Abstract
Background
Furosemide is the most common loop diuretic used worldwide. The off-label administration of furosemide bolus(es) for the prevention or to reverse acute kidney injury (AKI) is widespread but not supported by available evidence. We conducted a meta-analysis of randomized trials (RCTs) to investigate whether bolus furosemide to prevent or treat AKI is detrimental on patients’ survival.
Methods
Electronic databases were searched through October 2017 for RCTs comparing bolus furosemide administration versus any comparator in patients with or at risk for AKI. The primary endpoint was all-cause longest follow-up mortality. Secondary endpoints included new or worsening AKI, receipt of renal replacement therapy, length of hospital stay, and peak serum creatinine after randomization.
Results
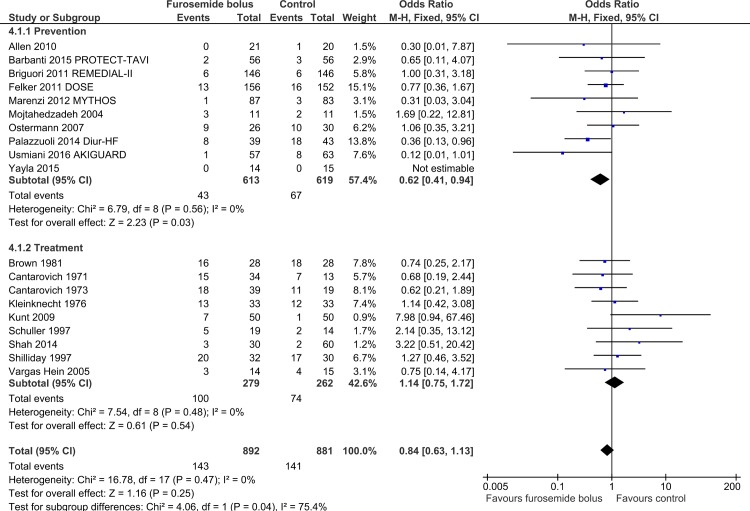
A total of 28 studies randomizing 3,228 patients were included in the analysis. We found no difference in mortality between the two groups (143/892 [16%] in the furosemide group versus 141/881 [16%] in the control group; odds ratio [OR], 0.84; 95% confidence interval [CI], 0.63 to 1.13; p = 0.25). No significant differences in secondary outcomes were found. A significant improvement in survival was found in the subgroup of patients receiving furosemide bolus(es) as a preventive measure (43/613 [7.0%] versus 67/619 [10.8%], OR 0.62; 95% CI, 0.41 to 0.94; p = 0.03)
Conclusions
Intermittent furosemide administration is not associated with an increased mortality in patients with or at risk for AKI, although it may reduce mortality when used as a preventive measure. Future high-quality RCTs are needed to define the role of loop diuretics in AKI prevention and management.
Trial registration
The study protocol was registered on PROSPERO database for systematic reviews (Registration no. CRD42017078607 – http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017078607).
Introduction
Acute kidney injury (AKI) is a common complication in hospitalized patients, with an overall estimated incidence of around 10%, increasing up to 60% among critically ill patients admitted to an intensive care unit (ICU) [1]. Of these, 6% develop severe AKI, with approximately 70% receiving acute renal replacement therapy (RRT)[2]. A relationship between renal failure and increased short- and long-term morbidity and mortality is well recognized across several clinical settings [1,3].
Currently, there are few interventions or medications that can alter the clinical course of AKI and favorably modify the outcome of critically ill patients once AKI occurred [4,5].
The use of loop diuretics in critically ill patients with AKI is a long standing and widespread clinical practice [6]. The rationale for the use of loop diuretics includes many aspects of their action, including an increase of tubular flow, a reduction in oxygen consumption and ischemic injury, and a reduction of TNF-induced apoptosis [7,8,9].
Furosemide remains the most common loop diuretic prescribed in critically ill patients [6,10,11]. Experimental studies have suggested that the infusion of low doses of furosemide can reduce the apoptosis phenomena induced by ischemia/reperfusion and gene transcription associated therewith [12,13].
On one hand, some small studies have suggested that diuretics can reduce the severity of acute renal failure transforming it from oliguric to not oliguric, reducing the duration of AKI, improving the speed of recovery of renal function and probably reducing the need of renal replacement treatment [14,15,16,17]. Furthermore, furosemide may also be helpful in the management of volume overload and electrolyte homeostasis, which could be ultimately related to AKI outcome [18,19].
On the other hand, several observational studies suggested that the use of diuretics in critically ill patients with AKI might not be associated with improvement in clinically relevant outcomes, and may even increase mortality [10,11]. A possible detrimental effect of loops diuretics administration has been suggested also for heart failure patients [20]. In addition, some in vitro studies, where blood mononuclear cells were stimulated with lipopolysaccharide, have revealed that high concentrations of furosemide could have cytotoxic and immunosuppressive effects characterized by reduced expression of interleukin-6, interleukin-8, and tumor necrosis factor-alpha [21].
In clinical practice, it is common experience that intermittent (bolus) furosemide administration is frequently the first strategy applied by clinicians when facing patients with early AKI, especially when oligo-anuria is present. However, such strategy is not currently supported by evidence-based medicine, and, in some settings, has been associated with harm [22,23].
Thus, we decided to carry out a systematic review and meta-analysis of all randomized clinical trials ever performed on furosemide bolus versus any comparator in any clinical AKI setting to evaluate its effect on survival and on clinically relevant outcomes.
Methods
This study is a systematic review and meta-analysis conducted in keeping with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [24,25,26]. The study protocol was registered on PROSPERO database for systematic reviews (Registration no. CRD42017078607) [27]. The PRISMA Checklist is available as S1 Checklist.
This study was supported by a grant from the Italian Medicines Agency (AIFA–Grant no. FARM12JFX9). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Search strategy
Pertinent studies were searched independently in BioMedCentral, PubMed, EMBASE, and the Cochrane Central Register of clinical trials by four trained investigators (search last updated October 15th, 2017). The PubMed search strategy used to include any randomized study ever performed with furosemide in patients with or at risk for AKI [28] is available in S1 Appendix. In addition, we employed backward snowballing (i.e., scanning of references of retrieved articles and pertinent reviews) and contacted the international experts and the manufacturers for further studies. No language restriction was enforced.
Study selection
References, obtained from database and literature searches, were examined first at a title/abstract level independently by four investigators, with divergences resolved by consensus, and then, if potentially pertinent, were retrieved as complete articles.
The following inclusion criteria were used for potentially relevant studies: (1) random allocation to treatment, (2) comparison between furosemide bolus versus any comparator, and (3) critically ill patients. There was no restriction on dose or time of administration. The exclusion criteria were as follows: (1) non-adult studies, (2) studies with a non-parallel design (e.g., crossover) randomized trials, (3) duplicate publications either acknowledged or not (in this case we referred to the first article published while we retrieved data from the article with the longest follow-up available, (4) non-human experimental studies, (5) studies with no data on outcome of interests; and (6) oral furosemide administration.
Two investigators independently assessed the compliance to selection criteria and selected the studies for the final analysis, with divergences finally resolved by consensus.
