Abstract
Objectives
Salvage total laryngectomies (STL) are not a homogeneous group. Most will fall into two groups: i) Patients with previous AJCC stage I/II larynx cancer who have had radiotherapy to the larynx only (STL‐LOR), or ii) Patients who have had previous AJCC stage III/IV larynx cancer and subsequent radiotherapy to the larynx and draining nodal basins with concurrent cisplatin chemotherapy (STL‐CRT). We aimed to compare PCF rates following STL in these two groups.
Methods
A retrospective review of the department's cohort between January 2010 and August 2015 was conducted.
Results
Seventy‐seven patients underwent total laryngectomy for larynx cancer between January 2010 and August 2015. There were 10 post‐laryngectomy fistulas (13.0%). Three of these occurred in the 38 patients undergoing primary total laryngectomy (PTL), and seven in the 39 patients undergoing STL, rates of 7.9% and 17.9%, respectively. Twenty‐two patients had received radiation to the larynx alone without chemotherapy (STL‐LOR) for initial Stage I/II disease. Eleven patients had received laryngeal and neck irradiation plus cisplatin chemotherapy (STL‐CRT) for initial stage III/IV disease. Of the 22 STL‐LOR patients, two developed PCF (9.1%). Of the 11 STL‐CRT patients, five developed PCF. There was no difference in the rate of PCF between PTL and STL‐LOR. There was a statistically significant increase in PCF in STL‐CRT versus PTL (p = .009) and in PCF in STL‐CRT versus STL‐LOR (p = .027).
Conclusion
Salvage laryngectomies are often treated as a homogenous group. We demonstrate that PCF rates vary significantly depending on preoperative radiation fields and the use of chemotherapy.
Level of Evidence
2b.
Keywords: Salvage surgery, laryngectomy, Radiotherapy, chemotherapy
INTRODUCTION
Pharyngocutaneous fistula (PCF) is the most frequent serious complication following total laryngectomy (TL) with rates varying from 2.6% to 65.5%.1 PCF is defined as dehiscence of the pharyngeal closure with resultant leakage of saliva in communication with the skin.1 Patient morbidity results from prolonged hospitalization, delayed oral feeding, and the risk of additional surgery while patient mortality can result from an increased risk of catastrophic vascular hemorrhage and delays to commencement of adjuvant radiotherapy and is challenging to manage in most cases.2 The collective impact on the cost of healthcare provision is great.3
Salvage total laryngectomy (STL) describes a laryngectomy performed following previous curative intent radiation therapy with or without chemotherapy. Paydarfar et al.4 and Liang et al.5 highlighted the greater than two‐fold increased risk in PCF with STL. This can be understood when considering the altered microvascular structure of irradiated tissue that negatively impacts wound healing.6
Salvage Laryngectomies Are Not a Homogenous Group
The main differences lie in extent of volume irradiated (larynx +/‐ elective cervical nodal volumes), dose fractionation schedule, and addition of concurrent chemotherapy. Furthermore, within the chemotherapy group some will receive a platinum‐based chemotherapeutic agent while those not suitable will often receive cetuximab.
In many centers, those patients with T1 and/or T2 N0 laryngeal tumors (AJCC stage I/II) who are felt to be unsuitable for surgical intervention will receive radiation treatment to the larynx. Depending on the distribution of the disease, these early larynx cancer patients will often not require elective treatment to the neck which can be monitored clinically. Modern radiotherapy protocols ensure very accurate delivery of the radiation dose to the larynx with only minimal dose delivery to the surrounding tissues. These patients do not require chemotherapy. Those individuals within this group who fail larynx only radiotherapy (LOR) or develop future recurrent disease and who are unsuitable for partial laryngeal surgery will have salvage total laryngectomy. It is therefore of interest to assess whether this cohort having larynx only radiotherapy (STL‐LOR) is at altered risk of PCF compared to those having primary total laryngectomy (PTL) and compared to those having comprehensive radiotherapy to the larynx and bilateral neck with concurrent cisplatin chemotherapy (STL‐CRT).
In order to reduce the risk of PCF, many units, including our own, now use non‐irradiated vascularized tissue from outside the field to reinforce or buttress the pharyngeal repair even when augmentation is not independently required. Pectoralis Major myofascial flaps (PMMF), for example, have been shown to reduce rates of PCF in STL.7 Some authors suggest that PMMF has very low morbidity while others point out that it is not without risk. These risks include shoulder dysfunction, impaired post‐laryngectomy speech, and excessive muscle bulk.8, 9 As such, it is important to identify whether STL‐LOR patients are at increased risk of PCF such that unnecessary flaps can be avoided.
