ABSTRACT
Bacterial choline degradation in the human gut has been associated with cancer and heart disease. In addition, recent studies found that a bacterial microcompartment is involved in choline utilization by Proteus and Desulfovibrio species. However, many aspects of this process have not been fully defined. Here, we investigate choline degradation by the uropathogen Escherichia coli 536. Growth studies indicated E. coli 536 degrades choline primarily by fermentation. Electron microscopy indicated that a bacterial microcompartment was used for this process. Bioinformatic analyses suggested that the choline utilization (cut) gene cluster of E. coli 536 includes two operons, one containing three genes and a main operon of 13 genes. Regulatory studies indicate that the cutX gene encodes a positive transcriptional regulator required for induction of the main cut operon in response to choline supplementation. Each of the 16 genes in the cut cluster was individually deleted, and phenotypes were examined. The cutX, cutY, cutF, cutO, cutC, cutD, cutU, and cutV genes were required for choline degradation, but the remaining genes of the cut cluster were not essential under the conditions used. The reasons for these varied phenotypes are discussed.
IMPORTANCE Here, we investigate choline degradation in E. coli 536. These studies provide a basis for understanding a new type of bacterial microcompartment and may provide deeper insight into the link between choline degradation in the human gut and cancer and heart disease. These are also the first studies of choline degradation in E. coli 536, an organism for which sophisticated genetic analysis methods are available. In addition, the cut gene cluster of E. coli 536 is located in pathogenicity island II (PAI-II536) and hence might contribute to pathogenesis.
KEYWORDS: microcompartment, carboxysome, choline, E. coli 536
INTRODUCTION
Hundreds of bacterial species produce complex proteinaceous organelles known as bacterial microcompartments (MCPs) (1–9). MCPs are among the largest known multiprotein complexes. They are typically 100 to 150 nm in diameter and are built from thousands of protein subunits of 10 to 20 different types. The function of MCPs is to optimize metabolic pathways by sequestering toxic or poorly retained metabolic intermediates and by increasing enzymatic reaction rates (7–9). Overall, MCPs consist of metabolic pathways encapsulated within a selectively permeable protein shell. This architecture allows the selective confinement of toxic pathway intermediates that would otherwise damage DNA and cytoplasmic components (10–14). It also prevents the loss of intermediates that would normally diffuse out of the cell resulting in the loss of valuable carbon (8, 11, 15). In addition, MCPs concentrate enzymes together with their substrates, increasing reaction rates, with the most notable example being the carboxysome MCP, which is used to enhance CO2 fixation by RubisCO via a carbon dioxide-concentrating mechanism (8).
Bacterial MCPs are present in many ecologically diverse and important bacteria and have a number of potential biotechnology applications. Bioinformatics analyses indicate that MCPs are produced by about 20% of bacteria distributed across 11 kingdom-level taxa, and physiological studies have shown that MCPs are involved in 10 or more metabolic processes ranging from carbon dioxide fixation to the catabolism of 1,2-propanediol, ethanolamine, choline, glycerol, rhamnose, fucose, and fucoidan (15–22). In addition, MCPs have been linked to pathogenesis in Salmonella and Listeria spp. (23–26), as well as to heart disease and cancer in humans due to their metabolic roles in the gut microbiome (27–30). Moreover, several labs have begun to develop MCPs as a platform for protein-based containers for use in renewable chemical production, drug delivery, and the expression of toxic proteins (31–42). However, as of yet, only three MCP types have been studied in any detail: the 1,2-propanediol utilization (Pdu) MCP, the ethanolamine utilization (Eut) MCP, and the carboxysome. Hence, a great deal still remains to be learned about the physiology and ecology of diverse MCPs, as well as their associations with human health and their potential biotechnology applications.
The genes for MCPs are typically found in large operons that encode both the shell proteins and the enzymes for the encapsulated metabolic pathway (1–9). The pathway enzymes vary according to the substrate metabolized which defines the MCP type. The shells of MCPs are primarily built from bacterial microcompartment (BMC) domain proteins and a bacterial microcompartment vertex (BMV) protein (43–47). MCP operons typically encode a single BMV protein that is thought to help impart curvature to the shell and multiple BMC domain proteins which fulfill various functional roles (44, 47). Hexameric BMC domain proteins have central pores that mediate the selective transport of small molecules across the MCP shell (43, 48, 49). The trimeric class of BMC domain proteins includes members proposed to have allosterically gated pores for the transport of larger molecules, as well as members with FeS clusters thought to mediate electron transfer across the MCP shell (50–57). Moreover, BMC domains are found fused to a variety of protein domains of unknown function, and MCP operons show extensive variation in the number and types of BMC domain proteins they encode. Presumably, this diversity is needed for the optimal function of varied MCP types operating in diverse host organisms. However, the specific functions of many BMC domain proteins are unknown.
Recently, a choline utilization (cut) gene cluster was identified in Desulfovibrio desulfuricans (58). This cluster included a gene for a glycyl-radical choline TMA-lyase (CutC), as well as multiple genes for bacterial MCP shell proteins. Physiological and genetic studies demonstrated that choline TMA lyase and the cut gene cluster were required for choline utilization by Desulfovibrio alaskensis and Proteus mirabilis (58–61). Bioinformatic analyses showed that cut gene clusters are widely but unevenly distributed among bacteria (58, 61). Studies also indicated that the cutC gene is diagnostic for choline-degrading bacteria and provides a useful marker for metagenomic analyses (61). In addition, recent electron microscopy of P. mirabilis and fitness studies in D. alaskensis G20 substantiated that a bacterial MCP was involved in choline degradation (59, 60). However, many aspects of choline degradation, including its physiological role, regulation, and mechanisms have not been investigated fully.
In this report, we characterize choline degradation by Escherichia coli 536. E. coli 536 is a uropathogen, and in this organism, the choline gene cluster is found in a pathogenicity island (PAI-II536) (62).
RESULTS
Choline utilization by E. coli 536.
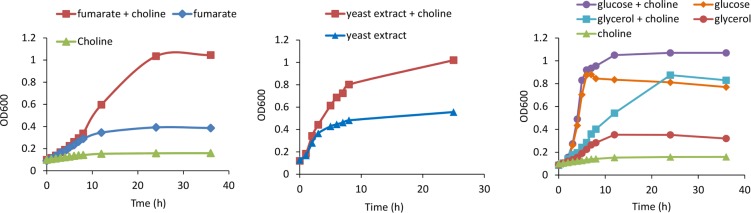
Prior bioinformatic analyses identified a choline utilization (cut) gene cluster in PAI-II536 (58, 61, 62). This cut cluster encodes a putative choline TMA lyase (CutC) which is diagnostic for choline utilization, as well as multiple homologs of MCP shell proteins, suggesting that a bacterial MCP is involved in choline degradation in E. coli 536. Growth studies were conducted to determine whether E. coli 536 is indeed capable of choline degradation. Choline did not serve as a sole carbon and energy source under aerobic or anaerobic conditions. However, under anaerobic conditions, choline simulated the growth of E. coli 536 on minimal medium that also contained fumarate or small amounts of yeast extract (0.2%) (Fig. 1). Choline also stimulated anaerobic growth of E. coli 536 on medium containing glucose and glycerol (Fig. 1). In further tests, E. coli 536 was unable to use choline as a carbon source for anaerobic respiration with nitrate, trimethylamine-N-oxide (TMAO), or dimethyl sulfoxide (DMSO) as terminal electron acceptors. As a positive control, E. coli 536 was grown on glycerol in combination with nitrate, TMAO, or DMSO as terminal electron acceptors. Growth was positive under these conditions. We also tested E. coli 536 for anaerobic respiration of choline or glycerol with tetrathionate as a terminal electron acceptor but with negative results. For these studies, Salmonella enterica serovar Typhimurium LT2 was used as a positive control and was found to grow well by anaerobic respiration of glycerol with tetrathionate as terminal electron acceptor (63).
FIG 1.
