Abstract
Testicular germ cell tumours (TGCTs) are a heterogeneous group of neoplasms, mostly affecting young men. Curability rates are high and adequate treatment relies on careful and accurate pathological and clinical assessment. Indeed, TGCTs' histopathological subtyping is critical for adequate therapeutic decision. Considering the limitation of currently available serum biomarkers, novel candidates have been proposed, most notably miR-371a-3p, which outperformed classical serum markers, but no detailed information concerning TGCT subtype was available. Thus, we carried out evaluation of miR-371a-3p expression levels among TGCT subtypes using a consecutive cohort of tissue samples. MiR-371a-3p discriminated TGCTs from control tissues with high sensitivity and specificity (AUC = 0.99). Furthermore, seminomas displayed higher miR-371a-3p expression levels compared to non-seminomatous TGCTs, which also showed significant differences among them. Nonetheless, prepubertal TGCTs depicted lower miR-371a-3p expression levels than postpubertal TGCTs. Globally, miR-371a-3p expression levels decreased in parallel with progressive cell differentiation. We concluded that miR-371a-3p is TGCTs-specific and it might be clinically useful for early detection and disease monitoring.
This article is part of a discussion meeting issue ‘Frontiers in epigenetic chemical biology’.
Keywords: testicular germ cell tumour, microRNAs, biomarker, miR-371a-3p, postpubertal, prepubertal
1. Background
Testicular germ cell tumours (TGCTs) are a diversified group of neoplasms derived from a primitive germ cell, depicting a wide variety of histological subtypes [1]. Although relatively rare, representing less than 1% of all cases of male malignancies, incidence has been increasing over the past decades [2] and, currently, TGCTs are the most common tumours diagnosed in men between 20 and 35 years, representing approximately 95% of malignant testicular neoplasms [3]. The interest in TGCTs has grown significantly in recent years, mainly due to improved disease curability rates, even at advanced stages [2]. Despite high survival rates, biomarkers that might improve early disease detection and monitoring could have a significant clinical impact owing to the limitations of currently available serological markers [4]. Indeed, the specificity and sensitivity of serum α-fetoprotein (AFP), β-subunit of human chorionic gonadotropin (βHCG) and lactate dehydrogenase (LDH) are not ideal, with only 60% of patients exhibiting increased levels of these markers. For instance, in seminomas (SEs), AFP levels are within the normal range: only 25% display high βHCG and LDH is non-specific, being elevated in other conditions [5–7].
miRNAs are small RNA molecules that do not translate into peptides or proteins, but are involved in several essential biological processes, such as cell cycle, proliferation, differentiation, apoptosis and tumour development [8]. Not only is miRNA expression profile altered in many diseases, including urological cancer, but miRNAs' patterns differ between malignant and benign tissues as well as among different tumour entities, highlighting their potential as biomarkers in various malignancies [9]. Increased miR-371–373 cluster expression seems to be a consistent miRNA signature of testicular germ cell neoplasms [10,11]. It was previously shown that the miR-371–373 cluster has an oncogenic potential in TGCTs due to its ability to mask the effect of mutated p53, by inhibiting Ras-induced oncogenic senescence in TGCTs [12]. Subsequently, it was demonstrated that this cluster was significantly upregulated in TGCTs [11]. The mechanisms underlying this upregulation have been elucidated and a feedback loop between the miR-371–373 cluster and Wnt/β-catenin signalling pathway was revealed. Remarkably, stem cells showed higher miR-371–373 cluster expression than differentiated cells [13] and because Wnt signalling plays a critical role in regulating cell stemness, the miR-371–373 cluster appears to contribute to maintenance of stem-cell status [14].
Previous studies have shown that serum levels of all three miRNAs from the miR-371–373 cluster (miR-371a-3p, miR-372 and miR-373–3p) were significantly higher in patients when compared to healthy male individuals (controls). Among them, miR-371a-3p displayed higher expression in SE and non-seminomatous tumours (NST), demonstrating the largest post-operative decrease and outperforming classical TGCTs serum biomarkers [8,15]. Furthermore, significantly higher miR-371a-3p levels were found in testicular vein blood (TVB) than in the peripheral blood, indicating that the primary source for miR-371a-3p upregulation was a testicular tumour itself [16]. Finally, miR-371a-3p expression in primary tissues of TGCTs has been previously reported, as well [10,17,18]. Hence, miR-371a-3p has been recognized as a promising candidate biomarker for patients with TGCTs [6]. Nevertheless, data on differential expression of miR-371a-3p among TGCT subtypes are lacking and this may impact on its performance as biomarker. We, thus, aimed to characterize miR-371a-3p expression profile across TGCTs subtypes, comparing with normal testis, emphasizing putative differences between seminomatous and non-seminomatous TGCTs, as well as between prepubertal and postpubertal TGCTs.