In addition to salvage surgery, other risks for the development of PCF have also been analyzed. Preoperative albumin as a marker of nutritional status has been found as a significant predictor of PCF level when below 40 g/L.10, 11, 12
Similarly, comorbid chronic obstructive pulmonary disease (COPD) is a recognized predictor of PCF in the literature.11, 13 Surprisingly active smoking up until surgery has consistently been found to be nonpredictive of PCF.5, 13 Commencement of oral feeding after surgery is a source of significant controversy.14, 15, 16
Perioperative antibiotic prophylaxis is almost universal given the clean‐contaminated nature of the operation. There is, however, striking variability in choice of agent and duration of treatment as evidenced in a 2015 survey of UK surgeons.17 A methodologically robust trial, albeit a small one, demonstrated a remarkable reduction in the PCF rate with 10 days of metronidazole administration in the perioperative period.18
Prophylaxis against gastroesophageal reflux disease (GORD) has also been demonstrated to reduce the PCF rate.19, 20, 21
Other positive predictors have included performance of concurrent neck dissection,4, 13 advanced tumor size,5, 12, 13 positive surgical margins,5, 12, 13, 22 low postoperative hemoglobin,4, 5, 12, 13, 23 and preoperative tracheostomy.4, 12, 23
In addition to the primary aim of the study, we also performed a full analysis of other potential causative factors for PCF in our laryngectomy population.
MATERIALS AND METHODS
A retrospective review of the department's cohort was conducted. Ethics approval was granted by the Royal Brisbane & Women's Hospital Human Research Ethics Committee. Notes were trawled for data including head and neck multidisciplinary meeting and specialist clinic correspondence and admission documentation. All final histology reports and contrast medium swallow examinations were reviewed by the authors to define potential predictors under investigation and confirm the diagnosis of PCF.
PATIENTS
We analyzed all patients undergoing total laryngectomy for an indication of laryngeal cancer between January 2010 and August 2015. Excluded from the study cohort were patients undergoing pharyngolaryngectomy or laryngectomy with partial pharyngectomy rather than total laryngectomy or an indication other than laryngeal cancer. None of the included patients had any other reconstruction over and above our standard three‐layered primary closure of the pharyngeal tissues following removal of the larynx. No free flaps were used in any of the analyzed patients. Prior to 2015, PMMF flaps were not used in our institution to reduce rates of PCF, and thus groups are readily comparable for PCF rates without confounding from the use of flaps.
The records of 77 consecutive patients undergoing total laryngectomy were retrospectively reviewed. Patient, disease, and perioperative data were recorded for analysis. Patient data included age, sex, smoking habit, comorbid diabetes, comorbid COPD, and preoperative albumin.
Disease data included indication, tumor, and nodal stage. In cases of salvage laryngectomy, initial treatment, time interval until salvage surgery, radiation dosage, and fields were recorded. Perioperative data included intraoperative performance of concomitant neck dissection, cricopharyngeal myotomy, three‐layer pharyngeal closure, and primary tracheoesophageal puncture. Postoperative use of metronidazole, anti‐skin commensal antibiotic, and proton pump inhibitor (PPI) were analyzed. Margin status, postoperative hemaglobin, postoperative day‐of contrast medium swallow, and local wound complications were also assessed.
The outcome of interest, PCF, was determined radiographically and clinically. A contrast medium swallow was performed prior to commencement of oral feeding as routine in all patients following total laryngectomy in addition to clinical assessment of the wound. Patients were also followed‐up at postoperative specialist clinic appointments with clinical assessment assisted by repeat contrast medium swallow examinations as needed. Radiographic evidence of a sinus tract greater than two centimeters or frank fistulas with saliva draining onto the skin were considered evidence of PCF. Details of subsequent management of fistulas, be it conservative or operative, were recorded for reporting purposes. Salvage laryngectomy patients with N+ necks received ipsilateral modified radical neck dissection. Those without nodal disease did not receive neck dissection if the neck had received prior irradiation.
STATISTICAL ANALYSIS
Statistical analysis was carried out using SPSS Statistics software package version 20 (Armonk, NY: IBM Corp). A p‐value ≤ .05 was considered statistically significant.
Descriptive statistics for continuous variables were reported as mean and standard deviation for normally distributed variables or median and interquartile range for non‐normally distributed variables. Categorical variables were reported as n (%).
