Abstract
Significant racial and ethnic disparities in maternal morbidity and mortality exist in the United States. Black women are three to four times more likely to die a pregnancy-related death as compared with white women. Growing research indicates that quality of healthcare, from preconception through postpartum care, may be a critical lever for improving outcomes for racial and ethnic minority women. This article reviews racial and ethnic disparities in severe maternal morbidities and mortality, underlying drivers of these disparities, and potential levers to reduce their occurrence.
Keywords: disparities, severe maternal morbidity, maternal mortality, quality improvement, race
Introduction
Non-Hispanic black women are three to four times more likely to die from pregnancy-related causes than Non-Hispanic white women.1 This disparity in maternal death, defined within 1 year of pregnancy “caused by a pregnancy complication, a chain of events initiated by pregnancy, or the aggravation of unrelated condition by the physiologic effects of pregnancy,2 has existed for over a century and has actually widened over the last hundred years.3,4 Currently, it represents the largest disparity among all the conventional population perinatal health measures.1 Non-Hispanic black women have had the fastest rate of increase in maternal deaths between 2007 and 2014 and have maternal death rates up to 12 times higher in some cities than Non-Hispanic white women.5,6 Pregnancy-related mortality is also elevated among Native Americans/Native Alaskans, Asians/Pacific Islanders, and for certain subgroups of Hispanic women including Puerto Ricans in specific regions within the United States.6–8
For every maternal death, 100 women suffer a severe obstetric morbidity, a life threatening diagnosis or undergo a lifesaving procedure during their delivery hospitalization.9 Severe maternal morbidity affects greater than 60,000 women annually in the United States and has been on the rise over the last few decades.9,10 Severe morbidity poses an enormous risk to women’s health and well-being and similar to pregnancy-related mortality, racial and ethnic minority women have higher rates of severe maternal morbidity events.11,12 In fact, Non-Hispanic black women have the highest rates for 22 of 25 severe morbidity indicators used by the Center for Disease Control (CDC) to monitor population estimates for severe maternal morbidity.11
Given these disturbing trends growing attention has focused on reducing racial and ethnic disparities in maternal morbidity and mortality rates in the United States. The fact that the risk of a pregnancy-related death for black women in some regions of the United States is similar to risk for women in some developing countries has alarmed health professionals, patients, and policy makers. The American College of Obstetricians, and Gynecologists, Society for Maternal Fetal Medicine, the Council on Patient Safety in Women’s Health Care, and Maternal Child Health Bureau are a few of the many health advocacy and health professional organizations now committed to policies to reduce these disparities.13
To date a great deal of research has demonstrated that nearly half of severe maternal morbidity events and maternal deaths are preventable making quality of healthcare a critical lever to address racial and ethnic disparities in their occurrence.14 Research by our team has focused on understanding how quality of hospital care, specifically delivery and hospital care, contributes to racial and ethnic disparities in severe maternal morbidity and mortality rates. Others have investigated how preconception care, antenatal care, and postpartum care may contribute to racial/ethnic disparities in maternal health outcomes. This article reviews racial and ethnic disparities in severe maternal morbidities and mortality, underlying drivers of these disparities, and potential levers to reduce these disparities throughout the continuum of maternity care. In this review, I refer to Non-Hispanic black as black and Non-Hispanic white as white.
Conceptual Model
Disparities, defined as “differences that result in a particular type of health difference that is closely linked with economic, social, or environmental disadvantage,”15 are complex and are the result of numerous factors including social, environmental, biologic, genetic, behavioral, as well as healthcare factors. Below is a model demonstrating pathways to racial and ethnic disparities in severe maternal morbidity and mortality.(See Figure 1).
Figure 1.
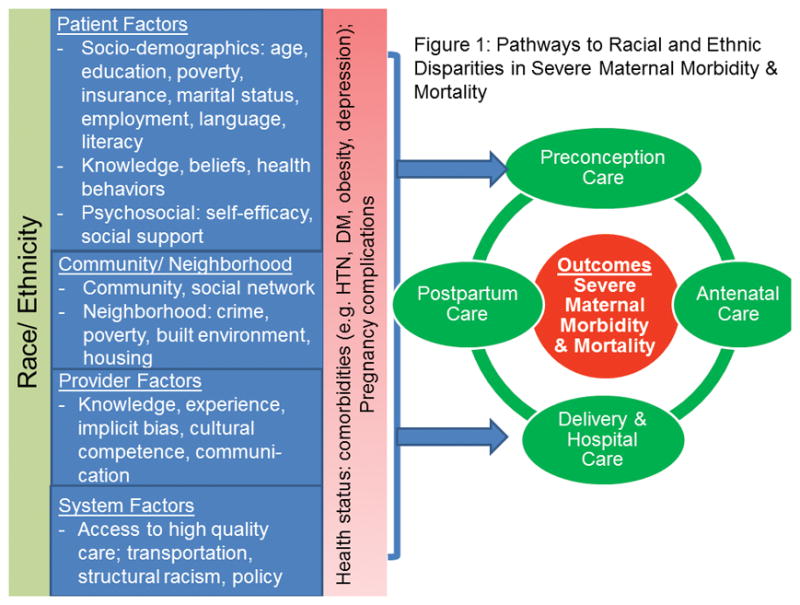
Conceptual Model
The model highlights the importance of social determinants and includes patient factors (e.g., socioeconomic status, race and ethnicity, gender, behaviors, beliefs, biology, genetics), community and neighborhood factors (e.g., social networks and built environment, housing,), provider factors (e.g., knowledge, implicit bias, communication) and system factors (e.g., access to high quality care, structural racism, social and political policies, healthcare institutions).16,17 The combination of these factors results in maternal health status including the presence of clinical comorbidities and pregnancy complications which may or may not lead to severe maternal morbidity or mortality. The cycle of preconception, antenatal, delivery, and postpartum care are closely linked and care at each phase across the continuum can impact severe maternal morbidity and mortality. The social significance of race is indisputable and racial disparities in maternal health have roots in inequalities in the provision of healthcare at the system level, the provider level, and the patient level.18
Persistent Racial/Ethnic Disparities in Severe Maternal Morbidity and Mortality in the United States