2. Methods
(a). Patients and sample collection
Archival, formalin-fixed, paraffin-embedded tissue from 119 patients with TGCTs (103 germ cell neoplasia in situ (GCNIS)-related—68 pure SE and 35 NST (five pure embryonal carcinoma (EmbrCa), two pure postpubertal-type teratoma (TE) and 28 mixed germ cell tumours)—and 16 GCNIS-unrelated (four prepubertal-type yolk-sac tumour (YST) and 12 prepubertal-type TE), diagnosed and treated with orchiectomy at Portuguese Oncology Institute of Porto, Portugal, between 2005 and 2016) was selected for this study. Additionally, control samples deriving from 15 patients subjected to orchiectomy due to inflammatory or benign testicular pathology were included. All histological slides were retrieved, reviewed by an experienced uropathologist, re-classified according to 2016 World Health Organization recommendations [19] and staged according to TNM classification 7th edition [20]. Moreover, the International Germ Cell Consensus Classification (a prognosis factor-based staging system developed by the International Germ Cell Cancer Collaborative Group (IGCCCG)) was applied to all malignant TGCTs [21]. Representative areas of all tumours, including different components of mixed TGCTs, were identified and selectively collected for subsequent RNA extraction. These areas were then individually considered for the purposes of comparisons among TGCT subtypes. Thus, analysed tissue samples comprised 138 GCNIS-related TGCTs—68 pure SE and 70 NST (five pure EmbrCa and two pure postpubertal-type TE, as well as 12 SE, 17 EmbrCa, 13 postpubertal-type YST, six choriocarcinoma (CH) and 14 postpubertal-type TE as components of mixed germ cell tumours), four prepubertal-type YST and 12 prepubertal-type TE.
(b). MicroRNA expression analysis
Total RNA from formalin-fixed paraffin-embedded (FFPE) tissue samples was extracted using Norgen's FFPE RNA/DNA Purification Plus Kit (Norgen, Canada), according to manufacturer's instructions. Reverse transcription (RT) was performed to a total of 10 ng in a final volume of 15 µl, using Taqman™ MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). The reaction was carried out for miR-371a-3p using stem-loop-primers from the relevant TaqMan microRNA assays (miR-371a-3p, Assay ID 002124, Applied Biosystems). Reverse transcription was performed in a Veriti® Thermal Cycler (Applied Biosystems®). Thermal-cycling conditions consisted of: 30 min at 16°C, 30 min at 42°C and 5 min at 85°C. Samples were then stored at −20°C.
Quantitative real-time PCR (RT-qPCR) was performed in a 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA), according to the recommended protocol. RNU48 (Assay ID 001006) was used as internal control [22] and relative expression of targets tested in each sample was obtained using the formula: relative expression = target gene mean quantity/RNU48 mean quantity. The obtained ratio was then multiplied by 1000 for easier tabulation.
(c). Statistical analysis
Differences in miR-371a-3p expression levels among TGCT subtypes were assessed using non-parametric tests, i.e. the Kruskal–Wallis test, followed by Mann–Whitney U-test for pairwise comparisons, when appropriate. p-values below 0.05 were considered statistically significant and Bonferroni correction was applied to pairwise comparisons.
To assess the discriminative power of miR-371a-3p expression levels among TGCT subtypes, received operating characteristic (ROC) curves were constructed and biomarker performance parameters (sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy) were calculated. Cut-off was established based on the highest value obtained in ROC curve analysis.
Statistical analyses were performed using SPSS Statistics 24 (IBM-SPSS, IL, USA) and graphics were constructed using GraphPad 6 Prism (GraphPad Software, USA).
3. Results
(a). Clinical characterization and association between miR-371a-3p expression levels and standard clinicopathological parameters
Median ages of patients at diagnosis for GCNIS-related TGCTs, GCNIS-unrelated TGCTs and controls were 31, 16 and 49 years, respectively. Most of the patients (65%) with GCNIS-related TGCTs presented with clinical stage I (67/103), whereas only 10.7% (11/103) were at stage III. According to prognostic-based staging, 25.2% (26/103) of the cases were classified as good prognosis, 5.8% (6/103) as intermediate prognosis and 3.9% (4/103) as poor prognosis. Relevant clinical and pathological data are presented in table 1 and electronic supplementary material, table S1. Serum tumour markers' levels were available for most patients, both pre- and post-operatively (table 2).
Table 1.
Clinical and pathological data of all TGCT patients and controls used in present study. TGCT, testicular germ cell tumour; GCNIS, germ cell neoplasia in situ; IGCCCG, International Germ Cell Cancer Collaborative Group; n.a, not applicable.