Univariate analyses to assess predictors of PCF were carried out using statistical tests appropriate to the variable. Continuous variables were analyzed using the independent T‐test if normally distributed. Alternatively, the nonparametric Mann‐Whitney U test was used for non‐normally distributed continuous variables. Categorical variables were dichotomized for the purposes of univariate analysis and analyzed using Fisher's exact test.
RESULTS
There was a total of 10 postlaryngectomy fistulas in the cohort of 77 patients representing an overall incidence rate of 13.0%. Three of these occurred in the 38 patients undergoing PTL (7.9%), the remaining 7 in the 39 patients undergoing STL (17.9%).
Considering the STL‐LOR cohort alone, however, only 2 out of 22 patients developed a PCF (9.1%), while out of the 11 patients who had received laryngeal and neck irradiation plus cisplatin‐based chemotherapy (STL‐CRT), 5 developed a PCF (Table 1, Fig. 1)
Table 1.
Patients Developing PCF by Treatment Groups.
| Groups | Overall | PCF | No‐PCF | PCF rate (%) |
|---|---|---|---|---|
| All patients | 77 | 10 | 67 | 13.0 |
| PTL | 38 | 3 | 35 | 7.9 |
| STL‐LOR | 22 | 2 | 20 | 9.1 |
| STL‐CRT (Cisplatin) | 11 | 5 | 6 | 45.5 |
PTL = primary total laryngectomy; STL‐LOR = Salvage total laryngectomy with radiation to the larynx only and no chemotherapy; STL‐CRT‐Cis = salvage total laryngectomy with larynx and neck radiation plus cisplatin chemotherapy.
Figure 1.
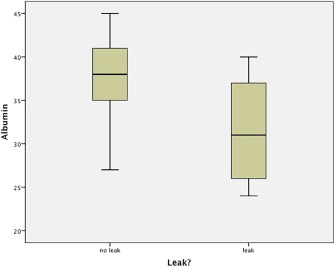
Comparison of the preoperative albumin level with PCF formation.
PCF = pharyngocutaneous fistula
A small minority, six STL patients, did not fit into either of these two categories. Two patients had radiation to the larynx and neck without chemotherapy. One patient had cisplatin chemotherapy and radiation to the larynx only without radiation to the neck. Three patients had larynx and bilateral neck irradiation with cetuximab chemotherapy. None of these six “intermediate risk” STL patients developed a PCF. There was no difference in the rate of PCF between PTL and STL‐LOR. There was a statistically significant increase in PCF in STL‐CRT versus PTL (p = .009) and in PCF in STL‐CRT versus STL‐LOR (p = .027). (Table 2)
Table 2.
Statistical Analysis of Laryngectomy Treatment Groups.
| Group comparisons | p‐value |
|---|---|
| PTL vs. STL‐LOR | .999 |
| PTL vs. STL‐CRT | .009 |
| STL‐LOR vs. STL‐CRT | .027 |
Data available for only 63 PCF (‐) in active smoking; 64 PCF (‐) in diabetic and COPD; 65 PCF (‐) in preoperative albumin; 9 PCF (+) in cricopharyngeal myotomy; 58 PCF (‐) and 9 PCF (+) in metronidazole and anti‐skin commensal antibiotic; 57 PCF (‐) and 9 PCF (+) in PPI.
PCF = pharyngocutaneous fistula; PTL = primary total laryngectomy; STL‐LOR = Salvage total laryngectomy with radiation to the larynx only and no chemotherapy; STL‐CRT = salvage total laryngectomy with larynx and neck radiation
Seven of the 10 fistulas were managed conservatively while three were managed operatively with vascularized local myocutaneous flaps. Pectoralis major was used in two cases and sternocleidomastoid in the other.
Univariate analysis was conducted analyzing patient characteristics, disease characteristics, and perioperative factors as predictors of PCF (Table 3).
Table 3.
