Abstract
Growing interest in the bactericidal effect of graphene and graphene-derived nanomaterials has led to the investigation and effective publication of the bactericidal effects of the substratum, many of which present highly conflicting material. The nature of bacterial cell death on graphene bio-interfaces, therefore, remains poorly understood. Here, we review recent findings on the bactericidal effect of graphene and graphene-derived nanomaterials, and proposed mechanisms of cell inactivation, due to mechanical contact with graphene materials, including lipid extraction, physical damage to membranes and pore formation.
Keywords: graphene, bactericidal effect, bio-interfaces
1. Introduction
Graphene is a two-dimensional, single atomic layer of hexagonally arranged sp2 bonded carbon atoms. The two-dimensional honeycomb lattice is the basic building block for all other graphitic materials [1]. Over the past 50 years, graphene and graphene-derived materials have been studied extensively due to their excellent electrical conductivity, mechanical strength and optical absorption properties [1–4]. Graphene and graphene oxide (GO) are easily fabricated through the chemical exfoliation of graphite oxide (GtO) to produce atomically thin flakes. In fact, GtO has offered researchers the potential to fabricate large-scale, chemically modified graphene materials [5,6]. Graphene-derived materials, such as graphene, GO and reduced GO (rGO), all present with differing thermal, optical, electrical and mechanical properties and have proved versatility in being processed into a wide range of new materials with distinctly different properties [7]. Differences can be accounted for by introduced defects into the graphitic structure during the reduction of GO and uncontrolled chemical functionalisation or adsorption of impurities, dramatically changing its properties [6].
Recently, the strong antibacterial activity of graphene materials has been brought to light [8–11]. The first reported instances of the antimicrobial activity of graphene showed very high rates of cell inactivation against both Gram-negative and Gram-positive bacteria (table 1). These initial investigations into graphene–bio-interfaces revealed that direct contact between bacterial cells and the ‘sharp’ edges of the graphene nanowalls were responsible for severe membrane deformation and the efflux of cytoplasmic material [9,10] due to a so-called mechano-bactericidal effect: an atom-thin layer of graphene acts as a blade to cut the cell membrane. Another suggested mechanism is induced oxidation. GO contains a range of reactive oxygen functional groups (epoxy, hydroxyl and carboxyl), which makes it less hydrophobic and readily dispersed in water due to electrostatic repulsions and enhanced solvation provided by attached oxygen species [2]. Therefore, antibacterial activity has been the focus of many investigations where bacteria are available to interact with GO nanosheets dispersed in aqueous media. GO dispersed in buffer solution was shown to inactivate 98.5% of Escherichia coli within a 2 h incubation and similarly, within 1 h, 84% of Staphylococcus aureus were observed to be dead (table 1) [9,10]. Measurements of the intracellular material leaked into solution after incubation on nanostructured graphene surfaces were undertaken by measuring the concentration of RNA [10]. Similar bactericidal activity has also been observed for copper oxide ‘nanoflakes’ [17]. Therefore, the deposition of graphene and GO nanomaterials onto metal substrata such as stainless steel has made provision for effective antibacterial surfaces. Graphene-derived nanomaterials have since been deposited onto fabrics and filters and hybridized with other antimicrobial agents such as nanocomposites for use in biomedical applications and water disinfection [12,13,18,19].
Table 1.
Bactericidal activity and cell-inactivation effect at bio-interface of graphene-derived materials.
| graphene-based nanomaterial | bacteria | indicative antibacterial activitya | cell-inactivation effect | source |
|---|---|---|---|---|
| graphene and graphene oxide nanowalls | E. coli | 59% | cell membrane damage | [10] |
| S. aureus | 84% | |||
| graphene oxide nanosheets and reduced graphene oxide |
E. coli | 98.5% | loss of cellular integrity | [9] |
| graphene oxide nanosheets | E. coli | 89% | ROS-independent oxidative stress | [8] |
| graphene oxide fabric | E. coli | 98% | cell membrane damage | [12] |
| PVK–graphene nanocomposite | E. coli | 91% | direct contact | [13] |
| B. subtilis | 98% | |||
| graphene oxide and reduced graphene oxide | P. aeruginosa | 87% | loss of cell viability due to oxidative stress and DNA fragmentation | [14] |
| graphene oxide films | E. coli | 89% | suggested that the edges of GO are not an integral part of its antimicrobial mechanism | [15] |
| graphene oxide nanosheet | E. coli | 97.7% | ‘wrapping’ effect of large GO nanosheets removing bacteria from access to available nutrients | [16] |
aAntibacterial efficacy should be regarded as indicative only as different protocols are used in different laboratories.
The large volume of research conducted in this area, due to the previously reported antimicrobial properties of graphene nanomaterials, has produced conflicting and often opposing results and stirred up much debate regarding the mechanism of bactericidal action of graphene and relative materials [14,20,21]. It is apparent that a substantial amount of the disagreement in the literature arises from the lack of a standard of antibacterial efficiency estimation. This needs to be established to encourage more consistency among published results. Here, we review recent findings on the bactericidal effect of graphene and graphene-derived materials focusing on mechano-bactericidal effect and provide a critical review of the proposed mechanisms of cell inactivation on graphene bio-interfaces.
2. Antimicrobial activity of graphene-derived nanomaterials
A graphene suspension in nutrient broth failed to show any microbial activity over time; in fact, an enhancement in cell growth was reported as a function of turbidity [22]. By contrast, other studies using the same experimental conditions show high rates of inactivation of Pseudomonas aeruginosa [14,23]. In response to this ongoing debate, Palmieri et al. have confirmed that, in the presence of salts, GO forms aggregates which remove cutting edges from interaction with bacteria [11], thus impeding damage of cells. This makes GO aggregates less efficient than single GO sheets; therefore, antimicrobial activity becomes concentration-dependent. Additionally, assuming weak interaction between GO and bacteria, the GO was proposed as scaffolding to enhance bacterial growth [11,20,22]. It has been further hypothesized that studies conducted using nutrient broth may account for the loss of antibacterial activity due to the GO basal planes readily adsorbing a variety of molecules from suspension via non-covalent interactions, thereby removing them from interaction with bacterial cells [21]. Another possible mechanism for the bactericidal action of graphene nanosheets proposes the destructive extraction of lipids from the lipid bilayer [24]. Nevertheless, the continuous publication of inconsistent results and debate over bactericidal mechanism demonstrates the need for further elucidation of the interaction between graphene nanomaterials and bacteria which eventuate in cell death.
