Abstract
This study with poplar (Populus tremula × Populus alba) cuttings was aimed to test the hypothesis that sulfate uptake is regulated by demand-driven control and that this regulation is mediated by phloem-transported glutathione as a shoot-to-root signal. Therefore, sulfur nutrition was investigated at (a) enhanced sulfate demand in transgenic poplar over-expressing γ-glutamylcysteine (γ-EC) synthetase in the cytosol and (b) reduced sulfate demand during short-term exposure to H2S. H2S taken up by the leaves increased cysteine, γ-EC, and glutathione concentrations in leaves, xylem sap, phloem exudate, and roots, both in wild-type and transgenic poplar. The observed reduced xylem loading of sulfate after H2S exposure of wild-type poplar could well be explained by a higher glutathione concentration in the phloem. In transgenic poplar increased concentrations of glutathione and γ-EC were found not only in leaves, xylem sap, and roots but also in phloem exudate irrespective of H2S exposure. Despite enhanced phloem allocation of glutathione and its accumulation in the roots, sulfate uptake was strongly enhanced. This finding is contradictory to the hypothesis that glutathione allocated in the phloem reduces sulfate uptake and its transport to the shoot. Correlation analysis provided circumstantial evidence that the sulfate to glutathione ratio in the phloem may control sulfate uptake and loading into the xylem, both when the sulfate demand of the shoot is increased and when it is reduced.
Sulfur ranks fifth or sixth by quantity of nutrient elements in plants (Cram, 1990). In its reduced form, i.e. in the oxidation state-II, sulfur is mainly found as a structural and functional component of proteins. Sulfur is mostly available to plants in the oxidized form of sulfate in the soils and must thus be reduced and assimilated into the sulfur-containing amino acids Cys and Met to fulfil requirements of protein synthesis. The processes of both reduction and assimilation are often restricted to mature leaves (Brunold, 1990), but especially in trees, considerable reduction and assimilation may also occur in the roots. The supply of stem tissues and young developing leaves with reduced sulfur from assimilatory sulfate reduction in the roots is particularly notable in trees (Herschbach and Rennenberg, 1997).
Sulfate supply at the whole plant level may be controlled by regulation of sulfate uptake by the roots whereas the supply to the shoot may be determined by xylem loading of sulfate in the root. In herbaceous plants, several pieces of evidence indicate that sulfate uptake and subsequent loading into the xylem are regulated by phloem allocation of glutathione. When glutathione was supplied to excised tobacco roots, sulfate uptake and loading into the xylem stream were inhibited (Herschbach and Rennenberg, 1991). Exposure of the shoot to atmospheric H2S enhanced the glutathione contents of leaves and roots and, simultaneously, diminished sulfate uptake and xylem loading (Herschbach et al., 1995a, 1995b; Rennenberg and Herschbach, 1996; De Kok et al., 1997, 1998). Since H2S can be used as a source of reduced sulfur by the leaves (De Kok, 1990; Rennenberg and Herschbach, 1996; De Kok et al., 1997, 1998), it was concluded from these experiments that glutathione can signal the sulfur status of the shoot to the root (Rennenberg, 1995; Rennenberg and Herschbach, 1995). Consistent with this view Lappartient and Touraine (1996) found that in canola, glutathione transported via the phloem to the roots reduced sulfate uptake by the roots. Apparently, phloem-mediated shoot-to-root allocation of glutathione executes demand-driven control of sulfate uptake and/or loading into the xylem stream by the roots in herbaceous plants.
In perennial plants regulation of sulfate nutrition seems to be much more complex. Storage and mobilization processes require seasonal changes in the regulation of sulfate uptake and xylem loading of sulfate that are at least partially independent of the sulfur status of the shoot (Herschbach and Rennenberg, 1997; Schulte et al., 1998). These processes are reflected by seasonal changes in the sulfur composition and contents of xylem sap along the tree axis (Schupp et al., 1991; Rennenberg et al., 1994; Schneider et al., 1994). Because of the relatively long distances between root and shoot and the corresponding time delay, shoot-to-root signaling via phloem transport of glutathione may not be an exclusive signal in the control of sulfate uptake and loading into the xylem in adult trees. This conclusion is supported by a lack of basipetal phloem transport of glutathione fed to the needles of spruce trees (Schupp et al., 1992; Blaschke et al., 1996). However, basipetal phloem transport of glutathione has been observed in seedlings of several deciduous tree species (Herschbach and Rennenberg, 1995, 1996; Schulte et al., 1998). However, this transport was not necessarily connected with the regulation of sulfate nutrition. In mycorrhizal and non-mycorrhizal beech, sulfate uptake by excised roots was inhibited by Cys and Met, but not by glutathione; xylem loading of sulfate was enhanced rather than decreased in beech seedlings when glutathione or Cys were fed (Kreuzwieser et al., 1996; Kreuzwieser and Rennenberg, 1998). In excised poplar (Populus tremula × Populus alba) roots sulfate uptake and loading into the xylem were inhibited by glutathione (Van der Zalm et al., 2000). Hence, it has been suggested that the model of demand-driven control of sulfur nutrition by phloem transport of glutathione from the shoot to the root cannot easily be transferred from herbaceous to perennial plant species (Herschbach and Rennenberg, 1997).
In the present study two experimental approaches were used to test the model of demand-driven control. In a first approach we reduced the sulfate demand of poplar shoots by H2S fumigation; under these conditions significant amounts of H2S are taken up by the leaves and are incorporated into organic sulfur compounds, whereas assimilatory sulfate reduction declines (Brunold, 1990; De Kok, 1990). In a second approach the sulfate demand of poplar shoots was enhanced by over-expression of γ-glutamyl-Cys (γ-EC) synthetase (γ-ECS) in the cytosol. In contrast to recent experiments with tobacco plants that over-expressed γ-ECS in the chloroplasts (Creissen et al., 1999), transgenic poplar that over-expressed γ-ECS in the cytosol displayed no symptoms of oxidative stress (Noctor et al., 1998). Over-expression of γ-ECS in the cytosol may overcome Cys limitation of glutathione synthesis and enhanced amounts of glutathione and γ-EC are synthesized in the leaves (Noctor et al., 1996). The sulfate requirement for enhanced γ-EC and glutathione synthesis in poplar lines over-expressing γ-ECS must increase the sulfate demand. In both experimental approaches, sulfate and reduced sulfur compounds were analyzed in leaves, roots, phloem exudates, and xylem saps; in addition, sulfate uptake and xylem loading of sulfate of excised poplar roots were determined.
RESULTS
Developmental Stage of Wild-Type and Transgenic Poplar during H2S Exposure
Both wild-type and transgenic poplar over-expressing the bacterial gene of γ-ECS in the cytosol had developed 11 to 17 leaves at the end of the fumigation experiment. Although transgenic and wild-type poplar were in the same age and from the same batch of micropropagated trees, transgenic plants were slightly greater in shoot fresh weight and height than wild-type plants irrespective of H2S fumigation but were similar in root fresh weight, shoot to root ratio, or shoot and root fresh weight to dry weight ratio (Table I). Short-term exposure to H2S did not significantly alter any of the growth parameters (Table I).
Table I.
Developmental stage of wild-type and transgenic (over-expressing γ-ECS in the cytosol, line ggs 28) poplar after H2S exposure
| Wild Type | Wild Type | Transgenic | Transgenic | |
|---|---|---|---|---|
| 0 μL L−1 H2S | 0.25 μL L−1 H2S | 0 μL L−1 H2S | 0.25 μL L−1 H2S | |
| Shoot fresh wt (g) | 5.0 ± 1.5a | 5.4 ± 1.3a,b | 6.4 ± 1.6b,c | 6.8 ± 2.2c |
| Root fresh wt (g) | 1.2 ± 0.6a | 1.7 ± 0.5a | 1.7 ± 0.5a | 2.0 ± 0.9a |
| Shoot to root ratio | 3.7 ± 0.6a | 3.3 ± 0.4a | 3.6 ± 0.5a | 3.6 ± 0.4a |
| Shoot (fresh wt/dry wt) | 4.7 ± 0.3a | 4.8 ± 0.6a | 4.4 ± 0.2a | 4.7 ± 0.6a |
| Root (fresh wt/dry wt) | 9.9 ± 1.4a | 9.9 ± 2.5a | 10.0 ± 1.1a | 10.3 ± 0.9a |
| Plants height (cm) | 18.3 ± 3.0a | 20.8 ± 3.7a | 22.5 ± 3.9a,b | 25.5 ± 4.4b |
| No. of leaves | 13 ± 2a | 13 ± 1a | 14 ± 3a | 15 ± 2a |
Transgenic and wild-type poplar from one batch of micropropagated trees were either exposed to 0 or 0.25 μL L−1 H2S for 48 h during a 3-week period of the experiments. The data shown are means ± sd of seven to 14 plants of each poplar line and each treatment. Significant differences at P < 0.05 between treatments are indicated with different indices.