Effect of choline on anaerobic growth of E. coli 536. Cells were grown on NCE minimal medium supplemented with 1 mM MgSO4, 50 μM ferric citrate, and the following: choline (1%), fumarate (50 mM), yeast extract (0.2%), glucose (10 mM), and/or glycerol (10 mM). Cultures were incubated anaerobically in sealed tubes at 37°C with shaking. All growth curves were repeated three or more times with nearly identical results.
Choline utilization among the ECOR collection.
The E. coli Reference (ECOR) collection is a set of 72 natural isolates of E. coli intended to represent the genetic diversity of this species (64). To test the ECOR collection for the ability to use choline, MacConkey-choline indicator medium was used. On this medium, positive strains are red due to acid production from choline. Among 72 ECOR strains tested, 5 (6.9%) strains were positive for choline utilization in this test. They were ECOR 38-41 and 64 (see Fig. S1 in the supplemental material).
The five choline-positive ECOR strains were tested for the presence of the choline TMA lyase gene by PCR, as described previously (61). PCR amplification followed by DNA sequencing indicated that each positive ECOR strain had a cutC TMA lyase gene. Thus, it is likely that five ECOR strains use the choline TMA lyase pathway of choline degradation. We also note that MacConkey-choline agar provides a simple test for bacterial choline degradation that should prove helpful in various studies of choline metabolism. Even though choline metabolism occurs only in the absence of oxygen, the MacConkey-choline plates were incubated under aerobic conditions. This works because as colonies expand, their centers become anaerobic.
E. coli 536 produces bacterial microcompartments during growth on choline.
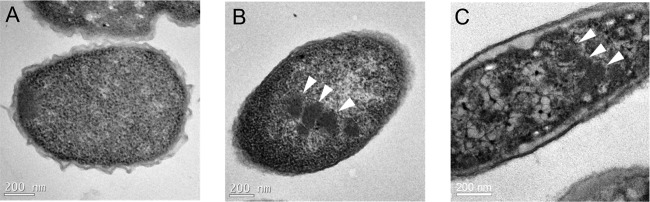
The presence of MCP structural genes in the cut operon of E. coli 536 suggested that a bacterial MCP might be involved in choline degradation in this organism. To test this possibility, E. coli 536 was grown anaerobically on LB medium with and without choline, and thin sections were prepared and examined by transmission electron microscopy. Protein complexes similar in size and appearance to bacterial MCPs were observed in cells grown in the presence of choline but not in its absence (Fig. 2).
FIG 2.
Electron microscopy images of E. coli 536 grown in the presence of choline. (A and B) E. coli 536 grown anaerobically on yeast extract (A) or yeast extract supplemented with choline (B). (C) S. enterica grown under conditions where Pdu MCPs form. Arrowheads point to the microcompartments.
Overall structure of the E. coli 536 cut operon.
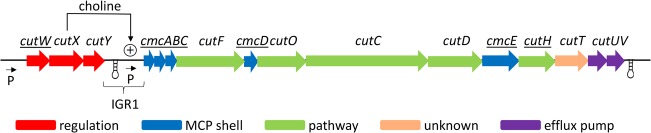
E. coli 536 has a type II choline utilization gene cluster similar to that of P. mirabilis (59) (Fig. 3). This cluster is composed of 16 genes all transcribed in the same direction. At the ends of the operon, flanking the cutW and cutV genes, are intergenic regions (the distance to the nearest ORF) of 302 and 295 bp, respectively. Each gene in the cut cluster is separated by ≤53 bp, with the exception of the cutY and cmcA genes, where there is an intergenic region of 519 bp (IGR1). The IGR1 region contains no predicted genes transcribed in the same direction as the main cut operon and only one small putative gene encoding a protein of 86 amino acids transcribed in the reverse direction of the main cut operon, based on GeneMark.hmm analyses (65). We also note that the start site of the cutD gene was likely misidentified during annotation of the genome available through the PATRIC website. Analyses with both BLASTP and the GeneMark.hmm software indicate that the start of the cutD gene is a GTG 159 bp upstream of the proposed ATG start site (65, 66). Analysis with RegRNA 2.0 (67) predicts two intrinsic terminators: one that starts 147 bp downstream of the cutY stop codon (TGAGCAAAAGCGCATTTTTT) and a second that begins 80 bp downstream of the cutV gene (GCGGCTTCCACAACAGGGAGCCGTTTTCTT). Overall, analyses of the cut gene cluster suggests the existence of two operons: one consisting of three genes, cutW-cutX-cutY, and a second main operon consisting of 13 genes, cmcA-cmcB-cmcC-cutF-cmcD-cutO-cmcC-cutD-cmcE-cutH-cutT-cutU-cutV.
FIG 3.
The cut gene cluster of E. coli 536. Bioinformatic analyses indicated that the choline utilization (cut) gene cluster of E. coli 536 is composed of two operons: a three-gene operon consisting of cutW-cutX-cutY and a main choline operon consisting of cmcA-cmcB-cmcC-cutF-cmcD-cutO-cmcC-cutD-cmcE-cutH-cutT-cutU-cutV. General gene functions are indicated by color. Underlined gene names indicate gene deletions that did not impair growth stimulation by choline (Table 1). The genes (proposed function) shown are cutW (transcriptional regulator), cutX (transcriptional regulator), cutY (regulation), cmcA, cmcB, cmcC, cmcD, and cmcE (MCP shell proteins), cutF (aldehyde dehydrogenase), cutO (alcohol dehydrogenase), cutC (choline TMA lyase), cutD (choline TMA lyase-activating enzyme), cutH (phosphotransacylase), cutT (unknown), cutU (efflux pump), and cutV (efflux pump).
For this report, we named previously unnamed choline utilization genes. We used both cut and cmc acronyms. This was done to prevent conflicts with the gene names previously used for type I choline gene clusters (61). The acronym cmc is for choline microcompartment. Each cmc gene has homology to MCP shell proteins.
Genes in the cut operon are required for choline degradation by E. coli 536.
Each gene of the cut cluster and IGR1 was deleted individually by linear recombination of PCR products (68). In each case, the entire gene (except for the ribosome binding region of the upstream gene [∼20 bp]) was deleted (68). The target gene was initially replaced with a chloramphenicol resistance marker, which was removed with the FLP recombinase, leaving behind only an FLP recombination target (FRT) site (68). This approach is designed to produce nonpolar deletions (68). In some cases, we attempted to replace the target gene with a kanamycin resistance cassette. This worked poorly in our study due to a large number of false positives.
For each mutant, anaerobic growth in liquid medium (0.2% yeast extract, 50 μM ferric citrate, and 1% choline) and on MacConkey-choline plates was used to test for the ability to utilize choline. The results of these tests are summarized in Table 1. Growth curves and MacConkey tests are shown in Fig. S2 and S3. Deletions of cutX, cutY, IGR1, cutF, cutO, cutC, cutD, cutU, and cutV eliminated choline utilization in anaerobic liquid cultures. All of these deletions also prevented the production of a red color on MacConkey-choline indicator plates, except cutO::frt and IGR1::frt, which reduced but did not eliminate color formation. Individual deletions of seven choline utilization genes (cutW, cutT, cmcA, cmcB, cmcC, cmcD, and cmcE) did not noticeably impair choline degradation in liquid medium or on MacConkey-choline indicator plates. The reasons for these various phenotypes are addressed in the Discussion.
TABLE 1.
