Abstract
A series of hydroxamic acids linked by different lengths to a chiral imidazo-ketopiperazine scaffold were synthesized. The compounds with linker lengths of 6 and 7 carbon atoms were the most potent in histone deacetylase (HDAC) inhibition, and were specific submicromolar inhibitors of the HDAC1, HDAC6 and HDAC8 isoforms. A docking model for the binding mode predicts binding of the hydroxamic acid to the active site zinc cation and additional interactions between the imidazo-ketopiperazine and the enzyme rim. The compounds were micromolar inhibitors of the MV4-11, THP-1 and U937 cancer cell lines. Increased levels of histone H3 and tubulin acetylation support a cellular mechanism of action through HDAC inhibition.
This article is part of a discussion meeting issue ‘Frontiers in epigenetic chemical biology’.
Keywords: zinc metalloenzymes, epigenetics, histone deacetylases, enzyme inhibitors, peptidomimetics
1. Introduction
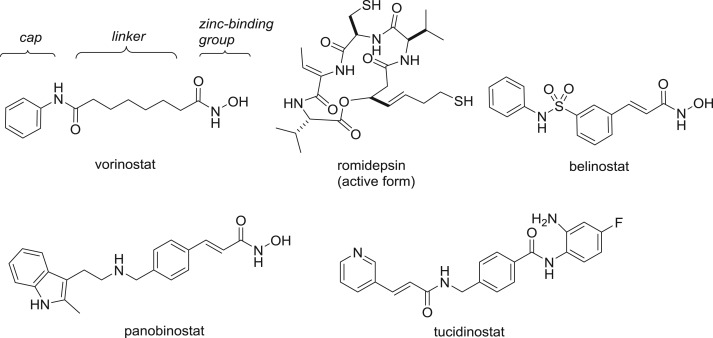
The zinc-dependent histone deacetylases (HDACs) are a major target for epigenetic drug discovery, with five HDAC inhibitors approved for the treatment of haematological cancers [1,2]. These drugs (figure 1), as well as others in clinical development, share a common pharmacophore composed of a zinc-binding group and a cap connected by a linker [3]. The first approved HDAC inhibitor vorinostat features a hydroxamic acid, and indeed this bidentate chelating functionality remains the most popular choice for the zinc-binding group [4]. Although alternatives such as sulfonamides and carboxylic acids are successful zinc-binding groups against other enzyme targets, they have seldom yielded HDAC inhibitors with submicromolar levels of activity [5]. A rare exception is the marine natural product azumamide E [6,7]. Azumamide E, like the clinically approved drug romidepsin and other macrocyclic peptide or depsipeptide natural product HDAC inhibitors, contain relatively weak monodentate zinc-binding groups such as carboxylic acids, thiols and ketones. Nevertheless, these compounds often bind to the HDAC enzymes with higher affinity than synthetic hydroxamic acids due to the additional binding interactions between the macrocyclic cap and the enzyme rim [8–10]. Furthermore, the size of the macrocycle facilitates discrimination between the 11 human HDAC isoforms. While vorinostat is a pan-HDAC inhibitor, the larger peptides exhibit varying degrees of isoform selectivity that may be important for the avoidance of side effects in therapeutic applications.
Figure 1.
Approved HDAC inhibitors, with the common pharmacophore indicated for vorinostat.
Our previous studies on the total synthesis of the romidepsin family of HDAC inhibitors and their structure–activity (SAR) relationships demonstrate that considerable structural variation of the scaffold is tolerated without compromising biological activity [11–17]. In this work, we ask the question whether high HDAC affinity and selectivity can be achieved within a smaller non-macrocyclic framework. We had several basic requirements in our search for a new scaffold: (i) reduction of molecular weight and H-bond donors and acceptors compared to the macrocycles to enhance cell permeability; (ii) retention of chirality to ensure a non-flat topology that can form isoform-selective interactions with the chiral enzyme rim; (iii) accessibility from commercial building blocks available with high diversity; and (iv) ability to introduce a hydroxamic acid to favour active site interactions with the zinc cation.