H2S Uptake, Photosynthesis, and Transpiration
Transpiration and the rate of photosynthesis were similar in both wild-type and transgenic poplar, respectively (Table II). Poplar shoots formed a sink for atmospheric H2S. At 0.25 μL L−1 the rates of H2S uptake of wild-type and transgenic poplar were similar (Table II). H2S fumigation did not significantly influence the rate of photosynthesis or transpiration in either wild-type or transgenic plants (Table II).
Table II.
Influence of H2S exposure on the rates of photosynthesis and transpiration of wild-type and transgenic poplar and H2S uptake rates during the photoperiod
| Wild Type | Wild Type | Transgenic | Transgenic | |
|---|---|---|---|---|
| 0 μL L−1 H2S | 0.25 μL L−1 H2S | 0 μL L−1 H2S | 0.25 μL L−1 H2S | |
| Photosynthesis [μmol CO2 g fresh wt−1 h−1] | 137 ± 21a | 146 ± 21a | 126 ± 15a | 141 ± 14a |
| Transpiration [mmol H2O g fresh wt−1 h−1] | 37.7 ± 5.9a,b | 40.3 ± 4.5a | 36.5 ± 3.1a,b | 34.6 ± 5.1b |
| H2S uptake [nmol H2S g fresh wt−1 h−1] | – | 223 ± 26a | – | 210 ± 20a |
Plants were exposed to H2S for 48 h. The temperature during the measurements was 20°C ± 1°C and the photon flux density was 380 ± 20 μmol m−2 s−1 (within the 400–700 nm range). The data shown are means ± sd of six plants of each poplar line and each treatment. Significant differences at P < 0.05 between treatments are indicated with different indices.
Sulfur Compounds in Poplar Leaves
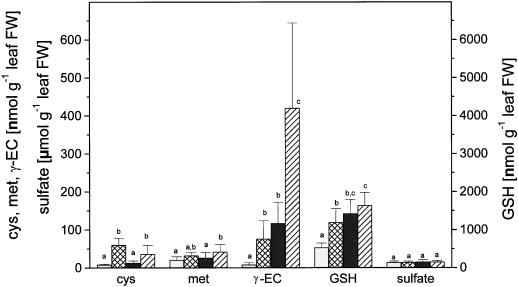
Sulfate and reduced soluble sulfur compounds were determined in the leaves of wild-type and transgenic poplar (Fig. 1). As previously published (Noctor et al., 1996), Cys concentrations did not differ significantly between the lines. Similar results were obtained for Met and sulfate. In the leaves of transgenic poplar, γ-EC concentrations were 14 times and glutathione concentrations more than twice those in leaves of the wild type (Fig. 1) as also observed by Noctor et al. (1996). Exposure to H2S had differing effects on concentrations of thiols, Met, and sulfate in leaves of wild-type and transgenic poplar (Fig. 1). Cys and Met concentrations were enhanced in the leaves of both poplar lines to a similar extent, whereas γ-EC concentrations of the leaves increased more markedly in transgenic lines as compared with the wild type in response to H2S fumigation (Fig. 1). Similar results were observed for glutathione, but the glutathione concentration of 1,425 ± 371 nmol g−1 fresh weight in the leaves of transgenic poplar exposed to ambient air was not significantly different from that of 1,642 ± 338 nmol g−1 fresh weight in leaves of transgenic poplar exposed to H2S. Apparently, glutathione concentrations in the leaves of the transgenic poplar were already close to maximum without H2S exposure (Rennenberg, 1997). Further increases in glutathione in leaves in response to H2S may be limited either by glutathione synthesis (Rennenberg, 1997) or glutathione export (Herschbach et al., 1998). Sulfate concentrations of the leaves were not affected by H2S fumigation in either wild-type or transgenic lines (Fig. 1).
Figure 1.
Influence of H2S exposure on thiol, Met, and sulfate in leaves of wild-type and transgenic (γ-ECS over-expressing in the cytosol) poplar (line ggs 28). Six- to 8-week-old wild-type poplar (□, ambient air; ▩, H2S) and transgenic poplar (▪, ambient air; ▨, H2S) were exposed for 48 h to ambient air or 0.25 μL L−1 H2S, respectively. Young mature, fully expanded leaves were analyzed for thiol, Met, and sulfate. The data shown are means ± sd with leaves from six to seven poplar plants each treatment. Significant differences at P < 0.05 between treatments are indicated with different indices. Note: 10-fold higher scale for glutathione (GSH) and the micromole unit for sulfate and the nanomole unit for reduced sulfur. FW, Fresh weight.
Sulfur Compounds in Phloem Exudates
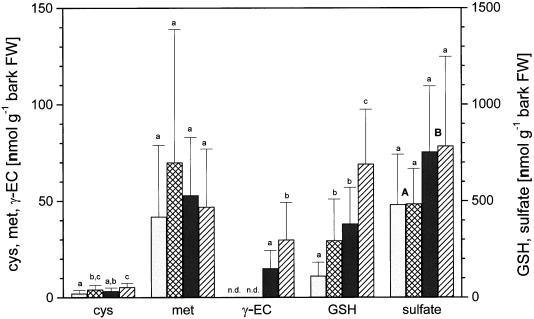
Over-expression of γ-ECS increased the concentrations of γ-EC, glutathione, and sulfate in phloem exudates (Fig. 2) as previously found by Herschbach et al. (1998). γ-EC increased more than 10-fold, glutathione 3.4-fold, and sulfate 1.6-fold. Cys and Met concentrations in phloem exudates were unchanged. Exposure to H2S increased concentrations of thiols in phloem exudates but not those of Met or sulfate (Fig. 2). Cys concentrations of phloem exudates significantly increased in response to H2S fumigation in both wild-type and transgenic poplar. γ-EC was only detected in phloem exudates of transgenic poplar and increased 2-fold in concentration in response to H2S exposure. Glutathione concentrations were significantly increased in phloem exudates of wild-type as well as transgenic poplar plants fumigated with H2S.
Figure 2.
Influence of H2S exposure on thiol, Met, and sulfate in shoot phloem exudates of wild-type and transgenic poplar. Six- to 8-week-old wild-type poplar (□, ambient air; ▩, H2S) and transgenic poplar (▪, ambient air; ▨, H2S) were exposed for 48 h to ambient air or 0.25 μL L−1 H2S, respectively. The data shown are means ± sd with phloem exudates from six to seven poplar plants each. Significant differences at P < 0.05 between treatments are indicated with different indices. Significant differences between the two poplar lines were indicated with A and B. n.d., Not detected. Note: 10-fold higher scale for sulfate and glutathione (GSH). FW, Fresh weight.
Sulfur Compounds in Lateral Roots
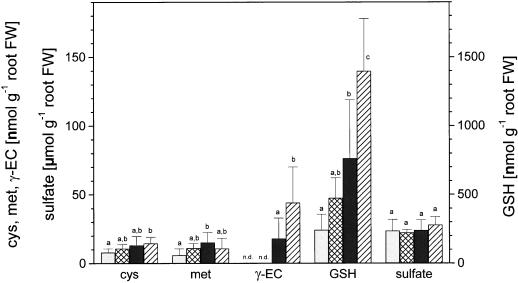
Compared to poplar leaves, sulfate concentrations in lateral roots were generally greater and thiol and Met contents generally lower, independent of the poplar line studied and the treatment applied. γ-ECS over-expression increased concentrations of Cys in roots by approximately 2-fold, of γ-EC by more than 10-fold, and of glutathione by approximately 3-fold (Fig. 3). Also, Met concentrations in roots were significantly increased by γ-ECS over-expression, whereas sulfate concentrations were not (Fig. 3). Exposure to H2S had no effect on Cys, Met, or sulfate concentrations independent of the poplar line analyzed (Fig. 3), whereas γ-EC and glutathione concentrations in the roots of transgenic poplar were about twice those in control plants. In wild-type poplar glutathione concentrations in roots increased slightly, but not significantly by H2S fumigation; γ-EC was not detected in wild-type poplar irrespective of H2S fumigation (Fig. 3).
Figure 3.