Phenotypes of cut and cmc mutants
| Genotypea | Function of closest homolog of deleted gene | MacConkey-choline plateb | Choline utilizationc | Protein product (accession no.) |
|---|---|---|---|---|
| Wild type | + | + | ||
| ΔcutW::frt | Transcriptional regulator | + | + | WP_000926369.1 |
| ΔcutX::frt | Transcriptional regulator | − | − | WP_001270145.1 |
| ΔcutY::frt | Unknown | − | − | WP_001217008.1 |
| ΔIGR1::frt | Intergenic region (519 bp) | +/− | − | |
| ΔcmcA::frt | BMC protein | + | + | WP_000502008.1 |
| ΔcmcB::frt | BMC protein | + | + | WP_000502010.1 |
| ΔcmcC::frt | BMC protein | + | + | WP_001206281.1 |
| ΔcutF::frt | Aldehyde dehydrogenase | − | − | WP_000570989.1 |
| ΔcmcD::frt | BMV protein | + | + | WP_000599365.1 |
| ΔcutO::frt | Alcohol dehydrogenase | +/− | − | WP_001288714.1 |
| ΔcutC::frt | Choline TMA lyase | − | − | WP_000035052.1 |
| ΔcutD::frt | Choline TMA lyase-activating enzyme | − | − | WP_001275637.1 |
| ΔcmcE::frt | BMC protein | + | + | WP_001086628.1 |
| ΔcutH::frt | Phosphotransacetylase | + | + | WP_000564322.1 |
| ΔcutT::frt | Unknown | + | + | WP_000139438.1 |
| ΔcutU::frt | Efflux pump | − | − | WP_000095890.1 |
| ΔcutV::frt | Efflux pump | − | − | WP_000481835.1 |
The wild-type strain was E. coli 536.
MacConkey-choline is MacConkey agar base supplemented with 0.8% choline chloride (pH 7.0).
Choline utilization was measured as growth stimulation by choline in liquid medium containing 0.2% yeast extract, 50 μM Fe citrate, and 1% choline chloride (Fig. S2).
The cut operon of E. coli 536 is induced by choline supplementation.
To examine the regulation of the choline operon of E. coli 536, we used the cutF gene as a reporter for induction of the main cut operon (Fig. 3). The cutF gene encodes a close homolog of aldehyde dehydrogenases (ALD) whose production can be measured by a relatively easy enzyme assay (69). To test for induction of the cutF gene by choline, wild-type E. coli 536 was grown anaerobically without or with 1% choline, and ALD assays were performed (Table 2). Extracts from cells grown anaerobically without or with choline had activities of 100 ± 19 and 810 ± 121 nm · min−1 · mg−1, respectively. In contrast, controls showed that the ALD activities in a ΔcutF mutant were 70 ± 7 and 100 ± 20 nm · min−1 · mg−1 without and with choline, respectively. Together, these results indicated that choline induced expression of the cutF gene and that CutF activity would be a useful reporter for induction of the main cut operon.
TABLE 2.
Effect of selected mutations on induction of the main cut operon using the CutF aldehyde dehydrogenase as a reporter
| Strain | Aldehyde dehydrogenase (CutF) activity (nm · min−1 · mg−1)a |
|||
|---|---|---|---|---|
| Anaerobic |
Aerobic |
|||
| None | Choline | None | Choline | |
| Wild type | 100 ± 19 | 810 ± 121 | 10 ± 10 | 50 ± 21 |
| ΔcutF mutant | 70 ± 7 | 100 ± 20 | 10 ± 10 | 10 ± 5 |
| ΔcutW mutant | 70 ± 3 | 730 ± 118 | 10 ± 6 | 20 ± 5 |
| ΔcutX mutant | 70 ± 19 | 100 ± 10 | 10 ± 1 | 20 ± 15 |
| ΔcutY mutant | 60 ± 7 | 60 ± 2 | 4 ± 3 | 6 ± 4 |
Each strain was grown anaerobically or aerobically on LB, 1 mM MgSO4, and 50 μM ferric citrate without or with choline. Cell extracts were prepared, and ALD activity was measured as described in Materials and Methods.
CutX is a positive regulator of the main cut operon.
We also looked at expression of the CutF ALD in strains carrying a ΔcutW, ΔcutX, or cutY mutation (Table 2). The CutX protein has sequence similarity to transcriptional regulators. A cutX deletion mutant was grown anaerobically on medium without or with choline. Cells extracts were prepared, and the ALD activities were measured to be 70 ± 19 and 100 ± 10 nm · min−1 · mg−1, respectively. Hence, in contrast to the wild-type strain, choline did not significantly induce expression of the CutF ALD in a cutX deletion strain (Table 2). These results (in conjunction with the finding the CutX has a predicted DNA-binding domain) indicate that cutX encodes a transcriptional activator that induces the main cut operon in response to choline supplementation.
We also looked at the effects of a cutW deletion on expression of the cut operon. Like CutX, the CutW protein has sequence similarity to transcriptional regulatory proteins. However, in contrast to the results obtained with the cutX deletion, a cutW deletion did not significantly affect induction of the cut operon by choline supplementation. Extracts prepared from ΔcutW cells grown without and with choline produced 70 ± 3 and 730 ± 118 nm · min−1 · mg−1 of ALD activity compared to the wild type, which produced 100 ± 19 and 810 ± 121 nm · min−1 · mg−1 of ALD activity, respectively.
CutY is also needed for induction of the main cut operon.
Bioinformatic analyses indicate that cutW, cutX, and cutY are in the same operon (Fig. 3). Unlike CutW and CutX, the CutY protein lacks sequence similarity to known transcription factors. Hence, it was somewhat unexpected that a cutY deletion prevented induction of the cut operon by choline under anaerobic conditions (Table 2). Cell extracts prepared from ΔcutY cells grown without or with choline produced 60 ± 7 and 60 ± 2 nm · min−1 · mg−1 of ALD activity compared to the wild type, which produced 100 ± 19 and 810 ± 121 nm · min−1 · mg−1 of ALD activity. Presumably, cutY has a role in the regulation of the cut operon, and some possibilities are considered in Discussion.
The cut operon is not induced under aerobic conditions.
The effects of oxygen on induction of the cut operon were investigated (Table 2). Strains carrying ΔcutW, ΔcutX, cutY, and ΔcutF mutations as well as the wild type were grown aerobically without or with choline, cell extracts were prepared, and CutF ALD activities were measured. The activities, without and with choline, respectively, were as follows: wild type, 10 ± 10 and 50 ± 21 nm · min−1 · mg−1; ΔcutF mutant, 10 ± 10 and 10 ± 5 nm · min−1 · mg−1; ΔcutX mutant, 10 ± 1 and 20 ± 15 nm · min−1 · mg−1; ΔcutW mutant, 10 ± 6 and 20 ± 5 nm · min−1 · mg−1, and ΔcutY mutant, 4 ± 3 and 6 ± 4 nm · min−1 · mg−1. Controls showed that the CutF ALD was stable in the presence of oxygen (within experimental error, it retained >95% activity after ∼24 h in air). Hence, we infer that the cut operon is not induced substantially in the presence of oxygen and that cutW, cutX, and cutY deletions do not substantially affect aerobic expression.
Complementation studies.
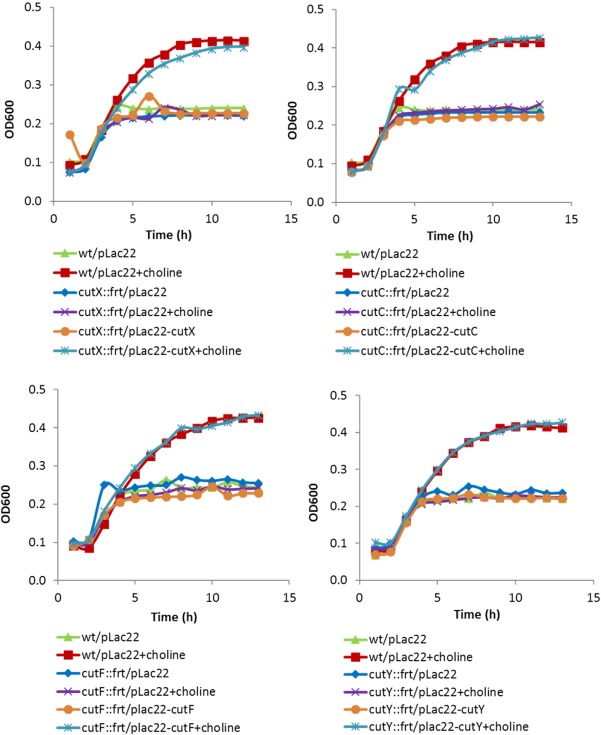
Complementation studies were performed for four key cut mutants, ΔcutC, ΔcutF, ΔcutX, and ΔcutY. For these studies, we tested whether expression of the corresponding gene from plasmid pLac22 would restore growth simulation by choline to wild-type levels. For all four mutants, full complementation was observed (Fig. 4). These results established that the phenotypes observed for the cutC (choline TMA lyase), cutF (aldehyde dehydrogenase), cutX (transcriptional regulator), and cutY deletions were a consequence of that mutation and not an unexpected mutation inadvertently introduced during strain construction. For these tests, growth curves were performed using a microplate reader inside an anaerobic chamber, as described in Materials and Methods.