Black women are more likely to die than white women and have higher case-fatality rates from a range of conditions though the leading causes of maternal death for black and white women are similar.2,3 Cardiovascular conditions, cardiomyopathy and other medical conditions contributed to 40.9% and 46.8% of pregnancy-related deaths among white and black women and 25.5% of Hispanic women for deaths from 2011–2013 while 39.5% of pregnancy–related deaths among Hispanics and 48.9% among women from other racial/ethnic backgrounds were attributable to hemorrhage, infection, and hypertensive disorders of pregnancy.2 Black women experience higher mortality from cardiomyopathy, hypertensive disorders of pregnancy, and hemorrhage while Hispanic women have an increased risk of death due to hypertensive disorders.7,19 A national study which investigated pregnancy-related mortality among black versus white women found that black women had a case-fatality rate 2.4 to 3.3 times higher than that of white women for five specific pregnancy complications including preeclampsia, eclampsia, abruptio placentae, placenta previa, and postpartum hemorrhage.20 In a review of North Carolina maternal deaths, black versus white women had higher ratios of pregnancy related death from cardiomyopathy, hemorrhage, and respiratory complications with relative risks of 4.6 (95% CI 2.2–9.9), 4.9 (95% CI 1.2–19.4) and 6.1 (95% CI 1.2–31.3) respectively.19 The leading cause of death in Hispanic women following pregnancy was hypertensive disease placing them at a 3-fold increased risk of death due to this complication.7 In a study investigating factors associated with mortality in pregnant women with severe morbidity, researchers found among women with a diagnosis of pregnancy induced hypertension, black and Hispanic women were 9.9 (95% CI: 4.4–22.2), and 7.9 (95% CI: 3.2–19.6) times more likely to die than were white women; and among women with a diagnosis of hemorrhage, black and Hispanic women were 4.7 (95% CI: 1.3–16.8) and 3.7 (95% CI:0.8–16.70) times more likely to die than were white women. Even after adjustment black and Hispanic women with pregnancy induced hypertension were much more likely to die (adjusted relative risk of 8.5; 95% CI: 3.2–22.5 and 6.1; 95% CI: 2.3–16.6) respectively.21 Other studies demonstrate women with preeclampsia have higher rates of maternal and obstetric complications and experience higher adjusted mortality than white women.22
Similar to maternal mortality rates, rates of severe maternal morbidity are higher in racial and ethnic minority women.12,23,24 In a population-based case-control study using linked birth certificate and state discharge abstract data, investigators reported an increased risk for severe maternal morbidity among all ethnic minority women versus whites with the highest rate being reported for blacks.12 Black, Hispanic, Asian/Pacific Islander, and American Indian/Alaska Native women had 2.1, 1.3, 1.2 and 1.7 times higher rates of severe morbidity compared with white women in data from seven states.11 Similarly population-based data from New York City demonstrate that severe maternal morbidity rates were higher among black and Hispanic versus white women (4.2% and 2.7% versus 1.5%, respectively).23,25
Not only do black women have higher case-fatality rates but they also are more likely to experience comorbid illnesses and pregnancy complications. Black women have higher rates of certain types of hemorrhage,26 preeclampsia - even among women without preexisting chronic hypertension,27 asthma,28 cardiac events, and infections. Using national delivery data our team documented that black versus white women had elevated rates of pregnancy-induced and chronic hypertension, asthma, placental disorders, gestational diabetes, preexisting diabetes, and blood disorders.29 Black women experienced higher rates for 11 of 17 comorbidities examined using population-based data from New York City including diseases such as asthma, hypertension and diabetes.23 While the overall burden of complications is higher for blacks than whites and rates for Asians and Hispanics are similar to whites,30 studies suggest that rates of postpartum hemorrhage, third and fourth degree lacerations, and major puerperal infections are higher among Asians than whites. Hispanic women have greater odds of postpartum hemorrhage, diabetes, and major puerperal infections than whites.30,31 In a study investigating severe maternal morbidity rates among whites and Hispanics, Hispanic women experienced 8/11 comorbidities more frequently than white women.25 Data also suggest that racial and ethnic minority women, particularly black women, develop these conditions at earlier ages, are less likely to have their conditions adequately managed, and more likely to have complications and mortality from these conditions.32
In addition to chronic illness and pregnancy complications other known risk factors for pregnancy related mortality and severe morbidity are advanced maternal age, no prenatal care, lower levels of educational attainment, and low socioeconomic status.3,29,33 However, the increased risk of maternal death among racial and ethnic minority women appears to be, at least in part, independent of sociodemographic risk.34 Adjustment for sociodemographic and reproductive factors has not explained the racial gap in pregnancy-related mortality in most studies. For instance, in one study, adjustment for maternal age, income, hypertension, gestational age at delivery, and receipt of prenatal care only reduced odds ratios for pregnancy-related mortality from 3.07 (95% CI 2.0–4.54) to 2.65 (95% CI 1.73–4.07).19 Another study found the largest racial disparity among women with the lowest risk of pregnancy-related disease.3 Data suggest that a web of factors including higher prevalence of comorbidities and pregnancy complications, lower socioeconomic status, and less access to prenatal care, contribute to but do not fully explain the elevated rates of severe maternal morbidity and mortality among racial and ethnic minority women.
Delivery Hospital Quality and Disparities
Recent data suggests that a sizeable portion of racial and ethnic disparities in severe maternal morbidity and mortality may be explained by variation in hospital quality. Several recent investigations have found that racial and ethnic minority women deliver in different and lower quality hospitals than whites.23,35 Using national data, we found that 75% of black deliveries in the United States occurred in a quarter of hospitals, whereas only 18% of whites delivered in those same hospitals.35 Further, hospitals that disproportionately cared for black deliveries had higher risk-adjusted severe maternal morbidity rates for both black and white women in those hospitals.
In a series of studies we conducted using population-based data from New York City, we found that black women were more likely to deliver in hospitals with higher risk-adjusted severe maternal morbidity rates and that racial differences in the distribution of deliveries may contribute to the black/white disparities in severe maternal morbidity rates.23,29 Severe maternal morbidity rates varied 6-fold across New York City hospitals and the cumulative distribution of deliveries among hospitals ranked from lowest to highest morbidity rates differed for black and white mothers (p<.001) (See Figure 2).23
Figure 2.