Univariate Analysis of Predictors for PCF Formation for All Patients Undergoing Total Laryngectomy.
| PCF (+) | PCF (‐) | p‐value | |
|---|---|---|---|
| Age (years) | 66.4 [9.7] | 64.8 [10.6] | .65 |
| Male gender (n) | 10 (100) | 60 (89.6) | .59 |
| Active smoker (n) | 6 (60) | 25 (39.7) | .31 |
| Diabetic (n) | 0 (0) | 11 (17.2) | .34 |
| COPD (n) | 6 (60) | 21 (32.8) | .16 |
| Preoperative albumin (g/L) | 31.8 [6.0] | 37.6 [4.3] | .005 |
| Salvage surgery (n) | 7 (70) | 32 (47.8) | .31 |
| High tumor stage (n) at time of surgery | 5 (50) | 49 (73.1) | .15 |
| Positive nodal stage (n) | 4 (40) | 21 (31.3) | .72 |
| Concurrent neck dissection (n) | 4 (40) | 26 (38.8) | .999 |
| Cricopharyngeal myotomy (n) | 6 (66.7) | 54 (80.6) | .39 |
| Involved surgical margins (n) | 0 (0) | 1 (1.5) | .999 |
| Primary tracheoesophageal puncture (n) | 1 (10) | 35 (52.2) | .02 |
| Postoperative hemaglobin (g/L) | 93.3 [19.0] | 106.3 [16.0] | .02 |
| Swallow day (day) | 12 {4} | 10 {3} | .06 |
| Metronidazole (n) | 7 (77.8) | 46 (79.3) | .999 |
| Anti‐skin commensal antibiotic (n) | 7 (77.8) | 27 (46.6) | .15 |
| Proton pump inhibitor (PPI) (n) | 6 (66.7) | 24 (42.1) | .28 |
| Local wound hematoma (n) | 3 (30) | 3 (4.5) | .03 |
| Values are mean [standard deviation], median {interquartile range} or n (%) | |||
| PCF = pharyngocutaneous fistula |
Bold font indicates positive correlation, italic font indicates negative correlation.
With univariate analysis, preoperative albumin (Fig. 1), postoperative hemaglobin (Fig. 2), and local wound hematoma were significant predictors of PCF formation. Surprisingly, 10% versus 52.2% of patients who had a primary tracheoesophageal puncture (TOP) developed PCF. This is likely due to patient selection for primary TEP.
Figure 2.
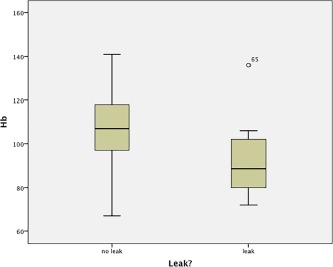
Comparison of the postoperative hemaglobin level with PCF formation.
PCF = pharyngocutaneous fistula
When only patients undergoing salvage laryngectomy were considered, the same factors for PCF formation were significant on univariate analysis. Patients who developed fistulas and those who did not had even more marked differences in predictors of preoperative albumin, postoperative hemoglobin, and wound hematoma formation.
DISCUSSION
Salvage laryngectomy is defined as a laryngectomy performed following previous curative intent radiation therapy with or without chemotherapy. This definition includes two very different groups of patients. In the modern era, many patients with T1 and T2 N0 (Stage I/II) larynx cancers will receive larynx‐only radiotherapy (LOR) with observation of the neck. This definitive radiotherapy to the larynx results in a very high cure rate with very low morbidity. Newer treatment approaches utilize intensity modulated radiotherapy to treat the larynx while sparing the adjacent carotid arteries and achieves 88% local control rate at three years for T1–2N0 glottic SCC.24 This highly accurate localized treatment has been taken one step further by a Dutch group who limited radiotherapy to the single involved vocal cord and reported a two‐year local control rate of 100%.25 Some of these T1/T2 patients will not be cured by radiotherapy and some will subsequently undergo salvage total laryngectomy (STL‐LOR).
Patients with stage III/IV larynx cancer will be treated with larynx and neck irradiation with added chemotherapy (most commonly cisplatin) in an organ preservation protocol which is expected to produce 68% locoregional control rate at five years.26, 27 These two groups do not have the same risk of surgical complications following total laryngectomy. It is useful in practical terms to separate these two groups when analyzing PCF rates. It helps us to create a framework when establishing those patients who might benefit from flap reinforcement of the pharyngeal repair. This is the first study to single out the important cohort of patients who have had LOR and demonstrate clearly that this group has no increased risk of PCF.
Other studies have looked at the risk of radiotherapy versus chemoradiotherapy with mixed results. Some have shown no increased risk of PCF with the addition of chemotherapy. Weber et al.28 did not find any difference in the major complication rate for patients receiving radiotherpy (RT) alone compared to those receiving Chemoradiotherapy (CXRT). However, their study only included failures following stage III/IV disease. Thus, their radiation only cohort is likely very different to our own. Similarly, Klozar et al.29 found no difference between XRT and CXRT but no information about radiation fields is available.