FIG 4.
Complementation of key choline mutants used in this study (cutX, cutC, cutF. and cutY mutants). These growth tests were performed using a microplate reader. The growth medium used for complementation studies was NCE supplemented with 0.75% yeast extract, 175 mM NaCl, 50 μM ferric citrate, and 10 mM MgCl2, with or without 1% choline chloride (pH 7.0). Media also contained 50 μM IPTG to induce gene expression from pLac22.
DISCUSSION
A study by Craciun and Balskus identified a glycyl-radical choline TMA lyase and a cut gene cluster used for bacterial choline degradation (58). Further studies determined that cut clusters are widely but unevenly distributed across Proteobacteria, Firmicutes, Actinobacteria, and Fusobacteria (61). Two types of cut clusters were identified: one that has ∼20 genes (type I), and a second that has ∼16 genes (type II) (61). The two types include a similar complement of choline degradative enzymes, suggesting a common pathway of choline catabolism. Both also include genes for the formation of bacterial MCPs, although these genes vary substantially (58–61). Based on the gene content of the cut cluster, as well as biochemical studies, a pathway for choline degradation has been proposed (Fig. 5) (58, 59, 61). Degradation begins with choline entering the lumen of the Cut MCP, where it is converted to TMA and acetaldehyde by choline TMA lyase (CutC), supported by its activating enzyme (CutD) (58). TMA is likely excreted and not used as a nitrogen source (59). Acetaldehyde is converted to acetate and ethanol. This pathway generates 1 ATP, an electron sink (acetaldehyde), and a source of acetyl coenzyme A (acetyl-CoA). Based on analogy with the 1,2-propanediol, ethanolamine, and carboxysome MCPs, the likely function of the choline MCP is to increase pathway flux and to sequester acetaldehyde to prevent toxicity and carbon loss (11, 12, 15).
FIG 5.
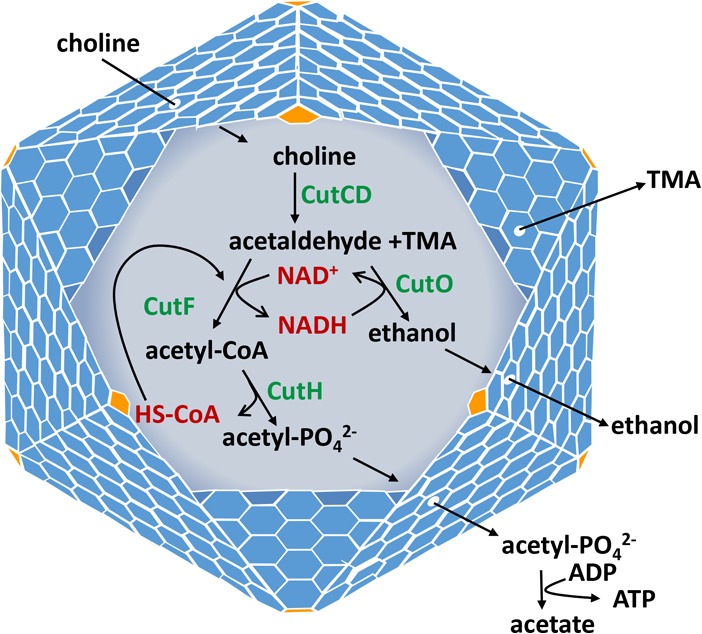
Model for the bacterial MCP used for choline degradation by E. coli 536. Choline diffuses through the protein shell and enters the lumen of the Cut MCP, where it is converted to acetaldehyde and TMA. The acetaldehyde is further metabolized to ethanol and acetate, generating 1 ATP to support growth. Based on analogy with other MCPs, its proposed function is to sequester acetaldehyde to prevent toxicity and/or diffusive loss through the cell envelope. CutCD, choline TMA lyase and its activating enzyme; CutF, acetaldehyde dehydrogenase; CutO, alcohol dehydrogenase; CutH, phosphotransacetylase; TMA, trimethylamine.
In this study, we investigated choline degradation in E. coli 536. This organism has a type II cut gene cluster. Studies showed that choline stimulated the growth of E. coli 536 on glucose, glycerol, fumarate, or small amounts of yeast extract, similar to the results obtained with P. mirabilis (59). Here, we also tested for anaerobic respiration of choline with nitrate, DMSO, and TMAO, three compounds E. coli is known to use as terminal electron acceptors (70); however, the results were negative. The results were also negative for aerobic growth on choline as a sole carbon source and for anaerobic respiration of choline with tetrathionate as terminal electron acceptor. Tetrathionate was of interest because it can serve as a terminal electron acceptor for the anaerobic respiration of ethanolamine and 1,2-propanediol, and both of these processes involve a bacterial MCP (15). Overall, the growth studies conducted here show that E. coli 536 degrades choline by fermentation and possibly by anaerobic respiration with fumarate as a terminal electron acceptor, although the latter possibility will need to be substantiated by further studies to demonstrate electron transport phosphorylation.
For this report, the organization of the cut gene cluster of E. coli 536 was examined (Fig. 3). The cut cluster then begins with two genes that encode proteins with homology to transcriptional regulators, cutW and cutX, and a third gene (cutY) that encodes a hypothetical protein. Given that cutW, cutX, and cutY coding regions are separated by 22 bp or overlap by 1 bp, respectively, we propose that the cutW, cutX, and cutY genes form an operon (Fig. 3). Downstream of cutY is a 519-bp intergenic region, followed by the cmcA-cmcB-cmcC-cutF-cmcD-cutO-cmcC-cutD-cmcE-cutH-cutT-cutU-cutV genes, which likely form the main cut operon, since the largest intergenic region here is only 52 bp (Fig. 3).
Regulation of the cut cluster was investigated using the cutF gene as a transcriptional reporter for the main cut operon. The results indicated that the main cut operon was induced by choline supplementation (Table 2). A similar result was obtained for P. mirabilis by transcriptomics, which showed that each gene in the main cut operon was induced by choline supplementation (59). Here, we expanded on prior work by looking at possible regulatory roles for CutW, CutX, and CutY (CutW and CutX both have homology to transcriptional regulators). The results showed that choline did not induce the cut operon in a ΔcutX mutant. In contrast, a ΔcutW mutant had no effect on choline induction under similar conditions. Based on these studies, we hypothesize that CutX is a positive transcriptional regulator that binds choline (or a downstream metabolite) and induces transcription of the main cut operon by binding to the intergenic region between cutY and cmcA (although no binding studies were performed here). We also found that a cutY deletion prevented induction of the main cut operon. This result was somewhat unexpected, since CutY lacks recognizable homology to transcriptional regulators. However, since both CutY and CutX are required for induction of the cut operon, these proteins might work together to mediate operon induction. The results also showed that choline does not induce the cut operon under aerobic conditions; however, the regulatory systems involved were not identified. CutW, CutX, or CutY might respond to or be inactivated by oxygen. Alternatively, oxygen control might be mediated by global regulators, such as Arc or Fnr (71, 72).