| clinicopathologic features | TGCT patients |
controls | |
|---|---|---|---|
| GCNIS-related cases | GCNIS-unrelated cases | ||
| patients, n | 103 | 16 | 15 |
| age median (range) | 31 (13–52) | 16 (1–35) | 49 (16–86) |
| histological subtype | |||
| seminoma | 68 | n.a. | n.a. |
| embryonal carcinoma | 5 | n.a. | n.a. |
| postpubertal-type yolk-sac | 0 | n.a. | n.a. |
| choriocarcinoma | 0 | n.a. | n.a. |
| postpubertal-type teratoma | 2 | n.a. | n.a. |
| mixed germ cell tumours: | 28 | n.a. | n.a. |
| seminoma components | 12 | n.a. | n.a. |
| embryonal carcinoma components | 17 | n.a. | n.a. |
| yolk-sac tumour components | 13 | n.a. | n.a. |
| choriocarcinoma components | 6 | n.a. | n.a. |
| teratoma components | 14 | n.a. | n.a. |
| prepubertal-type teratoma | n.a. | 12/16 | n.a. |
| prepubertal-type yolk-sac | n.a. | 4/16 | n.a. |
| group staging | |||
| I | 67 | n.a. | n.a. |
| II | 24 | n.a. | n.a. |
| III | 11 | n.a. | n.a. |
| prognostic-based staging (according to IGCCCG classification), for metastatic diseasea | |||
| not applicable (non-metastatic) | 67 | n.a. | n.a. |
| good | 26 | n.a. | n.a. |
| intermediate | 6 | n.a. | n.a. |
| poor | 4 | n.a. | n.a. |
aInformation was not available in some TGCT cases.
Table 2.
Serum tumour marker levels of all TGCT cases used in this study. AFP, α-fetoprotein; βHCG, β-subunit of human chorionic gonadotropin; LDH, lactate dehydrogenase; GCNIS, germ cell neoplasia in situ.
| serum tumour markers | pre-surgery | post surgery |
|---|---|---|
| GCNIS-related cases | ||
| AFP elevated | 20/97 (21%) | 12/80 (15%) |
| βHCG elevated | 41/95 (43%) | 16/80 (20%) |
| LDH elevated | 32/82 (39%) | 15/67 (22%) |
| GCNIS-unrelated cases | ||
| AFP elevated | 4/5 (80%) | 3/3 (100%) |
| βHCG elevated | 2/13 (15%) | 0/3 (0%) |
| LDH elevated | 4/11 (36%) | 1/4 (25%) |
Expression levels of miR-371a-3p did not associate with serum marker levels or patients’ age (data not shown). No association was found between miR-371a-3p expression levels and TNM staging or prognostic-based staging, either.
(b). Assessment of miR-371a-3p expression levels in TGCTs and controls
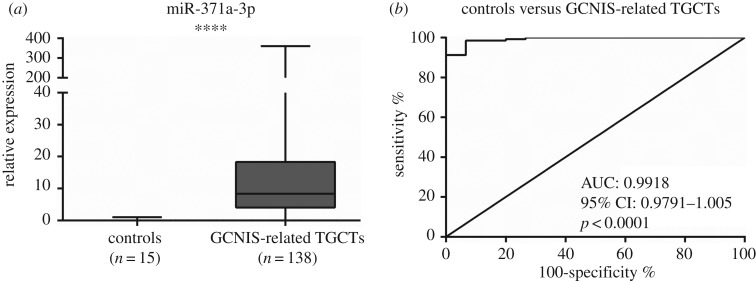
To verify whether miR-371a-3p expression was TGCT-specific, expression levels in normal and neoplastic tissue samples were evaluated (table 3) and these levels were significantly higher in TGCT tissues compared to controls (figure 1a; p < 0.0001).
Table 3.
Distribution of miR-371a-3p expression levels. SE, seminoma; NST, non-seminomatous tumours; IQR, interquartile range; EmbrCa, embryonal carcinoma; YST, postpubertal-type yolk-sac tumour; CH, choriocarcinoma; TE, postpubertal-type teratoma; mixed TGCTs, subtypes as components of mixed germ cell tumours.
| controls median (IQR) | pure SE median (IQR) | NST median (IQR) | ||
|---|---|---|---|---|
| 0.01 (0–0.03) | 11.33 (5.32–33.01) | 5.48 (2.42–11.94) | ||
| NST subtypes median (IQR) | ||||
| EmbrCa components in mixed TGCTs | YST components in mixed TGCTs | CH components in mixed TGCTs | TE components in mixed TGCTs | SE components in mixed TGCTs |
| 8.72 (4.33–69.68) | 4.16 (2.86–9.19) | 6.61 (3.25–9.27) | 0.87 (0.24–2.36) | 4.96 (3.04–7.38) |
| Prepubertal TGCTs median (IQR) | ||||
| 0.06 (0.02–3.09) | ||||
Figure 1.
Box-plots of miR-371a-3p expression levels in normal tissue testis (controls) and TGCTs (tumours) samples (a) and respective receiver operating characteristic curve (b). AUC, area under the curve.
Subsequently, ROC curve analysis was performed (figure 1b), revealing an area under the curve (AUC) of 0.99. Using the cut-off value derived from this analysis (0.0875), miR-371a-3p expression distinguished GCNIS-related TGCTs from normal testicular tissue with 92.2% sensitivity, 93.3% specificity and 92.3% accuracy (table 4).
Table 4.
