Abstract
Objective
Thyroid orbitopathy is a poorly understood extrathyroidal manifestation of Graves' disease that can cause disfiguring proptosis and vision loss. Orbital decompression surgery for Graves' orbitopathy (GO) can address both cosmetic and visual sequelae of this autoimmune condition. Endonasal endoscopic orbital decompression provides unmatched visualization and access to inferomedial orbital wall and orbital apex. This review examines the state of the art approaches employed in endonasal endoscopic orbital decompression for GO.
Methods
Review of literature evaluating novel surgical maneuvers for GO.
Results
Studies examining the efficacy of endonasal endoscopic orbital decompression are heterogenous and retrospective in design; however, they reveal this approach to be a safe and effective technique in the management of GO.
Conclusion
Subtle variations in endoscopic techniques significantly affect postsurgical outcomes and can be tailored to the specific clinical indication in GO making endonasal endoscopic decompression the most versatile approach available.
Level of Evidence
NA.
Keywords: Orbital decompression, endoscopic orbital surgery, Graves' ophthalmopathy, proptosis
INTRODUCTION
Graves' orbitopathy (GO), occurring in up to 50% of patients, represents the most common extrathyroidal manifestation of Graves' disease.1 The proptosis results from infiltration and deposition of collagen and glycosaminoglycan in the retrobulbar and extra‐ocular connective tissues. Edema, vascular congestion, fatty hyperproliferation, muscular hypertrophy and fibrosis increase intraorbital volume and pressure. Clinical ramifications include severe proptosis, lid retraction with exposure keratitis, diplopia, compressive myopathy, and in severe cases, compressive optic neuropathy (CON) and vision loss. GO is typically characterized by two phases—an active inflammatory phase followed by a chronic, or fibrotic, phase.2
The 2016 European Group on Graves' Orbitopathy (EUOGO) guidelines for GO management recommend high‐dose IV glucocorticoids as first line therapy for moderate‐to‐severe and active GO.3 Rituximab, radiotherapy, and cyclosporine represent second‐line options. Indications for orbital decompression surgery include urgent vision‐threatening optic neuropathy in active‐disease phase and elective decompression for mild exposure keratitis, diplopia, and disfiguring exophthalmos after six months of disease inactivity.3 Leong et al. performed a systematic review including studies from 1990 to 2008 and found that the most common indications for surgery were cosmesis (42.4%), CON (40.6%), and exposure keratitis (7.9%).4
Orbital decompression surgery was first described by Dollinger in 1911 and performed through a lateral approach.5 Since then, multiple variations of the surgery aimed at increasing orbital volume and reducing compressive effects on the orbital structures and ophthalmic nerve by removing the surrounding bony walls, periorbita and/or fat have been developed (Fig. 1). The superior orbital roof decompression has been avoided due to the potential complications of cerebrospinal fluid leak and pulsating proptosis resulting from transmission of intracranial pressure changes. The trans‐antral technique which removes the inferior and medial orbital walls via a Caldwell‐Luc antrostomy was popularized by Walsh and Ogura in the 1950s.6 While effective a reducing proptosis, bothersome complications like permanent infraorbital nerve hypesthesia, oroantral fistula, facial swelling, and dental issues were not uncommon.7, 8, 9, 10 Furthermore, post‐operative diplopia rates reached 65% due to the “setting sun” phenomenon caused by hypoglobus.11 Similar complications plague so‐called “minimal access” external approaches, including transconjunctival, transpalpebral, and transcaruncular approaches.12, 13, 14
Figure 1.
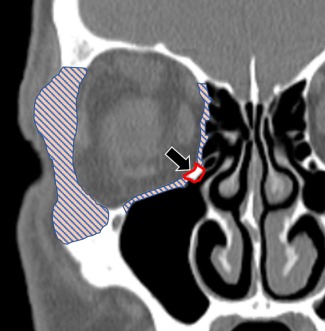
Coronal CT scan showing areas of orbital decompression as represented by the pattern filled area. The black arrow points to the inferomedial orbital strut (outlined) that is preserved by many authors to reduce diplopia.