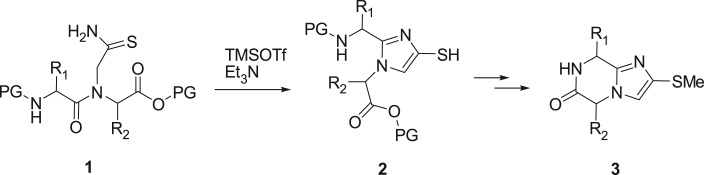
The above factors led us to consider an imidazo-ketopiperazine scaffold as a potential solution. This compact bicyclic heterocycle complies with guidelines for oral bioavailability while incorporating two chiral centres that originate from readily available amino acid starting materials. A further attractive feature was the resemblance to diketopiperazines, a privileged scaffold around which we have constructed compound libraries by solution and solid-phase synthesis [18–22]. The imidazo-ketopiperazine was first reported by Bischoff and coworkers, as part of their study on an imidazole cis-amide bond peptidomimetic [23]. The dipeptides 1 (figure 2) containing N-glycinyl thioamide substituents underwent dehydrative cyclization to the imidazole 2 under conditions similar to those developed by Hopkins [24]. In one example with l-Ala-l-Ala (R1 = R2 = Me), 2 was further cyclized to the imidazo-ketopiperazine 3. We envisioned that an adaptation of this route by elongation of the thioalkyl group to incorporate a zinc-binding group would provide a template for HDAC inhibition with the imidazo-ketopiperazine core serving as the cap.
Figure 2.
The Bischoff synthesis of an imidazo-ketopiperazine scaffold from a dipeptidyl thioamide.
2. Methods
Detailed experimental procedures for compound synthesis and characterization data, protocols for biochemical HDAC enzyme assays and cell-based assays, and molecular docking are provided in the electronic supplementary material.
3. Results and discussion
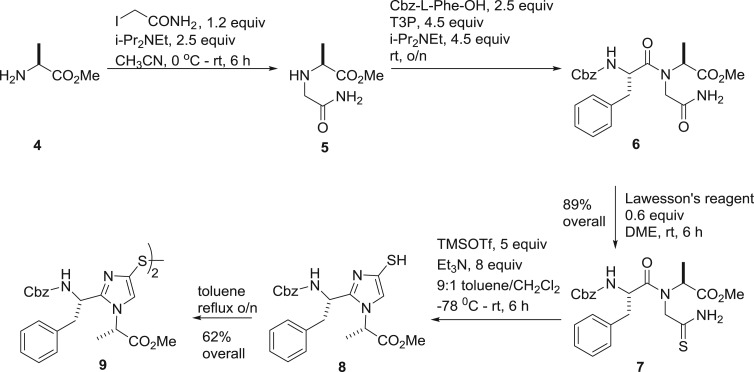
Our first compounds featured the l-Phe-l-Ala version of 2. In a telescoped one-pot sequence of three reactions (scheme 1), l-Ala methyl ester 4 was alkylated to give glycinyl amide 5 which was condensed with Cbz-l-Phe using propanephosphonic acid anhydride (T3P) as coupling agent to provide dipeptide 6. In the same pot, the solvent was evaporated off and the residue redissolved in 1,2-dimethoxyethane followed by treatment with Lawesson's reagent to effect selective thionation. The product thioamide 7 was isolated in 89% overall yield over the three operations. The dehydrative cyclization of thioamide 7 with trimethylsilyl triflate afforded thiol 8, which was conveniently stored as the disulfide 9 obtained by refluxing in toluene.
Scheme 1.
Synthesis of the imidazolyl thiol 8 and the corresponding disulfide 9.