Performance of miR-371a-3p expression levels as biomarker in specific settings. PPV, positive predictive value; NPV, negative predictive value.
| test | sensitivity % | specificity % | PPV % | NPV % | accuracy % |
|---|---|---|---|---|---|
| controls versus GCNIS-related TGCTs | 92.2 | 93.3 | 99.3 | 53.8 | 92.3 |
| pure SE versus SE in mixed TGCTs | 83.3 | 66.2 | 30.3 | 95.7 | 68.8 |
| controls versus postpubertal TE | 87.5 | 93.3 | 93.3 | 87.5 | 90.3 |
| controls versus prepubertal TGCTs | 68.8 | 80.0 | 78.6 | 70.6 | 74.2 |
(c). Differential expression of miR-371a-3p among TGCT subtypes and discriminative power
To ascertain miRNA-371a-3p differential expression in tissue samples, comparative analyses between TGCTs and normal testicular tissues, as well as among TGCT subtypes were carried out.
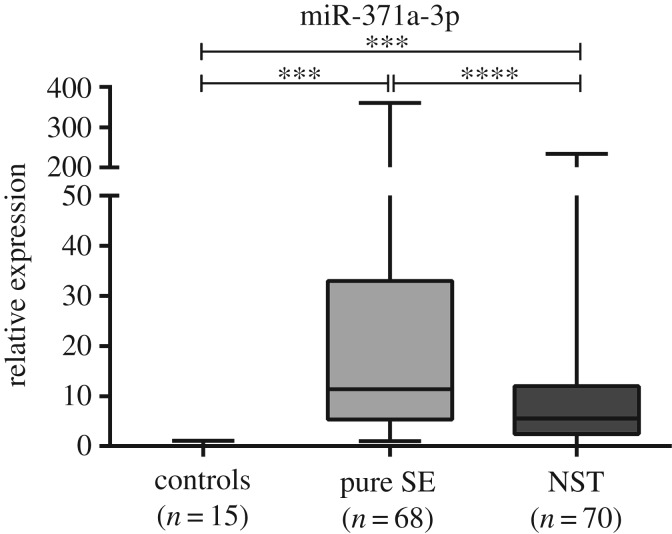
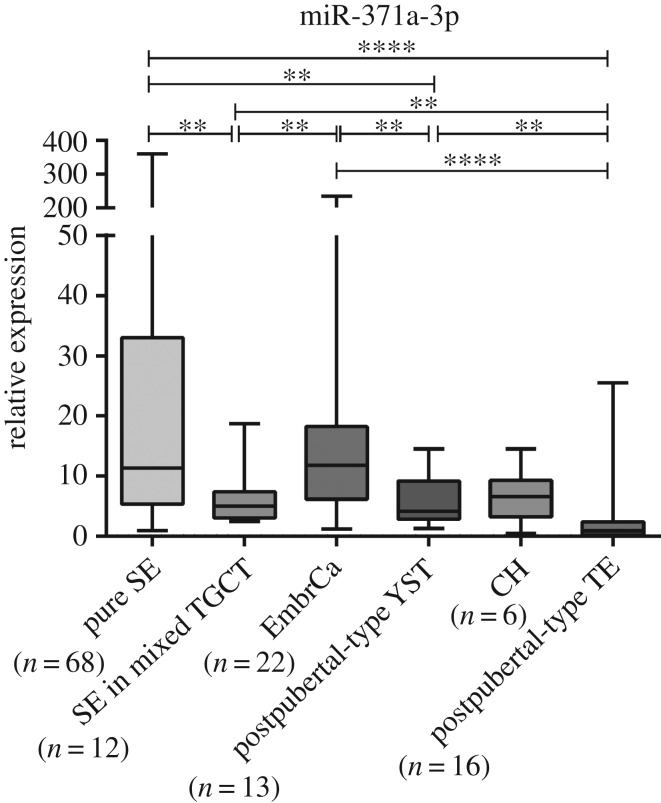
Both pure SE and NST displayed higher miR-371a-3p expression levels than controls (p < 0.001) (figure 2). Pure SE was the most common TGCT in this series (n = 68), displaying the highest miRNA-371a-3p expression levels, which were significantly higher than those of NST (p < 0.0001) (figures 2 and 3). Among NST, EmbrCa displayed the highest miR-371a-3p expression levels, whereas those of postpubertal TE were the lowest, significantly differing from the remainder NST subtypes (figure 3).
Figure 2.
Box-plots of miR-371a-3p expression levels in normal testis tissue (controls), pure seminomas (pure SE) and non-seminomatous tumours (NST) samples. ***p < 0.001; ****p < 0.0001.
Figure 3.
Box-plot of miR-371a-3p expression levels between the different subtypes of TGCTs. **p < 0.01; ****p < 0.0001.
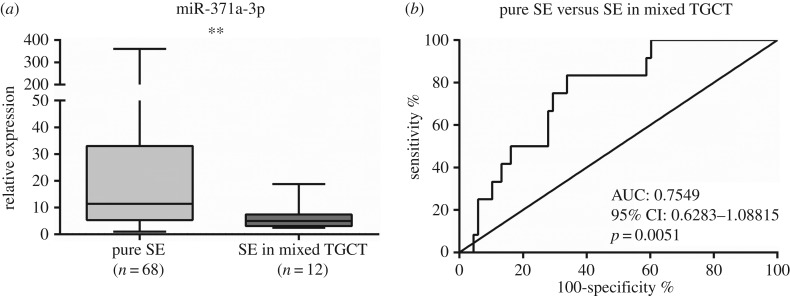
Interestingly, differences in miR-371a-3p expression levels between pure SE and SE in mixed TGCTs (p < 0.01) were depicted (figure 4a). Using the calculated cut-off value (7.869), ROC curve analysis disclosed an AUC of approximately 0.75 (figure 4b). The discriminatory performance was rather modest, with only 30% PPV (table 4).