The implementation of endonasal endoscopic techniques in orbital decompression surgery has allowed easier access and superior visualization of the medial and inferomedial orbital walls while also avoiding undesirable complications attendant with external approaches. Still, inferomedial orbital wall decompression is associated with some of the highest rates of postoperative diplopia.15 As such, endoscopic approaches alone can also result in postoperative, new‐onset diplopia as seen with other techniques. Endoscopic decompression can also be a component of a “balanced decompression,” where the medial and lateral walls (2‐wall decompression) or the medial, lateral, and inferior walls (3‐wall decompression) are removed with or without fat. A balanced decompression can offset the globe's shift in axis and reduce the incidence of postoperative diplopia and dystopia.5, 13 Graham et al. showed that balanced decompression of the medial and lateral walls could reduce new‐onset diplopia rates to as low as 10%.13
This review will focus on the latest advances in endonasal endoscopic techniques that allow for more precise and individualized decompression surgery with improved outcomes. We performed a comprehensive literature search for studies evaluating variations of endoscopic orbital decompression techniques specifically for Graves' orbitopathy using the PubMed database. Search terms included combinations of the following phrases: “endoscopic”; “orbital decompression”; “Graves orbitopathy”; “thyroid eye disease”; and “thyroid orbitopathy”. Additionally, each article's references were reviewed to identify additional publications that were not retrieved via the above search terminology. Studies written in English published up to September 2017 were reviewed. Summary of findings compiled in Table 1.
Table 1.
Summary of Outcomes from Selected Studies Employing Different Endoscopic Decompression Techniques. Reduction in proptosis values were reported as a mean. CON = compressive optic neuropathy; IOS = inferomedial orbital strut; NR = not reported.
| Technique details | Reduction in proptosis (mm) | Diplopia resolution | New‐onset diplopia | Reported complications | Citations |
|---|---|---|---|---|---|
| Endoscopic 2‐wall decompression without IOS preservation | |||||
| Endo medial and inferior wall removal | 3.06–4.7 | 0–5% | 20–50% | Epiphora, acute sinusitis34 | 21, 34, 35 |
| Endoscopic 2‐wall decompression with IOS preservation | |||||
| Endo medial and inferior wall removal | 1.63–4.6 | 0–12% | 0–47% | Periorbital hematoma36 | 19, 21, 36 |
| Endoscopic balanced 3‐wall decompression with IOS preserved | |||||
| Combined endo and external medial, inferior and lateral wall removal | 3.4–5.0 | 11–44% | 0–16% | V2 anesthesia, corneal abrasion15, epistaxis, epiphora, sinusitis, chemosis17 | 15, 17, 21 |
| Endoscopic sling preservation | |||||
| Endo preservation of medial periorbital sling as part of combined, balanced approach | 5.1 | 50–57% | 0–6% | See Yao et al.17 | 16, 17 |
| Endoscopic orbital fat decompression | |||||
|
Endo medial wall and intraconal fat removal IOS and sling preserved |
6.2 | NR | 0% | Epistaxis | 32 |
| Endoscopic selective decompression for CON | |||||
|
Posterior medial wall decompression IOS preserved some with floor removal |
2.2–3.1 | NR28, 6.7%15 | 0% | See Kingdom et al.15 | 15, 29 |
The Endoscopic Orbital Sling Technique
Citing a high incidence of postoperative diplopia with endoscopic techniques, Metson et al. introduced the orbital sling technique. The authors describe leaving a 1 cm strip of periorbita along the entire length of the medial wall dissection to support the medial rectus and prevent its prolapse (Fig. 2). The initial study population included 13 patients undergoing decompression for exposure keratitis or cosmesis and 24 historical controls who had not undergone a sling technique decompression. All patients underwent a standard total ethmoidectomy, maxillary antrostomy, sphenoidotomy, and resection of middle turbinate to allow for maximal decompression. Twelve patients in the study group had a concurrent lateral wall decompression. They did not note the rate of lateral wall decompression for the control group. Notably, the inferomedial strut (IOS, discussed in detail below) was removed in all patients. There was no new‐onset or worsening diplopia in the study group compared to 16.7% new‐onset and 20% worsening diplopia in the control group. Reduction in proptosis remained unchanged between the two groups (5.1 mm mean reduction). Notably, the authors noted a resolution of proptosis in 50% of the sling group patients.16 In a subsequent retrospective study including 73 patients undergoing 115 decompressions, the use of the orbital sling technique was associated with decreased new‐onset diplopia and increased likelihood of resolution in preoperative diplopia.17 The results of these studies indicate that preservation of a medial periorbital sling is an effective technique in cases where maximal decompression is not necessary and vision is not threatened. Moreover, the studies suggest that the orbital sling can serve as a support mechanism that reduces the inferomedial displacement of the globe to prevent new‐onset or worsening diplopia without affecting reduction in proptosis.