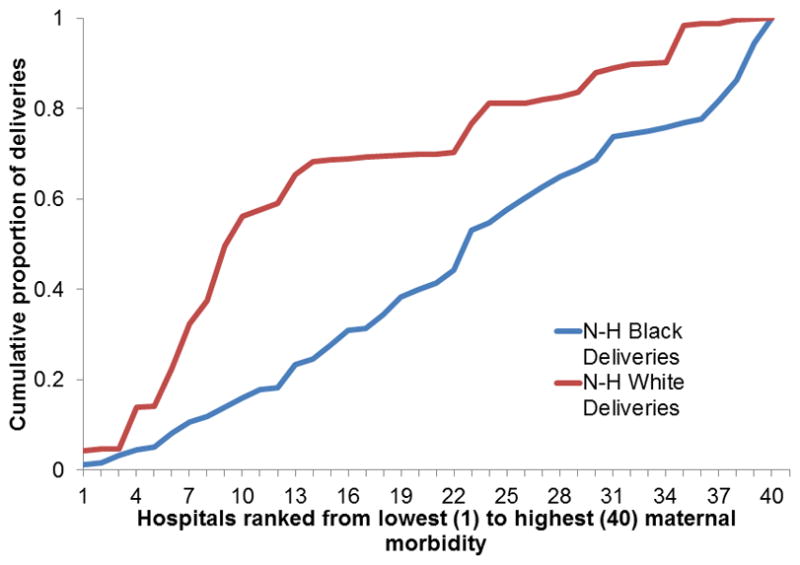
Cumulative distributions of deliveries according to hospital, ranked from lowest to highest morbidity ratio for black and white mothers
The majority of white deliveries (65.3%) occurred in the lowest tertile for severe morbidity compared with 23.3% of all black deliveries. We utilized a simulation method to quantify the impact of delivery location on the disparity and estimated that if black women delivered at the same hospitals as white women, nearly 1000 black women could avoid severe morbid events during their delivery hospitalizations which would reduce the black severe maternal morbidity rate from 4.2% to 2.9%. We conducted similar analyses for Hispanic versus white women in New York City.25 If Hispanic mothers went to hospitals in the same proportion as white mothers we estimate that 485 few morbid events would occur among Hispanic women in New York City and the Hispanic severe maternal morbidity rate would be reduced from 2.7% to 2.3%. Others have also found that black-serving hospitals performed worse than other hospitals on delivery-related indicators using data from seven states.36
The results in obstetrics are similar to a number of studies in other areas of medicine which have demonstrated that minorities receive care in different and lower quality hospitals than whites.24,37 Studies of acute myocardial infarction, stroke, and very low birthweight neonatal mortality, have shown that black patients tend to receive care in hospitals with higher mortality rates and lower rates of effective evidence-based medical treatments compared with white patients.37–39 These studies suggest that place matters and there is growing attention to improving quality of care during delivery hospitalization to reduce disparities in maternal mortality and morbidity. It also important to note that racial and ethnic disparities have been documented both between hospitals and within hospitals and improvements in quality of care may address both of these causes of disparities.
Antenatal Care and Disparities
Early and adequate prenatal care is thought to promote healthy pregnancies through screening and management of a woman’s risk factors and health conditions and to promote health behaviors during pregnancy. A number of studies have demonstrated an association between fewer prenatal visits and poorer pregnancy outcomes such as low birthweight, preterm birth, and infant mortality.40,41 The association of antenatal care with maternal outcomes is less established. However, data do suggest that no or few prenatal visits are associated with maternal death and severe maternal morbidity.19,23,25 Among pregnancy related deaths from 2011–2013 in the United States for whom onset of prenatal care was known, 24.5% had started their prenatal care in the 2nd or 3rd trimester and 8.5% had not received any care.2
Timing and receipt of prenatal care vary greatly by race and ethnicity. First trimester prenatal care initiation was highest for white and Asian women (79 % and 78% respectively), followed by multiple race and Hispanic women (71 and 69%, respectively), and was lowest for black, American Indian/Alaska Native, and Native Hawaiian/other Pacific Island women (64%, 59%, and 55% respectively) in 2012.42 Population based studies have documented that black and Hispanic women are nearly four times and two times respectively to receive 0–5 prenatal care visits as compared with white women.23,25 Insurance status, availability of transportation, and other social factors likely play a role in receipt and timing of prenatal care.
Antenatal access to maternal fetal medicine specialists and medicine subspecialists is recommended to improve outcomes among pregnant women with chronic illness and pregnancy-related complications.43–45 Studies have shown that the density of maternal fetal medicine specialists is significantly and inversely associated with maternal mortality ratios.46 Appropriate availability of specialized services beyond maternal fetal medicine specialist (e.g. cardiologists, infection disease specialists, etc.) and multidisciplinary care are also important for healthy pregnancies.47 Less frequent visits to a maternal fetal medicine specialists or indicated medicine subspecialists for women with chronic illness may results in adverse pregnancy outcomes,48 and many women do not see a subspecialist when one is necessary. Many women are not referred to a maternal fetal medicine specialists despite having chronic conditions.49 In a survey, 31% of generalist ob/gyns were not satisfied with the maternal fetal medicine services available to them for their patients.50 The extent of disparities in access to maternal fetal medicine specialists and indicated medicine subspecialists for high risk pregnant women remains unknown.
Delay of prenatal care initiation has been associated with endorsement of experiences of racism.51 Although experiences of racism, discrimination, and stress have been the focus of research on perinatal outcomes, few studies have examined their association with maternal health outcomes.52 Studies have shown black and American Indian/Alaska Native women report the highest number of stressful life events in the year before birth.53 Higher levels of self-reported and biologic measures of chronic stress have been reported for black versus white pregnant women.54 The Listening to Mothers III survey found that 40% of participants experienced communication issues, and nearly one quarter perceived discrimination during birth hospitalization.55 Black or Hispanic race/ethnicity was associated with nearly three times higher odds of discrimination due to race, language, or culture.55
Given the significant racial and ethnic disparities in timeliness and utilization of prenatal care and the fact that racial and ethnic minority women are more likely to have many chronic illnesses (including chronic stress) that place them at a higher risk of severe maternal morbidity and mortality, access to high quality antenatal care including availability of maternal fetal medicine specialists and medicine subspecialists for certain chronic illnesses is likely an important part of the pathways explaining disparities.
Preconception Care, Postpartum Care, and Disparities
The notion that improving preconception health can improve pregnancy outcomes by improving the overall health of women as well as reproductive planning is well accepted.56 The elevated rates of obesity, hypertension, diabetes, and chronic illness among racial and ethnic minority women and the strong link between these comorbidites and adverse maternal outcomes highlights the crucial need for a focus on women’s health before conception. Nearly one-half of pregnancies are unintended which underscores the need for a strong focus on contraception.