3. The role of surface chemistry?
Factors that may influence the antimicrobial activity of graphene nanomaterials include material properties (electronic structure, size, surface area, material stiffness, purity and lateral dimensions) and surface chemistry [25]. Surface chemical properties can be tuned by changing oxidation or reduction degrees or through doping and surface functionalization, and even before functionalization, the surface chemical properties between members of the graphene nanomaterial family vary in terms of hydrophilicity/hydrophobicity, stability and dispersability in aqueous environments. Additionally, due to the different methods to produce graphite (Gt) derivatives, impurities can be introduced into the graphitic structure, such as chemical additives. Chemical reduction removes various attached hydrophilic functional groups (carboxyls, hydroxyls and epoxyls) on the GO nanomaterial. Graphene is a single, atomic plane of Gt, obtained through mechanical or chemical exfoliation of Gt. GO can further be obtained from exfoliation of GtO. GO is then a single atomic plane of graphene with carboxylic groups attached to the peripherals of the sheet, and hydroxyl and epoxy groups attached to the basal plane. Further chemical or mechanical treatments can result in rGO possessing fewer oxygen-rich functional groups, which can completely change surface properties [26].
The role of surface chemistry contributing to the bactericidal action of GO nanowalls was originally investigated by reducing GO, which introduced changes into the graphitic structure, altering surface chemistry properties [10]. It was found that GO and rGO nanowalls possessed considerable antibacterial activity against both Gram-negative and Gram-positive bacteria; rGO exhibited substantially higher antibacterial activity, supposedly due to a stronger interaction between a bacterial cell membrane (which is slightly negative) and the edges of the nanowalls [27] resulting in enhanced cell-substratum contact. Additionally, it was speculated that the difference between the antibacterial activity observed for E. coli and S. aureus (table 1) can be attributed to the existence of an outer membrane around the Gram-negative species which provides the cell with more protection when compared with the Gram-positive bacterium [10,11].
A systematic study comprising Gt, GtO, GO and reduced GO showed that GO and rGO exhibited higher antibacterial activity against P. aeruginosa than Gt [14]. A similar study, investigating the same range of graphene nanomaterials towards E. coli, observed that GO displayed the highest bactericidal efficiency, although GO and rGO both outperformed Gt and GtO. The nominal size of Gt and GtO particles in dispersion was approximately 6 µm, whereas GO and rGO particles were approximately 0.3–2 µm.
The differing antibacterial activity between graphene materials in their various forms may be attributed to the ability of the material to stably disperse in aqueous media. Aggregation of the carbon-based engineered nanomaterials occurs commonly in this environment. Gt and GtO particles are much larger than GO and rGO nanosheets, as outlined above. rGO particles/nanosheets become larger than GO in dispersion due to the aggregation of the rGO particles. In contrast to Gt, GtO and rGO which aggregate into three-dimensional particles, GO remains as a two-dimensional nanosheet with a thickness of approximately 1 nm [8,28]. This leads to the hypothesis that, because of different aggregation conditions, small carbon-based materials may potentially penetrate cells, while larger aggregates of material may attach to the outside. It is apparent from studies that the distinct antibacterial activities of graphene nanomaterials are dependent on the aggregation state, and dispersability, of each respective material [8,14]. Santos et al. [13] showed that a poly(N-vinylcarbazole) (PVK) and graphene nanocomposite achieved higher numbers of cell death than graphene alone most likely due to the better dispersion of graphene nanocomposite in the presence of PVK.
The role of oxidative stress in determining the antibacterial mechanism of graphene surfaces is often claimed to be responsible for the nanomaterials’ extensive cytotoxicity [29]. Reactive oxygen species (ROS) include the superoxide radical (O2–), hydrogen peroxide and the hydroxyl radical. These molecules are important in triggering cellular processes, particularly apoptosis, and it has been suggested that excess generation of ROS will permeabilize the mitochondrial membrane, disrupting cellular respiratory processes, and initiate programmed cell death [29].
Studies undertaken by Liu et al., however, provided additional evidence of the mechanical bactericidal action of graphene nanomaterials (figure 1) and showed via the cell proliferation assay that relatively little superoxide anion is produced by graphene materials under liquid conditions, therefore invalidating the role of oxidative stress as a result of the production of ROS influencing the mechanism of cell death [8]. It was also reported that an increase in the extent of oxidation of a bacterial antioxidant (glutathione; GSH) corresponding to an increase in the concentration of graphene nanosheet incubated with bacterial suspension might point towards graphene materials being capable of mediating ROS-independent oxidation [8,14,30]. This link, however, is weak and there is a lack of direct experimental evidence reported to support this hypothesis. In fact, experimental evidence suggests that material properties, geometry and size of flakes are much more important when determining bactericidal properties than surface chemical properties. Liu et al. showed that the size-dependent antibacterial activity did not stem from the oxidation capacity of GO by comparing the ability of GO nanosheets of differing lateral dimensions to oxidize GSH [1].
Figure 1.
SEM images showing E. coli cells incubated without graphene nanomaterials (a,b), cells incubated with GO suspension (c,d) and cells incubated with a reduced GO suspension (e,f). SEM micrographs reveal the change in cellular morphology upon interaction in suspension with graphene nanomaterials. Reproduced with permission from Liu et al. [8] (Copyright 2011 American Chemical Society).
The metallicity of the substratum, and the ability of the graphene to act as a pathway for electron transfer, has also been purported as a potential explanation for the high rates of cell death observed on graphene nanomaterials [30]. Graphene produced by chemical vapour deposition on Cu and Ge substrata was reported to be highly antibacterial against both S. aureus and E. coli; however, graphene grown on an SiO2 substratum did not effectively inhibit the adhesion of bacterial cells [31]. Cell lysis and cytoplasmic leakage were recorded for bacteria incubated on Cu-graphene and Ge-graphene; however, bacteria incubated on SiO2-graphene incurred no significant membrane damage. As Liu et al. [8] suggested, the phenomenon can be explained by separate antibacterial effects at work. The conductive properties of both Cu and Ge substrata, overlaid with a graphene monolayer, can act as an excellent circuit for electron transfer. Electrons from the bacterial membrane are thought to be transported through the graphene and accepted by the conductive substratum under a negative membrane potential. This would disrupt the electron transfer within the cell, impeding ATP production which consequently leads to cell death [31,32]. This theory was further verified by incubating bacteria on a thin GO film, which did not possess exposed sharp edges [15]. The GO films possessed an antibacterial efficiency of 89% [15].
