Abstract
Aim:
The aim of the study is to find the incidence of analgesic and opioid use in pain associated in HNC patient undergoing radiation therapy.
Background:
Radiation therapy with concurrent chemotherapy has become the standard of care in head and neck cancer. Acute toxicity like mucositis and dysphagia has increased with aggressive therapy. Pain is an invariable accompaniment of oropharyngeal mucositis, which leads to decreased quality of life and treatment break.
Materials and Methods:
This is a retrospective review of radiation charts of head and neck patients treated from January 2013 to June 2017 at St. John's Medical college and Hospital, Bengaluru.
Results:
A total of 138 (92%) patients required analgesia during the radiation course. The analgesic consumption started increasing from week 2, peaked at week 5, persist for 6 weeks and started declining after week 10. 52% patients required opioids, especially from week 4 to week 8. 15% of patients required Morphine, the maximum use in week 6 to week 8. The use of chemotherapy (P = 0.031), presence of grade 3 mucositis (P = 0.010) and grade 3 dysphagia (P = 0.001) were significantly associated with severe pain (use of strong opioids). All 80 (100%) patients receiving concurrent chemotherapy required analgesia. More than 80% patients required opioids and one fourth required strong analgesic in concurrent chemotherapy group.
Conclusion:
More than 90% of all head and neck cancer patient undergoing radiation therapy experience therapy related pain for more than 6 weeks. 53% of the patients require opioids and 15% require strong opioids. The use of concurrent chemotherapy was significantly associated with severe pain.
Keywords: Head-and-neck cancer, mucositis pain, opioid, radiation mucositis
INTRODUCTION
Head-and-neck cancer (HNC) remains the most common cancer in India and constitutes 25% of cancer burden. Most patients present in advanced stage. In general, surgery is the primary modality in oral cavity and radiation therapy in pharyngeal and laryngeal cancer. Radiation therapy is the most common therapy either in curative or palliative treatment of HNC patients.
In search of cure, cancer-directed therapy has become more and more aggressive over time. Combined modality treatment is the cornerstone of modern cancer therapy. Concurrent chemoradiation (CCRT) is the standard of care in the majority of HNC patients. The absolute benefit of chemotherapy is to the tune of 6.5% at 5 years when given concurrently to radiation therapy in HNC.[1] Along with increased survival, CCRT comes with increased acute toxicities. As pointed out by Bentzen and Trotti,[2] the toxicity pattern has not been well documented with CCRT and it has already become the standard of care in clinical practice. The incidence of mucositis increased by 2-fold when chemotherapy was added to radiation therapy.[3,4] With increased incidence of oropharyngeal mucositis, dysphagia, aspiration, and pain are invariable accompaniments. These lead to decreased quality of life, increased cost, treatment break, and incomplete treatment.
The advancement of radiation technology especially intensity modulated radiation therapy has decreased late toxicities like xerostomia and spinal cord injury but acute mucositis and associated pain remains a concern. At present, only maintaining good oral hygiene and controlling pain are the treatment for mucositis. In a review of 33 studies on radiation mucositis, the incidence of pain was 69%, and only a single study with small sample size (n = 30) mentioned the incidence of opioid use about 53%.[5] The opioid consumption is very low in India compared with high-income countries. The policies and practice change are required for cancer pain management in India.[6]
The authors believe underestimation of pain and underutilization of opioids in radiation mucositis in HNC patients in the present era of aggressive treatment strategies. In this study, we intend to find incidence and pattern of analgesic and opioid use in pain due to radiation mucositis in HNC patients.
MATERIALS AND METHODS
The aim of the study is to find the incidence of analgesic and opioid use in pain associated with HNC patient undergoing radiation therapy. This retrospective review includes HNC patients treated between January 2013 and June 2017 at St. John's Medical College, Bengaluru, Karnataka, India. All HNC patients on radiation therapy are being reviewed twice a week during radiation therapy as per department protocol and followed on a monthly basis till 6 months of starting radiation therapy. At every contact, acute toxicity, pain, analgesic use, and other relevant parameters are recorded in radiation therapy chart. Acute toxicity and pain were graded as per Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. We mined all records belonging to HNC patients and collected different parameters required.