A recent study examining preconception risk factors associated with adverse birth outcomes (including obesity, at-risk drinking, smoking, diabetes, and frequent mental distress) found that the majority of women (52%) had at least one risk factor and nearly 20% had two or more risk factors.56 Further, significant racial/ethnic disparities in prevalence rates were documented with American Indian and Alaska Native women having the highest rates of four of five preconception risk factors (at-risk drinking, smoking, diabetes, and mental distress). Black women had the highest rates of obesity, and Hispanics had the second highest rates of diabetes. Two or more risk factors was also highest among women who were American Indian and Alaska Native. Clearly there is evidence that many of these same risk factors put pregnant women at risk for severe maternal morbidity and mortality. Assistance in addressing both risk factors and chronic illnesses prior to becoming pregnant is crucial if we aim to reduce disparities.
Unintended pregnancies have been linked to multiple adverse perinatal outcomes and significant racial/ethnic disparities exist in untended pregnancies rates with black and Hispanic women having higher unintended pregnancy rates as compared with white women.57 In fact black women have over twice the rate of unintended pregnancies as compared with white women. Though the association between unintended pregnancies with severe maternal morbidity and mortality is less studied, unintended pregnancies are associated with adverse perinatal outcomes including maternal depression.58
Postpartum care is important for monitoring the health of women with chronic illness and as a means to link vulnerable women with the health system. Postpartum follow-up within 7–10 days of delivery is recommended for patients with hypertensive disorders of pregnancy.59 Women with poor maternal outcomes are at increased risk for recurrence in their next pregnancy and are at increased risk of chronic illness in later life. For example, women with preeclampsia have an increased risk of cardiovascular disease,60 and women with gestational diabetes have a seven-fold increased risk of later Type 2 Diabetes,61 and an elevated risk of hypertension.62,63 Although the postpartum period presents an opportunity to intervene to improve this trajectory, the proportion of low-income women who attend a postpartum visit is low.64,65 No prenatal care and late entry into antepartum care are correlated with not attending the postpartum visit.66 Many of these women face barriers such as cost, transportation, childcare, psychological distress, communication with provider, and knowledge.67–69
Levers to Reduce Disparities
There is an urgent need to improve quality in obstetrics overall, as well as the care we deliver to racial and ethnic minority women.70 This can be done by improving quality across the care continuum. As black and other racial and ethnic minority women have worse access to preconception and prenatal care as well as deliver in lower quality hospitals, overall improvements are likely to yield high benefits for these groups. We must also implement interventions that specifically target racial and ethnic disparities in maternal health and healthcare. The Council on Patient Safety in Women’s Health Care and the Alliance for Innovation in Maternal Health (AIM Program) recently published the “Reduction of Peripartum Racial/Ethnic Disparities Patient Safety Bundle”. This bundle provides action steps that institutions and clinicians can implement in an effort to reduce disparities in maternal morbidity and mortality.13 The bundle considers steps across the care continuum.
Improving Delivery and Hospital Care Through Quality Initiatives
A significant proportion of severe maternal morbidity and mortality events are preventable. Provider factors such as inappropriate or delay in diagnosis or treatment and system factors such as communication failures and policies/procedures not in place or not followed are common preventability factors.71,72 Preventability of these events maybe higher among black than white women.71 In a study of maternal deaths, 46% of black and 33% of white maternal deaths were considered potentially preventable.71 Improved quality of care is often considered the most important factor in preventing these events.71,72
A number of quality initiatives aimed at standardizing delivery care will likely improve care at all hospitals and especially the lowest performing hospitals, which serve a disproportionate number of racial and ethnic minority women. The implementation of safety bundles (e.g. hemorrhage, venous thrombolic disease, hypertension) are important steps to improving care to women at all hospitals.73 Protocols, checklists, triggers (such as maternal early warning criteria), simulation trainings, the provision of coordinated care and crew resource management, team training, staff training, credentialing, and the promotion of a safety culture have all been recommended in recent reviews.73–77
Education and Communication about Disparities
As recommended in the AIM bundle as well as others, educating clinicians and staff about racial and ethnic disparities in maternal outcomes, the importance of shared-decision making, cultural competency, and implicit bias are important steps to address disparities in care. The results of a recent Society for Maternal Fetal Medicine survey demonstrated an inconsistency between providers’ willingness to acknowledge disparities in their practices and their consideration of implicit bias; 84% of respondents agreed that disparities impact their practice but only 29% believed personal biases affected how they care for patients.78 There are a number of tools about implicit bias and some studies show improvement in implicit bias with physician education and increased awareness of their biases.79
Another step to enhancing the experience of racial and ethnic minority women is staff education about effective communication skills. There are toolkits referenced by AIM that address these fundamental issues in clinical care.13 The only way to make progress in the reduction of disparities is by measuring them. Implementation of a disparities dashboard, which stratifies quality metrics by race and ethnicity, is a useful tool which allows hospitals and healthcare systems to become aware of disparities within their hospitals and to monitor their performance on quality metrics for groups with higher risks of poor outcomes.80 This should be accompanied by quality improvement activities to target disparities. The bundle also emphasizes the importance of building a culture of equity similar to the recent focus on a culture of safety. Multidisciplinary maternal mortality and severe maternal morbidity reviews are recommended. There is also growing evidence that programs that partner with communities may have a substantial impact on improving quality and reducing disparities.81
New Models of Antenatal Care
Prenatal care utilization is thought to be important for promoting healthy pregnancies but its association with maternal outcomes is not well established.52 There is a current trend toward patient-centered care in obstetrics and enhanced models of prenatal care are being evaluated by the Centers for Medicare and Medicaid Services (CMS) primarily with an interest in lowering preterm birth rates. Although there is insufficient evidence to determine whether any of the three models impact maternal outcomes and reduce disparities two of these approaches are of particular interest. One of the three enhanced models of prenatal care is CenteringPregnancy, a group prenatal care model, which has been implemented across the country. A randomized trial testing this model versus standard prenatal care among low-risk pregnant adolescents found a 33% lower preterm birth rate in the CenteringPregnancy group and these results were more pronounced among black versus white participants.82 However, these results were not duplicated in a 14 center cluster randomized trial recently.83 The impact of Centering on reducing disparities in severe maternal morbidity and mortality requires further study.
