Abstract
Background
Although vitamin D (vitD) deficiency is a common problem in pregnant women, in China, few studies have focused on the relationship between maternal vitD deficiency throughout the three trimesters and subsequent neonatal outcomes in China.
Methods
Between 2015 and 2016, maternal serum and neonate cord blood samples were collected from 1978 mother-neonate pairs from Liuzhou city.
Results
The mean concentrations of 25-hydroxy vitD (25(OH)D) were 16.17±6.27 and 15.23±5.43 ng/ml in the mother and neonate groups, respectively, and the prevalence values of vitD deficiency in the two groups were 78.18% and 83.27%, respectively. Logistic regression showed that maternal vitD deficiency independently increased the risk of gestational diabetes mellitus (GDM) (adjust OR, aOR 1.08; P = 0.026). A relatively lower risk of vitD deficiency was observed in the third trimester than in the first and second trimester (aOR 0.80; P = 0.004). VitD-calcium cosupplementation during pregnancy improves the vitD deficiency in both the maternal and neonatal groups (aOR 0.56, 0.66; P<0.001 and 0.021, respectively). Maternal vitD deficiency significantly increased the risk of neonatal low birth weight (LBW) (aOR 2.83; P = 0.005) and small-for-gestational-age (SGA) (aOR 1.17; P = 0.015). There was a positive correlation between maternal and neonatal vitD deficiency (r = 0.879, P<0.001). VitD supplementation during pregnancy significantly reduced the risk of giving birth to LBW infants (OR = 0.47, 95%CI = 0.33–0.68, P<0.001).
Conclusions
Further research focusing on the consumption of vitD with calcium during pregnancy and the consequential clinical outcomes in Chinese pregnant women is warranted.
Introduction
Vitamin D (vitD) is a well-known secosteroid hormone for its classical functions, such as skeletal health and bone metabolism[1]. Recently, there has been considerable recognition of the importance of its role in modifying the immune system and regulating cell proliferation and cell differentiation[2,3]. VitD deficiency was defined as a serum 25-hydroxy vitD (25-OH D) concentration less than 20 ng/ml, while vitD insufficiency was defined as a 25-(OH)-D concentration less than 30 ng/ml[4]. Due to the function of vitD, many concerns have been raised regarding important impacts of vitD deficiency and the association risk of diseases such as chronic kidney disease[5], cystic fibrosis[6], obesity[7], etc.
An abundance of epidemiological evidence links vitD deficiency and insufficiency to a variety of adverse maternal and neonatal outcomes including preeclampsia, hypertension, gestational diabetes mellitus (GDM), spontaneous abortion, intrauterine growth restriction (IUGR), small size for gestational age (SGA), low birth weight (LBW) and premature birth[8–13]. It was reported that maternal vitD deficiency is considered an important biomarker which can change the glucocorticoid-related parameters in placenta. Maternal vitD deficiency induces the placental and fetal glucocorticoid exposure thus leads to the adverse outcome of fetal growth restriction eventually[14]. However, other observational studies have shown no association between the vitD status and adverse pregnancy outcomes[15,16].
Although there is growing evidence that vitD deficiency and insufficiency are associated with negative pregnancy and infant outcomes, evidence for the correlation of hypovitaminosis D with the potential risk to maternal-neonatal pairs is limited. The objective of the present study was to explore the correlation of maternal-neonatal pairs with vitD status and determine whether maternal vitD deficiency may increase the risk of adverse neonatal outcomes.
Subjects and methods
Study design and subjects
The Liuzhou Birth Cohort Study was a prospective population-based study with a birth cohort that recruited 2000 pregnant women between 2015 and 2016 at Liuzhou Maternity and Child Health Care Hospital. The inclusion criteria were mother-singleton-offspring pairs for whom serum samples from mothers and umbilical cord blood samples from neonates were obtained. Because there were reports[14,15] described the association between vitD deficiency and the risk of GDM, so this cohort included GDM. The exclusion criteria were maternal and neonatal endocrine disorders, such as diabetes mellitus (type 1 or 2) except GDM, thyroid disease, renal and cardiovascular disease, and a history of spontaneous abortion. Eight pregnant women who gave birth to twins, four women who had abortions, two women who had fetal deaths, and eight women who withdrew were excluded from this study. A total of one thousand nine hundred and seventy eight mother-singleton-offspring pairs were included in this study.
Ethics statement
The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Liuzhou Maternity and Child Health Care hospital. In addition, all the participants were given written informed consent in the study. All procedures and methods involving human samples were in accordance with approved guidelines.
Measurement of the serum 25(OH)D level
Blood samples were collected and sent to the clinical laboratory for the vitD measurement. Serum 25(OH)D levels were measured using an electrochemiluminescence immunoassay (ECLIA) kit (Roche Elecsys 10100/201 system, Germany) according to manufacturer’s instructions as described previously [17].
Definitions
Season was defined by the following groups: spring (March to May), summer (June to August), autumn (September to November), winter (December to February).
SGA was calculated as birth weight below the 10th percentile for gestational age[18].
IUGR was defined as a failure of the fetus to achieve its optimal growth potential according to the criteria adopted by the American College of Obstetricians and Gynecologists (ACOG)[19].
Preeclampsia was defined as the presence of hypertension (systolic blood pressure ≥140mmHg and/or diastolic blood pressure ≥90mmHg) after 20 weeks of gestation with the presence of proteinuria (≥0.3g/24h or ≥1+ on dipstick) in previously normotensive women, according to the criteria adopted by ACOG[20].
