Abstract
Purpose
To utilize results from the Ocular Hypertension Study that can aid patients and clinicians to make evidence based decisions about the management of ocular hypertension.
Design
1636 participants with ocular hypertension were randomized to treatment with topical ocular hypotensive treatment or close observation. The goal of treatment was a 20% reduction in intraocular pressure and an intraocular pressure of ≤ 24 mmHg. After a mean of 7.5 years, participants in the observation group also received treatment. Visual field tests that were performed twice a year and optic disc photographs once a year.
Results
At 60 months, the cumulative frequency of developing POAG was 4.4% in the medication group and 9.5% in the observation group (hazard ratio for medication, 0.40; 95% CI, 0.27–0.59; P <.0001). At 13 years the cumulative proportion of participants who developed POAG was 0.22 (95% CI 0.19–0.25) in the original observation group and 0.16 (95% CI 0.13–0.19) in the original medication group (complimentary log-log x2 P = .009). A 5 factor model (older age, higher IOP, thinner central cornea thickness, larger cup/disc ratio and higher visual field pattern standard deviation) separated participants at high and low risk of developing POAG.
Conclusion
Clinicians and patients can make evidence based decisions about the management of ocular hypertension using the risk model and considering patient age, medical status, life expectancy and personal preference.
Elevated intraocular pressure (IOP) is a leading risk factor for the development of primary open angle glaucoma (POAG) and the only modifiable risk factor at present. Patients with elevated IOP and no signs of POAG on standard clinical tests are referred to as having ocular hypertension (OHT). It is estimated that 4–7% of the people in the United States above age 40 years have OHT1. Managing this large group of people is time consuming and costly for patients, clinicians and society. The high prevalence of OHT and its relationship to the potentially blinding disease, POAG, raises several important questions including: How often should ocular hypertensive patients be examined? What diagnostive tests should be performed? Can we identify OHT patients at high-risk vs low risk for developing POAG? How many OHT patients will develop visual limitations due to POAG? Finally, which ocular hypertensive patients might benefit from early preventative treatment? This perspective summarizes results from the Ocular Hypertension Treatment Study (OHTS) that can aid patients and clinicians to make evidence based decisions about the management of OHT.
OHTS Phase 1 (Februay 1994 – June 2002)
OHTS phase 1 randomized 1636 ocular hypertensive participants to ocular hypotensive medication or close observation2. 25% of the participants were self-identified African Americans. The entry criteria are listed in the Table. The goal of treatment was to reduce IOP by 20% or more and to reach an IOP ≤24 mmHg. Clinicians could utilize any topical ocular hypotensive medication approved for use in the United States. Participants were seen twice a year for visual field tests and once a year for stereoscopic optic disc photographs. Masked readers at the respective reading centers reviewed the visual fields and optic disc photographs. If two consecutive sets of the optic disc photographs or three consecutive sets of visual fields demonstrated change, the case was reviewed by a masked endpoint committee to determine whether the change was due to POAG or not. The Endpoint Committee reviewed medical history and visual fields and optic disc photographs for both eyes from baseline to current.
Table.
| Inclusion Criteria |
| IOP in one eye of ≥ 24 mm Hg and ≤ 32 mm Hg. |
| IOP in fellow eye of ≥ 21 mm Hg and ≤ 32 mm Hg. |
| Age 40 to 80 years. |
| Normal and reliable Humphrey 30-2 visual fields for both eyes as determined by the Visual Field Reading Center. |
| Normal optic discs in both eyes on clinical examination and on photographs as determined by the Optic Disc Reading Center. |
OHTS Phase I had two Major Goals
To determine the safety and efficacy of topical ocular hypotensive medication in delaying or preventing the onset of POAG in ocular hypertensive patients.
To determine baseline clinical and demographic factors that predict which patients would or would not develop POAG.