Another promising model is enhanced prenatal care at maternity care homes. The North Carolina Division of Health implemented a program called Pregnancy Medical Home for low-income pregnant women in 2011. The program provides case management for high-risk pregnant Medicaid patients. The program has received attention because of the declines in black maternal mortality rates in the state.84 CMS is this evaluating this model of prenatal care at a number of sites across the country.85
Enhanced prenatal care for high risk pregnant women may be an important addition. For example, the use of programs like CenteringPregnancy for pregnant women with hypertension or gestational diabetes might be a useful tool but additional study is required. In addition improving access to maternal fetal medicine specialists and medicine subspecialists is important for pregnant women with chronic illness and pregnancy-related complications. 43–45,86 A multidisciplinary approach during antenatal and delivery care is recommended for women with pregnancies complicated by preexisting medical or mental health conditions, and maybe especially important for racial and ethnic minority women who have higher prevalence rates of these conditions.59,87
Reinforcing Preconception and Postpartum Care
Preconception care is an important window to target disparities in maternal morbidity and mortality. Given the elevated rates of obesity, hypertension, diabetes, and chronic illness among racial and ethnic minority women and the strong link between these comorbidites and adverse maternal outcomes, a focus on preconception care is crucial. Rates of unintended pregnancies are higher among black women, and these pregnancies are associated with elevated risk of adverse outcomes. Contraception is an important strategy to reduce maternal mortality and morbidity especially for women with select medical conditions. For example pregnancy is not advised with certain high risk cardiac conditions which confer elevated risk of maternal mortality.59 Preconception care is important for optimizing clinical care and for giving women reproductive choice.88 Preconception counselling is important to identify maternal risk factors, such as obesity, hypertension, and tobacco use, to optimize clinical health prior to pregnancy, and to review use of medications in an effort to potentially avoid exposure to a teratogen during conception and pregnancy.88
There is growing recognition of the importance of postpartum care to improve the health of women during the inter-pregnancy interval and for long-term health. Significant racial/ethnic disparities in receipt of postpartum care are evidence and likely have implications for disparities in maternal health and the long term health of women. New models of postpartum care are being developed. Our team combined a delivery system and payment system reform in an effort to reduce disparities in high risk postpartum care. The program includes a social worker/care management component and payment system redesign. Preliminary results show improved rates of postpartum care and high satisfaction among low-income racial and ethnic minority women.65 Additional postpartum programs utilizing patient navigators have also shown promise for improved retention in postpartum care, contraception uptake, depression screening, and vaccination among low income minority women.86
Conclusion
Significant racial and ethnic disparities in maternal outcomes exist in the United States and there is an urgent need to reduce disparities. A growing body of research acknowledges the role that structural racism plays in generating these disparities. The complex nature of racial and ethnic disparities in severe maternal morbidity and mortality rates requires a multipronged approach to reduce their occurrence. A comprehensive approach to quality improvement throughout the care continuum (from preconception to postpartum and inter-pregnancy care) is required to reduce racial and ethnic disparities in severe maternal morbidity and mortality rates.
Acknowledgments
Funding/Support. This study was supported by the National Institute on Minority Health and Health Disparities under Award Number R01MD007651.
I thank Jennifer Zeitlin, DSc, MA for input on a draft of this manuscript (permission was granted for this acknowledgement).
Footnotes
Role of the Funder/Sponsor: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Callaghan WM. Overview of maternal mortality in the United States. Semin Perinatol. 2012 Feb;36(1):2–6. doi: 10.1053/j.semperi.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-Related Mortality in the United States, 2011–2013. Obstet Gynecol. 2017 Aug;130(2):366–373. doi: 10.1097/AOG.0000000000002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saftlas AF, Koonin LM, Atrash HK. Racial disparity in pregnancy-related mortality associated with livebirth: can established risk factors explain it? Am J Epidemiol. 2000 Sep 1;152(5):413–419. doi: 10.1093/aje/152.5.413. [DOI] [PubMed] [Google Scholar]
- 4.Singh GK. Maternal Mortality in the United States, 1935–2007: Substantial Racial/Ethnic, Socioeconomic, and Geographic Disparities Persist. [Accessed August 26, 2017];A 75th Anniversary Publication. 2010 https://www.hrsa.gov/ourstories/mchb75th/mchb75maternalmortality.pdf.
- 5.Moaddab A, Dildy GA, Brown HL, et al. Health Care Disparity and State-Specific Pregnancy-Related Mortality in the United States, 2005–2014. Obstet Gynecol. 2016 Oct;128(4):869–875. doi: 10.1097/AOG.0000000000001628. [DOI] [PubMed] [Google Scholar]
- 6.New York City Department of Health and Mental Hygiene; Bureau of Maternal and Child Health. Pregnancy-Associated Mortality New York City, 2006–2010. New York: 2015. [Google Scholar]
- 7.Hopkins FW, MacKay AP, Koonin LM, Berg CJ, Irwin M, Atrash HK. Pregnancy-related mortality in Hispanic women in the United States. Obstet Gynecol. 1999 Nov;94(5 Pt 1):747–752. doi: 10.1016/s0029-7844(99)00393-2. [DOI] [PubMed] [Google Scholar]
- 8.CDC. Pregnancy-related deaths among Hispanic, Asian/Pacific Islander, and American Indian/Alaska Native women--United States, 1991–1997. MMWR Morb Mortal Wkly Rep. 2001 May 11;50(18):361–364. [PubMed] [Google Scholar]
- 9.Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120(5):1029–1036. doi: 10.1097/aog.0b013e31826d60c5. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. [Accessed August 27, 2017];Severe Maternal Morbidity in the United States. 2017 http://www.cdc.gov/reproductivehealth/MaternalInfantHealth/SevereMaternalMorbidity.html.
- 11.Creanga AA, Bateman BT, Kuklina EV, Callaghan WM. Racial and ethnic disparities in severe maternal morbidity: a multistate analysis, 2008–2010. Am J Obstet Gynecol. 2014 May;210(5):435.e431–438. doi: 10.1016/j.ajog.2013.11.039. [DOI] [PubMed] [Google Scholar]
- 12.Gray KE, Wallace ER, Nelson KR, Reed SD, Schiff MA. Population-based study of risk factors for severe maternal morbidity. Paediatr Perinat Epidemiol. 2012 Nov;26(6):506–514. doi: 10.1111/ppe.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Council on Patient Safety in Women’s Health Care. Alliance for Innovation on Maternal Health (AIM) [Accessed August 26, 2017];Safe Health Care for Every Woman. 2015 http://www.safehealthcareforeverywoman.org/aim.php.