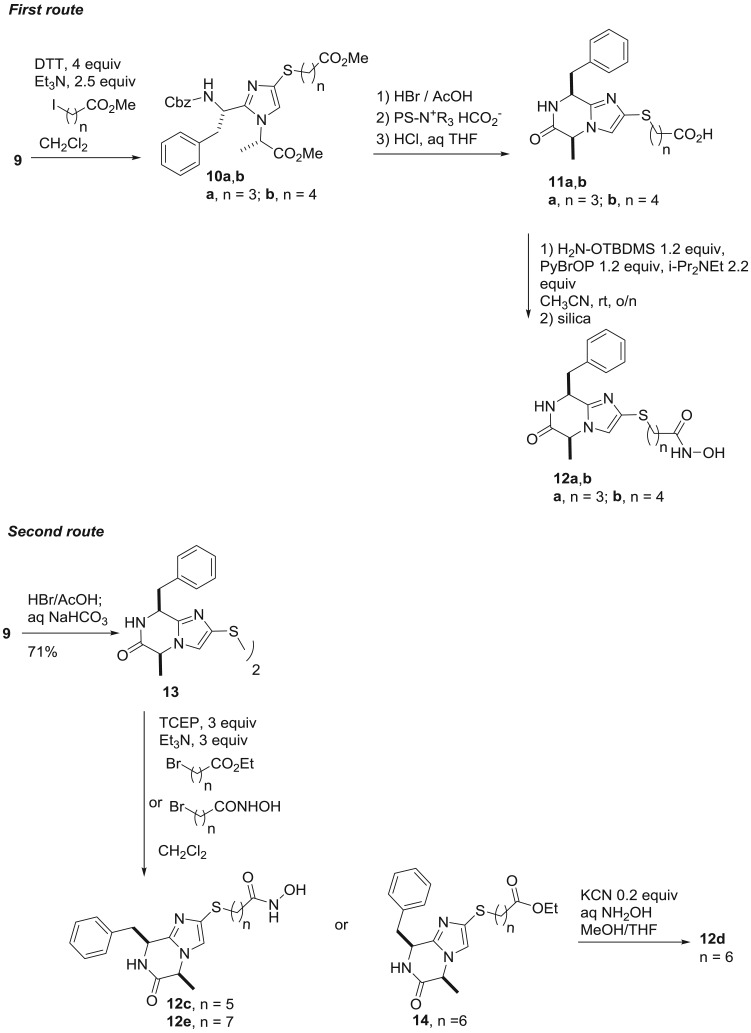
When desired, the thiol was regenerated from disulfide 9 by reduction and we then elaborated the compound to the imidazo-ketopiperazine by two sequences that differ in the order of the steps (scheme 2). In the first route, thiol 9 was alkylated with two ω-halo-esters, leading to the thioethers 10a and 10b, varying in the length of the linker. Removal of the N-terminal Cbz protecting group gave the protonated amines, which were liberated to the free base by neutralization with a polystyrene-supported formate ion exchange resin. The free amine spontaneously cyclized to the imidazo-ketopiperazine and the sidechain ester was then hydrolyzed to afford the carboxylic acids 11a and 11b. A number of methods were investigated for the conversion of the carboxylic acid to the corresponding hydroxamic acid. The most reliable proved to be coupling with the protected tert-butyldimethylsilyl ether of hydroxylamine. Afterwards, the silyl ether was conveniently removed by stirring the crude reaction mixture with silica, thus avoiding the need for a separate deprotection step. By this method, we obtained hydroxamic acids 12a and 12b with linker lengths n = 3 and n = 4.
Scheme 2.
Synthesis of hydroxamic acids 12a–e from disulfide 9.
While the above route was also applicable to compounds with longer linker lengths, an alternative provided higher overall yields. In this sequence, the disulfide 9 was first cyclized to the imidazo-ketopiperazine 13. The disulfide was reduced and directly alkylated with ω-halo-hydroxamic acids to give 12c and 12e with linker lenghts n = 5 and n = 7. For the hydroxamic acid 12d with a linker length n = 6, alkylation with an ester afforded the intermediate 14, which was converted to the hydroxamic acid by cyanide catalyzed nucleophilic displacement with hydroxylamine.
With the hydroxamic acids 12a–e in hand, we were ready to evaluate whether the imidazo-ketopiperazine cap was compatible with HDAC inhibition. The initial profiling involved biochemical assays against two HDAC isoforms, the class I nuclear isoform HDAC1 and the class II cytoplasmic isoform HDAC6. We were pleased to find that all five compounds have micromolar or submicromolar IC50 values against these two isoforms (table 1). As expected from the SAR of other HDAC inhibitors, the activity is profoundly influenced by the linker and the optimum was reached with the longer six and seven carbon linkers present in 12d and 12e. These were additionally tested, together with 12a, against HDAC8 and 12d in particular exhibited submicromolar activity. Gratifyingly, the preliminary data suggested that selective inhibition of HDAC isoforms can be achieved with our chiral imidazo-ketopiperazine heterocyclic cap.
Table 1.