Figure 4.
Box-plot (a) of miR-371a-3p expression levels in pure seminoma (pure SE) and SE in mixed germ cell tumours samples and respective ROC curve (b). AUC, area under the curve.
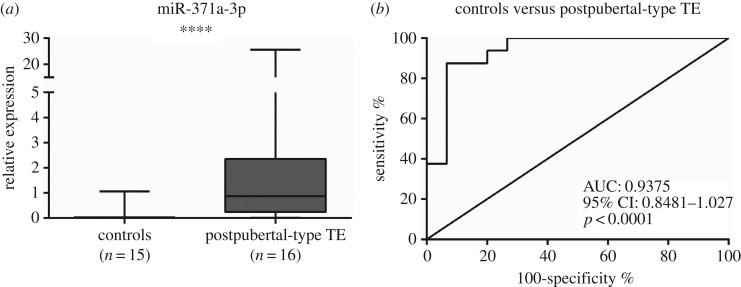
Among postpubertal TGCTs, TE displayed the lowest miR-371a-3p expression levels but these were still significantly higher than those of normal testicular tissue (figure 5a; p < 0.0001). ROC curve analysis disclosed an AUC close to 0.93 (figure 5b) and using a cut-off value of 0.0875, miR-371a-3p accurately distinguished postpubertal TE from controls (table 4).
Figure 5.
(a) Box-plot of miR-371a-3p expression levels in controls and postpubertal teratoma samples and (b) respective ROC curve. AUC, area under the curve.
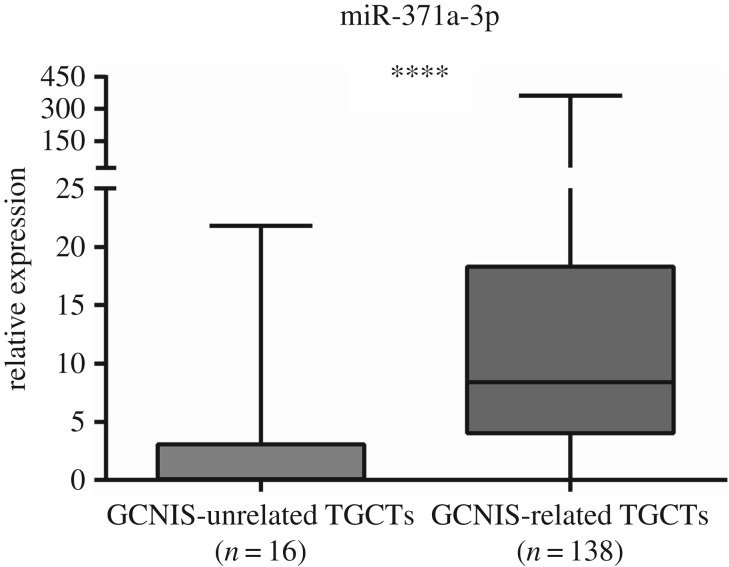
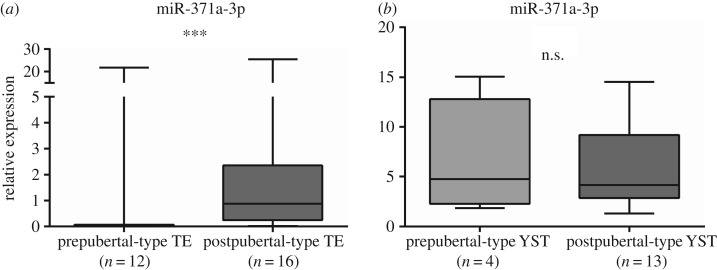
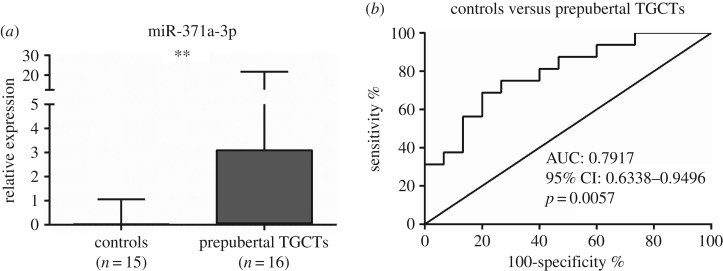
Globally, miR-371a-3p expression levels were significantly higher in GCNIS-related TGCTs compared to GCNIS-unrelated TGCTs (p=0.0066) (figure 6). Considering the specific subtypes, miR-371a-3p expression was significantly higher in postpubertal versus prepubertal TE (p = 0.0008) (figure 7a), whereas no significant differences were apparent between postpubertal and prepubertal YST (figure 7b). Furthermore, miR-371a-3p expression levels were significantly higher in prepubertal TGCTs compared to controls (p < 0.0001) (figure 8). ROC curve analysis revealed an AUC of approximately 0.80 and using the calculated cut-off of 0.0274, miR-371a-3p expression discriminated prepubertal TGCTs from normal testicular tissue with 69% sensitivity and 80% specificity (table 4).