Influence of H2S exposure on thiol, Met, and sulfate in roots of wild-type and transgenic poplar. Six- to 8-week-old wild-type poplar (□, ambient air; ▩, H2S) and transgenic poplar (▪, ambient air; ▨, H2S) were exposed for 48 h to ambient air or 0.25 μL L−1 H2S, respectively. The data shown are means ± sd with lateral roots from six to seven poplar plants each treatment. Significant differences at P < 0.05 between treatments are indicated with different indices. n.d., Not detected. Note: 10-fold higher scale for glutathione (GSH) and the micromole unit for sulfate and nanomole unit for reduced sulfur. FW, Fresh weight.
Sulfur Compounds in Xylem Sap
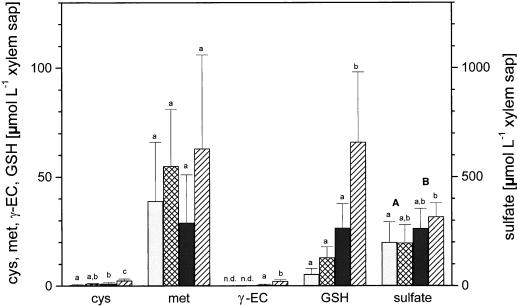
Compared to wild-type poplar over-expression of γ-ECS enhanced concentrations of Cys in xylem sap more than 2-fold, of γ-EC more than 10-fold, and of glutathione about 5-fold (Fig. 4). Also, sulfate concentrations in the xylem sap of transgenic poplar were increased compared with the wild-type control (Fig. 4). Exposure to H2S doubled the concentration of Cys in xylem sap in both wild-type and transgenic poplar (Fig. 4). H2S exposure increased concentrations of glutathione by more than 2-fold in both wild-type and transgenic poplar and increased concentrations of γ-EC by approximately 4-fold in transgenic poplar (Fig. 4). Sulfate concentrations in xylem sap were not affected by H2S exposure irrespective of poplar line (Fig. 4).
Figure 4.
Influence of H2S exposure on thiol, Met, and sulfate in shoot xylem saps of wild-type and transgenic poplar. Six- to 8-week-old wild-type poplar (□, ambient air; ▩, H2S) and transgenic poplar (▪, ambient air; ▨, H2S) were exposed for 48 h to ambient air or 0.25 μL L−1 H2S, respectively. The data shown are means ± sd with xylem saps from six to seven poplar plants each treatment. Significant differences at P < 0.05 between treatments are indicated with different indices. n.d., Not detected. Note: 10-fold higher scale for sulfate. GSH, glutathione.
Sulfate Uptake and Xylem Loading of Sulfate
Independent of H2S exposure, over-expression of γ-ECS led to a significant increase in sulfate uptake by excised non-mycorrhizal poplar roots. Xylem loading of sulfate was only slightly but not significantly enhanced by over-expression of γ-ECS (Table III). The proportion of sulfate taken up that was loaded into the xylem stream was similar in wild-type and transgenic plants. Exposure of the shoot to H2S did not change sulfate uptake by the roots either in wild-type or transgenic poplar (Table III). In wild-type but not in transgenic poplar xylem loading of sulfate was significantly diminished upon H2S exposure (Table III). Correspondingly, the proportion of the sulfate taken up that was loaded into the xylem decreased significantly.
Table III.
Sulfate uptake and xylem loading of sulfate in excised roots from wild-type and transgenic poplar exposed to H2S
| Wild Type | Wild Type | Transgenic | Transgenic | |
|---|---|---|---|---|
| 0 μL L−1 H2S | 0.25 μL L−1 H2S | 0 μL L−1 H2S | 0.25 μL L−1 H2S | |
| Sulfate uptake (nmol g fresh wt−1 h−1) | 129 ± 45a,A | 118 ± 37a,A | 152 ± 43a,B | 164 ± 35a,B |
| Xylem loading (nmol g fresh wt−1 h−1) | 8.5 ± 4.2b | 4.1 ± 1.1a | 10.0 ± 4.3b | 8.1 ± 2.9b |
| Xylem loading (% of total uptake) | 6.4 ± 2.5b | 3.6 ± 0.9a | 6.5 ± 1.7b | 5.0 ± 1.6a |
Excised poplar roots were pre-incubated in the incubation chamber described by Herschbach and Rennenberg (1991) with 0.1 mm sulfate for 2 h and subsequently exposed to 0.1 mm [35S]sulfate. After 4 h of incubation radioactivity in root segments and in the solution of the exudation compartment were measured by liquid scintillation counting. The data shown are means ± sd with seven poplar plants of each poplar line and each treatment. Significant differences between treatments at P < 0.05 are indicated with different small letters; differences between poplar lines are indicated with capital letters.
Correlation Analysis
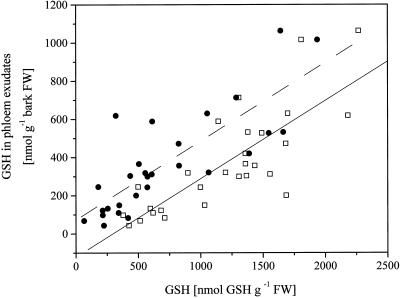
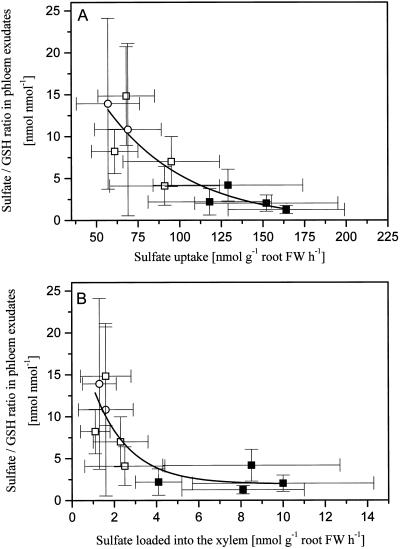
Glutathione concentrations in phloem exudates increased with increasing glutathione concentrations in leaves (Fig. 5). Glutathione concentrations in phloem exudates were also closely related to glutathione concentrations in the roots (Fig. 5). Remarkably, the slope of both regressions was identical. Apparently, glutathione concentrations in roots are largely determined by the production of glutathione in the leaves and transported to the roots via the phloem. For the poplar lines studied and the range of applied treatments, concentrations of glutathione in phloem exudate or roots, and sulfate uptake and loading into xylem were not related (data not shown). However, the sulfate-to-glutathione ratio in phloem exudates declined in a clear-cut, non-linear fashion with both increasing sulfate uptake and loading into xylem (Fig. 6, A and B). Correlation coefficients for exponential models of both sulfate uptake (r = 0.89) and xylem loading (r = 0.84) at P < 0.5 were strong.
Figure 5.
Correlation between glutathione (GSH) in phloem exudates and leaves or roots. Glutathione concentration in phloem exudates from individual plants were plotted against their corresponding glutathione contents in leaves (□) and roots (●) independent of the treatment and of the poplar line used. Linear fits of the glutathione concentration in phloem exudates against the glutathione concentration (a) in the leaves (straight line) with y = −121 + 0.41x and r2 = 0.628 and (b) in the roots (broken line) with y = 73 + 0.41x and r2 = 0.656 from one experiment (wild type and line ggs28) are given. FW, Fresh weight.
Figure 6.
Correlation between the sulfate to glutathione (GSH) ratio in phloem exudates and the sulfate uptake or the xylem loading of sulfate. The mean value of the sulfate/glutathione ratio in phloem exudates from one poplar line and treatment is plotted against the corresponding mean value of the sulfate uptake rate (A) and rate of xylem loading of sulfate (B). The exponential fits y = −0.22 + 13e(−(x − 57)/50) with χ2 = 6.5 and r2 = 0.795 (A) and y = 1.9 + 10.9e(−(x − 1.1)/1.7) with χ2 = 9.2 and r2 = 0.709 were performed with the data from three independent experiments, first wild type plus line ggs28 (▪), second wild type and line ggs 11 (□), and an additional experiment performed with the first wild type (○). FW, Fresh weight.
DISCUSSION
In the present study, the hypothesis of glutathione-mediated, demand-driven control of sulfur nutrition, previously suggested for herbaceous plants (Rennenberg, 1995; Rennenberg and Herschbach, 1995; Lappartient and Touraine, 1996), was tested with young poplar trees. Rates of sulfate uptake by excised non-mycorrhizal poplar roots were 3 to 4 times greater than those by excised, non-mycorrhizal roots of beech (Kreuzwieser et al., 1996), twice as great as found for oak (Seegmüller et al., 1996), but still significantly less than previously reported for herbaceous plants (Herschbach and Rennenberg, 1991; Herschbach et al., 1995a, 1995b). In comparison to oak and beech, poplar grows more quickly and may, therefore, have a greater demand for sulfate. However, rates of loading of sulfate into the xylem were similar in poplar (this study), oak (Seegmüller et al., 1996), and beech seedlings (Kreuzwieser et al., 1996), suggesting that enhanced sulfate reduction in roots may contribute to increased demand for sulfate by poplar.