Data abstraction and study endpoint
Baseline characteristics, procedural, and outcome data were abstracted independently by four trained investigators; the divergences were resolved by consensus. Specifically, we extracted potential sources of significant clinical heterogeneity, such as study design, sample size, clinical setting/indication, furosemide bolus dose, control treatment, and follow-up duration, as well as primary study endpoint and other key outcomes. Corresponding author of original authors were contacted in cases of missing data on outcome of interests.
Risk of bias assessment
The internal validity and risk of bias of included trials was appraised by two independent reviewers according to the Risk of Bias Assessment Tool developed by the Cochrane collaboration [29, 30] that assesses the adequacy of randomization sequence generation, the concealment of treatment allocation, blinding of participating subjects, treating personnel and outcome assessors, complete reporting of outcome, possible selective outcome reporting, possible other sources of bias, and provide a final judgement on the overall risk of bias. Publication bias were assessed by visually inspecting funnel plots for pooled analyses containing >10 studies [31].
Data analysis and synthesis
Computations were performed with RevMan (Review Manager, Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014; available at http://community.cochrane.org/tools/review-production-tools/revman-5/revman-5-download). Hypothesis of statistical heterogeneity was tested by means of Cochran Q test, with statistical significance set at the two-tailed 0.10 level, whereas extent of statistical consistency was measured with I2, defined as 100% X (Q-df)/Q, where Q is Cochran’s heterogeneity statistic and the degrees of freedom (df). Binary outcomes from individual studies were analyzed to compute individual and pooled odds ratio (OR) with pertinent 95% confidence intervals (CIs), fitting a fixed-effect model in case of low statistical inconsistency (I2 <25%) or with random-effect model (which better accommodates clinical and statistical variations) in case of moderate or high statistical inconsistency (I2 ≥25%). Weighted mean difference (WMD) and 95% CIs were computed for continuous variables using the same methods as just described. For individual studies reporting continuous outcomes as median and range or median and interquartile range, mean and standard deviation were estimated using equations elaborated by Wan and colleagues [32].
The primary endpoint was all-cause longest follow-up mortality. The pre-specified secondary endpoint were 28/30-days mortality, new/worsening AKI, receipt of RRT, length of hospital stay, and peak serum creatinine after randomization. Outcomes were defined as per-original author’s definition.
Sensitivity analyses were performed by sequentially removing each study and reanalyzing the remaining dataset (producing a new analysis for each study removed), by selecting an individual subset (defined by setting, control drug, and indication for treatment [e.g., prevention versus treatment of AKI]), and by analyzing only data from studies with low risk of bias.
Statistical significance was set at the two-tailed 0.05 level for hypothesis testing. Unadjusted p values are reported throughout.
We performed pre-defined trial sequential analysis (TSA) [33,34], with the intent of maintaining an overall 5% risk of type I error and a 10% risk of type II error, at a power of 90%. We assumed a relative risk reduction of 15% for each outcome. The analysis was conducted using the control event proportion derived from the present meta-analyses. The resulting required information size was further diversity (D2)-adjusted; in case of D2 = 0, we performed a sensitivity analysis assuming a D2 = 25%. Fixed-effect model was employed. We used the TSA software (TSA Viewer [Computer program], version 0.9.5.5 Beta, Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, 2016.).
Results
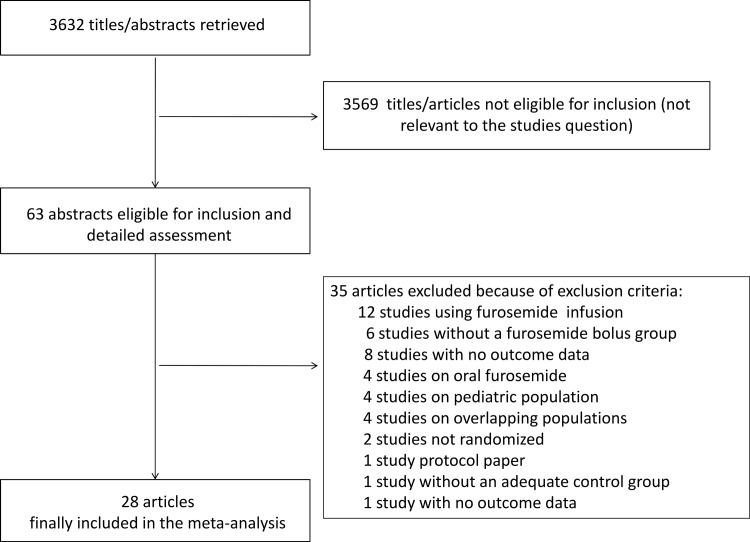
A total of 3632 references were examined. After exclusions of non-pertinent studies, a total of 63 studies were retrieved as complete articles. Of these, 35 were further excluded, as they did not met inclusion criteria. Details of excluded studies together with reason for exclusion are available in the Table A in S1 Appendix. Finally, a total of 28 studies randomizing 3228 patients were included in the analysis (Fig 1) [15–17,22,35–57].
Fig 1. Examined studies.
Trials’ characteristics
Characteristics of included trials are described in Table 1. Included trials were performed in the settings of AKI either in ICU or general ward, cardiac surgery, acutely decompensated heart failure, and contrast-induced AKI.
Table 1. Study characteristics.
| First author | Year | Setting | Multi center | Control treatment | N treatment group | N control group | Prevention or treatment? |
|---|---|---|---|---|---|---|---|
| Ad N [35] | 2002 | Cardiac surgery | No | Infusion furosemide | 39 | 36 | Prevention |
| Allen LA [36] | 2010 | Acute decompensated heart failure | No | Infusion furosemide | 21 | 20 | Prevention |
| Bayat F [37] | 2015 | Cardiac surgery | No | Standard care | 42 | 42 | Prevention |
| Barbanti M [38] | 2015 | Contrast-Induced AKI | No | Standard care | 56 | 56 | Prevention |
| Briguori C [39] | 2011 | Contrast-Induced AKI | Yes | Standard care | 146 | 146 | Prevention |
| Brown CB [40] | 1981 | AKI/ICU | No | Infusion furosemide | 28 | 28 | Treatment |
| Cantarovich F [15] | 1973 | AKI/ICU | No | Standard care | 39 | 19 | Treatment |
| Copeland JG [41] | 1983 | Cardiac surgery | No | Infusion furosemide | 9 | 9 | Prevention |
| Dussol B [42] | 2006 | Contrast-Induced AKI | No | 0.9% Saline | 79 | 77 | Prevention |
| Felker BM [43] | 2011 | Acute decompensated heart failure | No | Infusion furosemide | 156 | 152 | Prevention |
| Gu CQ [44] | 2013 | Contrast-induced AKI | Yes | Standard care | 422 | 437 | Prevention |
| Karayannopoulos S [16] | 1974 | AKI/ICU | No | Standard care | 10 | 10 | Treatment |
| Kleinknecht D [17] | 1976 | AKI/ICU | No | Placebo | 33 | 33 | Treatment |
| Kunt AT [45] | 2009 | Cardiac surgery | No | Infusion furosemide | 50 | 50 | Treatment |
| Llorens P [46] | 2014 | Acute decompensated heart failure | No | Infusion furosemide | 73 | 36 | Prevention |
| Marenzi G [47] | 2012 | Contrast-Induced AKI | Yes | Standard care | 87 | 83 | Prevention |
| Mojtahedzadeh M [48] | 2004 | AKI/ICU | No | Infusion furosemide | 11 | 11 | Prevention |
| Ostermann M [49] | 2007 | AKI/ICU | No | Infusion furosemide | 26 | 30 | Prevention |
| Palazzuoli A [50] | 2014 | Acute decompensated heart failure | No | Infusion furosemide | 39 | 43 | Prevention |
| Schuller D [51] | 1997 | AKI/ICU | No | Infusion furosemide | 19 | 14 | Treatment |
| Shah RA [52] | 2014 | Acute decompensated heart failure | No | Infusion furosemide | 30 | 60 | Treatment |
| Shilliday IR [53] | 1997 | AKI/ICU | No | Placebo | 32 | 30 | Treatment |
| Solomon R [22] | 1994 | Contrast-Induced AKI | No | Saline (± mannitol) | 25 | 53 | Prevention |
| Thomson MR [54] | 2010 | Acute decompensated heart failure | No | Infusion furosemide | 30 | 26 | Treatment |
| Usmiani T [55] | 2016 | Contrast-Induced AKI | No | Standard care | 57 | 63 | Prevention |
| Vargas Hein O [56] | 2005 | Cardiac surgery | No | Torsemide | 14 | 15 | Treatment |
| Yayla Ç [57] | 2015 | Acute decompensated heart failure | No | Infusion furosemide | 14 | 15 | Prevention |
In 14 trials control treatment was represented by continuous furosemide infusion, in 12 trials by placebo/standard treatment, and in 2 further trials by an active pharmacological comparator. Fifteen trials administered furosemide bolus as a preventive measure in patients who had not yet developed AKI, while 11 as treatment of established AKI.