Figure 6.
Box-plots of miR-371a-3p expression levels in GCNIS-unrelated TGCT samples and GCNIS-related TGCT samples. ****p < 0.0001.
Figure 7.
Box-plot of miR-371a-3p expression levels in prepubertal-type TE and postpubertal-type TE as component of mixed TGCTs samples (a) and box-plot of miR-371a-3p expression levels in Prepubertal-type YST and Postpubertal-type YST as component of mixed TGCTs samples (b).
Figure 8.
(a) Box-plot of miR-371a-3p expression levels in controls and prepubertal TGCTs samples and (b) ROC curve. AUC, area under the curve.
4. Discussion
TGCTs are very heterogeneous neoplasms; accurate subtype discrimination is critical for therapeutic decisions and contributes to reducing mortality and improving quality of life. These outcomes are also influenced by early diagnosis and appropriate follow-up, enabling timely therapeutic intervention. However, biomarkers fit to accomplish these goals are notably lacking in clinical practice. Indeed, classical serum markers have limited sensitivity and specificity, entailing the search for novel biomarkers. In this vein, epigenetic-based biomarkers, more specifically, serum miR-371a-3p levels, have recently been shown to outperform classical serum markers both for disease detection and monitoring [8,15]. For the successful implementation of this promising biomarker, however, it is not only necessary to confirm those preliminary findings in larger patient cohorts, but also to identify possible flaws related to differential expression of miR-371a-3p among TGCT subtypes.
In this study, we confirmed that miR-371a-3p expression levels might, indeed, be useful markers for TGCT diagnosis/detection as it accurately discriminated TGCTs from normal testicular tissue, in line with previous studies performed both in tissue and plasma samples [5,16]. Importantly, the discriminatory power remained when controls were compared to SE or NST, which parallels previous findings in serum [23]. Thus, our results confirm the superior diagnostic specificity of miR-371a-3p for TGCT detection compared to classical serum tumour markers [9], especially in patients with SE, for whom classical markers demonstrated very limited sensitivity [5]. Although we did not find significant association between miR-371a-3p expression and clinicopathological parameters such as stage and prognostic grouping, others have reported such associations, with metastasized patients displaying higher miR-371a-3p levels [5,9]. However, direct and immediate comparison with our results must be done with caution because those studies used serum samples, whereas we used FFPE tissue samples, with individual selection of each subtype of every mixed TGCT in separate, though differences could also be due to dissimilarities of patient cohorts. We hypothesize that higher miR-371a-3p serum levels might be due to increased tumour burden and a more elevated number of circulating tumour cells in metastasized patients, increasing the production and release of this microRNA.
Unlike previous studies [5], we observed significant differences in miR-371a-3p expression between SE and NST, although different sample types were assessed. Indeed, this result might be due to sample source (serum versus tissues), as Dieckmann and co-workers reported that no correlation was found between miR-371a-3p expression levels in tumour tissue and serum [15]. As we did not have access to serum samples from our cohort, it is not possible to draw a definitive conclusion. Moreover, Dieckmann and co-workers have found NST stage I to have significantly higher miR-371a-3p levels than SE in the same stage. These results contrast with ours. However, again, careful analysis is mandatory: the proportion of EmbrCa subtype among NST is different between studies, with these authors acknowledging that a high proportion of this subtype might explain their results; also, we have analysed each component (SE, EmbrCa, YST, CH and TE) as a unit, and therefore, analysis is not directly comparable to that achieved in serum studies. In fact, evaluation of differential expression of miR-371a-3p among individual TGCT subtypes disclosed other significant differences, most notably a progressive decrease from SE to postpubertal TE, with EmbrCa, YST and CH displaying intermediate levels. Although the cause for this variation remains elusive, the association with the degree of tissue differentiation is quite striking, as previously pointed out [5]. Indeed, miR-371–373 cluster expression has been reported as specific to stem cells [13]. Hence, less differentiated histological subtypes (like SE and EmbrCa, which are biologically closer to stem cells) would be expected to depict higher miR-371a-3p expression compared to postpubertal TE, the more differentiated TGCTs subtype [24]. In this sequence, it is noteworthy that pure SE displayed significantly higher miR-371a-3p expression levels than SE foci retrieved from mixed TGCTs. This is an interesting finding because no differences are depicted at morphological level, although SE foci from mixed TGCTs already demonstrate alterations in miR-371a-3p expression that bring them closer to EmbrCa and other NST subtypes. Theoretically, this might be useful to complement histopathological assessment of TGCTs, as low miR-371a-3p expression levels in an otherwise typical pure SE might indicate the possibility of mixed TGCTs, prompting more thorough tissue sampling. However, the discriminative power was quite modest, limiting its routine use.