Each gene in the cut cluster of E. coli 536 was deleted individually by lambda Red recombineering (68). For each mutant, choline degradation was tested by growth on liquid medium and color formation on MacConkey-choline indicator plates (Table 1; see Fig. S2 and S3 in the supplemental material). Deletions of cutX, cutY, IGR1, cutF, cutO, cutC, cutD, cutU, and cutV eliminated growth stimulation by choline in liquid culture, indicating that these genes are required for choline degradation. The cutF, cutO, cutC, and cutD genes all encode metabolic enzymes (or homologs thereof) used to degrade choline. CutX and CutY are required for induction of the main cut operon, as described above. IGR1 likely includes cis-acting elements needed for operon induction. CutU and CutV are homologs of efflux pumps that extrude amines (73). Presumably, CutU and CutV are needed to pump TMA out of the cell to prevent inhibition, although the reasons behind TMA inhibition are currently unknown to us. A deletion of the cutH gene did not significantly reduce choline degradation. CutH has homology to phosphotransacetylase enzymes. E. coli is known to express the Pta phosphotransacetylase constitutively (74). This enzyme might be able to replace the CutH enzyme for choline degradation. However, this finding was somewhat surprising, since MCP-associated Pta enzymes are required for internal cofactor recycling in the eut and pdu MCPs, and the deletion of their encoding genes results in reduced growth on MCP substrates (75).
Deletion of each of the five MCP shell genes of the cut operon individually (cmcA, cmcB, cmcC, cmcD, and cmcE) did not noticeably impair choline degradation under the conditions used here. Based on analogy with other MCPs, the cut MCP is presumed to sequester acetaldehyde to prevent toxicity or diffusive loss (11, 12). For this study, choline degradation was measured under anaerobic conditions in sealed tubes, which would prevent diffusive loss of acetaldehyde. Furthermore, aldehyde toxicity has primarily been observed as DNA damage (12, 14), which was not measured here. Thus, it was not particularly surprising to us that the deletion of cut MCP shell genes lacked a phenotype in the studies performed here. Further work will be needed to verify the specific physiological function of the cut MCP.
Electron microscopy was used to test whether E. coli 536 forms MCPs during growth on choline. The results indicated that this organism forms MCPs in the presence of choline but not in its absence (Fig. 2). The Cut MCPs formed by E. coli 536 were similar in size and shape to the Pdu MCPs formed by S. enterica during growth on 1,2-propanediol (Fig. 2). Prior studies showed that P. mirabilis (which also has a type I gene cut gene cluster) forms MCPs during growth on choline (59). Hence, two studies have indicated that a bacterial MCP is produced for choline degradation.
To better evaluate the distribution of choline degradation among E. coli strains, we screened the ECOR collection using MacConkey agar supplemented with choline. The ECOR collection consists of 72 E. coli strains that are representative of the genetic variation of the species as a whole (64). Five of 72 ECOR strains were found to degrade choline, and all five strains also tested positive for choline TMA lyase by PCR. Two of the choline-positive ECOR strains were isolated from patients with urinary tract infections (UTIs), and three strains were from feces of healthy individuals. We know of no specific connection between choline degradation and E. coli 536 pathogenesis, although choline does induce virulence factors in enterohemorrhagic E coli (76).
MATERIALS AND METHODS
Chemicals and reagents.
Antibiotics, NAD+, and coenzyme A were from the Sigma Chemical Company (St. Louis, MO). Choice Taq Blue master mix was from Denville Scientific (Holliston, MA). KOD Hot Start master mix was from EMD Millipore (Billerica, MA). Isopropyl-β-d-1-thiogalactopyranoside (IPTG) was from Diagnostic Chemicals Limited (Charlottetown, Prince Edward Island, Canada). Restriction enzymes and T4 DNA ligase were from New England BioLabs (Beverly, MA). Bacterial protein extraction reagent (B-PERII), 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF), DNase, choline chloride, and other reagents were from Fisher Scientific (Pittsburgh, PA).
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 3. Biosafety level 2 (BSL2) precautions were used for all growth studies. The rich media used were lysogeny broth (LB), also known as Luria-Bertani medium (Becton, Dickinson and Company, Franklin Lakes, NJ) (77), and Terrific broth (MP Biomedicals, Solon, OH). MacConkey-choline indicator plates were prepared using a Difco MacConkey agar base supplemented with 0.8% choline chloride from a 50% stock solution that was adjusted to pH 7.0. The liquid medium used was no-carbon-E (NCE) medium (78) supplemented with 1 mM MgSO4, 50 μM ferric citrate, and one or more of the following: 1% choline chloride (pH 7.0), 0.2% yeast extract, 50 mM disodium fumarate (pH 7.0), 10 mM glucose, and/or 10 mM glycerol. Anaerobic growth curves were performed using 18 by 150-mm serum tubes sealed with butyl rubber stoppers. Serum tubes (containing 6 ml of medium) were inoculated to 120 μl of overnight culture grown aerobically on LB supplemented with 10 mM MgSO4. Tubes were moved inside an anaerobic chamber (Coy Laboratory Products, Grass Lake, MI) allowed to degas, sealed, and removed. The sealed tubes were incubated at 37°C with shaking at 275 rpm in an Innova 2400 shaker (New Brunswick Scientific). Optical density was measured using a Spectronic 20D+ spectrophotometer (Thermo Fisher Scientific). For complementation studies, growth curves were determined using a Synergy HT microplate reader (BioTek, Winooski, VT) as previously described (79), with the modification that the microplate reader was placed inside an anaerobic chamber. The growth medium used for complementation studies was NCE supplemented with 0.75% yeast extract, 175 mM NaCl, 50 μM ferric citrate, and 10 mM MgCl2, with or without 1% choline chloride (pH 7.0).
TABLE 3.
Strains used in this study
| Straina | Genotype | Source |
|---|---|---|
| E. coli 536 (BE2278) | Wild type | Gift from Harry L. T. Mobley |
| BE2346 | ΔcutW::frt | This study |
| BE2309 | ΔcutX::frt | This study |
| BE2348 | ΔcutY::frt | This study |
| BE2310 | ΔIGR1::frt | This study |
| BE2354 | ΔcmcA::frt | This study |
| BE2306 | ΔcmcB::frt | This study |
| BE2308 | ΔcmcC::frt | This study |
| BE2307 | ΔcutF::frt | This study |
| BE2352 | ΔcmcD::frt | This study |
| BE2305 | ΔcutO::frt | This study |
| BE2433 | ΔcutC::frt | This study |
| BE2362 | ΔcutD::frt | This study |
| BE2350 | ΔcmcE::frt | This study |
| BE2358 | ΔcutH::frt | This study |
| BE2356 | ΔcutT::frt | This study |
| BE2364 | ΔcutU::frt | This study |
| BE2360 | ΔcutV::frt | This study |
| BE2531 | E. coli 536/pLac22 | This study |
| BE2532 | ΔcutX::frt/pLac22-cutX | This study |
| BE2533 | ΔcutX::frt/pLac22 | This study |
| BE2534 | ΔcutC::frt/pLac22-cutC | This study |
| BE2535 | ΔcutC::frt/pLac22 | This study |
| BE2556 | ΔcutF::frt/pLac22-cutF | This study |
| BE2557 | ΔcutF::frt/pLac22 | This study |
All mutants used in this study are derivatives of E. coli 536.
Construction of chromosomal mutations.
Chromosomal deletions were made using the lambda Red recombinase, as described previously (68, 79). Chloramphenicol resistance was selected (kanamycin selection gave many false-positive colonies). Chloramphenicol resistance markers were removed using the FLP recombinase, as described previously (68). This leaves behind an 82-nucleotide scar (an FRT site). All mutations were verified by colony PCR amplification across the scar, followed by DNA sequencing.
Electron microscopy.
Double-strength LB medium (40 g/liter) supplemented with 10 mM MgSO4 and 50 μM ferric citrate was dispensed into two sterile test tubes (2 ml/tube), and one tube was supplemented with 1% choline chloride. Both tubes were inoculated with 20 μl of an aerobic LB overnight culture of E. coli 536 and incubated statically for 8 h in an anaerobic chamber. Inside an anaerobic chamber, 300 μl of this starter culture was used to inoculate 30 ml of similar medium in 50-ml sterile tubes. These cultures were incubated in the anaerobic chamber for ∼16 h at 37°C, statically. While still working inside the anaerobic chamber, cells were dispensed into 50-ml centrifuge tubes, capped tightly, and centrifuged at 10,000 × g using a Beckman JA-10 rotor. After centrifugation, the supernatants were decanted inside that anaerobic chamber. Cells were removed from the chamber, quickly resuspended in 2% paraformaldehyde plus 0.3% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2), and incubated for 30 min at room temperature. After fixation, cells were washed three times with 0.1 M cacodylate buffer (pH 7.2). Imbedding, sectioning, and electron microscopy were carried out as described previously (79).