Mortality data were available for 19 trials, with four trials reporting 28/30 days mortality. Data on receipt of RRT were available from 14 trials, data on new onset/worsening AKI from 16 trials, length of hospital stay in 7 trials and peak serum creatinine following randomization in 6 trials.
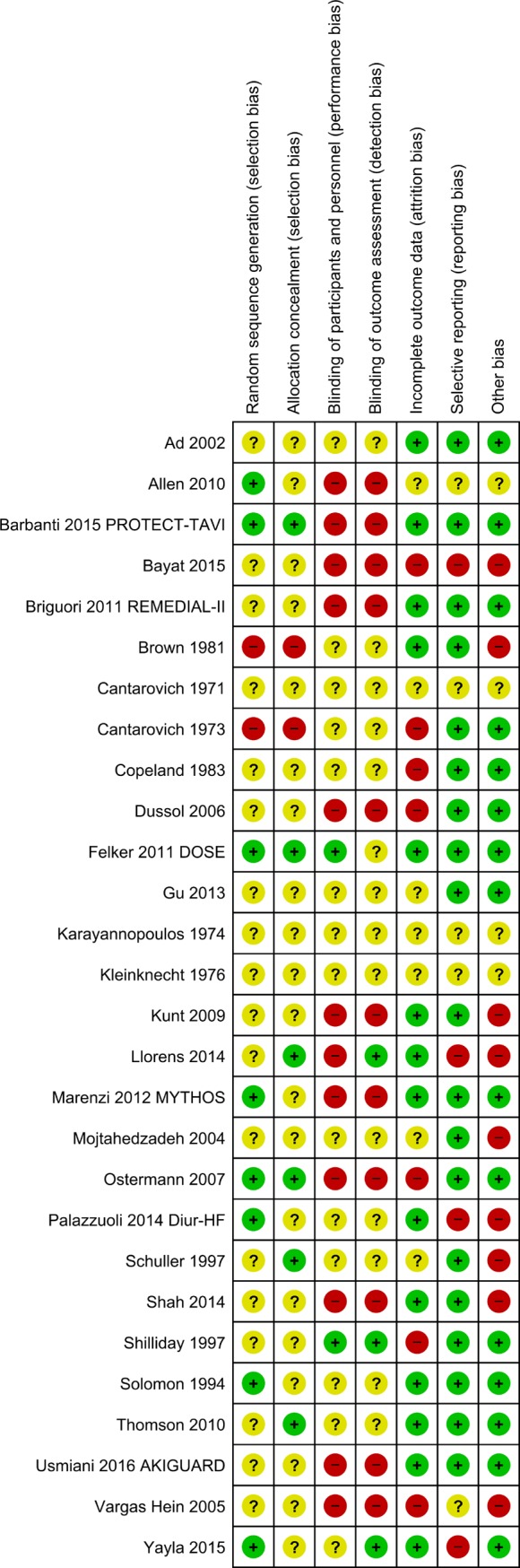
Overall, risk of bias analysis showed that none of included trials was at low risk of bias. A total of 19 trials were considered at high risk of bias, and 9 at unclear risk of bias (Fig 2 and Figure A in S1 Appendix)
Fig 2. Studies bias.
Studies results are reported in the text and in S1 Appendix.
All-cause longest follow-up mortality
Overall, a total of 143/892 (16%) patients assigned to the furosemide bolus group died, as compared with 141/881 (16%) assigned to the control group, with no difference between the two groups (OR = 0.84; 95% CI 0.63 to 1.13; p-value = 0.25; I2 = 0%) (Table 2) (Figure B in S1 Appendix). Trial sequential analysis was inconclusive with only 25.79% of the information size accrued, suggesting the need for more randomized controlled trial(s) to establish firm evidence on the beneficial or detrimental effect on survival of furosemide bolus over control (OR 0.84, TSA-adjusted 95% CI, 0.46, 1.54) (Figure C in S1 Appendix). In particular, TSA estimated that the required information size would be 6874 randomized patients to show a 15% relative risk reduction.
Table 2. Overall results.
| Analysis | Treatment group | Control group | OR/MD | 95% CI | p-value for effect | p-value for heterogeneity | I2 (%) |
|---|---|---|---|---|---|---|---|
| Longest f-up mortality, n–events/N (%) | 143/892 (16%) | 141/881 (16%) | 0.84 | 0.63 to 1.13 | 0.25 | 0.47 | 0 |
| 28/30 days mortality–events/N (%) | 16/282 (8.8%) | 11/312 (3.5%) | 1.62 | 0.78 to 3.35 | 0.20 | 0.27 | 23 |
| New/worsening AKI–events/N (%) | 179/1335 (13.4%) | 243/1333 (18.2%) | 0.72 | 0.47 to 1.10 | 0.13 | 0.001 | 60 |
| Need for RRT–events/N (%) | 78/843 (9.3%) | 94/842 (11.6%) | 0.49 | 0.21 to 1.15 | 0.10 | 0.15 | 32 |
| Hospital LOS, days–mean ± SD | 0.17 | -1.04 to 1.39 | 0.78 | 0.003 | 70 | ||
| Peak serum creatinine, mg/dl | 0.10 | -0.12 to 0.33 | 0.36 | < 0.001 | 98 |
CI: confidence interval; MD: mean difference; OR: odds ratio; AKI: acute kidney injury; RRT: renal replacement therapy; LOS, length of hospital stay.
A subgroup effect was identified only for studies administrating furosemide as prevention versus treatment, with a favorable effect in “prevention” trials (OR = 0.62; 95% CI 0.41 to 0.94; p-value = 0.03; I2 = 0%, with 9 studies included), and a neutral effect in “treatment” trials (OR = 1.14; 95% CI 0.75 to 1.72; p-value = 0.54; I2 = 0%, with 9 studies included), with a p-value between groups = 0.04. (Fig 3). Conversely, a subgroup effect depending on control treatment or clinical setting was not identified (Figures D and E in S1 Appendix). Trend towards subgroup differences were identified when stratifying analysis by control treatment and treatment indication (prevention vs treatment) (Figure D and Table D in S1 Appendix).