Interestingly, previous studies using plasma samples revealed that miR-371a-3p is overexpressed in both SE and NST alike, with the exception of TE [1,5,23,25]. Our data show that within NST, miR-371a-3p expression significantly varies among the various subtypes, but this variation is small, limiting its potential as subtype discriminator. Contrary to previous reports, postpubertal TE displayed significantly lower (although still detectable) miR-371a-3p expression levels compared to the other subtypes. Nevertheless, these low levels could impair its performance as global TGCT biomarker, as miR-371a-3p expression levels in postpubertal TE approached those of normal testicular tissue. Remarkably, however, those levels were sufficient to discriminate postpubertal TE from controls, confirming its potential. This result is of clinical importance as postpubertal TE is present in approximately 50% of mixed TGCTs and it constitutes the most common subtype represented in recurrent disease [6]. Thus, the use of miR-371a-3p expression for disease monitoring is not likely to be impaired.
According to the currently accepted tumorigenesis model of TGCTs, GCNIS-related and GCNIS-unrelated TGCTs constitute different disease entities [19]. In our cohort, GCNIS-unrelated TGCTs are only represented by prepubertal YST and TE, as no spermatocytic tumour was identified in this consecutive series. Interestingly, miR-371a-3p expression levels were significantly higher in GCNIS-related TGCTs, although this global result masks some differences between specific subtypes. Indeed, prepubertal TE disclosed significantly lower miR-371a-3p expression levels than postpubertal TE (although still significantly higher than those of controls), whereas no differences were depicted for pre- and postpubertal YST. Despite morphological similarities (especially in YST), clinical behaviour is quite distinct: prepubertal-type YST shows only 6% relapses when managed on surveillance protocols and prepubertal TE usually displays a benign disease course, whereas postpubertal counterparts are distinctly malignant [19]. The association between miR-371a-3p expression levels and cell differentiation might partially explain these findings [13]. Indeed, pre- and postpubertal YST are morphologically indistinct, while prepubertal TE (which displays the lowest miR-371a-3p expression levels among all TGCTs) usually corresponds to mature-type tissues, while postpubertal TE may show a variable degree of tissue immaturity.
Overall, we found that miR-371a-3p is differentially expressed among TGCTs, with variable but significantly higher levels compared to normal testicular tissue. Pure SE displayed the highest expression levels and, globally, decreased expression seemed to parallel cell differentiation. Moreover, GCNIS-related TGCTs displayed higher miR-371a-3p expression levels compared to GCNIS-unrelated. Thus, our results confirm the specificity of this biomarker, already ascertained in serum. Indeed, it might be clinically useful both for early detection and disease monitoring, outperforming AFP and βHCG, especially in SE. Nevertheless, it is important to determine whether the biomarker performance of miR-371a-3p in plasma might be affected by TGCT subtype.
Supplementary Material
Acknowledgement
The authors are grateful to the Department of Urology of the Portuguese Oncology Institute of Porto.
Ethics
All patients enrolled signed informed consent. This study was approved by institutional review board (CES 12R/2017).
Data accessibility
This article has no additional data.
Authors' contributions
Conceived and designed the experiments: R.H. and C.J. Performed the experiments: B.V.-S., D.B.-S., J.L., A.L.C., R.G., M.C. Analysed the data: B.V.-S., D.B.-S., J.L., R.H., C.J. Contributed reagents/material/analysis: I.B., J.O., R.H., C.J.
Competing interests
None of the authors has any conflict of interest to declare.
Funding
This work was supported by a Grant from Research Center of Portuguese Oncology Institute of Porto (FBGEBC-CI-IPOP-27-2016).
References
- 1.Gillis AJ, et al. 2013. Targeted serum miRNA (TSmiR) test for diagnosis and follow-up of (testicular) germ cell cancer patients: a proof of principle. Mol. Oncol. 7, 1083–1092. ( 10.1016/j.molonc.2013.08.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. 2015. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386. ( 10.1002/ijc.29210) [DOI] [PubMed] [Google Scholar]
- 3.Feldman DR. 2015. Update in germ cell tumours. Curr. Opin Oncol. 27, 177–184. [DOI] [PubMed] [Google Scholar]
- 4.Costa AL, Lobo J, Jeronimo C, Henrique R. 2017. The epigenetics of testicular germ cell tumors: looking for novel disease biomarkers. Epigenomics 9, 155–169. ( 10.2217/epi-2016-0081) [DOI] [PubMed] [Google Scholar]
- 5.Dieckmann KP, et al. 2017. Serum levels of MicroRNA miR-371a-3p: a sensitive and specific new biomarker for germ cell tumours. Eur. Urol. 71, 213–220. ( 10.1016/j.eururo.2016.07.029) [DOI] [PubMed] [Google Scholar]