A 75g oral glucose tolerance test (75g OGTT) was performed between 24–48 weeks of gestation. GDM was defined as the fasting plasma glucose ≥92mg/dl (5.1mmol/L) or 1h plasma glucose ≥180mg/dl (10.0mmol/L), or 2h plasma glucose ≥153mg/dl (8.5mmol/L), according to the recommendations of reference[21].
Weight of birth was measured within 15 minutes after birth. Low birth weight was defined as those newborns with birth weight less than 2500g.
Minority ethnic group: There are 56 different ethnic groups in China. Han and Zhuang are the two major ethnic groups in Guangxi, which consist of about 90% of the population in Guangxi. The minority ethnic group is defined as any other ethnic groups except Han and Zhuang.
Data collection
VitD status based on laboratory and clinical records from two electronic databases was reviewed. The following data were extracted: maternal age, pre-pregnancy BMI, gestational age, season of blood draw, trimester, GDM, preeclampsia, vitD supplemental during pregnancy, vitD-calcium cosupplementation during pregnancy, birth weight, infant gender, preterm delivery, IUGR, SGA, neonatal/maternal serum 25(OH)D levels
Statistical analysis
SPSS software version 20.0 (SPSS Inc. Chicago, IL, USA) and R (http://www.R-project.org) were used for data analyses. Nonparametric methods were used to compared variables of which were not normally distributed. The Kruskal-Wallis rank test was used to compare the differences between trimesters, seasons of maternal blood draw, birth season, and of 25(OH)D measurements. Comparisons of associations of the 25(OH)D level with different groups of related factors were performed using univariate analysis methods. The following variables were entered as predictors in the model: maternal age, pre-pregnancy BMI, gestational age, season of blood draw, trimester, GDM, preeclampsia, vitD supplemental during pregnancy, vitD-calcium cosupplementation during pregnancy, birth weight, infant gender, preterm delivery, IUGR, SGA, neonatal/maternal vitD deficiency. All variables with P<0.10 were selected for inclusion in the multivariate logistic regression model to identify predisposing risk factors that are associated with maternal and neonatal vitD deficiency. P values of <0.05 were considered significant. All P values were corrected by Bonferroni’s method for multiple testing.
Results
25(OH)D concentrations
Table 1 shows the quartile values of 25(OH)D (Q1 = 11.92 ng/ml, Q2 = 14.84 ng/ml, and Q3 = 18.85 ng/ml) of the maternal vitD status and (Q1 = 11.40 ng/ml, Q2 = 14.38 ng/ml, and Q3 = 18.01 ng/ml) of the neonatal vitD status. Maternal-neonatal pairs of participants had a median cord blood 25(OH)D concentration of 16.17 ng/ml and 15.23 ng/ml, respectively. Overall, 79.18% and 83.27% of respective Liuzhou maternal-neonatal participant pairs had serum 25(OH)D levels <20 ng/ml and 96.41% and 97.98% had levels <30 ng/ml (Table 1).
Table 1. VitD level and the prevalence of vitD deficiency in maternity and neonates (n = 1978).
| Variable | Maternal 25(OH)D | Neonatal 25(OH)D |
|---|---|---|
| Q1 | 11.92 | 11.40 |
| Q2 | 14.84 | 14.38 |
| Q3 | 18.85 | 18.01 |
| Q4 | 53.80 | 47.04 |
| Min (ng/ml) | 1.28 | 2.68 |
| Max (ng/ml) | 53.80 | 47.04 |
| Mean±SD (ng/ml) | 16.17±6.27 | 15.23±5.43 |
| The prevalence of vitD deficiency (%) [25(OH)D<20 ng/ml] | 79.18 | 83.27 |
| The prevalence of vitD insufficiency (%) [<30(ng/ml)] | 96.41 | 97.98 |
Q: quarter
Maternal characteristics and vitD status
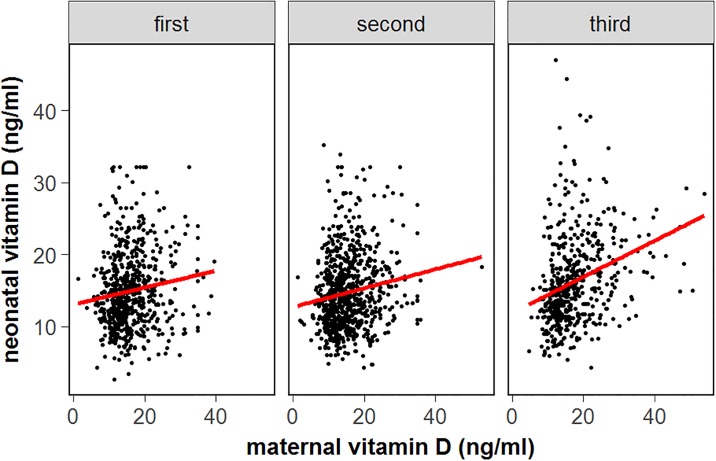
Evaluation of the potential risk factors explored in this study revealed that season was a determinant of maternal vitD status. Compared to spring, a significant increase in maternal 25(OH)D concentrations was observed in the summer, autumn and winter seasons; the odds ratio (OR) and 95% confidential interval (95% CI) were 0.31 (0.22–0.44), 0.33 (0.23–0.48), and 0.60 (0.40–0.90), respectively (Table 2, Fig 1). The maternal 25(OH)D concentration was significantly higher in the third trimester than in the first two trimesters; the OR values (95% CI) were 1.52 (1.15–1.99) and 1.54 (1.17–2.04), respectively (Table 2, Fig 2). In the multivariate regression model, maternal vitD deficiency independently increased the GDM risk, the OR (95% CI) was 1.08 (1.04–1.10) (P = 0.026) (Table 3). A relatively lower risk of vitD deficiency was observed in the third trimester than in the first and second trimester (adjusted OR = 0.80, 95% CI, 0.69–0.93; P = 0.004). Maternal 25(OH)D concentration was inversely associated with SGA risk, the OR (95% CI) was 1.17 (1.03–1.32) (P = 0.015).