Figure 2.
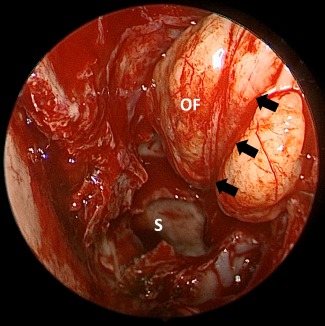
The orbital sling technique. Endoscopic view of an orbital sling technique. Middle turbinate has been resected. Black arrows show the orbital sling. OF = orbital fat; S = sphenoid sinus.
Endoscopic Preservation of the Inferomedial Strut
The inferomedial strut is a conceptual structure formed by bones that make up the inferior and medial orbital walls. It serves as a major support mechanism preventing the inferomedial displacement of orbital contents and the resultant shift in globe axis that can result in diplopia or dystopia. Anteriorly, it is formed by thick maxillary bone at the orbital rim, centrally, it is formed by the junction of the thin maxillary bone and ethmoid lamina, and posteriorly towards the orbital apex, and it is made up of the thick triangulated junction of the palatine and ethmoid bones (Fig. 3). The anterior IOS contains strong fibrous attachments to the globe and act as the major support mechanism for the orbit.18 Goldberg first described preservation of the IOS via a transconjunctival approach,12 but this was quickly translated to the endoscopic approach.19 However, until recently, preservation of the IOS was considered by many a technically challenging pursuit, as it limits access to the orbital floor during endoscopic decompression.16
Figure 3.
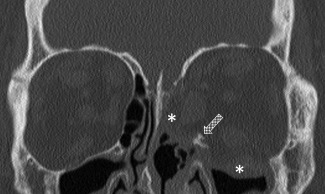
Coronal CT scan on a patient that underwent a medial and inferior wall orbital decompression with the preservation of the inferomedial orbital strut (patterned arrow). Asterisks show the orbital contents displaced into the sinonasal cavities.
Bleier et al. recently described an endoscopic technique for isolated orbital floor decompression with preservation of the entire IOS. In a proof‐of‐concept study, the authors employ angled frontal sinus instruments and cite a 100% successful completion rate in their series of 12 consecutive patients.20 Finn et al. subsequently published a retrospective review in 26 patients (45 orbits) that evaluated diplopia and proptosis outcomes after 2‐ and 3‐wall decompressions with or without IOS preservation. Forty percent of the patients in this study were decompressed for CON. The entire IOS was preserved, except in select cases of CON, exposure keratopathy, and disfiguring proptosis. The authors found IOS preservation was associated with decreased new‐onset diplopia, a high incidence (36%) of diplopia resolution, and a mean of 3.39 mm reduction in proptosis. The authors' results advocate for a balanced, 3‐wall decompression with strut preservation to promote maximum proptosis reduction while minimizing diplopia.21
Yao et al. conducted a retrospective review of 73 patients undergoing 115 balanced decompressions of the medial and lateral orbital walls. Clinical indications for surgery included exophthalmos, exposure keratopathy, gaze restriction, and optic neuropathy. In this study, the authors describe a modified endoscopic inferomedial strut (mIOS) preservation technique in which the posterior IOS is removed endoscopically, but the anterior strut is left intact (Fig. 4). The authors also performed an orbital sling procedure, except in patients with disfiguring exophthalmos (exophthalmopathy measured >28 mm) or threatened vision loss. Mean proptosis reduction achieved was 5.0 +/‐ 2.1 mm, and the preservation of an orbital sling did not influence the degree of reduction in proptosis. Diplopia resolved in 26% of cases, and 57% of patients undergoing sling technique had resolution in their diplopia compared to 17% in the non‐sling group. The authors in this study advocate for the orbital sling technique in select patients as a measure to resolve pre‐operative diplopia.17 This study also highlights the importance of posterior, inferior orbital strut removal in augmenting the degree of proptosis reduction without increasing the risk of diplopia.