Our analysis included only laryngectomy patients with the exclusion of those having partial or complete pharyngectomy. Some papers included TL, pharyngolaryngectomy, and/or partial pharyngectomy with free flap reconstruction in their cohorts.7, 30 Some have included patients having oesophagopharyngolaryngectomy31 with others then generalizing the findings to laryngectomy patients. Indeed, meta‐analyses looking at postlaryngectomy PCF32 have included papers analyzing exclusively pharyngolaryngectomy and free tissue transfer33 when comparing radiotherapy and chemoradiotherapy groups. Findings for pharyngolaryngectomy and laryngectomy with partial pharyngectomy cannot be generalized to total laryngectomy without free flap reconstruction.
Meta‐analyses which found no difference between radiotherapy and chemoradiotherapy groups have included large numbers of patients with pedicled and free flap reconstructions which in itself alters the risk of PCF.9, 34, 35 It is not always clear whether CTRT groups had more flaps than RT groups and this may represent a confounding factor.
Other studies which have findings very similar to our own include Suslu et al. 2017.1 Patients having flap reconstruction were not included. They found the rate of fistula in the XRT and induction chemo SL‐ICT group had rates of fistula which were not significantly higher than PTL. In contrast, patients having TL‐CRT had a PCF rate significantly higher than the PTL group (p = .004). Interestingly, their rates of fistula in both STL‐XRT and STL‐CXRT groups were very similar to our own.
Ganly et al.36 ensured that patients having pharyngolaryngectomy were excluded from their study. Forty‐six percent of their patients had RT and 54% had CTRT. They specified that RT was the initial treatment for early stage disease (AJCC Stage I/II), whereas CTRT was the treatment of choice for stage III/IV. Like us, they found no significant difference in the frequency of complications in the STL‐RT group compared to the PTL group. There was, however, increased risk of PCF in the STL‐CTRT group (p = .012). They found CTRT was the only significant predictor of total complications including PCF.
From our study, it is evident that those having STL‐LOR are not at increased risk of PCF. As previously mentioned, this has implications when analyzing papers which have looked at the benefits of PMMF flaps. It would be helpful if STL‐LOR patients were excluded from these studies if their risk of fistula is no different from primary laryngectomy. If STL‐LOR patients are included disproportionately in control groups, the papers may underestimate the value of these flaps.
Most papers analyzing the use of flaps in STL specified that the patients had preoperative RT or CRT but the fields of the RT were not significantly discussed or analyzed and may or may not have contained STL‐LOR patients.7, 35, 37, 38, 39, 40 Many also mentioned the advanced stage of disease at time of surgery but not the stage of disease prior to initial XRT +/‐ chemotherapy, which would have had implications for the preoperative treatment protocols. Gil et al.41 included both XRT and CXRT salvage cases. They had more CXRT patients in the flap group which they appropriately acknowledged and factor into their discussion. Withrow et al.42 specified inclusion criteria having both XRT alone and CXRT, but clarified that all patients received wide field radiation treatment and was therefore unlikely to include STL‐LOR patients. Only Oosthuizen et al.43 included only CXRT salvage patients.
The chronicity of papers examining the subject may be of importance. Advances in RT with regards to radiation fields, delivery, and dosing may be resulting in current patients having STL‐RT having no increase in PCF.
Although most of our salvage laryngectomy patients fell into our two study groups, some patients lay somewhere in between—either having radiotherapy to the larynx and neck without chemotherapy and a very small proportion having chemoradiotherapy to the larynx alone without neck irradiation. The DAHANCA study44 suggested that within RT, only group field size was predictive of fistula. This has not been demonstrated convincingly. However, if we were to simply compare radiation with chemoradiation, we risk creating a disparate group of radiation‐only patients. As it stands, we only had two patients who had larynx and neck radiotherapy without cisplatin. Neither of these two patients developed PCF but with only two patients it is not possible to draw any safe conclusions about these patients. Thus, in identifying a low‐risk salvage laryngectomy group we decided to exclude those having radiation to a wide field and only included those having radiation to the larynx alone.
Whether those having chemoradiotherapy with a platinum‐based agent are at higher risk of complications in salvage surgery than those having cetuximab is of significant debate.45, 46 Thus, for similar reasons to those above, in assessing a high risk group, we have selected those having cisplatin‐based chemotherapy. The number of patients falling outside the two groups was not large enough to evaluate in terms of risk of PCF.