Despite numerous published works ‘confirming’ the fate of bacterial cells incubated in suspensions containing graphene nanomaterials, or on graphene substrata, the reported results still give rise to debate over the bactericidal mechanism of graphene-related nanomaterials. The conflicting data suggest that the ‘confirmed’ mechanisms published are still merely speculations and the true bactericidal properties of graphene remain to be fully elucidated.
4. Mechano-bactericidal action of graphene nanosheets: recent advances in understanding graphene and graphene-derived nanomaterial bio-interfaces
In further studies of bacterial cells incubated with GO nanosheets and other graphene materials in dispersion, GO nanosheets were observed to destroy E. coli cells through mechanical action [8,33,34]. It was found that, in solution, the antibacterial activity of graphene nanomaterials is concentration-dependent, i.e. higher concentrations of graphene nanosheets result in a higher incidence of cell death, and dispersion-dependent (more stably dispersed nanomaterials achieved higher rates of inactivation) [8–10,13,16,28]. More significantly, the size and aggregation of graphene materials have been found to have impact on the bactericidal efficiency of these surfaces and materials in suspension. It was predicted by Sanchez et al. that material properties, such as surface area, layer number and lateral dimension, would be important in determining the biological interactions of graphene-derived nanomaterials. Lateral dimension, for example, is particularly relevant for cellular uptake [25]. Liu et al. [16] reported that the antibacterial activity of GO nanosheets of differing lateral dimensions (size differed by more than 100 times) showed large GO sheets exhibiting higher antibacterial activity against E. coli than smaller sheets. The lateral size of GO nanosheets also seemed to have impact on the concentration and time-dependent inactivation of bacterial cells. This study reported the use of GO nanosheets with dimensions of more than 9 µm2; however, this is much larger than particle sizes reported in other studies. The conclusion that bactericidal activity stems from ‘wrapping’ of the bacterium and isolation from nutrient in media must be considered as a stand-alone case.
Another separate study, which also investigated the impact of GO nanosheet size, determined that GO-based surface coatings were more efficient antimicrobial surfaces when the GO nanosheets were smaller. They also confirmed the ‘wrapping’ mechanism of large GO nanosheets in suspension [35].
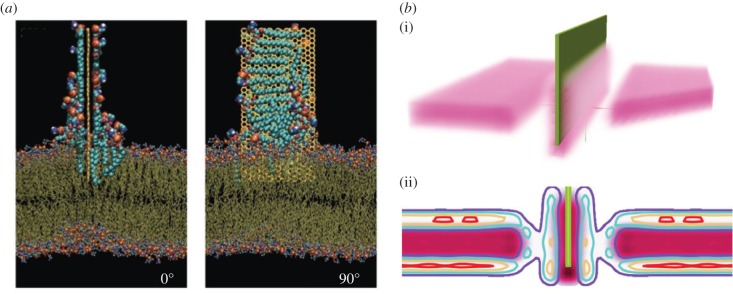
Recent rationalization of the killing effect, where the degree of lipophilicity of graphene and GO nanosheets, has been proposed as a factor influencing the extent of damage to bacterial cell membranes [24,36,37]. Owing to the carbon nature of graphene, graphene layers are able to interact with lipids in the cellular membrane. The interactions between graphene surface and lipid tails can be strong enough to overcome lipid–lipid attractions, enabling physical membrane disruption. The tails of phospholipids present in the bacterial cell bilayer have been observed to interact with graphene, allowing insertion of the graphene into the membrane which, in turn, may lead to extraction of phospholipids following the insertion of graphene nanosheets into the cell membrane, and creation of pores, altering osmotic pressure and culminating in cell inactivation [24,36]. Molecular dynamic simulations performed to test the formation of hybridized graphene/cellular membrane super structures provided the first evidence of physical disruption of cellular membranes. The model illustrated how a graphene monolayer, encapsulated by phospholipids, is inserted into a cellular membrane through, firstly, interaction between the phospholipid micelle which initiates the formation of a bottle-neck vesicle which draws the graphene monolayer into the centre of the membrane bilayer. This process was calculated to take approximately 516 ns [38].
Wang et al. proposed that the graphene nanosheet would undergo a period of fluctuations under Brownian motion before approaching the membrane with one corner. Following contact, insertion and rotation occur which facilitate spontaneous entry into the membrane. Before insertion, the graphene nanosheet is wrapped by a monolayer of lipids, pulled from the top layer of the lipid bilayer, substantially altering the free energy barrier of graphene entry into the cell (figure 2a) [39].
Figure 2.
Two suggested mechanical impacts on the lipid membrane due to interaction with the sharp edge of a graphene flake: (a) extraction of the lipids from the membrane (adapted from Krishnamoorthy et al. [34]) and (b) pore formation due to reorientation of lipid tails to graphene surface; lipid heads are inverted into the pore. (i) Tails around graphene flake and (ii) volume fraction of lipids in the regime of pore formation. Adapted from Pham et al. [36].
Dissipative particle dynamics simulations performed by Guo et al. were used to reproduce the events at the lipid bilayer in the presence of graphene nanosheets with varying dimensions. Size- and edge-dependent effects were recorded, and small nanosheets with side lengths of 3.5 nm were observed to easily form the aforementioned ‘sandwich’ membrane/graphene superstructure, whereas larger nanostructures (side length = 14 nm) initiated the formation of hemisphere vesicles for encapsulation [40]. Disruption of the bilayer structure was found to be more significant with larger graphene nanosheets than smaller ones, suggesting higher cytotoxicity for cells. More recent research also focused on the size dependency of graphene nanosheets and their interaction with the lipid bilayer. Small sheets (0.9–5.2 nm) were able to enter the membrane without creating significant perturbation, while larger sheets (5.2–11.2 nm) would only cross the membrane in a particular orthogonal orientation or were adsorbed onto the membrane surface causing lipid displacement, as they relocated onto the graphene nanosheet, and pore formation, disrupting cell functioning [41]. Like all other models proposed, rotation and orientation of the nanosheet is deemed an important factor in translocation of graphene into the lipid bilayer, although preferred orientation is dependent on size [38,39,41,42].