Figure 4.
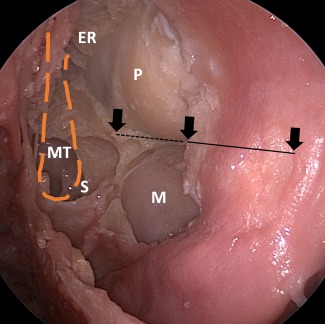
Endoscopic view of the left orbit following modified inferomedial orbital strut technique (IOS) (cadaver). The arrow represents the anterior, middle and posterior aspect of the IOS. The dotted line is the posterior half of the IOS that has been removed. ER = ethmoid roof; M = maxillary sinus; MT = previously resected area of the middle turbinate; P = periorbita; S = sphenoid sinus.
Selective Endoscopic Orbital Apex Decompression for Compressive Optic Neuropathy
Compressive optic neuropathy, occurring between 2–8.6% of patients with GO, is the most serious consequence of Graves' ophthalmopathy.22, 23 The onset can be heralded by visual field loss, changes in visual acuity, optic nerve edema, and afferent pupillary defect. Nerve compression occurs at the intracanalicular portion of the nerve formed by the two struts of the lesser wing of the sphenoid and the annulus of Zinn. High‐dose IV steroids are first line treatment for CON, and decompression surgery is indicated when medical therapy fails or in presence of recent‐onset choroidal folds and globe subluxation.3 The goal of decompressing the orbital apex is to reduce the compressive forces within the optic canal. Approaches for orbital apex decompression include transorbital, transantral coronal, external ethmoidectomy, and endonasal endoscopic approaches.24 The endonasal endoscopic technique provides unparalleled visualization of the orbital apex and represents an effective modality for improving visual acuity in patients with GO and CON. Traditional endoscopic apex decompression involves total sphenoethmoidectomy with inferomedial wall removal. Some endoscopic surgeons also advocate removal of optic canal bone 1cm posterior to the sphenoid face and, in some cases, incision of the nerve sheath.24, 25, 26 Mueller et al. has performed a morphometric analysis of the orbital process of the palatine bone and have noted that its surgical removal improves surgical exposure within the inferomedial apex.27 The indications for optic nerve decompression with sheath fenestration in the setting of CON are unclear. Schaefer et al. found that visual improvement occurred in 89% of CON patients undergoing endonasal endosopic orbital apex decompression combined with transconjunctival orbital floor decompression. In this study, the lateral wall of the sphenoid sinus anterior to the carotid artery was also removed to allow for optic nerve canal decompression, and the inferomedial strut was preserved.