Enzyme assays.
Overnight cultures (aerobic) were prepared in 3 ml of LB and 10 mM MgSO4 and incubated overnight at 37°C with shaking at 275 rpm. This culture (500 μl) was used to inoculate 50 ml of LB, 1 mM MgSO4, and 50 μM ferric citrate, with and without 1% choline chloride. Cells were incubated anaerobically at 37°C for ∼5 h to an optical density at 600 nm (OD600) of ∼1.0 or aerobically at 37°C for ∼2.5 h to an OD600 of ∼1.0. Cells were harvested by centrifugation at 5,000 × g for 10 min using a JA7.5 rotor and a Beckman Avanti J-25 centrifuge. The cells were washed with 10 ml of 50 mM Tris (pH 8.0), 300 mM NaCl, and 5% glycerol. Cells were lysed with 1 ml of 50% B-PERII in 100 mM Tris (pH 8.0), 300 mM KCl, 10 mM MgCl2, 1 mg/ml lysozyme, 0.04 mM AEBSF, and 0.05 mg/ml DNase. The lysate was centrifuged at 15,000 × g for 10 min at 4°C in a 5417R microcentrifuge (Eppendorf). The supernatant was collected, and its protein concentration was determined using Bio-Rad protein assay reagent and used for enzyme assays. Aldehyde dehydrogenase assays were performed by following the increase in absorbance at 340 nM in assay mixtures containing 1 mm NAD+, 0.5 mM coenzyme A, 1 mM dithiothreitol, 35 mM potassium phosphate (pH 8.0), 50 mM KCl, and an appropriate amount of enzyme, as previously described (69). Quantification was based on ε340 of 6.22 mM−1 · cm−1.
Molecular biology methods.
Agarose gel electrophoresis, plasmid purification, PCR, restriction digestion, ligation reactions, and electroporation were carried out using standard protocols, as described previously (16, 80). Taq or Phusion DNA polymerase (New England BioLabs) was used for amplification of chromosomal DNA and colony PCR. KOD DNA polymerase was used for amplification of plasmid templates. Plasmid DNA was purified using Qiagen products (Chatsworth, CA), according to the manufacturer's instructions. Following restriction digestion or PCR amplification, DNA was purified using Promega Wizard PCR preps (Madison, WI) or Qiagen gel extraction kits. For ligation of DNA fragments, T4 DNA ligase or NEBuilder HiFi DNA assembly master mix was used according to the manufacturer's instructions (New England BioLabs).
Complementation studies.
Genes used for complementation were cloned into plasmid pLac22, which allows for tight regulation of cloned genes by IPTG (81). Chromosomal genes were amplified using Phusion DNA polymerase and cloned between the BglII and HinDIII sites of pLac22. Cloning was accomplished either by designing PCR primers with BglII and HinDIII sites or ∼20 bp of homology for ligation with T4 DNA ligase or by Gibson assembly, respectively. Positive clones were identified by colony PCR, and the DNA sequences of all clones were verified.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grant AI081146 from the National Institutes of Health to T.A.B.
We thank Harry L. T. Mobley and Stephanie Himpsl for kindly providing E. coli 536. We also thank the ISU DNA Sequencing and Synthesis Facility for assistance with DNA analyses and the ISU Microscopy and Nanoimaging facility for help with electron microscopy.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00764-17.
REFERENCES
- 1.Abdul-Rahman F, Petit E, Blanchard JL. 2013. The distribution of polyhedral bacterial microcompartments suggests frequent horizontal transfer and operon reassembly. J Phylogenetics Evol Biol 1:1–7. [Google Scholar]
- 2.Axen SD, Erbilgin O, Kerfeld CA. 2014. A taxonomy of bacterial microcompartment loci constructed by a novel scoring method. PLoS Comput Biol 10:e1003898. doi: 10.1371/journal.pcbi.1003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jorda J, Lopez D, Wheatley NM, Yeates TO. 2013. Using comparative genomics to uncover new kinds of protein-based metabolic organelles in bacteria. Protein Sci 22:179–195. doi: 10.1002/pro.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zarzycki J, Erbilgin O, Kerfeld CA. 2015. Bioinformatic characterization of glycyl radical enzyme-associated bacterial microcompartments. Appl Environ Microbiol 81:8315–8329. doi: 10.1128/AEM.02587-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bobik TA. 2006. Polyhedral organelles compartmenting bacterial metabolic processes. Appl Microbiol Biotechnol 70:517–525. doi: 10.1007/s00253-005-0295-0. [DOI] [PubMed] [Google Scholar]
- 6.Bobik TA, Lehman BP, Yeates TO. 2015. Bacterial microcompartments: widespread prokaryotic organelles for isolation and optimization of metabolic pathways. Mol Microbiol 98:193–207. doi: 10.1111/mmi.13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chowdhury C, Sinha S, Chun S, Yeates TO, Bobik TA. 2014. Diverse bacterial microcompartment organelles. Microbiol Mol Biol Rev 78:438–468. doi: 10.1128/MMBR.00009-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rae BD, Long BM, Badger MR, Price GD. 2013. Functions, compositions, and evolution of the two types of carboxysomes: polyhedral microcompartments that facilitate CO2 fixation in cyanobacteria and some proteobacteria. Microbiol Mol Biol Rev 77:357–379. doi: 10.1128/MMBR.00061-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerfeld CA, Heinhorst S, Cannon GC. 2010. Bacterial microcompartments. Annu Rev Microbiol 64:391–408. doi: 10.1146/annurev.micro.112408.134211. [DOI] [PubMed] [Google Scholar]
- 10.Havemann GD, Sampson EM, Bobik TA. 2002. PduA is a shell protein of polyhedral organelles involved in coenzyme B(12)-dependent degradation of 1,2-propanediol in Salmonella enterica serovar Typhimurium LT2. J Bacteriol 184:1253–1261. doi: 10.1128/JB.184.5.1253-1261.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penrod JT, Roth JR. 2006. Conserving a volatile metabolite: a role for carboxysome-like organelles in Salmonella enterica. J Bacteriol 188:2865–2874. doi: 10.1128/JB.188.8.2865-2874.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sampson EM, Bobik TA. 2008. Microcompartments for B12-dependent 1,2-propanediol degradation provide protection from DNA and cellular damage by a reactive metabolic intermediate. J Bacteriol 190:2966–2971. doi: 10.1128/JB.01925-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brinsmade SR, Paldon T, Escalante-Semerena JC. 2005. Minimal functions and physiological conditions required for growth of Salmonella enterica on ethanolamine in the absence of the metabolosome. J Bacteriol 187:8039–8046. doi: 10.1128/JB.187.23.8039-8046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rondon RR, Horswill AR, Escalante-Semerena JC. 1995. DNA polymerase I function is required for the utilization of ethanolamine, 1,2-propanediol, and propionate by Salmonella Typhimurium LT2. J Bacteriol 177:7119–7124. doi: 10.1128/jb.177.24.7119-7124.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price GD, Badger MR. 1989. Isolation and characterization of high CO2-requiring-mutants of the cyanobacterium Synechococcus PCC7942: two phenotypes that accumulate inorganic carbon but are apparently unable to generate CO2 within the carboxysome. Plant Physiol 91:514–525. doi: 10.1104/pp.91.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bobik TA, Havemann GD, Busch RJ, Williams DS, Aldrich HC. 1999. The propanediol utilization (pdu) operon of Salmonella enterica serovar Typhimurium LT2 includes genes necessary for formation of polyhedral organelles involved in coenzyme B12-dependent 1,2-propanediol degradation. J Bacteriol 181:5967–5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kofoid E, Rappleye C, Stojiljkovic I, Roth J. 1999. The 17-gene ethanolamine (eut) operon of Salmonella typhimurium encodes five homologues of carboxysome shell proteins. J Bacteriol 181:5317–5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erbilgin O, McDonald KL, Kerfeld CA. 2014. Characterization of a planctomycetal organelle: a novel bacterial microcompartment for the aerobic degradation of plant saccharides. Appl Environ Microbiol 80:2193–2205. doi: 10.1128/AEM.03887-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sriramulu DD, Liang M, Hernandez-Romero D, Raux-Deery E, Lunsdorf H, Parsons JB, Warren MJ, Prentice MB. 2008. Lactobacillus reuteri DSM 20016 produces cobalamin-dependent diol dehydratase in metabolosomes and metabolizes 1,2-propanediol by disproportionation. J Bacteriol 190:4559–4567. doi: 10.1128/JB.01535-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shively JM, Ball F, Brown DH, Saunders RE. 1973. Functional organelles in prokaryotes: polyhedral inclusions (carboxysomes) of Thiobacillus neapolitanus. Science 182:584–586. doi: 10.1126/science.182.4112.584. [DOI] [PubMed] [Google Scholar]