Table 2. The correlations of independent factors with 25(OH)D in maternity by univariate analysis.
| Variable | OR | 95%CI | P value | |
|---|---|---|---|---|
| Maternal age(years) | 0.005 | |||
| <30 | 1179(59.61) | Reference | ||
| 30–34 | 534(26.99) | 0.78 | 0.60–1.02 | |
| ≥35 | 265(13.40) | 1.40 | 1.04–1.90 | |
| Maternal prepregnancy BMI | 0.705 | |||
| <23 | 1445(73.05) | Reference | ||
| ≥23 | 533(26.85) | 0.97 | 0.75–1.25 | |
| Gestational age (weeks) | 0.701 | |||
| <37 | 101(5.1) | Reference | ||
| 37–39 | 1073(54.25) | 1.08 | 0.76–1.32 | |
| 40+ | 804(40.64) | 1.07 | 0.86–1.35 | |
| Ethnic | 0.716 | |||
| Han | 1006(50.8) | Reference | ||
| Zhuang | 795(40.2) | 1.07 | 0.75–1.23 | |
| Minority | 177(9.0) | 1.41 | 0.83–1.56 | |
| Season of blood draw | 0.001 | |||
| Spring | 435(21.99) | Reference | ||
| Summer | 594(30.03) | 0.31 | 0.22–0.44 | 0.001 |
| Autumn | 533(26.85) | 0.33 | 0.23–0.48 | 0.001 |
| Winter | 416(21.03) | 0.60 | 0.40–0.90 | 0.014 |
| Trimester | 0.002 | |||
| First | 652(32.96) | 1.52 | 1.15–1.99 | 0.003 |
| Second | 804(40.65) | 1.54 | 1.17–2.04 | 0.001 |
| Third | 522(26.39) | Reference | ||
| GDM | <0.001 | |||
| No | 1811(91.6) | Reference | ||
| Yes | 167(8.4) | 1.06 | 1.03–1.09 | |
| PE | 0.302 | |||
| No | 1915(96.8) | Reference | ||
| Yes | 63(3.2) | 0.98 | 0.94–1.02 | |
| vitD supplemental during pregnancy | 0.001 | |||
| No | 878(44.39) | Reference | ||
| ≤6 times/week | 676(34.18) | 0.47 | 0.36–0.61 | 0.001 |
| ≥1 time/day | 424(21.43) | 0.15 | 0.23–0.41 | 0.001 |
| vitD-calcium cosupplementation during pregnancy | 0.001 | |||
| No | 858(43.38) | Reference | ||
| ≤6 times/week | 717(36.25) | 0.43 | 0.33–0.56 | 0.001 |
| ≥1 time/day | 403(20.37) | 0.29 | 0.22–0.39 | 0.001 |
| Birth weight (g) | 0.001 | |||
| <2500 | 130(6.57) | 2.75 | 1.32–3.01 | |
| ≥2500 | 1848(93.43) | Reference | ||
| Gender | 0.497 | |||
| Boy | 1046(52.88) | Reference | ||
| Girl | 932(47.12) | 0.93 | 0.75–1.15 | |
| Preterm delivery (<37 weeks) | 0.672 | |||
| No | 1839(92.97) | Reference | ||
| Yes | 139(7.03) | 0.91 | 0.59–1.41 | |
| IUGR | 0.106 | |||
| No | 1941(98.1) | Reference | ||
| Yes | 37(1.9) | 0.95 | 0.89–1.01 | |
| SGA | 0.012 | |||
| No | 1800(91.0) | Reference | ||
| Yes | 178(9.0) | 1.17 | 1.03–1.32 | |
| Neonatal vitD deficiency (<20ng/ml) | 0.001 | |||
| No | 331(16.73) | Reference | ||
| Yes | 1647(83.27) | 1.06 | 1.04–1.08 |
BMI: body mass index; GDM: gestational diabetes mellitus; PE: preeclampsia; IUGR: intrauterine growth restrictions; SGA: small-for gestational-age
Fig 1. Association between maternal 25(OH)D level during pregnancy and neonatal cord blood 25(OH)D level distributed by maternal blood drew season.
Fig 2. Association between maternal 25(OH)D level during pregnancy and neonatal cord blood 25(OH)D level distributed by trimester.
Table 3. The correlations of independent factors with 25(OH)D in maternity by multivariate analysis.
| Late Trimester | 0.80 | 0.69–0.93 | 0.004 |
| Gestational diabetes mellitus | 1.08 | 1.04–1.10 | 0.026 |
| vitD-calcium cosupplementation during pregnancy | 0.56 | 0.48–0.65 | <0.001 |
| Small-for gestational-age | 1.17 | 1.03–1.32 | 0.015 |
| Neonatal vitD deficiency | 1.92 | 1.47–2.49 | <0.001 |
VitD and vitD-calcium cosupplementation during pregnancy significantly reduced the risk of maternal vitD deficiency; the relative risks were 0.47 (95% CI, 0.36–0.61) and 0.15 (95% CI, 0.23–0.41) for vitD supplementation ≤6 times/week and ≥1 time/day, respectively. The same result was observed for vitD-calcium cosupplementation; for supplementation ≤6 times/week, the OR (95% CI) was 0.43 (0.33–0.56). For supplementation ≥1 time/day, this risk was reduced to 0.29 (95% CI, 0.22–0.39) compared to the group that did not take supplements. In the multivariate regression analysis, vitD-calcium cosupplementation during pregnancy increased the maternal vitD status; the OR (95% CI) was 0.56 (0.48–0.65) (P = <0.001) (Table 3).