Influence of linker length on inhibition of selected HDAC isoforms, data obtained from n = 1 experiments.
| compound, linker length n | HDAC1 IC50 (µM) | HDAC6 IC50 (µM) | HDAC8 IC50 (µM) |
|---|---|---|---|
| 12a, n = 3 | 24 | 4.6 | 3.4 |
| 12b, n = 4 | 3.6 | 0.9 | |
| 12c, n = 5 | 4.5 | 2.0 | |
| 12d, n = 6 | 0.9 | 0.1 | 0.1 |
| 12e, n = 7 | 0.8 | 0.3 | 2.8 |
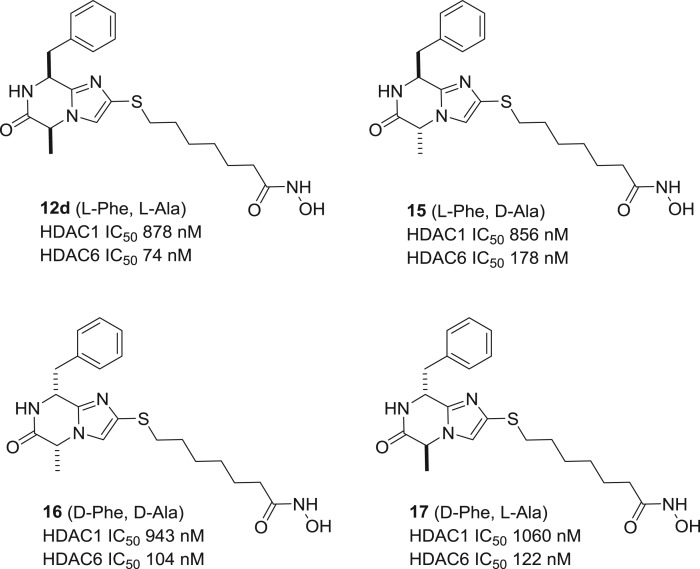
Since the imidazo-ketopiperazine scaffold contains two chiral centres, we were interested in the influence of stereochemistry on target affinity. Through a reaction sequence analogous to scheme 2, we carried out a stereochemical scan and prepared the three diastereomers 15–17 of hydroxamic acid 12d. While all four compounds show similar levels of activity and isoform selectivity between HDAC1 and HDAC6 (figure 3), it is possible that replacement of the Phe and Ala sidechains by other residues may result in significant differences in bioactivity between diastereomers.
Figure 3.
HDAC1 and HDAC6 inhibitory profile for the four diastereomers 12d, 15, 16 and 17.
In order to have a more detailed picture of the isoform selectivity, we submitted hydroxamic acid 12d for testing against all 11 human HDACs by the French CRO Cerep. At a test concentration of 10 µM, 12d had a remarkable degree of isoform selectivity and significantly inhibited only three isoforms, viz. HDAC1, HDAC6 and HDAC8 (table 2). Between these three isoforms, the determination of IC50 values revealed 12d to be most potent at HDAC6 inhibition, with a 13-fold degree of selectivity compared to HDAC8, the next highest inhibited isoform.
Table 2.
Percentage inhibition of individual HDAC isoforms by 12d, values are the mean of two measurements. IC50 values were determined for HDAC1, HDAC6 and HDAC8. Owing to differences in assay conditions, absolute values are not directly comparable with table 1.