All data entry and descriptive analysis were done in Microsoft Excel Worksheet. The maximum pain score is recorded in data sheet. The primary outcome is incidence of pain and opioid/strong opioids use in head-and-neck cancer patients undergoing radiation therapy. For evaluation of factors associated with severe pain (use of strong opioids), Chi-square test was used for categorical variables and t-test used for continuous variable. Chi-square test and t-test were done on STATA version 14, StataCorp LLC, Texas, USA. The various factors which were evaluated were: age (≤60 vs. >60 years), sex, Charlson Comorbidity Index, diabetes mellitus, site of primary tumor, tumor (T1, T2, T3, and T4), Node (N1, N2, and N3), stage grouping (I, II, II, IVA, and IVB), treatment modality (Radiation alone, Postoperative radiation, CCRT, Postoperative CCRT), RT dose received, use of concurrent chemotherapy, CCRT schedule (weekly vs. 3 weekly), Grade ≥ 3 mucositis, and grade ≥3 dysphagia.
RESULTS
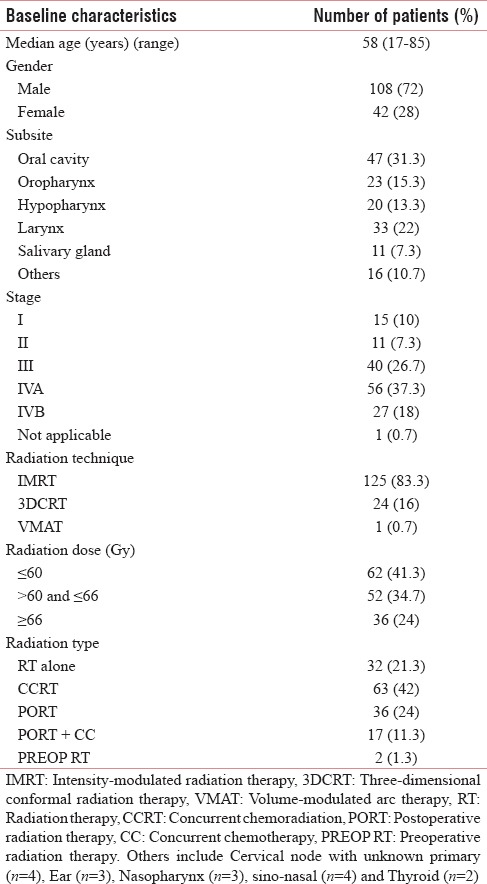
A total of 165 patients of HNC were treated at our center. Fifteen patients received palliative radiation therapy and were excluded from analysis. The baseline characteristics of patient, disease, and treatment received are depicted in Table 1. Majority of patients were male (72%). Nearly 82% of cancer belonged to Stage III, IVA, and IVB (%). Combined modality treatment was received by 78.2% of cases.
Table 1.
Baseline characteristics of patient, tumor, and treatment received (n=150)
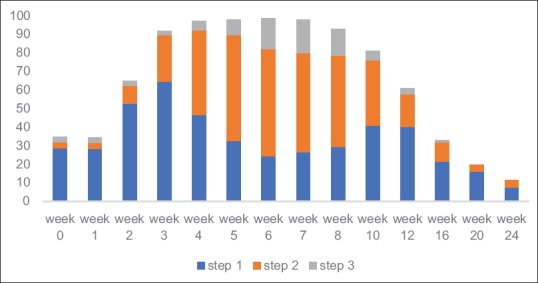
A total of 138 (92%) patients required analgesia during the radiation course. The most commonly used nonopioids were paracetamol and diclofenac. Tramadol and paracetamol were used in Step 2 and morphine and paracetamol were used in Step 3. The analgesic consumption started increasing from week 2, peaked at week 5, persisted for 6 weeks and started declining after week 10 [Figure 1]. The pattern of severity of pain followed severity of mucositis. More than 90% of patients required analgesia from week 3 to week 8. Even at 24 weeks of starting radiation, 11% patients required analgesics. Nearly 52% of patients required opioids, especially from week 4 to week 8. Almost 15% of patients required morphine, the maximum use in week 6 to week 8. Amitriptyline was used in 32 (21%) patients.
Figure 1.
Pattern of analgesic use for 24 weeks from start of radiation therapy. Step 1, 2, and 3, indicate WHO pain ladder (n=150)
The use of chemotherapy (P = 0.031), presence of Grade 3 mucositis (P = 0.010), and Grade 3 dysphagia (P = 0.001) were significantly associated with severe pain (use of strong opioids).