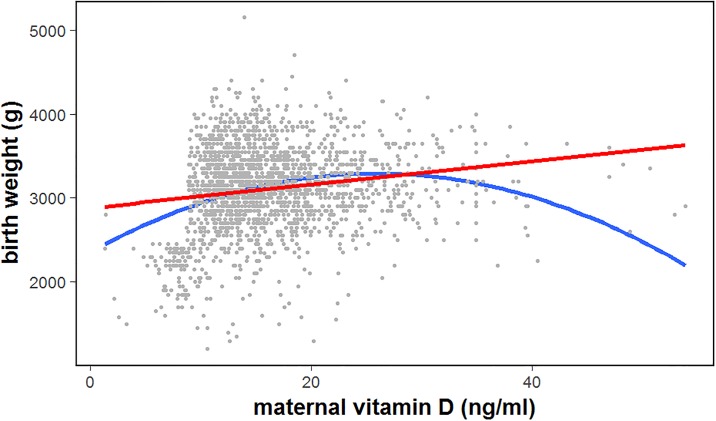
There was a positive correlation between the maternal vitD concentrations and neonatal birth weight (r = 0.522, P<0.001) (Fig 3). Maternal vitD deficiency significantly increased the risk of a low neonatal birth weight; the relative risk was 2.75 (95% CI, 1.32–3.01). Maternal vitD deficiency was an independent risk factor for neonatal vitD deficiency; the OR (95% CI) was 1.92 (1.47–2.49) (P<0.001).
Fig 3. Scatter plot of correlation between maternal 25(OH)D level during pregnancy and neonatal birth weight (r = 0.522, P<0.001).
Both the estimated regression line (red line) and a true curve line (blue line) demonstrate the inverse association between these two variables.
Neonatal characteristics and VitD status
The neonatal cord blood 25(OH)D was lower in birth seasons of spring and winter (P trend <0.001) (Table 4, Fig 4), but this relationship was no longer significant in the multivariate regression model. VitD and vitD-calcium cosupplementation during pregnancy significantly reduced the neonatal vitD deficiency risk; the relative risks were 0.47 (95% CI, 0.36–0.61) and 0.15 (95% CI, 0.23–0.41) for vitD supplementation ≤6 times/week and ≥1 time/day, respectively, and the relative risks were 0.43 (95% CI, 0.33–0.56) and 0.29 (95% CI, 0.22–0.39) for vitD-calcium cosupplementation ≤6 times/week and ≥1 time/day, respectively (Table 4). In the multivariate regression analysis, vitD-calcium cosupplementation was a protective factor associated with neonatal vitD deficiency; the OR (95% CI) was 0.66 (0.46–0.94) (P = 0.021) (Table 5).
Table 4. The correlations of independent factors with cord blood 25(OH)D in neonate by univariate analysis.
| Variable | n(%) | OR | 95%CI | P value |
|---|---|---|---|---|
| Maternal age(years) | 0.004 | |||
| <30 | 1179(59.61) | Reference | ||
| 30–34 | 534(26.99) | 1.18 | 0.89–1.55 | 0.243 |
| ≥35 | 265(13.40) | 1.73 | 1.25–2.39 | 0.001 |
| Maternal prepregnancy BMI | 0.530 | |||
| <23 | 1445(73.05) | Reference | ||
| ≥23 | 533(26.85) | 0.96 | 0.83–1.10 | |
| Gestational age (weeks) | 0.151 | |||
| <37 | 101(5.1) | Reference | ||
| 37–39 | 1073(54.25) | 1.02 | 0.77–1.25 | 0.167 |
| 40+ | 804(40.64) | 1.21 | 0.89–1.13 | 0.138 |
| Ethnic | 0.856 | |||
| Han | 1006(50.8) | Reference | ||
| Zhuang | 795(40.2) | 0.91 | 0.87–1.04 | |
| Minority | 177(9.0) | 1.05 | 0.93–1.21 | |
| Birth season | 0.001 | |||
| Spring | 549(27.76) | Reference | ||
| Summer | 653(33.01) | 0.50 | 0.36–0.69 | 0.001 |
| Autumn | 425(21.49) | 0.50 | 0.35–0.71 | 0.001 |
| Winter | 351(17.74) | 0.90 | 0.59–1.38 | 0.641 |
| GDM | 0.778 | |||
| No | 1811(91.6) | Reference | ||
| Yes | 167(8.4) | 0.99 | 0.97–1.03 | |
| PE | 0.196 | |||
| No | 1915(96.8) | Reference | ||
| Yes | 63(3.2) | 1.03 | 0.98–1.07 | |
| vitD supplemental during pregnancy | 0.003 | |||
| No | 878(44.39) | Reference | ||
| ≤6 times/week | 676(34.18) | 0.63 | 0.48–0.82 | 0.010 |
| ≥1 time/day | 424(21.43) | 0.71 | 0.52–0.97 | 0.030 |
| vitD-calcium cosupplementation during pregnancy | 0.001 | |||
| No | 858(43.38) | Reference | ||
| ≤6 times/week | 717(36.25) | 0.62 | 0.48–0.82 | 0.001 |
| ≥1 time/day | 403(20.37) | 0.61 | 0.44–0.83 | 0.002 |
| Birth weight (g) | 0.002 | |||
| <2500 | 130(6.57) | 3.23 | 1.56–6.67 | |
| ≥2500 | 1848(93.43) | Reference | ||
| Gender | 0.714 | |||
| Boy | 1046(52.88) | Reference | ||
| Girl | 932(47.12) | 1.05 | 0.83–1.32 | |
| Preterm delivery (<37 weeks) | 0.862 | |||
| No | 1839(92.97) | Reference | ||
| Yes | 139(7.03) | 1.04 | 0.66–1.64 | |
| IUGR | 0.264 | |||
| No | 1941(98.1) | Reference | ||
| Yes | 37(1.9) | 0.96 | 0.90–1.03 | |
| SGA | 0.215 | |||
| No | 1800(91.0) | |||
| Yes | 178(9.0) | |||
| Maternal vitD deficiency (<20ng/ml) | 0.001 | |||
| No | 412(20.83) | Reference | ||
| Yes | 1566(79.17) | 2.03 | 1.56–2.64 |
BMI: body mass index; GDM: gestational diabetes mellitus; PE: preeclampsia; IUGR: intrauterine growth restrictions; SGA: small-for gestational-age
Fig 4. Association between neonatal cord blood 25(OH)D level and maternal 25(OH)D level during pregnancy distributed by birth season.