This is a retrospective study, which makes it prone to selection bias. However, we feel this study is of significant value. None of the patients in this study had flaps because this was prior to our institution's routine use of PMMF flaps in salvage patients. All patients had their laryngeal defects closed primarily. Neck dissection did not increase the risk of PCF in our study. As such, the groups are easily comparable in terms of the preoperative intervention they received and the study demonstrates that patients having salvage laryngectomy following larynx‐only radiation (STL‐LOR) are not at increased risk of PCF compared to patients having PTL and as such do not warrant flap reinforcement to reduce PCF risk.
CONCLUSION
Defining low‐ and high‐risk groups within salvage laryngectomy is helpful when selecting which patients will benefit from surgical measures to reduce the risk of PCF. Patients having STL‐LOR are low risk for PCF and do not require flap reinforcement. Patients having STL‐CRT, especially those receiving platinum‐based chemotherapy, are at very high risk of fistula and should routinely have flap reinforcement to reduce the risk of PCF.
No financial Support or funding received
No conflict of interest to declare
BIBLIOGRAPHY
- 1. Suslu N, Senirli RT, Gunaydin RO, Ozer S, Karakaya J, Hosal AS. Pharyngocutaneous fistula after salvage laryngectomy. Acta Otolaryngol 2015;135:615–621. [DOI] [PubMed] [Google Scholar]
- 2. Papazoglou G, Doundoulakis G, Terzakis G, Dokianakis G. Pharyngocutaneous fistula after total laryngectomy: incidence, cause, and treatment. Ann Otol Rhinol Laryngol 1994;103:801–805. [DOI] [PubMed] [Google Scholar]
- 3. Parikh SR, Irish JC, Curran AJ, Gullane PJ, Brown DH, Rotstein LE. Pharyngocutaneous fistulae in laryngectomy patients: the Toronto. Hospital experience. J Otolaryngol 1998;27:136–140. [PubMed] [Google Scholar]
- 4. Paydarfar JA, Birkmeyer NJ. Complications in head and neck surgery: a meta‐analysis of postlaryngectomy pharyngocutaneous fistula. Arch Otolaryngol Head Neck Surg 2006;132:67–72. [DOI] [PubMed] [Google Scholar]
- 5. Liang JW, Li ZD, Li SC, Fang FQ, Zhao YJ, Li YG. Pharyngocutaneous fistula after total laryngectomy: A systematic review and meta‐analysis of risk factors. Auris Nasus Larynx 2015;42:353–359. [DOI] [PubMed] [Google Scholar]
- 6. Putten L, Bree R, Doornaert P, et al. Salvage surgery in post‐chemoradiation laryngeal. Acta Otorhinolaryngol Ital 2015;35:162–172. [PMC free article] [PubMed] [Google Scholar]
- 7. Righini C, Lequeux T, Cuisnier O, Morel N, Reyt E. The pectoralis myofascial flap in pharyngolaryngeal surgery after radiotherapy. Eur Arch Otorhinolaryngol 2005;262:357–361. [DOI] [PubMed] [Google Scholar]
- 8. Fung K, Teknos TN, Vandenberg CD, et al. (2007). Prevention of wound complications following salvage laryngectomy using free vascularized tissue. Head Neck 2007;29:425–430. [DOI] [PubMed] [Google Scholar]
- 9. Sharma S, Chaukar D, Laskar S, et al. Role of the pectoralis major myofascial flap in preventing pharyngocutaneous fistula following salvage laryngectomy. J Laryngol Otol 2016;130:860–864. [DOI] [PubMed] [Google Scholar]
- 10. Timmermans AJ, Lansaat L, Theunissen EA, Hamming‐Vrieze O, Hilgers FJ, van den Brekel MW. Predictive factors for pharyngocutaneous fistulization after total laryngectomy. Ann Otol Rhinol Laryngol 2014;123:153–161. [DOI] [PubMed] [Google Scholar]
- 11. Boscolo‐Rizzo P, De Cillis G, Marchiori C, Carpene S, Da Mosto MC. Multivariate analysis of risk factors for pharyngocutaneous fistula after total laryngectomy. Eur Arch Otorhinolaryngol 2008;265:929–936. [DOI] [PubMed] [Google Scholar]
- 12. Cecatto SB, Soares MM, Henriques T, Monteiro E, Moura CI. Predictive factors for the postlaryngectomy pharyngocutaneous fistula development: systematic review. Braz J Otorhinolaryngol 2014;80:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dedivitis RA, Aires FT, Cernea CR, Brandao LG. Pharyngocutaneous fistula after total laryngectomy: Systematic review of risk factors. Head Neck 2015;37:1691–1697. [DOI] [PubMed] [Google Scholar]
- 14. Rassekh CH, Haughey BH. Total laryngectomy and laryngopharyngectomy In: Flint PW, Haughey BH, Lund VJ, et al., ed. Cummings Otolaryngology Head & Neck Surgery. 5th ed Philadelphia: Elsevier; 2015. [Google Scholar]
- 15. Maclean J, Cotton S, Perry A. Variation in surgical methods used for total laryngectomy in Australia. J Laryngol Otol 2008;122:728–732. [DOI] [PubMed] [Google Scholar]
- 16. Martin SK. The effect of early oral feeding compared to standard oral feeding following total laryngectomy: a systematic. Adelaide, Australia: The University of Adelaide; 2013. Available at: https://digital.library.adelaide.edu.au/dspace/bitstream/2440/84473/9/01front.pdf. Accessed December 4, 2015.