Further simulations completed by the same group verified that successful transmembrane relocation of graphene nanosheets through a lipid bilayer is dependent on the degree of oxidation of the nanosheet and lateral size. In suspension, very small GO nanosheets cause very little membrane perturbation and tend to accumulate on the outside of the cell. By contrast, large GO sheets are able to pierce through the membrane [43]. For small (less than 8 nm) pristine graphene flakes with very little oxidation, interaction with the membrane was directed by contact between the membrane lipid tails and spontaneous insertion of the nanosheet into the membrane. According to the simulations, when the size of the graphene nanosheet is larger than 8 nm, interaction between the nanosheet and the membrane incurs the formation of a hemispherical lipid vesicle. With increasing oxidation of the basal plane, graphene–membrane interactions are mediated by the increased hydrophilicity and interaction mainly occurs with lipid head-groups [39]. Tu et al., in one of two models, suggested that extraction of phospholipids destroys the integrity of the membrane through removal of lipids and destabilization of the membrane structure. The geometric properties of nanosheets, such as sheet density, sharpness and orientation, may also influence the degree to which the graphene is inserted into the membrane [36,44]. Coarse-grained molecular dynamic simulations revealed that graphene will penetrate the cell membrane at the point of lowest energy barrier and when the atomically thin flake is in a particular orthogonal orientation which minimizes interactive free energy and is a thermodynamically preferred configuration [24,42,45]. Mao et al. also determined that, although pristine and nominally oxidized graphene nanosheets may orientate themselves to successfully pierce the lipid bilayer and be inserted into the membrane, heavily oxidized graphene (GO) nanosheets are faced with a high energy barrier for insertion; initially, nanosheets must be anchored onto the membrane before vibrations and spontaneous movement allows for the unoxidized graphene edges to come into contact with the lipid tail groups, facilitating insertion into the membrane [43]. Interestingly, it was also suggested that the bactericidal activity of GO nanosheets could be a purely spontaneous process (thermodynamically driven) with no need for energy consumption usually involved with the endocytosis process and vesiculation.
These conclusions were confirmed through further experimentation [24], showing the penetration of bacterial cell membranes occurring upon direct contact with the sharp edges of graphene nanomaterials.
TEM analysis of bacterial cells exposed to GO nanosheets in suspension shows destruction of the bacterial membrane and loss of intracellular substance, leading to the conclusion that irreversible damage to the membrane by graphene nanomaterials had occurred via some physical means (figure 3) [46]. Advanced microscopy methods show that Gram-negative bacteria are more susceptible to the action of GO nanosheets [28]. This finding is reiterated in the literature by research on the bactericidal activity of a range of other nanostructured materials due to the thinner cell membrane of Gram-negative bacteria which is less resistant to the ‘mechano-bactericidal’ action of nanostructured surfaces.
Figure 3.
TEM images of common dental pathogens post-exposure to GO nanosheets in dispersion. Micrographs reveal disruption of cellular morphology and leakage of cytoplasmic material due to physical membrane disruption by GO nanosheets. Reproduced with permission from American Chemical Society Publications (2015) [28].
In a study of graphene surfaces where the stacking orientation of graphene resulted in three separate nano-topographies [36], it was observed that graphene surfaces with an exposed edge length of 137.3 nm, density of graphene edge of 7.7 µm µm−2 and stack angle orientation of 62.1° were extremely bactericidal towards Gram-negative P. aeruginosa (87%) but not so much towards Gram-positive S. aureus. By contrast, graphene surfaces which were five times smoother exhibited much higher bactericidal activity against both types of cells. The authors concluded that the density of graphene edges is paramount in determining the effectiveness of the surface as a bactericidal material. Simulation studies revealed that the surface of the membrane lipid bilayer lifts to attach to a graphene sheet which, in turn, increases the contact area between the graphene and the hydrocarbon tails of the lipids (figure 2b). As per previous models [24,42], membrane penetration initially occurs through the rotation, vibrations and migration of the graphene edge into the lipid bilayers. Full penetration results in the formation of pores and a change in osmotic pressure within the cell which leads to swelling and eventual cell death [36]. Therefore, through multiple simulations and modelling proposed by various groups, the bactericidal mechanism has been suggested as the formation of pores following insertion of a graphene flake rather than the graphene nanowall acting as a ‘blade’ to ‘cut’ the bacterial membrane as has recently been suggested [11].
5. Biological effects of graphene materials
As graphene is made of covalently linked carbon atoms, graphene materials are among the strongest in the world. The rigidity and robustness of graphene materials greatly affect interactions with biological objects and raise the question of biocompatibility and toxicity. Most findings report the cytocompatability of graphene at low concentrations; however, there exists a strong correlation between increasing cytotoxicity and increasing concentration of graphene [9,13,14,19,47,48]. Graphene and GO have been shown to support the growth, proliferation and differentiation of a variety of cell lines [22,49,50]. Graphene materials have also been shown to allow for the spontaneous differentiation of mouse-induced pluri-potent stem cells and human mesenchymal stem cells [51,52]. However, the biological effects of graphene materials depend on the extent of functionalization of the graphene in order to reduce its toxicity. For example, reduced GO, or functionalized graphene (with polyethylene glycol/polyethyleneimine), has displayed higher cytocompatability when compared with cells treated with pristine graphene and GO due to the improved stability of the material in its reduced or functionalized state [53–57]. Hu et al. [58] observed that the toxicity of GO was largely diminished when incubated with bovine serum albumin due to the ability of the GO to adsorb protein. Additionally, the majority of cytotoxicity effects on mammalian cells show dose-dependent toxicity and size- and shape-dependent toxicity [59–63]. Cellular uptake of large GO particles by mouse myoblasts (0.6–0.8 µm diameter) was established to be an energy-driven process, phagocytosis. Because endocytosis is a temperature-dependent process, it was confirmed as the route of entry into the cells when no uptake was observed at 4°C. Smaller GO sheets (0.4 µm in diameter) were internalized via clathrin-mediated pathways [64]. In epithelial carcinoma lung cells, GO exhibited no cytotoxicity up to 100 µg ml−1 [60]; by contrast, studies involving graphene nanoribbons show cytotoxicity towards mammalian cells at concentrations as low as 1 µg ml−1 [65]. One explanation for the current disagreement in the literature regarding the cellular toxicity of graphene materials towards both bacteria and eukaryotic cells is that the physicochemical properties of GO and graphene, such as size, surface charge, particulate state, surface functional groups and residual precursors, are not always well controlled, although they are likely to have significant impact on biological/toxicological activity.