Table 5. The correlations of independent factors with cord blood 25(OH)D in neonate by multivariate analysis.
| Variable | OR | 95%CI | P value |
|---|---|---|---|
| VitD-calcium cosupplementation during pregnancy | 0.66 | 0.46–0.94 | 0.021 |
| Low birth weight | 2.83 | 1.36–5.89 | 0.005 |
| Maternal vitD deficiency | 1.79 | 1.36–2.35 | 0.001 |
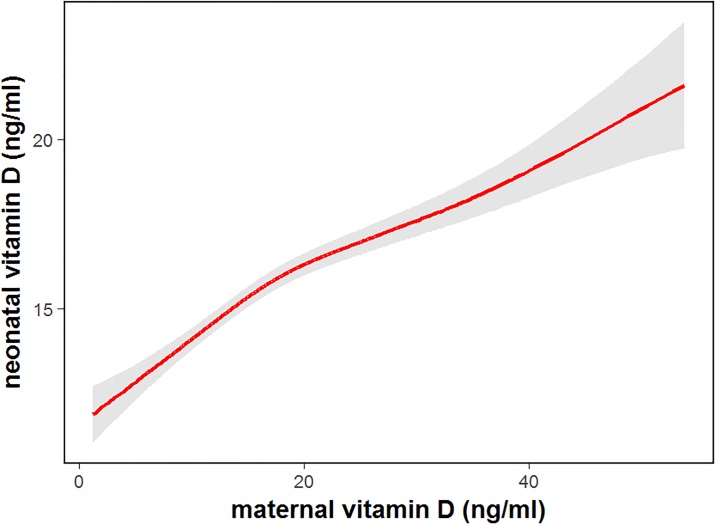
One hundred and thirty (6.57%) neonates had a LBW. The risk of vitD deficiency was much higher in neonates who had LBW than those who had a normal birth weight; the OR (95% CI) was 2.83 (1.36–5.89) (P = 0.005) (Table 5). The maternal vitD deficiency was also an independent risk factor for neonatal vitD deficiency; the OR (95% CI) was 1.79 (1.36–2.35) (P<0.001). There was a positive correlation between maternal and neonatal vitD deficiency (r = 0.879, P<0.001). The maternal vitD deficiency was a predictor for neonatal vitD deficiency, and the predictive model is shown in Fig 5. Maternal vitamin D supplementation played an important role in prevention of giving birth to LBW infants. In the vitD supplementation group, only 4.5% (50 women) gave birth to LBW infants, while 9.1% (80 women) who didn’t have vitD intervention gave birth to LBW infants. VitD supplementation during pregnancy significantly reduced the risk of giving birth to LBW infants (OR = 0.47, 95%CI = 0.33–0.68, P<0.001).
Fig 5. Neonatal cord blood 25(OH)D level was predicted by maternal 25(OH)D level modeled by restricted cubic splines.
The solid line is the predicted cord blood level, the gray shading is 95% confidence intervals.
Discussion
The recent establishment of a causal relationship between maternal vitD deficiency and adverse pregnancy outcomes is a matter of great public health concern[22–25]. The prevalence of maternal or neonatal vitD deficiencies has been extensively studied worldwide[8–10]. Few studies have been conducted on the vitD levels of both mothers and their newborns[11–16,21]. Our cohort study contains 1978 pairs of maternal-neonatal vitD samples and revealed that 79.18% of pregnant women and 83.27% of newborns had 25(OH)D levels below 20 ng/ml; for 25(OH)D levels below 30 ng/ml, which indicates vitD insufficiency, this figure increased to 96.41% in pregnant women and 97.98% in their newborns residing in Liuzhou, Western China. Although few studies have reported on the prevalence of the vitD status in pregnant women or newborns in China, their data agreed with ours, which indicated that a high prevalence of vitD deficiency occurred in both groups[26–29].