Plants can absorb and assimilate atmospheric H2S in their shoots, a pathway that may compete with pedospheric supply as sulfur sources for growth (De Kok et al., 1991, 1997; Stuiver and De Kok, 1997, 1998). Poplar foliage was a sink for atmospheric H2S in the present experiments and rates of H2S uptake by shoots of both wild-type and transgenic poplar were quite similar. H2S exposure generally results in a slight overload of reduced sulfur as is illustrated by increases in size and change in composition of the thiol pool, particularly in shoots (De Kok, 1990; De Kok et al., 1997; Stuiver and De Kok, 1997, 1998). Likewise, exposure of poplar to H2S resulted in enhanced thiol concentrations in both wild-type and transgenic poplar as evident from increased Cys, γ-EC, and glutathione concentrations in leaves (Fig. 1). Reduced amounts of sulfate for reduction and assimilation are thus required in leaves of poplar exposed to H2S. Since sulfate did not accumulate in poplar leaves upon exposure to H2S (Fig. 1), as also found for other plant species (De Kok, 1990), reduced allocation of sulfate to the leaves via the transpiration stream may be assumed. This view was supported in the present study by analysis of sulfate transport into and inside roots (Table IV). Xylem loading of sulfate, but not uptake was reduced in roots of wild-type and transgenic poplar exposed to H2S (Table III). Apparently, upon short-term H2S exposure, xylem loading of sulfate rather than sulfate uptake is down-regulated in response to a reduced sulfate demand by the leaves. Similar results have been previously obtained with herbaceous plants (Herschbach et al., 1995a, 1995b) indicating separate regulation of sulfate uptake and loading into the xylem stream. Potassium transport to the shoot is also regulated at the site of loading into the xylem stream (Engels and Marschner, 1992; Wegner and De Boer, 1997) and reduced K+ concentrations in xylem parenchyma cells are refilled by an increased K+ uptake (Wegner and De Boer, 1997).
Table IV.
Comparison between expected and observed effects of over-expression of γ-ECS and H2S fumigation in poplar
| Overexpression of
γ-ECS
|
H2S Fumigation
|
||||
|---|---|---|---|---|---|
| GSH | SO42− | GSH | SO42− | ||
| Leaves | E | + | − | + | 0 |
| F | + | 0 | + | 0 | |
| Phloem exudate | E | −/0 | − | + | 0 |
| F | + | + | + | 0 | |
| Roots | E | −/0 | − | + | 0 |
| F | + | +/0 | + | 0 | |
| Xylem sap | E | −/0 | + | + | − |
| F | + | + | + | 0 | |
| Sulfate uptake | E | + | − | ||
| F | + | 0 | |||
| Xylem loading | E | + | − | ||
| F | +/0 | − | |||
E, Expected; F, found; +, increasing concentration; 0, unchanged; −, reduced concentration.
The shoot-derived signal responsible for regulation of sulfate transport into and inside roots is still a matter of debate. In herbaceous plants, glutathione or Cys fed to the roots reduced sulfate uptake and loading into the xylem (Herschbach and Rennenberg, 1991, 1994; Lappartient and Touraine, 1996). Similar results were obtained for sulfate transporter transcripts. Reduced expression of a high-affinity sulfate transporter in barley was correlated with large concentrations of Cys, glutathione, and sulfate in roots (Smith et al., 1997). Vidmar et al. (1999) found that glutathione reduced the transcript of the high-affinity sulfate transporter in barley. A similar effect was observed with maize, whereas Cys rather than glutathione reduced the transcript of the plant-specific, high-affinity sulfate transporter (Bolchi et al., 1999). A low-affinity sulfate transporter was expressed under sulfur deficiency in Arabidopsis in the central cylinder, but not in the xylem, endodermis, cortex, and epidermis (Takahashi et al., 1997). This transporter was also down-regulated by glutathione (Lappartient et al., 1999).
In the present study with poplar, glutathione, Cys, and Met concentrations in roots increased in response to H2S fumigation (Fig. 3). Also glutathione and Cys concentrations in phloem sap increased under these conditions (Fig. 2) seemingly without inhibiting sulfate uptake (Table III). Apparently neither Cys nor glutathione regulated sulfur nutrition via sulfate uptake when shoot demand for sulfur was reduced. However, xylem loading of sulfate was inhibited under these conditions (Table III). Since both Cys and glutathione concentrations were increased in xylem saps and roots of plants exposed to H2S, we can neither definitively identify the reduced sulfur compound responsible for mediating inhibition of loading of sulfate into the xylem stream nor the location of the responsible metabolic pool of that reduced sulfur compound. However, since only glutathione concentrations in leaves and roots strictly correlated with glutathione concentrations in phloem exudates, this tripeptide seems to be a likely candidate as a shoot-to-root signal for mediating the control of sulfur nutrition at reduced demand. The strategy is similar for nitrogen. Increasing concentrations of amino acids (Gln, Glu, Asn, and Asp in beech and Arg and Ala in soybean) in phloem exudates correlate with a reduced nitrate uptake. These amino compounds are thought to be involved in adaptation of nitrogen nutrition to the demand (Muller and Touraine, 1992; Gessler et al., 1998).
In early investigations of regulation of sulfate uptake and transport, sulfate itself was suggested as a shoot-to-root signal (Jensén and König, 1982; Cram, 1983a, 1983b) as demonstrated for potassium, which regulates its own demand (Wegner and De Boer, 1997; White, 1997). Also, in recent studies high-sulfate concentrations in barley roots were correlated with slow rates of sulfate uptake (Smith et al., 1997). In the present investigation, reduced concentrations of sulfate in xylem sap and phloem exudate of plants exposed to H2S were not observed, although rates of xylem loading were diminished. Because we cannot distinguish between sulfate and reduced sulfur loaded into the xylem, it is possible that reduced sulfur rather than sulfate loaded into the xylem was reduced. Also other sulfate pools were not affected by H2S fumigation of wild-type plants (Table IV). It may therefore be concluded that changes in sulfate pools are not responsible for the regulation of sulfur nutrition under conditions of reduced demand in poplar plants.
Poplar plants over-expressing the bacterial gene of γ-ECS in the cytosol have a greater demand for sulfate in the shoot as compared with the wild type as evidenced by the observed increase in accumulation of reduced sulfur compounds. Hence, concentrations of sulfur compounds in different plant compartments and the rates of sulfate transport processes were compared between transgenic and wild-type plants to test the hypothesis of glutathione-mediated and demand-driven control of sulfate uptake and loading into the xylem. Biomass and its distribution among components and rates of photosynthesis did not differ between transgenic and wild-type poplar (Tables I and II). When transgenic poplar were exposed to ambient air, rates of sulfate uptake were significantly increased and rates of xylem loading of sulfate were slightly increased (Table III), reflecting increased sulfate demand of the shoot. Concentrations of reduced sulfur compounds in phloem exudates and in roots should be less in transgenic as compared with wild-type plants (Table IV) if the hypothesis of glutathione-mediated, demand-driven regulatory control is correct. Counter to this expectation glutathione concentrations in phloem exudates, roots, and xylem sap (Figs. 2–4; Table IV) and Cys concentrations in roots and xylem sap of transgenic poplar were greater than those in the wild type (Figs. 3 and 4).
We suggest the hypothesis of glutathione-mediated, demand-driven control of sulfate nutrition cannot be applied under conditions of enhanced sulfate demand. Since sulfate concentrations in phloem exudates, roots, and xylem sap were greater in transgenic than wild-type poplar, sulfate can also be excluded as the regulatory signal of enhanced demand (Table IV). These results are consistent with conclusions drawn from experiments on the regulation of sulfate uptake with barley (Smith et al., 1997) that include suggestions reduced sulfur may act as a negative metabolic regulator, in contrast to positive regulation of sulfate uptake exerted by other compounds than reduced sulfur. A comparable, but reversed mechanism was suggested for potassium. Enhanced K+ demand of the shoot seems to be signaled by a reduced K+ transport in the phloem (Wegner and De Boer, 1997). As a consequence xylem loading of K+ by a transporter sensitive to abscisic acid was increased (De Boer, 1999). Abscisic acid reduced K+ transport from xylem parenchyma cells into xylem and stimulates K+ uptake into xylem parenchyma cells (Roberts, 1998; De Boer, 1999). In studies on the regulation of sulfur nutrition hormonal compounds were not yet investigated and therefore cannot be excluded as additional regulatory factors.