- 17. Harris R, Ofo E, Cope D, et al. Current trends in antibiotic prophylaxis for laryngectomy in the UK ‐ a national survey. J Laryngol Otol 2015;129:63–67. [DOI] [PubMed] [Google Scholar]
- 18. Stathas T, Mallis A, Mastronikolis NS, et al. Pharyngocutaneous fistula complicating laryngectomy: can metronidazole help? ORL J Otorhinolaryngol Relat Spec 2011;73:291–294. [DOI] [PubMed] [Google Scholar]
- 19. Sarria Echegaray P, Tomas Barberan M, Mas Mercant S, Soler Vilarrasa R, Romaguera Lliso A. [Pharmacological prophylaxis of gastroesophageal reflux. Incidence of pharyngocutaneous fistula after total laryngectomy]. Acta Otorrinolaringol Esp 2000;51:239–242. [PubMed] [Google Scholar]
- 20. Seikaly H, Park P. Gastroesophageal reflux prophylaxis decreases the incidence of pharyngocutaneous fistula after total laryngectomy. Laryngoscope 1995;105:1220–1222. [DOI] [PubMed] [Google Scholar]
- 21. Stephenson KA, Fagan JJ. Effect of perioperative proton pump inhibitors on the incidence of pharyngocutaneous fistula after total laryngectomy: a prospective randomized controlled trial. Head Neck 2015;37:255–259. [DOI] [PubMed] [Google Scholar]
- 22. Saki N, Nikakhlagh S, Kazemi M. Pharyngocutaneous fistula after laryngectomy: incidence, predisposing factors, and outcome. Arch Iran Med 2008;11:314–317. [PubMed] [Google Scholar]
- 23. Benson EM, Hirata RM, Thompson CB, et al. Pharyngocutaneous fistula after total laryngectomy: a single‐institution experience, 2001–2012. Am J Otolaryngol 2015;36:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zumsteg ZS, Riaz N, Jaffery S, et al. Carotid sparing intensity‐modulated radiation therapy achieves comparable locoregional control to conventional radiotherapy in T1–2N0 laryngeal carcinoma. Oral Oncol 2015;51:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Al‐Mamgani A, Kwa SL, Tans L, et al. Single vocal cord irradiation: image guided intensity modulated hypofractionated radiation therapy for T1a glottic cancer: early clinical results. Int J Radiat Oncol Biol Phys 2015;93:337–343. [DOI] [PubMed] [Google Scholar]
- 26. Forastiere AA, Zhang Q, Weber RS, et al. Long‐term results of RTOG 91‐11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol 2012;31:845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blanchard P, Baujat B, Holostenco V, et al. Meta‐analysis of chemotherapy in head and neck cancer (MACH‐NC): a comprehensive analysis by tumour site. Radiother Oncol 2011;100:33–40. [DOI] [PubMed] [Google Scholar]
- 28. Weber RS, Berkey BA, Forastiere A, et al. Outcome of salvage total laryngectomy following organ preservation therapy: the Radiation Therapy Oncology Group trial 91‐11. Arch Otolaryngol Head Neck Surg 2003;129:44–49. [DOI] [PubMed] [Google Scholar]
- 29. Klozar J, Cada Z, Koslabova E. (2012). Complications of total laryngectomy in the era of chemoradiation. Eur Arch Otorhinolaryngol 2012;269:289–293. [DOI] [PubMed] [Google Scholar]
- 30. Basheeth N, O'Leary G, Sheahan P. Pharyngocutaneous fistula after salvage laryngectomy: impact of interval between radiotherapy and surgery, and performance of bilateral neck dissection. Head Neck 2014;36:580–584. [DOI] [PubMed] [Google Scholar]
- 31. Dirven R, Swinson BD, Gao K, Clark JR. The assessment of pharyngocutaneous fistula rate in patients treated primarily with definitive radiotherapy followed by salvage surgery of the larynx and hypopharynx. Laryngoscope 2009;119:1691–1695. [DOI] [PubMed] [Google Scholar]