GDM was the most common maternal complication with the prevalence of 4.1%-27.5% globally[30]. We observed a significant relationship between 25(OH)D deficiency and the risk of GDM. Our study is consistent with others indicating that there was an inverse association between 25(OH)D and risk of GDM[15]. Two nested case-control studies using a prospective designed confirmed that lower vitD status was associated with a significantly increased risk of subsequent GDM[31,32], one of which provided further evidence showed that 25(OH)D concentrations below the top quartile (<73.5 mmol/L) increased a 2-fold greater risk of GDM during pregnancy[32]. Our study, along with worldwide cohort studies[28,29] suggested that vitD may play a role in glucose tolerance, which will enable the design of proper interventions (e.g., vitD supplementation) to reduce the rates of some maternal complications.
Most of the recently published randomized controlled trials (RCTs) have focused on whether maternal vitD supplementation may influence maternal or neonatal vitD deficiency[33,34]. Few studies have focused on the positive maternal and fetal outcomes of consuming vitD with calcium during pregnancy[34]. Emerging evidence suggests that vitD sufficiency has a positive impact on the skeletal system and women who simultaneously supplement vitD and calcium during pregnancy may have a reduced risk of adverse outcomes while improving fetal growth[34,35]. The recently published meta-analysis with pooled 13 RCTs also confirmed that maternal vitD supplementation can increase circulating 25(OH)D levels, birth weight and birth length[36]. Our study showed a corresponding increase in the serum 25(OH)D and cord blood 25(OH)D levels with increasing use of vitD-calcium cosupplementation in pregnant women from Liuzhou. Our findings were further supported by Park et al[37], who found that there are higher maternal concentrations of vitD biomarkers in pregnant women who simultaneously supplement vitD and calcium in their third trimester to ensure sufficient calcium delivery to the fetus. As vitD deficiency is a common problem in both pregnant women and infants, our study highlights the positive effect of vitD-calcium cosupplementation during pregnancy on both the maternal and neonatal vitD levels.
VitD plays an important role in fetal growth due to its key interaction with Ca2+ homeostasis and parathyroid hormones. A significantly increased risk of low neonatal birth weight among populations with vitD deficiency during pregnancy is anticipated and consistent with existing trends in the literature[38]. Growing evidence has indicated that maternal vitD deficiency may impair fetal growth and lead to a series of adverse pregnancy outcomes, including preterm delivery, IUGR, SGA and neonatal LBW[11–16]. VitD deficiency during pregnancy was associated with nearly 2-fold greater odds of a LBW in our study. In agreement with our study, a significant positive association between maternal vitD deficiency and LBW has been reported in an Iranian population[38]. Our study showed that lower vitD status was associated with a significantly increased risk of subsequent delivered SGA neonates. In another study conducted in Caucasians, 25(OH)D concentrations were significantly lower in mothers who delivered neonates who were SGA[11], which was consistent with our results.
Our study reported that maternal vitD deficiency has a positive correlation with neonatal vitD deficiency, and maternal vitD deficiency significantly increased the risk for neonatal vitD deficiency, while the relative risk was nearly 2-fold. Reports from another Chinese population demonstrated similar results. A study conducted in Beijing indicated that neither mothers nor newborns had normal levels of 25(OH)D (>30 ng/ml) and that a significantly positive correlation between maternal and neonatal 25(OH)D concentrations has been demonstrated (r = 0.89, P<0.001). Meanwhile, severe maternal vitD deficiency was associated with a higher risk of LBW neonates[39], which was consistent with our results. The Anhui birth cohort study showed that the relative risk for a LBW in the maternal deficiency group was 12.31[40].
The data from our study indicated that pregnant women in the third trimester had higher vitD concentrations. VitD deficiency occurred in both the maternal and neonatal groups. A high prevalence of vitD deficiency in both groups was associated with low neonatal birth weight. The successful concurrent use of vitD and calcium contributed to high vitD levels in the maternal and neonatal populations.
Conclusions
In summary, our findings demonstrate the importance of interventions to ensure an adequate vitD level in pregnant women. Strategies should focus on different risk factors, and there should be continued efforts to increase the supplement with vitD and calcium during pregnancy which could in turn reduce the prevalence of vitD deficiency in both the maternal and neonatal groups.
Supporting information
(RAR)
Acknowledgments
This study was funded by the Guangxi Natural Science Foundation (2017GXNSFAA198312); Guangxi Medical and Health Self-funding Project (NoZ2013609, NoZ2012520, NoZ20170473, NoZ20170516) and the Liuzhou Science and Technology Bureau Project (No2014J030421, No2017BD20201). The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Data Availability
Data are available upon request. Data contain sensitive patient information and the overseeing ethics committee has imposed restrictions on making these data publicly available. For data requests, please contact the Ethics Committee of Liuzhou Maternity and Child Health Care Hospital: Lin Wang, email: 32531606@qq.com or the first author of this paper: LZWYL2006@163.com.