Similar to the mechano-bactericidal action of GO observed for bacterial cells, the cytotoxicity of GO has been reported to stem from its ability to affect the cellular integrity of mammalian cells through physical insertion through the phospholipid bilayer [42,66,67]. There have been reports of significant membrane deformation of mammalian cells in the presence of GO nanosheets as the cells attempt to internalize graphene nanomaterials [58,66]. A protein corona coating on GO was successfully used in order to mitigate the cytotoxicity of GO nanosheets towards lung epithelial cells, effectively retarding the insertion of the GO into the lipid bilayer [58,68]. Other explanations for cytotoxicity have been reported to arise from oxidative stress, DNA fragmentation and chromosomal aberrations [65,66]. Notably, the uptake of graphene nanosheets has been observed to be dependent on cell type. In a study of three separate cell lines (Saos-2 osteoblasts, HepG2 hepatoma cells and RAW-264.7 macrophages), three different endocytosis mechanisms were assessed. Graphene nanomaterials could be internalized through microtubules in osteoblasts, phagocytosis and clathrin-dependent mechanisms by hepatoma cells and macrophages internalized graphene through clathrin-dependent endocytosis only [69].
6. Conclusion and outlook
Understanding of the interactions between biological materials and graphene nanomaterial substrata is key to the fabrication of graphene-enabled biomedical tools and will help to manage possible cytotoxic effects arising from the increase in the use of graphene as an exciting tool within a wide array of industries ranging from photonics to medical devices. Graphene, GO and functionalized versions of these two materials show promise as effective antimicrobial agents with incredibly high bactericidal activities reported throughout the literature. Current understanding of the bactericidal mechanism is limited and remains under intense debate. In our opinion, the reasons for such discrepancies are (i) lack of precise protocol to define the bactericidal efficiency in different systems making it difficult to compare; (ii) the importance of size and geometry of graphene materials is underestimated; (iii) the aggregation of graphene materials, which depends on external conditions and concentration, has not received enough attention; (iv) lack of surface properties control in different fabrication techniques resulting in very different materials with the same name.
Theoretical modelling and simulations provide compelling evidence as to the physical perturbation of bacterial cell membranes, guided by the degree of lipophilicity of the graphene substrate. Membrane deformation occurs initially through the formation of pores and finally an imbalance of osmotic pressure and cell rupture.
In the future, the potential to capitalize on the unique electronic properties of graphene materials promises to establish new photo-electrochemical mechanisms for bactericidal action, which have yet to be explored. Graphene has a unique electron energy-band structure with a joint point in the dispersion diagram connecting the valence and conduction bands. This makes graphene able to absorb the entire spectrum of electromagnetic radiation and opens unique properties in thermal treatment, photo-activation and electron-hole pair generation.
The full potential and photo-electrochemical mechanisms of the bactericidal action of graphene-family materials could be elucidated in the near future by collective efforts employing Raman scattering (including surface-enhanced scattering) under electrical bias and photo-controlled excitation. In fact, currently, Raman is used as a tool to track morphological changes in bacteria, analysing the antibacterial action of GO on the molecular level [70]. Such measurements under chromatographic detection conditions will reveal products of occurring reactions, e.g. water splitting in simulated and real physiological conditions that are occurring on the graphene surface depending on its electrical potential and substrate (defining the energy-band diagram). Depending on the substratum and its electron affinity, graphene can open a band-gap in its electronic energy diagram and become an n- or p-type ‘semiconductor’. Manifestation of this will result in specific energy potential positions for electrons which creates oxidation/reduction pathways for electron transport, as seen with photo-activated TiO2 which leads to the production of oxidizing agents such as hydrogen peroxide H2O2 and 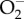 , which are known to cause oxidative stress in bacteria [71]. These future directions, which combine both surface chemistry and surface nano-topographical effects, promise to facilitate the use of graphene as an effective antimicrobial surface for use in a wide range of applications.
, which are known to cause oxidative stress in bacteria [71]. These future directions, which combine both surface chemistry and surface nano-topographical effects, promise to facilitate the use of graphene as an effective antimicrobial surface for use in a wide range of applications.
Data accessibility
No new data were collected in the course of this research.
Authors' contributions
All authors contributed to writing.
Competing interests
We declare we have no competing interests.
Funding
V.A.B. and E.P.I. acknowledge funding from Marie Curie Actions under EU FP7 Initial Training Network SNAL 608184 and support from the Research Hub for Australian Steel Manufacturing, Australian Research Council. D.P.L. is a recipient of a SUPRA scholarship.