At the end of 60 months, the cumulative frequency of developing POAG was 4.4% in the medication group and 9.5% in the observation group (hazard ratio for medication, 0.40; 95% CI, 0.27–0.59; P <.0001)3. This protective effect of treatment was statistically significant for both optic disc and visual field changes. OHTS provided clear evidence of treatment efficacy. We found no evidence of increased systemic or ocular risk associated with ocular hypotensive medication.
As anticipated, most of the early glaucomatous changes were detected on the optic disc photographs but about one third of the participants had their initial glaucomatous change detected on visual field tests3. Thus, it is necessary to monitor both functional and structural parameters as much as our patients might prefer to avoid visual field testing.
The second goal of OHTS Phase 1 was to identify baseline demographic and clinical factors that predict which patients would develop POAG. We determined that five baseline factors (older age, higher IOP, thinner central corneal thickness, larger cup/disc ratio and higher visual field pattern standard deviation) separated participants at high and low risk of developing POAG4. The model was subsequently confirmed by the European Glaucoma Prevention Study5 and in a study by Medeiros and coworkers6. The inclusion of age and IOP in the predictive model was not a surprise, but the inclusion of CCT was unexpected. The reason CCT is a good predictor of POAG is not entirely clear but we believe it is a biomarker for viscoelastic properties of the eye. Recent research suggests that another measure of the viscoelastic properties of the eye, corneal hysteresis, may be an even better predictor than CCT7. One could object to including cup/disc ratio and visual field PSD in the model as these factors are related to the outcome measures of POAG. However, when a clinician examines a patient for the first time he or she does not know if a cup/disc ratio of 0.5 represents a baseline measure or a change from an unknown baseline. Thus, including these measures is useful and appropriate. The OHTS predictive model has been widely adopted and is included in many preferred practice plans. It is interesting that race was not included in the multivariate risk model. We know that POAG is more common in African Americans but race drops out of the model when cup/disc ratio and CCT are included4. It is possible that race would have remained in the model if the sample size of OHTS were larger.
OHTS Phase 2 (June 2002–March 2009)
OHTS phase 1 was a proof of concept – early treatment decreased the incidence of POAG in ocular hypertensive individuals. Once lowering IOP had been proven effective in OHTS phase 1, it was important to determine when treatment should be initiated. One approach would be to treat every individual with elevated IOP. However, the potential benefit of treatment would have to outweigh the low conversion rate to POAG as well as the cost, inconvenience and potential adverse effects of treatment. A second approach would be to defer treatment until patients have early reproducible signs of POAG. However, delayed treatment may start a process of retinal nerve fiber degeneration that is less responsive to treatment i.e. patients who receive delayed treatment may be more likely to develop visual impairment or blindness in their lifetimes. A third approach would be to treat selected OHT patients who are at moderate to high risk for developing POAG. The best approach depends on whether there is a penalty for delayed treatment8.
There are two ways to measure whether delaying treatment is harmful by comparing early treatment and delayed treatment groups. The first is to determine if the cumulative incidence of POAG is greater in the delayed treatment group. The second is to determine if the subsequent course after diagnosis is worse in the delayed treatment group. In OHTS phase 2, participants previously randomized to treatment for a mean of 7.5 years remained on treatment for a mean of 5.5 additional years. Participants randomized to observation for a mean 7.5 years were then treated for a mean of 5.5 years. This created an early treatment group and a delayed treatment group. All the tests, measures and procedures remained the same as in OHTS phase 1. The cumulative frequency of participants who developed OAG over a median of 13 years is given in the Figure.
Figure 1.