- 6.Henrique R, Jerónimo C. 2017. Testicular germ cell tumors go epigenetics: will miR-371a-3p replace classical serum biomarkers? Eur. Urol. 71, 221–222. ( 10.1016/j.eururo.2016.08.013) [DOI] [PubMed] [Google Scholar]
- 7.Gilligan TD, et al. 2010. American society of clinical oncology clinical practice guideline on uses of serum tumor markers in adult males with germ cell tumors. J. Clin. Oncol. 28, 3388–3404. ( 10.1200/JCO.2009.26.4481) [DOI] [PubMed] [Google Scholar]
- 8.Belge G, Dieckmann KP, Spiekermann M, Balks T, Bullerdiek J. 2012. Serum levels of microRNAs miR-371-3: a novel class of serum biomarkers for testicular germ cell tumors? Eur. Urol. 61, 1068–1069. ( 10.1016/j.eururo.2012.02.037) [DOI] [PubMed] [Google Scholar]
- 9.Syring I, Bartels J, Holdenrieder S, Kristiansen G, Müller SC, Ellinger J. 2015. Circulating serum miRNA (miR-367-3p, miR-371a-3p, miR-372-3p and miR-373-3p) as biomarkers in patients with testicular germ cell cancer. J. Urol. 193, 331–337. ( 10.1016/j.juro.2014.07.010) [DOI] [PubMed] [Google Scholar]
- 10.Palmer RD, et al. 2010. Malignant germ cell tumors display common microRNA profiles resulting in global changes in expression of messenger RNA targets. Cancer Res. 70, 2911–2923. ( 10.1158/0008-5472.CAN-09-3301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillis A, et al. 2007. High-throughput microRNAome analysis in human germ cell tumours. J. Pathol. 213, 319–328. ( 10.1002/path.2230) [DOI] [PubMed] [Google Scholar]
- 12.Voorhoeve PM, et al. 2006. A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Cell 124, 1169–1181. ( 10.1016/j.cell.2006.02.037) [DOI] [PubMed] [Google Scholar]
- 13.Stadler B, et al. 2010. Characterization of microRNAs involved in embryonic stem cell states. Stem Cells Dev. 19, 935–950. ( 10.1089/scd.2009.0426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou A, Diao L, Xu H, Xiao Z, Li J, Zhou H, Qu L. 2012. β-Catenin/LEF1 transactivates the microRNA-371-373 cluster that modulates the Wnt/β-catenin-signaling pathway. Oncogene 31, 2968–2978. ( 10.1038/onc.2011.461) [DOI] [PubMed] [Google Scholar]
- 15.Dieckmann K, Spiekermann M, Balks T, Flor I, Löning T, Bullerdiek J, Belge G. 2012. MicroRNAs miR-371-3 in serum as diagnostic tools in the management of testicular germ cell tumours. Br. J. Cancer 107, 1754–1760. ( 10.1038/bjc.2012.469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spiekermann M, Belge G, Winter N, Ikogho R, Balks T, Bullerdiek J, Dieckmann KP. 2015. MicroRNA miR-371a-3p in serum of patients with germ cell tumours: evaluations for establishing a serum biomarker. Andrology 3, 78–84. ( 10.1111/j.2047-2927.2014.00269.x) [DOI] [PubMed] [Google Scholar]
- 17.Bing Z, Master S, Tobias J, Baldwin D, Xu X, Tomaszewski J. 2012. MicroRNA expression profiles of seminoma from paraffin-embedded formalin-fixed tissue. Virchows Arch. 461, 663–668. ( 10.1007/s00428-012-1325-9) [DOI] [PubMed] [Google Scholar]
- 18.Rijlaarsdam M, Agthoven T, Gillis A, Patel S, Hayashibara K, Lee K, Looijenga L. 2015. Identification of known and novel germ cell cancer-specific (embryonic) miRs in serum by high-throughput profiling. Andrology 3, 85–91. ( 10.1111/andr.298) [DOI] [PubMed] [Google Scholar]
- 19.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. 2016. The 2016 WHO classification of tumours of the urinary system and male genital organs–part a: renal, penile, and testicular tumours. Eur. Urol. 70, 93–105. ( 10.1016/j.eururo.2016.02.029) [DOI] [PubMed] [Google Scholar]
- 20.Edge SB, Compton CC. 2010. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 17, 1471–1474. ( 10.1245/s10434-010-0985-4) [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson PM, Read G. 1997. International germ cell consensus classification: a prognostic factor-based staging system for metastatic germ cell cancers. International germ cell cancer collaborative group. J. Clin. Oncol. 9, 207–209. ( 10.1016/S0936-6555(97)80001-5) [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Shi H, Li X, Chen G, Larsson C, Lui W.-O. 2017. miR-223-3p regulates cell growth and apoptosis via FBXW7 suggesting an oncogenic role in human testicular germ cell tumors. Int. J. Oncol. 50, 356–364. ( 10.3892/ijo.2016.3807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Agthoven T, Looijenga LH. 2017. Accurate primary germ cell cancer diagnosis using serum based microRNA detection (ampTSmiR test). Oncotarget 8, 58037 ( 10.18632/oncotarget.10867) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Looijenga LH, Stoop H, Biermann K. 2014. Testicular cancer: biology and biomarkers. Virchows Arch. 464, 301–313. ( 10.1007/s00428-013-1522-1) [DOI] [PubMed] [Google Scholar]
- 25.Murray MJ, et al. 2016. A pipeline to quantify serum and cerebrospinal fluid microRNAs for diagnosis and detection of relapse in paediatric malignant germ-cell tumours. Br. J. Cancer. 114, 151–162. ( 10.1038/bjc.2015.429) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.