| class I isoforms |
class IIa isoforms |
class IIb and IV isoforms |
|||
|---|---|---|---|---|---|
| HDAC1 | 57%, IC50 7.3 µM | HDAC4 | 4% | HDAC6 | 99%, IC50 160 nM |
| HDAC2 | 32% | HDAC5 | 23% | HDAC10 | 44% |
| HDAC3 | 38% | HDAC7 | 9% | HDAC11 | 10% |
| HDAC8 | 90%, IC50 2.1 µM | HDAC9 | 1% | ||
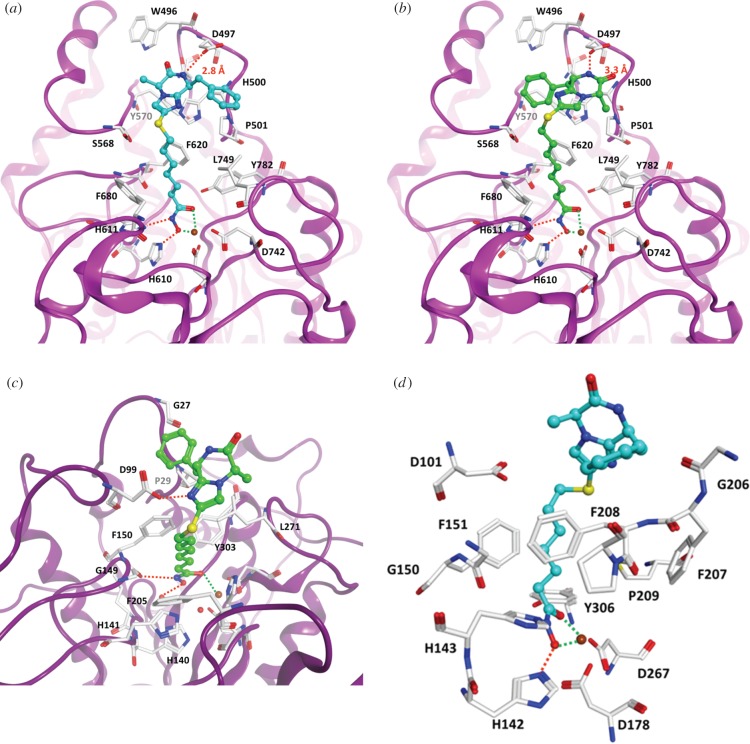
The observed selectivity for the three HDAC isoforms was modelled by docking the inhibitors into the enzyme active site. Bidentate chelation of the hydroxamic acid was observed for HDAC6 as well as HDAC8, as illustrated for compounds 12d and 12e (figure 4a,b). In addition, a hydrogen bond is predicted between the imidazo-ketopiperazine and the acidic D497 residue for the compounds with linker lengths of n = 6 and n = 7. Interestingly, the orientation of binding is ‘flipped’ between 12d and 12e with respect to the positioning of the phenyl and methyl groups. The availability of two binding modes may explain the relatively low differences in activity between the four diastereomers (figure 3). In the case of 12d, the terminal benzyl group attached to the imidazo-ketopiperazine is accommodated in the hydrophobic pocket formed between P501 and L749 (figure 4a). On the other hand, a weaker binding was predicted for HDAC1, with monodentate coordination of the hydroxamic acid (figure 4c), and is consistent with the experimentally observed reduced activity against this isoform. In the case of HDAC8, 12d is more solvent exposed and sticking out of the enzyme pocket, with the benzyl side-chain of the scaffold in interaction with F208 (figure 4d).
Figure 4.
Molecular docking of compounds (a) 12d and (b) 12e in the HDAC6 active site (PDB ID 5EDU). For comparison, the docking (c) of 12e in the HDAC1 active site (PDB 5ICN) and (d) of 12d in the HDAC8 active site (PDB ID 2V5X) is shown. Hydrogen bonds are shown as orange coloured dashed lines, coordination between the hydroxamic acid and the zinc ion (coloured brown) is shown as green coloured dashed lines. Conserved water molecules in the active site are shown as red spheres.
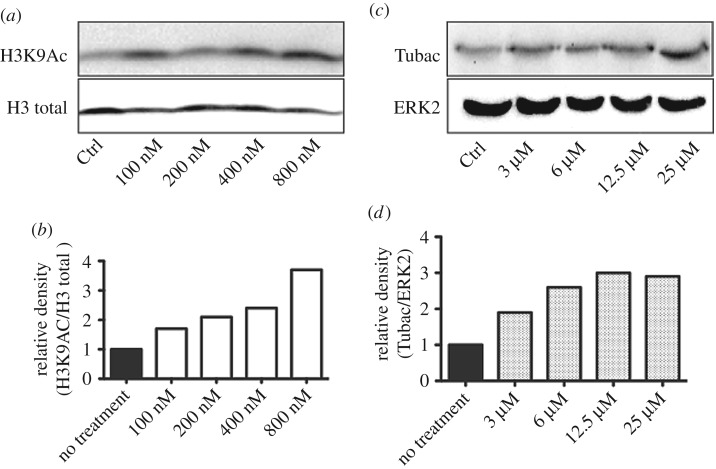
We profiled compounds 12c–e with the longer linkers in cellular assays for the growth inhibition of cancer cell lines. While compound 12c with the linker length n = 5 was relatively inactive (table 3), both 12d and 12e with linker lengths of n = 6 and n = 7 were micromolar inhibitors and the U937 lymphoma cell line was particularly sensitive to these compounds. Compound 12e was more active than 12d, and we believe this might be due to an increased lipophilicity affecting cellular uptake and efflux rather than intrinsic target affinity. Western blotting of U937 cell extracts treated with 12e demonstrated a dose-dependent increase in histone H3 and tubulin acetylation levels (figure 5), suggesting target engagement with both class I and class II HDAC isoforms. Given the activity profile (table 1), we believe the cellular effects are primarily due to the inhibition of the nuclear HDAC1 and HDAC8 as well as the cytoplasmic HDAC6.