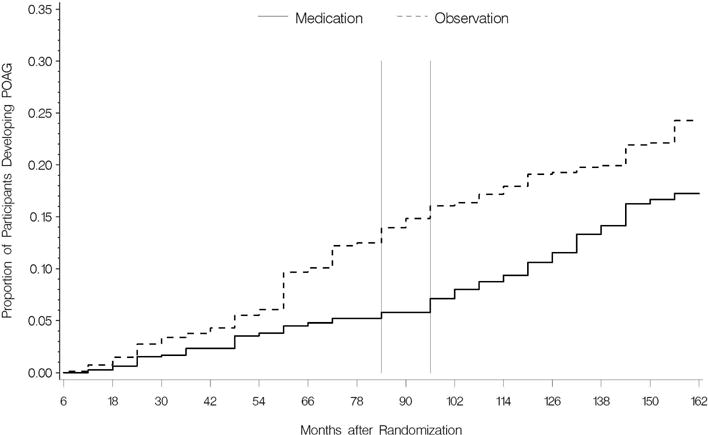
Cumulative probability of developing primary open-angle glaucoma (POAG) from February 1994 to March 2009 by randomization group.
The time between the two vertical lines indicate the initiation of medication in the original observation group.
The cumulative proportion of participants who developed POAG from randomization to 13 years of follow up was 0.22 (95% CI 0.19–0.25) in the original observation group and 0.16 (95% CI 0.13–0.19) in the original medication group (complimentary log-log x2 P = .009). The median time to develop POAG was 6.0 years in the observation group and 8.7 years in the medication group. The Figure demonstrates that the cumulative frequency curves diverge in phase 1 (HR for medication 0.42 95% CI 0 – 0.59) while the curves appear parallel in phase 2 (HR for medication, 1.06 95% CI 0.14 – 1.50 p=.77). These data demonstrate that the protective effect of medical treatment has a relatively rapid onset.
When the OHTS participants were divided into 3 equal sized groups on the basis of low, medium and high-risk using the prediction model mentioned above, we found that the percent protective effect of treatment was similar in the 3 groups but the absolute reduction in incidence was small in the low risk group and substantial in the high-risk group.
Another way to judge the effect of delayed treatment is to determine the subsequent course of participants in the observation group and the treatment group after the diagnosis of POAG. The slope of MD over time among participants who developed POAG did not differ by randomization group during the post POAG period (p=.23). With a median post-POAG follow-up of 3.5 years the PSD slopes of participants in the observation group were slightly worse than in the treatment group (p<.001). Thus, overall we can conclude that there is a modest penalty for delaying treatment in OHT subjects. This penalty is minimal for low risk patients and more substantial for high-risk patients8. This suggests that high-risk patients may benefit from more frequent examinations and from early treatment taking into consideration patient age, health status, life expectancy and personal preference. Conversely, most OHT patients at low risk could be followed at less frequent intervals without treatment.
Over the 13 year follow-up, 28% of African Americans and 16% of other participants developed POAG9. This difference occurred despite African Americans and others having similar initial and follow-up IOPs and similar treatment. OHTS participants, as is typical in many studies, were healthier, better educated and of higher socioeconomic status than the general population. OHTS provided all participants with ocular hypotensive medication free of charge. Thus, many of the common barriers to treatment were reduced and still African Americans had a worse prognosis. The worse prognosis is related to baseline risk factors of CCT and cup to disc ratio. African Americans are over represented in the baseline high-risk group and underrepresented in the low risk group.
OHTS Phase 3 (July 2015 – June 2020)
The OHTS project has been funded for a 20-year follow-up of the original study participants. We believe this information will provide further guidance to patients and clinicians about the appropriate frequency of follow-up and the benefit of early treatment. OHTS Phase 3 has 4 main objectives.
Determine the incidence and severity of POAG in the cohort.
Develop a 20-year prediction model including the previous factors identified and potential new risk factors. It is not clear of this approach is feasible or whether it is better to repeat the 5 year prediction model at various intervals.
Develop a model that identifies slow vs fast visual field progressors.
Determine the frequency and severity of self-reported limitations associated with POAG and correlate this with clinical findings. OHTS Phase 3 has the largest inception cohort of POAG ever reported and has a concurrent control group that underwent the same tests and measures for 20 years did not develop POAG.
Summary
On the basis of OHTS Phase I and 2, we recommend OHT patients and clinicians consider the following factors when deciding on management.