Also in the present study, sulfate uptake and loading into xylem seem to be regulated to the needs of the shoot irrespective of demand. Any signal that reduced xylem loading of sulfate at reduced demand, (e.g. as a consequence of H2S exposure of the shoot), was counteracted by another signal that stimulated both sulfate uptake and loading into the xylem at enhanced demand (e.g. in transgenic plants with enhanced thiol synthesis). From the present results it appears that the sulfate-to-glutathione ratio in the phloem rather than the concentration of the individual compounds best reflects both reduced and enhanced sulfate demand of the shoot (Fig. 6). This ratio declined non-linearly with both increasing sulfate uptake and increasing xylem loading of sulfate independent of H2S fumigation and independent of the poplar line studied. While these results provide circumstantial evidence (rather than a casual relationship), further investigations are required to test whether the sulfate-to-glutathione ratio can be considered the dominant shoot-to-root signal controlling sulfate uptake and loading into the xylem.
Finally, since nitrogen and sulfate assimilation may be coordinated by the Cys precursor O-acetyl-Ser (OAS; Ostrowski and Kredich, 1989; Brunold, 1993) we cannot exclude the possibility that enhanced sulfate assimilation in transgenic poplar stimulates nitrogen assimilation and, as a consequence, sulfate uptake. This possibility is supported by the observation that the concentrations of total free amino acid, mainly Gln, is generally greater in transgenic poplar as compared with wild-type plants (Noctor et al., 1997). The expression of a high-affinity, sulfate transporter is down-regulated at high-glutathione, -Cys, and -sulfate concentrations but this reduction is greatly counteracted by increased concentrations of OAS (Smith et al., 1997). Hence OAS is also a likely candidate for over-ruling the signal inhibiting xylem loading of sulfate in poplar under conditions of reduced demand. The finding that OAS stimulates sulfate uptake by excised mycorrhizal beech roots is consistent with this view (Kreuzwieser and Rennenberg, 1998) and further studies on the role of OAS in the regulation of sulfur nutrition are urgently required.
MATERIALS AND METHODS
Plant Material
The present experiments were performed with two, independent lines of wild-type poplar and two transgenic (Populus tremula × Populus alba ggs28 and ggs11) that over-expressed the bacterial gene γ-ECS in the cytosol (Strohm et al., 1995; Noctor et al., 1996; Arisi et al., 1997). Since similar results were obtained with different lines, data for one wild-type and one transgenic line (ggs28) are shown. Transgenic and wild-type poplar were micropropagated and cultivated under sterile conditions. After 4 weeks, cuttings were transferred into a soil mixture and grown in a greenhouse under long-day conditions in pots of 10 cm in height, length, and width (Strohm et al., 1995). The soil mixture consisted of 1 part of silica sand, particle size 0.06 to 0.2 mm, 1 part of sterilized commercial soil, and 2 parts of perlit (Agriperl, Perlite-Dämmstoff-GmbH, Dortmund, Germany). Plants were fertilized every 2 weeks with 200 mL of a 3 g L−1 solution of a commercial fertilizer (Hakaphos blau, COMPO GmbH, Munster, Germany; as declared by the manufactory the fertilizer contained: 15% [w/w] N, 10% [w/w] P2O5, 15% [w/w] K2O, 2% [w/w] MgO, 0.01% [w/w] B, 0.02% [w/w] Cu, 0.05% [w/w] Fe, 0.05% [w/w] Mn, 0.001% [w/w] Mo, and 0.015% [w/w] Zn). After 5 to 8 weeks of further growth, six to eight wild-type and transgenic plants were exposed either to ambient air or to H2S.
H2S Fumigation
Nine- to 12-week-old poplar plants were exposed for 48 h to 0.25 μL L−1 H2S or ambient air as described by Van der Kooij et al. (1997). Transgenic and wild-type poplar from the same batch were either exposed to H2S or ambient air during a 3-week period of the experiments. Before treatments were started the soil of the pots was covered with one layer of parafilm and, subsequently, with aluminum foil. Poplar plants were fumigated in 0.185-m3 size cylindrical (diameter 0.65 m) stainless steel cabinets with polycarbonate tops. The air temperature was controlled by adjusting the cabinet wall temperature. The air flow was 2.28 m3 h−1, and the air inside the cabinet was mixed by two fans placed at the bottom (59 m3 h−1 each). To avoid chamber effects, poplar plants from each treatment, ambient air or H2S fumigation, were exchanged after 24 h between the fumigation cabinets. Pressurized H2S (1,000 μL L−1 in nitrogen, Hoekloos, The Netherlands) was injected into the incoming air stream by electronic mass flow controllers (ASM, type AFC-260, Bilthoven, The Netherlands). The photoperiod was 16 h at a photon flux density of 380 ± 20 μmol m−2 s−1 (within the 400–700 nm range) with a Phillips HPL(R) N 400 W (Phillips, Eindhoven, The Netherlands) as light source. Day and night temperatures were approximately 20°C and approximately 18°C, respectively, and the relative humidity was 60% to 70%.
Determination of H2S Deposition, Transpiration, and Photosynthesis
The rates of H2S uptake, transpiration, and photosynthesis were derived from measurements of the differences in H2S, water, and CO2 concentrations between outlet and the inlet port of a fumigation and reference cabinet (containing pots without plants), rates of air flow through the cabinet, and the shoot weight as described previously (De Kok et al., 1991; Van der Kooij et al., 1997; Van der Kooij and De Kok, 1998). H2S concentrations were monitored with a SO2 analyzer (model 9850) equipped with a H2S converter (model 8770, Monitor Labs, Lear Siegler Measurement Controls Corporation, Englewood, CO). Measurements were corrected for controls containing pots with detached plants. Water and CO2 concentrations were measured with an infrared gas analyzer (ADC 225 MK2, Hoddesdon, UK).
Collection of Phloem Exudate
Phloem exudates were collected from slices of stem bark from six to eight wild-type and transgenic poplar plants from each treatment as described by Herschbach et al. (1998). Bark slices of approximately 150 mg fresh weight (1–2 cm2, 0.5–1.5 mm thick) were separated from the wood, washed in 2 mm EDTA, and allowed to equilibrate in different incubation solutions at 4°C. The incubation solution for thiols, i.e. Cys, γ-EC, and glutathione, contained 2 mm EDTA and 1 mm cyanide at pH 5.8. To prevent destruction of thiols by reactions with phenolic compounds, polyvinypolypyrrolidone (PVPP, Sigma, Deisenhoven, Germany) was added at a PVPP to bark fresh weight ratio of 2. For Met exudation bark slices were incubated in 2 mm EDTA, pH 6.8. Sulfate was measured in phloem exudates from bark slices equilibrated in distilled water. Patterns of exudation of Suc were determined as a control and were independent of the equilibration solution applied (data not shown). After 5 h, exudation was nearly complete as indicated by the release of Suc from the bark slices (Herschbach et al., 1998). From previous experiments, contamination of the phloem sap can be neglected under the experimental conditions applied (Schneider et al., 1996; Herschbach et al., 1998).
Xylem Sap Sampling
Xylem sap was collected from poplar shoots by the modification of the pressure chamber technique of Scholander et al. (1965) described by Rennenberg et al. (1996). Poplar shoots were cut 2 to 5 cm above the ground. Bark and cambium were removed at a length of 20 mm from the cut end. Shoots were fitted into the pressure chamber (Soil Moisture, Santa Barbara, CA) with 10 mm of the cut end protruding. Subsequently, the pressure in the chamber was raised slowly and the cut end was observed with a dissecting microscope. The pressure at which xylem sap first appeared at the cut end was recorded as the actual shoot water potential, and the initial exudate was discarded to avoid contamination. The pressure was then raised to 0.5 MPa over shoot water potential and kept constant for the following 2 min. The exuding xylem sap was collected in Eppendorf caps, frozen under liquid nitrogen, and stored until analysis at −80°C. Contamination with cellular compounds was measured by ATP analysis as described by Schneider et al. (1996) and was less than 1%, as previously reported for other plant species.
Collection of Leaves and Roots
For reduced sulfur and sulfate analysis, two young, fully expanded leaves were selected from each poplar plant. Lateral roots were washed in water to remove sand, soil, and perlite particles. Both leaves and roots were frozen in liquid nitrogen and stored at −80°C until analysis.