Funding Statement
This study was funded by the Guangxi Natural Science Foundation (2017GXNSFAA198312 and 2017AB49005); Guangxi Medical and Health Self-funding Project (No Z2013609, Z2012520, Z20170473, Z20170516) and the Liuzhou Science and Technology Bureau Project (No 2014J030421, No 2017BD20201). The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- 1.Al Nozha OM. Vitamin D and extra-skeletal health: causality or consequence. Int J Health Sci (Qassim). 2016;10(3):443–52. [PMC free article] [PubMed] [Google Scholar]
- 2.Bellavia D, Costa V, De Luca A, Maglio M, Pagani S, Fini M, et al. Vitamin D Level Between Calcium-Phosphorus Homeostasis and Immune System: New Perspective in Osteoporosis. Curr Osteoporos Rep. 2016. October 13 doi: 10.1007/s11914-016-0331-2 [DOI] [PubMed] [Google Scholar]
- 3.Cortes M, Chen MJ, Stachura DL, Liu SY, Kwan W, Wright F, et al. Developmental Vitamin D Availability Impacts Hematopoietic Stem Cell Production. Cell Rep. 2016;17(2):458–468. doi: 10.1016/j.celrep.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. ; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011; 96(7):1911–30. doi: 10.1210/jc.2011-0385 [DOI] [PubMed] [Google Scholar]
- 5.Obi Y, Hamano T, Isaka Y. Prevalence and prognostic implications of vitamin D deficiency in chronic kidney disease. Dis Markers. 2015;2015:868961 doi: 10.1155/2015/868961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lansing AH, McDonald C, Patel RA, Meihls S, Crowell K, Chatfield B, et al. Vitamin D deficiency in pediatric patients with cystic fibrosis: associated risk factors in the northern United States. South Med J. 2015;108(3):164–9. doi: 10.14423/SMJ.0000000000000254 [DOI] [PubMed] [Google Scholar]
- 7.Pereira-Santos M, Costa PR, Assis AM, Santos CA, Santos DB. Obesity and vitamin D deficiency: a systematic review and meta-analysis. Obes Rev. 2015;16(4):341–9. doi: 10.1111/obr.12239 [DOI] [PubMed] [Google Scholar]
- 8.Baca KM, Simhan HN, Platt RW, Bodnar LM. Low maternal 25-hydroxyvitamin D concentration increases the risk of severe and mild preeclampsia. Ann Epidemiol. 2016;26(12):853–857.e1. doi: 10.1016/j.annepidem.2016.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bärebring L, Bullarbo M, Glantz A, Leu Agelii M, Jagner Å, Ellis J, et al. Preeclampsia and Blood Pressure Trajectory during Pregnancy in Relation to Vitamin D Status. PLoS One. 2016;11(3):e0152198 doi: 10.1371/journal.pone.0152198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen LB, Jørgensen JS, Jensen TK, Dalgård C, Barington T, Nielsen J, et al. Vitamin D insufficiency is associated with increased risk of first-trimester miscarriage in the Odense Child Cohort. Am J Clin Nutr. 2015;102(3):633–8. doi: 10.3945/ajcn.114.103655 [DOI] [PubMed] [Google Scholar]
- 11.Gernand AD, Simhan HN, Caritis S, Bodnar LM. Maternal vitamin D status and small-for-gestational-age offspring in women at high risk for preeclampsia. Obstet Gynecol. 2014;123(1):40–8. doi: 10.1097/AOG.0000000000000049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin LL, Lu FG, Yang SH, Xu HL, Luo BA. Does Maternal Vitamin D Deficiency Increase the Risk of Preterm Birth: A Meta-Analysis of Observational Studies. Nutrients. 2016;8(5). doi: 10.3390/nu8050301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miliku K, Vinkhuyzen A, Blanken LM, McGrath JJ, Eyles DW, Burne TH, et al. Maternal vitamin D concentrations during pregnancy, fetal growth patterns, and risks of adverse birth outcomes. Am J Clin Nutr. 2016;103(6):1514–22. doi: 10.3945/ajcn.115.123752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei SQ. Vitamin D and pregnancy outcomes. Curr Opin Obstet Gynecol. 2014;26(6):438–47. doi: 10.1097/GCO.0000000000000117 [DOI] [PubMed] [Google Scholar]
- 15.Weinert LS, Silveiro SP. Vitamin D and its impact on maternal-fetal outcomes in pregnancy: A critical review. Matern Child Health J. 2015;19(1):94–101. [DOI] [PubMed] [Google Scholar]
- 16.Al-Shaikh GK, Ibrahim GH, Fayed AA, Al-Mandeel H. Impact of vitamin D deficiency on maternal and birth outcomes in the Saudi population: a cross-sectional study. BMC Pregnancy Childbirth. 2016;16:119 doi: 10.1186/s12884-016-0901-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li XH, Huang XP, Pan L, Wang CY, Qin J, Nong FW, et al. Vitamin D deficiency may predict a poorer outcome of IgA nephropathy. BMC Nephrol. 2016;17(1):164 doi: 10.1186/s12882-016-0378-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchant T, Willey B, Katz J, Clarke S, Kariuki S, ter Kuile F, et al. Neonatal mortality risk associated with preterm birth in East Africa, adjusted by weight for gestational age: individual participant level meta-analysis. PLoS Med. 2012;9(8):e1001292 doi: 10.1371/journal.pmed.1001292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Committee on Practice Bulletins—Gynecology, American College of Obstetricians and Gynecologists, Washington, DC 20090–6920, USA. Intrauterine growth restriction. Clinical management guidelines for obstetrician-gynecologists. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 2001;72(1):85–96. [PubMed] [Google Scholar]
- 20.ACOG Committee on Obstetric Practice. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 2002;77(1):67–75. [PubMed] [Google Scholar]
- 21.Zhou J, Su L, Liu M, Liu Y, Cao X, Wang Z, et al. Associations between 25-hydroxyvitamin D levels and pregnancy outcomes: a prospective observational study in southern China. Eur J Clin Nutr. 2014;68(8):925–30. doi: 10.1038/ejcn.2014.99 [DOI] [PubMed] [Google Scholar]
