi: 10.1182/blood-2015-02-629600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riihijarvi S, Fiskvik I, Taskinen M, Vajavaara H, Tikkala M, Yri O, et al. Prognostic influence of macrophages in patients with diffuse large B-cell lymphoma: a correlative study from a Nordic phase II trial. Haematologica. 2015;100:238–245. doi: 10.3324/haematol.2014.113472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wada N, Zaki MAA, Hori Y, Hashimoto K, Tsukaguchi M, Tatsumi Y, et al. Tumour-associated macrophages in diffuse large B-cell lymphoma: a study of the Osaka Lymphoma Study Group. Histopathology. 2012;60:313–319. doi: 10.1111/j.1365-2559.2011.04096.x. [DOI] [PubMed] [Google Scholar]
- 15.Scott DW, Gascoyne RD. The tumour microenvironment in B cell lymphomas. Nat Rev Cancer. 2014;14:517–534. doi: 10.1038/nrc3774. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.