- 14.Braveman P. What are health disparities and health equity? We need to be clear. Public Health Reports. 2014;129(1_suppl2):5–8. doi: 10.1177/00333549141291S203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ACOG Committee Opinion No. 649: Racial and Ethnic Disparities in Obstetrics and Gynecology. Obstet Gynecol. 2015 Dec;126(6):e130–134. doi: 10.1097/AOG.0000000000001213. [DOI] [PubMed] [Google Scholar]
- 16.Warnecke RB, Oh A, Breen N, et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health. 2008 Sep;98(9):1608–1615. doi: 10.2105/AJPH.2006.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilbourne AM, Switzer G, Hyman K, Crowley-Matoka M, Fine MJ. Advancing Health Disparities Research Within the Health Care System: A Conceptual Framework. Am J Public Health. 2006 Dec 1;96(12):2113–2121. doi: 10.2105/AJPH.2005.077628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dominguez TP. Race, racism, and racial disparities in adverse birth outcomes. Clin Obstet Gynecol. 2008 Jun;51(2):360–370. doi: 10.1097/GRF.0b013e31816f28de. [DOI] [PubMed] [Google Scholar]
- 19.Harper MA, Espeland MA, Dugan E, Meyer R, Lane K, Williams S. Racial disparity in pregnancy-related mortality following a live birth outcome. Ann Epidemiol. 2004 Apr;14(4):274–279. doi: 10.1016/S1047-2797(03)00128-5. [DOI] [PubMed] [Google Scholar]
- 20.Tucker MJ, Berg CJ, Callaghan WM, Hsia J. The Black–White Disparity in Pregnancy-Related Mortality From 5 Conditions: Differences in Prevalence and Case-Fatality Rates. American Journal of Public Health. 2007 Feb 01;97(2):247–251. doi: 10.2105/AJPH.2005.072975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg D, Geller SE, Studee L, Cox SM. Disparities in Mortality Among High Risk Pregnant Women in Illinois: A Population Based Study. Annals of Epidemiology. 2006 Jan 01;16(1):26–32. doi: 10.1016/j.annepidem.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Shahul S, Tung A, Minhaj M, et al. Racial Disparities in Comorbidities, Complications, and Maternal and Fetal Outcomes in Women With Preeclampsia/eclampsia. Hypertension in Pregnancy. 2015 Oct 02;34(4):506–515. doi: 10.3109/10641955.2015.1090581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howell EA, Egorova NN, Balbierz A, Zeitlin J, Hebert PL. Site of delivery contribution to black-white severe maternal morbidity disparity. Am J Obstet Gynecol. 2016 Aug;215(2):143–152. doi: 10.1016/j.ajog.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howell EA, Hebert P, Chatterjee S, Kleinman LC, Chassin MR. Black/white differences in very low birth weight neonatal mortality rates among New York City hospitals. Pediatrics. 2008 Mar;121(3):e407–415. doi: 10.1542/peds.2007-0910. [DOI] [PubMed] [Google Scholar]
- 25.Howell EA, Egorova NN, Janevic T, Balbierz A, Zeitlin J, Hebert PL. Severe Maternal Morbidity Among Hispanic Women in New York City: Investigation of Health Disparities. Obstet Gynecol. 2017;129(2):285–294. doi: 10.1097/AOG.0000000000001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rathore SS, McMahon MJ. Racial variation in the frequency of intrapartum hemorrhage. Obstet Gynecol. 2001 Feb;97(2):178–183. doi: 10.1016/s0029-7844(00)01121-2. [DOI] [PubMed] [Google Scholar]
- 27.Bryant AS, Seely EW, Cohen A, Lieberman E. Patterns of pregnancy-related hypertension in black and white women. Hypertens Pregnancy. 2005;24(3):281–290. doi: 10.1080/10641950500281134. [DOI] [PubMed] [Google Scholar]
- 28.Carroll KN, Griffin MR, Gebretsadik T, Shintani A, Mitchel E, Hartert TV. Racial differences in asthma morbidity during pregnancy. Obstet Gynecol. 2005 Jul;106(1):66–72. doi: 10.1097/01.AOG.0000164471.87157.4c. [DOI] [PubMed] [Google Scholar]
- 29.Howell EA, Egorova N, Balbierz A, Zeitlin J, Hebert PL. Black-white differences in severe maternal morbidity and site of care. Am J Obstet Gynecol. 2016;214(1):122.e121–122.e127. doi: 10.1016/j.ajog.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guendelman S, Thornton D, Gould J, Hosang N. Obstetric complications during labor and delivery: assessing ethnic differences in California. Womens Health Issues. 2006 Jul-Aug;16(4):189–197. doi: 10.1016/j.whi.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Cabacungan ET, Ngui EM, McGinley EL. Racial/Ethnic Disparities in Maternal Morbidities: A Statewide Study of Labor and Delivery Hospitalizations in Wisconsin. Matern Child Health J. 2011 Nov 22; doi: 10.1007/s10995-011-0914-6. [DOI] [PubMed] [Google Scholar]
- 32.Beckie TM. Ethnic and racial disparities in hypertension management among women. Semin Perinatol. 2017 Jun 7; doi: 10.1053/j.semperi.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol. 2010 Dec;116(6):1302–1309. doi: 10.1097/AOG.0b013e3181fdfb11. [DOI] [PubMed] [Google Scholar]
- 34.Berg CJ, Chang J, Callaghan WM, Whitehead SJ. Pregnancy-related mortality in the United States, 1991–1997. Obstet Gynecol. 2003 Feb;101(2):289–296. doi: 10.1016/s0029-7844(02)02587-5. [DOI] [PubMed] [Google Scholar]
- 35.Howell EA, Egorova N, Balbierz A, Zeitlin J, Hebert PL. Black-white differences in severe maternal morbidity and site of care. Am J Obstet Gynecol. 2016 Jan;214(1):122.e121–127. doi: 10.1016/j.ajog.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Creanga AA, Bateman BT, Mhyre JM, Kuklina E, Shilkrut A, Callaghan WM. Performance of racial and ethnic minority-serving hospitals on delivery-related indicators. Am J Obstet Gynecol. 2014 Jun 5; doi: 10.1016/j.ajog.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Morales LS, Staiger D, Horbar JD, et al. Mortality among very low-birthweight infants in hospitals serving minority populations. Am J Public Health. 2005 Dec;95(12):2206–2212. doi: 10.2105/AJPH.2004.046730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnato AE, Lucas FL, Staiger D, Wennberg DE, Chandra A. Hospital-level racial disparities in acute myocardial infarction treatment and outcomes. Med Care. 2005 Apr;43(4):308–319. doi: 10.1097/01.mlr.0000156848.62086.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stansbury JP, Jia H, Williams LS, Vogel WB, Duncan PW. Ethnic disparities in stroke: epidemiology, acute care, and postacute outcomes. Stroke. 2005 Feb;36(2):374–386. doi: 10.1161/01.STR.0000153065.39325.fd. [DOI] [PubMed] [Google Scholar]
- 40.Cox RG, Zhang L, Zotti ME, Graham J. Prenatal care utilization in Mississippi: racial disparities and implications for unfavorable birth outcomes. Matern Child Health J. 2011 Oct;15(7):931–942. doi: 10.1007/s10995-009-0542-6. [DOI] [PubMed] [Google Scholar]
- 41.Till SR, Everetts D, Haas DM. Incentives for increasing prenatal care use by women in order to improve maternal and neonatal outcomes. Cochrane Database Syst Rev. 2015 Dec 15;(12):CD009916. doi: 10.1002/14651858.CD009916.pub2. [DOI] [PMC free article] [PubMed]
- 42.U.S. Department of Health and Human Services, Health Resources and Services Administration, Maternal and Child Health Bureau. [Accessed August 26, 2017];Child Health USA. 2014 https://mchb.hrsa.gov/chusa14/health-services-financing-utilization/prenatal-care.html. 2017.