Fig 3. Furosemide prevention VS treatment subgroup.
Sequentially removing each trial did not change magnitude and direction of treatment effect (lowest OR = 0.78; 95% CI 0.58 to 1.05; p-value = 0.10; I2 = 0%, removing Kunt et al [45]; highest OR = 0.90; 95% CI = 0.67 to 1.21; p-value = 0.49; I2 = 0%, removing Usmiani et al [55]).
As no trial with low risk of bias was identified, pre-specified analysis including only low risk of bias trials was not performed.
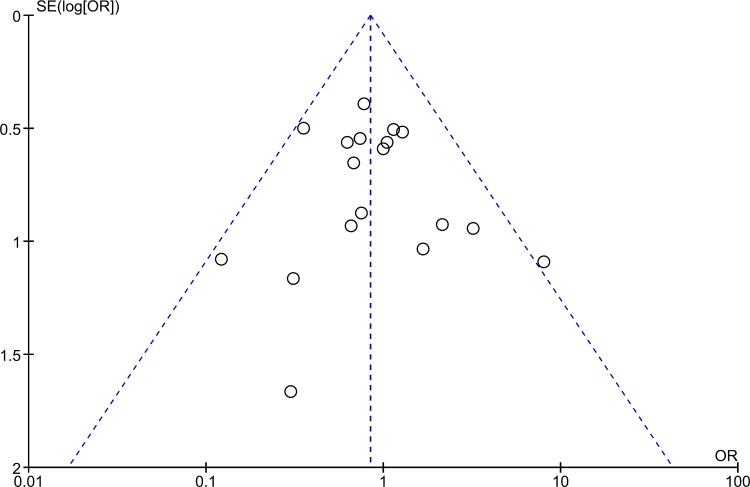
Visual inspection of funnel plot did not suggest possible presence of publication bias (Fig 4).
Fig 4. Funnel plot.
All-cause 28/30-days mortality
Overall, we found no difference in 28/30 days mortality between the treatment and the control group (16/282 [5.7%] in the furosemide bolus group versus 11/312 [3.5%] in the control group; OR = 1.62; 95% CI 0.78 to 3.35; p-value = 0.20; I2 = 23%, with 4 studies included) (Figures F to I in S1 Appendix).
New onset/worsening AKI
Overall, 179/1335 (13.4%) patients assigned to furosemide bolus administration experienced new onset or worsening of AKI, as compared with 243/1333 (18.2%) patients assigned to the control group (OR = 0.72; 95% CI = 0.47 to 1.10; p-value = 0.13; I2 = 60%, TSA inconclusive) (Figure J in S1 Appendix).
A subgroup effect was identified when analyzing trials according to the control treatment, with a favorable effect of furosemide bolus as compared with placebo/standard treatment and a harmful effect when compared with active pharmacological control (Figures K to M in S1 Appendix).
Receipt of RRT
Overall, 78/843 (9.3%) patients assigned to furosemide bolus administration received RRT, as compared with 94/842 (11.6%) patients assigned to the control group (OR = 0.49; 95% CI = 0.21 to 1.15; p-value = 0.10; I2 = 32%, TSA inconclusive) (Figure N in S1 Appendix).
A trend towards a subgroup effect was identified when analyzing trials according to setting, with a favorable effect of furosemide bolus in the setting of contrast-induced AKI and a trend towards a harmful effect in cardiac surgery (Figure P in S1 Appendix). In addition, in the subgroup of patients receiving preventive furosemide administration, we observed a significant reduction in receipt of RRT (Figure Q in S1 Appendix).
Length of hospital stay
Pooled analysis of the 6 trials that reported hospital length of stay did not show a statistically significant difference between furosemide bolus and control treatment (WMD = 0.17; 95% CI = -1.04 to 1.39; p-value = 0.78; I2 = 70%) (Figure R in S1 Appendix). A trend towards a subgroup difference was identified when comparing trials administering furosemide as a preventive measure as compared with trials administering it as treatment (Figures S to U in S1 Appendix).
Peak serum creatinine
Pooled analysis of the 6 trials reporting peak serum creatinine after randomization did not show a statistically significant difference between furosemide bolus and control treatment (WMD = 0.10; 95% CI = -0.12 to 0.33; p-value = 0.36; I2 = 98%) (Figure V in S1 Appendix). A subgroup effect was identified when analyzing trials according to the setting, with trials performed in cardiac surgery setting showing an increase in serum creatinine associated with bolus furosemide administration (Figures V to Y in S1 Appendix).
Contrast-induced AKI
Subgroup analyses comparing trials performed in the setting of contrast-induced AKI versus trials performed in all other settings did not identify a subgroup effect except in any of the analysed outcomes with the exception of all-cause 28/30-days mortality (p for interaction = 0.04) (Figures AB to AF in S1 Appendix).
Discussion
Key findings
In this meta-analysis of RCTs, we found that intermittent furosemide administration in patients with or at risk for AKI did not result in a lower mortality, reduced incidence or worsening of AKI, or decreased utilization of RRT. A trend towards a beneficial effect of intermittent furosemide administration was found when analyzing the subgroup of studies in which furosemide was administered to prevent AKI. This finding was consistent across different outcomes, with a similar beneficial effect on RRT utilization and a trend towards a beneficial effect on worsening AKI, length of hospital stay, and peak serum creatinine in “prevention” trials. However, TSA was inconclusive, suggesting no firm conclusions on the topic and the need of further high-quality studies on the topic.
Relationship to previous studies
The effect of loop diuretics on incidence and course of AKI has been a matter of debate and investigation for years. Accordingly, several RCTs and observational trials have been performed investigating the effect of diuretics and optimal diuretic strategy. As of today, convincing evidence that diuretic administration can alter per se the course of AKI or shorten renal recovery when RRT is needed is lacking [4,58,59].
In the largest meta-analysis performed so far on furosemide administration in patients with or at risk for AKI, the authors included a total of 11 studies and found no difference in mortality or RRT utilization between the furosemide and control group, for both AKI prevention and treatment [60]. Compared with this study, our meta-analysis specifically investigated the role of intermittent furosemide administration versus any comparator, including continuous furosemide infusion. In addition, we included a larger number of trials and patients, including some recent, large multicenter RCTs [42,46].
Other meta-analyses on the role of loop diuretics in prevention or treatment of AKI in different settings and with different inclusion criteria have been performed [61,62,63,64], all of which consistently found that loops diuretics administration was not associated with improved outcome in patients with AKI. Meta-analyses consistently confirmed a higher urine output associated with diuretics use [62,63] and some suggested a possible shorter duration of RRT [62] and number of dialysis treatments [63], although level of evidence was considered to be low. A positive effect of furosemide administration on RRT use was confirmed in our study, although limited to trials investigating preventive role of furosemide. Conversely, meta-analyses and small trials questioned the beneficial effect of furosemide on incidence or clinical course of AKI [37,61,65]. To clarify these issues, a pilot multicenter RCT on continuous furosemide administration versus placebo in critically ill patients with AKI (the SPARK study) was planned [66]. The study aimed at enrolling 216 patients with early AKI defined according to R-RIFLE criteria [67], with worsening AKI as primary endpoint. Unfortunately, the trial was interrupted early due to logistic problems and lack of funding after only 73 patients had been enrolled, and found no difference in the primary or any of secondary outcomes [68]. However, furosemide administration was associated with a higher risk of electrolyte abnormalities.