- 32. Hasan Z, Dwivedi RC, Gunaratne DA, et al. Systematic review and meta‐analysis of the complications of salvage total laryngectomy. Eur J Surg Oncol 2017;43:42–51. [DOI] [PubMed] [Google Scholar]
- 33. Miyamoto S, Sakuraba M, Nagamatsu S, Hayashi R. Salvage total pharyngolaryngectomy and free jejunum transfer. Laryngoscope 2011;121:947–951. [DOI] [PubMed] [Google Scholar]
- 34. Scotton W, Cobb R, Pang L, et al. Post‐operative wound infection in salvage laryngectomy: does antibiotic prophylaxis have an impact? Eur Arch Otorhinolaryngol 2012;269:2415–2422. [DOI] [PubMed] [Google Scholar]
- 35. Patel UA, Moore BA, Wax M, et al. Impact of pharyngeal closure technique on fistula after salvage laryngectomy. JAMA Otolaryngol Head Neck Surg 2013;139:1156–1162. [DOI] [PubMed] [Google Scholar]
- 36. Ganly I, Patel S, Matsuo J, et al. Postoperative complications of salvage total laryngectomy. Cancer 2005;103:2073–2081. [DOI] [PubMed] [Google Scholar]
- 37. Fung K, Teknos TN, Vandenberg CD, et al. Prevention of wound complications following salvage laryngectomy using free vascularized tissue. Head Neck 2007;29:425–430. [DOI] [PubMed] [Google Scholar]
- 38. Sousa AA, Castro SMDO, Porcaro‐Salles JM, et al. The usefulness of a pectoralis major myocutaneous flap in preventing salivary fistulae after salvage total laryngectomy. Braz J Otorhinolaryngol 2012;78:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Powell J, Ullal UR, Ahmed O, Ragbir M, Paleri V. Tissue transfer to post‐chemoradiation salvage laryngectomy defects to prevent pharyngocutaneous fistula: single‐centre experience. J Laryngol Otol 2014;128:365. [DOI] [PubMed] [Google Scholar]
- 40. Patel UA, Keni SP. Pectoralis myofascial flap during salvage laryngectomy prevents pharyngocutaneous fistula. Otolaryngol Head Neck Surg 2009;141:190–195. [DOI] [PubMed] [Google Scholar]
- 41. Gil Z, Gupta A, Kummer B, et al. The role of pectoralis major muscle flap in salvage total laryngectomy. Arch Otolaryngol Head Neck Surg 2009;135:1019–1023. [DOI] [PubMed] [Google Scholar]
- 42. Withrow KP, Rosenthal EL, Gourin CG, et al. Free tissue transfer to manage salvage laryngectomy defects after organ preservation failure. Laryngoscope 2007;117:781–784. [DOI] [PubMed] [Google Scholar]
- 43. Oosthuizen JC, Leonard DS, Kinsella JB. The role of pectoralis major myofascial flap in salvage laryngectomy: a single surgeon experience. Acta Otolaryngol 2012;132:1002–1005. [DOI] [PubMed] [Google Scholar]
- 44. Grau C, Johansen LV, Hansen HS, et al. Salvage laryngectomy and pharyngocutaneous fistulae after primary radiotherapy for head and neck cancer: a national survey from DAHANCA. Head Neck 2003;25:711–716. [DOI] [PubMed] [Google Scholar]
- 45. Léon X, Agöero A, López M, et al. Salvage surgery after local recurrence in patients with head and neck carcinoma treated with chemoradiotherapy or bioradiotherapy. Auris Nasus Larynx 2015;42:145–149. [DOI] [PubMed] [Google Scholar]
- 46. Suzuki H, Hanai N, Nishikawa D, Fukuda Y, Hasegawa Y. Complication and surgical site infection for salvage surgery in head and neck cancer after chemoradiotherapy and bioradiotherapy. Auris Nasus Larynx 2017;44:596–601. [DOI] [PubMed] [Google Scholar]