All eighty patients receiving concurrent chemotherapy required analgesia. Pain persisted for 8 weeks and started declining after 12 weeks of starting radiation therapy. More than 80% of patients required opioids and one-fourth required strong analgesic in CCRT group.
DISCUSSION
Pain during treatment of HNC patients is a common and troublesome issue. Almost 80% of patients experience pain during treatment and up to 36% have pain after 6 months of completing therapy.[7] In this study, 92% of patients had pain during therapy and 11% required analgesic after 6 months of starting radiation therapy. Severe pain was associated with the use of chemotherapy.
Various local and systemic strategies are used for pain management due to radiation mucositis. In a review by Trotter, there was insufficient evidence to recommend any optimal intervention for pain management in HNC.[8] Therefore, the current recommendation is to follow WHO pain ladder until further study proves otherwise. In the present study, the treating radiation oncologist followed the WHO pain ladder for radiation-induced mucositis pain management.
The study is retrospective in nature and is dependent on data recorded by treating physician in patient review chart. All patients' data were available in RT review charts. Patients' pain assessment was done by verbal or numerical rating scale, and analgesic was titrated accordingly. The pain assessment was done twice a week and as required during treatment and monthly after completing treatment. However, the pain rating scale was not documented in radiation review chart. The nonavailability of pain scale is the major limitation of the study. The use of topical agents, gabapentin and pregabalin, was not standardized and there was wide variation between treating oncologist in using these agents. Therefore, data regarding usage of these agents were not analyzed.
There is a lack of literature concerning management of pain in head-and-neck cancer patients undergoing radiation therapy and systemic therapy.[7] In review (n = 6181) by Trotti et al., only a single study (n = 30) stated the use of opioid to be 53%.[5] The reported incidence of opioid use and Grade 3–4 mucositis was 53% and 23%, respectively.[9] In the current study, incidence of opioid use and Grade 3–4 mucositis is 52% and 22%, respectively. The use of strong opioids was not mentioned and is 15% in the current study.
In a recent review, numerical rating scale or verbal rating scale or visual analog scale was recommended for pain assessment.[7] In the present study, verbal rating scale was used and documented as mild, moderate, or severe based on CTCAE 4.03. Daily pain assessment is also recommended for better pain control.[7] Pain assessment was done twice a week in our study.
This study highlights the requirement of analgesia during head and neck radiation therapy and describes the patterns of analgesic use. The radiation oncologists need to sensitize toward pain and analgesic requirement during head-and-neck radiation therapy.
CONCLUSION
More than 90% of all head-and-neck cancer patients undergoing radiation therapy experience therapy-related pain for >6 weeks. Almost 53% of the patients require opioids and 15% require strong opioids. The use of concurrent chemotherapy was significantly associated with severe pain.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.MACH-NC Collaborative Group. Pignon JP, le Maître A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Bentzen SM, Trotti A. Evaluation of early and late toxicities in chemoradiation trials. J Clin Oncol. 2007;25:4096–103. doi: 10.1200/JCO.2007.13.3983. [DOI] [PubMed] [Google Scholar]
- 3.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre JL, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–52. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 4.Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–44. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 5.Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother Oncol. 2003;66:253–62. doi: 10.1016/s0167-8140(02)00404-8. [DOI] [PubMed] [Google Scholar]
- 6.Lebaron V, Beck SL, Maurer M, Black F, Palat G. An ethnographic study of barriers to cancer pain management and opioid availability in India. Oncologist. 2014;19:515–22. doi: 10.1634/theoncologist.2013-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirabile A, Airoldi M, Ripamonti C, Bolner A, Murphy B, Russi E, et al. Pain management in head and neck cancer patients undergoing chemo-radiotherapy: Clinical practical recommendations. Crit Rev Oncol Hematol. 2016;99:100–6. doi: 10.1016/j.critrevonc.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Trotter PB, Norton LA, Loo AS, Munn JI, Voge E, Ah-See KW, et al. Pharmacological and other interventions for head and neck cancer pain: A systematic review. J Oral Maxillofac Res. 2013;3:e1. doi: 10.5037/jomr.2012.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bensadoun RJ, Franquin JC, Ciais G, Darcourt V, Schubert MM, Viot M, et al. Low-energy He/Ne laser in the prevention of radiation-induced mucositis. A multicenter phase III randomized study in patients with head and neck cancer. Support Care Cancer. 1999;7:244–52. doi: 10.1007/s005200050256. [DOI] [PubMed] [Google Scholar]