Several meta-analyses compared continuous versus intermittent furosemide administration in patients several clinical settings, both in adults and pediatric patients [69,70,71,72]. This meta-analyses yielded small differences in results depending on trial inclusion criteria, but consistently confirmed no improvement in major outcomes associated with any of the two strategies. The largest meta-analysis, including all RCTs performed on hospitalized patients (including pediatric and crossover studies), suggested that continuous administration might be associated with greater urine output [72], especially when preceded by a single bolus. Whether this translates in improved clinical outcome remains to be determined [73]. Compared with previously published meta-analyses, we focused on adult patients only and included a larger number of trials and comparators, while excluding crossover studies, as our meta-analysis aimed at investigating clinically relevant outcomes such as mortality.
Significance of study findings
Our study suggested that furosemide administration may have some benefits when used to prevent AKI, both in terms of mortality and need for RRT. However, we believe that our results should be interpreted with caution, as also suggested by TSA that was inconclusive due to too low information size. Indeed, positive results observed with furosemide administration are largely driven by trials performed in the setting of CI-AKI, and in particular by four trials investigating an automated fluid delivery system (RenalGuard, PLC Medical Systems, Milford, Massachusetts) which matches hydration with diuresis [41,42,50,58]. In these studies, furosemide administration to maintain a diuresis ≥ 300 mL/h with matched hydration was shown to prevent CI-AKI and subsequent need for RRT without major adverse events related to fluid overload [74]. Notably, volume expansion is currently the only widely recommended strategy to prevent CI-AKI [75,76] while furosemide was shown to have detrimental effect when not coupled with adequate hydration [22,23,45,77]. Accordingly, beneficial effect of RenalGuard system is probably related not on furosemide administration, but on the adequate diuresis achieved together with targeted volume expansion, thus limiting the risk of both fluid overload and dehydration. To further complicate the picture, published RCTs on RenalGuard system were all considered to be at high risk of bias, thereby downgrading level of evidence of this strategy [74], while the effectiveness of volume expansion in preventing CI-AKI has been recently challenged [78].
The role of fluid overload on incidence and pathogenesis of AKI and organ dysfunction in critically ill and surgical patients has been largely investigated in recent years [19,79,80,81]. It is now generally recognized that also excessive fluid overload and high central venous pressure causes renal congestion and impaired kidney perfusion, both in heart failure and critically ill patients. In this context, positive effects observed with furosemide administration in patients with AKI may be related to an indirect mechanism mediated by fluid removal, rather than a direct positive effect of loop diuretics. Accordingly, recent guidelines recommend diuretics use to optimize patient volume status [4,35]. A major problem in fluid management in patients with AKI is that both hypovolemia and hypervolemia are associated with AKI development and progression [79,80]. As a consequence, the same diuretic that might improve renal function in fluid-overloaded patient may have detrimental effect on kidney perfusion if a patient is or become volume-depleted. This dual effect can explain the controversial results obtained so far by studies investigating the role of diuretics in prevention or treatment of AKI.
It is a common clinical observation that reversal of oligo-anuria with furosemide administration is frequently associated with improvement in renal function. A furosemide stress test to assess AKI severity and likelihood of progression has been recently developed and validated [82,83]. Chawla et al. showed that a diuresis of at least 200 mL in two hours following a 1–1.5 mg/kg furosemide bolus was associated with reduced AKI progression. This might occur because adequate diuretic response to furosemide required adequate renal perfusion, active secretion of the drug in the tubule, and absence of urinary flow obstruction, all indicative of less severe injury and adequate renal reserve. Thereby, this simple test could globally evaluate kidney function and renal reserve. Therefore, in clinical practice, reversal of oligo-anuria with furosemide is more likely a marker of reduced kidney injury and/or dysfunction, rather than of a beneficial effect exerted by furosemide.
Limitations of the study
Our study has some limitations, which are characteristics of all aggregate data meta-analyses [24,84]. First, we included several studies performed in different setting, with different aims and different control groups. However, we also performed several subgroup analyses, which helped us to better define the influence of each subgroup on overall results. Second, all of the included trials were considered to carry an unclear or a high risk of bias, thereby reducing quality of evidence that our meta-analysis can provide. Nevertheless, identifying lack of high-quality trials and gaps in evidence on a topic is also an objective of systematic reviews and meta-analyses. In a similar context, meta-analyses should be considered hypothesis-generating rather than confirmatory. Third, some of the included studies were performed decades ago. Finally, we focused on adult patients receiving intermittent furosemide administration. Therefore, our results may not apply to continuous furosemide administration or pediatric population, although previous meta-analyses did not suggest that a different effect in this setting is expected.
Future studies and prospects
The role of furosemide and, diuretics administration in patients with or at risk for AKI still needs to be clearly determined. While it is generally accepted that available diuretics are unlikely to exert a direct kidney-protective effect in real-world clinical practice, an indirect effect through optimization of volume status can not be excluded. Future trials should better address this issue. An ideal trial should investigate whether optimization of volume status with diuretics reduces AKI development or progression. However, such a study is unlikely to be conducted mainly due to organizing and ethical reasons. Alternatively, it may be interesting to compare a diuretic-based versus an early-RRT-based fluid management strategy in AKI.
Conclusions
Randomized trials showed that intermittent furosemide administration is not associated with an overall improvement in survival or other major outcomes in patients with or at risk for AKI, although it may reduce mortality and RRT utilization when used as a preventive measure. However, these findings are largely influenced by a specific subset of trials performed in CI-AKI setting and likely attributable to the concomitant management protocol investigated in these trials. Furthermore, low risk of bias trials are lacking. Future high quality trials are needed to confirm the role of loop diuretics in AKI prevention and management, although ethical issues may limit feasibility.
Supporting information
Checklist for transparent and complete reporting of systematic reviews and meta-analyses.
(PDF)
Supplementary Appendix including.
(DOCX)
Acknowledgments
Dr. Bagshaw is supported by a Canada Research Chair in Critical Care Nephrology.