Analysis of Thiols
Thiols in phloem exudates were analyzed as previously described by Herschbach et al. (1998). For this purpose, phloem exudates were centrifuged at 16,000g and 4°C for 10 min. Aliquots of 300 μL of the supernatant were adjusted to pH 8.3 ± 0.2 by adding 100 μL of 1 m CHES (2-[cyclohexylamino]-ethansulfonacid), pH 8.4. Reduction of thiols was initiated by addition of 20 μL of 15 mm dithiothreitol (DTT) and terminated after 60 min by addition of 30 μL of 30 mm monobromobimane (mBBr) for derivatization. After 15 min, derivatization was stopped by acidification with 50 μL of 30% (v/v) acetic acid to stabilize mBBr-thiol derivatives. Aliquots of 100 μL were used for HPLC analysis. Thiols in xylem sap were analyzed according to Schupp et al. (1991). For this purpose, 50 μL of distilled water and 50 μL of 1 m CHES, pH 8.5, was added to 50 μL of xylem sap. Thiols were reduced by addition of 10 μL of 15 mm DTT, incubated for 60 min, derivatized by addition of 15 μL of 30 mm mBBr, incubated for 15 min, and finally stabilized by addition of 150 μL of acetic acid (10%, v/v). Aliquots of 100 to 200 μL were used in HPLC analysis. Thiols in leaves and roots were homogenized under liquid nitrogen and extracted as described by Strohm et al. (1995). Approximately 50 mg of frozen powder were transferred into precooled (4°C) vials containing 1.5 mL of 0.1 n HCl and 80 mg of insoluble PVPP. Samples were centrifuged at 16,000g and 4°C for 15 min. Aliquots of 120 μL of the supernatant were adjusted to pH 8.3 ± 0.2 with 180 μL of 200 mm CHES, pH 9.3. Oxidized thiols were reduced for 60 min by adding 30 μL of 5 mm DTT. Derivatization was performed with 20 μL of 30 mm mBBr for 15 min. Subsequently, thiol derivatives were stabilized with 80 μL of acetic acid (30%, v/v). Aliquots of 15 to 150 μL were used for to HPLC analysis. Thiol derivatives were separated and quantified by fluorescence detection as described by Schupp and Rennenberg (1988). Peaks were identified and quantified using a standard solution containing 0.2 mm Cys, 0.1 mm γ-EC, and 1 mm glutathione in 0.01 m HCl.
Met Analysis
For Met analysis in phloem exudates 1.3-mL aliquots were freeze-dried (Herschbach et al., 1998). The dried material was resuspended with 100 μL of 0.2 m sodium citrate buffer, pH 3.35, and 70-μL aliquots were analyzed using an amino acid analyzer (Biochrom, Pharmacia LKB, Freiburg, Germany). Aliquots of 50 μL of xylem sap were analyzed directly for Met. Met in tissue samples was extracted according to Winter et al. (1992). For this purpose, 500-mg aliquots of powdered tissue were homogenized in 0.6 mL of HEPES (4-[2-hydroxyethyl]-1-piperazineethanesulfonic acid) buffer (20 mm HEPES, 5 mm EGTA, and 10 mm NaF, pH 7.0) plus 5 mL of methanol:chloroform (3.5:1.5, v/v). The homogenate was incubated at 4°C for 30 min. Met was extracted twice with 3 mL of double-distilled water. Aqueous phases were combined, freeze-dried (Alpha 2–4 and LDC-1 M, Christ, Osterode, Germany) and dissolved in 100 μL of 0.2 m sodium citrate buffer, pH 3.35. A 70-μL aliquot of each sample was analyzed using the amino acid analyzer. Met was separated on a cation exchange column (PEEK column, 100 × 4.6 mm, Laborbedarf und Analysetechnik Karin Grüning, Olching, Germany) using a sodium citrate pH gradient. The flow of 0.2 m sodium citrate was 16.1 mL h−1 and the pH increased from 3.35 to 4.25 within 24 min. Thereafter, the column was regenerated within 8 min using 0.4 m NaOH, 2.7 mm EDTA, and equilibrated within 19 min with 0.2 m sodium citrate at pH 3.35. Separated Met was derivatized, post-column, with ninhydrin. The absorption of ninhydrin derivatives was recorded at 570 nm. Peaks were identified and quantified using a standard solution containing 500 μm Met.
Sulfate Analysis
Aliquots of 1.5 mL of phloem exudate or xylem sap were incubated for 60 min with 20 mg of PVPP at 4°C and analyzed for sulfate by anion-exchange chromatography with an automatic ion analyzer (DX 100, Dionex, Idstein, Germany). Sulfate was extracted from tissue samples powdered under liquid nitrogen in a mortar. Aliquots of 150 mg were suspended in 2 mL of twice-distilled water containing 20 mg of insoluble PVPP to remove phenolic compounds. After shaking for 1 h at 4°C, samples were boiled for 15 min, and centrifuged for 5 min and then for 10 min, at 16,000g and 4°C (Centrifuge 5402, Eppendorf, Engelsdorf, Germany). The clear supernatant was used for sulfate analysis by anion-exchange chromatography. In all samples anions were separated on a IonPac column (AS9-SC, 250 × 4 mm; Dionex) eluted with a mixture of 1.8 mm Na2CO3 and 1.7 mm NaHCO3 at a flow rate of 1.1 mL min−1. Sulfate was detected by a conductivity detector module (CDM, Dionex).
Uptake and Xylem Loading of Sulfate
Uptake and xylem loading of sulfate were measured as described by Herschbach and Rennenberg (1991). Poplar roots were washed with water to remove sand, perlite, and soil particles. Then roots were cut with a razor blade under transport medium consisting of 5 mm bis-tris-propane buffer pH 7.0, 0.5 mm CaCl2, and 0.1 mm K2SO4. To measure uptake, xylem loading, and exudation of 35SO42− with excised roots, we used the modification of the incubation chamber of Pitman (1971) described by Herschbach and Rennenberg (1991). Six poplar roots were placed horizontally in the incubation chamber. The cut ends were bathed in 10 mL of transport medium in the exudation compartment, and root tips in 85 mL of transport medium, containing 0.1 mm K2[35S]O4, in the uptake compartment. These two compartments were separated by a third (buffer compartment) filled with 9 mL of transport medium. The roots were fixed between the compartments with plastibase (Bristol Meyers Squibb GmbH, Regensburg, Germany). The uptake compartment was stirred, and each compartment was covered with slides. For equilibration, roots were pre-incubated with transport medium for 2 h followed by 4 h of exposure to [35S]sulfate. The transport medium in all compartments was renewed immediately before labeled sulfate was added as carrier-free [35S]sulfate (Amersham, Hertogenbosch, The Netherlands; 0.9 × 104 to 1.6 × 104 kBq). The final concentration of sulfate was 0.1 mm with 1.1 × 106 to 1.9 × 106 kBq mmol−1. After a 4-h incubation at room temperature uptake and xylem loading was terminated. For this purpose, three 0.5-mL aliquots of transport medium from each compartment were transferred into scintillation vials. Subsequently, the incubation compartment was washed 3 times for 20 s each time with 50 mL of transport medium without [35S]SO42−. Root segments from each chamber compartment were then cut with a razor blade and were transferred separately into scintillation vials.
Analysis of 35S
For liquid scintillation counting 4 mL of scintillation fluid (OptiPhase HiSafe 2, Wallac Oy, Turku, Finland) was added to the sampled transport media. Root samples were digested in 3 mL of tissue solubilizer (Soluene 350, Canberra Packard, Frankfurt) for 2 d at 70°C with a maximum of 150 mg fresh weight per sample. Subsequently, samples were bleached with 200 to 400 μL of H2O2 (30%–35%, v/v) after addition of 200 μL of isopropanol. After 1 d at root temperature 10 mL of liquid scintillation fluid (OptiPhase HiSave 3, Canberra Packard) was added. 35S was detected by liquid scintillation counting (2000 CA, Tri-CARB, Packard Instruments, Chicago).
Data Analysis
Net uptake of sulfate into the roots was calculated as the sum of radioactivity in each root segment plus the radioactivity exported out of the cut end of the roots (Herschbach and Rennenberg, 1991). Radioactivity in the root segments of the exudation and the buffer compartment plus the radioactivity in the solution of the exudation compartment was defined as the amount of “sulfate loaded into the xylem.” Experiments were performed with six to eight poplar plants each. Linear and exponential fits were performed with Microcal Origin (Microcal Software Version 5.0, Northampton, MA). Statistical analysis was performed using the Duncan's multi-factorial analysis with SPSS (SPSS for Windows, Release 7.0, Chicago) or Student's t test.