Interestingly, many GO patients with CON may not suffer from significant proptosis,28 and as such, may not warrant extensive wall decompression. Furthermore, the true efficacy of optic nerve canal drilling, bone removal and/or sheath fenestration is debated, rendering attendant risks of diplopia, nerve damage, CSF leak, and meningitis, seemingly unnecessary. Still, some view CON as a contraindication to IOS and orbital sling preservation.16, 21 Chu et al. describe a technique of orbital apex decompression where only the lamina papyracea from sphenoid face to the basal lamella was removed.29 The entire inferomedial strut was preserved, no other wall was decompressed, and the optic nerve canal bone was left intact. Six orbits without preoperative diplopia were decompressed in this fashion. All had improved or stabilized visual acuity; all but one patient had reversal of their RAPD; all dyschromatopsia had resolved in affected patients; average globe recession was 3.1 mm; and there was no new‐onset diplopia. The study was limited by small sample size (6 globes). In a later retrospective review involving 77 patients and 114 orbits, Kingdom et al. targeted a more conservative posterior medial wall decompression to 15 patients (23 eyes) with CON. The IOS was preserved in all patients via a transconjunctival floor decompression. All but one patient had stable‐to‐improved visual acuity in the group with CON. There was no new‐onset diplopia and only one case of worsening diplopia. Mean reduction in proptosis was 2.2 (0–5 mm range). Together, these studies provide a proof‐of‐principle series validating a more conservative and personalized technique in patients with Graves'‐associated CON.
Endoscopic orbital fat decompression
The increased retro‐orbital fat characteristic in GO represents a critical driving factor of disease severity. Olivari described transpalpebral intraorbital fat removal as a decompression maneuver in 1988 and since then, this technique has proven safe and effective for a variety of clinical indications in GO.30, 31 Traditionally an external procedure, intraorbital fat removal has long been within the purview of the oculoplastic surgeon. More recently, intra‐orbital fat removal has been performed via a endonasal endoscopic approach in GO. Lv et al. have very recently described an endoscopic trans‐ethmoidal fat decompression technique.32 The authors perform: 1) standard sphenoethmoidectomy, 2) removal of medial wall periorbital, and 3) creation of an orbital sling, which prevents medial rectus prolapse but allows manipulation of fat for removal. The IOS is preserved in this study. A blunt‐tipped low‐powered suction cutting device is then used to remove extraconal and intraconal fat around extraocular muscles. The authors retrospectively evaluated outcomes employing this technique in 43 patients (72 orbits) with CON. Ninety‐five percent demonstrated improvements in visual acuity, and the mean proptosis reduction was 6.2 +/‐ 1.2 mm. Furthermore, symmetry was achieved within 2 mm in 90.7% of patients. They cite no new‐onset diplopia and two patients with worsening diplopia. This study provides evidence for another highly effective and versatile approach to GO decompression via a totally endonasal endoscopic route.32
Conclusions and Future Directions
Orbital decompression surgery is not standardized, and literature reviewing techniques reveal considerable heterogeneity. A systematic review published in 2009 cited 15 different approaches.4 Similarly, a prospective study performed over a single year conducted by the EUOGO highlighted 18 different approaches across 11 different centers.7 This marked heterogeneity underscores a recent evolution in orbital decompression surgery characterized by refinement in techniques. Endonasal endoscopic orbital decompression provides unequaled access to the inferomedial orbital wall and apex while avoiding many risks of external approaches. The investigations highlighted in this review reveal a trend towards a more personalized approach towards decompression, where effects of decompressive maneuvers are tailored to decompression areas targeting each individual's needs. Unfortunately, the overwhelming majority of literature published on this subject is retrospective in nature and heterogenous in design. Thus, a comparison of outcomes across techniques has not yet proven feasible.2, 4, 33 Nonetheless, the available literature supports the rapid ascension of the endonasal endoscopic approach as one of the more versatile techniques in orbital decompression surgery.