- 21.Talarico TL, Casas IA, Chung TC, Dobrogosz WJ. 1988. Production and isolation of reuterin, a growth inhibitor produced by Lactobacillus reuteri. Antimicrob Agents Chemother 32:1854–1858. doi: 10.1128/AAC.32.12.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petit E, LaTouf WG, Coppi MV, Warnick TA, Currie D, Romashko I, Deshpande S, Haas K, Alvelo-Maurosa JG, Wardman C, Schnell DJ, Leschine SB, Blanchard JL. 2013. Involvement of a bacterial microcompartment in the metabolism of fucose and rhamnose by Clostridium phytofermentans. PLoS One 8:e54337. doi: 10.1371/journal.pone.0054337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchrieser C, Rusniok C, Kunst F, Cossart P, Glaser P, Listeria Consortium. 2003. Comparison of the genome sequences of Listeria monocytogenes and Listeria innocua: clues for evolution and pathogenicity. FEMS Immunol Med Microbiol 35:207–213. doi: 10.1016/S0928-8244(02)00448-0. [DOI] [PubMed] [Google Scholar]
- 24.Conner CP, Heithoff DM, Julio SM, Sinsheimer RL, Mahan MJ. 1998. Differential patterns of acquired virulence genes distinguish Salmonella strains. Proc Natl Acad Sci U S A 95:4641–4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heithoff DM, Conner CP, Hentschel U, Govantes F, Hanna PC, Mahan MJ. 1999. Coordinate intracellular expression of Salmonella genes induced during infection. J Bacteriol 181:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joseph B, Przybilla K, Stuhler C, Schauer K, Slaghuis J, Fuchs TM, Goebel W. 2006. Identification of Listeria monocytogenes genes contributing to intracellular replication by expression profiling and mutant screening. J Bacteriol 188:556–568. doi: 10.1128/JB.188.2.556-568.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. 2013. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. 2011. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karlsson FH, Fak F, Nookaew I, Tremaroli V, Fagerberg B, Petranovic D, Backhed F, Nielsen J. 2012. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun 3:1245. doi: 10.1038/ncomms2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bae S, Ulrich CM, Neuhouser ML, Malysheva O, Bailey LB, Xiao L, Brown EC, Cushing-Haugen KL, Zheng Y, Cheng TY, Miller JW, Green R, Lane DS, Beresford SA, Caudill MA. 2014. Plasma choline metabolites and colorectal cancer risk in the Women's Health Initiative Observational Study. Cancer Res 74:7442–7452. doi: 10.1158/0008-5472.CAN-14-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frank S, Lawrence AD, Prentice MB, Warren MJ. 2013. Bacterial microcompartments moving into a synthetic biological world. J Biotechnol 163:273–279. doi: 10.1016/j.jbiotec.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Kim EY, Tullman-Ercek D. 2013. Engineering nanoscale protein compartments for synthetic organelles. Curr Opin Biotechnol 24:627–632. doi: 10.1016/j.copbio.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Orner BP. (ed). 2015. Protein cages: methods and protocols. Springer, New York, NY. [Google Scholar]
- 34.Tsai SJ, Yeates TO. 2011. Bacterial microcompartments insights into the structure, mechanism, and engineering applications. Prog Mol Biol Transl Sci 103:1–20. doi: 10.1016/B978-0-12-415906-8.00008-X. [DOI] [PubMed] [Google Scholar]
- 35.Held M, Kolb A, Perdue S, Hsu SY, Bloch SE, Quin MB, Schmidt-Dannert C. 2016. Engineering formation of multiple recombinant Eut protein nanocompartments in E. coli. Sci Rep 6:24359. doi: 10.1038/srep24359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yung MC, Bourguet FA, Carpenter TS, Coleman MA. 2017. Re-directing bacterial microcompartment systems to enhance recombinant expression of lysis protein E from bacteriophage ϕX174 in Escherichia coli. Microb Cell Fact 16:71. doi: 10.1186/s12934-017-0685-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawrence AD, Frank S, Newnham S, Lee MJ, Brown IR, Xue WF, Rowe ML, Mulvihill DP, Prentice MB, Howard MJ, Warren MJ. 2014. Solution structure of a bacterial microcompartment targeting peptide and its application in the construction of an ethanol bioreactor. ACS Synth Biol 3:454–465. doi: 10.1021/sb4001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quin MB, Perdue SA, Hsu SY, Schmidt-Dannert C. 2016. Encapsulation of multiple cargo proteins within recombinant Eut nanocompartments. Appl Microbiol Biotechnol 100:9187–9200. doi: 10.1007/s00253-016-7737-8. [DOI] [PubMed] [Google Scholar]
- 39.Lassila JK, Bernstein SL, Kinney JN, Axen SD, Kerfeld CA. 2014. Assembly of robust bacterial microcompartment shells using building blocks from an organelle of unknown function. J Mol Biol 426:2217–2228. doi: 10.1016/j.jmb.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez-Esquer CR, Newnham SE, Kerfeld CA. 2016. Bacterial microcompartments as metabolic modules for plant synthetic biology. Plant J 87:66–75. doi: 10.1111/tpj.13166. [DOI] [PubMed] [Google Scholar]
- 41.Young EJ, Burton R, Mahalik JP, Sumpter BG, Fuentes-Cabrera M, Kerfeld CA, Ducat DC. 2017. Engineering the bacterial microcompartment domain for molecular scaffolding applications. Front Microbiol 8:1441. doi: 10.3389/fmicb.2017.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Held M, Quin MB, Schmidt-Dannert C. 2013. Eut bacterial microcompartments: insights into their function, structure, and bioengineering applications. J Mol Microbiol Biotechnol 23:308–320. doi: 10.1159/000351343. [DOI] [PubMed] [Google Scholar]
- 43.Kerfeld CA, Sawaya MR, Tanaka S, Nguyen CV, Phillips M, Beeby M, Yeates TO. 2005. Protein structures forming the shell of primitive bacterial organelles. Science 309:936–938. doi: 10.1126/science.1113397. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka S, Kerfeld CA, Sawaya MR, Cai F, Heinhorst S, Cannon GC, Yeates TO. 2008. Atomic-level models of the bacterial carboxysome shell. Science 319:1083–1086. doi: 10.1126/science.1151458. [DOI] [PubMed] [Google Scholar]
- 45.Sutter M, Greber B, Aussignargues C, Kerfeld CA. 2017. Assembly principles and structure of a 6.5-MDa bacterial microcompartment shell. Science 356:1293–1297. doi: 10.1126/science.aan3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeates TO, Jorda J, Bobik TA. 2013. The shells of BMC-type microcompartment organelles in bacteria. J Mol Microbiol Biotechnol 23:290–299. doi: 10.1159/000351347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wheatley NM, Gidaniyan SD, Liu Y, Cascio D, Yeates TO. 2013. Bacterial microcompartment shells of diverse functional types possess pentameric vertex proteins. Protein Sci 22:660–665. doi: 10.1002/pro.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park J, Chun S, Bobik TA, Houk KN, Yeates TO. 2017. Molecular dynamics simulations of selective metabolite transport across the propanediol bacterial microcompartment shell. J Phys Chem B 121:8149–8154. doi: 10.1021/acs.jpcb.7b07232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chowdhury C, Chun S, Pang A, Sawaya MR, Sinha S, Yeates TO, Bobik TA. 2015. Selective molecular transport through the protein shell of a bacterial microcompartment organelle. Proc Natl Acad Sci U S A 112:2990–2995. doi: 10.1073/pnas.1423672112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crowley CS, Cascio D, Sawaya MR, Kopstein JS, Bobik TA, Yeates TO. 2010. Structural insight into the mechanisms of transport across the Salmonella enterica Pdu microcompartment shell. J Biol Chem 285:37838–37846. doi: 10.1074/jbc.M110.160580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pang A, Warren MJ, Pickersgill RW. 2011. Structure of PduT, a trimeric bacterial microcompartment protein with a 4Fe-4S cluster-binding site. Acta Crystallogr D Biol Crystallogr 67:91–96. doi: 10.1107/S0907444910050201. [DOI] [PubMed] [Google Scholar]