Table 3.
Cell growth inhibition by compounds 12c–e in MV4-11, THP-1 and U937 cell lines.
| compound | cell line | IC50 (µM, n = 3), 72 h | IC50 (µM, n = 3) 96 h |
|---|---|---|---|
| 12c | MV4-11 | >25 | >25 |
| THP-1 | >25 | >25 | |
| U937 | >25 | >25 | |
| 12d | MV4-11 | 4.5 ± 0.3 | 6.8 ± 0.4 |
| THP-1 | 10.4 ± 0.3 | 9.0 ± 0.3 | |
| U937 | 0.9 ± 0.2 | 0.5 ± 0.7 | |
| 12e | MV4-11 | 1.7 ± 0.2 | 2.6 ± 0.7 |
| THP-1 | 1.7 ± 0.2 | 1.7 ± 0.6 | |
| U937 | 0.1 ± 0.02 | 0.3 ± 0.2 |
Figure 5.
Western blot analysis of (a) acetylated histone H3K9 and (b) acetylated tubulin and their relative loading controls (H3 total and ERK2), after treatment of U937 cells with HDAC inhibitor 12e. Western blot signals were quantified by densitometry using Image Lab 6.0 (BIORAD) and signal intensities plotted against the loading controls (b,d).
4. Conclusion
We report the imidazo-ketopiperazine scaffold as a new ‘cap’ for the assembly of potent and isoform-selective HDAC inhibitors. The scaffold contains two chiral centres and is readily accessible from amino acid precursors. Evaluation of the compounds revealed 12d and 12e to be submicromolar inhibitors of HDAC6, and a docking model is proposed for the binding interactions between these compounds and HDAC6, HDAC8 and HDAC1. In the case of 12d, screening against the full panel of HDACs revealed HDAC6 selectivity of at least 10-fold over all other isoforms. In cell-based assays, the compounds were micromolar inhibitors of cancer cell lines and 12e displayed a dose-dependent increase in histone and tubulin acetylation levels. Further analogues in this series are currently under investigation.
Supplementary Material
Data accessibility
Details for all experimental procedures have been uploaded as part of the electronic supplementary material.
Authors' contributions
B.L. and R.N. carried out the compound synthesis, under the supervision of L.B. and A.G., respectively. J.S. and A.C. assayed compounds against HDAC1, HDAC6 and HDAC8 under the supervision of M.J., with HDAC8 provided by M.M. and C.R. J.M. conducted the molecular docking under the supervision of W.S. Cellular assays were performed by M.T.B. The project was conceived and designed by L.B. and A.G. All authors contributed to the analysis and interpretation of data, drafting the article, revision and final approval of the version to be published.
Competing interests
We declare we have no competing interests.