- 22.Wei SQ, Qi HP, Luo ZC, Fraser WD. Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2013;26(9):889–99. doi: 10.3109/14767058.2013.765849 [DOI] [PubMed] [Google Scholar]
- 23.Kiely ME, Zhang JY, Kinsella M, Khashan AS, Kenny LC. Vitamin D status is associated with uteroplacental dysfunction indicated by pre-eclampsia and small-for-gestational-age birth in a large prospective pregnancy cohort in Ireland with low vitamin D status. Am J Clin Nutr. 2016;104(2):354–61. doi: 10.3945/ajcn.116.130419 [DOI] [PubMed] [Google Scholar]
- 24.Toko EN, Sumba OP, Daud II, Ogolla S, Majiwa M, Krisher JT, et al. Maternal Vitamin D Status and Adverse Birth Outcomes in Children from Rural Western Kenya. Nutrients. 2016;8(12). doi: 10.3390/nu8120794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanieh S, Ha TT, Simpson JA, Thuy TT, Khuong NC, Thoang DD, et al. Maternal vitamin D status and infant outcomes in rural Vietnam: a prospective cohort study. PLoS One. 2014;9(6):e99005 doi: 10.1371/journal.pone.0099005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu P, Tong SL, Hu WB, Hao JH, Tao RX, Huang K, et al. Cord Blood 25-hydroxyvitamin D and Fetal Growth in the China-Anhui Birth Cohort Study. Sci Rep. 2015;5:14930 doi: 10.1038/srep14930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu X, Wang W, Wei Z, Ouyang F, Huang L, Wang X, et al. Vitamin D status and related factors in newborns in Shanghai, China. Nutrients. 2014;6(12):5600–10. doi: 10.3390/nu6125600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yun C, Chen J, He Y, Mao D, Wang R, Zhang Y, et al. Vitamin D deficiency prevalence and risk factors among pregnant Chinese women. Public Health Nutr. 2017;20(10):1746–1754. doi: 10.1017/S1368980015002980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu T, Liu TJ, Ge X, Kong J, Zhang LJ, Zhao Q. High prevalence of maternal vitamin D deficiency in preterm births in northeast China, Shenyang. Int J Clin Exp Pathol. 2015;8(2):1459–65. [PMC free article] [PubMed] [Google Scholar]
- 30.Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract. 2014;103(2):176–85. doi: 10.1016/j.diabres.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 31.Zhang C, Qiu C, Hu FB, David RM, van Dam RM, Bralley A, et al. Maternal plasma 25-hydroxyvitamin D concentrations and the risk for gestational diabetes mellitus. PLoS One. 2008;3(11):e3753 doi: 10.1371/journal.pone.0003753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parlea L, Bromberg IL, Feig DS, Vieth R, Merman E, Lipscombe LL. Association between serum 25-hydroxyvitamin D in early pregnancy and risk of gestational diabetes mellitus. Diabet Med. 2012;29(7):e25–32. doi: 10.1111/j.1464-5491.2011.03550.x [DOI] [PubMed] [Google Scholar]
- 33.Sahoo SK, Katam KK, Das V, Agarwal A, Bhatia V. Maternal vitamin D supplementation in pregnancy and offspring outcomes: a double-blind randomized placebo-controlled trial. J Bone Miner Metab. 2017;35(4):464–471. doi: 10.1007/s00774-016-0777-4 [DOI] [PubMed] [Google Scholar]
- 34.Palacios C, De-Regil LM, Lombardo LK, Peña-Rosas JP. Vitamin D supplementation during pregnancy: Updated meta-analysis on maternal outcomes. J Steroid Biochem Mol Biol. 2016;164:148–155. doi: 10.1016/j.jsbmb.2016.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karamali M, Asemi Z, Ahmadi-Dastjerdi M, Esmaillzadeh A. Calcium plus vitamin D supplementation affects pregnancy outcomes in gestational diabetes: randomized, double-blind, placebo-controlled trial. Public Health Nutr. 2016;19(1):156–63. doi: 10.1017/S1368980015000609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pérez-López FR, Pasupuleti V, Mezones-Holguin E, Benites-Zapata VA, Thota P, Deshpande A, et al. Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2015;103(5):1278–88.e4. doi: 10.1016/j.fertnstert.2015.02.019 [DOI] [PubMed] [Google Scholar]
- 37.Park H, Brannon PM, West AA, Yan J, Jiang X, Perry CA, et al. Maternal vitamin D biomarkers are associated with maternal and fetal bone turnover among pregnant women consuming controlled amounts of vitamin D, calcium, and phosphorus. Bone. 2017;95:183–191. doi: 10.1016/j.bone.2016.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khalessi N, Kalani M, Araghi M, Farahani Z. The Relationship between Maternal Vitamin D Deficiency and Low Birth Weight Neonates. J Family Reprod Health. 2015;9(3):113–7. [PMC free article] [PubMed] [Google Scholar]
- 39.Song SJ, Si S, Liu J, Chen X, Zhou L, Jia G, et al. Vitamin D status in Chinese pregnant women and their newborns in Beijing and their relationships to birth size. Public Health Nutr. 2013;16(4):687–92. doi: 10.1017/S1368980012003084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen YH, Fu L, Hao JH, Yu Z, Zhu P, Wang H, et al. Maternal vitamin D deficiency during pregnancy elevates the risks of small for gestational age and low birth weight infants in Chinese population. J Clin Endocrinol Metab. 2015;100(5):1912–9. doi: 10.1210/jc.2014-4407 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(RAR)
Data Availability Statement
Data are available upon request. Data contain sensitive patient information and the overseeing ethics committee has imposed restrictions on making these data publicly available. For data requests, please contact the Ethics Committee of Liuzhou Maternity and Child Health Care Hospital: Lin Wang, email: 32531606@qq.com or the first author of this paper: LZWYL2006@163.com.