ACKNOWLEDGMENTS
We thank Dr. Monika Schulte and Ulrike Heizmann for technical support during the experiments and Prof. Mark Adams for critical reading of the manuscript. The skillful technical assistance of Tanja Hartmann, Ulrike Hanemann, and Tatja Dopatka is gratefully acknowledged.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (contract nos. Re 515/6 and He 3003/1).
LITERATURE CITED
- Arisi A-CM, Noctor G, Foyer C, Jouanin L. Modification of thiol contents in poplar (Populus tremula × P. alba) overexpressing enzymes involved in glutathione synthesis. Planta. 1997;203:362–372. doi: 10.1007/s004250050202. [DOI] [PubMed] [Google Scholar]
- Blaschke L, Schneider A, Herschbach C, Rennenberg H. Reduced sulfur allocation from three-year-old needles of Norway spruce (Picea abies[Karst] L.) J Exp Bot. 1996;47:1025–1032. [Google Scholar]
- Bolchi A, Petrucco S, Tenca PL, Foroni C, Ottonello S. Coordinate modulation of maize sulfate permease and ATP sulfurylase mRNAs in response to variations in sulfur nutritional status: stereospecific down regulation by l-cysteine. Plant Mol Biol. 1999;39:527–537. doi: 10.1023/a:1006148815106. [DOI] [PubMed] [Google Scholar]
- Brunold C. Reduction of sulfate to sulfide. In: Rennenberg H, Brunold C, De Kok LJ, Stulen I, editors. Sulfur Nutrition and Sulfur Assimilation in Higher Plants. The Hague, The Netherlands: SPB Academic Publishing; 1990. pp. 13–31. [Google Scholar]
- Brunold C. Regulatory interactions between sulfate and nitrate assimilation. In: De Kok LJ, Stulen I, Rennenberg H, Brunold C, Rauser WE, editors. Sulfur Nutrition and Assimilation in Higher Plants. The Hague, The Netherlands: SPB Academic Publishing; 1993. pp. 61–75. [Google Scholar]
- Cram WJ. Characteristics of sulfate transport across plasmalemma and tonoplast of carrot root cells. Plant Physiol. 1983a;72:204–211. doi: 10.1104/pp.72.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cram WJ. Sulfate accumulation is regulated at the tonoplast. Plant Sci Lett. 1983b;31:329–338. [Google Scholar]
- Cram WJ. Uptake and transport of sulfate. In: Rennenberg H, Brunold C, De Kok LJ, Stulen I, editors. Sulfur Nutrient and Sulfur Assimilation in Higher Plants. The Hague, The Netherlands: SPB Academic Publishing; 1990. pp. 3–11. [Google Scholar]
- Creissen G, Firmin J, Fryer M, Kular B, Leyland N, Reynolds H, Pastori G, Wellburn F, Baker N, Wellburn A, Mullineaux P. Elevated glutathione biosynthetic capacity in the chloroplasts of transgenic tobacco plants paradoxically causes increased oxidative stress. Plant Cell. 1999;11:1277–1291. doi: 10.1105/tpc.11.7.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer Potassium translocation into the roots xylem. Plant Biol. 1999;1:36–45. [Google Scholar]
- De Kok LJ. Sulfur metabolism in plants exposed to atmospheric sulfur. In: Rennenberg H, Brunold C, De Kok LJ, Stulen I, editors. Sulfur Nutrition and Sulfur Assimilation in Higher Plants. The Hague, The Netherlands: SPB Academic Publishing; 1990. pp. 111–130. [Google Scholar]
- De Kok LJ, Rennenberg H, Kuiper PJC. The internal resistance in spinach shoots to atmospheric H2S is determined by metabolic processes. Plant Physiol Biochem. 1991;29:463–470. [Google Scholar]
- De Kok LJ, Stuiver CEE, Rubinigg M, Westerman S, Grill D. Impact of atmospheric sulfur deposition on sulfur metabolism in plants: H2S as sulfur source deprived Brassica oleraceaL. Botanica Acta. 1997;110:411–419. [Google Scholar]
- De Kok LJ, Stuiver CEE, Stulen I. Impact of atmospheric H2S on plants. In: De Kok LJ, Stulen I, editors. Responses of Plant Metabolism to Air Pollution and Global Change. Leiden, The Netherlands: Backhuys Publishers; 1998. pp. 51–63. [Google Scholar]
- Engels C, Marschner H. Adaption of potassium translocation into the shoot of maize (Zea mays) to shoot demand: evidence for xylem loading as a regulation step. Physiol Plant. 1992;86:263–268. [Google Scholar]
- Gessler A, Schultze M, Schrempp S, Rennenberg H. Interaction of phloem-translocated amino compounds with nitrate net uptake by the roots of beech (Fagus sylvatica) seedlings. J Exp Bot. 1998;49:1529–1537. [Google Scholar]
- Herschbach C, De Kok LJ, Rennenberg H. Net uptake of sulfate and its transport to the shoot in spinach plants fumigated with H2S or SO2: does atmospheric sulfur affect the ‘inter-organ’ regulation of sulfur nutrition. Botanica Acta. 1995a;108:41–46. [Google Scholar]
- Herschbach C, De Kok LJ, Rennenberg H. Net uptake of sulfate and its transport to the shoot in tobacco plants fumigated with H2S or SO2. Plant Soil. 1995b;175:75–84. [Google Scholar]
- Herschbach C, Jouanin L, Rennenberg H. Overexpression of γ-glutamylcysteine synthetase, but not of glutathione synthetase elevates glutathione allocation in the phloem of transgenic poplar (Populus tremula × Populus alba) trees. Plant Cell Physiol. 1998;39:447–451. [Google Scholar]
- Herschbach C, Rennenberg H. Influence of glutathione (GSH) on sulfate influx, xylem loading and exudation in excised tobacco roots. J Exp Bot. 1991;42:1021–1029. [Google Scholar]
- Herschbach C, Rennenberg H. Influence of glutathione (GSH) on net uptake of sulfate and sulfate transport in tobacco plants. J Exp Bot. 1994;45:1069–1076. [Google Scholar]
- Herschbach C, Rennenberg H. Long-distance transport of 35S-sulfur in 3-year-old beech trees (Fagus sylvatica) Physiol Plant. 1995;95:379–386. [Google Scholar]
- Herschbach C, Rennenberg H. Storage and re-mobilization of sulfur in beech trees (Fagus sylvatica) Physiol Plant. 1996;98:125–132. [Google Scholar]
- Herschbach C, Rennenberg H. Sulfur nutrition in conifers and deciduous trees. In: Rennenberg H, Eschrich W, Ziegler H, editors. Trees: Contributions to Modern Tree Physiology. Leiden, The Netherlands: Backhuys Publishers; 1997. pp. 293–311. [Google Scholar]
- Jensén P, König T. Development of regulation mechanisms for SO42−influx in spring wheat roots. Physiol Plant. 1982;55:459–464. [Google Scholar]
- Kreuzwieser J, Herschbach C, Rennenberg H. Sulfate uptake and xylem loading of non-mycorrhizal excised roots of young Fagus sylvaticatrees. Plant Physiol Biochem. 1996;34:409–416. [Google Scholar]
- Kreuzwieser J, Rennenberg H. Sulfate uptake and xylem loading of mycorrhizal beech roots. New Phytol. 1998;140:319–329. doi: 10.1046/j.1469-8137.1998.00266.x. [DOI] [PubMed] [Google Scholar]
- Lappartient AG, Touraine B. Demand-driven control of root ATP sulfurylase activity and SO42−uptake in intact canola: the role of phloem-translocated glutathione. Plant Physiol. 1996;111:147–157. doi: 10.1104/pp.111.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappartient AG, Vidmar JJ, Leustek T, Glass ADM, Touraine B. Inter-organ signaling in plants: regulation of ATP sulfurylase and sulfate transporter genes expression in roots mediated by phloem-translocated compound. Plant J. 1999;18:89–95. doi: 10.1046/j.1365-313x.1999.00416.x. [DOI] [PubMed] [Google Scholar]
- Muller B, Touraine B. Inhibition of NO3−uptake by various phloem-translocated amino acids in soybean seedlings. J Exp Bot. 1992;43:617–623. [Google Scholar]
- Noctor G, Arisi A-CM, Jouanin L, Kunert KJ, Rennenberg H, Foyer CH. Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot. 1998;49:623–647. [Google Scholar]