- 42.Antony KM, Dildy GA. Postpartum hemorrhage: The role of the Maternal–Fetal Medicine specialist in enhancing quality and patient safety. Semin Perinatol. 2013 Aug 01;37(4):246–256. doi: 10.1053/j.semperi.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Safi LM, Tsiaras SV. Update on Valvular Heart Disease in Pregnancy. Current Treatment Options in Cardiovascular Medicine. 2017 Aug 05;19(9):70. doi: 10.1007/s11936-017-0570-2. [DOI] [PubMed] [Google Scholar]
- 45.D’Alton ME, Bonanno CA, Berkowitz RL, et al. Putting the “M” back in maternal–fetal medicine. Am J Obstet Gynecol. 2013 Jun 01;208(6):442–448. doi: 10.1016/j.ajog.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan SA, Hill EG, Newman RB, Menard MK. Maternal-fetal medicine specialist density is inversely associated with maternal mortality ratios. Am J Obstet Gynecol. 2005 Sep 01;193(3):1083–1088. doi: 10.1016/j.ajog.2005.05.085. [DOI] [PubMed] [Google Scholar]
- 47.Russo M, Krenz EI, Hart SR, Kirsch D. Multidisciplinary Approach to the Management of Placenta Accreta. The Ochsner Journal. 2011 Spring;11(1):84–88. [PMC free article] [PubMed] [Google Scholar]
- 48.Eden RD, Penka A, Britt DW, Landsberger EJ, Evans MI. Re-evaluating the role of the MFM specialist: Lead, follow, or get out of the way. The Journal of Maternal-Fetal & Neonatal Medicine. 2005 Jan 01;18(4):253–258. doi: 10.1080/14767050500246292. [DOI] [PubMed] [Google Scholar]
- 49.Vintzileos AM, Ananth CV, Smulian JC, Scorza WE, Knuppel RA. Defining the relationship between obstetricians and maternal-fetal medicine specialists. Am J Obstet Gynecol. 2001 Oct 01;185(4):925–930. doi: 10.1067/mob.2001.117348. [DOI] [PubMed] [Google Scholar]
- 50.Wenstrom K, Erickson K, Schulkin J. Are obstetrician-gynecologists satisfied with their maternal-fetal medicine consultants? A survey. Am J Perinatol. 2012 Sep;29(8):599–608. doi: 10.1055/s-0032-1311984. [DOI] [PubMed] [Google Scholar]
- 51.Gadson A, Akpovi E, Mehta PK. Exploring the social determinants of racial/ethnic disparities in prenatal care utilization and maternal outcome. Semin Perinatol. 2017 doi: 10.1053/j.semperi.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 52.Lu MC, Chen B. Racial and ethnic disparities in preterm birth: the role of stressful life events. Am J Obstet Gynecol. 2004 Sep;191(3):691–699. doi: 10.1016/j.ajog.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 53.Borders AE, Wolfe K, Qadir S, Kim KY, Holl J, Grobman W. Racial/ethnic differences in self-reported and biologic measures of chronic stress in pregnancy. J Perinatol. 2015 Aug;35(8):580–584. doi: 10.1038/jp.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Attanasio L, Kozhimannil KB. Patient-reported Communication Quality and Perceived Discrimination in Maternity Care. Med Care. 2015 Oct;53(10):863–871. doi: 10.1097/MLR.0000000000000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slaughter-Acey JC, Caldwell CH, Misra DP. The Influence of Personal and Group Racism on Entry Into Prenatal Care Among-African American Women. Women’s Health Issues. 2013 Nov 01;23(6):e381–e387. doi: 10.1016/j.whi.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Denny CH, Floyd RL, Green PP, Hayes DK. Racial and ethnic disparities in preconception risk factors and preconception care. J Womens Health (Larchmt) 2012 Jul;21(7):720–729. doi: 10.1089/jwh.2011.3259. [DOI] [PubMed] [Google Scholar]
- 57.Finer LB, Zolna MR. Declines in Unintended Pregnancy in the United States, 2008–2011. N Engl J Med. 2016 Mar 03;374(9):843–852. doi: 10.1056/NEJMsa1506575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abajobir AA, Maravilla JC, Alati R, Najman JM. A systematic review and meta-analysis of the association between unintended pregnancy and perinatal depression. Journal of Affective Disorders. 2016 Mar 01;192:56–63. doi: 10.1016/j.jad.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 59.Abir G, Mhyre J. Maternal mortality and the role of the obstetric anesthesiologist. Best Practice & Research Clinical Anaesthesiology. 2017 Mar 01;31(1):91–105. doi: 10.1016/j.bpa.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 60.Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V. Preeclampsia and Future Cardiovascular Risk among Women: A Review. J Am Coll Cardiol. 2014 Feb 21; doi: 10.1016/j.jacc.2014.02.529. [DOI] [PubMed] [Google Scholar]
- 61.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009 May 23;373(9677):1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 62.Bentley-Lewis R, Powe C, Ankers E, Wenger J, Ecker J, Thadhani R. Effect of race/ethnicity on hypertension risk subsequent to gestational diabetes mellitus. Am J Cardiol. 2014 Apr 15;113(8):1364–1370. doi: 10.1016/j.amjcard.2014.01.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tobias DK, Hu FB, Forman JP, Chavarro J, Zhang C. Increased risk of hypertension after gestational diabetes mellitus: findings from a large prospective cohort study. Diabetes Care. 2011 Jul;34(7):1582–1584. doi: 10.2337/dc11-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Bocanegra HT, Braughton M, Bradsberry M, Howell M, Logan J, Schwarz EB. Racial and ethnic disparities in postpartum care and contraception in California’s Medicaid program. Am J Obstet Gynecol. 2017 doi: 10.1016/j.ajog.2017.02.040. [DOI] [PubMed] [Google Scholar]