- 52.Cai F, Sutter M, Cameron JC, Stanley DN, Kinney JN, Kerfeld CA. 2013. The structure of CcmP, a tandem bacterial microcompartment domain protein from the beta-carboxysome, forms a subcompartment within a microcompartment. J Biol Chem 288:16055–16063. doi: 10.1074/jbc.M113.456897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klein MG, Zwart P, Bagby SC, Cai F, Chisholm SW, Heinhorst S, Cannon GC, Kerfeld CA. 2009. Identification and structural analysis of a novel carboxysome shell protein with implications for metabolite transport. J Mol Biol 392:319–333. doi: 10.1016/j.jmb.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka S, Sawaya MR, Phillips M, Yeates TO. 2009. Insights from multiple structures of the shell proteins from the beta-carboxysome. Protein Sci 18:108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson MC, Cascio D, Leibly DJ, Yeates TO. 2015. An allosteric model for control of pore opening by substrate binding in the EutL microcompartment shell protein. Protein Sci 24:956–975. doi: 10.1002/pro.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thompson MC, Crowley CS, Kopstein J, Bobik TA, Yeates TO. 2014. Structure of a bacterial microcompartment shell protein bound to a cobalamin cofactor. Acta Crystallogr F Struct Biol Commun 70:1584–1590. doi: 10.1107/S2053230X1402158X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takenoya M, Nikolakakis K, Sagermann M. 2010. Crystallographic insights into the pore structures and mechanisms of the EutL and EutM shell proteins of the ethanolamine-utilizing microcompartment of Escherichia coli. J Bacteriol 192:6056–6063. doi: 10.1128/JB.00652-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Craciun S, Balskus EP. 2012. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci U S A 109:21307–21312. doi: 10.1073/pnas.1215689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jameson E, Fu T, Brown IR, Paszkiewicz K, Purdy KJ, Frank S, Chen Y. 2016. Anaerobic choline metabolism in microcompartments promotes growth and swarming of Proteus mirabilis. Environ Microbiol 18:2886–2898. doi: 10.1111/1462-2920.13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuehl JV, Price MN, Ray J, Wetmore KM, Esquivel Z, Kazakov AE, Nguyen M, Kuehn R, Davis RW, Hazen TC, Arkin AP, Deutschbauer A. 2014. Functional genomics with a comprehensive library of transposon mutants for the sulfate-reducing bacterium Desulfovibrio alaskensis G20. mBio 5:e01041-14. doi: 10.1128/mBio.01041-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martínez-del Campo A, Bodea S, Hamer HA, Marks JA, Haiser HJ, Turnbaugh PJ, Balskus EP. 2015. Characterization and detection of a widely distributed gene cluster that predicts anaerobic choline utilization by human gut bacteria. mBio 6:e00042-15. doi: 10.1128/mBio.00042-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dobrindt U, Blum-Oehler G, Nagy G, Schneider G, Johann A, Gottschalk G, Hacker J. 2002. Genetic structure and distribution of four pathogenicity islands (PAI I536 to PAI IV536) of uropathogenic Escherichia coli strain 536. Infect Immun 70:6365–6372. doi: 10.1128/IAI.70.11.6365-6372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Price-Carter M, Tingey J, Bobik TA, Roth JR. 2001. The alternative electron acceptor tetrathionate supports B12-dependent anaerobic growth of Salmonella enterica serovar Typhimurium on ethanolamine or 1,2-propanediol. J Bacteriol 183:2463–2475. doi: 10.1128/JB.183.8.2463-2475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ochman H, Selander RK. 1984. Standard reference strains of Escherichia coli from natural populations. J Bacteriol 157:690–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lukashin AV, Borodovsky M. 1998. GeneMark.hmm: new solutions for gene finding. Nucleic Acids Res 26:1107–1115. doi: 10.1093/nar/26.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 67.Chang TH, Huang HY, Hsu JB, Weng SL, Horng JT, Huang HD. 2013. An enhanced computational platform for investigating the roles of regulatory RNA and for identifying functional RNA motifs. BMC Bioinformatics 14(Suppl 2):S4. doi: 10.1186/1471-2105-14-S2-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leal NA, Havemann GD, Bobik TA. 2003. PduP is a coenzyme-a-acylating propionaldehyde dehydrogenase associated with the polyhedral bodies involved in B12-dependent 1,2-propanediol degradation by Salmonella enterica serovar Typhimurium LT2. Arch Microbiol 180:353–361. doi: 10.1007/s00203-003-0601-0. [DOI] [PubMed] [Google Scholar]
- 70.Unden G, Bongaerts J. 1997. Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochim Biophys Acta 1320:217–234. doi: 10.1016/S0005-2728(97)00034-0. [DOI] [PubMed] [Google Scholar]
- 71.Iuchi S, Lin EC. 1988. arcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc Natl Acad Sci U S A 85:1888–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shaw DJ, Guest JR. 1982. Amplification and product identification of the fnr gene of Escherichia coli. J Gen Microbiol 128:2221–2228. [DOI] [PubMed] [Google Scholar]
- 73.Kücken D, Feucht H, Kaulfers P. 2000. Association of qacE and qacEDelta1 with multiple resistance to antibiotics and antiseptics in clinical isolates of Gram-negative bacteria. FEMS Microbiol Lett 183:95–98. [DOI] [PubMed] [Google Scholar]
- 74.Wolfe AJ. 2005. The acetate switch. Microbiol Mol Biol Rev 69:12–50. doi: 10.1128/MMBR.69.1.12-50.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y, Jorda J, Yeates TO, Bobik TA. 2015. The PduL phosphotransacylase is used to recycle coenzyme A within the Pdu microcompartment. J Bacteriol 197:2392–2399. doi: 10.1128/JB.00056-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gonyar LA, Kendall MM. 2014. Ethanolamine and choline promote expression of putative and characterized fimbriae in enterohemorrhagic Escherichia coli O157:H7. Infect Immun 82:193–201. doi: 10.1128/IAI.00980-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bertani G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol 62:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berkowitz D, Hushon JM, Whitfield HJ Jr, Roth J, Ames BN. 1968. Procedure for identifying nonsense mutations. J Bacteriol 96:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sinha S, Cheng S, Sung YW, McNamara DE, Sawaya MR, Yeates TO, Bobik TA. 2014. Alanine scanning mutagenesis identifies an asparagine-arginine-lysine triad essential to assembly of the shell of the Pdu microcompartment. J Mol Biol 426:2328–2345. doi: 10.1016/j.jmb.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 81.Warren JW, Walker JR, Roth JR, Altman E. 2000. Construction and characterization of a highly regulable expression vector, pLAC11, and its multipurpose derivatives, pLAC22 and pLAC33. Plasmid 44:138–151. doi: 10.1006/plas.2000.1477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.