The authors are grateful to Omar Saleh, MD, Sofia Beatrice Pellegrini, RN, Giuseppe Ponzetta, RN, for their contribution in study conduction and manuscript review.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Supported by a grant from the Italian Medicines Agency (AIFA – Grant no. FARM12JFX9, http://www.agenziafarmaco.gov.it/) received by Prof. Tiziana Bove. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bellomo R, Ronco C, Mehta RL, Asfar P, Boisramé-Helms J, Darmon M, et al. Acute kidney injury in the ICU: from injury to recovery: reports from the 5th Paris International Conference. Ann Intensive Care. 2017;7:49 doi: 10.1186/s13613-017-0260-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. ; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005; 294:813–8. doi: 10.1001/jama.294.7.813 [DOI] [PubMed] [Google Scholar]
- 3.Doyle JF, Forni LG. Acute kidney injury: short-term and long-term effects. Crit Care. 2016;20:188 doi: 10.1186/s13054-016-1353-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joannidis M, Druml W, Forni LG, Groeneveld ABJ, Honore PM, Hoste E, et al. Prevention of acute kidney injury and protection of renal function in the intensive care unit: update 2017: Expert opinion of the Working Group on Prevention, AKI section, European Society of Intensive Care Medicine. Intensive Care Med. 2017;43:730–749. doi: 10.1007/s00134-017-4832-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landoni G, Bove T, Székely A, Comis M, Rodseth RN, Pasero D, et al. Reducing mortality in acute kidney injury patients: systematic review and international web-based survey. J Cardiothorac Vasc Anesth. 2013;27:1384–98. doi: 10.1053/j.jvca.2013.06.028 [DOI] [PubMed] [Google Scholar]
- 6.Bagshaw SM, Delaney A, Jones D, Ronco C, Bellomo R. Diuretics in the management of acute kidney injury: a multinational survey. Contrib Nephrol. 2007;156:236–49. doi: 10.1159/000102089 [DOI] [PubMed] [Google Scholar]
- 7.Kramer HJ, Schüürmann J, Wassermann C, Düsing R. Prostaglandin-independent protection by furosemide from oliguric ischemic renal failure in conscious rats. Kidney Int. 1980;17:455–64. [DOI] [PubMed] [Google Scholar]
- 8.Bayati A, Nygren K, Källskog O, Wolgast M. The effect of loop diuretics on the long-term outcome of post-ischaemic acute renal failure in the rat. Acta Physiol Scand. 1990;139:271–9. doi: 10.1111/j.1748-1716.1990.tb08924.x [DOI] [PubMed] [Google Scholar]
- 9.Heyman SN, Rosen S, Epstein FH, Spokes K, Brezis ML. Loop diuretics reduce hypoxic damage to proximal tubules of the isolated perfused rat kidney. Kidney Int. 1994;45:981–5. [DOI] [PubMed] [Google Scholar]
- 10.Mehta RL, Pascual MT, Soroko S, Chertow GM; PICARD Study Group. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA. 2002;288:2547–53. [DOI] [PubMed] [Google Scholar]
- 11.Uchino S, Doig GS, Bellomo R, Morimatsu H, Morgera S, Schetz M, et al. ; Beginning and Ending Supportive Therapy for the Kidney (B.E.S.T. Kidney) Investigators. Diuretics and mortality in acute renal failure. Crit Care Med. 2004;32:1669–77. [DOI] [PubMed] [Google Scholar]
- 12.Aravindan N, Aravindan S, Riedel BJ, Weng HR, Shaw AD. Furosemide prevents apoptosis and associated gene expression in a rat model of surgical ischemic acute renal failure. Ren Fail. 2007;29:399–407. doi: 10.1080/08860220701263671 [DOI] [PubMed] [Google Scholar]
- 13.Aravindan N, Shaw A. Effect of furosemide infusion on renal hemodynamics and angiogenesis gene expression in acute renal ischemia/reperfusion. Ren Fail. 2006;28:25–35. [DOI] [PubMed] [Google Scholar]
- 14.Cantarovich F, Fernandez JC, Locatelli A, Perez Loredo J. Frusemide in high doses in the treatment of acute renal failure. Postgrad Med J. 1971;47:Suppl:13–7. [PubMed] [Google Scholar]
- 15.Cantarovich F, Galli C, Benedetti L, Chena C, Castro L, Correa C, et al. High dose frusemide in established acute renal failure. Br Med J. 1973;4:449–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karayannopoulos S. Letter: High-dose frusemide in renal failure. Br Med J. 1974; 2: 278–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleinknecht D, Ganeval D, Gonzalez-Duque LA, Fermanian J. Furosemide in acute oliguric renal failure. A controlled trial. Nephron. 1976;17:51–8. doi: 10.1159/000180710 [DOI] [PubMed] [Google Scholar]
- 18.Teixeira C, Garzotto F, Piccinni P, Brienza N, Iannuzzi M, Gramaticopolo S, et al. ; NEFROlogia e Cura INTensiva (NEFROINT) investigators. Fluid balance and urine volume are independent predictors of mortality in acute kidney injury. Crit Care. 2013;17:R14 doi: 10.1186/cc12484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grams ME, Estrella MM, Coresh J, Brower RG, Liu KD; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network. Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol. 2011;6:966–73. doi: 10.2215/CJN.08781010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damman K, Kjekshus J, Wikstrand J, Cleland JG, Komajda M, Wedel H, et al. Loop diuretics, renal function and clinical outcome in patients with heart failure and reduced ejection fraction. Eur J Heart Fail. 2016;18:328–36. doi: 10.1002/ejhf.462 [DOI] [PubMed] [Google Scholar]
- 21.Yuengsrigul A, Chin TW, Nussbaum E. Immunosuppressive and cytotoxic effects of furosemide on human peripheral blood mononuclear cells. Ann Allergy Asthma Immunol. 1999;83(6 Pt 1):559–66. [DOI] [PubMed] [Google Scholar]
- 22.Solomon R, Werner C, Mann D, D'Elia J, Silva P. Effects of saline, mannitol, and furosemide on acute decreases in renal function induced by radiocontrast agents. N Engl J Med. 1994;331:1416–20. doi: 10.1056/NEJM199411243312104 [DOI] [PubMed] [Google Scholar]
- 23.Weinstein JM, Heyman S, Brezis M. Potential deleterious effect of furosemide in radiocontrast nephropathy. Nephron. 1992;62:413–5. doi: 10.1159/000187090 [DOI] [PubMed] [Google Scholar]
- 24.Greco T, Zangrillo A, Biondi-Zoccai G, Landoni G. Meta-analysis: pitfalls and hints. Heart Lung Vessel. 2013;5:219–25. [PMC free article] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097 doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100 doi: 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bove T, Landoni G, Zangrillo A. Furosemide in critically ill patients with or at risk of acute kidney injury. A meta-analysis of randomized trials. Furosemide in critically ill patients with or at risk of acute kidney injury. A meta-analysis of randomized trials. PROSPERO 2017 CRD42017078607. [DOI] [PMC free article] [PubMed]
- 28.Biondi-Zoccai GG, Agostoni P, Abbate A, Testa L, Burzotta F. A simple hint to improve Robinson and Dickersin's highly sensitive PubMed search strategy for controlled clinical trials. Int J Epidemiol. 2005;34:224–5; author reply 225. doi: 10.1093/ije/dyh311 [DOI] [PubMed] [Google Scholar]
- 29.Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011.