BIBLIOGRAPHY
- 1. Clauser L, Galie M, Sarti E, Dallera V. Rationale of treatment in Graves ophthalmopathy. Plast Reconstr Surg 2001;108(7):1880–1894. [DOI] [PubMed] [Google Scholar]
- 2. Boboridis KG, Uddin J, Mikropoulos DG, et al. Critical appraisal on orbital decompression for thyroid eye disease: a systematic review and literature search. Adv Ther 2015;32(7):595–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bartalena L, Baldeschi L, Boboridis K, et al. The 2016 European Thyroid Association/European Group on Graves' Orbitopathy Guidelines for the Management of Graves' Orbitopathy. Eur Thyroid J 2016;5(1):9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leong SC, Karkos PD, Macewen CJ, White PS. A systematic review of outcomes following surgical decompression for dysthyroid orbitopathy. Laryngoscope 2009;119(6):1106–1115. [DOI] [PubMed] [Google Scholar]
- 5. Shepard KG, Levin PS, Terris DJ. Balanced orbital decompression for Graves' ophthalmopathy. Laryngoscope 1998;108(11 Pt 1 ):1648–1653. [DOI] [PubMed] [Google Scholar]
- 6. Walsh TE, Ogura JH. Transantral orbital decompression for malignant exophthalmos. Laryngoscope 1957;67(6):544–568. [DOI] [PubMed] [Google Scholar]
- 7. Warren JD, Spector JG, Burde R. Long‐term follow‐up and recent observations on 305 cases of orbital decompression for dysthyroid orbitopathy. Laryngoscope 1989;99(1):35–40. [DOI] [PubMed] [Google Scholar]
- 8. DeSanto LW. The total rehabilitation of Graves' ophthalmopathy. Laryngoscope 1980;90(10 Pt 1):1652–1678. [PubMed] [Google Scholar]
- 9. Murray JP. Complications after treatment of chronic maxillary sinus disease with Caldwell‐Luc procedure. Laryngoscope 1983;93(3):282–284. [DOI] [PubMed] [Google Scholar]
- 10. DeFreitas J, Lucente FE. The Caldwell‐Luc procedure: institutional review of 670 cases: 1975–1985. Laryngoscope 1988;98(12):1297–1300. [DOI] [PubMed] [Google Scholar]
- 11. Long JA, Baylis HI. Hypoglobus following orbital decompression for dysthyroid ophthalmopathy. Ophthal Plast Reconstr Surg 1990;6(3):185–189. [DOI] [PubMed] [Google Scholar]
- 12. Goldberg RA, Shorr N, Cohen MS. The medical orbital strut in the prevention of postdecompression dystopia in dysthyroid ophthalmopathy. Ophthal Plast Reconstr Surg 1992;8(1):32–34. [DOI] [PubMed] [Google Scholar]
- 13. Graham SM, Brown CL, Carter KD, Song A, Nerad JA. Medial and lateral orbital wall surgery for balanced decompression in thyroid eye disease. Laryngoscope 2003;113(7):1206–1209. [DOI] [PubMed] [Google Scholar]
- 14. Cruz AA, Leme VR. Orbital decompression: a comparison between trans‐fornix/transcaruncular inferomedial and coronal inferomedial plus lateral approaches. Ophthal Plast Reconstr Surg 2003;19(6):440–445; discussion 445. [DOI] [PubMed] [Google Scholar]
- 15. Kingdom TT, Davies BW, Durairaj VD. Orbital decompression for the management of thyroid eye disease: An analysis of outcomes and complications. Laryngoscope 2015;125(9):2034–2040. [DOI] [PubMed] [Google Scholar]
- 16. Metson R, Samaha M. Reduction of diplopia following endoscopic orbital decompression: the orbital sling technique. Laryngoscope 2002;112(10):1753–1757. [DOI] [PubMed] [Google Scholar]
- 17. Yao WC, Sedaghat AR, Yadav P, Fay A, Metson R. Orbital decompression in the endoscopic age: the modified inferomedial orbital strut. Otolaryngol Head Neck Surg 2016;154(5):963–969. [DOI] [PubMed] [Google Scholar]
- 18. Kim JW, Goldberg RA, Shorr N. The inferomedial orbital strut: an anatomic and radiographic study. Ophthal Plast Reconstr Surg 2002;18(5):355–364. [DOI] [PubMed] [Google Scholar]