- Noctor G, Arisi A-CM, Jouanin L, Valadier M-H, Roux Y, Foyer C. Light dependent modulation of foliar glutathione synthesis and associated amino acid metabolism in poplar overexpressing γ-glutamylcysteine synthetase. Planta. 1997;202:357–369. [Google Scholar]
- Noctor G, Strohm M, Jouanin L, Kunert K-J, Foyer CH, Rennenberg H. Synthesis of glutathione in leaves of transgenic poplar overexpression γ-glutamylcysteine synthetase. Plant Physiol. 1996;112:1071–1078. doi: 10.1104/pp.112.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski J, Kredich NM. Molecular characterization of the cysJIH promoters of Salmonella typhimurium and Escherichia coli: regulation by cysB protein and N-acetyl-l-serine. J Bacteriol. 1989;171:130–140. doi: 10.1128/jb.171.1.130-140.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman MG. Uptake and transport of ions in barley seedlings: I. Estimation of chloride fluxes in cells of excised roots. Aust J Biol Sci. 1971;24:407–421. [Google Scholar]
- Rennenberg H. Processes involved in glutathione metabolism. In: Wallsgrove RM, editor. Amino Acids and Their Derivatives in Higher Plants: Biosynthesis and Metabolism. Cambridge, UK: Cambridge University Press; 1995. pp. 155–171. [Google Scholar]
- Rennenberg H. Molecular approaches to glutathione biosynthesis. In: Cram WJ, De Kok LJ, Stulen I, Brunold C, Rennenberg H, editors. Sulfur Metabolism in Higher Plants. Leiden, The Netherlands: Backhuys Publishers; 1997. pp. 59–70. [Google Scholar]
- Rennenberg H, Herschbach C. Sulfur nutrition of trees: a comparison of spruce (Picea abies L.) and beech (Fagus sylvaticaL.) Z Pflanzenernähr Bodenk. 1995;158:513–517. [Google Scholar]
- Rennenberg H, Herschbach C. Responses of plants to atmospheric sulfur. In: Iqbal JM, Yunus M, editors. Plant Responses to Air Pollution. New York: John Wiley & Sons; 1996. pp. 285–293. [Google Scholar]
- Rennenberg H, Schneider S, Weber P. Analysis of uptake and allocation of nitrogen and sulfur compounds by trees in the field. J Exp Bot. 1996;47:1491–1498. [Google Scholar]
- Rennenberg H, Schupp R, Glavac V, Jochheim H. Xylem sap composition of beech (Fagus sylvaticaL.) trees: seasonal changes in the axial distribution of sulfur compounds. Tree Physiol. 1994;14:541–548. doi: 10.1093/treephys/14.5.541. [DOI] [PubMed] [Google Scholar]
- Roberts SK. Regulation of K+channels in maize roots by water stress and abscisic acid. Plant Physiol. 1998;116:145–153. [Google Scholar]
- Schneider A, Schatten T, Rennenberg H. Exchange between phloem and xylem during long distance transport of glutathione in spruce trees (Picea abies[Karst.] L.) J Exp Bot. 1994;45:457–462. doi: 10.1093/jxb/erg146. [DOI] [PubMed] [Google Scholar]
- Schneider S, Gessler A, Weber P, Von Sengbusch D, Hanemann U, Rennenberg H. Soluble N compounds in trees exposed to high load of N: a comparison of spruce (Picea abies) and beech (Fagus sylvatica) grown under field conditions. New Phytol. 1996;134:103–114. [Google Scholar]
- Scholander PF, Hammel T, Bradstreet ED, Hemmingsen EA. Sap pressure in vascular plants. Science. 1965;148:339–345. doi: 10.1126/science.148.3668.339. [DOI] [PubMed] [Google Scholar]
- Schulte M, Herschbach C, Rennenberg H. Interactive effects of CO2, mycorrhization and drought stress on long distance transport of reduced sulfur in young pedunculate oak trees. Plant Cell Environ. 1998;21:917–926. [Google Scholar]
- Schupp R, Glavac V, Rennenberg H. Thiol composition of xylem sap of beech trees. Phytochemistry. 1991;30:1415–1418. [Google Scholar]
- Schupp R, Rennenberg H. Diurnal changes in the glutathione concentration of spruce needles (Picea abiesL.) Plant Sci. 1988;57:113–117. [Google Scholar]
- Schupp R, Schatten T, Willenbrink J, Rennenberg H. Long-distance transport of reduced sulfur in spruce (Picea abiesL.) J Exp Bot. 1992;43:1243–1250. [Google Scholar]
- Seegmüller S, Schulte M, Herschbach C, Rennenberg H. Interactive effects of mycorrhization and elevated atmospheric CO2 on sulfur nutrition of young pedunculate oak (Quercus roburL.) trees. Plant Cell Environ. 1996;19:418–426. [Google Scholar]
- Smith FW, Hawkesford MJ, Ealing PM, Clarkson DT, Vanden Berg PJ, Belcher AR, Warrilow AGS. Regulation of expression of a cDNA from barley roots encoding a high affinity sulfate transporter. Plant J. 1997;12:875–884. doi: 10.1046/j.1365-313x.1997.12040875.x. [DOI] [PubMed] [Google Scholar]
- Strohm M, Jouanin L, Kunert KJ, Pruvost C, Polle A, Foyer HC, Rennenberg H. Regulation of glutathione synthesis in leaves of transgenic poplar (Populus tremula x P. alba) overexpressing glutathione synthetase. Plant J. 1995;7:141–145. [Google Scholar]
- Stuiver CEE, De Kok LJ. Atmospheric H2S as sulfur source for sulfur deprived Brassica oleracea L. and Hordeum vulgareL. In: Cram WJ, De Kok LJ, Stulen I, Brunold C, Rennenberg H, editors. Sulfur Metabolism in Higher Plants, Molecular, Ecophysiological and Nutritional Aspects. Leiden, The Netherlands: Backhuys Publishers; 1997. pp. 293–294. [Google Scholar]
- Takahashi H, Yamazaki M, Sasakura N, Watanabe A, Leustek T, de Almeida-Engler J, Engler G, Van Montagu M, Saito K. Regulation of cysteine biosynthe-sis in higher plants: a sulfate transporter induced in sulfate-starved roots plays a central role in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1997;94:11102–11107. doi: 10.1073/pnas.94.20.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kooij TAW, De Kok LJ. Kinetics of deposition of SO2 and H2S to shoots of Arabidopsis thalianaL. In: De Kok LJ, Stulen I, editors. Responses of Plant Metabolism to Air Pollution and Global Change. Leiden, The Netherlands: Backhuys Publisher; 1998. pp. 479–481. [Google Scholar]
- Van der Kooij TAW, De Kok LJ, Haneklaus S, Schnug E. Uptake and metabolism of sulfur dioxide by Arabidopsis thaliana. New Phytol. 1997;135:101–107. doi: 10.1046/j.1469-8137.1997.00619.x. [DOI] [PubMed] [Google Scholar]
- Van der Zalm E, Schneider A, Rennenberg H. Characteristics and regulation of sulfate uptake and xylem loading by poplar roots (Populus tremula × P. alba) In: Brunold C, Rennenberg H, De Kok LJ, Stulen I, Davidian JC, editors. Sulfur Nutrition and Sulfur Assimilation in Higher Plants: Molecular, Biochemical and Physiological Aspects. Bern, Switzerland: Paul Haupt; 2000. pp. 277–282. [Google Scholar]
- Vidmar JJ, Schjoerring JK, Touraine B, Glass ADM. Regulation of the hvst1 gene encoding a high-affinity sulfate transporter from Hordeum vulgare. Plant Mol Biol. 1999;40:883–892. doi: 10.1023/a:1006230131841. [DOI] [PubMed] [Google Scholar]
- Wegner LH, De Boer AH. Properties of two outward-rectifying channels in root xylem parenchyma cells suggest a role in K+homeostasis and long-distance signaling. Plant Physiol. 1997;115:1707–1719. doi: 10.1104/pp.115.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ. The regulation of K+ influx into roots of rye (Secale cereale L.) seedlings by negative feedback via the K+flux from shoot to root in the phloem. J Exp Bot. 1997;48:2063–2073. [Google Scholar]
- Winter H, Lohaus G, Heldt HW. Phloem transport of amino acids in relation to their cytosolic levels in barley leaves. Plant Physiol. 1992;99:996–1004. doi: 10.1104/pp.99.3.996. [DOI] [PMC free article] [PubMed] [Google Scholar]