- 30.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928 doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002 doi: 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 32.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135 doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008;61:64–75. doi: 10.1016/j.jclinepi.2007.03.013 [DOI] [PubMed] [Google Scholar]
- 34.Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol. 2008;61:763–9. doi: 10.1016/j.jclinepi.2007.10.007 [DOI] [PubMed] [Google Scholar]
- 35.Ad N, Suyderhoud JP, Kim YD, Makary MA, DeGroot KW, Lue HC, et al. Benefits of prophylactic continuous infusion of furosemide after the maze procedure for atrial fibrillation. J Thorac Cardiovasc Surg. 2002;123:232–6. [DOI] [PubMed] [Google Scholar]
- 36.Allen LA, Turer AT, Dewald T, Stough WG, Cotter G, O'Connor CM. Continuous versus bolus dosing of Furosemide for patients hospitalized for heart failure. Am J Cardiol. 2010;105:1794–7. doi: 10.1016/j.amjcard.2010.01.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bayat F, Faritous Z, Aghdaei N, Dabbagh A. A study of the efficacy of furosemide as a prophylaxis of acute renal failure in coronary artery bypass grafting patients: A clinical trial. ARYA Atheroscler.2015;11:173–8. [PMC free article] [PubMed] [Google Scholar]
- 38.Barbanti M, Gulino S, Capranzano P, Immè S, Sgroi C, Tamburino C, et al. Acute Kidney Injury With the RenalGuard System in Patients Undergoing Transcatheter Aortic Valve Replacement: The PROTECT-TAVI Trial (PROphylactic effecT of furosEmide-induCed diuresis with matched isotonic intravenous hydraTion in Transcatheter Aortic Valve Implantation). JACC Cardiovasc Interv. 2015;8:1595–604. doi: 10.1016/j.jcin.2015.07.012 [DOI] [PubMed] [Google Scholar]
- 39.Briguori C, Visconti G, Focaccio A, Airoldi F, Valgimigli M, Sangiorgi GM, et al. ; REMEDIAL II Investigators. Renal Insufficiency After Contrast Media Administration Trial II (REMEDIAL II): RenalGuard System in high-risk patients for contrast-induced acute kidney injury. Circulation. 2011;124:1260–9. doi: 10.1161/CIRCULATIONAHA.111.030759 [DOI] [PubMed] [Google Scholar]
- 40.Brown CB, Ogg CS, Cameron JS. High dose frusemide in acute renal failure: a controlled trial. Clin Nephrol. 1981;15:90–6. [PubMed] [Google Scholar]
- 41.Copeland JG, Campbell DW, Plachetka JR, Salomon NW, Larson DF. Diuresis with continuous infusion of furosemide after cardiac surgery. Am J Surg. 1983;146:796–9. [DOI] [PubMed] [Google Scholar]
- 42.Dussol B, Morange S, Loundoun A, Auquier P, Berland Y. A randomized trial of saline hydration to prevent contrast nephropathy in chronic renal failure patients. Nephrol Dial Transplant. 2006;21:2120–6. doi: 10.1093/ndt/gfl133 [DOI] [PubMed] [Google Scholar]
- 43.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, et al. ; NHLBI Heart Failure Clinical Research Network. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu GQ, Lu R, Cui W, Liu F, Zhang Y, Yang XH, et al. Low-dose furosemide administered with adequate hydration reduces contrast-induced nephropathy in patients undergoing coronary angiography. Cardiology. 2013;125:69–73. doi: 10.1159/000350648 [DOI] [PubMed] [Google Scholar]
- 45.Kunt AT, Akgün S, Atalan N, Bitir N, Arsan S. Furosemide infusion prevents the requirement of renal replacement therapy after cardiac surgery. Anadolu Kardiyol Derg. 2009;9:499–504. [PubMed] [Google Scholar]
- 46.Llorens P, Miró Ò, Herrero P, Martín-Sánchez FJ, Jacob J, Valero A et al. Clinical effects and safety of different strategies for administering intravenous diuretics in acutely decompensated heart failure: a randomised clinical trial. Emerg Med J. 2014;31:706–13. doi: 10.1136/emermed-2013-202526 [DOI] [PubMed] [Google Scholar]
- 47.Marenzi G, Ferrari C, Marana I, Assanelli E, De Metrio M, Teruzzi G,. Prevention of contrast nephropathy by furosemide with matched hydration: the MYTHOS (Induced Diuresis With Matched Hydration Compared to Standard Hydration for Contrast Induced Nephropathy Prevention) trial. JACC Cardiovasc Interv. 2012;5:90–7. doi: 10.1016/j.jcin.2011.08.017 [DOI] [PubMed] [Google Scholar]
- 48.Mojtahedzadeh M, Salehifar E, Vazin A, Mahidiani H, Najafi A, Tavakoli M, et al. Comparison of hemodynamic and biochemical effects of furosemide by continuous infusion and intermittent bolus in critically ill patients. J Infus Nurs. 2004;27:255–61. [DOI] [PubMed] [Google Scholar]
- 49.Ostermann M, Alvarez G, Sharpe MD, Martin CM. Frusemide administration in critically ill patients by continuous compared to bolus therapy. Nephron Clin Pract. 2007;107:c70–6. doi: 10.1159/000108641 [DOI] [PubMed] [Google Scholar]
- 50.Palazzuoli A, Pellegrini M, Ruocco G, Martini G, Franci B, Campagna MS, et al. Continuous versus bolus intermittent loop diuretic infusion in acutely decompensated heart failure: a prospective randomized trial. Crit Care. 2014;18:R134 doi: 10.1186/cc13952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schuller D, Lynch JP, Fine D. Protocol-guided diuretic management: comparison of furosemide by continuous infusion and intermittent bolus. Crit Care Med. 1997;25:1969–75. [DOI] [PubMed] [Google Scholar]
- 52.Shah RA, Subban V, Lakshmanan A, Narayanan S, Udhayakumaran K, Pakshirajan B, et al. A prospective, randomized study to evaluate the efficacy of various diuretic strategies in acute decompensated heart failure. Indian Heart J. 2014;66:309–16. doi: 10.1016/j.ihj.2014.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shilliday IR, Quinn KJ, Allison ME. Loop diuretics in the management of acute renal failure: a prospective, double-blind, placebo-controlled, randomized study. Nephrol Dial Transplant. 1997;12:2592–6. [DOI] [PubMed] [Google Scholar]
- 54.Thomson MR, Nappi JM, Dunn SP, Hollis IB, Rodgers JE, Van Bakel AB. Continuous versus intermittent infusion of furosemide in acute decompensated heart failure. J Card Fail. 2010;16:188–93. doi: 10.1016/j.cardfail.2009.11.005 [DOI] [PubMed] [Google Scholar]
- 55.Usmiani T, Andreis A, Budano C, Sbarra P, Andriani M, Garrone P, et al. AKIGUARD (Acute Kidney Injury GUARding Device) trial: in-hospital and one-year outcomes. J Cardiovasc Med (Hagerstown). 2016;17:530–7. [DOI] [PubMed] [Google Scholar]
- 56.Vargas Hein O, Staegemann M, Wagner D, von Heymann C, Martin M, Morgera S, et al. Torsemide versus furosemide after continuous renal replacement therapy due to acute renal failure in cardiac surgery patients. Ren Fail. 2005;27:385–92. [DOI] [PubMed] [Google Scholar]
- 57.Yayla Ç, Akyel A, Canpolat U, Gayretli Yayla K, Eyiol A, Akboğa MK, et al. Comparison of three diuretic treatment strategies for patients with acute decompensated heart failure. Herz. 2015;40:1115–20. doi: 10.1007/s00059-015-4327-y [DOI] [PubMed] [Google Scholar]