References
- 1.Geim AK, Novoselov KS. 2007. The rise of graphene. Nat. Mat. 6, 183–191. ( 10.1038/nmat1849) [DOI] [PubMed] [Google Scholar]
- 2.Gao X, Zhao Y, Chen Z. 2013. From graphene to graphene oxide and back. In Graphene chemistry: theoretical perspectives (eds Jiang D, Chen Z), pp. 291–317, 1st edn New York, NY: John Wiley & Sons, Ltd. [Google Scholar]
- 3.Dreyer DR, Park S, Bielawski CW, Ruoff RS. 2010. The chemistry of graphene oxide. Chem. Soc. Rev. 39, 228–240. ( 10.1039/B917103G) [DOI] [PubMed] [Google Scholar]
- 4.Loh KP, Bao Q, Ang PK, Yang J. 2010. The chemistry of graphene. J. Mater. Chem. 20, 2277 ( 10.1039/b920539j) [DOI] [Google Scholar]
- 5.Zhu Y, Murali S, Cai W, Li X, Suk JW, Potts JR, Ruoff RS. et al. 2010. Graphene and graphene oxide: synthesis, properties, and applications. Adv. Mater. 22, 3906–3924. ( 10.1002/adma.201001068) [DOI] [PubMed] [Google Scholar]
- 6.Gómez-Navarro C, Meyer JC, Sundaram RS, Chuvilin A, Kurasch S, Burghard M, Kern K, Kaisers U. 2010. Atomic structure of reduced graphene oxide. Nano Lett. 10, 1144–1148 ( 10.1021/nl9031617) [DOI] [PubMed] [Google Scholar]
- 7.Compton OC, Nguyen ST. 2010. Graphene oxide, highly reduced graphene oxide, and graphene: versatile building blocks for carbon-based materials. Small 6, 711–723. ( 10.1002/smll.200901934) [DOI] [PubMed] [Google Scholar]
- 8.Liu S, Zeng TH, Hofmann M, Burcombe E, Wei J, Jiang R, Kong J, Chen Y. 2011. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: membrane and oxidative stress. ACS Nano 5, 6971–6980. ( 10.1021/nn202451x) [DOI] [PubMed] [Google Scholar]
- 9.Hu W, Peng C, Luo W, Lv M, Li X, Li D, Huang Q, Fan C. 2010. Graphene-based antibacterial paper. ACS Nano 4, 4317–4323. ( 10.1021/nn101097v) [DOI] [PubMed] [Google Scholar]
- 10.Akhavan O, Ghaderi E. 2010. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 4, 5731–5736. ( 10.1021/nn101390x) [DOI] [PubMed] [Google Scholar]
- 11.Palmieri V, et al. 2017. Bacteria meet graphene: modulation of graphene oxide nanosheet interaction with human pathogens for effective antimicrobial therapy. ACS Biomater. Sci. Eng. 3, 619–627. ( 10.1021/acsbiomaterials.6b00812) [DOI] [PubMed] [Google Scholar]
- 12.Zhao J, Deng B, Lv M, Li J, Zhang Y, Jiang H, Peng C, Li J, Shi J, Huang Q, Fan C. 2013. Graphene oxide-based antibacterial cotton fabrics. Adv. Healthcare Mater. 2, 1259–1266. ( 10.1002/adhm.201200437) [DOI] [PubMed] [Google Scholar]
- 13.Santos CM, Mangadlao J, Ahmed F, Leon A, Advincula RC, Rodrigues DF. 2012. Graphene nanocomposite for biomedical applications: fabrication, antimicrobial and cytotoxic investigations. Nanotechnology 23, 395101 ( 10.1088/0957-4484/23/39/395101) [DOI] [PubMed] [Google Scholar]
- 14.Gurunathan S, Woong Han J, Abdal Daye A, Eppakayala V, Kim J-H. 2012. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomedicine. 2012, 5901–5914. ( 10.2147/IJN.S37397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mangadlao JD, Santos CM, Felipe MJL, de Leon ACC, Rodrigues DF, Advincula RC. 2015. On the antibacterial mechanism of graphene oxide (GO) Langmuir–Blodgett films. Chem. Commun. 51, 2886–2889. ( 10.1039/C4CC07836E) [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Hu M, Zeng TH, Wu R, Jiang R, Wei J, Wang L, Kong J, Chen Y. 2012. Lateral dimension-dependent antibacterial activity of graphene oxide sheets. Langmuir 28, 12 364–12 372. ( 10.1021/la3023908) [DOI] [PubMed] [Google Scholar]
- 17.Akhavan O, Azimirad R, Safa S, Hasani E. 2011. CuO/Cu(OH)2 hierarchical nanostructures as bactericidal photocatalysts. J. Mater. Chem. 21, 9634 ( 10.1039/c0jm04364h) [DOI] [Google Scholar]
- 18.Bao Q, Zhang D, Qi P. 2011. Synthesis and characterization of silver nanoparticle and graphene oxide nanosheet composites as a bactericidal agent for water disinfection. J. Colloid Interface Sci. 360, 463–470. ( 10.1016/j.jcis.2011.05.009) [DOI] [PubMed] [Google Scholar]
- 19.Mazaheri M, Akhavan O, Simchi A. 2014. Flexible bactericidal graphene oxide–chitosan layers for stem cell proliferation. Appl. Surf. Sci. 301, 456–462. ( 10.1016/j.apsusc.2014.02.099) [DOI] [Google Scholar]
- 20.Chen H-Q, Gao D, Wang B, Zhao R-F, Guan M, Zheng L-N, Zhou X-y, Chai Z-f, Feng W-y. et al. 2014. Graphene oxide as an anaerobic membrane scaffold for the enhancement of B. adolescentis proliferation and antagonistic effects against pathogens E. coli and S. aureus. Nanotechnology 25, 165101 ( 10.1088/0957-4484/25/16/165101) [DOI] [PubMed] [Google Scholar]
- 21.Hui L, Piao J-G, Auletta J, Hu K, Zhu Y, Meyer T, Liu H, Yang L. 2014. Availability of the basal planes of graphene oxide determines whether it is antibacterial. ACS Appl. Mater. Interfaces 6, 13 183–13 190. ( 10.1021/am503070z) [DOI] [PubMed] [Google Scholar]
- 22.Ruiz ON, Fernando KAS, Wang B, Brown NA, Luo PG, McNamara ND, Vangsness M, Sun Y-P, Bunker CE. 2011. Graphene oxide: a nonspecific enhancer of cellular growth. ACS Nano 5, 8100–8107. ( 10.1021/nn202699t) [DOI] [PubMed] [Google Scholar]
- 23.Gurunathan S, Han JW, Dayem AA, Eppakayala V, Park M-R, Kwon D-N, Kim J-H. 2013. Antibacterial activity of dithiothreitol reduced graphene oxide. J. Ind. Eng. Chem. 19, 1280–1288. ( 10.1016/j.jiec.2012.12.029) [DOI] [Google Scholar]
- 24.Tu Y. et al 2013. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotech. 8, 968 ( 10.1038/nnano.2013.275) [DOI] [PubMed] [Google Scholar]