Funding
We gratefully acknowledge funding from the following sources: B.L. and L.B. (Rouen) were funded by the INTERREG ISCE:Chem (Ref: 1917/4061) selected under the European Cross-border Cooperation Programme INTERREG IV A France (Channel)—England, co-funded by the ERDF and the Centre Universitaire Normand de Chimie Organique ‘Crunch’ network. R.N. and A.G. (Norwich) were funded by the European Union's Seventh Framework Programme for Research, Technological Development and Demonstration under Grant Agreement 602080 (A-ParaDDisE) and the University of East Anglia. M.T.B. (Marseille) was funded by La Ligue Contre le Cancer and the Institut National de la Santé et de la Recherche Médicale (INSERM). J.S., A.C. and M.J. (Freiburg) were funded by the European Union's Seventh Framework Programme for Research, Technological Development and Demonstration under Grant Agreement 241865 (SEtTReND) and 602080 (A-ParaDDisE), and the Deutsche Forschungsgemeinschaft (DFG, Ju295/13-1). M.M. and C.R. (Strasbourg) were funded by the European Union's Seventh Framework Programme for Research, Technological Development and Demonstration under Grant Agreement 241865 (SEtTReND) and 602080 (A-ParaDDisE), and the Centre National de la Recherche Scientifique (CNRS), the Institut National de la Santé et de la Recherche Médicale (INSERM) and the Université de Strasbourg. J.M. and W.S. (Halle) were funded by the European Union's Seventh Framework Programme for Research, Technological Development and Demonstration under Grant Agreement 241865 (SEtTReND) and 602080 (A-ParaDDisE), and the Deutsche Forschungsgemeinschaft (DFG, Si868/13-1). A.G., M.J., W.S and M.T.B. are members of the COST Action CM1406 ‘Epigenetic Chemical Biology’ and acknowledge financial support from the network.
References
- 1.Zwergel C, Stazi G, Valente S, Mai A. 2016. Histone deacetylase inhibitors: updated studies in various epigenetic-related diseases . J. Clin. Epigenet. 2, 1–7. [Google Scholar]
- 2.Manal M, Chandrasekar MJN, Priya JG, Nanjan MJ. 2016. Inhibitors of histone deacetylase as antitumor agents: a critical review. Bioorg. Chem. 67, 18–42. ( 10.1016/j.bioorg.2016.05.005) [DOI] [PubMed] [Google Scholar]
- 3.Roche J, Bertrand P. 2016. Inside HDACs with more selective HDAC inhibitors. Eur. J. Med. Chem. 121, 451–483. ( 10.1016/j.ejmech.2016.05.047) [DOI] [PubMed] [Google Scholar]
- 4.Shen S, Kozikowski AP. 2016. Why hydroxamates may not be the best histone deacetylase inhibitors—what some may have forgotten or would rather forget? ChemMedChem 11, 15–21. ( 10.1002/cmdc.201500486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawai K, Nagata N. 2012. Metal–ligand interactions: an analysis of zinc binding groups using the Protein Data Bank. Eur. J. Med. Chem. 51, 271–276. ( 10.1016/j.ejmech.2012.02.028) [DOI] [PubMed] [Google Scholar]
- 6.Nakao Y, et al. 2006. Azumamides A-E: histone deacetylase inhibitory cyclic tetrapeptides from the marine sponge Mycale izuensis. Angew. Chem. Int. Ed. Engl. 45, 7553–7557. ( 10.1002/anie.200602047) [DOI] [PubMed] [Google Scholar]
- 7.Wen S, Carey KL, Nakao Y, Fusetani N, Packham G, Ganesan A. 2007. Total synthesis of azumamide A and azumamide E, evaluation as histone deacetylase inhibitors, and design of a more potent analogue. Org. Lett. 9, 1105–1108. ( 10.1021/ol070046y) [DOI] [PubMed] [Google Scholar]
- 8.Maolanon AR, Kristensen HM, Leman LJ, Ghadiri MR, Olsen CA. 2017. Natural and synthetic macrocyclic inhibitors of the histone deacetylase enzymes. Chembiochem 18, 5–49. ( 10.1002/cbic.201600519) [DOI] [PubMed] [Google Scholar]
- 9.Ganesan A. 2016. Romidepsin and the zinc-binding thiol family of natural product HDAC inhibitors. In Successful drug discovery, vol. 2 (eds J Fischer WE, Childers), pp. 13–30. Weinheim, Germany: Wiley. [Google Scholar]