- 19. Wright ED, Davidson J, Codere F, Desrosiers M. Endoscopic orbital decompression with preservation of an inferomedial bony strut: minimization of postoperative diplopia. J Otolaryngol 1999;28(5):252–256. [PubMed] [Google Scholar]
- 20. Bleier BS, Lefebvre DR, Freitag SK. Endoscopic orbital floor decompression with preservation of the inferomedial strut. Int Forum Allergy Rhinol 2014;4(1):82–84. [DOI] [PubMed] [Google Scholar]
- 21. Finn AP, Bleier B, Cestari DM, et al. A retrospective review of orbital decompression for thyroid orbitopathy with endoscopic preservation of the inferomedial orbital bone strut. Ophthal Plast Reconstr Surg 2017;33(5):334–339. [DOI] [PubMed] [Google Scholar]
- 22. Nadeau S, Pouliot D, Molgat Y. Orbital decompression in Graves' orbitopathy: a combined endoscopic and external lateral approach. J Otolaryngol 2005;34(2):109–115. [DOI] [PubMed] [Google Scholar]
- 23. Graham SM, Carter KD. Combined‐approach orbital decompression for thyroid‐related orbitopathy. Clin Otolaryngol Allied Sci 1999;24(2):109–113. [DOI] [PubMed] [Google Scholar]
- 24. Pletcher SD, Sindwani R, Metson R. Endoscopic orbital and optic nerve decompression. Otolaryngol Clin North Am 2006;39(5):943–958, vi. [DOI] [PubMed] [Google Scholar]
- 25. Schaefer SD, Soliemanzadeh P, Della Rocca DA, et al. Endoscopic and transconjunctival orbital decompression for thyroid‐related orbital apex compression. Laryngoscope 2003;113(3):508–513. [DOI] [PubMed] [Google Scholar]
- 26. Luxenberger W, Stammberger H, Jebeles JA, Walch C. Endoscopic optic nerve decompression: the Graz experience. Laryngoscope 1998;108(6):873–882. [DOI] [PubMed] [Google Scholar]
- 27. Mueller SK, Freitag SK, Bleier BS. Morphometric analysis of the orbital process of the palatine bone and its relationship to endoscopic orbital apex surgery. Ophthal Plast Reconstr Surg 2017. Epub, ahead of print. [DOI] [PubMed] [Google Scholar]
- 28. Kazim M, Trokel SL, Acaroglu G, Elliott A. Reversal of dysthyroid optic neuropathy following orbital fat decompression. Br J Ophthalmol 2000;84(6):600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chu EA, Miller NR, Lane AP. Selective endoscopic decompression of the orbital apex for dysthyroid optic neuropathy. Laryngoscope 2009;119(6):1236–1240. [DOI] [PubMed] [Google Scholar]
- 30. Olivari N. [Transpalpebral decompression operation in endocrine orbitopathy (exophthalmos)]. Wien Med Wochenschr 1988;138(18):452–455. [PubMed] [Google Scholar]
- 31. Richter DF, Stoff A, Olivari N. Transpalpebral decompression of endocrine ophthalmopathy by intraorbital fat removal (Olivari technique): experience and progression after more than 3000 operations over 20 years. Plast Reconstr Surg 2007;120(1):109–123. [DOI] [PubMed] [Google Scholar]
- 32. Lv Z, Selva D, Yan W, Daniel P, Tu Y, Wu W. Endoscopical orbital fat decompression with medial orbital wall decompression for dysthyroid optic neuropathy. Curr Eye Res 2016;41(2):150–158. [DOI] [PubMed] [Google Scholar]
- 33. Boboridis KG, Bunce C. Surgical orbital decompression for thyroid eye disease. Cochrane Database Syst Rev 2011(12):CD007630. [DOI] [PubMed] [Google Scholar]
- 34. Malik R, Cormack G, MacEwen C, White P. Endoscopic orbital decompression for dyscosmetic thyroid eye disease. J Laryngol Otol 2008;122(6):593–597. [DOI] [PubMed] [Google Scholar]
- 35. Kennedy DW, Goodstein ML, Miller NR, Zinreich SJ. Endoscopic transnasal orbital decompression. Arch Otolaryngol Head Neck Surg 1990;116(3):275–282. [DOI] [PubMed] [Google Scholar]
- 36. Stiglmayer N, Mladina R, Tomic M, et al. Endonasal endoscopic orbital decompression in patients with Graves' ophthalmopathy. Croat Med J 2004;45(3):318–322. [PubMed] [Google Scholar]