- 25.Sanchez VC, Jachak A, Hurt RH, Kane AB. 2012. Biological interactions of graphene-family nanomaterials: an interdisciplinary review. Chem. Res. Toxicol. 25, 15–34. ( 10.1021/tx200339h) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park S, Ruoff RS. 2009. Chemical methods for the production of graphenes. Nat. Nanotechnol. 4, 217–224. ( 10.1038/nnano.2009.58) [DOI] [PubMed] [Google Scholar]
- 27.Salas EC, Sun Z, Lüttge A, Tour JM. 2010. Reduction of graphene oxideviabacterial respiration. ACS Nano. 4, 4852–4856. ( 10.1021/nn101081t) [DOI] [PubMed] [Google Scholar]
- 28.He J, Zhu X, Qi Z, Wang C, Mao X, Zhu C, He Z, Li M, Tang Z. et al. 2015. Killing dental pathogens using antibacterial graphene oxide. ACS Appl. Mater. Interfaces 7, 5605–5611. ( 10.1021/acsami.5b01069) [DOI] [PubMed] [Google Scholar]
- 29.Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. 2007. Mitochondria, oxidative stress and cell death. Apoptosis 12, 913–922. ( 10.1007/s10495-007-0756-2) [DOI] [PubMed] [Google Scholar]
- 30.Kim TI, Kwon B, Yoon J, Park I-J, Bang GS, Park Y, Seo Y-S, Choi S-Y. 2017. Antibacterial activities of graphene oxide–molybdenum disulfide nanocomposite films. ACS Appl. Mater. Interfaces 9, 7908–7917. ( 10.1021/acsami.6b12464) [DOI] [PubMed] [Google Scholar]
- 31.Li J, Wang G, Zhu H, Zhang M, Zheng X, Di Z, Liu X, Wang X. 2014. Antibacterial activity of large-area monolayer graphene film manipulated by charge transfer. Sci. Rep. 4, 4369 ( 10.1038/srep04359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao HY, Laurent S, Chen W, Akhavan O, Imani M, Ashkarran AA, Mahmoudi M. 2013. Graphene: promises, facts, opportunities, and challenges in nanomedicine. Chem. Rev. 113, 3407–3424. ( 10.1021/cr300335p) [DOI] [PubMed] [Google Scholar]
- 33.Tegou E, et al. 2016. Terms of endearment: bacteria meet graphene nanosurfaces. Biomaterials 89, 38–55. ( 10.1016/j.biomaterials.2016.02.030) [DOI] [PubMed] [Google Scholar]
- 34.Krishnamoorthy K, Veerapandian M, Zhang L-H, Yun K, Kim SJ. 2012. Antibacterial efficiency of graphene nanosheets against pathogenic bacteria via lipid peroxidation. J. Phys. Chem. C 116, 17 280–17 287. ( 10.1021/jp3047054) [DOI] [Google Scholar]
- 35.Perreault F, de Faria AF, Nejati S, Elimelech M. 2015. Antimicrobial properties of graphene oxide nanosheets: why size matters. ACS Nano 9, 7226–7236. ( 10.1021/acsnano.5b02067) [DOI] [PubMed] [Google Scholar]
- 36.Pham VTH, Truong VK, Quinn MDJ, Notley SM, Guo Y, Baulin VA, Al Kobaisi M, Crawford RJ, Ivanova EP. 2015. Graphene induces formation of pores that kill spherical and rod-shaped bacteria. ACS Nano 9, 8458–8467. ( 10.1021/acsnano.5b03368) [DOI] [PubMed] [Google Scholar]
- 37.Ji H, Sun H, Qu X. 2016. Antibacterial applications of graphene-based nanomaterials: recent achievements and challenges. Adv. Drug Deliv. Rev. 105, 176–189. ( 10.1016/j.addr.2016.04.009) [DOI] [PubMed] [Google Scholar]
- 38.Titov AV, Král P, Pearson R. 2010. Sandwiched graphene–membrane superstructures. ACS Nano. 4, 229–234. ( 10.1021/nn9015778) [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Wei Y, Shi X, Gao H. 2013. Cellular entry of graphene nanosheets: the role of thickness, oxidation and surface adsorption. RSC Adv. 3, 15776 ( 10.1039/c3ra40392k) [DOI] [Google Scholar]
- 40.Guo R, Mao J, Yan L-T. 2013. Computer simulation of cell entry of graphene nanosheet. Biomaterials 34, 4296–4301. ( 10.1016/j.biomaterials.2013.02.047) [DOI] [PubMed] [Google Scholar]
- 41.Dallavalle M, Calvaresi M, Bottoni A, Melle-Franco M, Zerbetto F. 2015. Graphene can wreak havoc with cell membranes. ACS Appl. Mater. Interfaces 7, 4406–4414. ( 10.1021/am508938u) [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Yuan H, von dem Bussche A, Creighton M, Hurt RH, Kane AB, Gao H. 2013. Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites. Proc. Natl Acad. Sci. USA 110, 12 295–12 300. ( 10.1073/pnas.1222276110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mao J, Guo R, Yan L-T. 2014. Simulation and analysis of cellular internalization pathways and membrane perturbation for graphene nanosheets. Biomaterials 35, 6069–6077. ( 10.1016/j.biomaterials.2014.03.087) [DOI] [PubMed] [Google Scholar]
- 44.Hegab HM, ElMekawy A, Zou L, Mulcahy D, Saint CP, Ginic-Markovic M. 2016. The controversial antibacterial activity of graphene-based materials. Carbon 105, 362–376. ( 10.1016/j.carbon.2016.04.046) [DOI] [Google Scholar]
- 45.Gao H. 2014. Probing mechanical principles of cell–nanomaterial interactions. J. Mech. Phys. Solids 62, 312–339. ( 10.1016/j.jmps.2013.08.018) [DOI] [Google Scholar]
- 46.Chen J, Wang X, Han H. 2013. A new function of graphene oxide emerges: inactivating phytopathogenic bacterium Xanthomonas oryzae pv. Oryzae. J. Nanopart. Res. 15, 1658. [Google Scholar]
- 47.Agarwal S, Zhou X, Ye F, He Q, Chen GCK, Soo J, Boey F, Zhang H, Chen P. 2010. Interfacing live cells with nanocarbon substrates. Langmuir 26, 2244–2247. ( 10.1021/la9048743) [DOI] [PubMed] [Google Scholar]
- 48.Gurunathan S, Kim J-H. 2016. Synthesis, toxicity, biocompatibility, and biomedical applications of graphene and graphene-related materials. Int. J. Nanomedicine 11, 1927–1945. ( 10.2147/IJN.S105264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryoo S-R, Kim Y-K, Kim M-H, Min D-H. 2010. Behaviors of NIH-3T3 fibroblasts on graphene/carbon nanotubes: proliferation, focal adhesion, and gene transfection studies. ACS Nano 4, 6587–6598. ( 10.1021/nn1018279) [DOI] [PubMed] [Google Scholar]