- 65.Howell EA, Padrón NA, Beane SJ, et al. Delivery and Payment Redesign to Reduce Disparities in High Risk Postpartum Care. Maternal and Child Health Journal. 2017 Mar 01;21(3):432–438. doi: 10.1007/s10995-016-2221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Siddiqui R, Bell T, Sangi-Haghpeykar H, Minard C, Levison J. Predictive factors for loss to postpartum follow-up among low income HIV-infected women in Texas. AIDS Patient Care STDS. 2014 May;28(5):248–253. doi: 10.1089/apc.2013.0321. [DOI] [PubMed] [Google Scholar]
- 67.DiBari JN, Yu SM, Chao SM, Lu MC. Use of postpartum care: predictors and barriers. Journal of pregnancy. 2014 doi: 10.1155/2014/530769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Henderson V, Stumbras K, Caskey R, Haider S, Rankin K, Handler A. Understanding factors associated with postpartum visit attendance and contraception choices: Listening to low-income postpartum women and health care providers. Maternal and child health journal. 2016;20(1):132–143. doi: 10.1007/s10995-016-2044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bryant AS, Haas JS, McElrath TF, McCormick MC. Predictors of compliance with the postpartum visit among women living in healthy start project areas. Maternal and Child Health Journal. 2006;10(6):511–516. doi: 10.1007/s10995-006-0128-5. [DOI] [PubMed] [Google Scholar]
- 70.Eichelberger KY, Doll K, Ekpo GE, Zerden ML. Black Lives Matter: Claiming a Space for Evidence-Based Outrage in Obstetrics and Gynecology. Am J Public Health. 2016 Oct;106(10):1771–1772. doi: 10.2105/AJPH.2016.303313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berg CJ, Harper MA, Atkinson SM, et al. Preventability of pregnancy-related deaths: results of a state-wide review. Obstet Gynecol. 2005 Dec;106(6):1228–1234. doi: 10.1097/01.AOG.0000187894.71913.e8. [DOI] [PubMed] [Google Scholar]
- 72.Lawton B, MacDonald EJ, Brown SA, et al. Preventability of severe acute maternal morbidity. Am J Obstet Gynecol. 2014 Jun;210(6):557.e551–556. doi: 10.1016/j.ajog.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 73.Arora KS, Shields LE, Grobman WA, D’Alton ME, Lappen JR, Mercer BM. Triggers, Bundles, Protocols, and Checklists - What Every Maternal Care Provider Needs to Know. Am J Obstet Gynecol. 2015 Oct 15; doi: 10.1016/j.ajog.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 74.Mhyre JM, D’Oria R, Hameed AB, et al. The maternal early warning criteria: a proposal from the national partnership for maternal safety. J Obstet Gynecol Neonatal Nurs. 2014 Nov-Dec;43(6):771–779. doi: 10.1111/1552-6909.12504. [DOI] [PubMed] [Google Scholar]
- 75.Clark SL. Strategies for reducing maternal mortality. Semin Perinatol. 2012 Feb;36(1):42–47. doi: 10.1053/j.semperi.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 76.Lappen JR, Seidman D, Burke C, Goetz K, Grobman WA. Changes in care associated with the introduction of a postpartum hemorrhage patient safety program. Am J Perinatol. 2013 Nov;30(10):833–838. doi: 10.1055/s-0033-1333674. [DOI] [PubMed] [Google Scholar]
- 77.Pettker CM, Grobman WA. Obstetric Safety and Quality. Obstet Gynecol. 2015 Jul;126(1):196–206. doi: 10.1097/AOG.0000000000000918. [DOI] [PubMed] [Google Scholar]
- 78.Jain J, Moroz L. Strategies to reduce disparities in maternal morbidity and mortality: Patient and provider education. Semin Perinatol. 2017 Jun 07; doi: 10.1053/j.semperi.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 79.Wheeler SM, Bryant AS. Racial and Ethnic Disparities in Health and Health Care. Obstet Gynecol Clin North Am. 2017 Mar;44(1):1–11. doi: 10.1016/j.ogc.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 80.Mass General Hospital Institute for Health Policy. [Accessed November 1, 2016];Improving Quality and Achievning Equity: A Guide for Hospital Leaders. http://www2.massgeneral.org/disparitiessolutions/z_files/disparities%20leadership%20guide_final.pdf.
- 81.Peek ME, Ferguson M, Bergeron N, Maltby D, Chin MH. Integrated community-healthcare diabetes interventions to reduce disparities. Curr Diab Rep. 2014 Mar;14(3):467. doi: 10.1007/s11892-013-0467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes: a randomized controlled trial. Obstet Gynecol. 2007 Aug;110(2 Pt 1):330–339. doi: 10.1097/01.AOG.0000275284.24298.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ickovics JR, Earnshaw V, Lewis JB, et al. Cluster Randomized Controlled Trial of Group Prenatal Care: Perinatal Outcomes Among Adolescents in New York City Health Centers. Am J Public Health. 2016 Feb;106(2):359–365. doi: 10.2105/AJPH.2015.302960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Belluz J. Black moms die in childbirth 3 times as often as white moms. [Accessed August 27, 2017];Except in North Carolina. 2017 https://www.vox.com/health-care/2017/7/3/15886892/black-white-moms-die-childbirth-north-carolina-less.
- 85.Center for Medicare and Medicaid Services. [Accessed August 28, 2017];Strong Start for Mothers and Newborns Initiative: Enhanced Prenatal Care Models. 2017 https://innovation.cms.gov/initiatives/Strong-Start-Strategy-2/
- 86.Yee LM, Martinez NG, Nguyen AT, Hajjar N, Chen MJ, Simon MA. Using a Patient Navigator to Improve Postpartum Care in an Urban Women’s Health Clinic. Obstet Gynecol. 2017 May;129(5):925–933. doi: 10.1097/AOG.0000000000001977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Obstetric Care Consensus No. 2: Levels of Maternal Care. Obstet Gynecol. 2015 Feb;125(2):502–515. doi: 10.1097/01.AOG.0000460770.99574.9f. [DOI] [PubMed] [Google Scholar]
- 88.Clapp MA, Bernstein SN. Preconception Counseling for Women With Cardiac Disease. Current Treatment Options in Cardiovascular Medicine. 2017 Aug 05;19(9):67. doi: 10.1007/s11936-017-0565-z. [DOI] [PubMed] [Google Scholar]