- 58.Kellum JA, Lameire N; KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17:204 doi: 10.1186/cc11454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ichai C, Vinsonneau C, Souweine B, Armando F, Canet E, Clec'h C, et al. ; Société française d’anesthésie et de réanimation (Sfar); Société de réanimation de langue française (SRLF); Groupe francophone de réanimation et urgences pédiatriques (GFRUP); Société française de néphrologie (SFN). Acute kidney injury in the perioperative period and in intensive care units (excluding renal replacement therapies). Ann Intensive Care. 2016;6:48 doi: 10.1186/s13613-016-0145-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ho KM, Power BM. Benefits and risks of furosemide in acute kidney injury. Anaesthesia. 2010;65:283–93. doi: 10.1111/j.1365-2044.2009.06228.x [DOI] [PubMed] [Google Scholar]
- 61.Ho KM, Sheridan DJ. Meta-analysis of frusemide to prevent or treat acute renal failure. BMJ. 2006;333:420 doi: 10.1136/bmj.38902.605347.7C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bagshaw SM, Delaney A, Haase M, Ghali WA, Bellomo R. Loop diuretics in the management of acute renal failure: a systematic review and meta-analysis. Crit Care Resusc. 2007;9:60–8. [PubMed] [Google Scholar]
- 63.Sampath S, Moran JL, Graham PL, Rockliff S, Bersten AD, Abrams KR. The efficacy of loop diuretics in acute renal failure: assessment using Bayesian evidence synthesis techniques. Crit Care Med. 2007;35:2516–24. [DOI] [PubMed] [Google Scholar]
- 64.Gandhi A, Husain M, Salhiyyah K, Raja SG. Does perioperative furosemide usage reduce the need for renal replacement therapy in cardiac surgery patients? Interact Cardiovasc Thorac Surg. 2012;15:750–5. doi: 10.1093/icvts/ivs208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lassnigg A, Donner E, Grubhofer G, Presterl E, Druml W, Hiesmayr M. Lack of renoprotective effects of dopamine and furosemide during cardiac surgery. J Am Soc Nephrol. 2000;11:97–104. [DOI] [PubMed] [Google Scholar]
- 66.Bagshaw SM, Gibney RT, McAlister FA, Bellomo R. The SPARK Study: a phase II randomized blinded controlled trial of the effect of furosemide in critically ill patients with early acute kidney injury. Trials. 2010;11:50 doi: 10.1186/1745-6215-11-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bagshaw SM, Gibney RTN, Kruger P, Hassan I, McAlister FA, Bellomo R. The effect of low-dose furosemide in critically ill patients with early acute kidney injury: A pilot randomized blinded controlled trial (the SPARK study). J Crit Care. 2017;42:138–146. doi: 10.1016/j.jcrc.2017.07.030 [DOI] [PubMed] [Google Scholar]
- 69.Zangrillo A, Cabrini L, Monti G, Turi S, Moizo E, Vinciguerra F, et al. Continuous infusion versus bolus injection of furosemide in critically ill patients. A systematic review and meta-analysis. Signa Vitae. 2011;6:58–63 [Google Scholar]
- 70.Zangrillo A, Cabrini L, Biondi-Zoccai GGL, Monti G, Turi S, Sheiban I, et al. Continuous infusion versus bolus injection of furosemide in pediatric patients after cardiac surgery: a meta-analysis of randomized studies. Signa Vitae. 2012;7:17–22. [Google Scholar]
- 71.Wu MY, Chang NC, Su CL, Hsu YH, Chen TW, Lin YF, et al. Loop diuretic strategies in patients with acute decompensated heart failure: a meta-analysis of randomized controlled trials. J Crit Care. 2014;29:2–9. doi: 10.1016/j.jcrc.2013.10.009 [DOI] [PubMed] [Google Scholar]
- 72.Alqahtani F, Koulouridis I, Susantitaphong P, Dahal K, Jaber BL. A meta-analysis of continuous vs intermittent infusion of loop diuretics in hospitalized patients. J Crit Care. 2014;29:10–7. doi: 10.1016/j.jcrc.2013.03.015 [DOI] [PubMed] [Google Scholar]
- 73.Palazzuoli A, Ruocco G, Vescovo G, Valle R, Di Somma S, Nuti R. Rationale and study design of intravenous loop diuretic administration in acute heart failure: DIUR-AHF. ESC Heart Fail. 2017;4:479–486. doi: 10.1002/ehf2.12226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Putzu A, Boscolo Berto M, Belletti A, Pasotti E, Cassina T, Moccetti T, et al. Prevention of Contrast-Induced Acute Kidney Injury by Furosemide With Matched Hydration in Patients Undergoing Interventional Procedures: A Systematic Review and Meta-Analysis of Randomized Trials. JACC Cardiovasc Interv. 2017;10:355–363. doi: 10.1016/j.jcin.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 75.Lameire N, Kellum JA; KDIGO AKI Guideline Work Group. Contrast-induced acute kidney injury and renal support for acute kidney injury: a KDIGO summary (Part 2). Crit Care. 2013;17:205 doi: 10.1186/cc11455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vanmassenhove J, Kielstein J, Jörres A, Biesen WV. Management of patients at risk of acute kidney injury. Lancet. 2017;389:2139–2151. doi: 10.1016/S0140-6736(17)31329-6 [DOI] [PubMed] [Google Scholar]
- 77.Majumdar SR, Kjellstrand CM, Tymchak WJ, Hervas-Malo M, Taylor DA, Teo KK. Forced euvolemic diuresis with mannitol and furosemide for prevention of contrast-induced nephropathy in patients with CKD undergoing coronary angiography: a randomized controlled trial. Am J Kidney Dis. 2009;54:602–9. doi: 10.1053/j.ajkd.2009.03.024 [DOI] [PubMed] [Google Scholar]
- 78.Nijssen EC, Rennenberg RJ, Nelemans PJ, Essers BA, Janssen MM, Vermeeren MA, et al. Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high risk of contrast-induced nephropathy (AMACING): a prospective, randomised, phase 3, controlled, open-label, non-inferiority trial. Lancet. 2017;389:1312–1322. doi: 10.1016/S0140-6736(17)30057-0 [DOI] [PubMed] [Google Scholar]
- 79.Perner A, Prowle J, Joannidis M, Young P, Hjortrup PB, Pettilä V. Fluid management in acute kidney injury. Intensive Care Med. 2017;43:807–815. doi: 10.1007/s00134-017-4817-x [DOI] [PubMed] [Google Scholar]
- 80.Prowle JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol. 2014;10:37–47. doi: 10.1038/nrneph.2013.232 [DOI] [PubMed] [Google Scholar]
- 81.Wang N, Jiang L, Zhu B, Wen Y, Xi XM; Beijing Acute Kidney Injury Trial (BAKIT) Workgroup. Fluid balance and mortality in critically ill patients with acute kidney injury: a multicenter prospective epidemiological study. Crit Care. 2015;19:371 doi: 10.1186/s13054-015-1085-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chawla LS, Davison DL, Brasha-Mitchell E, Koyner JL, Arthur JM, Shaw AD, et al. Development and standardization of a furosemide stress test to predict the severity of acute kidney injury. Crit Care. 2013;17:R207 doi: 10.1186/cc13015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koyner JL, Davison DL, Brasha-Mitchell E, Chalikonda DM, Arthur JM, Shaw AD, et al. Furosemide Stress Test and Biomarkers for the Prediction of AKI Severity. J Am Soc Nephrol. 2015;26:2023–31. doi: 10.1681/ASN.2014060535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Frieden TR. Evidence for Health Decision Making—Beyond Randomized, Controlled Trials. N Engl J Med. 2017;377:465–475. doi: 10.1056/NEJMra1614394 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Checklist for transparent and complete reporting of systematic reviews and meta-analyses.
(PDF)
Supplementary Appendix including.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.