- 50.Kalbacova M, Broz A, Kong J, Kalbac M. 2010. Graphene substrates promote adherence of human osteoblasts and mesenchymal stromal cells. Carbon 48, 4323–4329. ( 10.1016/j.carbon.2010.07.045) [DOI] [Google Scholar]
- 51.Chen GY, Pang DWP, Hwang SM, Tuan HY, Hu YC. 2012. A graphene-based platform for induced pluripotent stem cells culture and differentiation. Biomaterials 33, 418–427. ( 10.1016/j.biomaterials.2011.09.071) [DOI] [PubMed] [Google Scholar]
- 52.Nayak TR, et al. 2011. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano 5, 4670–4678. ( 10.1021/nn200500h) [DOI] [PubMed] [Google Scholar]
- 53.Gurunathan S, Han JW, Park JH, Eppakayala V, Kim J-H. 2014. Ginkgo biloba: a natural reducing agent for the synthesis of cytocompatible graphene. Int. J. Nanomedicine 2014, 363–377. ( 10.2147/IJN.S53538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wojtoniszak M, Chen X, Kalenczuk RJ, Wajda A, Łapczuk J, Kurzewski M, Drozdzik M, Chu PK, Borowiak-Palen E. 2012. Synthesis, dispersion, and cytocompatibility of graphene oxide and reduced graphene oxide. Colloids Surf. B 89, 79–85. ( 10.1016/j.colsurfb.2011.08.026) [DOI] [PubMed] [Google Scholar]
- 55.Feng L, Zhang S, Liu Z. 2011. Graphene based gene transfection. Nanoscale 3, 1252 ( 10.1039/c0nr00680g) [DOI] [PubMed] [Google Scholar]
- 56.Gurunathan S, Han J, Eppakayala V, Dayem A, Kwon D-N, Kim J-H. 2013. Biocompatibility effects of biologically synthesized graphene in primary mouse embryonic fibroblast cells. Nanoscale Res. Lett. 8, 393 ( 10.1186/1556-276X-8-393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sasidharan A, Panchakarla LS, Chandran P, Menon D, Nair S, Rao CNR, Koyakutty M. 2011. Differential nano-bio interactions and toxicity effects of pristine versus functionalized graphene. Nanoscale 3, 2461 ( 10.1039/c1nr10172b) [DOI] [PubMed] [Google Scholar]
- 58.Hu W, Peng C, Lv M, Li X, Zhang Y, Chen N, Fan C, Huang Q. 2011. Protein corona-mediated mitigation of cytotoxicity of graphene oxide. ACS Nano 5, 3693–3700. ( 10.1021/nn200021j) [DOI] [PubMed] [Google Scholar]
- 59.Mullick Chowdhury S, Lalwani G, Zhang K, Yang JY, Neville K, Sitharaman B. 2013. Cell specific cytotoxicity and uptake of graphene nanoribbons. Biomaterials 34, 283–293. ( 10.1016/j.biomaterials.2012.09.057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ali-Boucetta H, Bitounis D, Raveendran-Nair R, Servant A, Van den Bossche J, Kostarelos K. 2013. Graphene oxide: purified graphene oxide dispersions lack in vitro cytotoxicity and in vivo pathogenicity (Adv. Healthcare Mater. 3/2013). Adv. Healthcare Mater. 2, 512 ( 10.1002/adhm.201370014) [DOI] [PubMed] [Google Scholar]
- 61.Teo WZ, Chng ELK, Sofer Z, Pumera M. 2014. Cytotoxicity of exfoliated transition-metal dichalcogenides (MoS2, WS2, and WSe2) is lower than that of graphene and its analogues. Chem. A Eur. J. 20, 9627–9632. ( 10.1002/chem.201402680) [DOI] [PubMed] [Google Scholar]
- 62.Ahmadian H, Hashemi E, Akhavan O, Shamsara M, Hashemi M, Farmany A, Joupari MD. 2017. Apoptotic and anti-apoptotic genes transcripts patterns of graphene in mice. Mater. Sci. Eng. C 71, 460–464. ( 10.1016/j.msec.2016.09.073) [DOI] [PubMed] [Google Scholar]
- 63.Akhavan O, Ghaderi E, Akhavan A. 2012. Size-dependent genotoxicity of graphene nanoplatelets in human stem cells. Biomaterials 33, 8017–8025. ( 10.1016/j.biomaterials.2012.07.040) [DOI] [PubMed] [Google Scholar]
- 64.Mu Q, Su G, Li L, Gilbertson BO, Yu LH, Zhang Q, Sun Y-P, Yan B. 2012. Size-dependent cell uptake of protein-coated graphene oxide nanosheets. ACS Appl. Mater. Interfaces 4, 2259–2266. ( 10.1021/am300253c) [DOI] [PubMed] [Google Scholar]
- 65.Akhavan O, Ghaderi E, Emamy H, Akhavan F. 2013. Genotoxicity of graphene nanoribbons in human mesenchymal stem cells. Carbon 54, 419–431. ( 10.1016/j.carbon.2012.11.058) [DOI] [Google Scholar]
- 66.Lammel T, Boisseaux P, Fernández-Cruz M-L, Navas JM. 2013. Internalization and cytotoxicity of graphene oxide and carboxyl graphene nanoplatelets in the human hepatocellular carcinoma cell line Hep G2. Part. Fibre Toxicol. 10, 27 ( 10.1186/1743-8977-10-27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liao K-H, Lin Y-S, Macosko CW, Haynes CL. 2011. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Interfaces 3, 2607–2615. ( 10.1021/am200428v) [DOI] [PubMed] [Google Scholar]
- 68.Duan G, Kang S-g, Tian X, Garate JA, Zhao L, Ge C, Zhou R. 2015. Protein corona mitigates the cytotoxicity of graphene oxide by reducing its physical interaction with cell membrane. Nanoscale 7, 15 214–15 224. ( 10.1039/C5NR01839K) [DOI] [PubMed] [Google Scholar]
- 69.Linares J, Matesanz MC, Vila M, Feito MJ, Gonçalves G, Vallet-Regí M, Marques PAAP, Portolés MT. 2014. Endocytic mechanisms of graphene oxide nanosheets in osteoblasts, hepatocytes and macrophages. ACS Appl. Mater. Interfaces 6, 13 697–13 706. ( 10.1021/am5031598) [DOI] [PubMed] [Google Scholar]
- 70.Nanda SS, Yi DK, Kim K. 2016. Study of antibacterial mechanism of graphene oxide using Raman spectroscopy. Sci. Rep. 6, 4317 ( 10.1038/srep28443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Juodkazis K, Juodkazyte J, Jelmakas E, Kalinauskas P, Valsiunas I, Mecinskas P, Juodkazis S. 2010. Photoelectrolysis of water: solar hydrogen — achievements and perspectives. Opt. Express. 18, A147 ( 10.1364/OE.18.00A147) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were collected in the course of this research.