Early medical treatment of ocular hypertensive patients reduces the 5-year incidence of POAG by 60%.
A 5-factor model (age, IOP, CCT, C/D ratio and visual field PSD) has reasonable accuracy in distinguishing high from low risk OHT patients.
The absolute benefit of early treatment is greatest in high-risk OHT patients. Clinicians and patients can decide on the potential benefit of early treatment based on risk level and patient age, health status, life expectancy and personal preference.
There is little absolute benefit of early treatment in low risk OHT patients. Most OHT patients fall into this group and probably can be followed less frequently without treatment.
The cumulative risk of developing POAG in OHTS patients was linear over at least 15 years. There did not appear to be a period beyond which OHT patients no longer developed POAG.
African American OHT patients develop POAG at a higher rate despite similar baseline IOP, follow up IOP and treatment. The higher incidence of POAG was related to baseline cup/disc ratio and CCT. This does not imply that all African American OHT patients should be treated. The prediction formula can be utilized for African American patients to make evidence based decisions.
Starting medical treatment after the development of early signs of POAG has only a modest impact on the rate of visual field loss over the subsequent 5-year follow up. Some clinicians may choose not to treat any OHT patients until early glaucomatous damage is confirmed. However, this approach requires patients to return for follow-up visits and appropriate diagnostic tests in a timely fashion.
Given the wide variety of ocular hypotensive medications available, there appear to be safe, well-tolerated choices for reducing IOP in most OHT patients should clinicians, and patients reach this decision.
It is still necessary to assess structural and functional parameters in OHT patients to determine if POAG has developed and/or is progressing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mae O. Gordon, Department of Ophthalmology and Visual Sciences, Washington University School of Medicine, 660 S. Euclid Avenue, St. Louis, MO 63110
Michael A. Kass, Department of Ophthalmology and Visual Sciences, Washington University School of Medicine, 660 S. Euclid Avenue, St. Louis, MO 63110
References
- 1.Leibowitz HM, Krueger DE, Maunder LR, et al. The Framingham Eye Study monograph: An ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973–1975. Surv Ophthalmol. 1980;24(Suppl):335–610. [PubMed] [Google Scholar]
- 2.Gordon MO, Kass MA, for the Ocular Hypertension Treatment Study G The Ocular Hypertension Treatment Study: Design and Baseline Description of the Participants. Archives of Opthalmology. 1999;117:573–583. doi: 10.1001/archopht.117.5.573. [DOI] [PubMed] [Google Scholar]
- 3.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study – A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Archives of Ophthalmology. 2002;120(6):701–13. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 4.Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714–20. doi: 10.1001/archopht.120.6.714. discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 5.Miglior S, Zeyen T, Pfeiffer N, et al. Results of the European Glaucoma Prevention Study. Ophthalmology. 2005;112(3):366–75. doi: 10.1016/j.ophtha.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Medeiros FA, Weinreb RN, Sample PA, et al. Validation of a predictive model to estimate the risk of conversion from ocular hypertension to glaucoma. Arch Ophthalmol. 2005;123(10):1351–60. doi: 10.1001/archopht.123.10.1351. [DOI] [PubMed] [Google Scholar]
- 7.Congdon NG, Broman AT, Bandeen-Roche K, et al. Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am J Ophthalmol. 2006;141(5):868–75. doi: 10.1016/j.ajo.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Kass MA, Gordon MO, Gao F, et al. Delaying treatment of ocular hypertension: the ocular hypertension treatment study. Arch Ophthalmol. 2010;128(3):276–87. doi: 10.1001/archophthalmol.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higginbotham EJ, Gordon MO, Beiser JA, et al. The Ocular Hypertension Treatment Study: topical medication delays or prevents primary open-angle glaucoma in African American individuals. Arch Ophthalmol. 2004;122(6):813–20. doi: 10.1001/archopht.122.6.813. [DOI] [PubMed] [Google Scholar]