- 10.Ganesan A. 2015. Macrocyclic inhibitors of zinc-dependent histone deacetylases (HDACs). In Macrocycles in drug discovery (ed. Levin J.), pp. 109–140. Cambridge, UK: RSC. [Google Scholar]
- 11.Yurek-George A, Habens F, Brimmell M, Packham G, Ganesan A. 2004. Total synthesis of spiruchostatin A, a potent histone deacetylase inhibitor. J. Am. Chem. Soc. 126, 1030–1031. ( 10.1021/ja039258q) [DOI] [PubMed] [Google Scholar]
- 12.Doi T, Iijima Y, Shin-ya K, Ganesan A, Takahashi T. 2006. A total synthesis of spiruchostatin A. Tetrahedron Lett. 47, 1177–1180. ( 10.1016/j.tetlet.2005.12.031) [DOI] [Google Scholar]
- 13.Yurek-George A, et al. 2007. The first biologically active synthetic analogues of FK228, the depsipeptide histone deacetylase inhibitor. J. Med. Chem. 50, 5720–5726. ( 10.1021/jm0703800) [DOI] [PubMed] [Google Scholar]
- 14.Wen S, Packham G, Ganesan A. 2008. Macrolactamization versus macrolactonization: a practical total synthesis of FK228, the depsipeptide histone deacetylase inhibitor. J. Org. Chem. 73, 9353–9361. ( 10.1021/jo801866z) [DOI] [PubMed] [Google Scholar]
- 15.Iijima Y, Munakata A, Shin-ya K, Ganesan A, Doi T, Takahashi T. 2009. A solid-phase total synthesis of the cyclic depsipeptide HDAC inhibitor spiruchostatin A. Tetrahedron Lett. 50, 2970–2972. ( 10.1016/j.tetlet.2009.04.005) [DOI] [Google Scholar]
- 16.Benelkebir H, Marie S, Hayden A, Lyle J, Loadman P, Crabb SJ, Packham G, Ganesan A. 2011. Total synthesis of largazole and analogues: HDAC inhibition, antiproliferative activity and metabolic stability. Bioorg. Med. Chem. 19, 3650–3658. ( 10.1016/j.bmc.2011.02.024) [DOI] [PubMed] [Google Scholar]
- 17.Benelkebir H, Donlevy AM, Packham G, Ganesan A. 2011. Total synthesis and stereochemical assignment of burkholdac B, a depsipeptide HDAC inhibitor. Org. Lett. 13, 6334–6337. ( 10.1021/ol202197q) [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Usui T, Osada H, Ganesan A. 1999. Synthesis and evaluation of tryprostatin B and demethoxyfumitremorgin C analogues. J. Med. Chem. 43, 1577–1585. ( 10.1021/jm9905662) [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Ganesan A. 2000. The N-acyliminium Pictet-Spengler condensation as a multicomponent combinatorial reaction on solid phase and its application to the synthesis of demethoxyfumitremorgin C analogues. Org. Lett. 1, 1647–1649. ( 10.1021/ol991030d) [DOI] [Google Scholar]
- 20.Bonnet D, Ganesan A. 2002. Solid-phase synthesis of tetrahydro-β-carboline-hydantoins via the N-acyliminium Pictet-Spengler reaction and cyclative cleavage. J. Comb. Chem. 4, 546–548. ( 10.1021/cc020026h) [DOI] [PubMed] [Google Scholar]
- 21.Bonnet D, Margathe JF, Radford S, Pflimlin E, Riche S, Doman P, Hibert M, Ganesan A. 2012. Combinatorial aid for underprivileged scaffolds: solution and solid-phase strategies for a rapid and efficient access to novel aza-diketopiperazines (Aza-DKP). ACS Comb. Sci. 14, 323–334. ( 10.1021/co300015k) [DOI] [PubMed] [Google Scholar]
- 22.Regenass P, Bosc D, Riché S, Gizzi P, Hibert M, Karmazin L, Ganesan A, Bonnet D. 2017. Comparative study of the synthesis and structural and physicochemical properties of diketopiperazines vs aza-diketopiperazines. J. Org. Chem. 82, 3239–3244. ( 10.1021/acs.joc.6b02895) [DOI] [PubMed] [Google Scholar]
- 23.Petit S, Fruit C, Bischoff L. 2010. New family of peptidomimetics based on the imidazole motif. Org. Lett. 12, 4928–4931. ( 10.1021/ol102118u) [DOI] [PubMed] [Google Scholar]
- 24.Holler T, Ruan F, Spaltenstein A, Hopkins PB. 1989. Total synthesis of marine mercaptohistidines: ovothiols A, B, and C. J. Org. Chem. 54, 4570–4575. ( 10.1021/jo00280a022) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Details for all experimental procedures have been uploaded as part of the electronic supplementary material.